Origin and Reservoir Significance of Authigenic Minerals in Lacustrine Shales: A Case Study from the Paleogene Dongying Sag, Bohai Bay Basin, East China
Abstract
1. Introduction
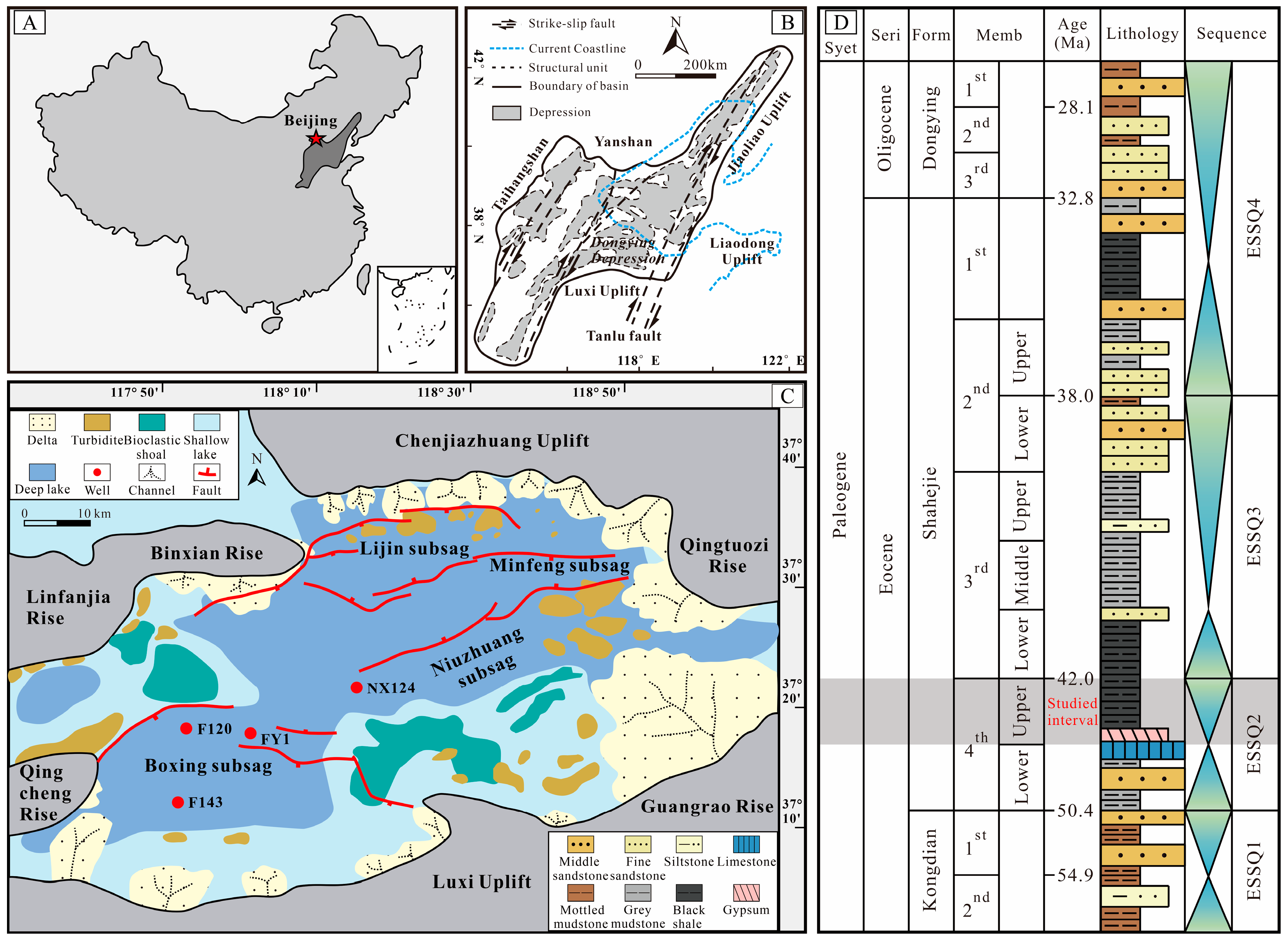
2. Geological Setting
3. Samples and Experimental Methods
3.1. Petrographic Analysis
3.2. Organic Geochemistry Analysis
3.3. Inorganic Geochemistry Analysis
3.4. SEM and EDS
3.5. Helium Porosity
4. Results
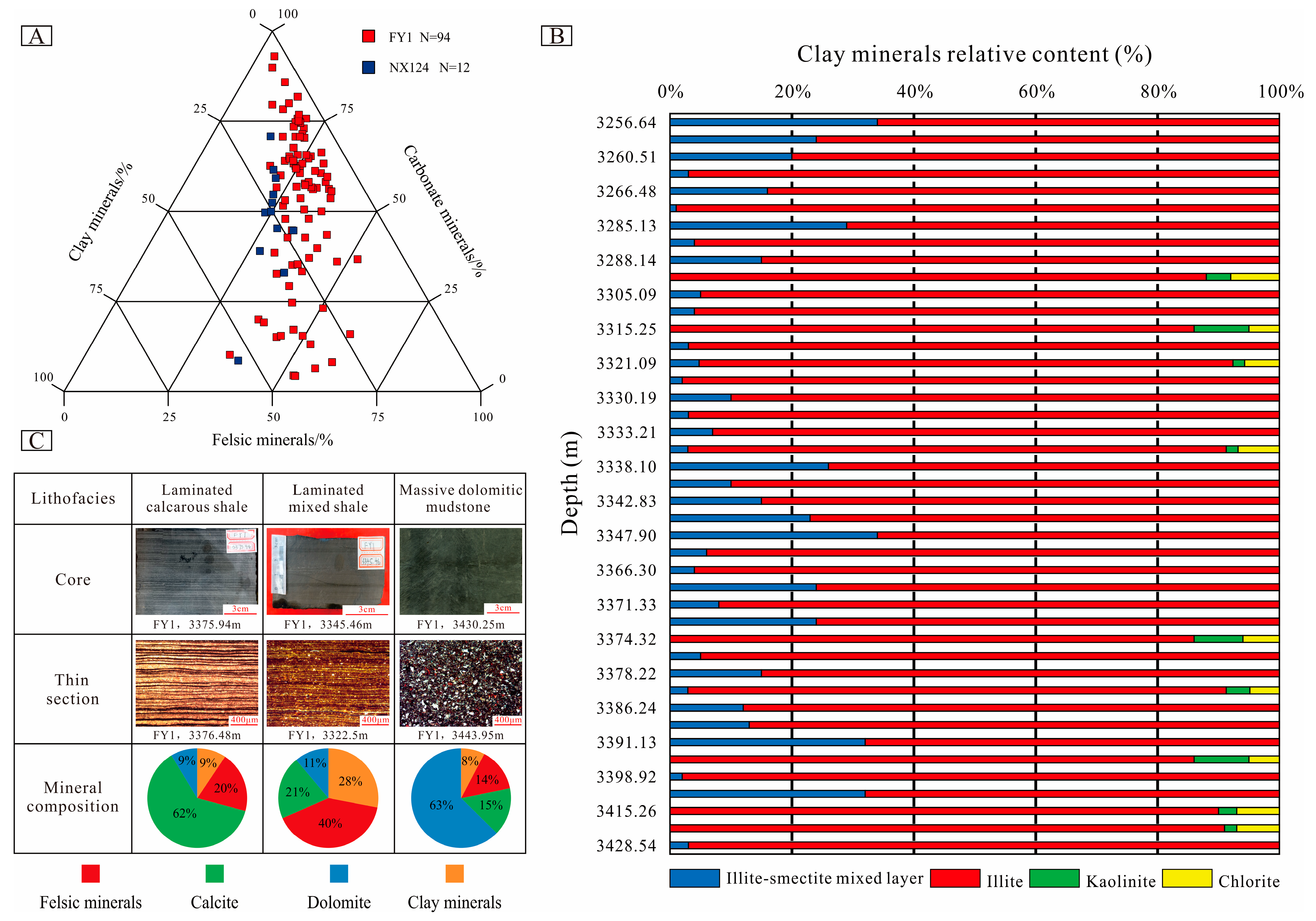
4.1. Petrologic Features and Lithofacies Types
4.2. Geochemical Characteristics
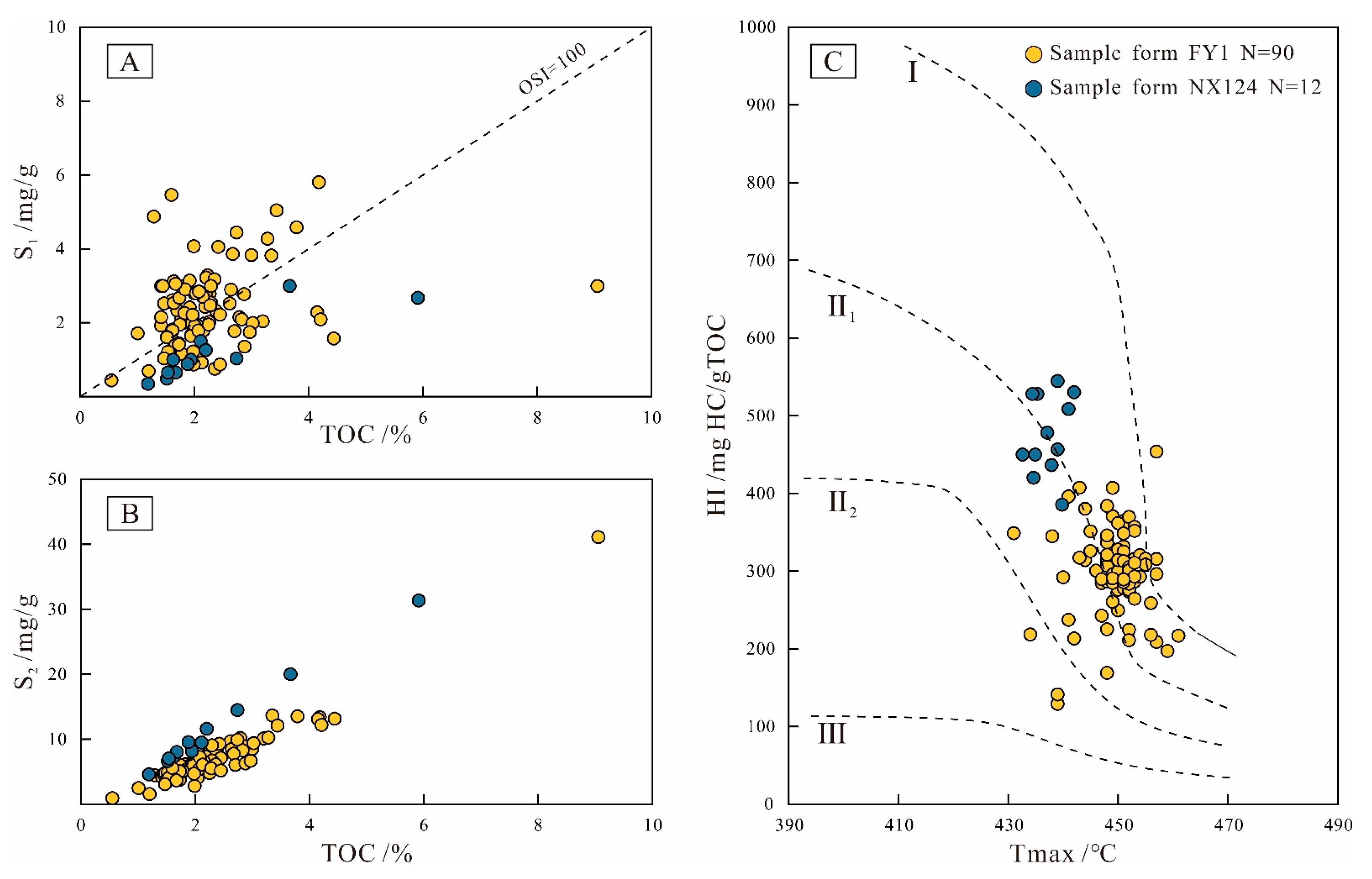
4.2.1. Organic Geochemistry
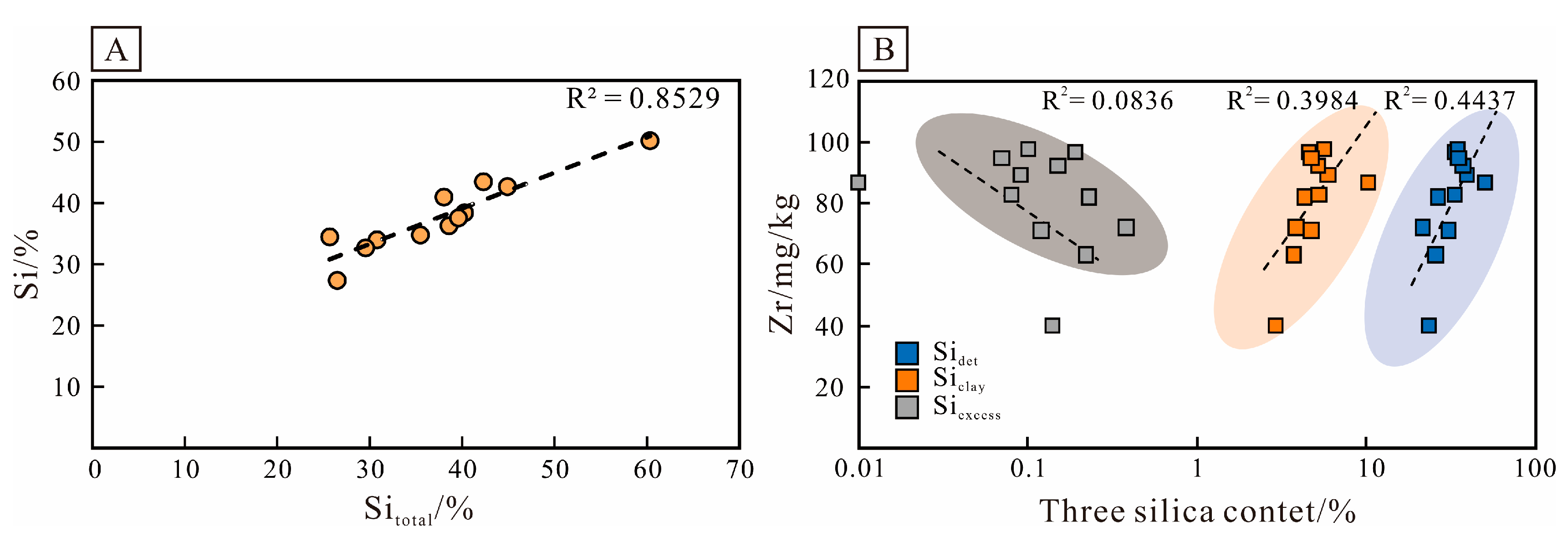
4.2.2. Elemental Geochemistry
4.3. Types and Characteristics of Authigenic Minerals
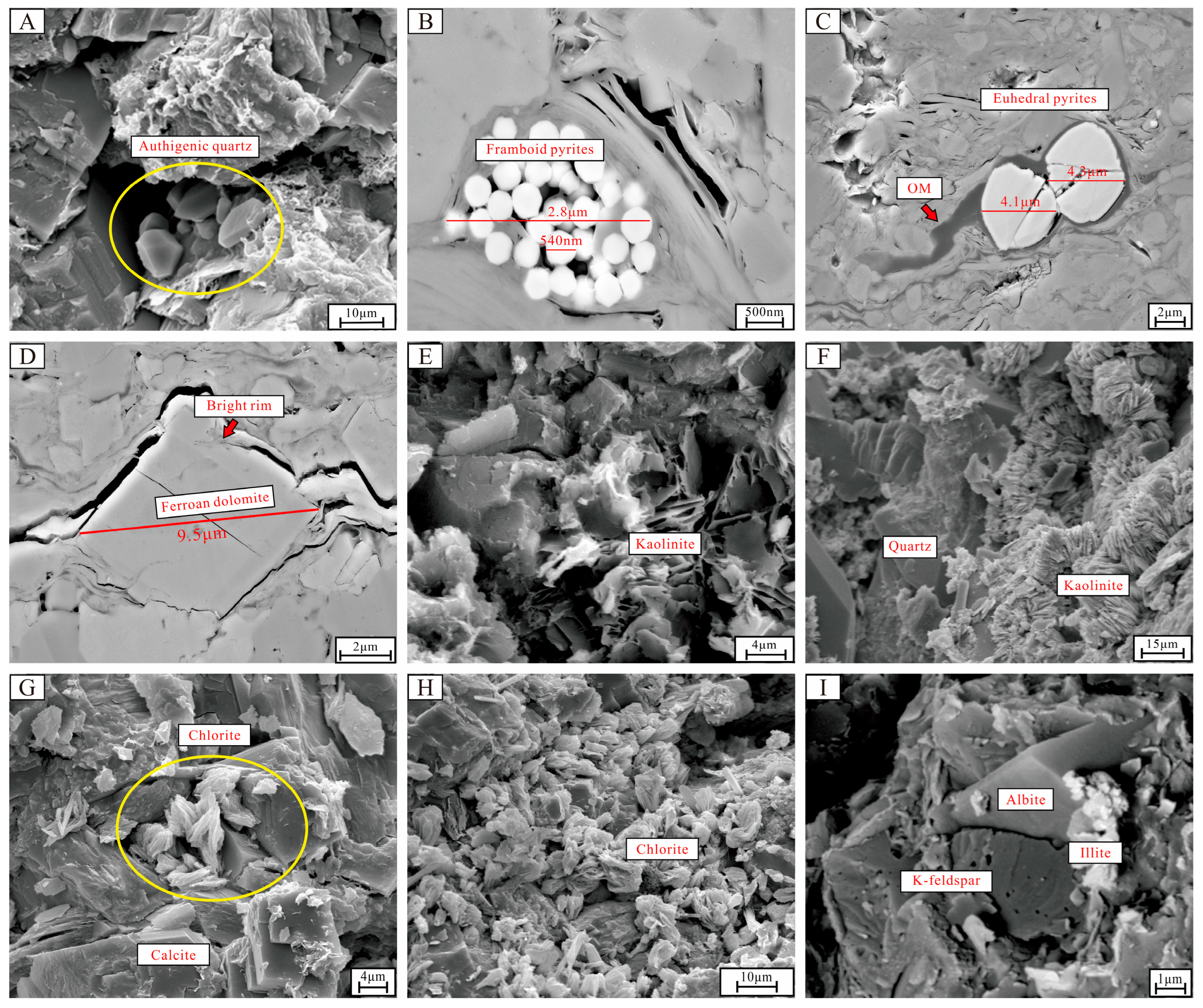
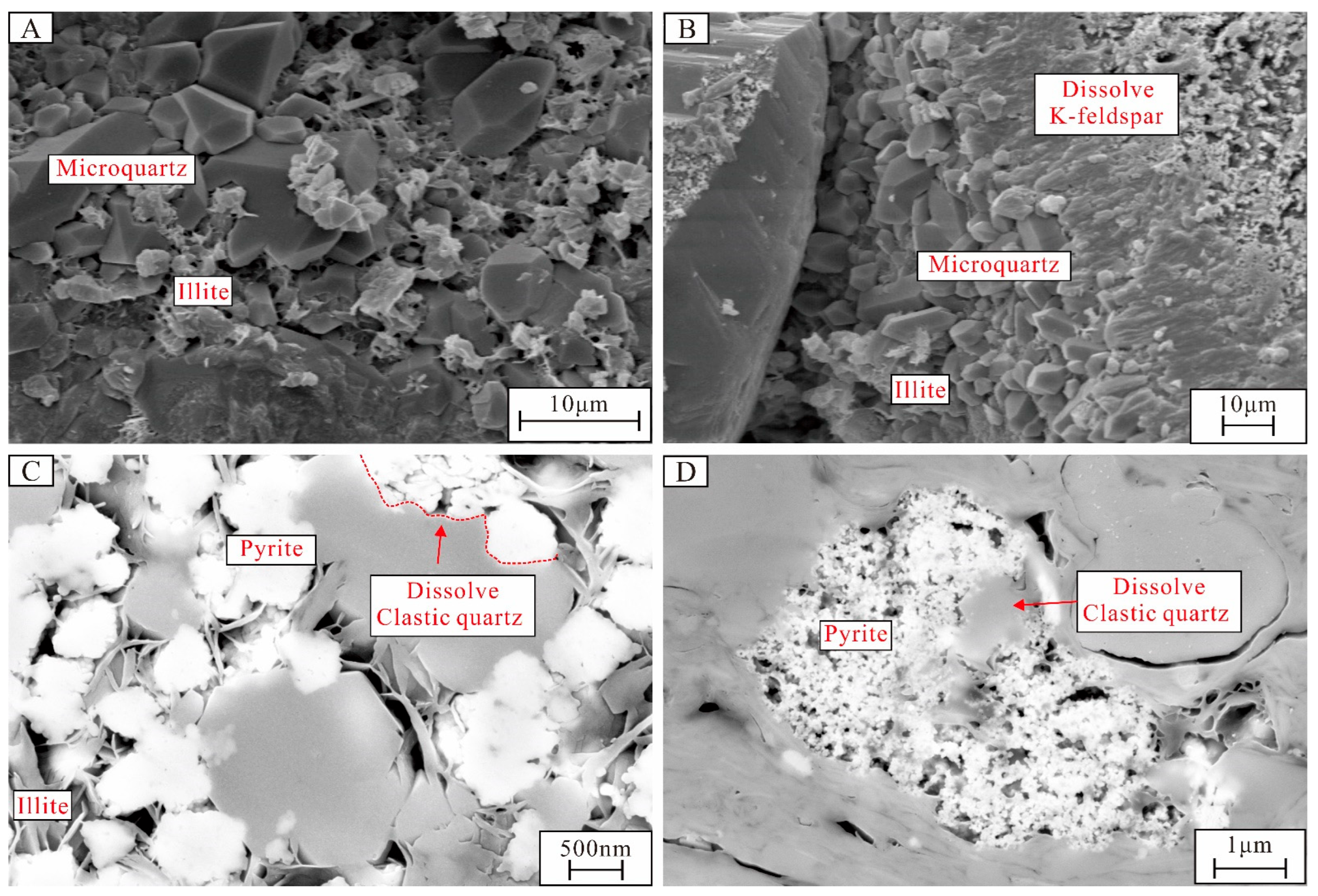
4.3.1. Characteristics of Authigenic Quartz
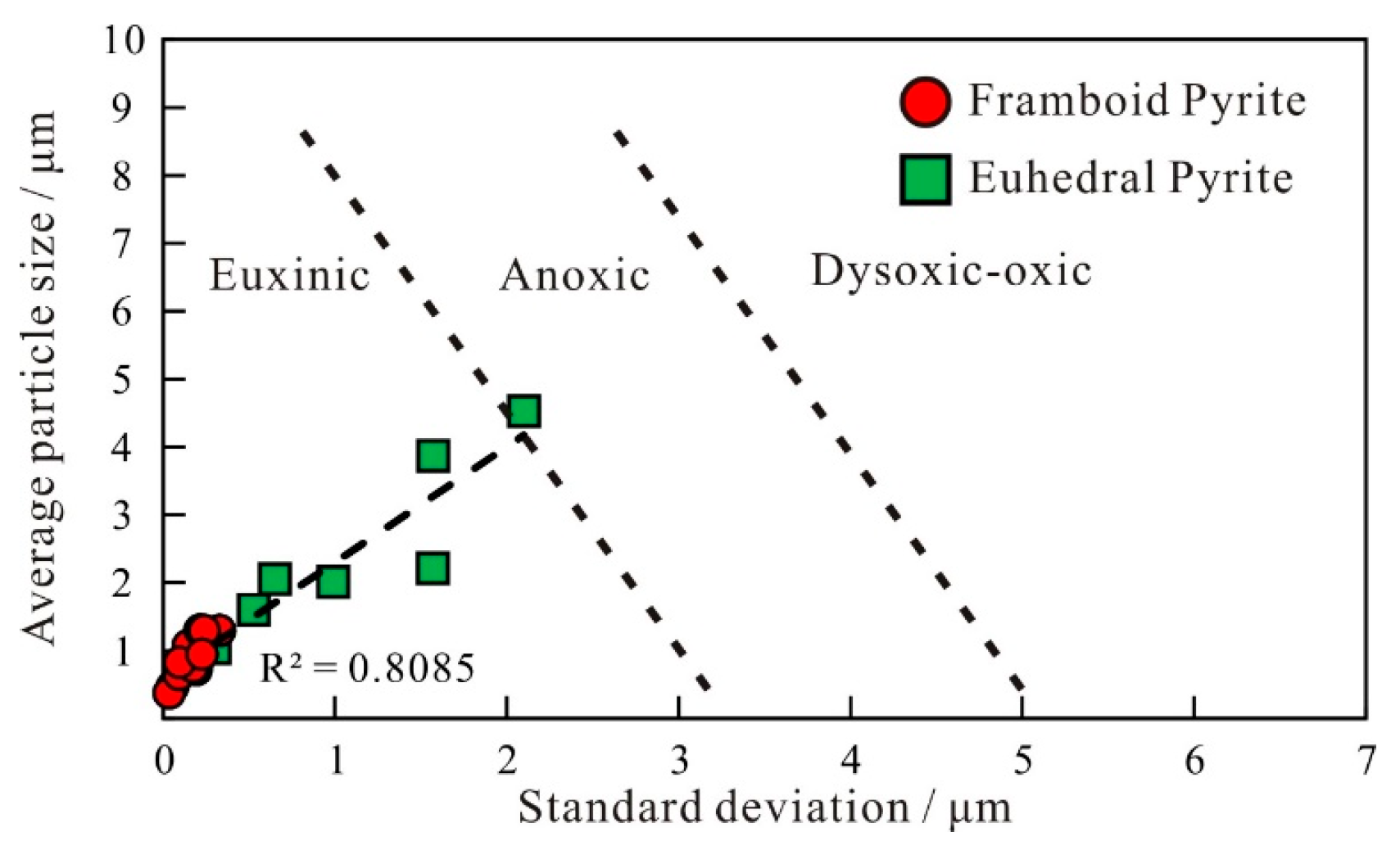
4.3.2. Characteristics of Pyrite
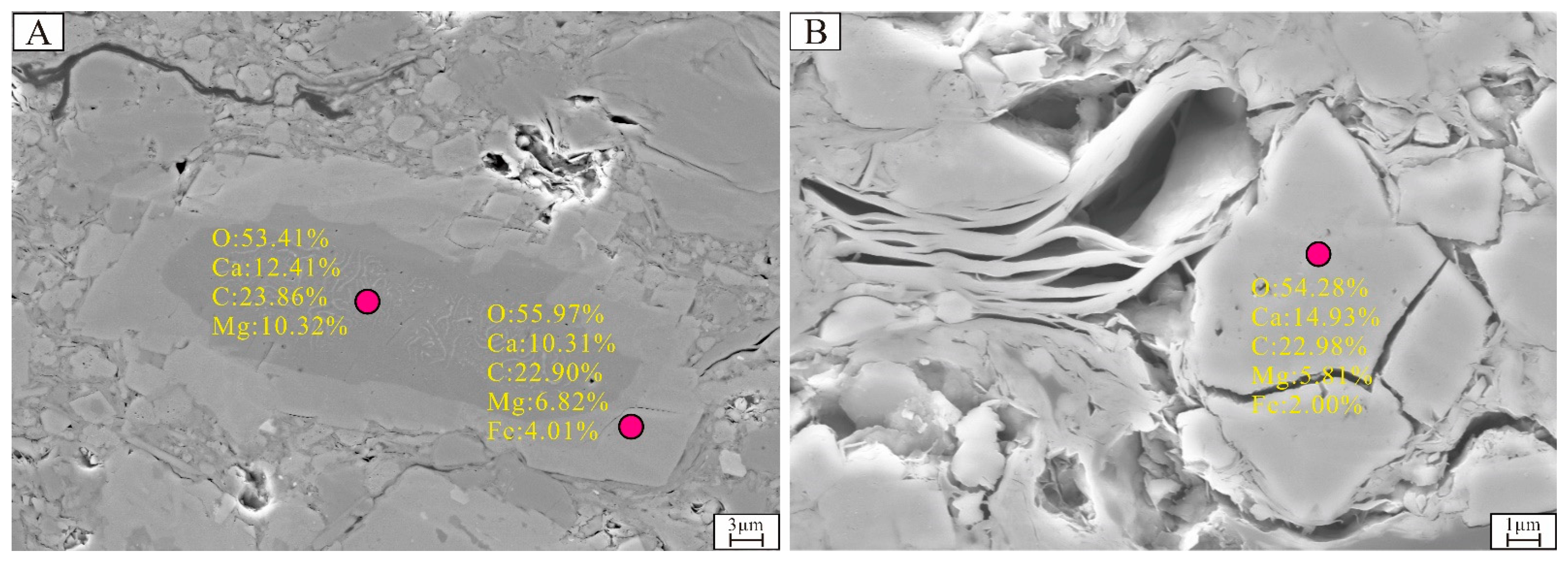
4.3.3. Characteristics of Ferroan Dolomite
4.3.4. Characteristics of Authigenic Clay Minerals
4.3.5. Characteristics of Albite
4.4. Porosity Characteristics
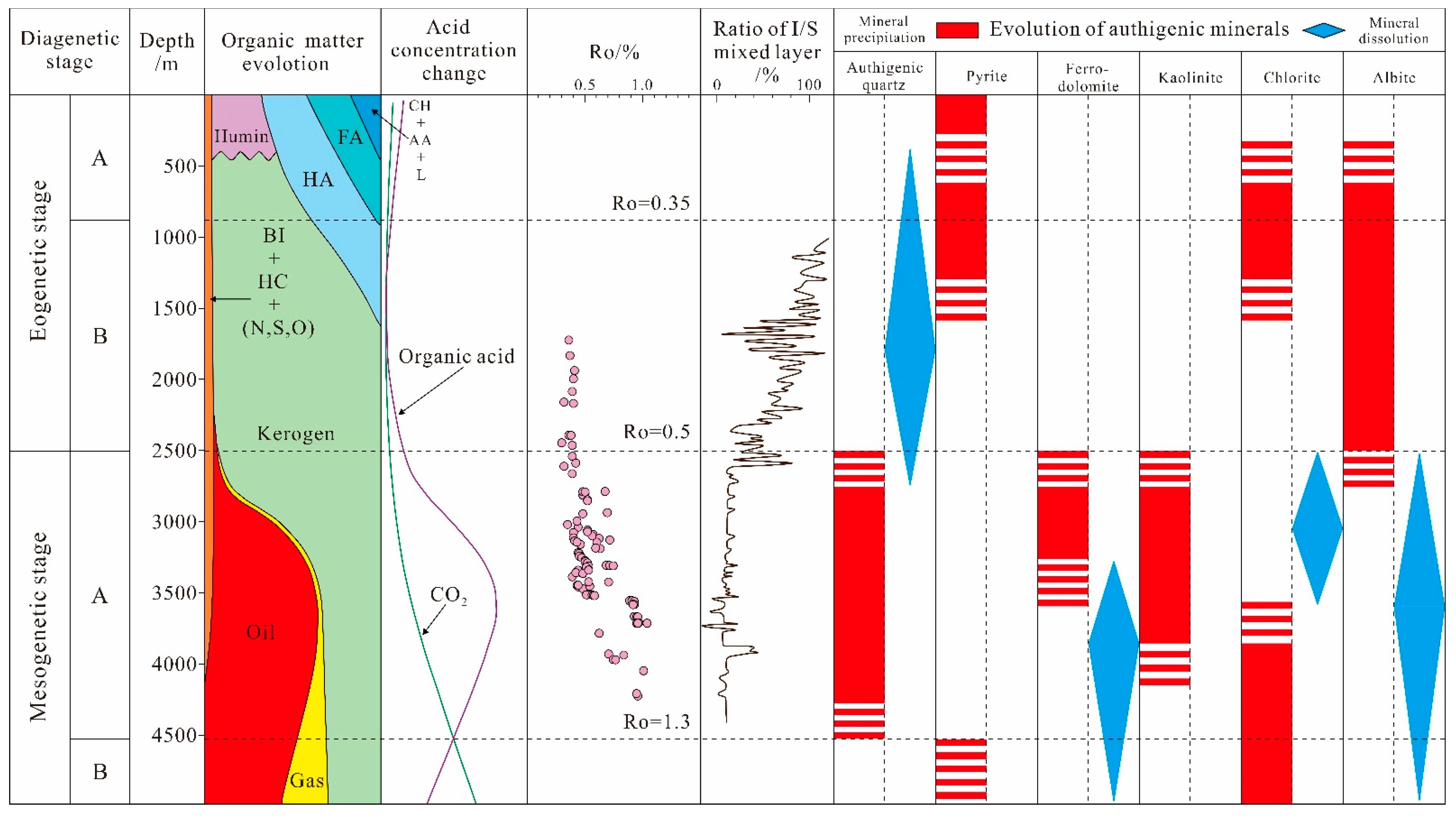
4.5. Diagenetic Stage
5. Discussion
5.1. The Origin of Authigenic Minerals
5.1.1. The Origin of Authigenic Quartz
5.1.2. The Origin of Pyrite
5.1.3. The Origin of Ferroan Dolomite
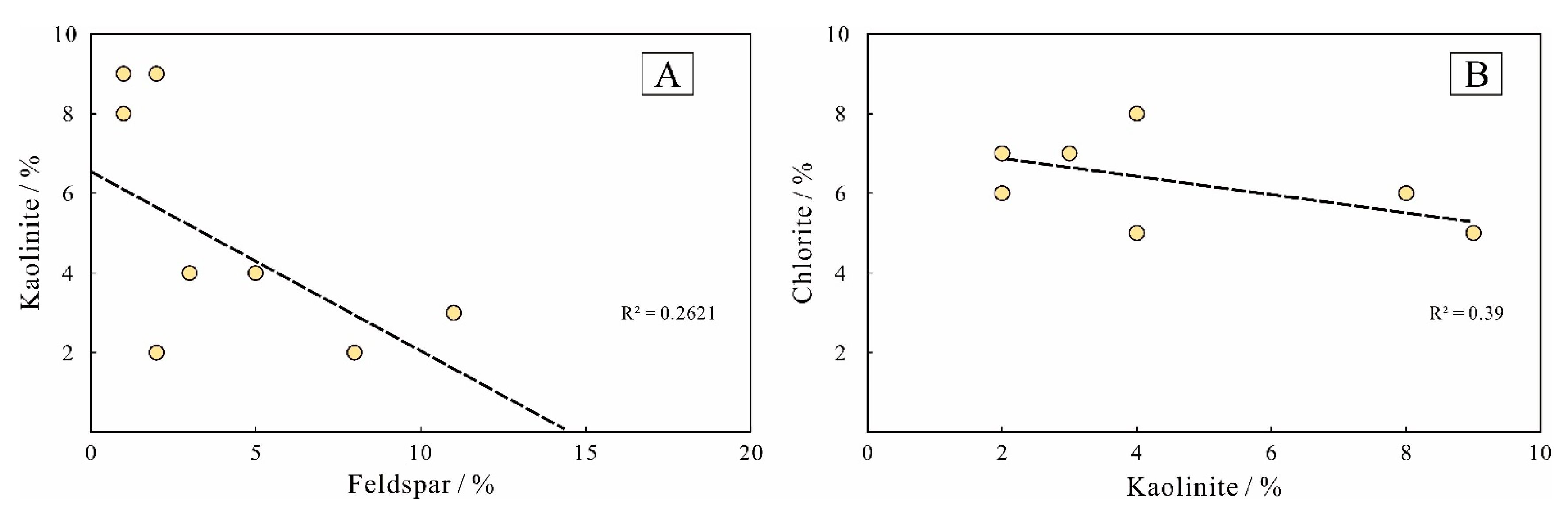
5.1.4. The Origin of Clay Minerals
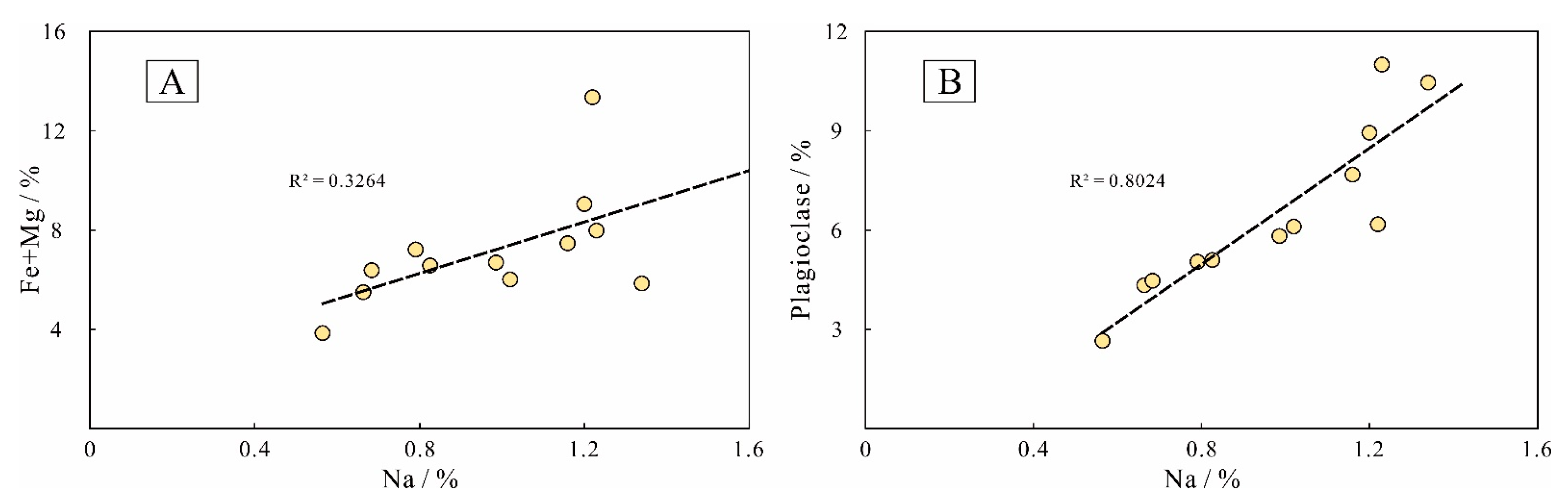
5.1.5. The Origin of Albite
5.2. The Diagenetic Evolution Sequence of Authigenic Minerals
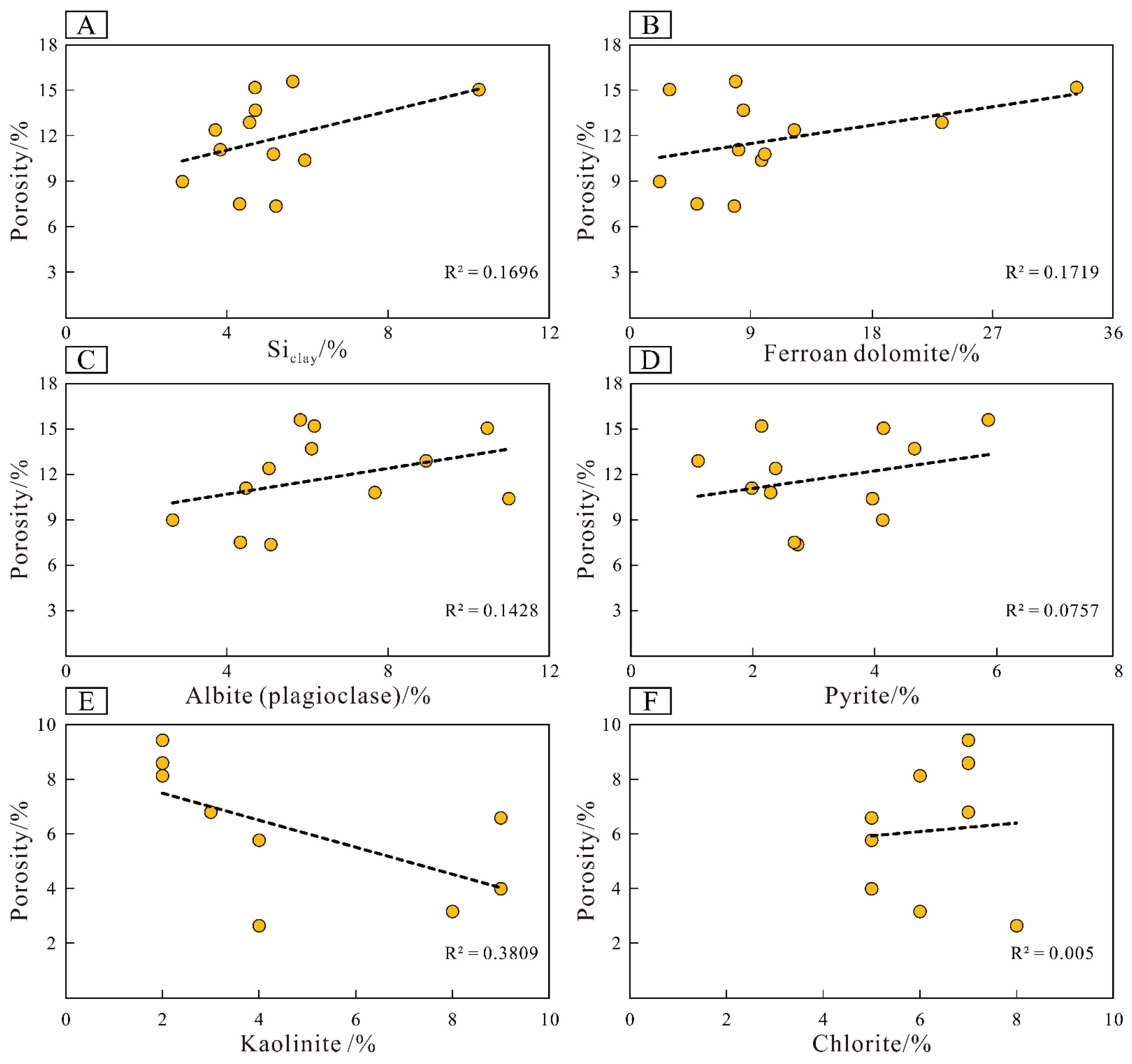
5.3. Implications for Shale Reservoir Quality
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giles, M.R.; Marshall, J.D. Constraints on the development of secondary porosity in the subsurface: Re-evaluation of processes. Mar. Pet. Geol. 1986, 3, 243–255. [Google Scholar] [CrossRef]
- Ronald, C.S.; Crossey, L.J.; Hagen, E.S.; Heasler, H.P. Organic-inorganic interactions and sandstone diagenesis. AAPG Bull. 1989, 73, 1–23. [Google Scholar]
- Giles, M.R.; Boer, R.B.d. Secondary porosity: Creation of enhanced porosities in the subsurface from the dissolution of carbonate cements as a result of cooling formation waters. Mar. Pet. Geol. 1989, 6, 261–269. [Google Scholar] [CrossRef]
- Thyne, G. A model for diagenetic mass transfer between adjacent sandstone and shale. Mar. Pet. Geol. 2001, 18, 743–755. [Google Scholar] [CrossRef]
- Taylor, T.R.; Giles, M.R.; Hathon, L.A. Sandstone diagenesis and reservoir quality prediction: Models, myths, and reality. AAPG Bull. 2010, 94, 1093–1132. [Google Scholar] [CrossRef]
- Han, S.; Yu, B.; Ruan, Z.; Bai, C.; Shen, Z.; Löhr, S.C. Diagenesis and fluid evolution in the third member of the Eocene Shahejie Formation, Bonan Sag, Bohai Bay Basin, China. Mar. Pet. Geol. 2021, 128, 105003. [Google Scholar] [CrossRef]
- Dill, H.G.; Luppold, F.W.; Techmer, A.; Chaodumrong, P.; Phoonphun, S. Lithology, micropaleontology and chemical composition of calcareous rocks of Paleozoic through Cenozoic age (Surat Thani Province, central Peninsular Thailand): Implications concerning the environment of deposition and the economic potential of limestones. J. Asian Earth Sci. 2004, 23, 63–89. [Google Scholar] [CrossRef]
- Poursoltani, M.R.; Gibling, M.R.; Pe-Piper, G. Diagenesis, burial history, and hydrocarbon potential of Cambrian sandstone in the northern continental margin of Gondwana: A case study of the Lalun Formation of central Iran. J. Asian Earth Sci. 2019, 172, 143–169. [Google Scholar] [CrossRef]
- Loucks, R.; Reed, R.; Ruppel, S.; Jarvie, D.M. Morphology, Genesis, and Distribution of Nanometer-Scale Pores in Siliceous Mudstones of the Mississippian Barnett Shale. J. Sediment. Res. 2009, 79, 848–861. [Google Scholar] [CrossRef]
- Milliken, K.L.; Rudnicki, M.; Awwiller, D.N.; Zhang, T. Organic matter-hosted pore system, Marcellus Formation (Devonian), Pennsylvania. AAPG Bull. 2013, 97, 177–200. [Google Scholar] [CrossRef]
- Taylor, K.G.; Macquaker, J.H.S. Diagenetic alterations in a silt- and clay-rich mudstone succession: An example from the Upper Cretaceous Mancos Shale of Utah, USA. Clay Miner. 2014, 49, 213–227. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, S.; Wang, Y.; Tan, M. Lithofacies types and reservoirs of Paleogene fine-grained sedimentary rocks Dongying Sag, Bohai Bay Basin, China. Pet. Explor. Dev. 2016, 43, 218–229. [Google Scholar] [CrossRef]
- Aplin, A.C.; Macquaker, J.H.S. Mudstone diversity: Origin and implications for source, seal, and reservoir properties in petroleum systems. AAPG Bull. 2011, 95, 2031–2059. [Google Scholar] [CrossRef]
- Ruppel, S.C.; Reed, R.M.; Loucks, R.G. Spectrum of pore types and networks in mudrocks and a descriptive classification for matrix-related mudrock pores. AAPG Bull. 2012, 96, 1071–1098. [Google Scholar]
- Schicker, A.; Gier, S.; Schieber, J.; Krois, P. Diagenesis of the Malmian Mikulov Formation source rock, Vienna Basin: Focus on matrix and pores. Mar. Pet. Geol. 2021, 129, 105082. [Google Scholar] [CrossRef]
- Gale, J.F.W.; Jon, E.O.; Laubach, S.E. Natural fractures in shale: A review and new observations. AAPG Bull. 2014, 98, 2165–2216. [Google Scholar] [CrossRef]
- Milliken, K.L.; Reed, R.M.; McCarty, D.K.; Bishop, J.; Lipinski, C.J.; Fischer, T.B.; Crousse, L.; Reijenstein, H. Grain assemblages and diagenesis in the Vaca Muerta Formation (Jurassic-Cretaceous), Neuquén Basin, Argentina. Sediment. Geol. 2019, 380, 45–64. [Google Scholar] [CrossRef]
- Cobbold, P.R.; Zanella, A.; Rodrigues, N.; Løseth, H. Bedding-parallel fibrous veins (beef and cone-in-cone): Worldwide occurrence and possible significance in terms of fluid overpressure, hydrocarbon generation and mineralization. Mar. Pet. Geol. 2013, 43, 1–20. [Google Scholar] [CrossRef]
- Sun, B.; Yang, W. Folded calcite cracks in noncalcareous shales: A window into shale diagenesis and hydrothermal influence. J. Sediment. Res. 2023, 93, 875–894. [Google Scholar] [CrossRef]
- Luo, T.; Guo, X.; He, Z.; Dong, T.; Tao, Z.; Yang, R.; Wang, K. Fluid evolution and gas enrichment in the Wufeng-Longmaxi shale reservoirs of the eastern Sichuan Basin, SW China. J. Asian Earth Sci. 2024, 259, 105905. [Google Scholar] [CrossRef]
- O’Brien, N.R. Fabric of Kaolinite and Illite Floccules. Clays Clay Miner. 1971, 19, 353–359. [Google Scholar] [CrossRef]
- Peltonen, C.; Marcussen, Ø.; Bjørlykke, K.; Jahren, J. Clay mineral diagenesis and quartz cementation in mudstones: The effects of smectite to illite reaction on rock properties. Mar. Pet. Geol. 2009, 26, 887–898. [Google Scholar] [CrossRef]
- Rodrigues, N.; Cobbold, P.R.; Loseth, H.; Ruffet, G. Widespread bedding-parallel veins of fibrous calcite (‘beef’) in a mature source rock (Vaca Muerta Fm, Neuquén Basin, Argentina): Evidence for overpressure and horizontal compression. J. Geol. Soc. 2009, 166, 695–709. [Google Scholar] [CrossRef]
- Peng, J.; Milliken, K.L.; Fu, Q. Quartz types in the Upper Pennsylvanian organic-rich Cline Shale (Wolfcamp D), Midland Basin, Texas: Implications for silica diagenesis, porosity evolution and rock mechanical properties. Sedimentology 2020, 67, 2040–2064. [Google Scholar] [CrossRef]
- Xiong, Z.; Cao, Y.; Liang, C.; Liu, K.; Wang, G.; Zhu, R.; Lei, P.; Wang, Y. Origin and significance of authigenic quartz and albite in lacustrine calcareous fine-grained sedimentary rocks. Mar. Pet. Geol. 2022, 143, 105799. [Google Scholar] [CrossRef]
- Jahren, J.; Bjorlykke, K. Open or closed geochemical systems during diagenesis in sedimentary basins: Constraints on mass transfer during diagenesis and the prediction of porosity in sandstone and carbonate reservoirs. AAPG Bull. 2012, 96, 2193–2214. [Google Scholar]
- Friis, H.; Molenaar, N.; Varming, T. Chlorite meniscus cement–implications for diagenetic mineral growth after oil emplacement. Terra Nova 2014, 26, 14–21. [Google Scholar] [CrossRef]
- Wilson, R.D.; Chitale, J.; Huffman, K.; Montgomery, P.; Prochnow, S.J. Evaluating the depositional environment, lithofacies variation, and diagenetic processes of the Wolfcamp B and lower Spraberry intervals in the Midland Basin: Implications for reservoir quality and distribution. AAPG Bull. 2020, 104, 1287–1321. [Google Scholar] [CrossRef]
- Liang, C.; Wu, J.; Cao, Y.; Liu, K.; Khan, D. Storage space development and hydrocarbon occurrence model controlled by lithofacies in the Eocene Jiyang Sub-basin, East China: Significance for shale oil reservoir formation. J. Pet. Sci. Eng. 2022, 215, 110631. [Google Scholar] [CrossRef]
- Yu, Z.; Chen, S.; Zhang, S.; Liu, X.; Tang, D.; Yan, J. Influence of diagenesis on reservoir performance of shale: A case study of the upper sub-member of Member 4 of Paleogene Shahejie Formation in Dongying sag. J. Palaeogeogr. (Chin. Ed.) 2022, 24, 771–784. [Google Scholar]
- Seewald, J.S. Organic–inorganic interactions in petroleum-producing sedimentary basins. Nature 2003, 426, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.G.; Macquaker, J.H.S.; Keller, M. Compositional controls on early diagenetic pathways in fine-grained sedimentary rocks: Implications for predicting unconventional reservoir attributes of mudstones. AAPG Bull. 2014, 98, 587–603. [Google Scholar]
- Raiswell, R. Evidence for surface reaction-controlled growth of carbonate concretions in shales. Sedimentology 1988, 35, 571–575. [Google Scholar] [CrossRef]
- Loucks, R.G.; Ruppel, S.C. Mississippian Barnett Shale: Lithofacies and depositional setting of a deep-water shale-gas succession in the Fort Worth Basin, Texas. AAPG Bull. 2007, 91, 579–601. [Google Scholar] [CrossRef]
- Wang, M.; Sherwood, N.; Li, Z.; Lu, S.; Wang, W.; Huang, A.; Peng, J.; Lu, K. Shale oil occurring between salt intervals in the Dongpu Depression, Bohai Bay Basin, China. Int. J. Coal Geol. 2015, 152, 100–112. [Google Scholar] [CrossRef]
- Macaulay, C.I.; Haszeldine, R.S.; Fallick, A.E. Distribution, chemistry, isotopic composition and origin of diagenetic carbonates; Magnus Sandstone, North Sea. J. Sediment. Res. 1993, 63, 33–43. [Google Scholar]
- Macquaker, J.H.S.; Taylor, K.G.; Gawthorpe, R.L. High-Resolution Facies Analyses of Mudstones: Implications for Paleoenvironmental and Sequence Stratigraphic Interpretations of Offshore Ancient Mud-Dominated Successions. J. Sediment. Res. 2007, 77, 324–339. [Google Scholar] [CrossRef]
- Soliman, M.F.; El Goresy, A. Framboidal and idiomorphic pyrite in the upper Maastrichtian sedimentary rocks at Gabal Oweina, Nile Valley, Egypt: Formation processes, oxidation products and genetic implications to the origin of framboidal pyrite. Geochim. Cosmochim. Acta 2012, 90, 195–220. [Google Scholar] [CrossRef]
- Dowey, P.J.; Taylor, K.G.; Hendry, J. Diagenetic mineral development within the Upper Jurassic Haynesville-Bossier Shale, USA. Sedimentology 2020, 67, 47–77. [Google Scholar] [CrossRef]
- Canfield, D.E.; Thamdrup, B.; Hansen, J.W. The anaerobic degradation of oraganic matter in Danish coastal sediments: Iron reduction, manganese reduction, and sulfate reduction. Geochim. Cosmochim. Acta 1993, 57, 3867–3883. [Google Scholar] [CrossRef]
- Milkov, A.V. Methanogenic biodegradation of petroleum in the West Siberian Basin (Russia): Significance for formation of giant Cenomanian gas pools. AAPG Bull. 2010, 94, 1485–1541. [Google Scholar] [CrossRef]
- Waliczek, M.; Machowski, G.; Więcław, D.; Konon, A.; Wandycz, P. Properties of solid bitumen and other organic matter from Oligocene shales of the Fore-Magura Unit in Polish Outer Carpathians: Microscopic and geochemical approach. Int. J. Coal Geol. 2019, 210, 103206. [Google Scholar] [CrossRef]
- Schlegel, M.E.; McIntosh, J.C.; Petsch, S.T.; Orem, W.H.; Jones, E.J.P.; Martini, A.M. Extent and limits of biodegradation by in situ methanogenic consortia in shale and formation fluids. Appl. Geochem. 2013, 28, 172–184. [Google Scholar] [CrossRef]
- Mastalerz, M.; Drobniak, A.; Schimmelmann, A. Porosity of Devonian and Mississippian New Albany Shale across a maturation gradient: Insights from organic petrology, gas adsorption, and mercury intrusion. AAPG Bull. 2013, 97, 1621–1643. [Google Scholar] [CrossRef]
- Tissot, B.; Durand, B.; Espitalié, J.; Combaz, A. Influence of Nature and Diagenesis of Organic Matter in Formation of Petroleum1. AAPG Bull. 1974, 58, 499–506. [Google Scholar]
- Curtis, M.E.; Cardott, B.J.; Sondergeld, C.H. Development of organic porosity in the Woodford Shale with increasing thermal maturity. Int. J. Coal Geol. 2012, 103, 26–31. [Google Scholar] [CrossRef]
- Pommer, M.; Milliken, K. Pore types and pore-size distributions across thermal maturity, Eagle Ford Formation, southern Texas. AAPG Bull. 2015, 99, 1713–1744. [Google Scholar] [CrossRef]
- Cai, J.; Du, J.; Song, M.; Lei, T.; Wang, X.; Li, Y. Control of clay mineral properties on hydrocarbon generation of organo-clay complexes: Evidence from high-temperature pyrolysis experiments. Appl. Clay Sci. 2022, 216, 106368. [Google Scholar] [CrossRef]
- Testa, G.; Lugli, S. Gypsum–anhydrite transformations in Messinian evaporites of central Tuscany (Italy). Sediment. Geol. 2000, 130, 249–268. [Google Scholar] [CrossRef]
- Ferrage, E.; Vidal, O.; Mosser-Ruck, R.g.; Cathelineau, M.; Cuadros, J. A reinvestigation of smectite illitization in experimental hydrothermal conditions: Results from X-ray diffraction and transmission electron microscopy. Am. Mineral. 2011, 96, 207–223. [Google Scholar] [CrossRef]
- Wang, G.; Ran, L.; Xu, J.; Wang, Y.; Ma, L.; Zhu, R.; Wei, J.; He, H.; Xi, Y.; Zhu, J. Technical development of characterization methods provides insights into clay mineral-water interactions: A comprehensive review. Appl. Clay Sci. 2021, 206, 106088. [Google Scholar] [CrossRef]
- Li, Y.; Yang, W.; Wang, Q.; Song, Y.; Jiang, Z.; Guo, L.; Zhang, Y.; Wang, J. Influence of the actively migrated diagenetic elements on the hydrocarbon generation potential in tuffaceous shale. Fuel 2019, 256, 115795. [Google Scholar] [CrossRef]
- Du, J.; Cai, J.; Zeng, X.; Zhang, Y.; Mu, X.; Wang, X. Depositional and diagenetic controls on hydrocarbon generation of mudstones through clay–organic matter interactions: A comparative study of the Dongpu Depression, Bohai Bay Basin, China. J. Asian Earth Sci. 2024, 262, 106000. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, M.; Misch, D.; Sachsenhofer, R.F.; Wu, Y.; Li, J. Mineral diagenesis in lacustrine organic-rich shales: Evolution pathways and implications for reservoir characteristics. J. Asian Earth Sci. 2024, 263, 106026. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, X.; Hu, Y. Orderly distribution and differential enrichment of hydrocarbon in oil-rich sags: A case study of Dongying Sag, Jiyang Depression, Bohai Bay Basin, East China. Pet. Explor. Dev. 2018, 45, 785–794. [Google Scholar] [CrossRef]
- Song, M.; Liu, H.; Wang, Y.; Liu, Y. Enrichment rules and exploration practices of Paleogene shale oil in Jiyang Depression, Bohai Bay Basin, China. Pet. Explor. Dev. 2020, 47, 225–235. [Google Scholar] [CrossRef]
- Qiu, N.; Zuo, Y.; Xu, W.; Chang, J. Meso-Cenozoic thermal structure of the lithosphere in the Bohai Bay Basin, eastern North China Craton: Thermal structure in Bohai Bay Basin. Geol. J. 2015, 51, 794–804. [Google Scholar] [CrossRef]
- Fu, C.; Li, S.; Li, S.; Xu, J. Tectonostratigraphic evolution of a rift basin and corresponding source-to-sink systems: Implications for the western Bohai Bay Basin, North China. Mar. Pet. Geol. 2022, 139, 105587. [Google Scholar] [CrossRef]
- Wu, Q.; Xian, B.; Gao, X.; Bai, Q.; Wang, Z.; Liu, J.; Chen, P.; Li, Y.; Rahman, N.U.; Tian, R.; et al. Differences of sedimentary triggers and depositional architecture of lacustrine turbidites from normal regression to forced regression: Eocene Dongying depression, Bohai Bay Basin, East China. Sediment. Geol. 2022, 439, 106222. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, Z.; Liang, C.; Baars, T.F.; Wang, Y.; Abels, H.A. Astronomical forcing of meter-scale organic-rich mudstone–limestone cyclicity in the Eocene Dongying sag, China: Implications for shale reservoir exploration. AAPG Bull. 2022, 106, 1557–1579. [Google Scholar] [CrossRef]
- Liang, C.; Cao, Y.; Wu, J.; Han, Y.; Liu, K.; Hao, F.; Khan, D.; Mei, J.; Zhang, S.; Wang, Y. Water depth–terrigenous input dynamic equilibrium controls the Eocene lacustrine shale laminae records in Jiyang depression, Bohai Bay Basin, East China. AAPG Bull. 2023, 107, 1987–2016. [Google Scholar] [CrossRef]
- Khan, D.; Zijun, L.; Qiu, L.; Kuiyuan, L.; Yongqiang, Y.; Cong, N.; Bin, L.; Li, X.; Habulashenmu, Y. Mineralogical and geochemical characterization of lacustrine calcareous shale in Dongying Depression, Bohai Bay Basin: Implications for paleosalinity, paleoclimate, and paleoredox conditions. Geochemistry 2023, 83, 125978. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, L.; Pu, X.; Jin, F.; Shi, Z.; Han, W.; Jiang, W.; Han, G.; Zhang, W.; Wang, H.; et al. Formation conditions and enrichment model of retained petroleum in lacustrine shale: A case study of the Paleogene in Huanghua depression, Bohai Bay Basin, China. Pet. Explor. Dev. 2020, 47, 856–869. [Google Scholar] [CrossRef]
- Wang, M.; Ma, R.; Li, J.; Lu, S.; Li, C.; Guo, Z.; Li, Z. Occurrence mechanism of lacustrine shale oil in the Paleogene Shahejie Formation of Jiyang Depression, Bohai Bay Basin, China. Pet. Explor. Dev. 2019, 46, 833–846. [Google Scholar] [CrossRef]
- Bjorlykke, K. Clay mineral diagenesis in sedimentary basins; a key to the prediction of rock properties; examples from the North Sea Basin. Clay Miner. 1998, 33, 15–34. [Google Scholar] [CrossRef]
- Merriman, R.J. Clay minerals and sedimentary basin history. Eur. J. Mineral. 2005, 17, 7–20. [Google Scholar] [CrossRef]
- Penghui, Z.; Zhiyong, C.H.E.N.; Lu, X.; YanJun, B.; Yan, F. The differential diagenetic evolution and its influencing factors of Lower Cambrian black rock series in the northwestern margin of Tarim Basin. Acta Petrol. Sin. 2020, 36, 3463–3476. [Google Scholar] [CrossRef]
- Li, K.; Xi, K.; Cao, Y.; Wang, Y.; Lin, M. Genesis of granular calcite in lacustrine fine-grained sedimentary rocks and its indication to volcanic-hydrothermal events: A case study of Permian Lucaogou Formation in Jimusar Sag, Junggar Basin, NW China. Pet. Explor. Dev. 2023, 50, 541–552. [Google Scholar] [CrossRef]
- Zhu, H.; Zhong, D.; Yao, J.; Sun, H.; Niu, X.; Liang, X.; You, Y.; Li, X. Alkaline diagenesis and its effects on reservoir porosity: A case study of Upper Triassic Chang 7 tight sandstones in Ordos Basin, NW China. Pet. Explor. Dev. 2015, 42, 51–59. [Google Scholar] [CrossRef]
- van de Kamp, P.C. Smectite-illite-muscovite transformations, quartz dissolution, and silica release in shales. Clays Clay Miner. 2008, 56, 66–81. [Google Scholar] [CrossRef]
- Niu, X.; Yan, D.; Zhuang, X.; Liu, Z.; Li, B.; Wei, X.; Xu, H.; Li, D. Origin of quartz in the lower Cambrian Niutitang Formation in south Hubei Province, upper Yangtze platform. Mar. Pet. Geol. 2018, 96, 271–287. [Google Scholar] [CrossRef]
- Wedepohl, K.H. Environmental influences on the chemical composition of shales and clays. Phys. Chem. Earth 1971, 8, 307–333. [Google Scholar] [CrossRef]
- Li, L.; Bao, Z.; Li, Z.; Chen, L.; Xu, X.; Li, Y.; Zhao, Y.; Song, X. Origin of quartz and its implications for different phase fluids in lacustrine shales from the Qingshankou Formation, southern Songliao Basin, China. Geoenergy Sci. Eng. 2024, 234, 212673. [Google Scholar] [CrossRef]
- Xi, Z.; Tang, S.; Lash, G.G.; Zhang, B.; Lin, D. Geochemical characteristics of organic carbon and pyrite sulfur in Ordovician-Silurian transition shales in the Yangtze Platform, South China: Implications for the depositional environment. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2021, 563, 110173. [Google Scholar] [CrossRef]
- Wilkin, R.T.; Barnes, H.L.; Brantley, S.L. The Size Distribution of Framboidal Pyrite in Modern Sediments—An Indicator of Redox Conditions. Geochim. Cosmochim. Acta J. Geochem. Soc. Meteorit. Soc. 1996, 60, 3897–3912. [Google Scholar] [CrossRef]
- Zuo, Q.; Xu, Y.; Zhang, Y. NanoSIMS sulfur isotope studies of pyrite from the Early Paleozoic marine shale: Implications for the sedimentary environment. Mar. Pet. Geol. 2021, 124, 104802. [Google Scholar] [CrossRef]
- Chao, L.; Shichao, J.; Yingchang, C.; Keyu, L.; Jing, W.; Fang, H. Characteristics, origins, and significance of pyrites in deep-water shales. Sci. China Earth Sci. 2024, 67, 313–342. [Google Scholar]
- Love, L.G.; Amstutz, G.C. Review of microscopic pyrite from the Devonian Chattanooga Shale and Rammelsberg Banderz. Fortschr Miner. 1966, 43, 277–309. [Google Scholar]
- Baronov, A.; Bufkin, K.; Shaw, D.W.; Johnson, B.L.; Patrick, D.L. A simple model of burst nucleation. Phys. Chem. Chem. Phys. PCCP 2015, 17, 20846–20852. [Google Scholar] [CrossRef]
- Steinike, K. A further remark on biogenic sulfides; inorganic pyrite spheres. Econ. Geol. 1963, 58, 998–1000. [Google Scholar] [CrossRef]
- Popa, R.; Kinkle, B.K.; Badescu, A. Pyrite Framboids as Biomarkers for Iron-Sulfur Systems. Geomicrobiol. J. 2004, 21, 193–206. [Google Scholar] [CrossRef]
- Ren, Y.; Yang, X.; Hu, X.; Wei, J.; Tang, C. Mineralogical and geochemical evidence for biogenic uranium mineralization in northern Songliao Basin, NE China. Ore Geol. Rev. 2022, 141, 104556. [Google Scholar] [CrossRef]
- Hao, F.; Zhang, X.; Wang, C.; Li, P.; Guo, T.; Zou, H.; Zhu, Y.; Liu, J.; Cai, Z. The fate of CO2 derived from thermochemical sulfate reduction (TSR) and effect of TSR on carbonate porosity and permeability, Sichuan Basin, China. Earth Sci. Rev. 2015, 141, 154–177. [Google Scholar] [CrossRef]
- Land, L.S. Failure to Precipitate Dolomite at 25 °C fromDilute Solution Despite 1000-Fold Oversaturation after32 Years. Aquat. Geochem. 1998, 4, 361–368. [Google Scholar] [CrossRef]
- Arvidson, R.S.; Mackenzie, F.T. The dolomite problem: Control of precipitation kinetics by temperature and saturation state. Am. J. Sci. 1999, 299, 257–288. [Google Scholar] [CrossRef]
- Roberts, J.A.; Bennett, P.C.; González, L.A.; Macpherson, G.L.; Milliken, K.L. Microbial precipitation of dolomite in methanogenic groundwater. Geology 2004, 32, 277–280. [Google Scholar] [CrossRef]
- Petrash, D.A.; Bialik, O.M.; Bontognali, T.R.R.; Vasconcelos, C.; Roberts, J.A.; McKenzie, J.A.; Konhauser, K.O. Microbially catalyzed dolomite formation: From near-surface to burial. Earth Sci. Rev. 2017, 171, 558–582. [Google Scholar] [CrossRef]
- Vasconcelos, C.; McKenzie, J.A. Microbial mediation of modern dolomite precipitation and diagenesis under anoxic conditions (Lagoa Vermelha, Rio de Janeiro, Brazil). J. Sediment. Res. 1997, 67, 378–390. [Google Scholar]
- Wright, D.T. The role of sulphate-reducing bacteria and cyanobacteria in dolomite formation in distal ephemeral lakes of the Coorong region, South Australia. Sediment. Geol. 1999, 126, 147–157. [Google Scholar] [CrossRef]
- Vasconcelos, C.; Warthmann, R.; McKenzie, J.A.; Visscher, P.T.; Bittermann, A.G.; Lith, Y.v. Lithifying microbial mats in Lagoa Vermelha, Brazil: Modern Precambrian relics? Sediment. Geol. 2006, 185, 175–183. [Google Scholar] [CrossRef]
- Deng, S.; Dong, H.; Lv, G.; Jiang, H.; Yu, B.; Bishop, M. Microbial dolomite precipitation using sulfate reducing and halophilic bacteria: Results from Qinghai Lake, Tibetan Plateau, NW China. Chem. Geol. 2011, 278, 151–159. [Google Scholar] [CrossRef]
- Hardie, L.A. On the Significance of Evaporites. Annu. Rev. Earth Planet. Sci. 1991, 19, 131–168. [Google Scholar] [CrossRef]
- Bojanowski, M.J. Authigenic dolomites in the Eocene-Oligocene organic carbon-rich shales from the Polish Outer Carpathians: Evidence of past gas production and possible gas hydrate formation in the Silesian basin. Mar. Pet. Geol. 2014, 51, 117–135. [Google Scholar] [CrossRef]
- Li, X.; Zhu, G.; Li, T.; Zhou, L.; Wu, Y.; Shen, B.; Ning, M. Conservative behavior of Mg isotopes in dolomite during diagenesis and hydrothermal alteration: A case study in the Lower Cambrian Qiulitage Formation, Gucheng area, Tarim Basin. Appl. Geochem. 2023, 148, 105540. [Google Scholar] [CrossRef]
- Mitchell, R.H.; Smith, D.L. Geology and mineralogy of the Ashram Zone carbonatite, Eldor Complex, Québec. Ore Geol. Rev. 2017, 86, 784–806. [Google Scholar] [CrossRef]
- Zhao, W.; Shen, A.; Qiao, Z.; Pan, L.; Hu, A.; Zhang, J. Genetic types and distinguished characteristics of dolomite and the origin of dolomite reservoirs. Pet. Explor. Dev. 2018, 45, 923–935. [Google Scholar] [CrossRef]
- Xiong, Z.; Cao, Y.; Liang, C. Characteristics and origin of crystalline dolomite: A case from Paleogene lacustrine fine-grained rocks in Jiyang depression, Bohai Bay Basin, China. Mar. Pet. Geol. 2024, 162, 106694. [Google Scholar] [CrossRef]
- Metwally, Y.M.; Chesnokov, E.M. Clay mineral transformation as a major source for authigenic quartz in thermo-mature gas shale. Appl. Clay Sci. 2012, 55, 138–150. [Google Scholar] [CrossRef]
- Mavromatis, V.; Meister, P.; Oelkers, E.H. Using stable Mg isotopes to distinguish dolomite formation mechanisms: A case study from the Peru Margin. Chem. Geol. 2014, 385, 84–91. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, T.; Liu, Q.; Zhang, S.; Zhang, L. Quantitative evaluation of potential organic-matter porosity and hydrocarbon generation and expulsion from mudstone in continental lake basins: A case study of Dongying sag, eastern China. Mar. Pet. Geol. 2015, 66, 906–924. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, L.; De Paolo, D.J.; Steefel, C.I. Chemical affinity and pH effects on chlorite dissolution kinetics under geological CO2 sequestration related conditions. Chem. Geol. 2015, 396, 208–217. [Google Scholar] [CrossRef]
- Lowson, R.T.; Brown, P.L.; Comarmond, M.C.J.; Rajaratnam, G. The kinetics of chlorite dissolution. Geochim. Cosmochim. Acta 2007, 71, 1431–1447. [Google Scholar] [CrossRef]
- Liao, R.; Chen, W.; Wang, N.; Zhang, J. Combined effects of temperature, mineral type, and surface roughness on chlorite dissolution kinetics in the acidic pH. Appl. Clay Sci. 2021, 201, 105931. [Google Scholar] [CrossRef]
- Smith, M.; Wolery, T.; Carroll, S. Kinetics of chlorite dissolution at elevated temperatures and CO2 conditions. Chem. Geol. 2013, 347, 1–8. [Google Scholar] [CrossRef]
- Wooldridge, L.J.; Worden, R.H.; Griffiths, J.; Utley, J.E.P.; Thompson, A. The origin of clay-coated sand grains and sediment heterogeneity in tidal flats. Sediment. Geol. 2018, 373, 191–209. [Google Scholar] [CrossRef]
- Zuo, R.; Cai, J.; Zeng, X.; Cheng, S. Detrital clay mineral input reconstructed based on weathering records and its influence on organic matter enrichment: A case study of the Paleogene Shahejie Formation in the Dongpu Sag, Bohai Bay Basin. J. Asian Earth Sci. 2024, 276, 106360. [Google Scholar] [CrossRef]
- Mosser, C. The use of B, Li and Sn in determining the origin of some sedimentary clays. Chem. Geol. 1983, 38, 129–139. [Google Scholar] [CrossRef]
- D’Ascanio, V.; Greco, D.; Menicagli, E.; Santovito, E.; Catucci, L.; Logrieco, A.F.; Avantaggiato, G. The role of geological origin of smectites and of their physico-chemical properties on aflatoxin adsorption. Appl. Clay Sci. 2019, 181, 105209. [Google Scholar] [CrossRef]
- Xin, C.; Jianhua, Z.; Jing, Y.; Keke, N.; Yuping, Y. Development and formation of Paleogene kaolinite, Bonan subsag. Pet. Explor. Dev. 2009, 36, 456–462. [Google Scholar]
- Beaufort, D.; Rigault, C.; Billon, S.; Billault, V.; Inoue, A.; Inoue, S.; Patrier, P. Chlorite and chloritization processes through mixed-layer mineral series in low-temperature geological systems—A review. Clay Miner. 2015, 50, 497–523. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, K.; Hou, J.; Yan, L.; Chen, F. Diagenetic controls on the quality of the Middle Permian Lucaogou Formation tight reservoir, southeastern Junggar Basin, northwestern China. J. Asian Earth Sci. 2019, 178, 139–155. [Google Scholar] [CrossRef]
- Velde, B. Compaction trends of clay-rich deep sea sediments. Mar. Geol. 1996, 133, 193–201. [Google Scholar] [CrossRef]
- Wang, F.; Feng, Z.; Wang, X.; Zeng, H. Effect of organic matter, thermal maturity and clay minerals on pore formation and evolution in the Gulong Shale, Songliao Basin, China. Geoenergy Sci. Eng. 2023, 223, 211507. [Google Scholar] [CrossRef]
- Milliken, K.L.; Zhang, T.; Chen, J.; Ni, Y. Mineral diagenetic control of expulsion efficiency in organic-rich mudrocks, Bakken Formation (Devonian-Mississippian), Williston Basin, North Dakota, U.S.A. Mar. Pet. Geol. 2021, 127, 104869. [Google Scholar] [CrossRef]
- Liang, C.; Cao, Y.; Liu, K.; Jiang, Z.; Wu, J.; Hao, F. Diagenetic variation at the lamina scale in lacustrine organic-rich shales: Implications for hydrocarbon migration and accumulation. Geochim. Cosmochim. Acta 2018, 229, 112–128. [Google Scholar] [CrossRef]
- Wang, X.; Liu, L.; Wang, Y.; Sheng, Y.; Zheng, S.; Wu, W.; Luo, Z. Comparison of the pore structures of Lower Silurian Longmaxi Formation shales with different lithofacies in the southern Sichuan Basin, China. J. Nat. Gas Sci. Eng. 2020, 81, 103419. [Google Scholar] [CrossRef]
- Fishman, N.S.; Hackley, P.C.; Lowers, H.; Hill, R.J.; Egenhoff, S.O.; Eberl, D.D.; Blum, A.E. The nature of porosity in organic-rich mudstones of the Upper Jurassic Kimmeridge Clay Formation, North Sea, offshore United Kingdom. Int. J. Coal Geol. 2012, 103, 32–50. [Google Scholar] [CrossRef]
- Wilson, R.D.; Schieber, J.; Nie, H.; Sun, C.; Liu, G.; Li, Y.; Schieber, J.; Fan, T.; Li, Z.; Schieber, J. The influence of primary and secondary sedimentary features on reservoir quality: Examples from the geneseo formation of New York, U.S.A. AAPG Mem. 2016, 112, 167–183. [Google Scholar]
- Dowey, P.J.; Taylor, K.G. Extensive authigenic quartz overgrowths in the gas-bearing Haynesville-Bossier Shale, USA. Sediment. Geol. 2017, 356, 15–25. [Google Scholar] [CrossRef]
- Lalonde, K.; Mucci, A.; Ouellet, A.; Gélinas, Y. Preservation of organic matter in sediments promoted by iron. Nature 2012, 483, 198–200. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, H.; Song, G.; Wang, Y.; Chen, S.; Zhang, S. Genesis and control factors of shale oil reserving space in Dongying sag. Acta Pet. Sin. 2016, 37, 1495–1507,1527. [Google Scholar]
- Davis, K.J.; Dove, P.M.; Yoreo, J.J.D. The Role of Mg2+ as an Impurity in Calcite Growth. Science 2000, 290, 1134–1137. [Google Scholar] [CrossRef] [PubMed]
- Klasa, J.; Ruiz-Agudo, E.; Wang, L.; Putnis, C.; Valsami-Jones, E.; Menneken, M.; Putnis, A. An atomic force microscopy study of the dissolution of calcite in the presence of phosphate ions. Geochim. Cosmochim. Acta 2013, 117, 115–128. [Google Scholar] [CrossRef]
- Naviaux, J.D.; Subhas, A.V.; Rollins, N.E.; Dong, S.; Berelson, W.M.; Adkins, J.F. Temperature dependence of calcite dissolution kinetics in seawater. Geochim. Cosmochim. Acta 2019, 246, 363–384. [Google Scholar] [CrossRef]
- Stefansson, A. Dissolution of primary minerals of basalt in natural waters I. Calculation of mineral solubilities from 0 °C to 350 °C. Chem. Geol. 2001, 172, 225–250. [Google Scholar] [CrossRef]
- Milliken, K. A Compositional Classification for Grain Assemblages in Fine-Grained Sediments and Sedimentary Rocks. J. Sediment. Res. 2014, 84, 1185–1199. [Google Scholar] [CrossRef]
- Yuan, G.; Cao, Y.; Gluyas, J.; Jia, Z. Reactive transport modeling of coupled feldspar dissolution and secondary mineral precipitation and its implication for diagenetic interaction in sandstones. Geochim. Cosmochim. Acta 2017, 207, 232–255. [Google Scholar] [CrossRef]
- Aagaard, P.; Egeberg, P.K.; Saigal, G.C.; Morad, S.; Bjorlykke, K. Diagenetic albitization of detrital K-feldspars in Jurassic, Lower Cretaceous and Tertiary clastic reservoir rocks from offshore Norway; II, Formation water chemistry and kinetic considerations. J. Sediment. Res. 1990, 60, 575–581. [Google Scholar] [CrossRef]
- Nicholson, P.H.; Esch, W.L.; Ajdukiewicz, J.M. Prediction of deep reservoir quality using early diagenetic process models in the jurassic norphlet formation, gulf of Mexico. AAPG Bull. 2010, 94, 1189–1227. [Google Scholar]
- Porten, K.; Kane, I.; Warchoł, M.; Southern, S.J. A Sedimentological Process-Based Approach to Depositional Reservoir Quality of Deep-Marine Sandstones: An Example from the Springar Formation, Northwestern Vøring Basin, Norwegian Sea. J. Sediment. Res. 2016, 86, 1269–1286. [Google Scholar] [CrossRef]
- Liu, D.; Yuan, P.; Liu, H.; Li, T.; Tan, D.; Yuan, W.; He, H. High-pressure adsorption of methane on montmorillonite, kaolinite and illite. Appl. Clay Sci. 2013, 85, 25–30. [Google Scholar] [CrossRef]



| Dividing Index | Number | Distribution Range (or Main Type) | Diagenetic Stage |
|---|---|---|---|
| Ro/% | 22 | 0.52–0.93 | Mesodiagegesis A |
| Tmax/°C | 90 | 431–461 °C | Mesodiagegesis A |
| Type and combination of clay minerals | 43 | (I/S + I + C) | Mesodiagegesis A |
| 43 | The ratio of I/S is 20% | Mesodiagegesis A | |
| 43 | Ordered I/S | Mesodiagegesis A | |
| 30 | Acicular and flocculent | Mesodiagegesis A | |
| Type and crystallinity of carbonate minerals | 30 | Sparry calcite | Mesodiagegesis A |
| 30 | Ferroan dolomite | Mesodiagegesis A | |
| Type of pores | 30 | Dissolved pores and recrystallized intergranular pores | Mesodiagegesis A |
| Color of sporopollen | 39 | Light yellow to black | Mesodiagegesis A |
| Samples | Depth/m | Al/% | Si/% | (Si/Al)total | Sidet/% | Siclay/% | Siexcess/% | Sitotal/% |
|---|---|---|---|---|---|---|---|---|
| NX124-2 | 3213.00 | 10.70 | 41.00 | 3.83 | 33.28 | 4.56 | 0.19 | 38.02 |
| NX124-4 | 3214.15 | 9.86 | 34.80 | 3.53 | 30.66 | 4.69 | 0.12 | 35.48 |
| NX124-7 | 3216.00 | 11.10 | 38.40 | 3.46 | 34.52 | 5.63 | 0.10 | 40.25 |
| NX124-10 | 3218.00 | 7.54 | 27.40 | 3.63 | 23.45 | 2.89 | 0.14 | 26.49 |
| NX124-15 | 3221.00 | 12.50 | 42.70 | 3.42 | 38.88 | 5.93 | 0.09 | 44.90 |
| NX124-19 | 3223.00 | 11.90 | 43.50 | 3.66 | 37.01 | 5.15 | 0.15 | 42.31 |
| NX124-23 | 3226.25 | 10.70 | 36.30 | 3.39 | 33.28 | 5.22 | 0.08 | 38.58 |
| NX124-28 | 3229.10 | 8.44 | 34.00 | 4.03 | 26.25 | 4.31 | 0.23 | 30.79 |
| NX124-32 | 3231.60 | 6.90 | 34.50 | 5.00 | 21.46 | 3.83 | 0.38 | 25.67 |
| NX124-38 | 3234.15 | 8.24 | 32.70 | 3.97 | 25.63 | 3.71 | 0.22 | 29.55 |
| NX124-47 | 3238.70 | 11.20 | 37.60 | 3.36 | 34.83 | 4.70 | 0.07 | 39.61 |
| NX124-57 | 3243.15 | 16.10 | 50.20 | 3.12 | 50.07 | 10.26 | 0.00 | 60.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, J.; Chen, S.; Yu, Z.; Zhang, P.; Feng, G. Origin and Reservoir Significance of Authigenic Minerals in Lacustrine Shales: A Case Study from the Paleogene Dongying Sag, Bohai Bay Basin, East China. Minerals 2025, 15, 493. https://doi.org/10.3390/min15050493
Yan J, Chen S, Yu Z, Zhang P, Feng G. Origin and Reservoir Significance of Authigenic Minerals in Lacustrine Shales: A Case Study from the Paleogene Dongying Sag, Bohai Bay Basin, East China. Minerals. 2025; 15(5):493. https://doi.org/10.3390/min15050493
Chicago/Turabian StyleYan, Jihua, Shiyue Chen, Zhiyun Yu, Pengfei Zhang, and Guozheng Feng. 2025. "Origin and Reservoir Significance of Authigenic Minerals in Lacustrine Shales: A Case Study from the Paleogene Dongying Sag, Bohai Bay Basin, East China" Minerals 15, no. 5: 493. https://doi.org/10.3390/min15050493
APA StyleYan, J., Chen, S., Yu, Z., Zhang, P., & Feng, G. (2025). Origin and Reservoir Significance of Authigenic Minerals in Lacustrine Shales: A Case Study from the Paleogene Dongying Sag, Bohai Bay Basin, East China. Minerals, 15(5), 493. https://doi.org/10.3390/min15050493





