Synthesis and Characterization of Na-X Zeolite Using a Natural Opaline Diatomite Rock from SE Spain
Abstract
1. Introduction
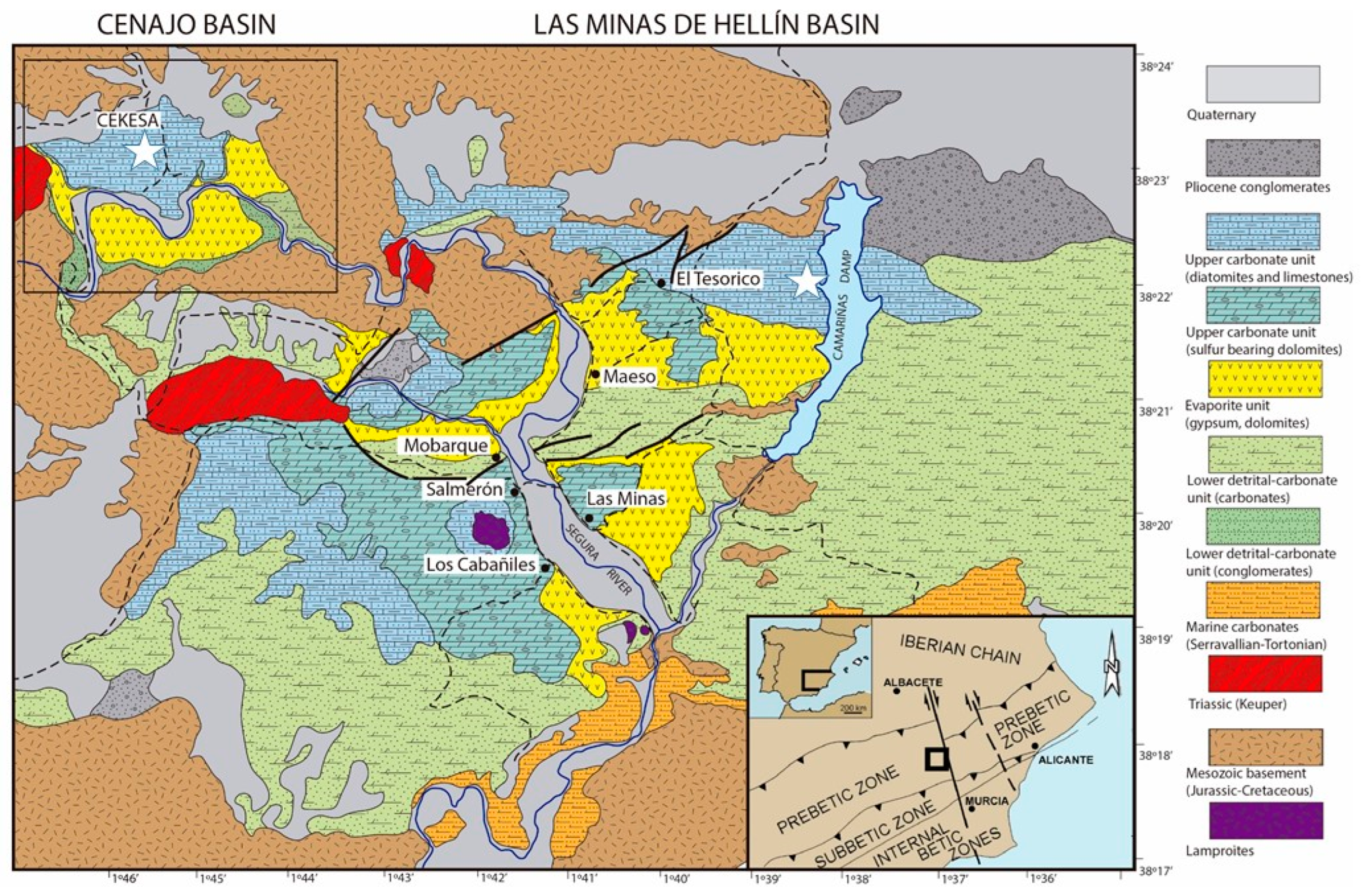
2. Diatomite Rocks: The “Albacete Diatomite”
3. Methods
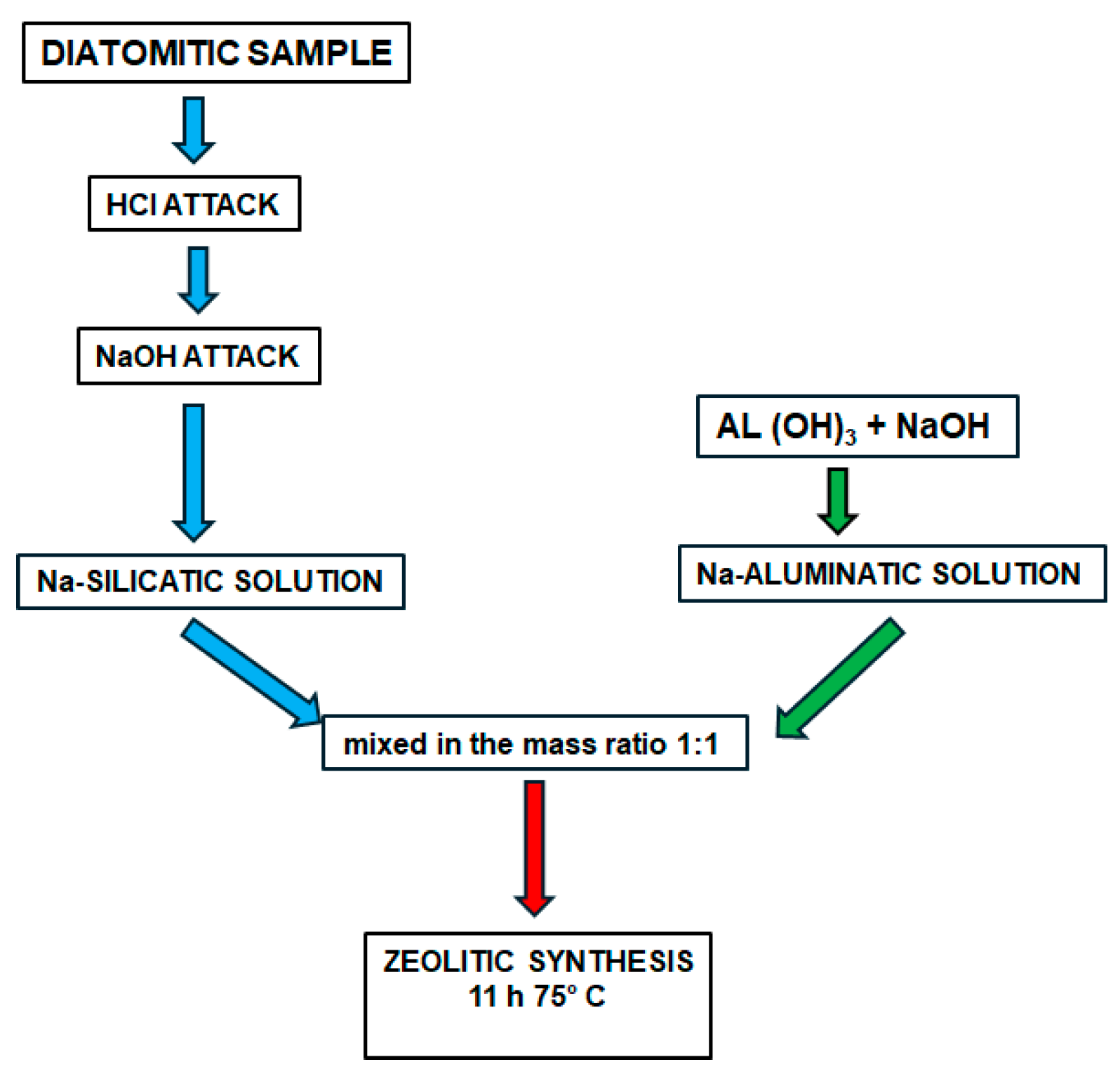
Preparation of Reagents from Natural Rocks and Zeolitic Synthesis
- Silicatic solution
- Aluminatic solution
- Zeolitic synthesis
4. Results and Discussion
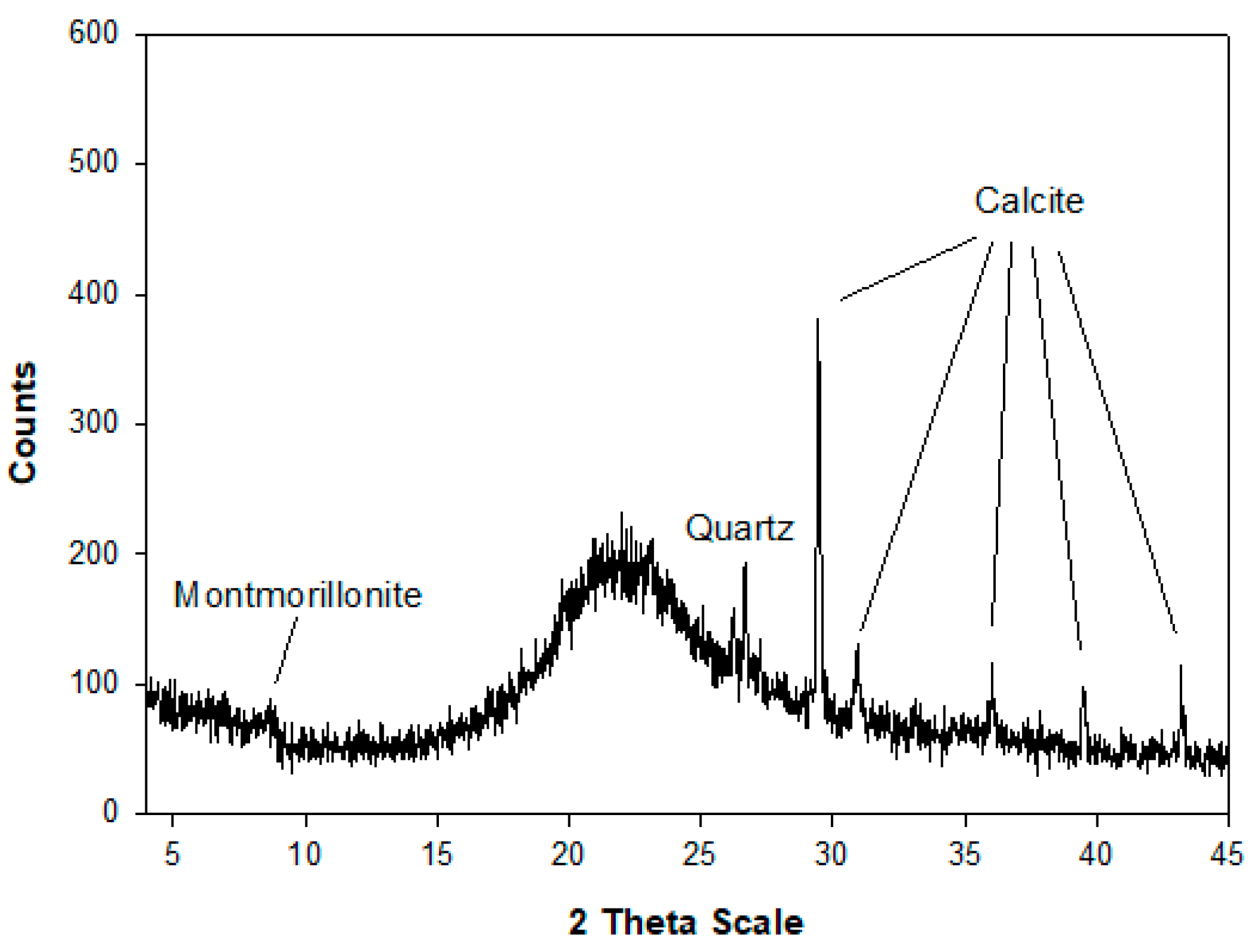
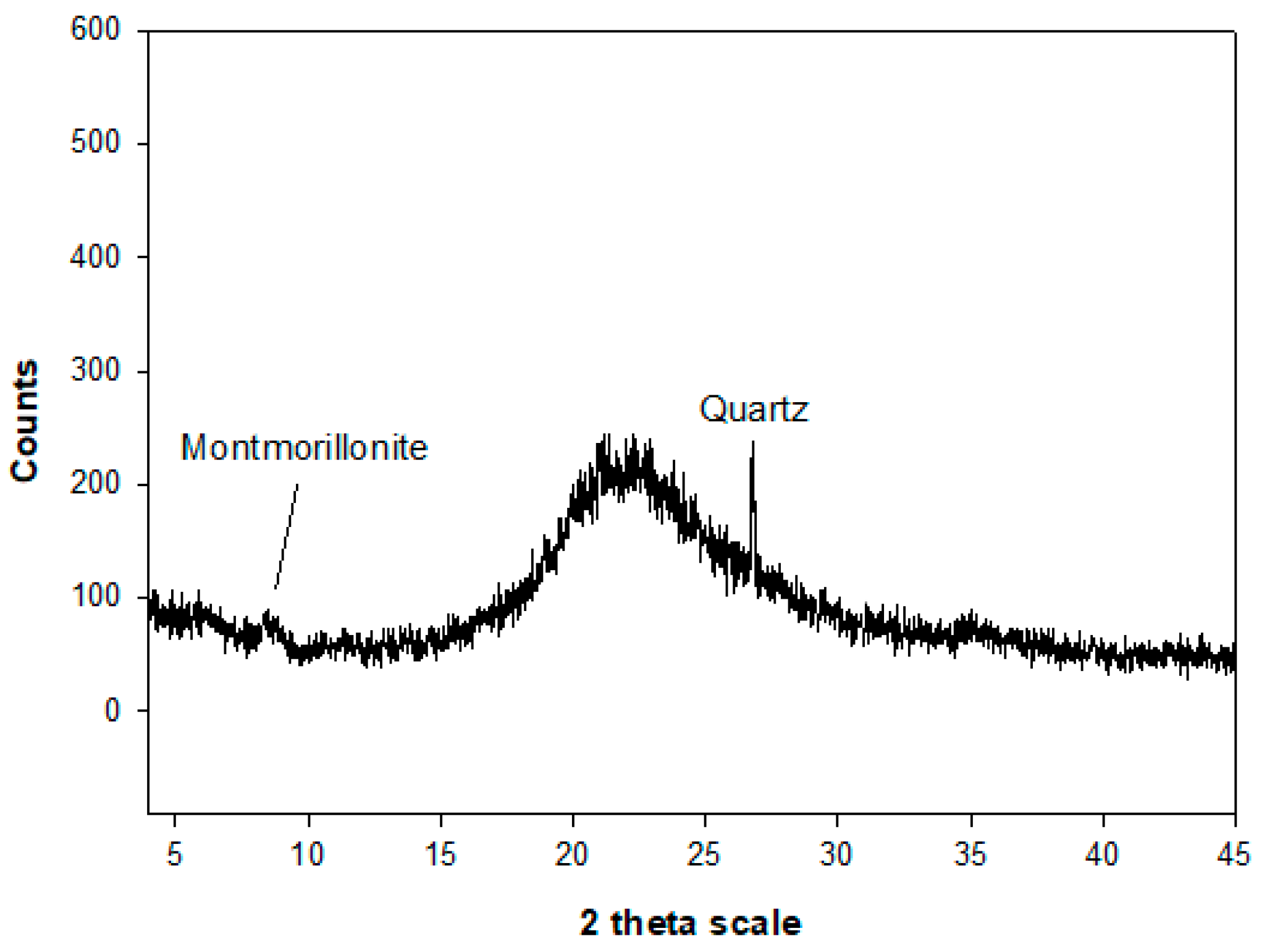
4.1. Mineralogical, Crystallographical and Chemical Characterisation of Na-X Zeolite
4.2. Textural and Physical Characterisation
4.3. Thermal Behaviour
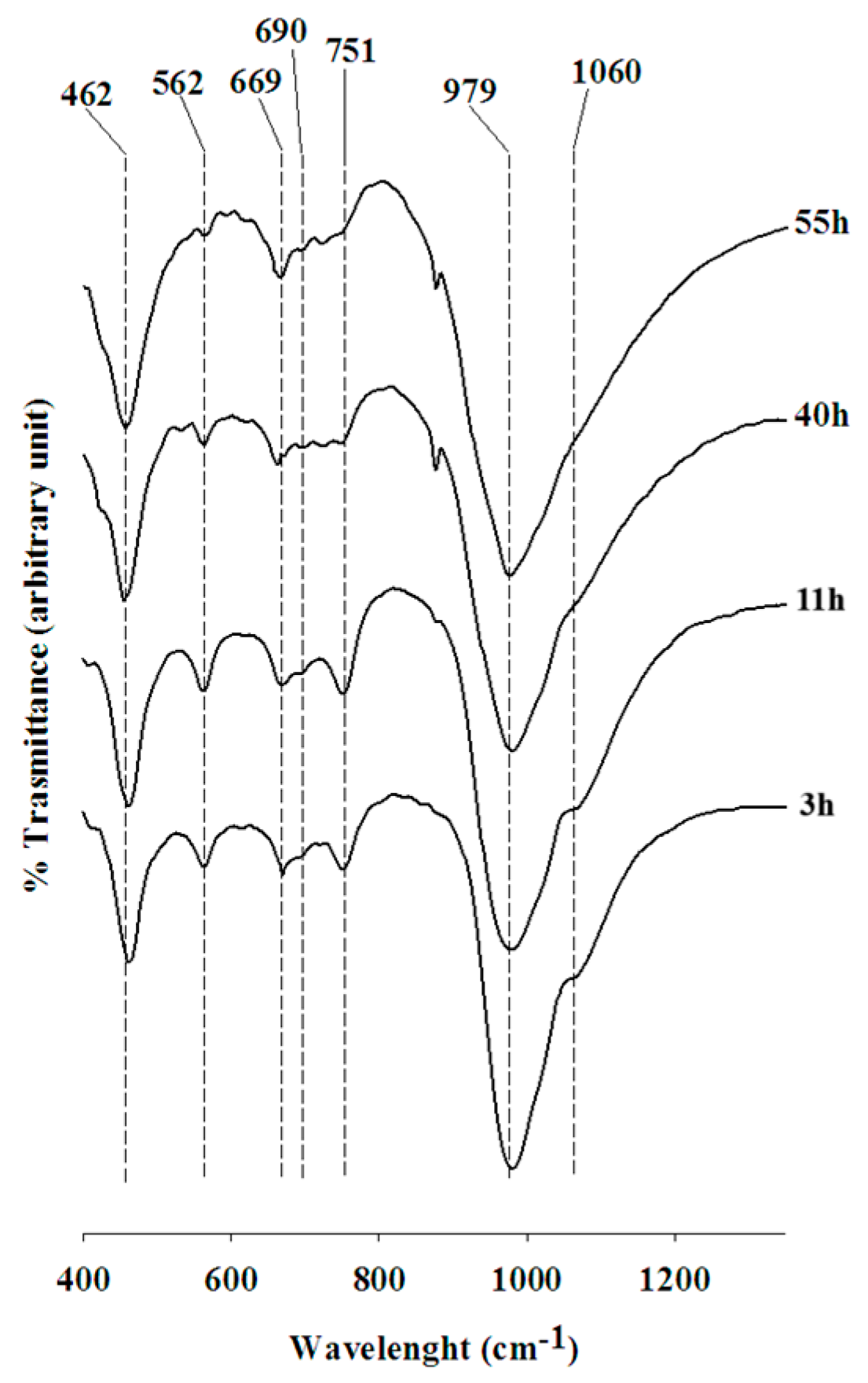
4.4. IR Spectroscopy
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beiersdorfer, R.E.; Ming, D.W.; Galindo, C. Solubility and cation exchange properties of zeoponic substrates. Microp. Mesopor. Mat. 2003, 61, 231–247. [Google Scholar] [CrossRef]
- Ackley, M.W.; Rege, S.U.; Saxena, H. Application of natural zeolites in the purification and separation of gases. Microp. Mesopor. Mat. 2003, 61, 25–42. [Google Scholar] [CrossRef]
- Perraki, T.; Kakali, G.; Kontoleon, F. The effect of natural zeolites on the early hydration of Portland cement. Microp. Mesopor. Mat. 2003, 61, 205–212. [Google Scholar] [CrossRef]
- Kowalak, S.; Jankowska, A. Application of zeolites as matrices for pigments. Microp. Mesopor. Mat. 2003, 61, 213–222. [Google Scholar] [CrossRef]
- Chang, H.L.; Shih, W.H. Synthesis of Zeolites A and X from Fly Ashes and TheirIon-Exchange Behavior with Cobalt Ions. Ind. Eng. Chem. Res. 2000, 39, 4185–4191. [Google Scholar] [CrossRef]
- Gesikiewicz-Puchalska, A.; Zgrzebnicki, M.; Michalkiewicz, B.; Kałamaga, A.; Narkiewicz, U.; Morawski, A.W.; Wrobel, R. Changes in Porous Parameters of the Ion Exchanged X Zeolite and Their Effect on CO2 Adsorption. Molecules 2021, 26, 7520. [Google Scholar] [CrossRef]
- Medykowska, M.; Wi´sniewska, M.; Chibowski, S. Fly Ash-Based Na-X Zeolite Application in Separation Process of Bovine Serum Albumin from Aqueous Solution in the Presence of Organic Substances with Anionic Character. Materials 2023, 16, 5201. [Google Scholar] [CrossRef]
- Zhou, H.; Korelskiy, D.; Leppajarvi, T.; Grahn, M.; Tanskanen, J.; Hedlund, J. Ultrathin zeolite X membranes for pervaporation dehydration of ethanol. J. Membr. Sci. 2012, 399, 106–111. [Google Scholar] [CrossRef]
- Li, X.S.; Remias, J.E.; Neathery, J.K.; Liu, K.L. NF/RO faujasite zeolite membrane- ammonia absorption solvent hybrid system for potential post-combustion CO2 capture application. J. Membr. Sci. 2011, 366, 220–228. [Google Scholar] [CrossRef]
- Perez, E.; Martin, L.; Rubio, C.; Urieta, J.S.; Piera, E.; Caballero, M.A.; Tellez, C.; Coronas, J. Encapsulation of alpha-Tocopheryl acetate into Zeolite Y for textile application. Ind. Eng. Chem. Res. 2010, 49, 8495–8500. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Desilets, F.; Felice, V.; Mecheri, B.; Licoccia, S.; Tavares, A.C. On the proton conductivity of Nafion-Faujasite composite membranes for low temperature direct methanol fuel cells. J. Power Sources 2011, 196, 9176–9187. [Google Scholar] [CrossRef]
- Felice, V.; Tavares, A.C. Faujasite zeolites as solid electrolyte for low temperature fuel cells. Solid State Ion. 2011, 194, 53–61. [Google Scholar] [CrossRef]
- Kulawong, S.; Youngjan, S.; Khemthong, P.; Chanlek, N.; Wittayakun, J.; Osakoo, N. Magnesium Impregnated on NaX Zeolite Synthesized from Cogon Grass Silica for Fast Production of ructose via Microwave-Assisted Catalytic Glucose Isomerization. Catalysts 2021, 11, 981. [Google Scholar] [CrossRef]
- Baši´c, A.; Penga, Ž.; Penga, J.; Kuzmani´c, N.; Svilovi´c, S. Zeolite NaX Mass and Propeller Agitator Speed Impact on Copper Ions Sorption. Processes 2023, 11, 264. [Google Scholar] [CrossRef]
- Ezzeddine, Z.; Batonneau-Gener, I.; Pouilloux, Y.; Hamad, H.; Saad, Z. Synthetic Nax Zeolite as a Very Efficient Heavy Metals Sorbent in Batch and Dynamic Conditions. Colloids Interfaces 2018, 2, 22. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, J.; Wang, Y.; Yang, M.; Li, Y.; Meng, C. Synthesis of zeolite X from low-grade bauxite. J. Chem. Technol. Biotechnol. 2013, 88, 1350–1357. [Google Scholar] [CrossRef]
- Breck, D.W. Zeolite molecular sieves. In Structure, Chemistry and Use; Robert E. Krieger Publishing Company: Malabar, FL, USA, 1984; p. 771. [Google Scholar]
- Aznar, A.J.; La Iglesia, A. Obtención de zeolitas a partir de arcillas aluminosas españolas. Boletín Geol. Y Min. 1985, 96, 541–549. [Google Scholar]
- Sanhueza, V.; Kelm, U.; Cid, R. Synthesis of molecular sieves from Chilean kaolinites: I. synthesis of NaA type zeolites. J. Chem. Technol. Biotechnol. 1999, 74, 358–363. [Google Scholar] [CrossRef]
- Novembre, D.; Gimeno, D.; Del Vecchio, A. Improvement in the synthesis conditions and studying the physicochemical properties of the zeolite Li-A (BW) obtained from a kaolinitic rock. Sci. Rep. 2020, 10, 5715–5723. [Google Scholar] [CrossRef]
- Novembre, D.; Gimeno, D.; Del Vecchio, A. Synthesis and characterization of Na-P1 (GIS) zeolite using a kaolinitic rock. Sci. Rep. 2021, 11, 4872–4883. [Google Scholar] [CrossRef]
- Novembre, D.; Gimeno, D. Synthesis and characterization of analcime (ANA) zeolite using a kaolinitic rock. Sci. Rep. 2021, 11, 13373–13382. [Google Scholar] [CrossRef]
- Abdmeziem-Hamoudi, K.; Siffert, B. Synthesis of molecular sieve zeolites from a smectite- type clay material. Appl. Clay Sci. 1989, 4, 1–9. [Google Scholar] [CrossRef]
- De Lucas, A.; Uguina, M.A.; Covian, I.; Rodriguez, L. Synthesis of 13X zeolite from calcined kaolin and sodium silicates for use in detergents. Ind. Eng. Chem. Res. 1992, 31, 2134–2140. [Google Scholar] [CrossRef]
- Feng, M.; Shi, Z.; Tong, Y.; Zhang, K. Facile Synthesis of Zeolite NaX from Natural Attapulgite Clay for Pb2+ Adsorption. Chemistry 2024, 6, 1217–1229. [Google Scholar] [CrossRef]
- Gualtieri, A.F. Synthesis of sodium zeolites from a natural halloysite. Phys. Chem. Miner. 2001, 28, 719–728. [Google Scholar] [CrossRef]
- Ibsaine, F.; Azizi, D.; Dionne, J.; Tran, L.H.; Coudert, L.; Pasquier, L.-C.; Blais, J.-F. Conversion of Aluminosilicate Residue Generated from Lithium Extraction Process to NaX Zeolite. Minerals 2023, 13, 1467. [Google Scholar] [CrossRef]
- Novembre, D.; Gimeno, D.; Marinangeli, L.; Tangari, A.C.; Rosatelli, G.; Ciulla, M.; di Profio, P. Rice Husk as Raw Material in Synthesis of NaA (LTA) Zeolite. Molecules 2024, 29, 4396. [Google Scholar] [CrossRef]
- Novembre, D.; Gimeno, D.; Marinangeli, L.; Tangari, A.C.; Rosatelli, G.; Ciulla, M.; di Profio, P. Synthesis and Characterization of Na-P1 (GIS) Zeolite Using Rice Husk. Molecules 2024, 29, 5596. [Google Scholar] [CrossRef]
- Takaaki, W.; Mioko, H.; Keiko, K.; Hiroji, I.; Osamu, T.; Kazuhiko, I.; Takashi, N.; Downs, R.T.; Rakovan, J.F. Zeolite synthesis from paper ash at low temperature (90 °C) with addition of diatomite. J. Hazard. Mater. 2006, B132, 244–252. [Google Scholar]
- Sanhueza, V.; Kelm, U.; Cid, R. Synthesis of mordenite from diatomite: A case of zeolite synthesis from natural material. J. Chem. Technol. Biotechnol. 2003, 78, 485–488. [Google Scholar] [CrossRef]
- Sanhueza, V.; Kelm, U.; Cid, R.; Lòpez-Escobar, L. Syntesis of ZSM-5 from diatomite: A case of zeolite syntesis from a natural material. J. Chem. Technol. Biotechnol. 2004, 79, 686–690. [Google Scholar] [CrossRef]
- Sanhueza, V.; Lòpez-Escobar, L.; Kelm, U.; Cid, R. Syntesis of a mesoporous material from two natural sources. J. Chem. Technol. Biotechnol. 2006, 81, 614–617. [Google Scholar] [CrossRef]
- Novembre, D.; Di Sabatino, B.; Gimeno, D.; Garcia Valles, M.; Martinez-Manent, S. Synthesis of Na-X zeolites from tripolaceous deposits (Crotone, Italy) and volcanic zeolitized rocks (Vico Volcano, Italy). Microp. Mesop. Mat. 2004, 75, 1–11. [Google Scholar] [CrossRef]
- Chaisena, A.; Rangsriwatananon, K. Syntesis of sodium zeolites from natural and modified diatomite. Mat. Lett. 2005, 59, 1474–1479. [Google Scholar] [CrossRef]
- Novembre, D.; Pace, C.; Gimeno, D. Syntheses and characterization of zeolites K-F and W type using a diatomite precursor. Mineral. Mag. 2014, 78, 1209–1225. [Google Scholar] [CrossRef]
- Vinaches, P.; da Silva Filho, S.H.; Souza, I.M.S.; Pergher, S.B.C. Diatomite incorporation in zeolite Fu-1. Mat. Lett. 2021, 293, 129697–129700. [Google Scholar] [CrossRef]
- Chandrasekar, S.; Pramada, P.N. Investigation on the synthesis of zeolite Na-X from Kerala kaolin. J. Por. Mat. 1999, 6, 283–297. [Google Scholar] [CrossRef]
- Rangsriwatananon, K.; Chaisena, A.; Thongkasam, C. Thermal and acid treatment on natural raw diatomite influencing in the synthesis of sodium zeolites. J. Porous Mater. 2008, 15, 499–505. [Google Scholar] [CrossRef]
- Du, Y.; Shia, S.; Daib, H. Water-bathing synthesis of high-surface-area zeolite P from diatomite. Particuology 2011, 9, 174–178. [Google Scholar] [CrossRef]
- Calvo, J.P. Los yacimientos de diatomita de España. Boletín Geológico y Minero. 1981, 92, 272–284. [Google Scholar]
- Calvo, J.P.; Elizaga, E. Diatomite deposits in Southeastern Spain: Geological and economical aspects. Ann. Inst. Geol. Publ. Hung. 1987, 70, 537–543. [Google Scholar]
- Griffiths, J. Spain minerals: Mixed fortunes. Ind. Miner. 1991, 285, 23–47. [Google Scholar]
- Regueiro, M.; Calvo, J.P.; Elizaga, E.; Calderón, V. Spanish diatomite: Geology and economics. Ind. Miner. 1993, 306, 57–67. [Google Scholar]
- Regueiro, M.; Calvo, J.P.; Elizaga, E.; Calderón, V. Diatomite deposits in Spain: An overview. In Proceedings of the VI International Flint Symposium, Abstracts Book, 93–99 ITGE, Madrid, Spain, 17–19 October 1991. [Google Scholar]
- Estadística Minera de España, Ministerio Para la Transición Ecológica y el Reto Demográfico. Available online: https://www.miteco.gob.es/content/dam/miteco/es/energia/files-1/mineria/Estadistica/DatosBibliotecaConsumer/2022/Estad%C3%ADstica%20Minera%20de%20Espa%C3%B1a%202022.pdf (accessed on 15 January 2025).
- Regueiro, M.; García-Ten, J.; Alonso-Jiménez, A. Synthesis of wollastonite from diatomite-rich marls and its potential in ceramic uses. Bol. Soc. Esp. Ceram Vidrio 2022, 61, 585–594. [Google Scholar] [CrossRef]
- Costafreda, J.L.; Martín, D.A.; Astudillo, B.; Presa, L.; Para, J.L.; Sanjuán, M.A. Diatomites from the Iberian Peninsula as Pozzolans. Materials 2023, 16, 3883. [Google Scholar] [CrossRef]
- García-Veigas, J.; Gimeno, D.; Pineda, V.; Cendón, D.I.; Sánchez-Román, M.; Artiaga, D.; Bembibre, G. The elemental sulfur ore deposit of Salmerón, Las Minas de Hellín basin. Late Miocene, SE Spain. Bol. Geol. Minero 2022, 133, 135–161. [Google Scholar] [CrossRef]
- Larson, A.C.; Von Dreele, R.B. GSAS: General Structure Analysis System. Document Laur 86-748; Los Alamos National Laboratory: Los Alamos, NM, USA, 1997. [Google Scholar]
- Toby, B.H. EXPGUI, a Graphical User Interface for GSAS. J. Appl. Crystallogr. 2001, 34, 210–213. [Google Scholar] [CrossRef]
- Olson, D.H. The crystal structure of dehydrated NaX. Zeolites 1995, 15, 439–443. [Google Scholar] [CrossRef]
- Felsche, J.; Luger, S.; Baerlocher, C. Crystal structures of the hydro-sodalite Na6[AlSiO4]6 8H2O and of the anhydrous sodalite Na6[AlSiO4]6. Zeolites 1986, 5, 367–372. [Google Scholar] [CrossRef]
- Novembre, D.; Gimeno, D.; d’Alessandro, N.; Tonucci, L. Hydrothermal synthesis and characterization of kalsilite by using a kaolinitic rock from Sardinia, Italy, and its application in the production of biodiesel. Mineral. Mag. 2018, 82, 961–973. [Google Scholar] [CrossRef]
- Fernandez-Turiel, J.L.; Gimeno, D.; Valero, F.; Rodríguez, J.J.; Carnicero, M.; Rodríguez, J.J. Factors influencing the quality of a surface water supply system: The Ter river, northeastern Spain. Fres. Environ. Bull. 2003, 12, 67–75. [Google Scholar]
- Imai, N.; Terashima, S.; Itoh, S.; Ando, A. 1994 compilation values for GSJ reference samples, Igneous Rocks series. Geochem. J. 1995, 29, 91–95. [Google Scholar] [CrossRef]
- Aulinas, M.; Gimeno, D.; Fernandez-Turiel, J.L.; Perez-Torrado, F.J.; Rodriguez-Gonzalez, A.; Gasperini, D. The Plio-Quaternary magmatic feeding system beneath Gran Canaria (Canary Islands, Spain): Constraints from thermobarometric studies. J. Geol. Soc. 2010, 167, 785–801. [Google Scholar] [CrossRef]
- Stamires, D.N. Properties of the Zeolite, Faujasite, Substitutional Series: A Review with New Data. Clays Clay Min. 1973, 21, 379–389. [Google Scholar] [CrossRef]
- Flaningen, E.M.; Khatami, H.A.; Szymanski, H.A. Infrared structural study of zeolite frameworks. Mol. Sieve Zeolites 1971, 16, 201–229. [Google Scholar]


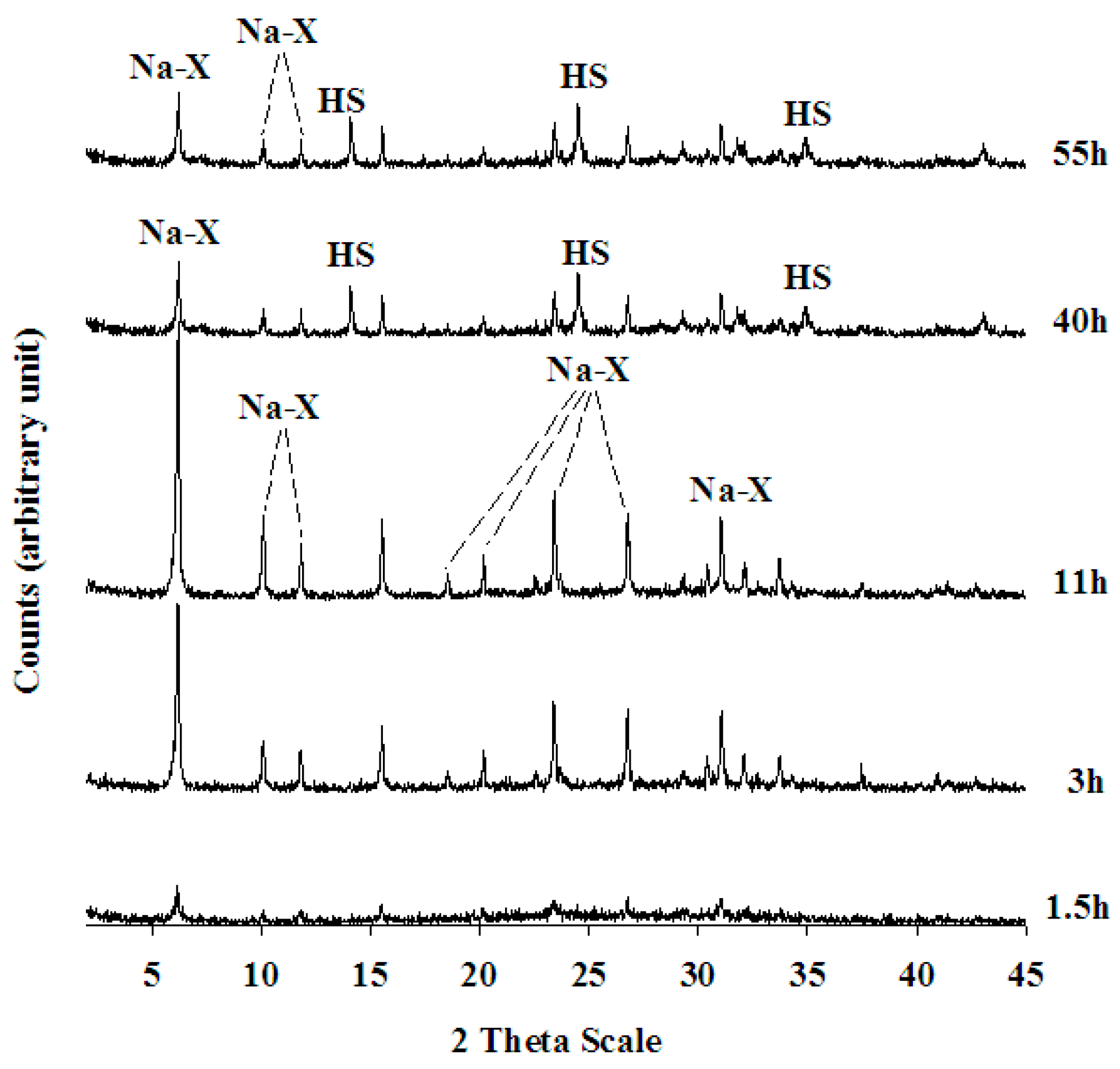
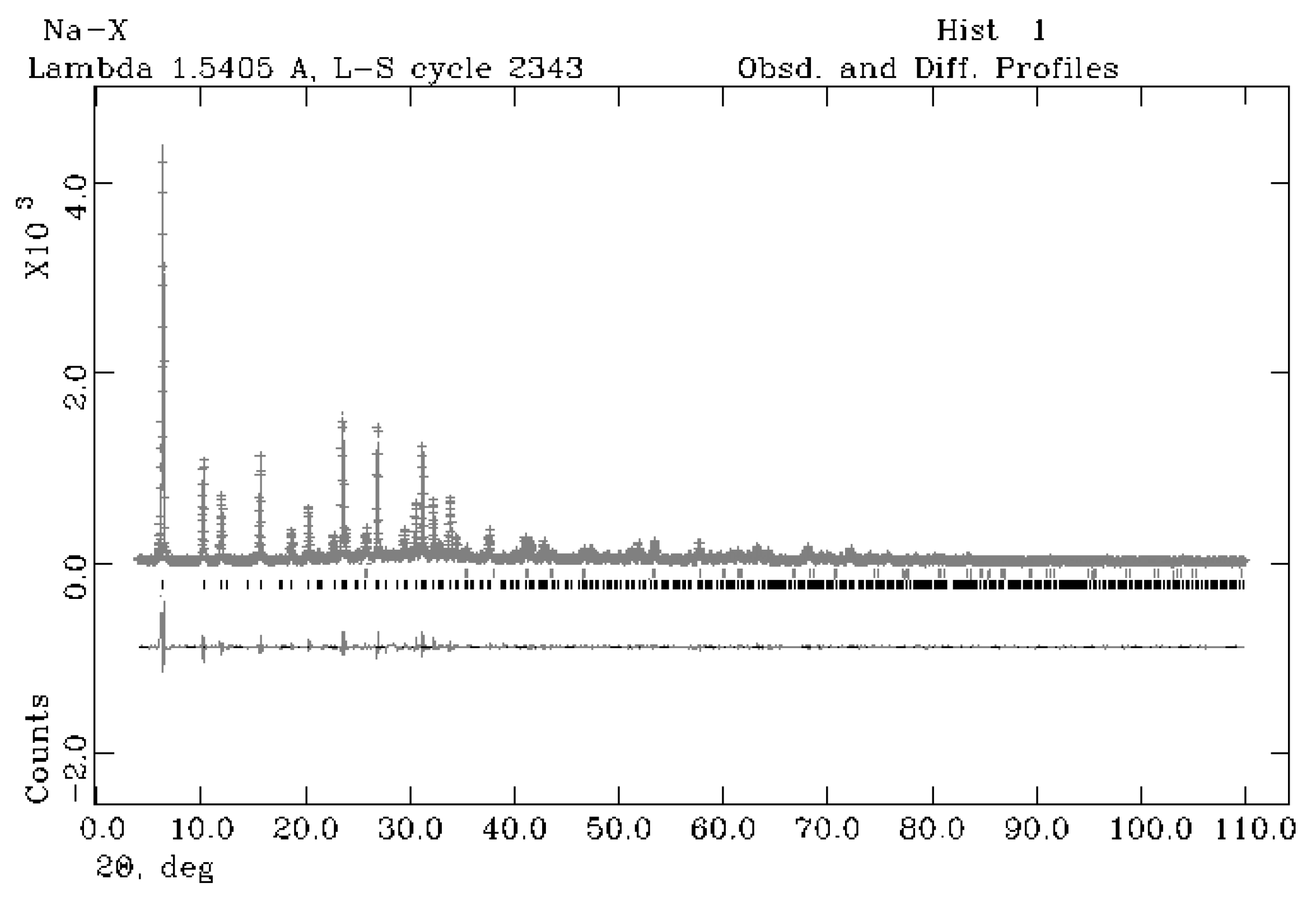
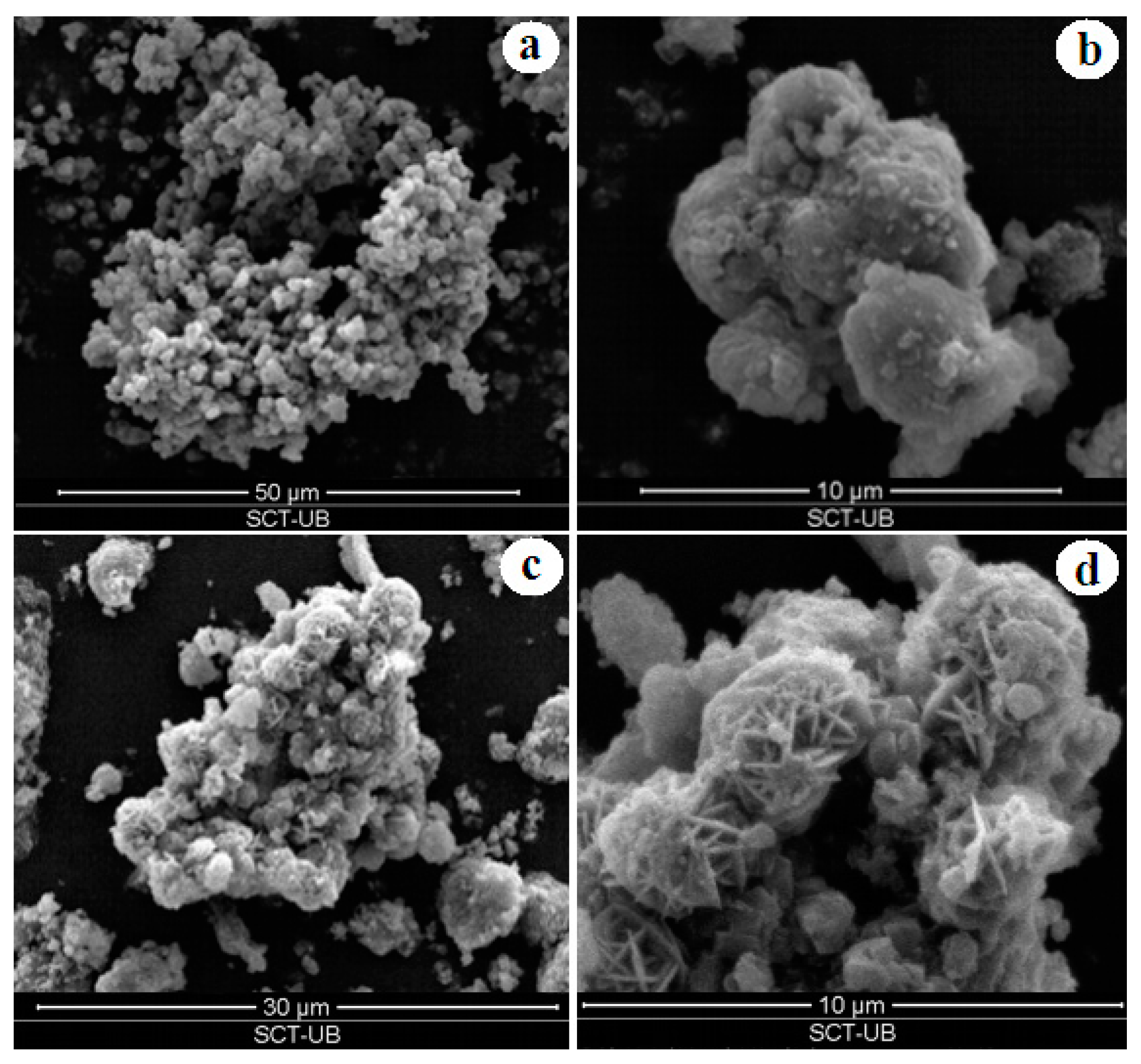


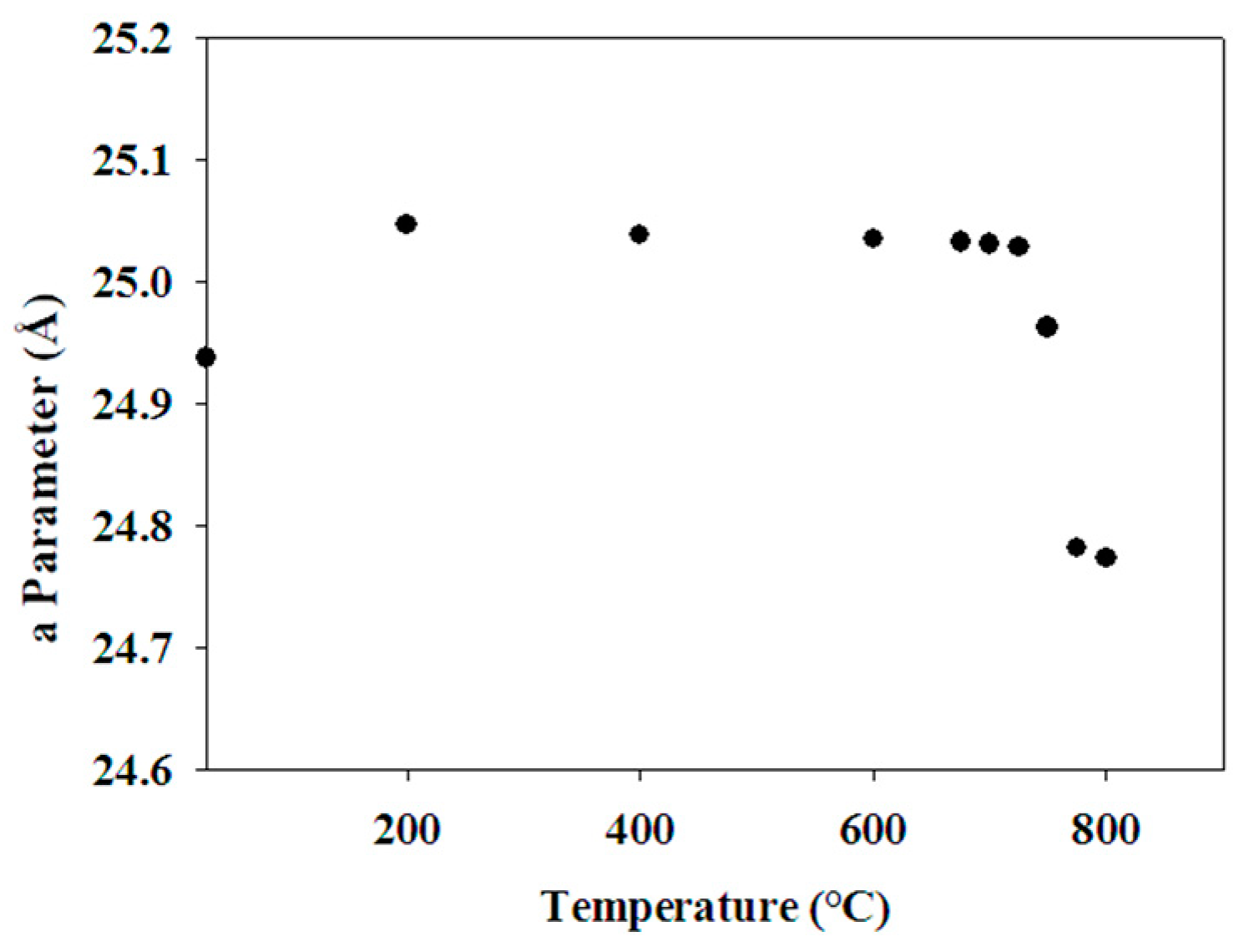
| 11 h | Na-X (*) | |
|---|---|---|
| SiO2 | 16.80 (0.37) | 1.36 |
| Al2O3 | 13.02 (0.18) | 12.77 |
| Fe2O3 | 0.02 (0.01) | / |
| CaO | 0.13 (0.01) | / |
| Na2O | 8 (0.09) | 11.27 |
| K2O | 0.03 (0.05) | / |
| Si/Al | 1.1 | 1.28 |
| Si/Si+Al | 0.52 | 0.56 |
| Sample + 10% Corundum Nist 676a | 75 °C–11 h |
|---|---|
| Rwp | 0.12 |
| Rp | 0.08 |
| CHI2 | 2.33 |
| space group Na-X | Fd-3 |
| a (Å) | 24.97 (32) |
| Na-X | 92.6 (14) |
| % amorphous | 7.4 (18) |
| Zeolite | Asymmetric Stretch | Symmetric Strech | Doulble Rings |
|---|---|---|---|
| Na-X (11 h) | 1060 (M)-979-(S) | 751 (M)-669 (W)-690 (M) | 562 (M) |
| Na-X (*) | 1060 (M)-971 (S) | 746 (M)-668 (W)-690 (M) | 560 (M) |
| Na-X (**) | 1065 (M)-982 (S) | 753 (M)-671 (W)-692 (M) | 566 (M) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novembre, D.; Gimeno, D. Synthesis and Characterization of Na-X Zeolite Using a Natural Opaline Diatomite Rock from SE Spain. Minerals 2025, 15, 238. https://doi.org/10.3390/min15030238
Novembre D, Gimeno D. Synthesis and Characterization of Na-X Zeolite Using a Natural Opaline Diatomite Rock from SE Spain. Minerals. 2025; 15(3):238. https://doi.org/10.3390/min15030238
Chicago/Turabian StyleNovembre, Daniela, and Domingo Gimeno. 2025. "Synthesis and Characterization of Na-X Zeolite Using a Natural Opaline Diatomite Rock from SE Spain" Minerals 15, no. 3: 238. https://doi.org/10.3390/min15030238
APA StyleNovembre, D., & Gimeno, D. (2025). Synthesis and Characterization of Na-X Zeolite Using a Natural Opaline Diatomite Rock from SE Spain. Minerals, 15(3), 238. https://doi.org/10.3390/min15030238








