Unified Phase Diagram and Competition-Coupling Mechanism for Pyrite Thermal Transformation
Abstract
1. Introduction
2. Re-Evaluating the Thermodynamic Boundaries
2.1. Analysis of Reported Phase Equilibria
- 1.
- Collaborative Control of Temperature and Oxygen Partial Pressure (pO2):
- 2.
- Non-Stoichiometry of Pyrrhotite (Fe1−XS):
- 3.
- Existence of Key Phase Transition Temperatures:
- Local Atmospheric Conditions:
- Influence of Mineral Occurrence Mode:
- Intra-Particle Temperature Gradients:
2.2. Thermodynamic Driving Force: Calculation ΔrGθ
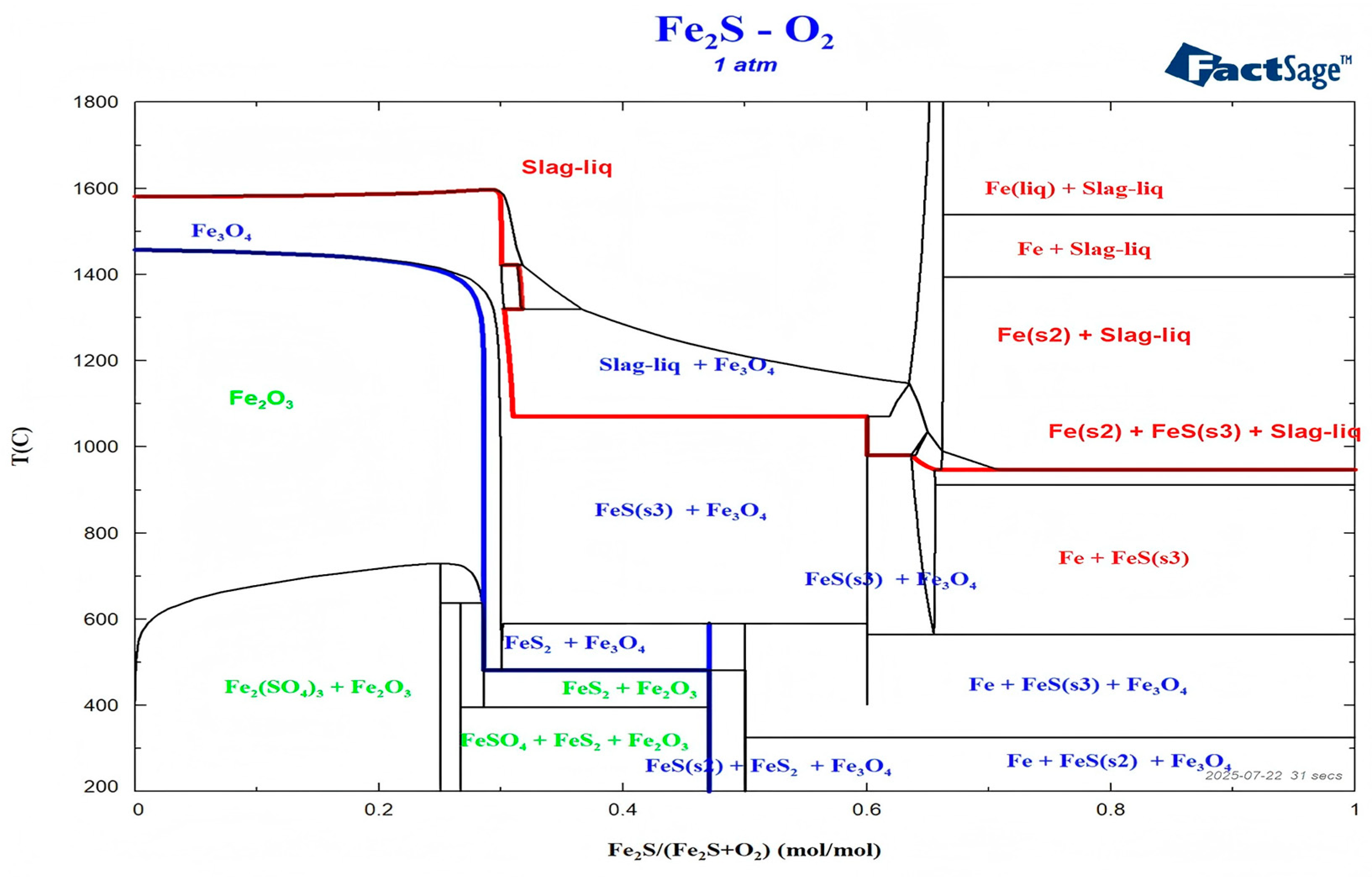
2.3. Calculation of Thermodynamic Phase Diagram
- 1.
- For FeS2/(FeS2 + O2) < 28.6% area (i.e., N (FeS2)/N (O2) < 0.4:1):
- 2.
- For 28.6% < FeS2/(FeS2 + O2) < 47.1% area (i.e., 0.4:1 < N (FeS2)/N (O2) < 0.89:1):
- 3.
- For 47.1% < FeS2/(FeS2 + O2) < 60% area (i.e., 0.89:1 < N (FeS2)/N (O2) < 1.5:1):
- 4.
- For 60% < FeS2/(FeS2 + O2) < 100% area (i.e., 1.5:1 < N (FeS2)/N (O2) < ∞):
- 5
- Sulfate area:
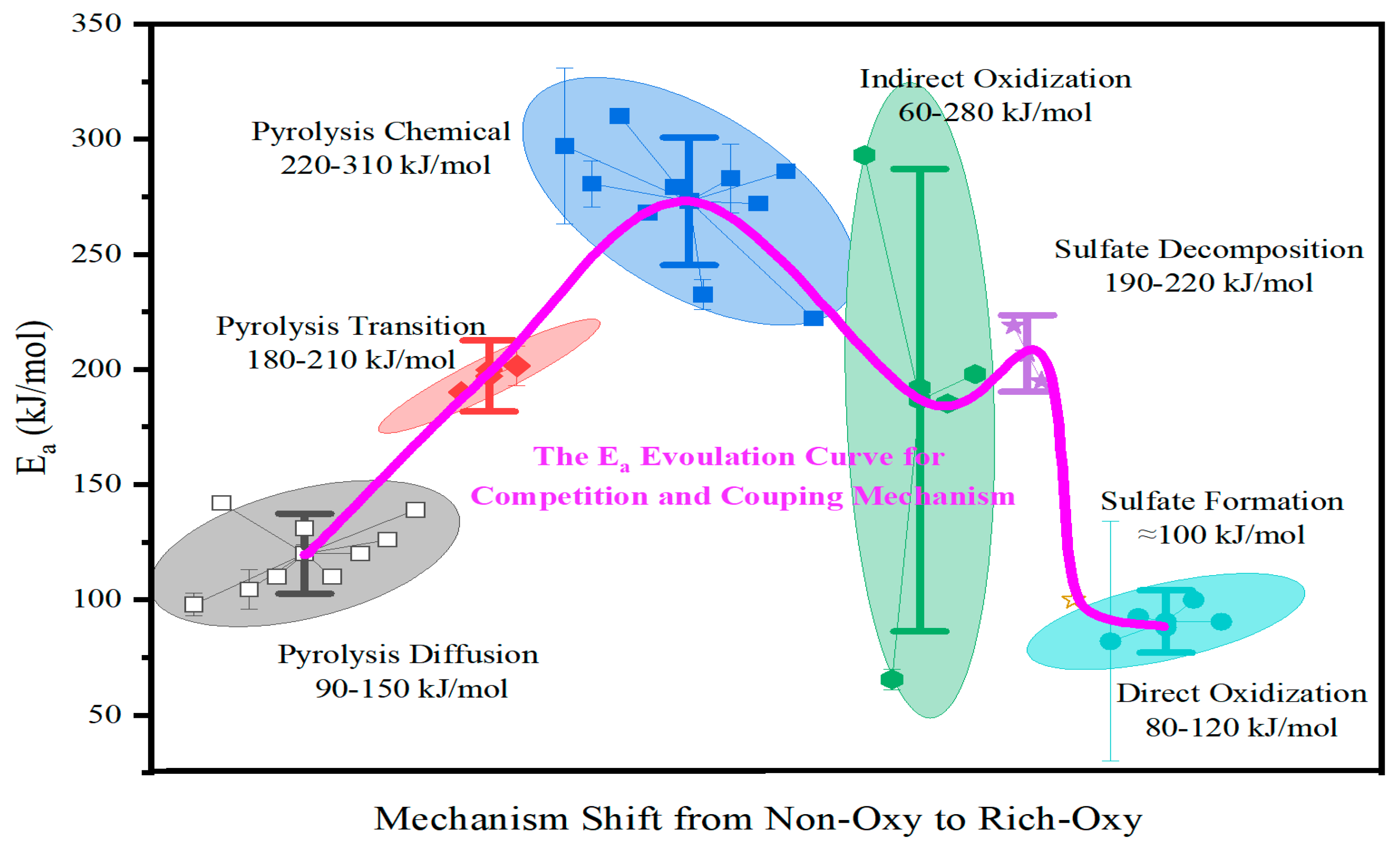
3. Reconciling Discrepant Kinetic Data
3.1. Reinterpreting Pyrolysis Mechanisms and Ea
- 1.
- Chemical Reaction Controlled Model (Intrinsic)
- 2.
- Diffusion Controlled Model (Apparent)
- 3.
- Two Steps and Transitional Model
3.2. Clarifying the Complex Oxidation Mechanisms and Ea
3.2.1. Predominant Oxidation Pathways
- 1.
- Direct Oxidation to Oxides:
- 2.
- Indirect Oxidation Via Fe1−XS:
- 3.
- Sulfate-Mediated Oxidation:
- 4.
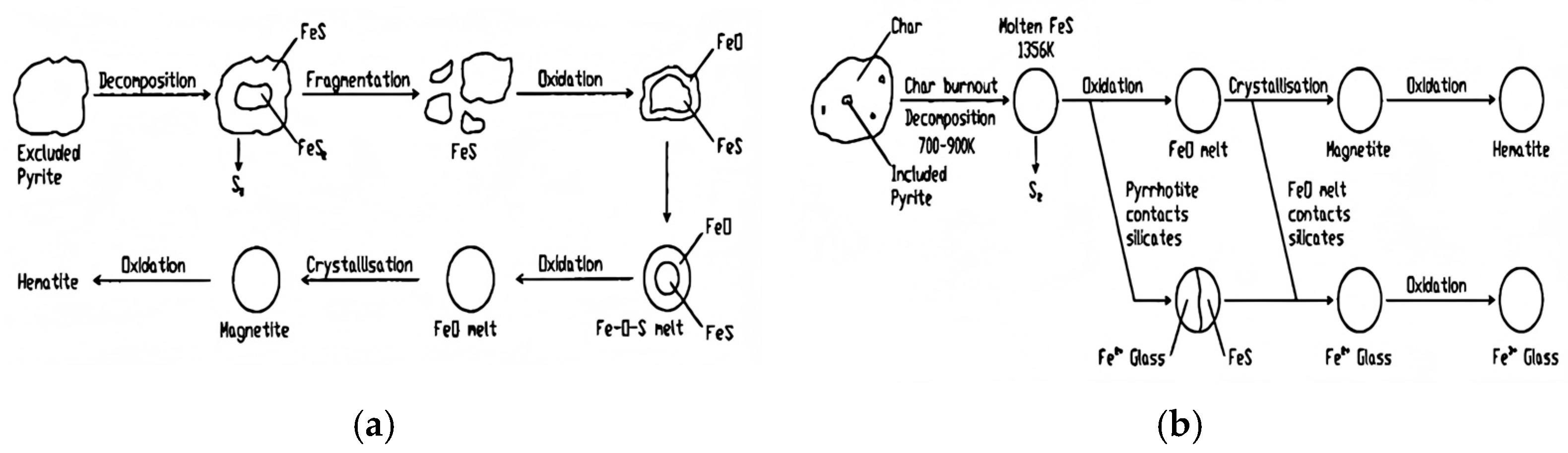
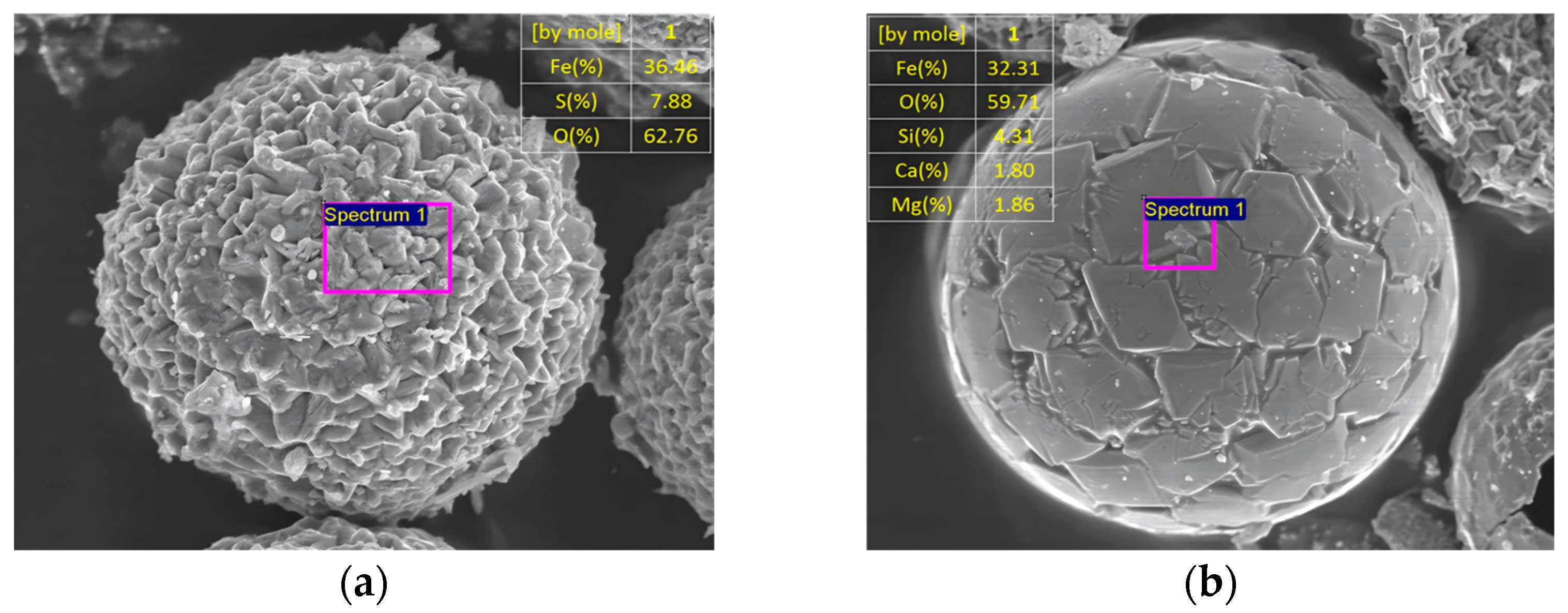
- Molten Phase-Dominated Transformation:
| No. | Author | Particle Size | Equipment | Atmosphere | Heating Rate | Model | Step1 Ea kJ/mol | Step2 Ea kJ/mol | Pathway | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | Gomes [146] | 7.39 μm | TGA | Air 10 mL/min | 2.5–20 °C/min. | Second-order | 470–580 °C:145.6 kJ/mol | FeS2 chemical decomposition | ||||
| 15–20 °C/min. | 2.5–7.5 °C/min. | First-order, | Area-contracting | 580–1000 °C: 33.2 | 580–1000 °C: 281.4 | Diffusion control in porous | The Fe2O3 layer inhabit reaction | |||||
| 4 | Yang [147] | Theoretical calculations | Density Functional Theory (DFT) | 197.96/175.83 | Surface oxidation Ea of 197.96 kJ/mol, Bulk sulfur migration Ea of 175.83 kJ/mol | |||||||
| No. | Author | Particle Size | Equipment | Atmosphere | Heating Rate | Model | Step1 Ea kJ/mol | Step2 Ea kJ/mol | Pathway |
|---|---|---|---|---|---|---|---|---|---|
| 5 | Concer [61] | TGA | Air 50 mL/min | 2.5, 5.0, 7.5, 10.0 K/min | Friedman | ~650 K 924 kJ/mol | ~770 K 451 kJ/mol | FeS2→Fe2(SO4)3→Fe2O3 | |
| 6 | Coombs [148,149] | 0.1–10 μm | TGA | Air 430 mL/min | 648–923 K 10.0 K/min | Friedman | 192 kJ/mol | Sulfate decomposition 219 kJ/mol | FeS2→Fe3O4 (low T), FeS2→Fe2(SO4)3→Fe2O3(mid T), and direct Fe2O3 formation (high T). |
| 7 | Ferrow [130] | 5–40 μm | TGA | Air | 200–380 °C | Weibull + Arrhenius | 100 kJ/mol | FeS2→FeSO4 below 380 °C. Fe2O3 occurs over 380 °C. | |
| 8 | Tian [129] | 74 μm | TGA | Air 50 mL/min | 10 °C/min | Avrami–Erofeev equation Inverse Jander equation | 194.81 kJ/mol | FeS2→FeSO4→Fe2O3 | |
3.2.2. Kinetic Model of Oxidation
3.2.3. Synthesis of Oxidation Activation Energy
3.3. Evidence for the Interplay Between Pyrolysis and Oxidation Kinetics
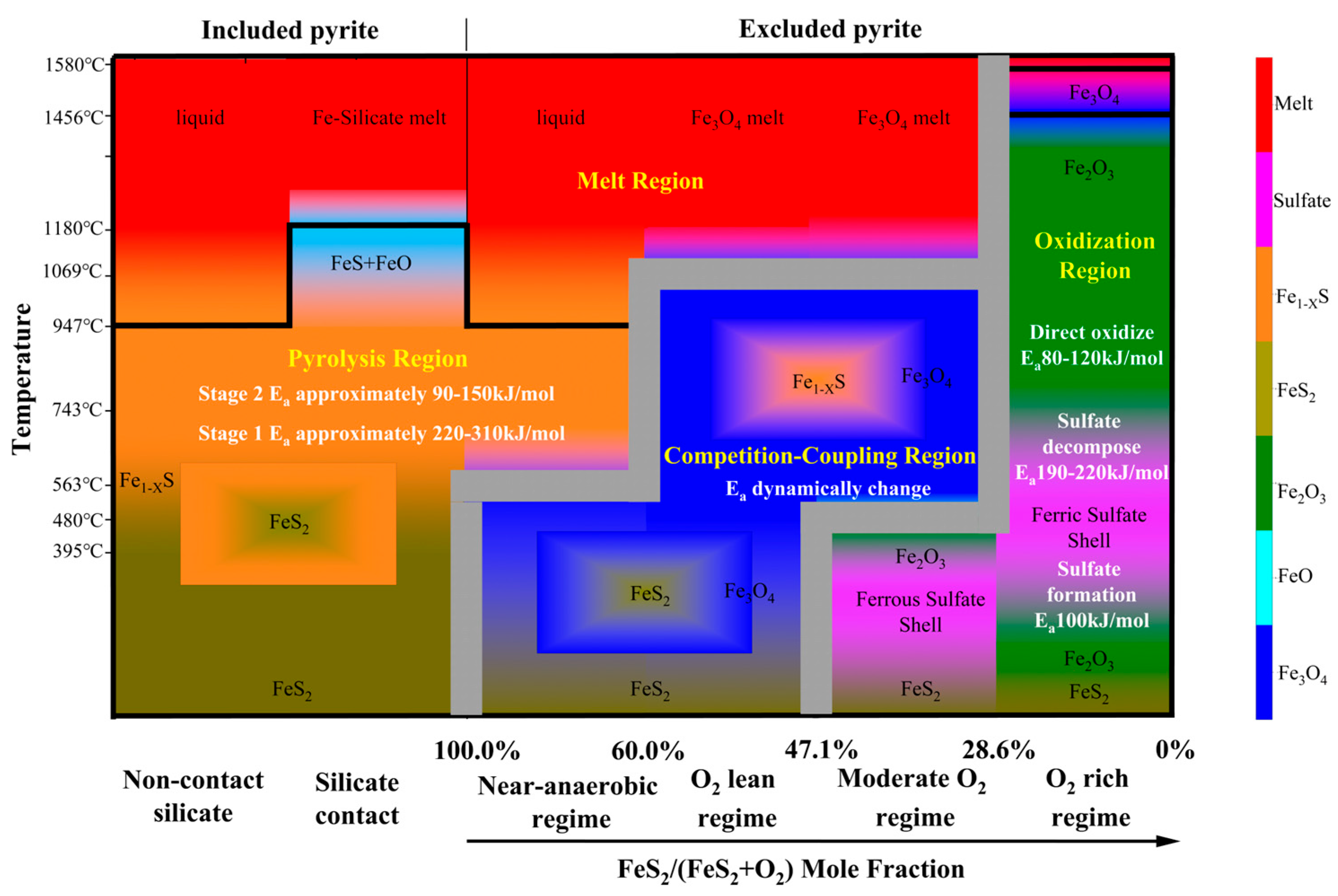
4. Proposing the Competition-Coupling Mechanism and Constructing the Unified Phase Diagram
4.1. The Principle of Competition-Coupling Mechanism
- Pyrolysis couples forward to oxidation: The initial pyrolysis of FeS2 generates a porous Fe1−XS intermediate, creating a “core-pore” structure. This porosity enhances the inward diffusion of oxygen, thereby facilitating the subsequent oxidation of the intermediate.
- Oxidation couples backward to pyrolysis: The oxidation of Fe1−XS can lead to the formation of a dense oxide or sulfate shell, resulting in a “core-pore-shell” morphology. This shell limits oxygen access to the core, which in turn promotes the continuation of pyrolysis internally.
4.2. A Predictive Phase Diagram: Bridging Thermodynamics and Kinetics
- 1.
- Pyrolysis-Dominated Region
- 2.
- Competition-Coupling Region
- 3.
- Oxidation-Dominated Region
- 4.
- Melt-Dominated Region
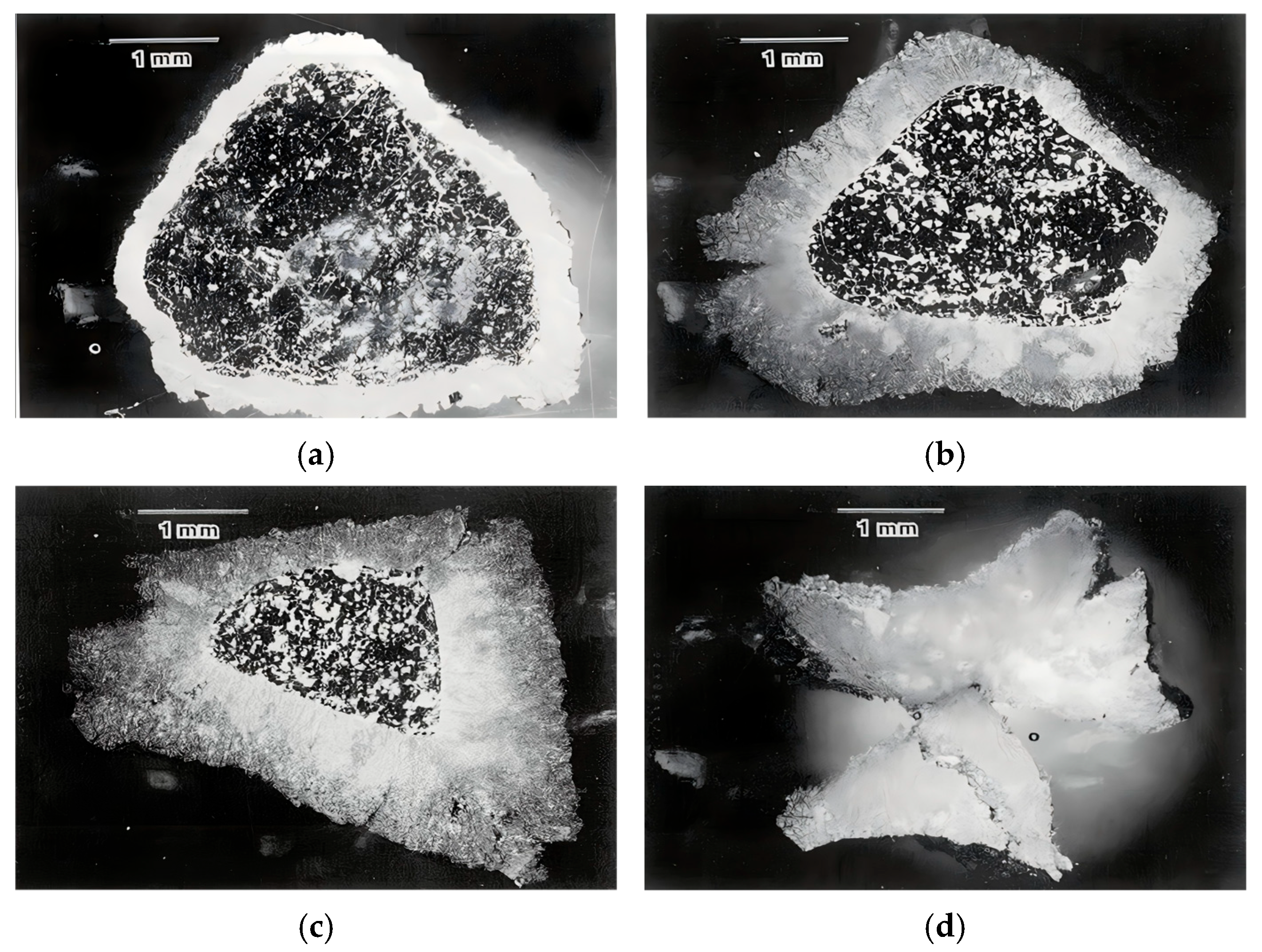
4.3. Practical Modulators: Particle Size, Heating Rate, and Occurrence Mode
- 1.
- Particle Fragmentation Effects:
- 2.
- Heating Rate Dependence:
- 3.
- Excluded vs. Included FeS2:
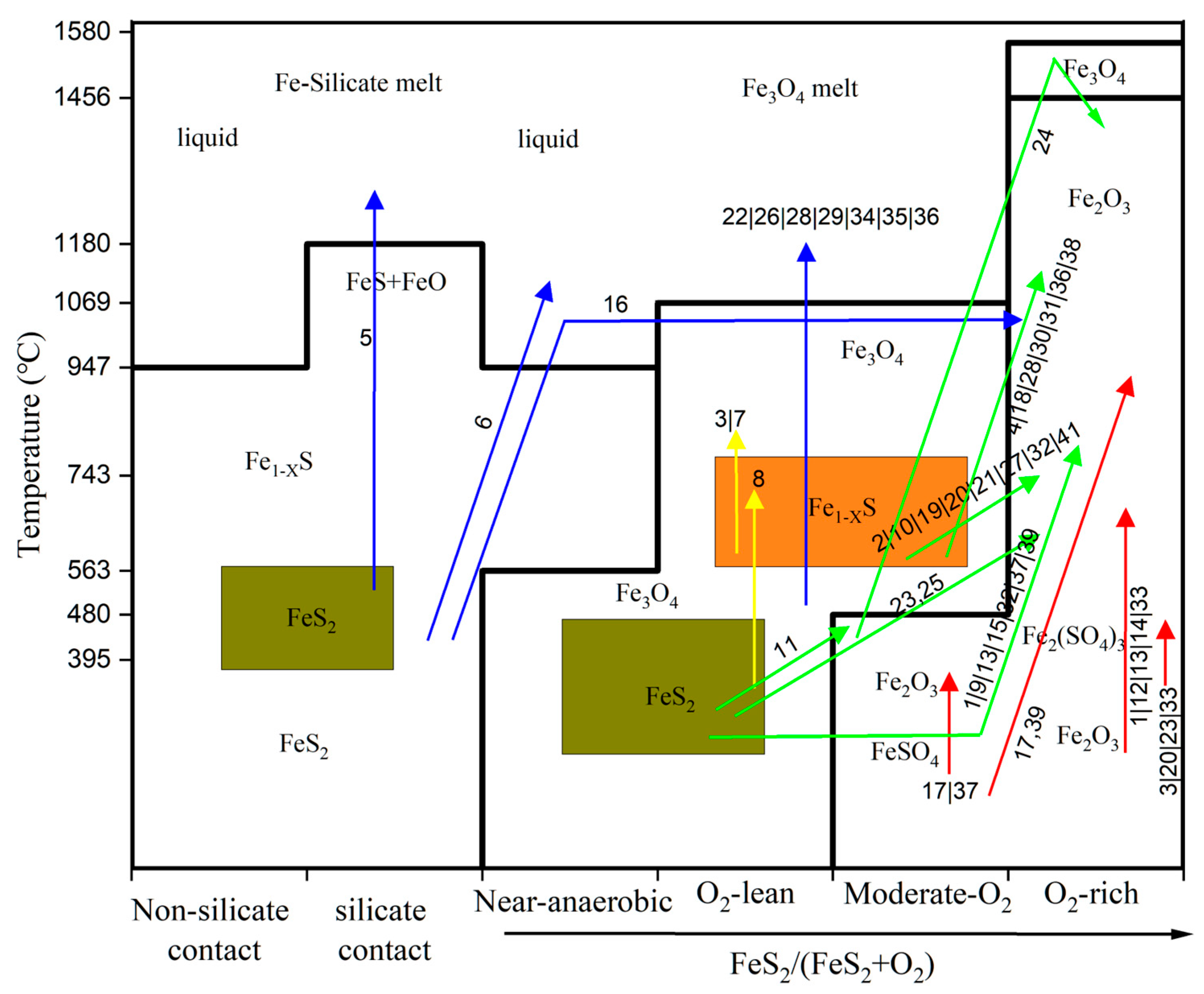
4.4. Validation: Mapping Pathways in the Literature onto the Phase Diagram
- 1.
- Direct and Sulfate-Mediated Oxidation (red arrowed line in the diagram):
- 2.
- Pyrrhotite (Fe1−XS)-Mediated Pathways (green arrowed line in the diagram):
- 3.
- Melt-Involving Pathways (blue arrowed line in the diagram):
- 4.
- Other Pathways (yellow arrowed line in the diagram):
5. Conclusions and Future Perspectives
5.1. Key Findings and Conclusions
- 1.
- Proposal of the Competition-Coupling Mechanism:
- 2.
- Reconciliation of Kinetic Data: Ea as a Dynamic Signature of Mixed Reaction.
- 3.
- Construction of Phase Diagram: Bridging Theory and Practice.
5.2. Future Challenges and Research Directions
- Quantifying Kinetic Phase Boundaries: Future research should employ techniques like HT-SEM, HT-XRD, and in situ XPS to quantitatively map how boundaries shift with particle size, heating rate, and gas flow, ultimately enabling the construction of a precise predictive diagram.
- Defining the Role of Sulfates: The critical switch between sulfate present as a temporary transitional intermediate or a final passivating layer requires clarification through surface analysis (XPS) and coupled TG-MS.
- Quantifying the Competition-Coupling Mechanism: Advanced kinetic analysis is required to distinguish whether pyrolysis and oxidation reactions are independent or strongly coupled.
5.3. Theoretical Limitations
- Pathways, Not End States: The phase diagram predicts the dominant kinetic pathway, rather than the final thermodynamic equilibrium. This explains why pyrolysis can prevail over thermodynamically favored oxidation in realistic.
- Apparent Ea as a Composite Metric: In the competition-coupling region, the apparent activation energy represents a blend of overlapping processes. It reflects the dynamic balance between pyrolysis and oxidation rather than a single elementary step.
- Static Situation Assumption: The phase diagram does not account for dynamic flow or particle fragmentation, which can instantaneously expose fresh surfaces and drastically alter the transformation pathway.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nie, Z.; Luo, J.; Tang, J.; Li, B.; Chen, B.; Gao, M.; Wang, X.; Guo, L. Pollution sources, characteristics and environmental risk assessment of heavy metals in surface water and sediments of typical pyrite mine in Southwest China. J. Environ. Sci. 2025, 157, 742–755. [Google Scholar] [CrossRef]
- Alhamed, M.; Wohnlich, S. Environmental impact of the abandoned coal mines on the surface water and the groundwater quality in the south of Bochum, Germany. Environ. Earth Sci. 2014, 72, 3251–3267. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Corpuz, R.D.; Igarashi, T.; Villacorte-Tabelin, M.; Alorro, R.D.; Yoo, K.; Raval, S.; Ito, M.; Hiroyoshi, N. Acid mine drainage formation and arsenic mobility under strongly acidic conditions: Importance of soluble phases, iron oxyhydroxides/oxides and nature of oxidation layer on pyrite. J. Hazard. Mater. 2020, 399, 122844. [Google Scholar] [CrossRef]
- Machado de Oliveira, C.; Gesser Müller, T.; Patricio Ferreira, L.; Prado Cechinel, M.A.; Peterson, M.; Raupp-Pereira, F. Valorization of iron pyrite from coal mining in southern Brazil. J. Environ. Chem. Eng. 2019, 7, 102931. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Machado, C.M.; Duarte, G.W.; Peterson, M. Beneficiation of pyrite from coal mining. J. Clean. Prod. 2016, 139, 821–827. [Google Scholar] [CrossRef]
- Park, I.; Tabelin, C.B.; Jeon, S.; Li, X.L.; Seno, K.; Ito, M.; Hiroyoshi, N. A review of recent strategies for acid mine drainage prevention and mine tailings recycling. Chemosphere 2019, 219, 588–606. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, L.; Liu, L.; Yang, Y.; Liu, Q. Iron removal from kaolinitic coal gangue via magnetic separation after oxidizing calcination with the crystal structure of kaolinite protected. Mater. Today Commun. 2023, 37, 107175. [Google Scholar] [CrossRef]
- Gao, J.; Sui, H.; Wu, S.; Zhang, R.; Zhang, M.; Cui, B.; Chu, H. Interaction study of oxygen and iron-sulfur clusters based on the density functional theory. Int. J. Chem. Eng. 2022, 2022, 9812188. [Google Scholar] [CrossRef]
- Luo, B.; Peng, T.; Sun, H. Innovative Methodology for Sulfur Release from Copper Tailings by the Oxidation Roasting Process. J. Chem. 2020, 2020, 8090846. [Google Scholar] [CrossRef]
- Yadollahi, A.; Abdollahi, H.; Ardejani, F.D.; Mirmohammadi, M.; Magdouli, S. Bio-oxidation behavior of pyrite, marcasite, pyrrhotite, and arsenopyrite by sulfur- and iron-oxidizing acidophiles. Bioresour. Technol. Rep. 2021, 15, 100699. [Google Scholar] [CrossRef]
- Sokić, M.; Marković, B.; Stanković, S.; Kamberović, Ž.; Štrbac, N.; Manojlović, V.; Petronijević, N. Kinetics of chalcopyrite leaching by hydrogen peroxide in sulfuric acid. Metals 2019, 9, 1173. [Google Scholar] [CrossRef]
- Leiva, E.; Cayazzo, M.; Dávila, L.; Torres, M.; Ledezma, C. Acid mine drainage dynamics from a paste tailing deposit: Effect of sulfate content on the consistency and chemical stability after storage. Metals 2021, 11, 860. [Google Scholar] [CrossRef]
- Mayoral, M.C.; Andrés, J.M.; Izquierdo, M.T.; Rubio, B. Pyrrhotite deposition through thermal projection to simulate iron sulphide slagging in oxyfuel combustion. Fuel 2012, 101, 197–204. [Google Scholar] [CrossRef]
- Mayoral, M.C.; Izquierdo, M.T.; Andrés, J.M.; Rubio, B. Mechanism of interaction of pyrite with hematite as simulation of slagging and fireside tube wastage in coal combustion. Thermochim. Acta 2002, 390, 103–111. [Google Scholar] [CrossRef]
- Bryers, R. Physical and chemical characteristics of pyrites and their influence on fireside problems in steam generators. J. Eng. Power 1976, 98, 517–527. [Google Scholar] [CrossRef]
- Hu, G.; Dam-Johansen, K.; Wedel, S.; Hansen, J.P. Decomposition and oxidation of pyrite. Prog. Energy Combust. Sci. 2006, 32, 295–314. [Google Scholar] [CrossRef]
- Jorgensen, F.R.A.; Moyle, F.J. Phases formed during the thermal analysis of pyrite in air. J. Therm. Anal. 1982, 25, 473–485. [Google Scholar] [CrossRef]
- Huffman, G.P.; Huggins, F.E.; Levasseur, A.A.; Chow, O.; Srinivasachar, S.; Mehta, A.K. Investigation of the transformations of pyrite in a drop-tube furnace. Fuel 1989, 68, 485–490. [Google Scholar] [CrossRef]
- Huffman, G.P.; Huggins, F.E.; Dunmyre, G.R. Investigation of the high-temperature behaviour of coal ash in reducing and oxidizing atmospheres. Fuel 1981, 60, 585–597. [Google Scholar] [CrossRef]
- Groves, S.; Williamson, J.; Sanyal, A. Decomposition of pyrite during pulverized coal combustion. Fuel 1987, 66, 461–466. [Google Scholar] [CrossRef]
- Bool, L.E.; Helble, J.J. Iron oxidation state and its effect on ash particle stickiness. In Applications of Advanced Technology to Ash-Related Problems in Boilers; Baxter, L., DeSollar, R., Eds.; Springer: Boston, MA, USA, 1996; pp. 281–292. [Google Scholar]
- Bool, L.E.; Peterson, T.W.; Wendt, J.O.L. The partitioning of iron during the combustion of pulverized coal. Combust. Flame 1995, 100, 262–270. [Google Scholar] [CrossRef]
- Srinivasachar, S.; Boni, A.A. A kinetic model for pyrite transformations in a combustion environment. Fuel 1989, 68, 829–836. [Google Scholar] [CrossRef]
- Srinivasachar, S.; Helble, J.J.; Boni, A.A. Mineral behavior during coal combustion 1. Pyrite transformations. Prog. Energy Combust. Sci. 1990, 16, 281–292. [Google Scholar] [CrossRef]
- Hong, Y.; Fegley, B. The kinetics and mechanism of pyrite thermal decomposition. Berichte Bunsenges. Phys. Chem. 1997, 101, 1870–1881. [Google Scholar] [CrossRef]
- Fegley, B. Why pyrite is unstable on the surface of venus. Icarus 1997, 128, 474–479. [Google Scholar] [CrossRef]
- Fegley, B.; Lodders, K.; Treiman, A.H.; Klingelhöfer, G. The rate of pyrite decomposition on the surface of venus. Icarus 1995, 115, 159–180. [Google Scholar] [CrossRef]
- McLennan, A.R.; Bryant, G.W.; Bailey, C.W.; Stanmore, B.R.; Wall, T.F. An experimental comparison of the ash formed from coals containing Pyrite and Siderite mineral in oxidizing and reducing conditions. Energy Fuels 2000, 14, 308–315. [Google Scholar] [CrossRef]
- McLennan, A.R.; Bryant, G.W.; Stanmore, B.R.; Wall, T.F. Ash formation mechanisms during pf combustion in reducing conditions. Energy Fuels 2000, 14, 150–159. [Google Scholar] [CrossRef]
- Wu, J.; Yu, D.; Zeng, X.; Yu, X.; Yu, G.; Han, J.; Liu, F.; Xu, M. Impacts of CO2 on the pyrite–kaolinite interaction and the product sintering strength. Proc. Combust. Inst. 2019, 37, 4479–4486. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, Q.; Zhang, S.; Wang, S.; Frost, R.L. Evolved gas analysis of coal-derived pyrite/marcasite. J. Therm. Anal. Calorim. 2014, 116, 887–894. [Google Scholar] [CrossRef]
- Wu, J.; Yu, D.; Yu, X.; Liu, F.; Chen, S.; Xu, M. High-temperature transformation of pyrite in CO2: Effects of residence time and the presence of O2. Proc. Combust. Inst. 2021, 38, 5493–5500. [Google Scholar] [CrossRef]
- Evangelou, V.P. Pyrite Oxidation and Its Control; CRC Press: Boca Raton, FL, USA, 1995; 293p. [Google Scholar]
- Darken, L.S.; Gurry, R.W. The system iron-oxygen. II. equilibrium and thermodynamics of liquid oxide and other phases. J. Am. Chem. Soc. 1946, 68, 798–816. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Q.; Liu, X.L.; Xu, B.; Yang, Y.B.; Jiang, T. A Thermodynamic analysis on the roasting of pyrite. Minerals 2019, 9, 220. [Google Scholar] [CrossRef]
- Waldner, P.; Pelton, A.D. Thermodynamic modeling of the Fe-S system. J. Phase Equilibria Diffus. 2004, 26, 23–38. [Google Scholar] [CrossRef]
- Lambert, J.M.; Simkovich, G.; Walker, P.L. The kinetics and mechanism of the pyrite-to-pyrrhotite transformation. Metall. Mater. Trans. B 1998, 29, 385–396. [Google Scholar] [CrossRef]
- Kullerud, G.; Yoder, H.S. Pyrite stability relations in the Fe-S system. Econ. Geol. 1959, 54, 533–572. [Google Scholar] [CrossRef]
- Jensen, E. Pyrrhotite: Melting relation and composition. Am. J. Sci. 1942, 240, 695–709. [Google Scholar] [CrossRef]
- Hillert, M.; Staffansson, L.-I. An analysis of the phase equilibria in the Fe−FeS system. Metall. Mater. Trans. B 1975, 6, 37–41. [Google Scholar] [CrossRef]
- Morimoto, N.; Gyobu, A.; Mukaiyama, H.; Izawa, E. Crystallography and stability of pyrrhotites. Econ. Geol. 1975, 70, 824–833. [Google Scholar] [CrossRef]
- Moreau, J.G.; Jõeleht, A.; Aruväli, J.; Heikkilä, M.J.; Stojic, A.N.; Thomberg, T.; Plado, J.; Hietala, S. Bulk synthesis of stoichiometric/meteoritic troilite (FeS) by high-temperature pyrite decomposition and pyrrhotite melting. Meteorit. Planet. Sci. 2022, 57, 588–602. [Google Scholar] [CrossRef]
- Nickel, E.H. Mineral Chemistry of Metal Sulfides. Mineral. Mag. 1979, 43, 186–187. [Google Scholar] [CrossRef]
- Jagadeesh, M.S.; Seehra, M.S. Thermomagnetic studies of conversion of pyrite and marcasite in different atmospheres (vacuum, H2, He and CO). J. Phys. D Appl. Phys. 1981, 14, 2153–2167. [Google Scholar] [CrossRef]
- Monteiro, J.L.F. Thermal decomposition of pyrite in a fluidized bed. Can. J. Chem. Eng. 1981, 59, 511–516. [Google Scholar] [CrossRef]
- Music, S.; Popovi, S.; Risti, M. Thermal decomposition of pyrite. J. Radioanal. Nucl. Chem. 1992, 162, 217–226. [Google Scholar] [CrossRef]
- Nakazawa, H. Phase relations and modulated structures of pyrrhotite Fe1−XS. Nihon Kessho Gakkaishi 1980, 22, 251–262. [Google Scholar] [CrossRef]
- Kissin, S.A.; Scott, S.D. Phase relations involving pyrrhotite below 350 degrees C. Econ. Geol. 1982, 77, 1739–1754. [Google Scholar] [CrossRef]
- Stull, D.R.; Prophet, H. JANAF Thermochemical Tables; National Institute of Standards and Technology: Gaithersburg, MD, USA, 1971. [Google Scholar]
- Cheng, H.; Liu, Q.; Huang, M.; Zhang, S.; Frost, R.L. Application of TG-FTIR to study SO2 evolved during the thermal decomposition of coal-derived pyrite. Thermochim. Acta 2013, 555, 1–6. [Google Scholar] [CrossRef]
- Zhang, X.; Kou, J.; Sun, C. A comparative study of the thermal decomposition of pyrite under microwave and conventional heating with different temperatures. J. Anal. Appl. Pyrolysis 2019, 138, 41–53. [Google Scholar] [CrossRef]
- Tian, C.; Rao, Y.; Su, G.; Huang, T.; Xiang, C.; Kordulis, C. The Thermal Decomposition Behavior of Pyrite-Pyrrhotite Mixtures in Nitrogen Atmosphere. J. Chem. 2022, 2022, 8160007. [Google Scholar] [CrossRef]
- Hoare, I.C.; Hurst, H.J.; Stuart, W.I.; White, T.J. Thermal decomposition of pyrite. Kinetic analysis of thermogravimetric data by predictor–corrector numerical methods. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1988, 84, 3071–3077. [Google Scholar] [CrossRef]
- Yan, J.; Xu, L.; Yang, J. A study on the thermal decomposition of coal-derived pyrite. J. Anal. Appl. Pyrolysis 2008, 82, 229–234. [Google Scholar] [CrossRef]
- Zhao, H.L.; Bai, Z.Q.; Guo, Z.X.; Kong, L.X.; Wei, Y.C.; Li, H.Z.; Bai, J.; Li, W. In situ study of the decomposition of pyrite in coal during hydropyrolysis. J. Anal. Appl. Pyrolysis 2021, 154, 105024. [Google Scholar] [CrossRef]
- Rau, H. Energetics of defect formation and interaction in pyrrhotite Fe1−xS and its homogeneity range. J. Phys. Chem. Solids 1976, 37, 425–429. [Google Scholar] [CrossRef]
- Ruan, S.; Wang, C.; Jie, X.; Yin, F.; Zhang, Y.; Yao, Z.; Chen, Y. Kinetics of pyrite multi-step thermal decomposition in refractory gold sulphide concentrates. J. Therm. Anal. Calorim. 2021, 147, 3689–3702. [Google Scholar] [CrossRef]
- Pemsler, J.; Lam, R.; Litchfield, J.; Dallek, S.; Larrick, B.; Beard, B. Discharge behavior and thermal stability of synthetic FeS2 cathode material. J. Electrochem. Soc. 1990, 137, 1. [Google Scholar] [CrossRef]
- Coats, A.W.; Bright, N.F.H. The kinetics of the thermal decomposition of pyrite. Can. J. Chem. 1966, 44, 1191–1195. [Google Scholar] [CrossRef]
- Hu, H.P.; Chen, Q.Y.; Yin, Z.L.; Zhang, P.M.; Zou, J.P.; Che, H.S. Study on the kinetics of thermal decomposition of mechanically activated pyrites. Thermochim. Acta 2002, 389, 79–83. [Google Scholar] [CrossRef]
- Concer, P.H.; Oliveira, C.M.D.; Montedo, O.R.K.; Angioletto, E.; Peterson, M.; Fiori, M.A.; Moreira, R.D.F.P. Kinetics of the oxidation reactions and decomposition of pyrite. Cerâmica 2017, 63, 39–43. [Google Scholar] [CrossRef]
- Charpentier, L.; Masset, P.J. Thermal decomposition of pyrite FeS2 under reducing conditions. In Proceedings of the 7th Pacific Rim International Conference on Advanced Materials and Processing, Cairns, Australia, 2–6 August 2010; pp. 2398–2401. [Google Scholar]
- Lv, W.; Yu, D.; Wu, J.; Zhang, L.; Xu, M. The chemical role of CO2 in pyrite thermal decomposition. Proc. Combust. Inst. 2015, 35, 3637–3644. [Google Scholar] [CrossRef]
- Boyabat, N.; Özer, A.K.; Bayrakçeken, S.; Gülaboğlu, M.Ş. Thermal decomposition of pyrite in the nitrogen atmosphere. Fuel Process. Technol. 2004, 85, 179–188. [Google Scholar] [CrossRef]
- Schwab, G.-M.; Philinis, J. Reactions of iron pyrite-its thermal decomposition, reduction by Hydrogen and air oxidation. J. Am. Chem. Soc. 1947, 69, 2588–2596. [Google Scholar] [CrossRef]
- Bu, X.N.; Xie, G.Y.; Chen, Y.R.; Ni, C. The Order of Kinetic Models in Coal Fines Flotation. Int. J. Coal Prep. Util. 2016, 37, 113–123. [Google Scholar] [CrossRef]
- Ni, C.; Bu, X.N.; Xia, W.C.; Peng, Y.L.; Xie, G.Y. Effect of slimes on the flotation recovery and kinetics of coal particles. Fuel 2018, 220, 159–166. [Google Scholar] [CrossRef]
- Jovanović, D. Kinetics of thermal decomposition of pyrite in an inert atmosphere. J. Therm. Anal. 1989, 35, 1483–1492. [Google Scholar] [CrossRef]
- Luganov, V.A.; Shabalin, V.I. Behaviour of Pyrite During Heating. Can. Metall. Q. 2013, 21, 157–162. [Google Scholar] [CrossRef]
- Toro, C.; Torres, S.; Parra, V.; Fuentes, R.; Castillo, R.; Díaz, W.; Reyes, G.; Balladares, E.; Parra, R. On the detection of spectral emissions of iron oxides in combustion experiments of pyrite concentrates. Sensors 2020, 20, 1284. [Google Scholar] [CrossRef]
- Aracena, A.; Jerez, O. Mechanism and kinetics of pyrite transformation at elevated temperatures. Physicochem. Probl. Miner. Process. 2021, 57, 127–139. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, H.; Yang, H.R.; Lv, J.F. Oxidation mechanism of pyrite concentrates (PCs) under typical circulating fluidized bed (CFB) roasting conditions and design principles of PCs’ CFB roaster. Chem. Eng. Process. Process Intensif. 2020, 153, 107944. [Google Scholar] [CrossRef]
- Lihui, L.; Qinfu, L.; Kenan, Z.; Shuai, Z.; Kuo, L.; Jintao, L.; Gaoyu, P. Thermal decomposition and oxidation of pyrite with different morphologies in the coal gangue of north China. J. Therm. Anal. Calorim. 2023, 148, 2039–2040. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Q.; Zhang, S.; Li, Y.; Yang, L. The thermal transformation behavior and products of pyrite during coal gangue combustion. Fuel 2022, 324, 124803. [Google Scholar] [CrossRef]
- Pelovski, Y.; Petkova, V. Investigation on thermal decomposition of pyrites part 1. J. Therm. Anal. Calorim. 1999, 56, 95–99. [Google Scholar] [CrossRef]
- Aracena, A.; Jerez, O.; Ortiz, R.; Morales, J. Pyrite oxidation kinetics in an oxygen-nitrogen atmosphere at temperatures from 400 to 500 °C. Can. Metall. Q. 2016, 55, 195–201. [Google Scholar] [CrossRef]
- McCarty, K.F.; Hamilton, J.C.; Boehme, D.R.; Nagelberg, A.S. In situ Raman spectroscopy of high temperature pyrite reactions related to deposit formation from coal. J. Electrochem. Soc. 1989, 136, 1223–1229. [Google Scholar] [CrossRef]
- Earnest, C. Descriptive oxidative profiles for pyrite in the low temperature ash component of coals by differential thermal analysis. Thermochim. Acta 1984, 75, 219–232. [Google Scholar] [CrossRef]
- Dunn, J.G. The oxidation of sulphide minerals. Thermochim. Acta 1997, 300, 127–139. [Google Scholar] [CrossRef]
- Dunn, J.G.; De, G.C.; O’Connor, B.H. The effect of experimental variables on the mechanism of the oxidation of pyrite: Part 2. oxidation of particles of size 90–125 μm. Thermochim. Acta 1989, 155, 135–149. [Google Scholar] [CrossRef]
- Dunn, J.G.; Mackey, L.C. The measurement of the ignition temperatures of commercially important sulfide minerals. J. Therm. Anal. 1992, 38, 487–494. [Google Scholar] [CrossRef]
- Labus, M. Pyrite thermal decomposition in source rocks. Fuel 2021, 287, 119529. [Google Scholar] [CrossRef]
- Schorr, J.R.; Everhart, J.O. Thermal behavior of pyrite and its relation to carbon and sulfur oxidation in clays. J. Am. Ceram. Soc. 1969, 52, 351–354. [Google Scholar] [CrossRef]
- Chandra, A.P.; Gerson, A.R. Pyrite (FeS2) oxidation: A sub-micron synchrotron investigation of the initial steps. Geochim. Cosmochim. Acta 2011, 75, 6239–6254. [Google Scholar] [CrossRef]
- Özdemir, Ö.; Banerjee, S.K. High temperature stability of maghemite (γ-Fe2O3). Geophys. Res. Lett. 1984, 11, 161–164. [Google Scholar] [CrossRef]
- Feitknecht, W.; Gallagher, K.J. Mechanisms for the oxidation of Fe3O4. Nature 1970, 228, 548–549. [Google Scholar] [CrossRef]
- Chen, H.K.; Li, B.Q.; Zhang, B.J. Decomposition of pyrite and the interaction of pyrite with coal organic matrix in pyrolysis and hydropyrolysis. Fuel 2000, 79, 1627–1631. [Google Scholar] [CrossRef]
- Li, H.-Y.; Zhang, S.-H. Detection of mineralogical changes in pyrite using measurements of temperature-dependence susceptibilities. Chin. J. Geophys. 2005, 48, 1454–1461. [Google Scholar] [CrossRef]
- Prasad, A.; Singru, R.M.; Biswas, A.K. Study of the roasting of pyrite minerals by Mössbauer spectroscopy. Phys. Status Solidi (A) 1985, 87, 267–271. [Google Scholar] [CrossRef]
- Jerzy Tomeczek, H.P. Kinetics of mineral matter transformation during coal combustion. Fuel 2002, 81, 1251–1258. [Google Scholar] [CrossRef]
- Helble, J.; Neville, M.; Sarofim, A.F. Aggregate formation from vaporized ash during pulverized coal combustion. Symp. (Int.) Combust. 1988, 21, 411–417. [Google Scholar] [CrossRef]
- Helble, J.J.; Sarofim, A.F. Influence of char fragmentation on ash particle size distributions. Combust. Flame 1989, 76, 183–196. [Google Scholar] [CrossRef]
- Helble, J.J.; Srinivasachar, S.; Boni, A.A. Factors influencing the transformation of minerals during pulverized coal combustion. Prog. Energy Combust. Sci. 1990, 16, 267–279. [Google Scholar] [CrossRef]
- Huffman, G.P.; Huggins, F.E.; Shah, N.; Shah, A. Behavior of basic elements during coal combustion. Prog. Energy Combust. Sci. 1990, 16, 243–251. [Google Scholar] [CrossRef]
- Wang, X.S.; Ma, T.D.; Tang, Y.G.; Gupta, R.; Schobert, H.H.; Zhang, J.Y. Thermal transformation of coal pyrite with different structural types during heat treatment in air at 573–1473 K. Fuel 2022, 327, 124918. [Google Scholar] [CrossRef]
- Blachere, J. Desulfurization of pyrite. J. Am. Ceram. Soc. 1966, 49, 590–593. [Google Scholar] [CrossRef]
- Schoenlaub, R.A. Oxidation of pyrite. J. Am. Ceram. Soc. 1969, 52, 40–43. [Google Scholar] [CrossRef]
- Wang, L.; Pan, Y.X.; Li, J.H.; Qin, H.F. Magnetic properties related to thermal treatment of pyrite. Sci. China Ser. D-Earth Sci. 2008, 51, 1144–1153. [Google Scholar] [CrossRef]
- Thorpe, A.N.; Senftle, F.E.; Alexander, C.C.; Dulong, F.T. Oxidation of pyrite in coal to magnetite. Fuel 1984, 63, 662–668. [Google Scholar] [CrossRef]
- Nishihara, K. Studies on the oxidation of pyrite:(part 1) thermal decomposition of pyrite. Mem. Fac. Eng. 1958, 20, 285–306. [Google Scholar]
- Nishihara, K.; Kondo, Y. Studies on the oxidation of pyrite III: The intermediate products in the oxidation of pyrite. Fac. Eng. Kyoto Univ. 1959, 21, 214–228. [Google Scholar]
- Bhargava, S.K.; Garg, A.; Subasinghe, N.D. In situ high-temperature phase transformation studies on pyrite. Fuel 2009, 88, 988–993. [Google Scholar] [CrossRef]
- Wang, L.; Fan, B.W.; He, Y.T.; Li, P.; Yin, D.Q.; Hu, Y.H. Characteristics of minerals and their associations of transformation processes in pyrite at elevated temperatures: An X-ray diffraction study. Ironmak. Steelmak. 2013, 41, 147–152. [Google Scholar] [CrossRef]
- Colombo, U.; Gazzarrini, F.; Lanzavecchia, G.; Sironi, G. Magnetite oxidation: A proposed mechanism. Science 1965, 147, 1033. [Google Scholar] [CrossRef]
- Davis, B.L.; Rapp, G.; Walawender, M.J. Fabric and structural characteristics of the martitization process. Am. J. Sci. 1968, 266, 482–496. [Google Scholar] [CrossRef]
- Elder, T. Particle-size effect in oxidation of natural magnetite. J. Appl. Phys. 1965, 36, 1012–1013. [Google Scholar] [CrossRef]
- Feitknecht, W.; Mannweiler, U. Der Mechanismus der Umwandlung von γ-zu α-Eisensesquioxid [1]. Helv. Chim. Acta 1967, 50, 570–581. [Google Scholar] [CrossRef]
- Minyuk, P.; Tyukova, E.; Subbotnikova, T.; Kazansky, A.Y.; Fedotov, A. Thermal magnetic susceptibility data on natural iron sulfides of northeastern Russia. Russ. Geol. Geophys. 2013, 54, 464–474. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, C.; Li, B.; Wang, C.; Chen, X.; Wei, J.; Yu, Q. Clarifying the decomposition process of pyrite and SO2 release in the cyclone preheater of a dry rotary cement kiln system. J. Clean. Prod. 2019, 241, 118422. [Google Scholar] [CrossRef]
- Lv, W.; Yu, D.; Wu, J.; Yu, X.; Du, Y.; Xu, M. A mechanistic study of the effects of CO2 on pyrrhotite oxidation. Proc. Combust. Inst. 2017, 36, 3925–3931. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, Y.; Yao, H. Mineral behavior during oxy-fuel combustion. In Oxy-Fuel Combustion; Elsevier: Amsterdam, The Netherlands, 2018; pp. 129–150. [Google Scholar]
- Yu, D.; Yu, X.; Wu, J.; Han, J.; Liu, F.; Pan, H. A comprehensive review of ash issues in oxyfuel combustion of coal and biomass: Mineral matter transformation, ash formation, and deposition. Energy Fuels 2021, 35, 17241–17260. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, P.; Cheng, H.; Liu, Q. Thermal phase transition of pyrite from coal: Implication for the environmental impact of sulfur pollution. J. Therm. Anal. Calorim. 2018, 134, 2391–2396. [Google Scholar] [CrossRef]
- Hansen, J.P.; Jensen, L.S.; Wedel, S.; Dam-Johansen, K. Decomposition and oxidation of pyrite in a fixed-bed reactor. Ind. Eng. Chem. Res. 2003, 42, 4290–4295. [Google Scholar] [CrossRef]
- Shkodin, V.G.; Abishev, D.N.; Kobzhasov, A.K.; Malyshev, V.P.; Mangutova, R.F. The question of thermal decomposition of pyrite. J. Therm. Anal. 1978, 13, 49–53. [Google Scholar] [CrossRef]
- Pysh’Yev, S.; Gayvanovych, V.; Pattek-Janczyk, A.; Stanek, J. Oxidative desulphurisation of sulphur-rich coal. Fuel 2004, 83, 1117–1122. [Google Scholar] [CrossRef]
- Hausen, D.M. Reversible reactions between pyrite and pyrrhotite in S02. J. Miner. 1991, 43, 31–34. [Google Scholar] [CrossRef]
- Thomas, P.S.; Hirschausen, D.; White, R.E.; Guerbois, J.P.; Ray, A.S. Characterisation of the oxidation products of pyrite by thermogravimetric and evolved gas analysis. J. Therm. Anal. Calorim. 2003, 72, 769–776. [Google Scholar] [CrossRef]
- Almeida, C.M.V.B.; Giannetti, B.F. Comparative study of electrochemical and thermal oxidation of pyrite. J. Solid State Electrochem. 2002, 6, 111–118. [Google Scholar] [CrossRef]
- Banerjee, A.C.; Rangaswamy, P.; Sood, S. Mechanism of oxidation of iron pyrite in dynamic air. Therm. Anal. 1980, 2, 241–246. [Google Scholar] [CrossRef]
- Banerjee, A.C. Mechanism of oxidation of iron pyrites. J. Chem. Soc. D Chem. Commun. 1971, 1006–1007. [Google Scholar] [CrossRef]
- Paulik, F.; Paulik, J.; Arnold, M. Kinetics and mechanism of the decomposition of pyrite under conventional and quasi-isothermal–quasi-isobaric thermoanalytical conditions. J. Therm. Anal. 1982, 25, 313–325. [Google Scholar] [CrossRef]
- Eneroth, E.; Koch, C.B. Crystallite size of haematite from thermal oxidation of pyrite and marcasite—Effects of grain size and iron disulphide polymorph. Miner. Eng. 2003, 16, 1257–1267. [Google Scholar] [CrossRef]
- Cole, D.A.; Simmons, G.W.; Herman, R.G.; Klier, K.; Czakó-Nagy, I. Transformations of iron minerals during coal oxidation. Fuel 1987, 66, 1240–1248. [Google Scholar] [CrossRef]
- Descostes, M.; Mercier, F.; Beaucaire, C.; Zuddas, P.; Trocellier, P. Nature and distribution of chemical species on oxidized pyrite surface: Complementarity of XPS and nuclear microprobe analysis. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2001, 181, 603–609. [Google Scholar] [CrossRef]
- Usher, C.R.; Paul, K.W.; Narayansamy, J.; Kubicki, J.D.; Sparks, D.L.; Schoonen, M.A.; Strongin, D.R. Mechanistic aspects of pyrite oxidation in an oxidizing gaseous environment: An in situ HATR-IR isotope study. Environ. Sci. Technol. 2005, 39, 7576–7584. [Google Scholar] [CrossRef]
- Kennedy, T.; Sturman, B.T. The oxidation of iron (II) sulphide. J. Therm. Anal. 1975, 8, 329–337. [Google Scholar] [CrossRef]
- Rimstidt, J.D.; Vaughan, D.J. Pyrite oxidation: A state-of-the-art assessment of the reaction mechanism. Geochim. Cosmochim. Acta 2003, 67, 873–880. [Google Scholar] [CrossRef]
- Tian, C.; Rao, Y.; Su, G.; Huang, T.; Niu, Y. Effects of Pyrrhotite on the Combustion Behavior and the Kinetic Mechanism of Pyrite-Pyrrhotite Mixture Powders in the Air. Int. J. Chem. Eng. 2023, 2023, 9567708. [Google Scholar] [CrossRef]
- Ferrow, E.A.; Mannerstrand, M.; Sjöberg, B. Reaction kinetics and oxidation mechanisms of the conversion of pyrite to ferrous sulphate: A Mössbauer spectroscopy study. Hyperfine Interact. 2005, 163, 109–119. [Google Scholar] [CrossRef]
- Ferrow, E.A.; Sjöberg, B.A. Oxidation of pyrite grains: A mössbauer spectroscopy and mineral magnetism study. Hyperfine Interact. 2006, 163, 95–108. [Google Scholar] [CrossRef]
- Li, Y.; Gao, X.; Wu, H. Ash cenosphere from solid fuels combustion. Part 2: Significant role of ash cenosphere fragmentation in ash and particulate matter formation. Energy Fuels 2013, 27, 822–829. [Google Scholar] [CrossRef]
- Li, Y.; Wu, H. Ash cenosphere from solid fuels combustion. Part 1: An investigation into Its formation mechanism using Pyrite as a model fuel. Energy Fuels 2012, 26, 130–137. [Google Scholar] [CrossRef]
- Senior, C.L.; Srinivasachar, S. Viscosity of ash particles in combustion systems for prediction of particle sticking. Energy Fuels 2002, 9, 277–283. [Google Scholar] [CrossRef]
- Wall, T.F. Mineral matter transformations and ash deposition in pulverised coal combustion. Symp. (Int.) Combust. 1992, 24, 1119–1126. [Google Scholar] [CrossRef]
- Nowok, J.W.; Benson, S.A.; Jones, M.L.; Kalmanovitch, D.P. Sintering behaviour and strength development in various coal ashes. Fuel 1990, 69, 1020–1028. [Google Scholar] [CrossRef]
- Fryda, L.E.; Sobrino, C.; Glazer, M.; Bertrand, C.; Cieplik, M.K. Study of ash deposition during coal combustion under oxyfuel conditions. Fuel 2012, 92, 308–317. [Google Scholar] [CrossRef]
- Asaki, Z.; Kondo, Y. Oxidation kinetics of iron sulfide in the form of dense plate, pellet and single particle. J. Therm. Anal. 1989, 35, 1751–1759. [Google Scholar] [CrossRef]
- Asaki, Z.; Mori, S.; Ikeda, M.; Kondo, Y. Oxidation of pyrrhotite particles falling through a vertical tube. Metall. Trans. B 1985, 16, 627–638. [Google Scholar] [CrossRef]
- Tenbrink, H.; Eenkhoorn, S.; Hamburg, G. A fundamental investigation of the flame kinetics of coal pyrite. Fuel 1996, 75, 945–951. [Google Scholar] [CrossRef]
- Jassim, E.; Benson, S.A.; Bowman, F.M.; Seames, W.S. The influence of fragmentation on the behavior of pyrite particles during pulverized coal combustion. Fuel Process. Technol. 2011, 92, 970–976. [Google Scholar] [CrossRef]
- Wu, H.W.; Li, Y. Ash cenosphere fragmentation during pulverised pyrite combustion: Importance of cooling. Proc. Combust. Inst. 2019, 37, 2773–2780. [Google Scholar] [CrossRef]
- Huang, F.; Zhang, L.-Q.; Yi, B.-J.; Xia, Z.-J.; Zheng, C.-G. Transformation pathway of excluded mineral pyrite decomposition in CO2 atmosphere. Fuel Process. Technol. 2015, 138, 814–824. [Google Scholar] [CrossRef]
- Huang, F.; Xin, S.; Mi, T.; Zhang, L. Study of pyrite transformation during coal samples heated in CO2 atmosphere. Fuel 2021, 292, 120269. [Google Scholar] [CrossRef]
- Yu, D.X.; Zhao, L.; Zhang, Z.Y.; Wen, C.; Xu, M.H.; Yao, H. Iron transformation and ash fusibility during coal combustion in air and O2/CO Medium. Energy Fuels 2012, 26, 3150–3155. [Google Scholar] [CrossRef]
- Gomes, T.; da Rosa, R.; Cargnin, M.; Quadri, M.B.; Peterson, M.; de Oliveira, C.M.; da Rosa Rabelo, N.; Angioletto, E. Pyrite roasting in modified fluidized bed: Experimental and modeling analysis. Chem. Eng. Sci. 2022, 261, 117977. [Google Scholar] [CrossRef]
- Yang, Y.J.; Liu, J.; Liu, F.; Wang, Z. Comprehensive evolution mechanism of SO2 formation during pyrite oxidation. Proc. Combust. Inst. 2019, 37, 2809–2819. [Google Scholar] [CrossRef]
- Coombs, P.; Munir, Z. The mechanism of oxidation of ferrous sulfide (FeS) powders in the range of 648 to 923 K. Metall. Trans. B 1989, 20, 661–670. [Google Scholar] [CrossRef]
- Coombs, P.; Munir, Z. The decomposition of iron (III) sulfate in air. J. Therm. Anal. 1989, 35, 967–976. [Google Scholar] [CrossRef]
- Vázquez, M.; Moreno-Ventas, I.; Raposo, I.; Palma, A.; Díaz, M.J. Kinetic of pyrite thermal degradation under oxidative environment. J. Therm. Anal. Calorim. 2019, 141, 1157–1163. [Google Scholar] [CrossRef]
- Mao, Y.; Bu, X.; Peng, Y.; Tian, F.; Xie, G. Effects of simultaneous ultrasonic treatment on the separation selectivity and flotation kinetics of high-ash lignite. Fuel 2020, 259, 116270. [Google Scholar] [CrossRef]
- Flagan, R.C. Submicron particles from coal combustion. Symp. (Int.) Combust. 1979, 17, 97–104. [Google Scholar] [CrossRef]
- Ni, C.; Xie, G.Y.; Jin, M.G.; Peng, Y.L.; Xia, W.C. The difference in flotation kinetics of various size fractions of bituminous coal between rougher and cleaner flotation processes. Powder Technol. 2016, 292, 210–216. [Google Scholar] [CrossRef]
- Hawkins, A.B. Engineering implications of the oxidation of pyrite: An overview, with particular reference to Ireland. In Implications of Pyrite Oxidation for Engineering Works; Springer International Publishing AG: Cham, Switzerland, 2014; pp. 1–98. [Google Scholar]
- Baxter, L.L. Char fragmentation and fly ash formation during pulverized-coal combustion. Combust. Flame 1992, 90, 174–184. [Google Scholar] [CrossRef]
- Baxter, L.L.; Mitchell, R.E. The release of iron during the combustion of Illinois No. 6 coal. Combust. Flame 1992, 88, 1–14. [Google Scholar] [CrossRef]
- Yani, S.; Zhang, D.K. An experimental study into pyrite transformation during pyrolysis of Australian lignite samples. Fuel 2010, 89, 1700–1708. [Google Scholar] [CrossRef]
- Bailey, C.W.; Bryant, G.W.; Matthews, E.M.; Wall, T.F. Investigation of the high-temperature behavior of excluded siderite grains during pulverized fuel combustion. Energy Fuels 1998, 12, 464–469. [Google Scholar] [CrossRef]
- Sheng, C.D.; Lin, J.; Li, Y.; Wang, C. Transformation behaviors of excluded pyrite during O2/CO combustion of pulverized coal. Asia-Pac. J. Chem. Eng. 2010, 5, 304–309. [Google Scholar] [CrossRef]
- Vuthaluru, H.B.; Eenkhoorn, S.; Hamburg, G.; Heere, P.G.T.; Kiel, J.H.A. Behaviour of iron-bearing minerals in the early stages of pulverised coal conversion processes. Fuel Process. Technol. 1998, 56, 21–31. [Google Scholar] [CrossRef]
- Zhao, H.-L.; Bai, Z.-Q.; Yan, J.-C.; Bai, J.; Li, W. Transformations of pyrite in different associations during pyrolysis of coal. Fuel Process. Technol. 2015, 131, 304–310. [Google Scholar] [CrossRef]
- Komraus, J.L.; Popiel, E.S.; Mocek, R. Chemical transformations of ferruginous minerals during the process of oxidation of hard coal. Hyperfine Interact. 1990, 58, 2589–2592. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Q.; Li, Y.; Cao, H.; Kang, X. Occurrence of iron in the minerals of carboniferous coal gangue of the Pingshuo open-pit mine, North China. Clays Clay Miner. 2022, 70, 695–711. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, H.; Liu, Q.; Yang, H. Experimental studies on phase transformation during pyrite concentrate oxidation under circulating fluidized bed (CFB) roasting conditions. Ind. Eng. Chem. Res. 2011, 50, 14168–14174. [Google Scholar] [CrossRef]
- Zeng, T.; Helble, J.J.; Bool, L.E.; Sarofim, A.F. Iron transformations during combustion of Pittsburgh no. 8 coal. Fuel 2009, 88, 566–572. [Google Scholar] [CrossRef]


| No. | Reaction | Equation | ΔrGθ at 500 K kJ/mol | ΔrGθ at 1000 K kJ/mol |
|---|---|---|---|---|
| 1 | FeS2 = 8/7Fe0.875S + 3/7S2(g) | −0.1219 T + 109.0817 | 48.13 | −12.82 |
| 2 | FeS2 = FeS + 1/2S2(g) | −0.1375 T + 140.2344 | 71.48 | 2.73 |
| 3 | S + 2O2 = 2SO2 | 0.1465 T − 723.7860 | 572.48 | 1003.73 |
| 4 | FeS2 + 11/4O2(g) = 1/2Fe2O3 + 2SO2(g) | 0.0756 T − 832.7516 | −794.95 | −757.15 |
| 5 | FeS2 + 7/4O2(g) = 1/2Fe2O3 + SO2(g) | 0.1407 T− 611.2840 | −540.93 | −470.58 |
| 6 | FeS2 + 8/3O2(g) = 1/3Fe3O4 + 2SO2(g) | 0.0519 T − 792.2288 | −766.28 | −740.33 |
| 7 | FeS2 + O2(g) = FeS + SO2(g) | −0.0647 T − 221.4677 | −253.82 | −286.17 |
| 8 | FeS2 + 6/7O2(g) = 8/7Fe0.875S + 6/7SO2(g) | −0.0592 T − 201.0982 | −230.70 | −260.30 |
| 9 | FeS2 + 3O2(g) = FeSO4 + SO2(g) | 0.2920 T − 1050.5365 | −904.54 | −758.54 |
| 10 | FeS2 + 7/2O2(g) = 1/2Fe2(SO4)3 + SO2(g) | 0.4859 T − 1265.3779 | −1022.43 | −779.48 |
| 11 | Fe0.875S + 53/32O2(g) = 7/16Fe2O3 + SO2(g) | 0.1192 T − 553.6720 | −494.07 | −434.47 |
| 12 | Fe0.875S + 19/12O2(g) = 7/24Fe3O4 + SO2(g) | 0.0985 T − 518.6612 | −469.41 | −420.16 |
| 13 | Fe0.875S + 1/8O2(g) = 7/8FeS + 1/8SO2(g) | −0.038 T − 18.0233 | −37.02 | −56.02 |
| 14 | Fe0.875S + O2(g) = 7/8FeSO4 + 1/8SO2(g) | 0.3099 T − 744.6433 | −589.69 | −434.74 |
| 15 | Fe0.875S + 47/16O2(g) = 7/16Fe2(SO4)3 + 5/16SO2(g) | 0.4938 T − 1127.0820 | −880.18 | −633.28 |
| 16 | FeS + 5/3O2(g) = 1/3Fe3O4 + SO2(g) | 0.1166 T − 570.7611 | −512.46 | −454.16 |
| 17 | FeS + 2O2(g) = FeSO4 | 0.3566 T − 829.0689 | −650.77 | −472.47 |
| 18 | FeS + 5/2O2(g) + 1/2SO2(g) = 1/2Fe2(SO4)3 | 0.5505 T − 1043.9102 | −768.66 | −493.41 |
| 19 | Fe3O4 + 1/4O2(g) = 3/2Fe2O3 | 0.0722 T − 121.5684 | −85.47 | −49.37 |
| 20 | Fe3O4 + O2(g) + 3SO2(g) = 3FeSO4 | 0.7201 T − 774.9232 | −414.87 | −54.82 |
| 21 | Fe3O4 + 5/2O2(g) + 9/2SO2(g) = 3/2Fe2(SO4)3 | 1.3018 T − 1419.4472 | −768.55 | −117.65 |
| 22 | Fe2O3 + 1/2O2(g) + 2SO2(g) = 2FeSO4 | 0.4319 T − 435.5699 | −219.62 | −3.67 |
| 23 | Fe2O3 + 3/2O2(g) + 3SO2(g) = Fe2(SO4)3 | 0.8197 T − 865.2525 | −455.40 | −45.55 |
| 24 | FeSO4 + 1/2O2(g) + 1/2SO2(g) = 1/2Fe2(SO4)3 | 0.1939 T − 214.8413 | −117.89 | −20.94 |
| No. | Author | Particle Size | Equipment | Atmosphere | Heating Rate | Model | Ea Value, kJ/mol | Mechanism |
|---|---|---|---|---|---|---|---|---|
| 1 | Hong [25] | 2 × 0.05 cm slice | Vertical tube | He, N2, 75 mL/min | Isothermal 400–590 °C | Linear dynamics | 297 ± 34 | Lattice decomposition |
| CO2, 75 mL/min | 275± 10 | Reacted with CO2 | ||||||
| 2 | Coats [59] | 0.25 inches cylinder | Vertical Quartz Tube | Ar 180 mL/min | Isothermal 600–653 °C | McKewan model | 270.6–290.7 | Chemically controlled |
| 3 | Pannetier (as cited from [16,25]) | Weight Loss | Vacuum | Isothermal 451–476 °C | Linear kinetics | 310 | ||
| 4 | Hu [60] | <1 mm | TG-DTG | Dynamic Ar | 1000 °C, 2.5, 5, 7.5, 15 K/min | Friedman | 268 | Lattice defects pyrolysis |
| 5 | Concer [61] | TGA | N2 50 mL/min | 2.5, 5.0, 7.5, 10.0 K/min. | Friedman | 279.2 | Volatilization of sulfur | |
| 6 | Pemsler [58] | 70–100, 100–140, 140–200, 270–325 mesh | TG | He 100–250 mL/min | Isothermal 500, 525, 550, 575, 600 °C None-isothermal: 2, 5, 10, 20 °C/min | Shrinking Core Model/First-Order | 226–239 | Chemical Reaction Control |
| 7 | Charpentier [62] | <63 μm | TGA/DSC | Ar 50 mL/min | 2–100 °C/min | Firedman | 250–350 (average 283 kJ/mol) | Chemical Reaction Control |
| No. | Author | Particle Size | Equipment | Atmosphere | Heating Rate | Model | Ea Value, kJ/mol | Mechanism |
|---|---|---|---|---|---|---|---|---|
| 8 | Lv [63] | 63–75 μm | TG | N2, 3000 mL/min | Isothermal 675–800 °C | Shrinking/3D diffusion | 103 | Step 1: 675–725 °C, Surface desulfurization Step 2: 725–800 °C, Diffusion desulfurization |
| 93 | ||||||||
| 9 | Fegley [27] | slice | High-Temperature Tube | CO2 | Isothermal, 390, 416, 468, 500, 530 °C 2.5–7 cm/min | Linear dynamics, Shrinking core | 142 | Desorption of sulfur |
| 100 ppm CO-CO2 | 156 | |||||||
| Ar-CO2 | 120 | |||||||
| 1.1% CO-CO2 | 153 | |||||||
| CO-CO2-SO2 | 141 | |||||||
| 10 | Boyabat [64] | 0.425–1.4 mm | Tube | N2 1670 cm/min | Non-Isothermal 400–800 °C | Shrinking core | 113 | Heat transfer at low-temperature Mass transfer at high-temperature |
| 96 | ||||||||
| 11 | Udintsev (as cited from [25]) | Vacuum, Ar | Isothermal 400–750 °C | Linear kinetics | 110 | |||
| 12 | Schwab [65] | 0.01–0.1 mm | Air, CO2, H2 | 400–650 °C | Linear kinetics | 125–138 | ||
| 13 | Zhukovskii (as cited from [16,25]) | Weight Loss | Vacuum, N2 | Isothermal 450–690 °C | Linear kinetics | 110 | ||
| 14 | Samal (as cited from [16,25]) | Weight Loss | Vacuum | Isothermal 486–554 °C | Linear kinetics | 120 |
| No. | Author | Particle Size | Equipment | Atmosphere | Heating Rate | Model | Ea Value, kJ/mol | Mechanism |
|---|---|---|---|---|---|---|---|---|
| 15 | Jovanović [68] | 0.072 mm | thermobalance | N2 | Isothermal 600, 660, 700, 750, 800, 850 °C | Farrar-Smith | 272 | Initially chemical reactions, later diffusion. |
| Diffusion control equation | 126 | |||||||
| 16 | Hoare [53] | 0.58 mg 220 mg particles | Stanton-Redcroft TG761/Cahn RG | N2 35 mL/min and 200 mL/min | 3 °C/min non-isothermal | Shrinking core, Ginstling-Brounshtein | 286 | Step 1: Chemical reaction Step 2: Solid-state diffusion |
| 190 | ||||||||
| 17 | Zhang [51] | 0.058 mm | tubular | N2 1 L/min | 10 °C/min, 450, 500, 600, 700 °C | Coats-Redfern | 199.76 | 600–700 °C |
| Microwave oven | 60 °C/min | 172.62 | 500–600 °C | |||||
| 18 | Lambert [37] | 210 × 250 μm 44 × 53 μm | TGA | Vacuum | Shrinking | 222 | S2 molecule form | |
| 139 | Desorption of S2 molecule | |||||||
| 19 | Luganov [69] | <0.1 mm | Thermal Analyzer | Ar 8–10 L/min | 10 °C/min 20–900 °C | Firedman | 193–210 | Chemical control at high temperatures Diffusion control at low temperature |
| No. | Author | Particle Size | Equipment | Atmosphere | Heating Rate | Model | Step1 Ea kJ/mol | Step2 Ea kJ/mol | Pathway |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Hong [25] | 2 × 1 × 0.05 cm slice | Vertical tube | O2-CO2, 75 mL/min | Isothermal 400–590 °C | Linear dynamics | 392–460 °C 82 ± 52 | 484–538 °C 293 ± 52 | Oxidized layer hinders reaction under 460 °C |
| CO2, CO-CO2, 75 mL/min | 260–275 | Rate constants similar with inert gases | |||||||
| 2 | Aracena [71,76] | 12.3, 16.0, 22.7, 33.8 μm | Vertical tube | O2, 5.07–28.69 kpa,1000 mL/min | Isothermal 550–800 °C | Initial slope | 70.1 kJ/mol | / | Temperature accelerates the first stage; Oxygen pressure impacts on second stage |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Xie, G.; Sha, J. Unified Phase Diagram and Competition-Coupling Mechanism for Pyrite Thermal Transformation. Minerals 2025, 15, 1139. https://doi.org/10.3390/min15111139
Liu M, Xie G, Sha J. Unified Phase Diagram and Competition-Coupling Mechanism for Pyrite Thermal Transformation. Minerals. 2025; 15(11):1139. https://doi.org/10.3390/min15111139
Chicago/Turabian StyleLiu, Mingrui, Guangyuan Xie, and Jie Sha. 2025. "Unified Phase Diagram and Competition-Coupling Mechanism for Pyrite Thermal Transformation" Minerals 15, no. 11: 1139. https://doi.org/10.3390/min15111139
APA StyleLiu, M., Xie, G., & Sha, J. (2025). Unified Phase Diagram and Competition-Coupling Mechanism for Pyrite Thermal Transformation. Minerals, 15(11), 1139. https://doi.org/10.3390/min15111139





