Construction of Structured Hydrotalcite Supported with Silver Halide and Its Enhanced Visible Light Photocatalytic Degradation of Methyl Orange
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Catalysts
2.3. Characterization of Catalysts
2.4. Degradation Experiment of HPG
3. Results and Discussion
3.1. Morphological and Microstructural Characterization
3.1.1. XRD Analysis
3.1.2. BET Analysis
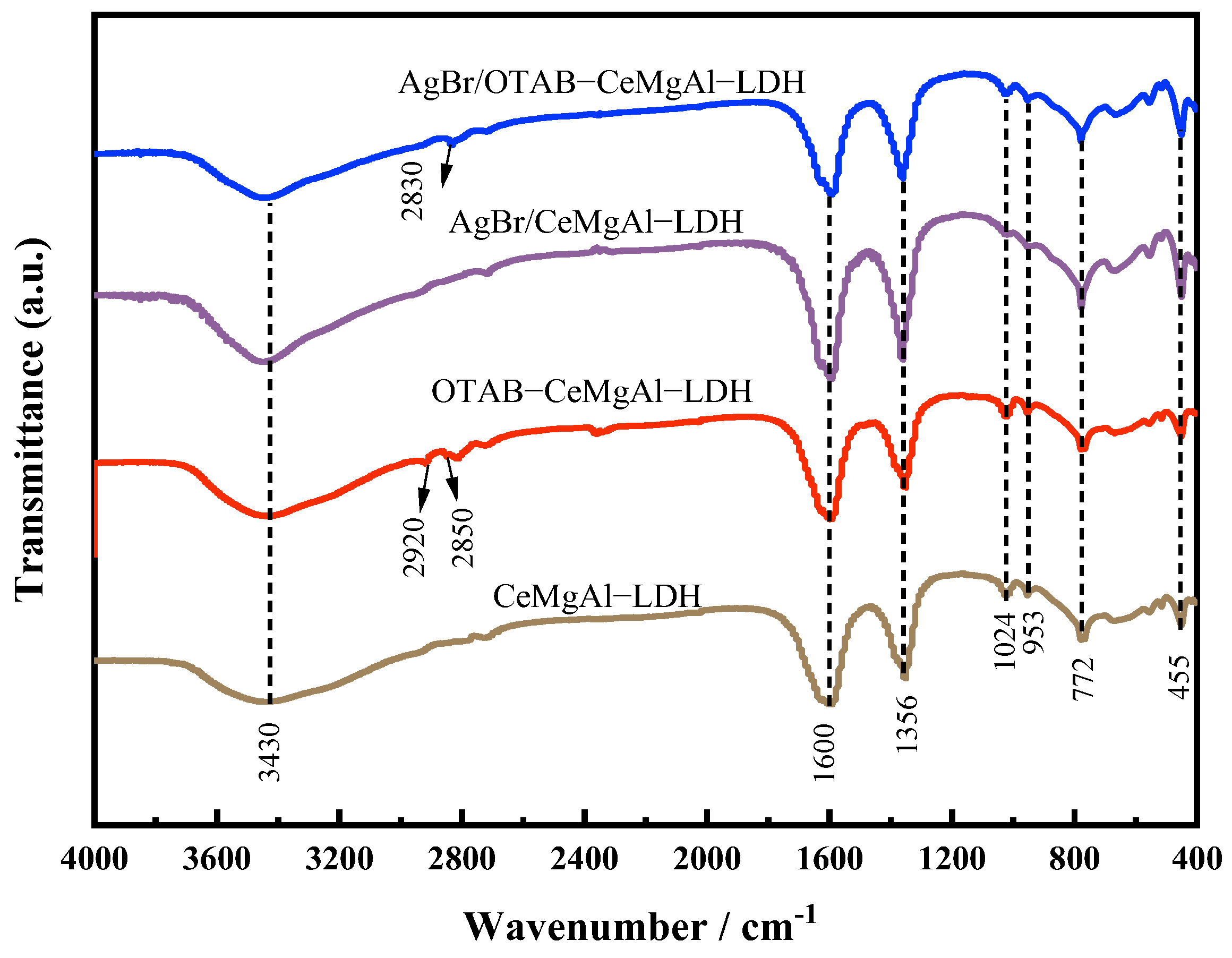
3.1.3. FT-IR Analysis
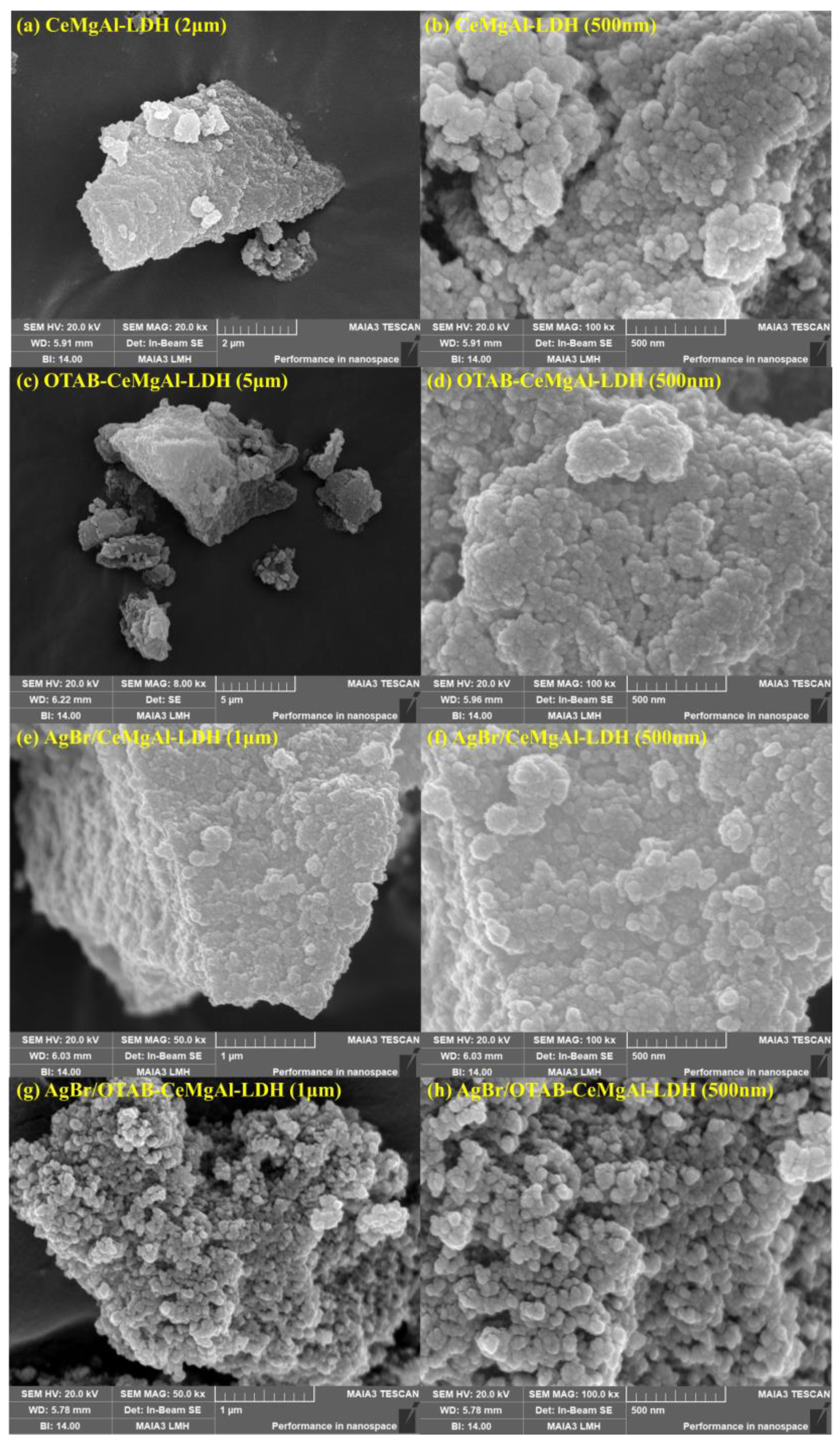
3.1.4. SEM Analysis
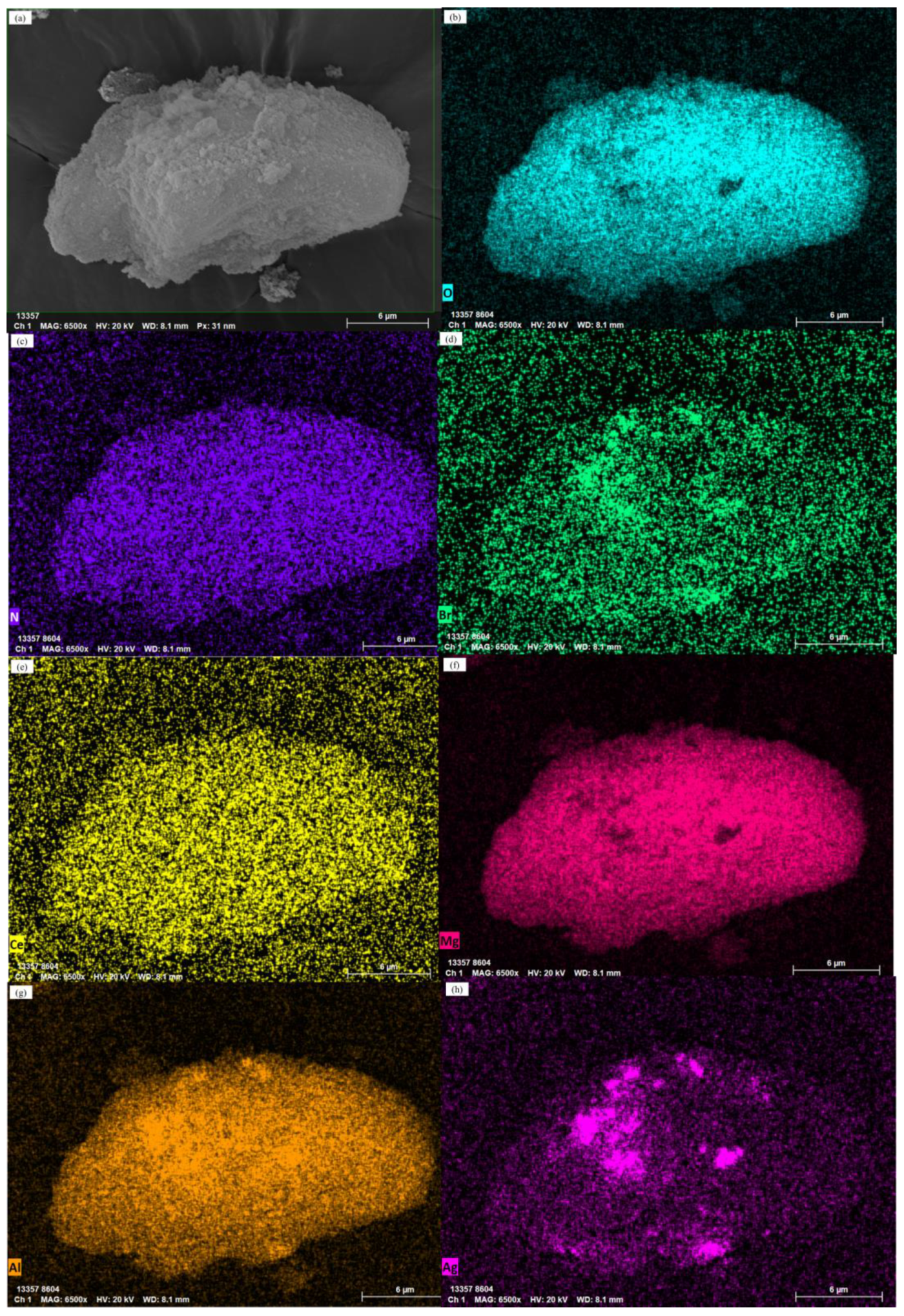
3.1.5. EDS Analysis
3.2. Optical Properties Characterization
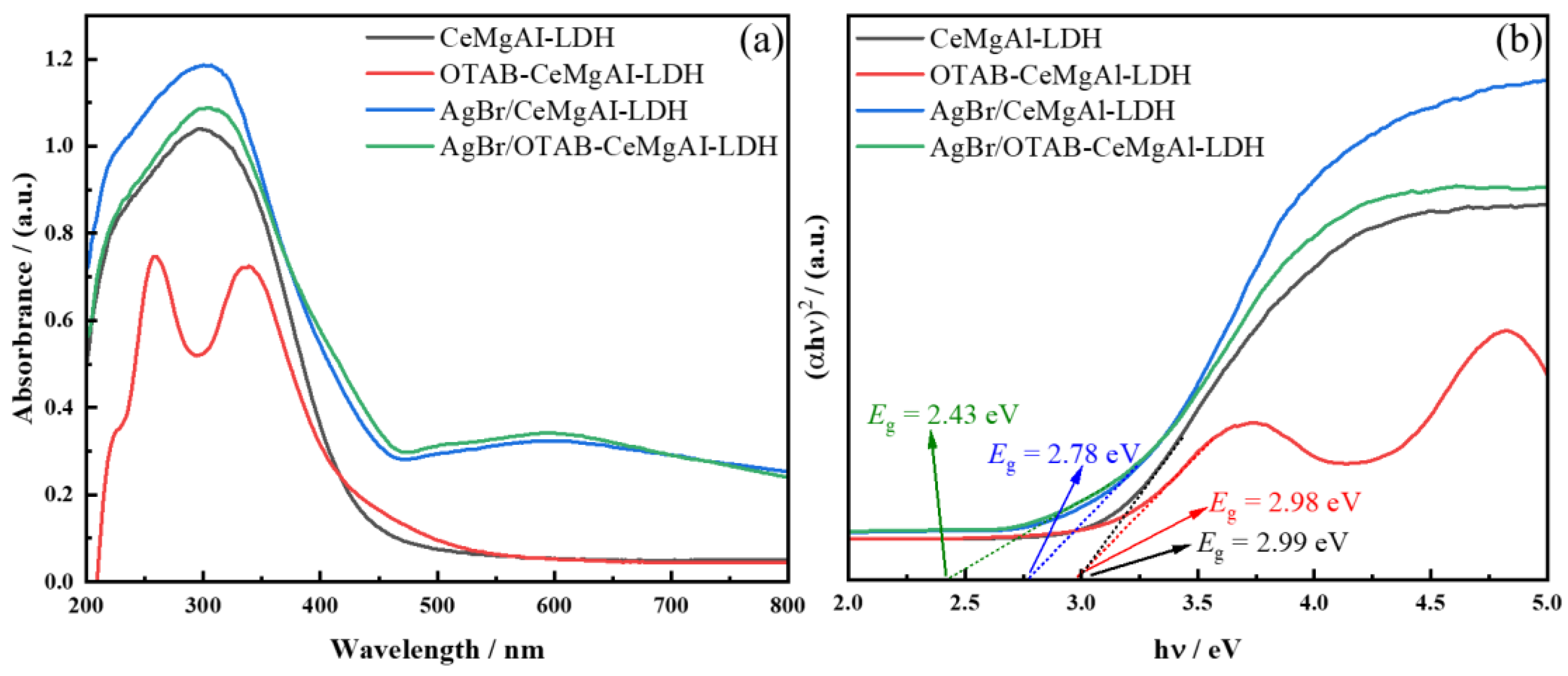
3.2.1. UV-Vis DRS Analysis
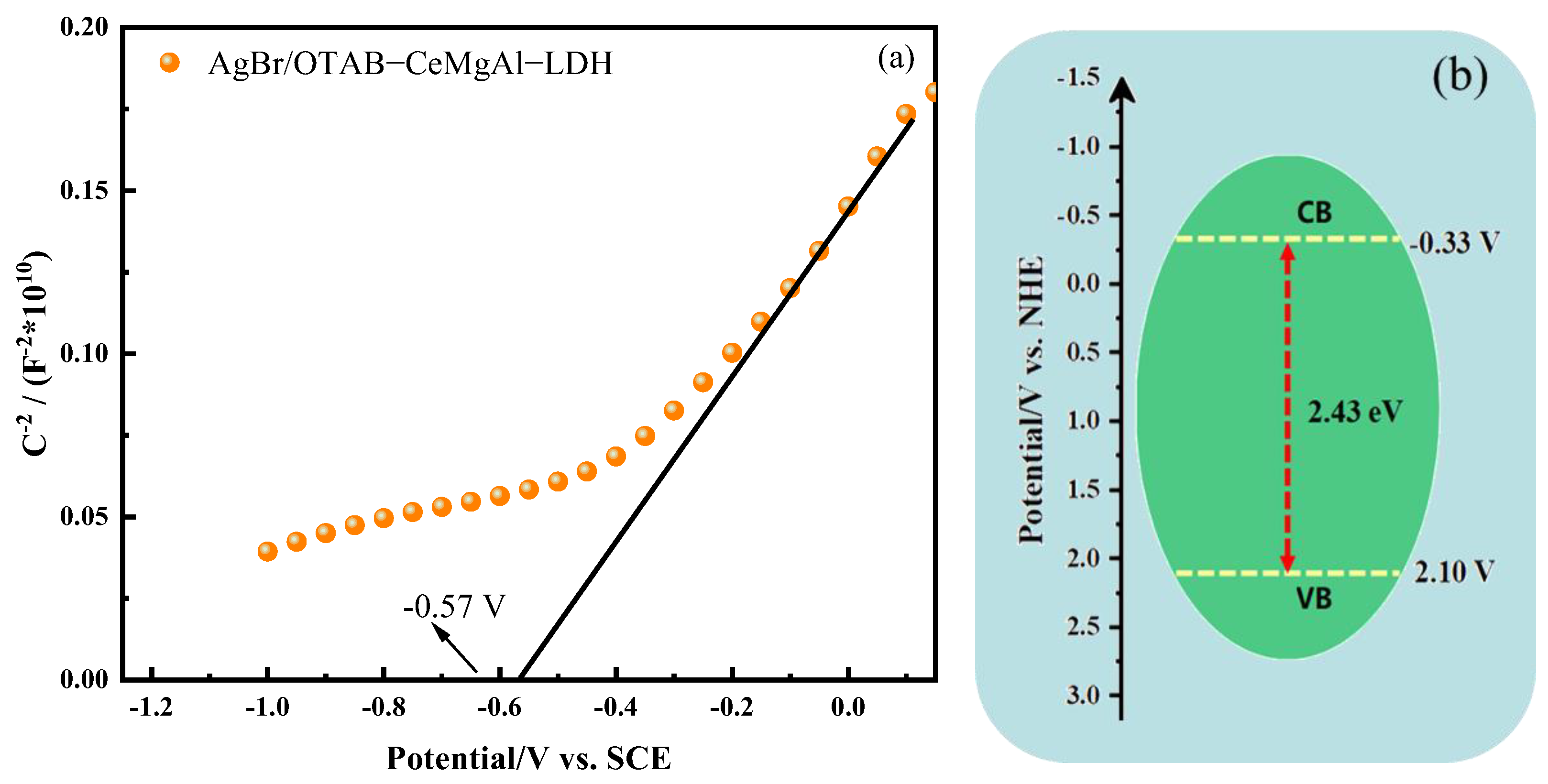
3.2.2. Electrochemical Analysis
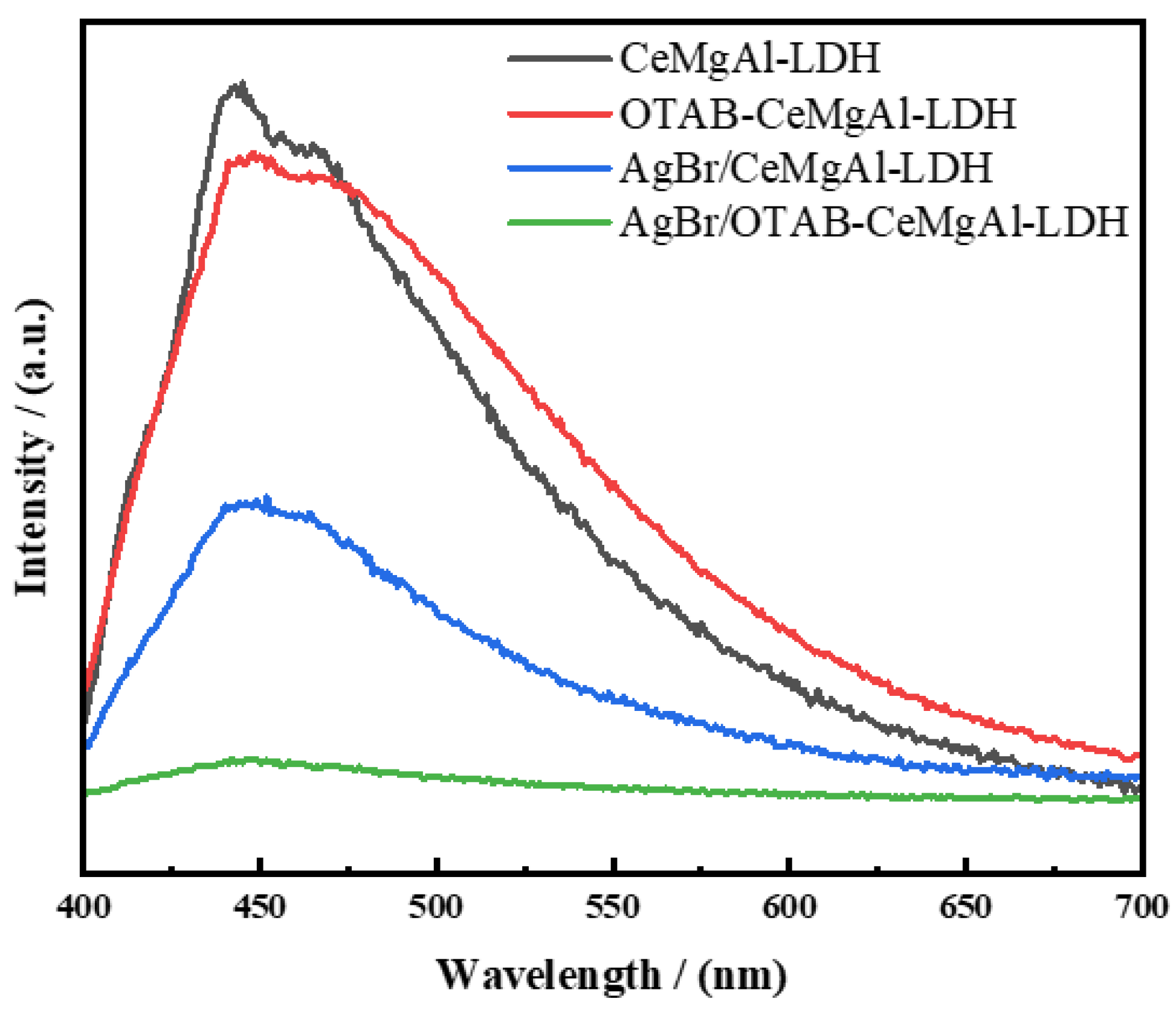
3.2.3. PL Analysis
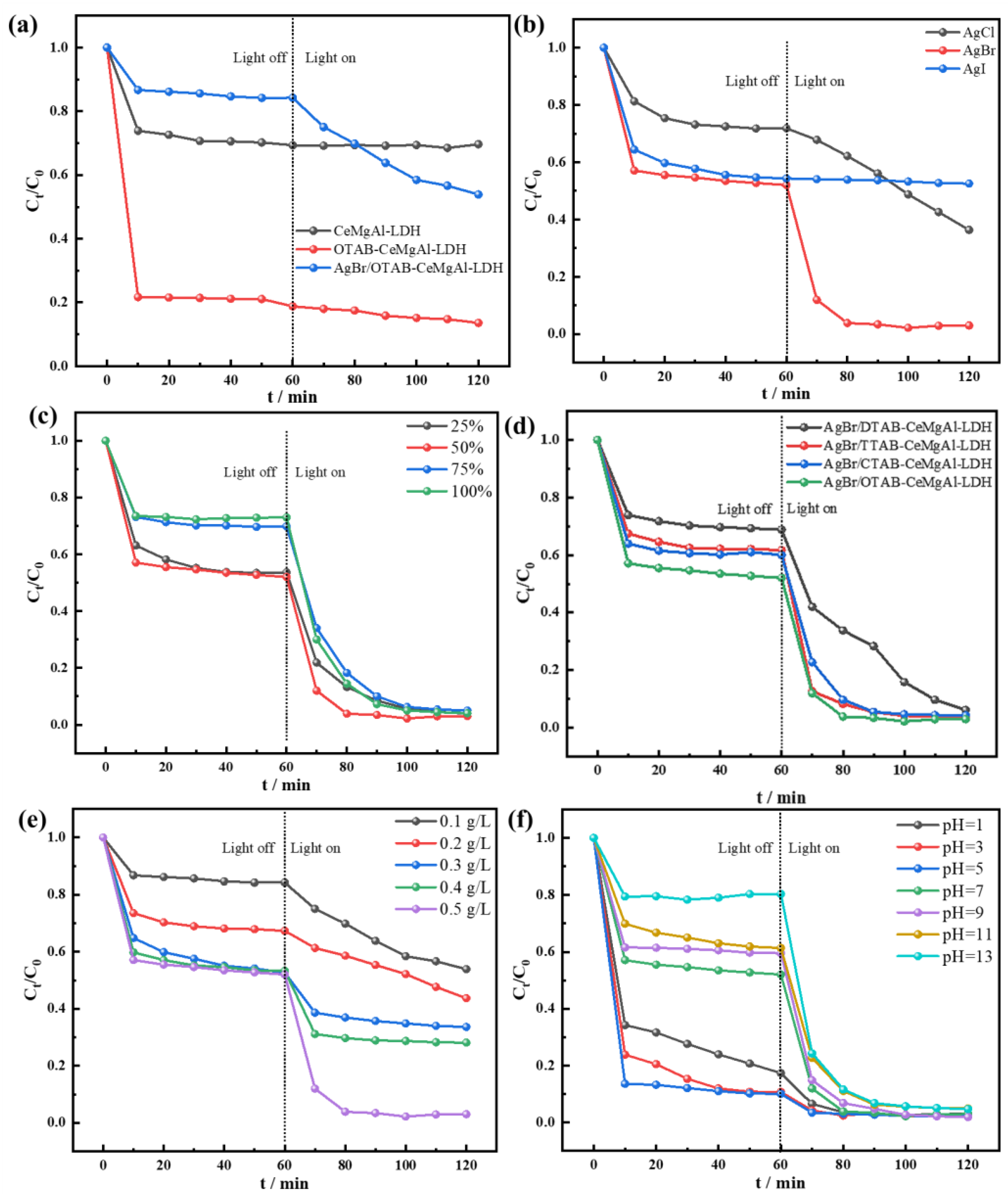
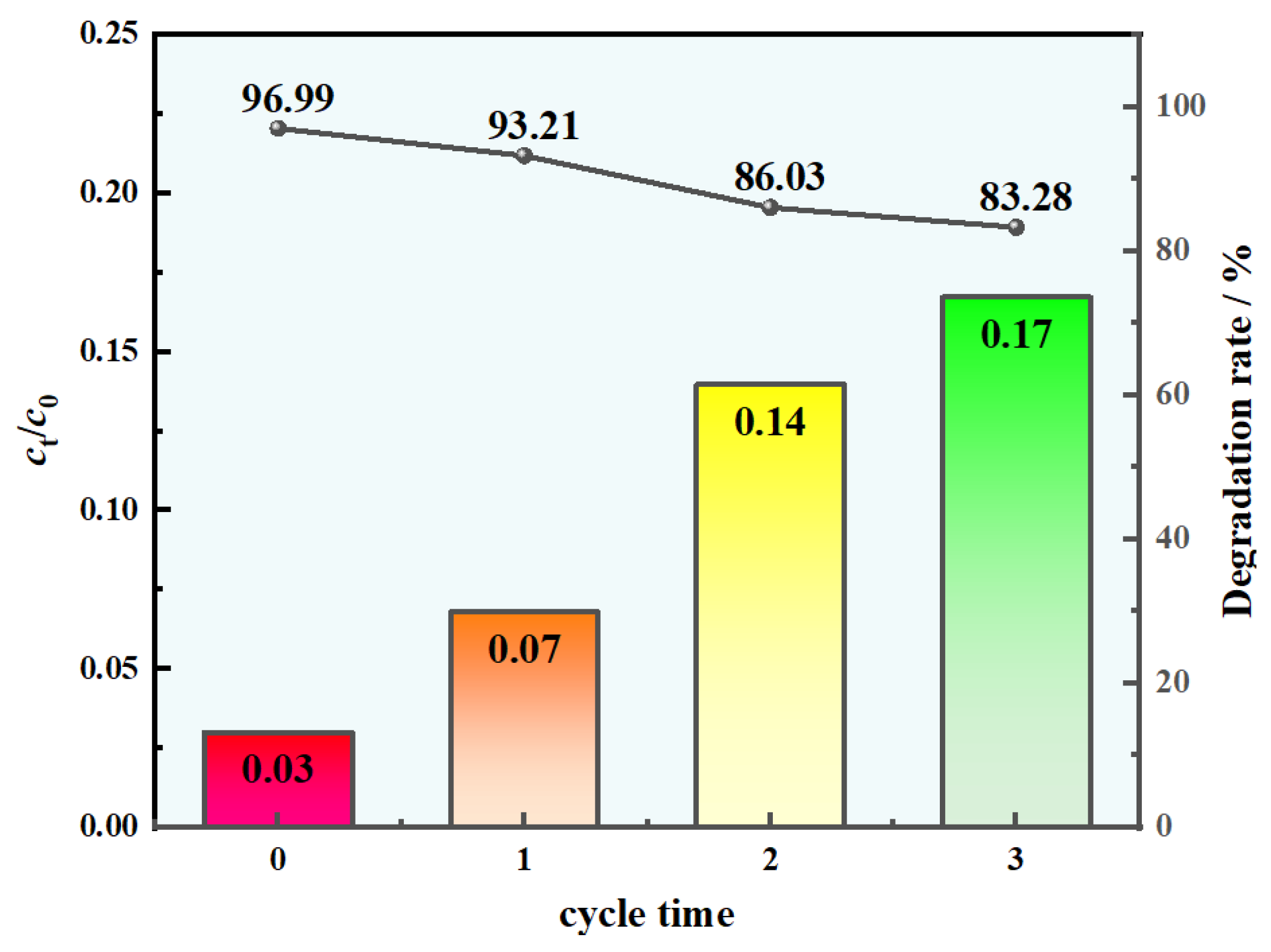
3.3. Catalytic Properties Investigation
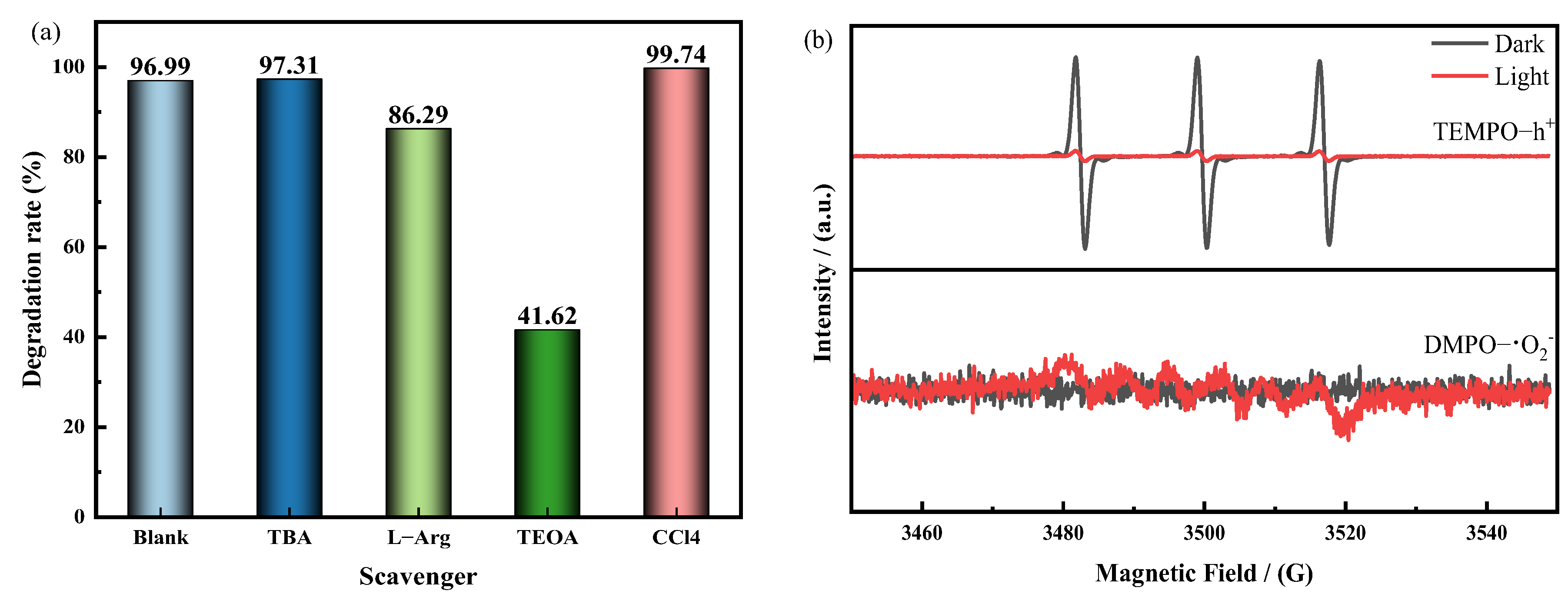
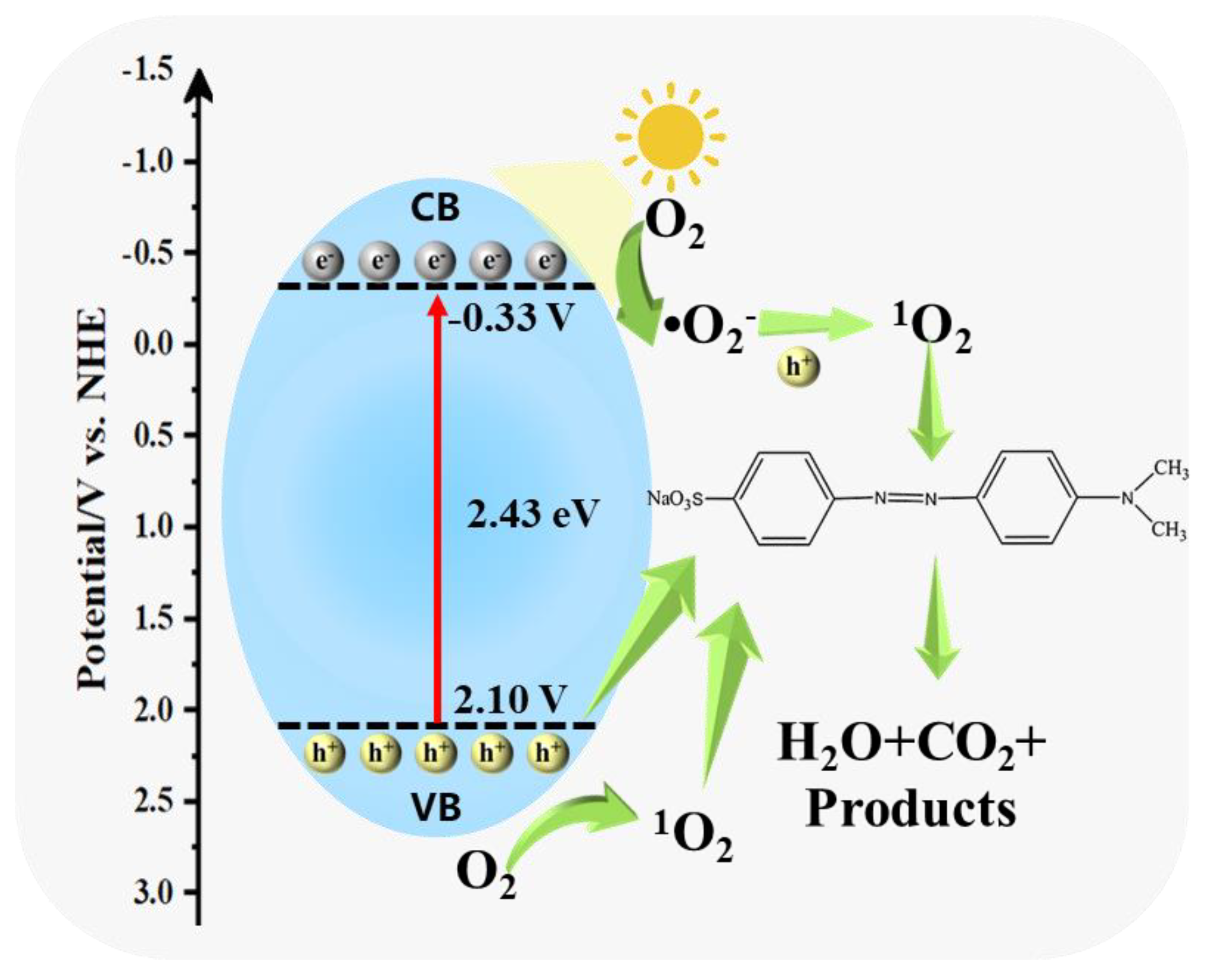
3.4. Photocatalytic Mechanism Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Y.; He, C. High salt permeation nanofiltration membranes based on NMG-assisted polydopamine coating for dye/salt fractionation. Desalination 2017, 413, 29–39. [Google Scholar] [CrossRef]
- Nyankson, E.; Amedalor, R.; Chandrabose, G.; Coto, M.; Krishnamurthy, S.; Kumar, R.V. Microwave-and formaldehyde-assisted synthesis of Ag–Ag3PO4 with enhanced photocatalytic activity for the degradation of rhodamine B dye and crude oil fractions. ACS Omega 2020, 5, 13641–13655. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.D.; Singh, A.; Khan, M.Z.; Tabraiz, S.; Sheikh, J. Current perspectives, recent advancements, and efficiencies of various dye-containing wastewater treatment technologies. J. Water Process Eng. 2023, 53, 103579. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, H.; Xue, G.; Liu, Y.; Chen, S.; Jia, C. A critical review of the aniline transformation fate in azo dye wastewater treatment. J. Clean. Prod. 2021, 321, 128971. [Google Scholar] [CrossRef]
- Ranjbari, A.; Yu, J.; Kim, J.; Kim, J.; Park, M.; Kim, K.H.; Heynderickx, P.M. Fundamental kinetic modeling of dye sensitization photocatalysis by oxygen vacancy enriched ZnO for the quantification of degradation by catalyst or dye sensitizer. Appl. Surf. Sci. 2024, 659, 159867. [Google Scholar] [CrossRef]
- Solayman, H.M.; Hossen, M.A.; Abd Aziz, A.; Yahya, N.Y.; Leong, K.H.; Sim, L.C.; Monir, M.U.; Zoh, K.D. Performance evaluation of dye wastewater treatment technologies: A review. J. Environ. Chem. Eng. 2023, 11, 109610. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Q.; Wei, Y.; Wei, W.; Du, W.; Zhang, J.; Chen, G.; Slaný, M. Preparation and swelling inhibition of mixed metal hydroxide to bentonite clay. Minerals 2022, 12, 459. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Q.; Du, W.; Li, Y.; Zhang, J.; Zhang, J.; Matejdes, M.; Slaný, M.; Gang, C. Multi-mixed metal hydroxide as a strong stratigraphic nanoclay inhibitor in solid-free drilling fluid. Nanomaterials 2022, 12, 3863. [Google Scholar] [CrossRef]
- Darabi, R.; Ghorbani-HasanSaraei, A.; Masoomzadeh, S.; Sefidan, A.M.; Gulbagca, F.; Tiri, R.N.E.; Al-Khafaji, A.H.Z.; Altuner, E.E.; Sen, F.; Davarnia, B.; et al. Enhanced photocatalytic performance of auto-combusted nanoparticles for photocatalytic degradation of azo dye under sunlight illumination and hydrogen fuel production. Chemosphere 2023, 336, 139266. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.L.; Ho, L.N.; Ong, S.A.; Wong, Y.S.; Voon, C.H.; Khalik, W.F.; Yusoff, N.A.; Nordin, N. Enhanced electricity generation and degradation of the azo dye Reactive Green 19 in a photocatalytic fuel cell using ZnO/Zn as the photoanode. J. Clean. Prod. 2016, 127, 579–584. [Google Scholar] [CrossRef]
- Bi, N.; Zheng, H.; Zhu, Y.; Jiang, W.; Liang, B. Visible-light-driven photocatalytic degradation of non-azo dyes over Ag2O and its acceleration by the addition of an azo dye. J. Environ. Chem. Eng. 2018, 6, 3150–3160. [Google Scholar] [CrossRef]
- Pan, C.; Mao, Z.; Yuan, X.; Zhang, H.; Mei, L.; Ji, X. Heterojunction Nanomedicine. Adv. Sci. 2022, 9, 2105747. [Google Scholar] [CrossRef] [PubMed]
- Ranjbari, A.; Demeestere, K.; Walgraeve, C.; Kim, K.H.; Heynderickx, P.M. Novel kinetic modeling of photocatalytic degradation of ethanol and acetaldehyde in air by commercial and reduced ZnO: Effect of oxygen vacancies and humidity. Chemosphere 2024, 358, 142118. [Google Scholar] [CrossRef] [PubMed]
- Vendramini, D.D.S.; Benatto, V.G.; Ashtiani, A.M.; La Porta, F.D.A. Photocatalytic Applications of SnO2 and Ag2O-Decorated SnO2 Coatings on Cement Paste. Catalysts 2023, 13, 1479. [Google Scholar] [CrossRef]
- Li, M.; Shah, N.H.; Zhang, P.; Chen, P.; Cui, Y.; Jiang, Y.; Wang, Y. Mechanism, modification and application of silver-based photocatalysts. Mater. Today Sustain. 2023, 22, 100409. [Google Scholar] [CrossRef]
- Zhang, S.; Song, S.; Gu, P.; Ma, R.; Wei, D.; Zhao, G.; Wen, T.; Jehan, R.; Hu, B.; Wang, X. Visible-light-driven activation of persulfate over cyano and hydroxyl group co-modified mesoporous g-C3N4 for boosting bisphenol A degradation. J. Mater. Chem. A 2019, 7, 5552–5560. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Q.; Chang, X.; Du, W.; Zhang, F.; Kuruc, M.; Slaný, M.; Chen, G. Use of highly dispersed mixed metal hydroxide gel compared to bentonite based gel for application in drilling fluid under ultra-high temperatures. Gels 2023, 9, 513. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xie, X.; Li, C.; Wang, H.; Wang, L. The role of soft colloidal templates in the shape evolution of flower-like MgAl-LDH hierarchical microstructures. RSC Adv. 2015, 5, 29757–29765. [Google Scholar] [CrossRef]
- Sun, H.; Chu, Z.; Hong, D.; Zhang, G.; Xie, Y.; Li, L.; Shi, K. Three-dimensional hierarchical flower-like Mg–Al-layered double hydroxides: Fabrication, characterization and enhanced sensing properties to NOx at room temperature. J. Alloys Compd. 2016, 658, 561–568. [Google Scholar] [CrossRef]
- Shao, M.; Han, J.; Wei, M.; Evans, D.G.; Duan, X. The synthesis of hierarchical Zn–Ti layered double hydroxide for efficient visible-light photocatalysis. Chem. Eng. J. 2011, 168, 519–524. [Google Scholar] [CrossRef]
- Xia, S.J.; Liu, F.X.; Ni, Z.M.; Xue, J.L.; Qian, P.P. Layered double hydroxides as efficient photocatalysts for visible-light degradation of Rhodamine B. J. Colloid Interface Sci. 2013, 405, 195–200. [Google Scholar] [CrossRef]
- Jiao, Z.; Liu, Z.; Ma, Z. Rodlike AgI/Ag2Mo2O7 heterojunctions with enhanced visible-light-driven photocatalytic activity. ACS Omega 2019, 4, 7919–7930. [Google Scholar] [CrossRef]
- Wang, K.; Miao, C.; Liu, Y.; Cai, L.; Jones, W.; Fan, J.; Li, D.; Feng, J. Vacancy enriched ultrathin TiMgAl-layered double hydroxide/graphene oxides composites as highly efficient visible-light catalysts for CO2 reduction. Appl. Catal. B: Environ. 2020, 270, 118878. [Google Scholar] [CrossRef]
- Luo, Y.; Han, Y.; Hua, Y.; Xue, M.; Yu, S.; Zhang, L.; Yin, Z.; Li, X.; Ma, X.; Wu, H.; et al. Step scheme nickel-aluminium layered double hydroxides/biochar heterostructure photocatalyst for synergistic adsorption and photodegradation of tetracycline. Chemosphere 2022, 309, 136802. [Google Scholar] [CrossRef] [PubMed]
- Thakur, P.; Raizada, P.; Singh, P.; Kumar, A.; Khan, A.A.P.; Asiri, A.M. Exploring recent advances in silver halides and graphitic carbon nitride-based photocatalyst for energy and environmental applications. Arab. J. Chem. 2020, 13, 8271–8300. [Google Scholar] [CrossRef]
- Chnadel, N.; Dutta, V.; Sharma, S.; Raizada, P.; Hosseini-Bandegharaei, A.; Kumar, R.; Singh, P.; Thakur, V.K. Z-scheme photocatalytic dye degradation on AgBr/Zn(Co)Fe2O4 photocatalysts supported on nitrogen-doped graphene. Mater. Today Sustain. 2020, 9, 100043. [Google Scholar] [CrossRef]
- Miao, X.; Shen, X.; Wu, J.; Ji, Z.; Wang, J.; Kong, L.; Liu, M.; Song, C. Fabrication of an all solid Z-scheme photocatalyst g-C3N4/GO/AgBr with enhanced visible light photocatalytic activity. Appl. Catal. A-Gen. 2017, 539, 104–113. [Google Scholar] [CrossRef]
- Jonjana, S.; Phuruangrat, A.; Thongtem, T.; Thongtem, S. Synthesis, analysis and photocatalysis of AgBr/Bi2MoO6 nanocomposites. Mater. Lett. 2016, 172, 11–14. [Google Scholar] [CrossRef]
- Li, B.; He, J. Multiple effects of dodecanesulfonate in the crystal growth control and morphosynthesis of layered double hydroxides. J. Phys. Chem. C 2008, 112, 10909–10917. [Google Scholar] [CrossRef]
- Tang, Y.; Bai, B.; Wu, Y.; Yang, B.; Zhou, L.; Qu, C. Enhanced Removal of Sulfonated Lignite from Oilfield Wastewater by Soft Colloidal Templated Porous Structure of MgAl-LDH. Water Air Soil Pollut. 2024, 235, 188. [Google Scholar] [CrossRef]
- Zhou, L.; Slaný, M.; Bai, B.; Du, W.; Qu, C.; Zhang, J.; Tang, Y. Enhanced Removal of Sulfonated Lignite from Oil Wastewater with Multidimensional MgAl-LDH Nanoparticles. Nanomaterials 2021, 11, 861. [Google Scholar] [CrossRef]
- Ranjbari, A.; Demeestere, K.; Kim, K.H.; Heynderickx, P.M. Oxygen vacancy modification of commercial ZnO by hydrogen reduction for the removal of thiabendazole: Characterization and kinetic study. Appl. Catal. B: Environ. 2023, 324, 122265. [Google Scholar] [CrossRef]
- Wang, X.R.; Cheng, H.M.; Gao, X.W.; Zhou, W.; Li, S.J.; Cao, X.L.; Yan, D. Intercalation assembly of kojic acid into Zn-Ti layered double hydroxide with antibacterial and whitening performances. Chin. Chem. Lett. 2019, 30, 919–923. [Google Scholar] [CrossRef]
- Fabbro, M.T.; Saliby, C.; Rios, L.R.; A La Porta, F.; Gracia, L.; Li, M.S.; Andrés, J.; Santos, L.P.S.; Longo, E. Identifying and rationalizing the morphological, structural, and optical properties of β-Ag2MoO4 microcrystals, and the formation process of Ag nanoparticles on their surfaces: Combining experimental data and first-principles calculations. Sci. Technol. Adv. Mater. 2015, 16, 065002. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Li, B.; Luo, K.; Shi, K.; Zhang, L.; Li, Y.; Yu, T.; Hu, W.; Xie, C.; et al. Restacked melon as highly-efficient photocatalyst. Nano Energy 2020, 77, 105124. [Google Scholar] [CrossRef]
- Liu, Z.; Jiang, Y.; Liu, X.; Zeng, G.; Shao, B.; Liu, Y.; Liu, Y.; Zhang, W.; Yan, M.; He, X. Silver chromate modified sulfur doped graphitic carbon nitride microrod composites with enhanced visible-light photoactivity towards organic pollutants degradation. Compos. Part B Eng. 2019, 173, 106918. [Google Scholar] [CrossRef]
- Yang, J.; Li, C.; Liang, D.; Liu, Y.; Li, Z.; Wang, H.; Huang, H.; Xia, C.; Zhao, H.; Liu, Y.; et al. Central-collapsed structure of CoFeAl layered double hydroxides and its photocatalytic performance. J. Colloid Interface Sci. 2021, 590, 571–579. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, M.F.; Bellato, C.R.; Miranda, L.D.; Milagres, J.L. Preparation of calcined hydrotalcite/TiO2-Ag composite and enhanced photocatalytic properties. Ceram. Int. 2017, 43, 1843–1852. [Google Scholar] [CrossRef]
- Guo, F.; Shi, W.; Wang, H.; Han, M.; Li, H.; Huang, H.; Liu, Y.; Kang, Z. Facile fabrication of a CoO/g-C3N4 p–n heterojunction with enhanced photocatalytic activity and stability for tetracycline degradation under visible light. Catal. Sci. Technol. 2017, 7, 3325–3331. [Google Scholar] [CrossRef]
- Ranjbari, A.; Kim, J.; Yu, J.; Kim, J.; Park, M.; Kim, N.; Demeestere, K.; Heynderickx, P.M. Effect of oxygen vacancy modification of ZnO on photocatalytic degradation of methyl orange: A kinetic study. Catal. Today 2024, 427, 114413. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, L.; Fugetsu, B. Morphology-controlled synthesis of sunlight-driven plasmonic photocatalysts Ag@AgX (X = Cl, Br) with graphene oxide template. J. Mater. Chem. A 2013, 1, 12536–12544. [Google Scholar] [CrossRef]
- Deng, H.; Wu, Y.; Li, L.; Wang, P.; Fang, K.; Li, J.; Hao, D.; Zhu, H.; Wang, Q.; Li, Q. Synergistic mechanisms for efficient and safe antibiotic removal: Effective adsorption and photo-catalytic degradation using aerogels. Sep. Purif. Technol. 2025, 354, 129455. [Google Scholar] [CrossRef]
- Liu, A.; Ma, X.; Shen, B.; Du, H.; Jiang, X.; Wu, Y.; Jin, Y.; Li, J.; Zhu, H.; Wang, Q. Sustainable dual-cathode photoelectro-Fenton system enabling oxidative and reductive removal of pollutants via visible light driving Fe sites conversion. Chem. Eng. J. 2024, 504, 158929. [Google Scholar] [CrossRef]
- Deng, L.; Shi, Z.; Peng, X.; Zhou, S. Magnetic calcinated cobalt ferrite/magnesium aluminum hydrotalcite composite for enhanced adsorption of methyl orange. J. Alloys Compd. 2016, 688, 101–112. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, M.; Li, Z.; Li, M. Visible light photocatalytic degradation of dyes by Ag3PO4/g-C3N4/CQDs composite. Surf. Interfaces 2024, 44, 103585. [Google Scholar] [CrossRef]
- Tang, T.; Jin, X.; Tao, X.; Huang, L.; Shang, S. Low-crystalline Ce-based bimetallic MOFs synthesized via DBD plasma for excellent visible photocatalytic performance. J. Alloys Compd. 2022, 895, 162452. [Google Scholar] [CrossRef]
- Mahmud, R.A.; Shafawi, A.N.; Ali, K.A.; Putri, L.K.; Rosli, N.I.M.; Mohamed, A.R. Graphene nanoplatelets with low defect density as a synergetic adsorbent and electron sink for ZnO in the photocatalytic degradation of Methylene Blue under UV–vis irradiation. Mater. Res. Bull. 2020, 128, 110876. [Google Scholar] [CrossRef]
- Jia, Z.; Chen, Q.; Li, C.; Li, Z.; Zhang, D.; Chu, R.; Wu, L. Facile in situ preparation of fibrous Ag/AgCl composites with efficient photocatalytic degradation of methyl orange undesr solar light. J. Phys. Chem. Solids 2020, 140, 109360. [Google Scholar] [CrossRef]

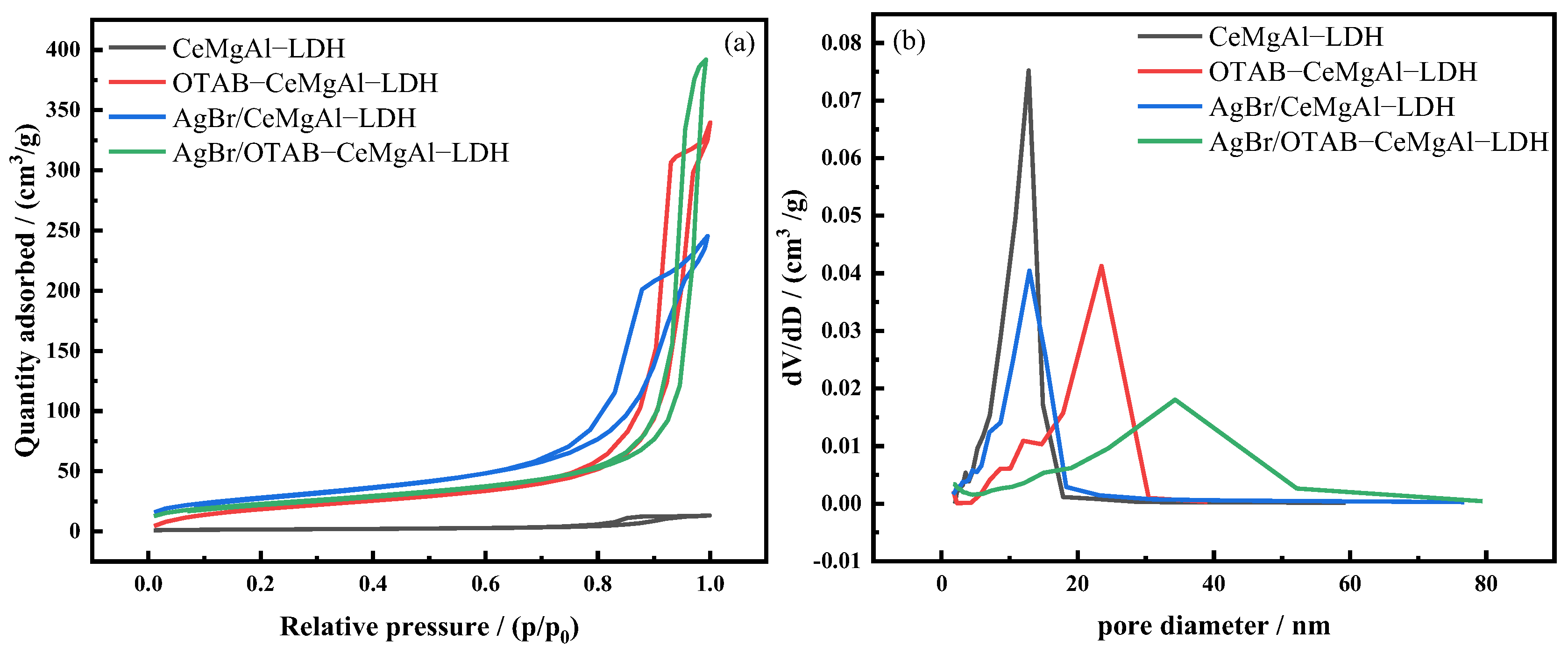
| Sample | Specific Area (m2/g) | Pore Volume (cm3/g) | Average Pore Width (nm) |
|---|---|---|---|
| CeMgAl-LDH | 73.99 | 0.53 | 28.14 |
| OTAB-CeMgAl-LDH | 132.89 | 0.03 | 13.87 |
| AgBr/CeMgAl-LDH | 62.18 | 0.61 | 29.51 |
| AgBr/OTAB-CeMgAl-LDH | 101.26 | 0.38 | 15.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Wang, C.; Yu, Z.; Yu, T.; Bai, B.; Chen, G.; Tang, Y. Construction of Structured Hydrotalcite Supported with Silver Halide and Its Enhanced Visible Light Photocatalytic Degradation of Methyl Orange. Minerals 2025, 15, 163. https://doi.org/10.3390/min15020163
Yang J, Wang C, Yu Z, Yu T, Bai B, Chen G, Tang Y. Construction of Structured Hydrotalcite Supported with Silver Halide and Its Enhanced Visible Light Photocatalytic Degradation of Methyl Orange. Minerals. 2025; 15(2):163. https://doi.org/10.3390/min15020163
Chicago/Turabian StyleYang, Jingwen, Chunhui Wang, Ziqi Yu, Tao Yu, Bingbing Bai, Gang Chen, and Ying Tang. 2025. "Construction of Structured Hydrotalcite Supported with Silver Halide and Its Enhanced Visible Light Photocatalytic Degradation of Methyl Orange" Minerals 15, no. 2: 163. https://doi.org/10.3390/min15020163
APA StyleYang, J., Wang, C., Yu, Z., Yu, T., Bai, B., Chen, G., & Tang, Y. (2025). Construction of Structured Hydrotalcite Supported with Silver Halide and Its Enhanced Visible Light Photocatalytic Degradation of Methyl Orange. Minerals, 15(2), 163. https://doi.org/10.3390/min15020163







