Radioiodide Sorption on Natural and Acid-Treated Zeolite
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Effect of Acid Treatment on the Composition and Properties of Zeolite
3.1.1. Iodide Sorption
3.1.2. pH Dependence of Iodide Sorption
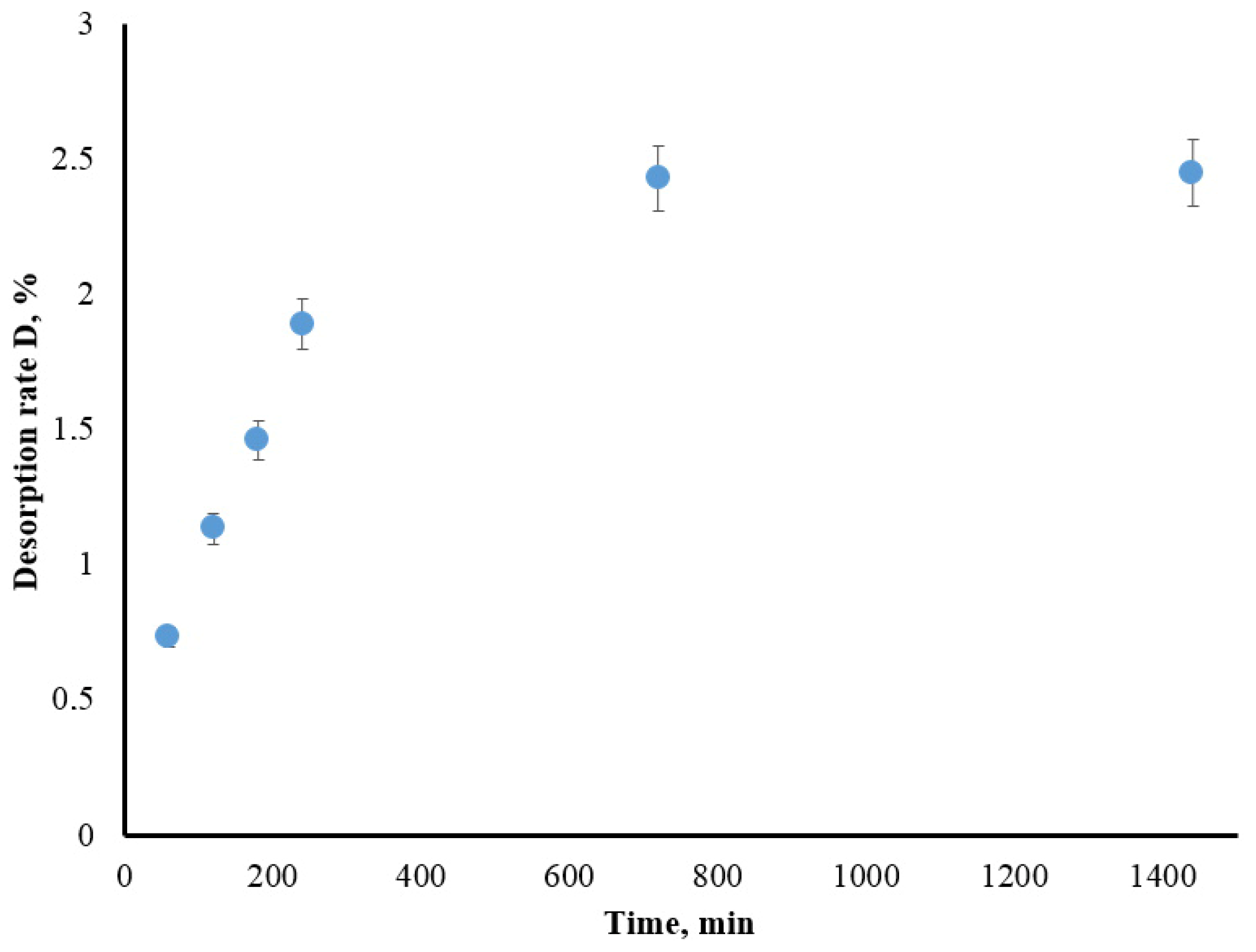
3.1.3. Iodide Desorption
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gottardi, G.; Galli, E. Natural Zeolites; Minerals and Rocks Series; Cambridge University Press: Cambridge, UK, 1985; Volume 18, p. 409. [Google Scholar] [CrossRef]
- Marantos, I.; Christidis, G.; Ulmanu, M. Zeolite Formation and Deposits. In Natural Zeolites Handbook; Bentham Science Publishers: Sharjah, United Arab Emirates, 2011; pp. 19–36. [Google Scholar]
- Ming, D.W.; Boettinger, J.L. Zeolites in Soil Environmental; De Gruyter: Berlin, Germany; Boston, MA, USA, 2001; pp. 323–346. [Google Scholar] [CrossRef]
- Belousov, P.; Kailachakov, P.; Rumyantseva, A. Zeolite Mineral Resource Base of Russia. Georesursy—Georesources 2024, 26, 260–274. [Google Scholar] [CrossRef]
- Burris, L.E.; Juenger, M.C.G. The effect of acid treatment on the reactivity of natural zeolites used as supplementary cementitious materials. Cem. Concr. Res. 2016, 79, 185–193. [Google Scholar] [CrossRef]
- Jeong, J.M.; Park, J.H.; Baek, J.H.; Hwang, R.H.; Jeon, S.G.; Yi, K.B. Effect of acid treatment of Fe-BEA zeolite on catalytic N2O conversion. Korean J. Chem. Eng. 2016, 34, 81–86. [Google Scholar] [CrossRef]
- Penafiel, M.E.; Jara-Cobos, L.; Flores, D.; Jerves, C.; Menendez, M. Enhancing adsorptive removal of diclofenac from aqueous solution: Evaluating organic and inorganic acid treatment of zeolite. Case Stud. Chem. Environ. Eng. 2024, 9, 100575. [Google Scholar] [CrossRef]
- Silva, M.; Lecus, A.; Lin, Y.; Corrao, J. Tailoring Natural Zeolites by Acid Treatments. J. Mater. Sci. Chem. Eng. 2019, 7, 26–37. [Google Scholar] [CrossRef]
- Zaiku, X.; Qingling, C.; Chengfang, Z.; Jiaqing, B.; Yuhua, C. Influence of Citric Acid Treatment on the Surface Acid Properties of Zeolite Beta. J. Phys. Chem. B 2000, 104, 2853–2859. [Google Scholar] [CrossRef]
- Velichkina, L.; Barbashin, Y.; Vosmerikov, A. Effect of Acid Treatment on the Properties of Zeolite Catalyst for Straight-Run Gasoline Upgrading. Catal. Res. 2021, 1, 16. [Google Scholar] [CrossRef]
- Side, S.; Putri, S.E.; Pratiwi, D.E.; Rahma, A.; Rahman, A. The Effect of Acid Treatment on The Characteristics of Modernite Zeolite. J. Sainsmat 2023, 12, 114–123. [Google Scholar] [CrossRef]
- Sulikowski, B. Dehydroxylation and catalytic activity of dealuminated y zeolites. React. Kinet. Catal. Lett. 1981, 16, 39–42. [Google Scholar] [CrossRef]
- Al-Nayili, A.; Albdiry, M.; Salman, N. Dealumination of Zeolite Frameworks and Lewis Acid Catalyst Activation for Transfer Hydrogenation. Arab. J. Sci. Eng. 2021, 46, 5709–5716. [Google Scholar] [CrossRef]
- Babic, V.; Koneti, S.; Moldovan, S.; Debost, M.; Gilson, J.-P.; Valtchev, V. Chromic acid dealumination of zeolites. Microporous Mesoporous Mater. 2022, 329, 111513. [Google Scholar] [CrossRef]
- Yoshioka, T.; Iyoki, K.; Yanaba, Y.; Okubo, T.; Wakihara, T. Dealumination of RHO zeolite by acid treatment and recrystallization with organic pore filler. J. Ceram. Soc. Jpn. 2024, 132, 45–49. [Google Scholar] [CrossRef]
- Belousov, P.; Semenkova, A.; Egorova, T.; Romanchuk, A.; Zakusin, S.; Dorzhieva, O.; Tyupina, E.; Izosimova, Y.; Tolpeshta, I.; Chernov, M.; et al. Cesium Sorption and Desorption on Glauconite, Bentonite, Zeolite, and Diatomite. Minerals 2019, 9, 625. [Google Scholar] [CrossRef]
- Milyutin, V.; Nekrasova, N.; Belousov, P.; Krupskaya, V.V. Sorption of Radionuclides 137Cs, 90Sr, and 233U on Various Natural Sorbents. Radiochemistry 2021, 63, 741–746. [Google Scholar] [CrossRef]
- Schroder, K.-P.; Sauer, J.; Leslie, M.; Richard, C.; Catlow, A.; Thomas, J.M. Bridging hydroxyl roups in zeolitic catalysts: A computer simulation of their structure, vibrational properties and acidity in protonated faujasites (H-Y zeolites). Chem. Phys. Lett. 1992, 188, 320–325. [Google Scholar] [CrossRef]
- Hosseinpour, E.; Rahbar-Kelishami, A.; Nabavi, M.S. Evaluation of alkaline and acidic modification of NaY zeolite for enhancing adsorptive removal of diclofenac sodium from aqueous solution. Surf. Interfaces 2023, 39, 102917. [Google Scholar] [CrossRef]
- Tsitsishvili, V.; Panayotova, M.; Mirdzveli, N.; Dzhakipbekova, N.; Panayotov, V.; Dolaberidze, N.; Nijaradze, M. Acid Resistance and Ion-Exchange Capacity of Natural Mixtures of Heulandite and Chabazite. Minerals 2023, 13, 364. [Google Scholar] [CrossRef]
- Wang, C.; Leng, S.; Guo, H.; Cao, L.; Huang, J. Acid and alkali treatments for regulation of hydrophilicity/hydrophobicity of natural zeolite. Appl. Surf. Sci. 2019, 478, 319–326. [Google Scholar] [CrossRef]
- Wang, C.; Leng, S.; Guo, H.; Yu, J.; Li, W.; Cao, L.; Huang, J. Quantitative arrangement of Si/Al ratio of natural zeolite using acid treatment. Appl. Surf. Sci. 2019, 498, 143874. [Google Scholar] [CrossRef]
- Barczyk, K.; Mozgawa, W.; Król, M. Studies of anions sorption on natural zeolites. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 133, 876–882. [Google Scholar] [CrossRef]
- Liu, S.; Ding, Y.; Li, P.; Diao, K.; Tan, X.; Lei, F.; Zhan, Y.; Li, Q.; Huang, B.; Huang, Z. Adsorption of the anionic dye Congo red from aqueous solution onto natural zeolites modified with N,N-dimethyl dehydroabietylamine oxide. Chem. Eng. J. 2014, 248, 135–144. [Google Scholar] [CrossRef]
- Radoor, S.; Karayil, J.; Parameswaranpillai1, J.; Siengchin, S. Removal of anionic dye congo red from aqueous environment using polyvinyl alcohol/sodium alginate/ZSM-5 zeolite membrane. Sci. Rep. 2020, 1, 15452. [Google Scholar] [CrossRef] [PubMed]
- Messaoudene, A.; Cheikh, S.; Hadadi, A.; Hamri, N.; Bollinger, J.-C.; Amrane, A.; Tahraoui, H.; Manseri, A.; Mouni, L. Adsorption Performance of Zeolite for the Removal of Congo Red Dye: Factorial Design Experiments, Kinetic, and Equilibrium Studies. Separations 2023, 10, 57. [Google Scholar] [CrossRef]
- Magomedbekov, E.P.; Merkushkin, A.O.; Obruchikov, A.V.; Pokalchuk, V.S. Trapping of Noble Radioactive Gases and their Decay Products under Static and Dynamic Conditions by Highly Porous Materials. Radioact. Waste 2021, 4, 33–37. [Google Scholar] [CrossRef]
- Obruchikov, A.; Merkushkin, A.; Magomedbekov, E.; Anurova, O. Radioiodide removal from air streams with impregnated UVIS® carbon fiber. Nucl. Eng. Technol. 2021, 53, 1717–1722. [Google Scholar] [CrossRef]
- Tyupina, E.; Pryadko, A. Bentonite-based sorbent modified with silver chloride with precipitation technique for capturing anionic radioiodide species. Sorpt. Chromatogr. Process. 2023, 23, 74–85. [Google Scholar] [CrossRef]
- Tyupina, E.; Pryadko, A. Use of silver-containing sorbents in anionic species of radioactive iodide management in nuclear industry and the methods of obtaining them. J. Radioanal. Nucl. Chem. 2024, 333, 599–613. [Google Scholar] [CrossRef]
- Blokhin, P.; Bogatov, S.; Boldyrev, K.; Sobolev, D. Sorption Influen e on Iodide-129 Release from the DGR’S Near Field. Radioact. Waste 2024, 1, 57–68. [Google Scholar] [CrossRef]
- Moore, D.M.; Reynolds, R.C., Jr. X-ray Diffraction and the Identification and Analysis of Clay Minerals, 2nd ed.; Oxford University Press: Oxford, UK; New York, NY, USA, 1999; p. 378. [Google Scholar]
- Post, J.E.; Bish, D.L. Rietveld refinement of crystal structures using powder X-ray diffraction data. Rev. Miner. Geochem. 1989, 20, 277–308. [Google Scholar]
- Doebelin, N.; Kleeberg, R. Profex: A graphical user interface for the Rietveld refinement program BGMN. J. Appl. Cryst. 2015, 48, 1573–1580. [Google Scholar] [CrossRef]
- Tucker, B.M. Laboratory Procedures for Cation Exchange Measurement on Soils. Available online: https://trid.trb.org/view/37268 (accessed on 15 February 2019).
- Eiler, R.K. Chemistry of Silica; MIR: Moscow, Russia, 1982; Volume 1, 416p. [Google Scholar]
- Malyavsky, N.I.; Pokidko, B.V. Workshop on Colloid Chemistry. In Method of Colorimetric Molybdate Analysis; IPC MITHT: Moscow, Russia, 2011; 36p. [Google Scholar]
- Komadel, P.; Schmidt, D.; Madejová, B. Alteration of smectites by treatments with hydrochloric acid and sodium carbonate solutions. Appl. Clay Sci. 1990, 5, 113–122. [Google Scholar] [CrossRef]
- Komadel, P. Structure and chemical characteristics of modified clays. In Natural Microporous Materials in Environmental Technology; Springer: Dordrecht, The Netherlands, 1999; pp. 3–18. [Google Scholar]
- He, H.; Guo, J.; Xie, X.; Lin, H.; Li, L. A microstructural study of acid-activated montmorillonite from Choushan, China. Clay Miner. 2002, 37, 337–344. [Google Scholar] [CrossRef]
- Foldvari, M. Handbook of Thermogravimetric System of Minerals and Its Use in Geological Practice; Geological Institute of Hungary: Budapest, Hungary, 2011; p. 180. [Google Scholar]



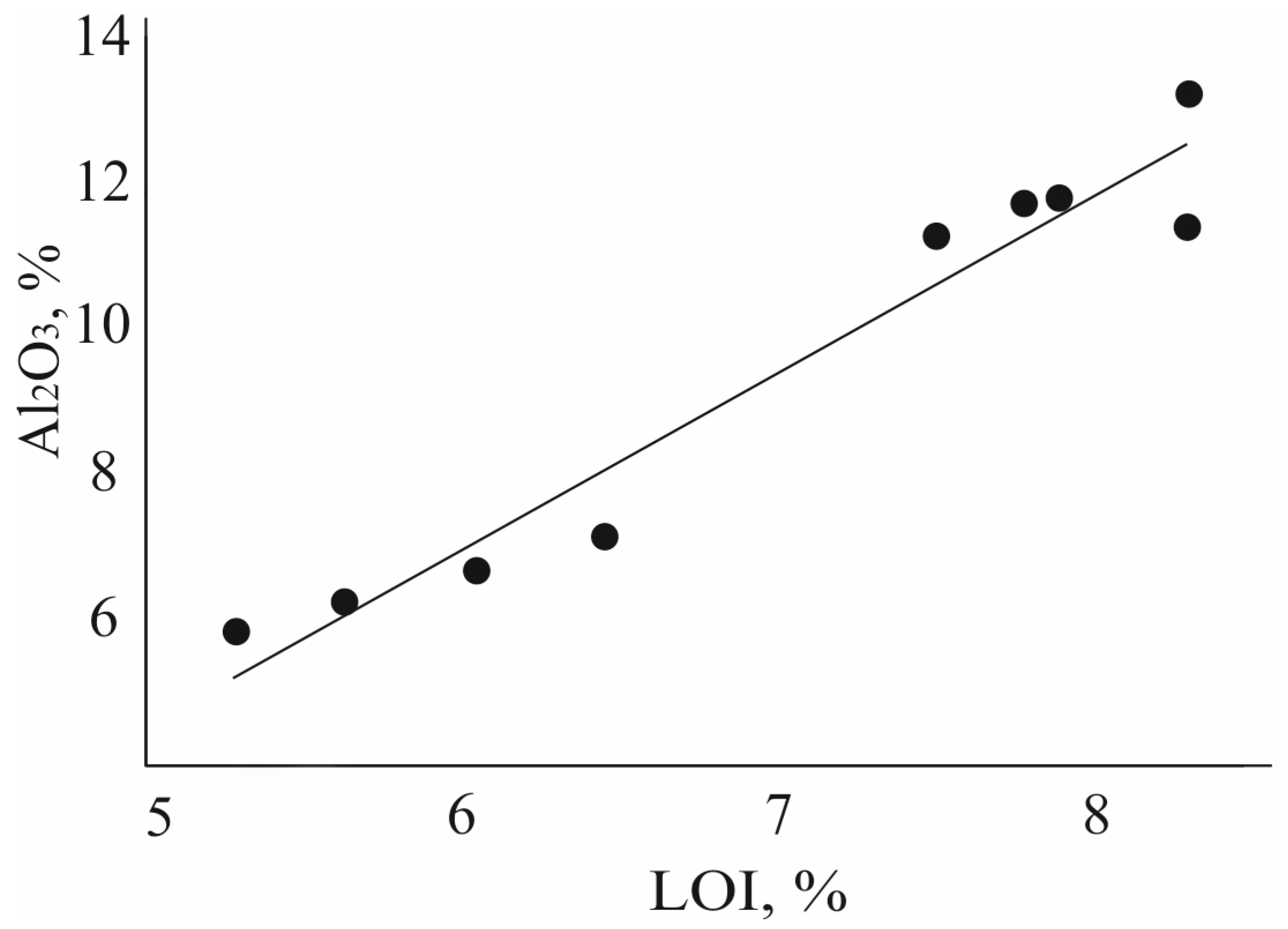

| Sample | LOI * | Na2O | MgO | Al2O3 | SiO2 | K2O | CaO | TiO2 | MnO | Fe2O3 |
|---|---|---|---|---|---|---|---|---|---|---|
| Z0 | 8.56 | 1.45 | 0.75 | 13.14 | 68.62 | 3.35 | 2.38 | 0.21 | 0.04 | 1.50 |
| Z1M20 | 7.42 | 0.47 | 0.70 | 11.72 | 73.59 | 2.24 | 2.08 | 0.17 | 0.03 | 1.40 |
| Z2M20 | 6.49 | 0.48 | 0.62 | 11.73 | 74.82 | 2.06 | 2.10 | 0.17 | 0.02 | 1.34 |
| Z4M20 | 5.79 | 0.45 | 0.65 | 11.37 | 75.88 | 2.03 | 2.04 | 0.19 | 0.02 | 1.41 |
| Z8M20 | 7.25 | 0.41 | 0.60 | 11.25 | 74.92 | 1.87 | 1.98 | 0.18 | 0.02 | 1.36 |
| Z1M90 | 5.25 | 0.37 | 0.30 | 7.15 | 83.26 | 1.95 | 0.55 | 0.19 | 0.01 | 0.94 |
| Z2M90 | 4.40 | 0.35 | 0.26 | 6.66 | 84.91 | 1.88 | 0.51 | 0.18 | 0.01 | 0.81 |
| Z4M90 | 4.64 | 0.37 | 0.21 | 6.23 | 85.41 | 1.77 | 0.46 | 0.18 | 0.01 | 0.68 |
| Z8M90 | 4.58 | 0.41 | 0.18 | 5.83 | 86.01 | 1.72 | 0.43 | 0.17 | 0.01 | 0.60 |
| Z8M90 after alkaline leaching | 6.93 | 0.92 | 0.16 | 10.45 | 78.93 | 1.5 | 0.41 | 0.17 | 0.01 | 0.52 |
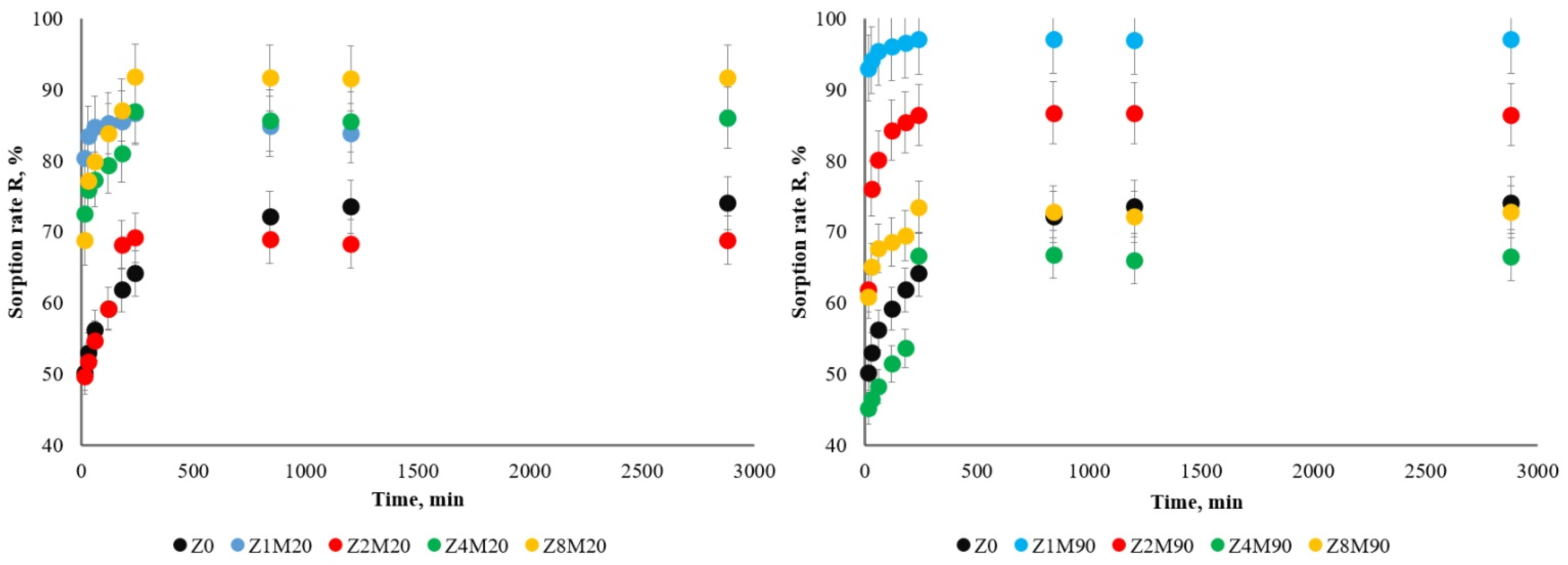
| Sample | R, % | Kd, mg/L |
|---|---|---|
| Z0 | 73.25 | 428 |
| Z1M20 | 84.92 | 821 |
| Z2M20 | 68.84 | 223 |
| Z4M20 | 86.00 | 619 |
| Z8M20 | 91.68 | 1110 |
| Z1M90 | 97.04 | 3254 |
| Z2M90 | 86.63 | 931 |
| Z4M90 | 66.50 | 200 |
| Z8M90 | 72.80 | 270 |
| Sample | pHinitial | R, % | Kd, mL/g |
|---|---|---|---|
| Z1M90 pH—2 | 2.26 | 99.81 | 52,600 |
| Z1M90 pH—4 | 4.24 | 99.35 | 15,337 |
| Z1M90 pH—6 | 6.35 | 97.04 | 3254 |
| Z1M90 pH—8 | 8.20 | 88.48 | 768 |
| Z1M90 pH—10 | 10.15 | 84.13 | 530 |
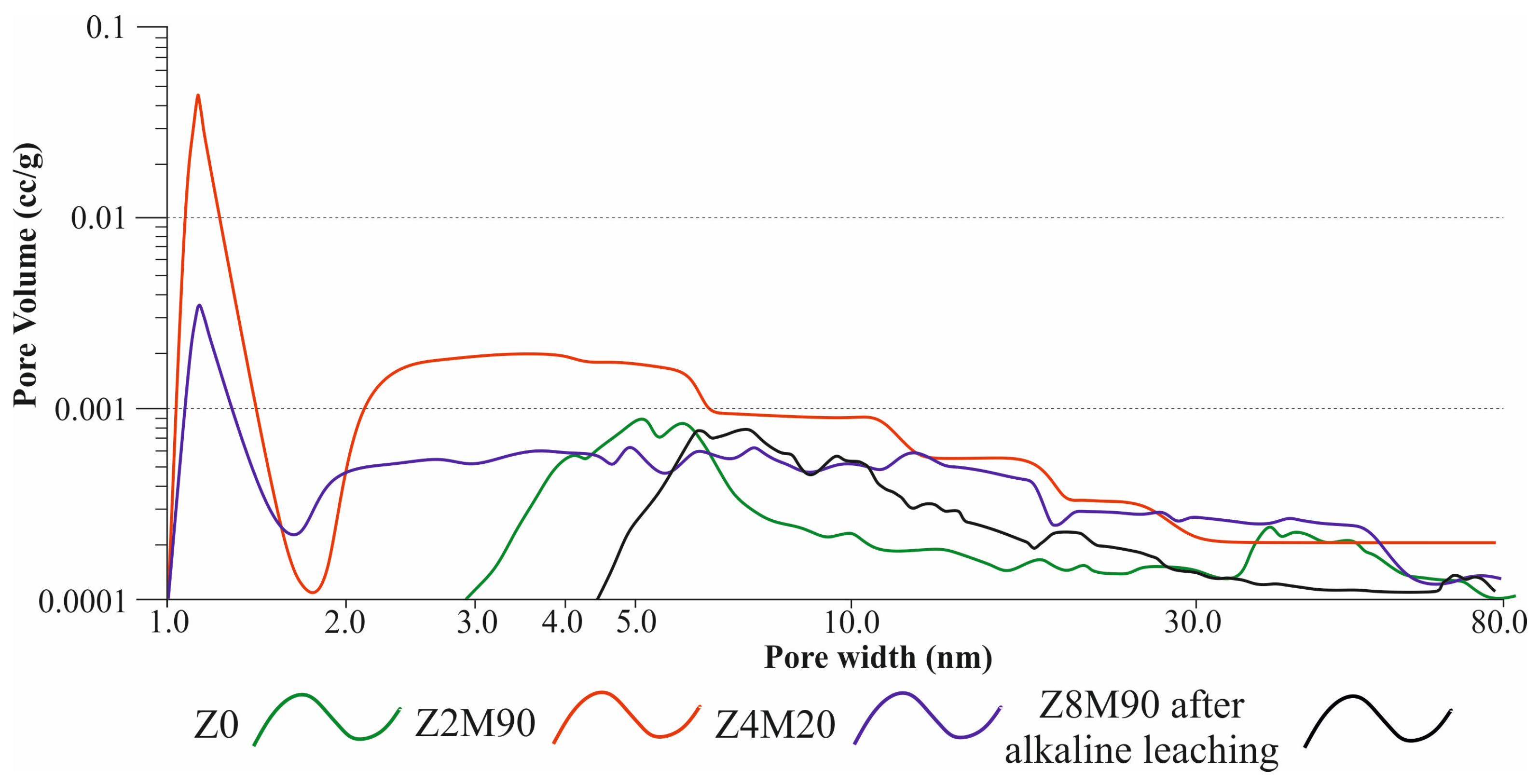
| Sample | Specific Surface Area SBET, m2/g | Pore Volume cm3/g, nm | Mean Pore Diameter, nm | T-Method Halsey, SBET, m2/g | ||
|---|---|---|---|---|---|---|
| Micropores | Meso/Macropores | Total | ||||
| Z0 | 14.0 | 0.053 | 5 | - | 14.0 | 14.0 |
| Z1M20 | 51.7 | 0.094 | <1 | 28.2 | 23.5 | 51.7 |
| Z2M20 | 38.6 | 0.081 | <1 | 17.1 | 21.5 | 38.6 |
| Z3M20 | 27.1 | 0.077 | 5 | 6.6 | 20.5 | 27.1 |
| Z8M20 | 29.7 | 0.068 | <1 | 12.6 | 17.1 | 29.7 |
| Z1M90 | 154.8 | 0.164 | <1 | 115.9 | 38.9 | 154.8 |
| Z2M90 | 177.8 | 0.183 | <1 | 134.4 | 43.4 | 177.8 |
| Z4M90 | 147.0 | 0.144 | 1 | 111.2 | 35.8 | 147.0 |
| Z8M90 | 154.4 | 0.151 | <1 | 116.5 | 37.9 | 154.4 |
| Z8M90 after alkaline leaching | 21.8 | 0.066 | 6 | - | 21.8 | 21.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belousov, P.; Tyupina, E.; Kozlov, P.; Izosimova, Y.; Tolpeshta, I.; Koroleva, T.; Pokidko, B.; Krupskaya, V.; Rumyantseva, A. Radioiodide Sorption on Natural and Acid-Treated Zeolite. Minerals 2025, 15, 494. https://doi.org/10.3390/min15050494
Belousov P, Tyupina E, Kozlov P, Izosimova Y, Tolpeshta I, Koroleva T, Pokidko B, Krupskaya V, Rumyantseva A. Radioiodide Sorption on Natural and Acid-Treated Zeolite. Minerals. 2025; 15(5):494. https://doi.org/10.3390/min15050494
Chicago/Turabian StyleBelousov, Petr, Ekaterina Tyupina, Pavel Kozlov, Yulia Izosimova, Inna Tolpeshta, Tatiana Koroleva, Boris Pokidko, Victoria Krupskaya, and Anastasia Rumyantseva. 2025. "Radioiodide Sorption on Natural and Acid-Treated Zeolite" Minerals 15, no. 5: 494. https://doi.org/10.3390/min15050494
APA StyleBelousov, P., Tyupina, E., Kozlov, P., Izosimova, Y., Tolpeshta, I., Koroleva, T., Pokidko, B., Krupskaya, V., & Rumyantseva, A. (2025). Radioiodide Sorption on Natural and Acid-Treated Zeolite. Minerals, 15(5), 494. https://doi.org/10.3390/min15050494









