Mechanism Research for the Influence of TiO2 Content on the Shape Transformation of Rutile Crystals
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Procedures
2.2.1. Experimental Procedure for the Influence of TiO2 Content on Rutile Shape and Rutile Settling
2.2.2. Experimental Procedure for Effect of TiO2 Content on the Isothermal Precipitation Kinetics of Rutile
3. Results and Discussion
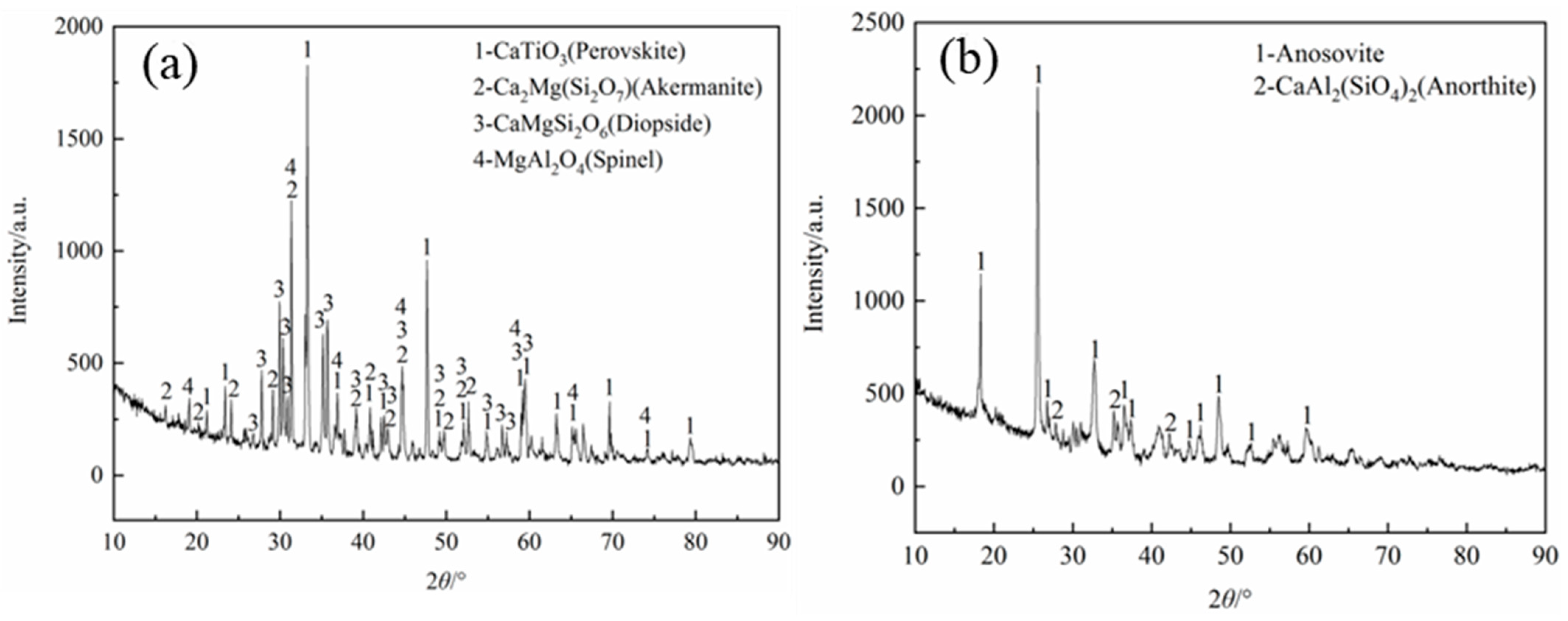
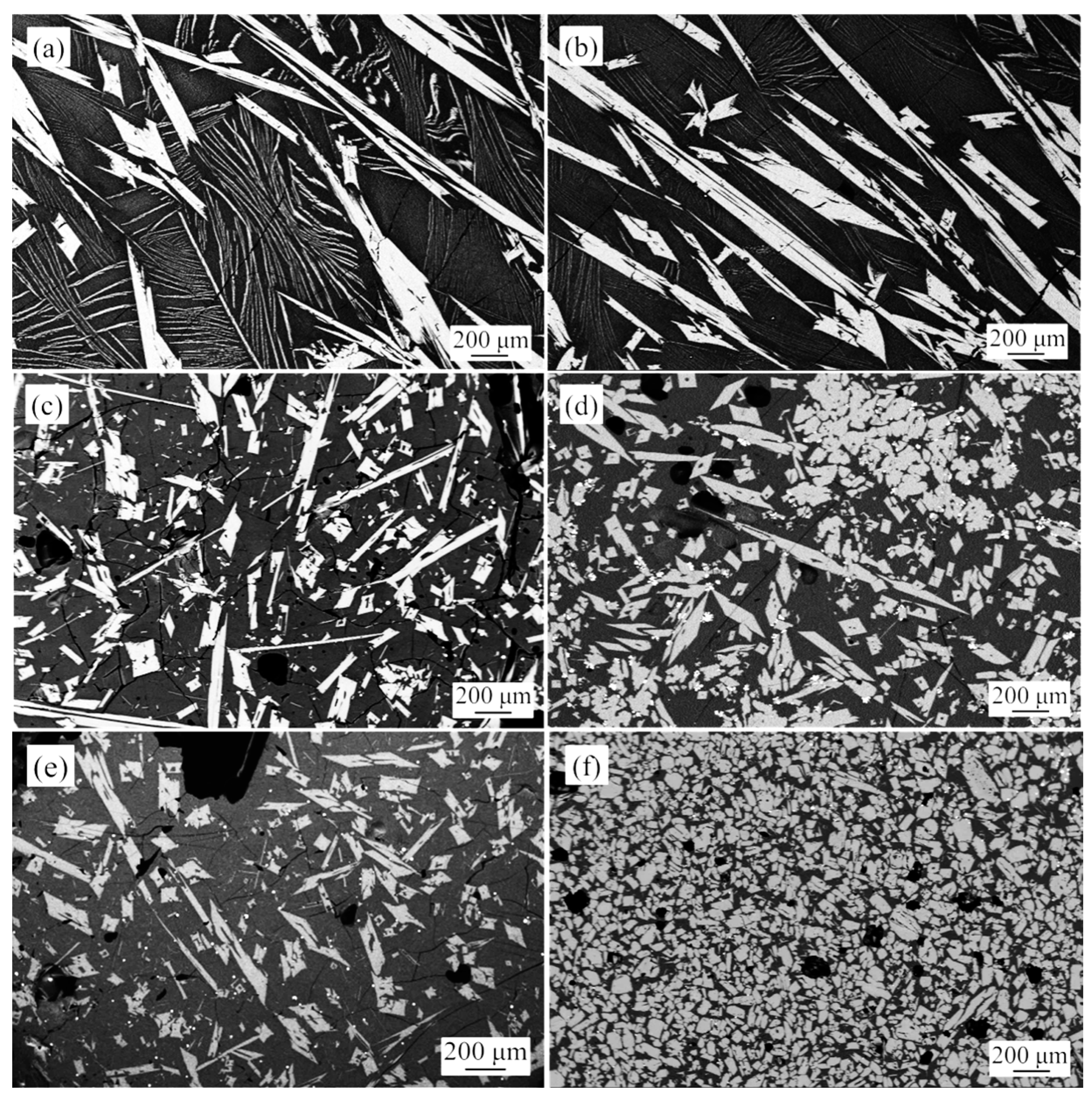
3.1. Influence of TiO2 Content on Rutile Shape and Rutile Settling
3.2. Influence of TiO2 Content on the Isothermal Precipitation Kinetics of Rutile
3.2.1. Theoretical Derivation of the Isothermal Precipitation Kinetics Equation of Rutile
3.2.2. Experimental Study on the Isothermal Precipitation Kinetics of Rutile
4. Conclusions
- While the TiO2 content of the raw materials increased from 27 to 47%, the shape transformation of the rutile crystal was as follows: cuboid → cube → sphere.
- The isothermal precipitation kinetics of the rutile crystal can be described by the JMAK Equation.
- When the TiO2 contents of the raw materials were 27, 37, and 47%, the growth index n was about 2, 3, and 4, respectively, indicating that the precipitation of the rutile crystal had a one-dimensional, two-dimensional, and three-dimensional growth. Thus, the shapes of the rutile crystals were a cuboid, a cube, and a sphere.
- The isothermal precipitation activation energy (absolute value) of the rutile crystal gradually decreased with an increase in the TiO2 content of the raw materials, implying that an increase in the TiO2 content of the raw materials is conducive to the precipitation of the rutile crystal.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yan, F.; Li, C.; Liang, B. A two-step sulfuric acid leaching process of Ti-bearing blast furnace slag. Chin. J. Process Eng. 2006, 3, 413–417. (In Chinese) [Google Scholar]
- Zhou, X.L.; Lu, X.G. Preparation of titanium alloy by direct reduction of Ti-bearing blast furnace slag. Chin. J. Nonferrous Met. 2010, 20, 1829–1835. (In Chinese) [Google Scholar]
- Lei, X.F.; Xue, X.X. Effect of sulfate on photocatalytic activity of titanium-bearing blast furnace slag. Mater. Rep. 2009, 23, 63–66. (In Chinese) [Google Scholar]
- Yang, H.; Zhao, S. Comprehensive utilization of BF slags in building materials. Conserv. Util. Miner. Resour. 2004, 1, 47–51. (In Chinese) [Google Scholar]
- Gao, Y.M.; Li, C.Y.; Li, Y.W. Analysis of carbothermal reduction of TiO2 and extraction of titanium carbonitride from the blast furnace slag bearing titania. J. Wuhan Univ. Sci. Technol. 2007, 30, 5–9. (In Chinese) [Google Scholar]
- Li, Y.H.; Lou, T.P.; Sui, Z.T. Selective enrichment of Ti component in Ti-bearing blast furnace slag and precipitation behavior of perovskite phase. Chin. J. Nonferrous Met. 2000, 5, 719–722. (In Chinese) [Google Scholar]
- Li, J.; Zhang, Z.T.; Zhang, M.; Guo, M.; Wang, X. The influence of SiO2 on the extraction of Ti element from Ti-bearing blast furnace slag. Steel Res. Int. 2011, 82, 607–614. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.H.; Zhang, W.; Li, G.Q. Thermodynamic analysis of extraction of synthetic rutile from modified slag. Ind. Eng. Chem. Res. 2013, 52, 4924–4931. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, L.; Zhang, J.H.; Feng, N.X. Crystallization and coarsening kinetics of rutile phase in modified Ti-bearing blast furnace slag. Ind. Eng. Chem. Res. 2012, 51, 12294–12298. [Google Scholar]
- Zhang, W.; Zhang, L.; Li, Y.H.; Li, X. An environmental procedure to extract titanium components and metallic iron from Ti-bearing blast furnace slag. Green Process. Synth. 2015, 4, 307–316. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, L.; Li, Y.H.; Li, X. Crystallization behavior and growing process of rutile crystals in Ti-bearing blast furnace slag. High Temp. Mater. Process. 2016, 35, 787–797. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.D.; Zhang, Z.T. Crystallization behavior of rutile in the synthesized Ti-bearing blast furnace slag using single hot thermocouple technique. ISIJ Int. 2011, 51, 1396–1402. [Google Scholar] [CrossRef]
- Sun, Y.Q.; Li, J.; Wang, X.D.; Zhang, Z.T. The effect of P2O5 on the crystallization behaviors of Ti-bearing blast furnace slags using single hot thermocouple technique. Metall. Mater. Trans. B 2014, 45, 1446–1455. [Google Scholar] [CrossRef]
- Du, Y.; Gao, J.T.; Lan, X.; Guo, Z.C. Selective precipitation and in situ separation of rutile crystals from titanium bearing slag melt in a super-gravity field. Cryst. Eng. Comm. 2018, 20, 3868–3876. [Google Scholar] [CrossRef]
- Du, Y.; Gao, J.T.; Lan, X.; Guo, Z.C. Recovery of rutile from Ti-bearing blast furnace slag through phase transformation and super-gravity separation for dielectric material. Ceram. Int. 2020, 46, 9885–9893. [Google Scholar] [CrossRef]
- Han, J.Q.; Zhang, J.H.; Chen, X.; Zhang, J.; Zhang, L.; Tu, G.F. Effect of rutile crystal shapes on its settlement. Trans. Nonferrous Met. Soc. China 2020, 30, 2848–2860. [Google Scholar] [CrossRef]
- Xu, B.P.; Yuan, G.Z.; Li, M.; Lei, Q.R.; You, B. Determination of low valence titanium (Ti2+, Ti3+) in vanadium titanium blast furnace slag. Iron Steel Vanadium Titan. 1989, 10, 61–66. [Google Scholar]
- Lasheen, T.A. Soda ash roasting of titania slag product from Rosetta ilmenite. Hydrometallurgy 2008, 93, 124–128. [Google Scholar] [CrossRef]
- Liu, S.S.; Guo, Y.F.; Qiu, G.Z.; Jiang, T.; Chen, F. Preparation of Ti-rich material from titanium slag by activation roasting followed by acid leaching. Trans. Nonferrous Met. Soc. China 2013, 23, 1174–1178. [Google Scholar] [CrossRef]
- Chen, G. Rapid synthesis of ruble TiO2 powders using microwave heating. J. Alloys Compd. 2015, 651, 503–508. [Google Scholar] [CrossRef]
- Borowiec, K.; Grau, A.E.; Gueguin, M.; Turgeon, J.F. Method to Upgrade Titania Slag and Resulting Product. U.S. Patent 5830420, 29 May 1997. [Google Scholar]
- Gueguin, M. Method of Preparing a Synthetic Rutile from a Titaniferous Slag Containing Magnesium Values. U.S. Patent 5063032, 5 November 1991. [Google Scholar]
- Yang, Y.H. Experimental Study on Preparation of Titanium Rich material from Titanium Slag in Electric Furnace; Kunming University of Science and Technology: Kunming, China, 2006. [Google Scholar]
- Borowiec, K. Sulphidization of solid titania slag. Scand. J. Metall. 1991, 20, 198–204. [Google Scholar]
- Lorenzo, A.T.; Arnal, M.L.; Albuerne, J.; Müller, A.J. DSC isothermal polymer crystallization kinetics measurements and the use of the Avrami equation to fit the data: Guidelines to avoid common problems. Polym. Test. 2007, 26, 222–231. [Google Scholar] [CrossRef]
- Kalu, P.N.; Waryoba, D.R. A JMAK-microhardness model for quantifying the kinetics of restoration mechanisms in inhomogeneous microstructure. Mater. Sci. Eng. A 2007, 464, 68–75. [Google Scholar] [CrossRef]
- Li, J.J.; Wang, J.C.; Xu, Q.; Yang, G.C. Comparison of Johnson-Mehl-Avrami-Kologoromov (JMAK) kinetics with a phase field simulation for polycrystalline solidification. Acta Mater. 2007, 55, 825–832. [Google Scholar] [CrossRef]
- Petrowsky, M.; Frech, R. Salt concentration dependence of the compensated Arrhenius equation for alcohol-based electrolytes. Electrochim. Acta 2010, 55, 1285–1288. [Google Scholar] [CrossRef]
- Chen, H.; Liu, N. Application of non-Arrhenius equations in interpreting calcium carbonate decomposition kinetics: Revisited. J. Am. Ceram. Soc. 2010, 93, 548–553. [Google Scholar] [CrossRef]




| Raw Materials | CaO | SiO2 | TiO2 | Ti2O3 | Al2O3 | MgO |
|---|---|---|---|---|---|---|
| 1 | 26.87 | 25.13 | 17.58 | 3.86 | 14.08 | 7.86 |
| 2 | 4.32 | 8.85 | 60.72 | 14.65 | 2.64 | 2.02 |
| No. | Ti-Bearing Blast Furnace Slag/g | Titanium Slag/g | SiO2/% | Oxidation Time/s | Total TiO2 Content/% |
|---|---|---|---|---|---|
| 1 | 407 | 93 | 18 | 168 | 27 |
| 2 | 315 | 185 | 10 | 168 | 37 |
| 3 | 222 | 278 | 8 | 126 | 47 |
| No. | The Content of Ti2O3/% |
|---|---|
| 1 | 1.14 |
| 2 | 1.27 |
| 3 | 1.23 |
| Method | Conditions | Advantages | Disadvantages |
|---|---|---|---|
| Sodium salt roasting–leaching [18] | 900–925 °C, 14%–25%H2SO4 | High-grade rutile | Corrosion of furnace lining |
| Phosphoric acid roasting–leaching [19] | 1000 °C, 15%H2SO4 | Medium-grade rutile | Corrosion of furnace lining |
| Microwave roasting–leaching [20] | 950 °C, 30%H3PO4 | Environmental protection in the roasting process | Low-grade rutile |
| Oxidation–reduction–leaching [21] | 800–1050 °C, 15%–22%HCl | Medium-grade rutile | High energy consumption |
| Oxidation–chlorination–leaching [22] | 800–850 °C, 10%–30%HCl | High-grade rutile | High energy consumption |
| Acid alkali combined leaching [23] | 30~180 g/L NaOH, 25 g/L HCl | Medium-grade rutile | More wastewater |
| Sulfation roasting–water leaching [24] | 600–1000 °C | Medium-grade rutile | Environmental pollution |
| TiO2 Content/% | Temperature/°C | k | n | R2/% |
|---|---|---|---|---|
| 27 | 1390 | 0.011 | 1.93 | 99.08 |
| 1410 | 0.0081 | 2.00 | 99.41 | |
| 1430 | 0.0065 | 2.00 | 99.05 | |
| 37 | 1390 | 0.0066 | 3.11 | 99.10 |
| 1410 | 0.0052 | 3.17 | 99.47 | |
| 1430 | 0.0039 | 3.21 | 99.25 | |
| 47 | 1390 | 0.0051 | 3.93 | 99.93 |
| 1410 | 0.0039 | 3.90 | 99.73 | |
| 1430 | 0.0032 | 4.05 | 99.79 |
| TiO2 Content/% | k0 | E/(kJ/mol) | R2/% |
|---|---|---|---|
| 27 | 9.4 × 10−13 | −320.7 | 96.76 |
| 37 | 1.3 × 10−12 | −308.8 | 99.61 |
| 47 | 1.4 × 10−11 | −273.2 | 96.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, J.; Zhang, L.; Yin, H.; Feng, Q.; Zhang, H. Mechanism Research for the Influence of TiO2 Content on the Shape Transformation of Rutile Crystals. Minerals 2025, 15, 449. https://doi.org/10.3390/min15050449
Han J, Zhang L, Yin H, Feng Q, Zhang H. Mechanism Research for the Influence of TiO2 Content on the Shape Transformation of Rutile Crystals. Minerals. 2025; 15(5):449. https://doi.org/10.3390/min15050449
Chicago/Turabian StyleHan, Jiqing, Li Zhang, Hongmei Yin, Qiuping Feng, and Hongsheng Zhang. 2025. "Mechanism Research for the Influence of TiO2 Content on the Shape Transformation of Rutile Crystals" Minerals 15, no. 5: 449. https://doi.org/10.3390/min15050449
APA StyleHan, J., Zhang, L., Yin, H., Feng, Q., & Zhang, H. (2025). Mechanism Research for the Influence of TiO2 Content on the Shape Transformation of Rutile Crystals. Minerals, 15(5), 449. https://doi.org/10.3390/min15050449






