Abstract
In this study, Raman spectroscopy is applied to determine the salinity of fluid inclusions in the H2O-NaCl-CO2 system. In the work, various systems are prepared, such as H2O-NaCl, H2O-CO2, and H2O-NaCl-CO2. For the H2O-NaCl system, the addition of NaCl salts decreases the intensity of the sub-band below 3330 cm−1 but increases the intensity of the sub-band above 3330 cm−1. According to the structural analysis of the H2O-NaCl system, the spectral changes are mainly related to the interactions between Cl− and water. After the Raman OH stretching bands are fitted into two sub-bands, the intensity ratio between them is used to calculate the Cl− concentrations (molarity scale) of NaCl solutions. Additionally, based on the measured Raman spectra, the effects of CO2 on water structure may be weak. It is reasonable to ignore the impact of dissolved CO2 on Raman OH stretching bands. The procedure above can be extended to quantitatively determine the Cl− molarity of the H2O-NaCl-CO2 system. To demonstrate its reliability, this method is applied to determine the salinity of synthetic and natural fluid inclusions containing CO2.
1. Introduction
It is well known that geological fluids play an important role in many geological processes, including metallogenesis, sedimentary diagenesis, metamorphism, and so on. They may be preserved as fluid inclusions in these processes. Analyzing salts and saline solutions in fluid inclusions is vital for understanding the formation and evolution of ore deposits and petroleum basins [1,2,3,4,5,6,7]. Consequently, it is important to determine the salinity of fluid inclusions.
Because microthermometry has high accuracy and can be operated easily, it is widely utilized to determine the salinity of fluid inclusion. This is based on the measurement of eutectic temperature and ice melting temperature [8,9] on the binary or ternary water–salt systems, such as H2O-NaCl, H2O-NaCl-KCl, and H2O-NaCl-CaCl2 systems. CO2 is an important component in many geological environments [9,10,11,12] and is often trapped within fluid inclusions. In the gaseous CO2-bearing fluid inclusions, the salinity is not obtained from ice melting temperatures but from clathrate melting temperatures [13]. However, due to the limited resolution of the microscope, it is difficult to determine the temperature of phase transition in small inclusions (diameter < 5 μm). Additionally, the formation of metastable phase assemblages and the complexity of system composition lead to the measured results deviating from the actual salinity [14,15,16,17]. Therefore, other analytical methods have been developed to measure the geochemistry of fluid inclusion.
Raman spectroscopy, with its non-contact and non-destructive nature, offers an efficient method for measuring the salinity of fluid inclusions. As salts are dissolved into liquid water, they become hydrated anions and cations. Although the Na+ and Cl− ions are non-Raman-active, the dissolved ions may lead to spectral changes in Raman OH stretching bands, which may be applied to determine the salinity of NaCl solutions. Bakker [14] and Baumgartner and Bakker [18] showed that Raman spectroscopy can be used to confirm microthermometric results and provide a more detailed analysis of phase changes.
To date, a few works have been carried out to determine the salinity of NaCl solution. In previous works [17,19], increasing the NaCl salinity mainly lowers the spectral intensities below 3300 cm−1 and enhances the intensities above 3300 cm−1. A skewing parameter, based on the integrated intensities of two defined spectral regions (2800–3300 cm−1 and 3300–3800 cm−1), was used to measure the salinity of H2O-NaCl systems. In Dubessy et al.’s work [20], based on the calculated area difference of Raman OH spectra between pure water and salt solution, this may be used to determine the chloride concentration (mol/kg H2O) of LiCl, NaCl, KCl, MgCl2, and CaCl2 systems. From the Baumgartner and Bakker [18] study, the Raman OH stretching bands are deconvoluted into three sub-bands, and the position shifts of sub-band Peak1 (3223 cm−1) may be utilized to determine the salinity (mass %) of H2O-NaCl system. Cl− is the main anion, but various cations are expected in natural fluid inclusion. Regarding the solutions containing Cl− ions, the spectral changes in OH stretching bands are mainly due to the dissolved Cl− ions [21]. It is important to determine the Cl− concentrations of aqueous solutions. In Sun et al. work [21], the Raman OH stretching bands are fitted into two sub-bands with Gaussian functions, and the intensity ratio between the two peaks may be utilized to quantitatively measure the Cl− concentrations (molarity scale) of H2O-NaCl, H2O-KCl, H2O-CaCl2, H2O-MgCl2, and H2O-NaCl-KCl-CaCl2 systems. In Wang et al. work [22], they found that the existence of CO32−, SO42− in the solutions could lead to the estimated salinity to be higher than the actual results, and the presence of CO2 had no significant effect on the measured results. From the above, further study is necessary to determine the salinity of the H2O-NaCl-CO2 system.
In this work, various systems are prepared, such as H2O-NaCl, H2O-CO2, and H2O-NaCl-CO2. Based on the measured Raman spectra, they are applied to quantitatively measure the salinity of the H2O-NaCl-CO2 system. In H2O-NaCl systems, the Cl− concentrations (molarity scale) can be quantitatively determined by analyzing the intensity ratio of the two fitted sub-bands in the Raman OH stretching bands. According to this study, the effects of dissolved CO2 on water structure may be weak. It is reasonable to ignore the effects of CO2 on Raman OH stretching bands. Therefore, the above method may be extended to quantitatively measure the Cl− concentrations of the H2O-NaCl-CO2 system. To demonstrate its reliability, it is applied to measure the salinity of synthetic and natural fluid inclusions containing CO2.
2. Materials and Methods
2.1. Samples
In this study, aqueous NaCl solutions were prepared at different NaCl mass %. Additionally, aqueous solutions of Na2CO3 and Na2SO4 were also prepared to examine the influence of dissolved carbonate (CO32−) and sulfate (SO42−) ions on the Raman OH stretching bands of water. Deionized water was used, and analytical-grade salts were employed without further purification.
Raman spectroscopy is used to determine the salinity of synthetic and natural fluid inclusions. Various synthetic fluid inclusions were prepared through fused silica capillaries [23,24], such as H2O-CO2 and H2O-NaCl-CO2 systems. A 5 cm long tube, sealed at one end, was filled with the liquid and centrifuged to force the liquid into the closed end. The open end of the tube was then connected to a pressure line, and the air was removed by evacuation. Gaseous CO2 was cryogenically added by immersing the sealed end of the tube in liquid nitrogen, allowing the gas to condense for several minutes. After evacuation, the open end of the tube was sealed by fusion using a hydrogen flame [23].
The natural fluid inclusions were taken from No.3 pegmatite, Koktokay area, Xinjiang autonomous region, China. The diameters of natural inclusions are in the range of 2 μm to 25 μm. The CO2 phase is estimated to occupy about 30–50 vol % of the whole inclusions. Additionally, three phases can be found in the natural fluid inclusions, such as vapor CO2, liquid CO2, and aqueous solutions.
2.2. Raman Quantitative Measurement
Raman spectra were collected using a Renishaw inVia Reflex confocal micro-Raman system in backscattering geometry with 532 nm laser excitation at 50 mW. The spectrometer, equipped with a 50 μm entrance slit and a 2400/mm grating, provided a resolution of approximately 0.9 cm−1. The objective lens magnification was 50×. The scanning time was 30 s, accumulation times were 3 times (30 s × 3). Raman spectral measurement was conducted at 295 K.
Regarding the Raman-active molecular vibration, the measured Raman intensity may be theoretically expressed as,
in which I is the measured Raman spectral intensity, K is the proportionality coefficient, N is the number of Raman-active molecules, σ is the Raman scattering cross-section, and IL is the incident laser intensity. From the equation, the measured Raman spectral intensity is influenced by both the number of Raman-active molecules present and the intensity of the incident light. Consequently, variations in measurement conditions, such as excitation source power, optical configuration, scanning time, and accumulation times (Figure 1), can affect the measured Raman intensity. This may be used to enhance the measured Raman spectral intensities. Additionally, this also indicates that the measured Raman intensity cannot be directly applied to quantitatively determine the molecular number.
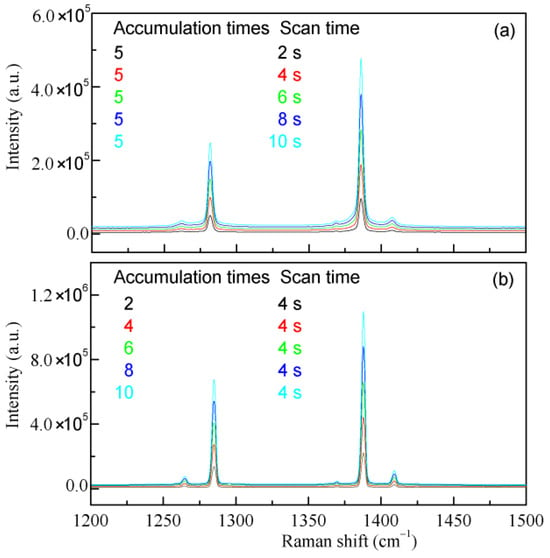
Figure 1.
The effects of measurement conditions on the Raman intensities. The Raman spectra of CO2 are measured under various scanning times (a) and accumulation times (b).
Prior to using Raman intensity for quantitative determination of solute concentrations, it is necessary to correct for the influence of Raman spectral measurement conditions. The intensity ratio (or relative intensity) between two Raman peaks provides an approach to the process of Raman quantitative measurement. According to Equation (1), the intensity ratio may be expressed as,
in which R means the reference sample. To eliminate the effects of measurement conditions on Raman spectral intensity, it is necessary to ensure the measurement conditions of the reference sample are the same as the analytical sample. By applying this method, solute concentrations in solutions can be quantitatively measured, where N represents the number of Raman-active molecules. In the Raman quantitative measurement, it is reasonable to use the units related to molecular numbers to express the solute concentrations, such as molarity.
Based on the measured Raman spectra, the intensity ratio (relative intensity) is calculated and used to determine the solute concentrations. Currently, this is divided into external and internal standards [25,26,27]. The standard means the Raman-active molecule, whose Raman spectrum is recorded under the measurement conditions the same as the analytical sample. For the external standard, the Raman spectra of the reference and analytical sample are separately recorded under the same measurement conditions. It is necessary to continuously measure the Raman spectra of them. It is indeed challenging to ascertain whether the measurement conditions of them are the same. An internal standard, a substance added (or incorporated) into the analytical sample, provides a reference point for intensity correction in Raman quantitative measurements. The Raman spectra include both the internal standard and the analytical sample, with a non-overlapping band of the internal standard serving as the reference. This method ensures identical measurement conditions for both the reference and the analytical sample, making it highly suitable for intensity correction.
At ambient conditions, the Raman OH stretching bands of water lie in the range of 2800–3800 cm−1 (Figure 2). Increasing NaCl concentrations decreases the intensity of the sub-band below 3330 cm−1 but increases the intensity of the sub-band above 3330 cm−1 (Figure 3). The changes in Raman OH stretching bands are dependent on salt concentrations, allowing for the measurement of salinity in H2O-NaCl systems.
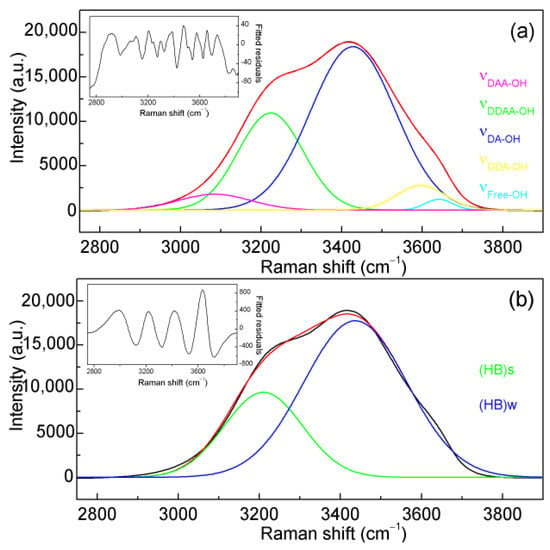
Figure 2.
Deconvolution of Raman OH stretching bands of water. (a) The Raman OH stretching bands of ambient water are fitted into five sub-bands attributed to OH vibrations engaged in various local hydrogen bonding interactions. (b) For salinity determination in NaCl solutions, the Raman OH stretching bands are fitted with two sub-bands. The fitted residuals are also shown.
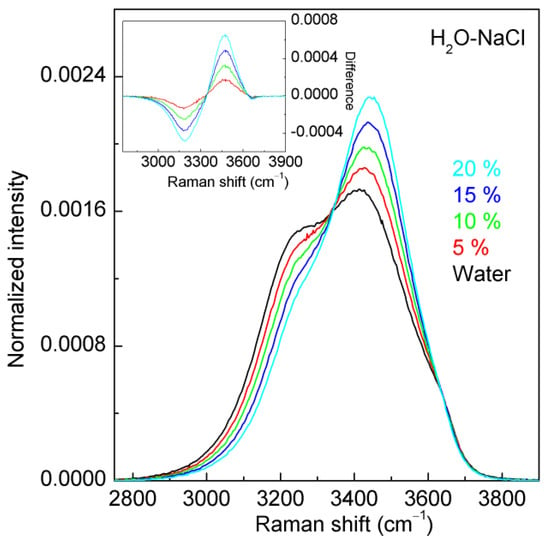
Figure 3.
The normalized intensity of the H2O-NaCl system. The inlet shows the normalized intensity difference between NaCl solutions and water. Dissolved NaCl salts predominantly decrease the intensity of the sub-band near 3220 cm−1 while increasing the intensity of the sub-band near 3450 cm−1. This is used to determine the Cl− molarity of NaCl solutions.
In this work, spectral analysis may be necessary. Raman spectra were processed using the Jandel Scientific Peakfit v 4.04 computer program. Raman spectra were smoothed to minimize noise, and their baselines were corrected using linear lines. To analyze the spectral changes, the Raman OH stretching band of water was fitted with two Gaussian sub-bands (Table 1). The ratio of intensities between these sub-bands can be used to determine the salinity of NaCl solutions.

Table 1.
The fitted parameters of Raman OH stretching bands of the H2O-NaCl system. The vibrational frequencies and intensities of two sub-bands are labeled as (HB)S and (HB)W, I(HB)S and I(HB)W. K means the intensity ratio between the two sub-bands. In comparison with pure water, the difference (ΔK) may be used to determine the Cl− concentrations (molar concentrations) of solutions.
Furthermore, normalized intensities were calculated to eliminate the influence of measurement conditions on spectral intensity, providing a more accurate representation of the spectral changes in the Raman OH stretching bands of water. In this study, the sum of Raman intensities in the range of the OH stretching band may be calculated, and the ratio of measured intensity to the sum is utilized to calculate the normalized intensity.
2.3. Microthermometry
Additionally, both Raman spectroscopy and microthermometry were used to determine the salinity of natural H2O-NaCl-CO2 fluid inclusions. In this work, microthermometric measurement was conducted using a THMSG 600 cooling and heating stage at the fluid inclusion laboratory, Institute of Geology and Geophysics, Chinese Academy of Sciences. The temperature range was from −120 °C to 40 °C, and temperature errors were ± 0.1 °C. In the study, the fluid inclusions were supercooled using liquid nitrogen and heated at the rate of 15 °C/min. When the temperature was close to the clathrate melting temperature (Tm,cla), the heating rate was kept at 0.1 °C/min to keep the clathrates in equilibrium with the system. The measured clathrate melting temperature (Tm,cla) was used to determine the salinity of the natural fluid inclusion [28].
3. Results
In this study, after the Raman OH stretching band is fitted into two peaks (Table 1), the intensity ratio between them (kH2O-NaCl, I(HB)w/I(HB)s) is used to measure the Cl− concentrations of H2O-NaCl system (Figure 2). Similar to our previous work [21], the corresponding intensity ratio (kwater) of pure water is also determined to eliminate the influence caused by the measurement conditions and spectral analysis process. In comparison with the intensity ratio of pure water (kwater), the difference (Δk, kH2O-NaCl− kwater) between NaCl solution and water is used to calculate the Cl− molarity (Table 1). Based on the Raman spectral measurement of the H2O-NaCl system, Cl− molarity of NaCl solutions can be expressed as (Figure 4),
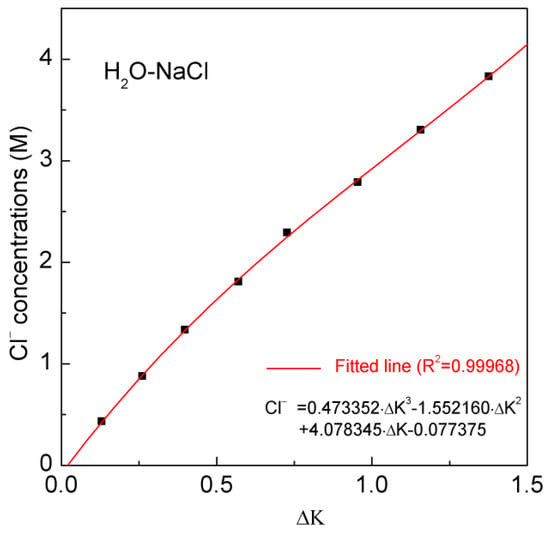
Figure 4.
The dependence of Cl− concentrations (M) on the calculated ΔK. The fitted equation is shown in a solid line.
In theory, the water structure may be influenced by the dissolved CO2. Before Raman spectroscopy is used to quantitatively measure the salinity of aqueous NaCl solutions, it is necessary to investigate the effects of CO2 on water structure (Figure 5). In comparison with pure water, only slight changes are found in the normalized intensity difference between the H2O-CO2 system and pure water. With increasing pressure, this leads to a slight increase in Raman intensities of the high wavenumber sub-band (>3600 cm−1) and a decrease in Raman intensities of the low wavenumber sub-band (<3200 cm−1). Additionally, slight spectral changes are also observed in the H2O-NaCl-CO2 system (Figure 6).
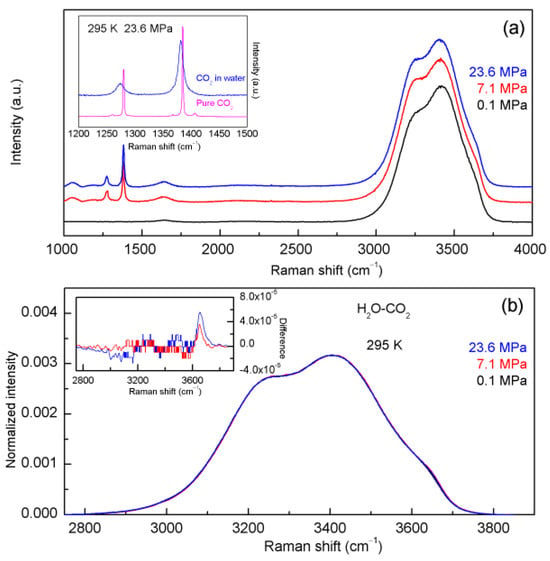
Figure 5.
(a) The Raman spectra of the H2O-CO2 system. (b) The normalized intensity of the Raman OH stretching band of the H2O-CO2 system. In comparison with pure water, only slight changes can be found in the normalized intensity difference between the H2O-CO2 system and pure water, which are shown in the inlet.
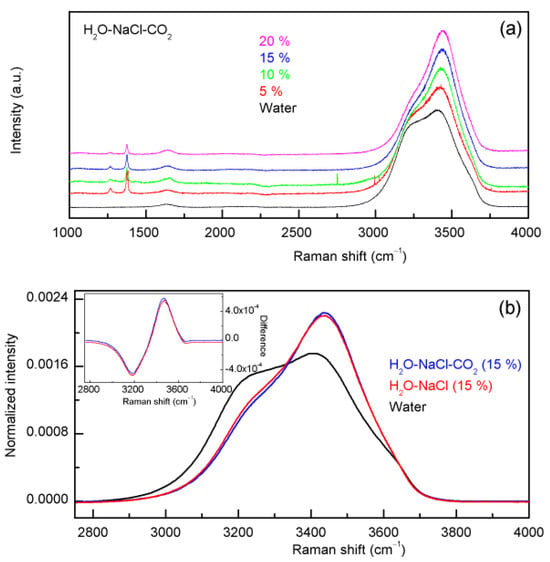
Figure 6.
(a) The Raman spectra of the H2O-NaCl-CO2 system. (b) Only slight changes are observed in the normalized intensity difference between H2O-NaCl-CO2 and H2O-NaCl systems, which are shown in the inlet.
It is found that the effects of dissolved CO2 on Raman OH vibrations are much weaker than those from dissolved NaCl salts (Figure 3).
Based on the Raman spectroscopic studies, in comparison with the effects of NaCl, it is reasonable to ignore the influence of dissolved CO2 on Raman OH stretching bands. Therefore, the intensity ratio, determined from the two fitted sub-bands of the H2O-NaCl system, may be reasonably used to determine the salinity of synthetic inclusions in the H2O-NaCl-CO2 system (Figure 7 and Table 2). It is found that the calculated Cl− molarity is close to the salinity of synthetic fluid inclusions.
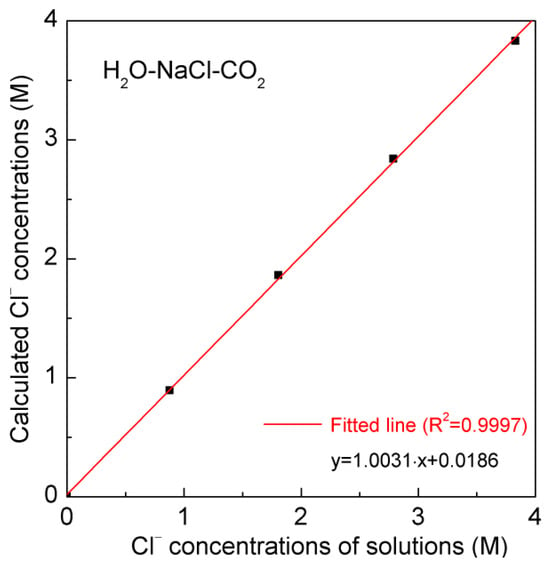
Figure 7.
The calculated Cl− concentrations of synthetic H2O-NaCl-CO2 system. The synthetic and calculated Cl− concentrations (molar concentrations) are, respectively, shown in the x- and y-axis.

Table 2.
Regarding H2O-NaCl-CO2 systems, the Raman spectral changes in Raman OH stretching bands are mainly due to the Cl− ions. Based on Equation (3), the Raman OH stretching bands may be deconvoluted into two sub-bands. The intensity ratio between them is utilized to determine the Cl− concentrations of H2O-NaCl-CO2 systems.
In the study, to demonstrate the reliability of the above equation, the salinity of large natural fluid inclusions (>5 μm) is also measured by Raman spectroscopy and microthermometry (Figure 8 and Table 3).
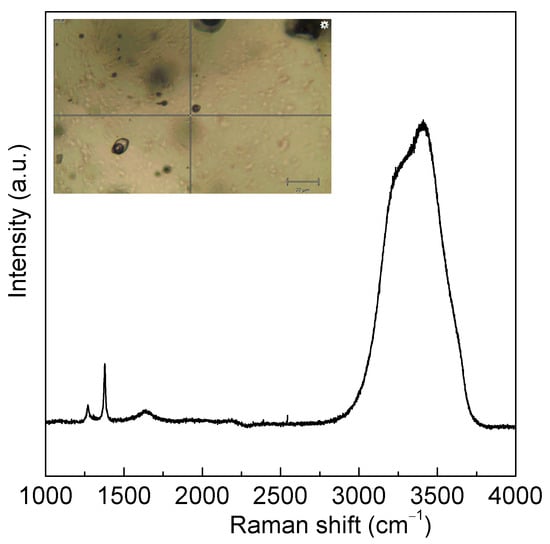
Figure 8.
Raman spectrum of natural fluid inclusion of H2O-NaCl-CO2 system from No. 3 pegmatite, Koktokay area, Xinjiang autonomous region, China. Based on the measured Raman spectra, only CO2 can be found in the bubble of fluid inclusion. Both gas and liquid CO2 phases are found in fluid inclusion. The salinity of large inclusions (>5 um) is measured by Raman spectroscopy and microthermometry.

Table 3.
The measured salinity of natural fluid inclusions of the H2O-NaCl-CO2 system. Both microthermometry and Raman spectroscopy are used to determine the salinity of large inclusions (diameter larger than 5 μm).
4. Discussion
To date, plenty of experimental measurements and theoretical works have been carried out to understand the structure of liquid water. Various structural models have been proposed, which are roughly partitioned into two categories: mixture and continuum models [29,30]. Mixture models posit the coexistence of two distinct structural types with a sharp boundary between them. In contrast, continuum models envision water as a continuous, three-dimensional network of hydrogen bonds with a wide range of O-H···O angles and distances. Unlike mixture models, these networks cannot be “broken” into separate molecular species.
The vibrational normal modes of a single H2O molecule consist of 2A1 (comprising the asymmetric stretching vibration ν1 at 3657.05 cm−1 and a bending vibration ν2 near 1595 cm−1) and B1 (the antisymmetric stretching vibration ν3 at 3755.97 cm−1) [31]. All these modes are Raman-active. Upon the formation of a hydrogen bond between two water molecules, electron redistribution occurs. The formation of hydrogen bonds increases the O-H bond lengths and reduces the H···O and O···O distances between water molecules [32], causing OH stretching vibrations to shift to lower wavenumbers.
In our Raman spectroscopic studies [33,34,35], as the three-dimensional hydrogen bonding occurs, OH vibrational frequencies may be related to the hydrogen-bonded networks in the first shell of a water molecule (local hydrogen bonding). Hydrogen bonding beyond this first shell has a negligible effect on OH vibrations. In ambient water, the Raman OH stretching band can be deconvoluted into five sub-bands (Figure 2), corresponding to OH vibrations involved in various local hydrogen-bonded networks: DDAA (double donor-double acceptor, tetrahedral hydrogen bonding), DDA (double donor-single acceptor), DAA (single donor-double acceptor), DA (single donor-single acceptor), and free OH vibrations, respectively [33,34,35].
According to Raman spectroscopic studies, a Local Statistical Model (LSM) is proposed, which posits that a water molecule interacts with the neighboring water molecules (within the first hydration shell) through different local hydrogen-bonded networks. At ambient conditions, various local hydrogen bondings may be found around a water molecule, contrasting with the mixture and continuum structural models of liquid water.
As NaCl salts are dissolved into water, they become the hydrated Na+ and Cl− ions. Molecular dynamics (MD) simulations indicate hydration numbers of 5.67 and 7.31 for Na+ and Cl−, respectively, at 0.5 M concentration. At 4 M concentration, these coordination numbers increase to 5.09 and 7.78, respectively [36]. The electrostatic interactions of Na+ and Cl− with the unoccupied orbitals of surrounding water molecules undoubtedly alter the water structure, as evident in the spectral changes observed in the Raman OH stretching bands of water (Figure 3).
Neutron scattering experiments and MD simulations consistently demonstrate that the influence of anions and cations on the surrounding water structure is primarily confined to the first hydration shell [37,38]. This finding aligns with other studies employing X-ray diffraction [39], X-ray absorption spectroscopy [40], femtosecond time-resolved infrared (fs-IR) vibrational spectroscopy [41,42], and optical Kerr-effect spectroscopy [43]. This may also be understood by the dependence of OH vibrations on the hydrogen bondings of water. As the three-dimensional hydrogen bondings appear, Raman OH vibrations are primarily influenced by the local hydrogen-bonded network of a water molecule [33,34,35], and dissolved solutes are anticipated to primarily affect the structure of the water layer directly adjacent to the solute (the first hydration shell).
The effects of Na+ and Cl− ions on water structure exhibit distinct characteristics. Ab initio MD simulations suggest that Na+ ions slightly disrupt the formation of donor hydrogen bonds [44]. This relatively weak electronic perturbation by Na+ ions is further supported by structural studies employing near-edge X-ray absorption spectroscopy at the Na edge [45] and Oxygen K-edge X-ray absorption spectra [40], as well as similar findings for other monovalent cations (Li+ and K+) [40]. In contrast, a significant intensity increase in the main edge region of the X-ray absorption spectrum is observed in the first solvation shell of Cl− [40]. These structural studies indicate that water-chloride interactions are primarily responsible for the observed spectral changes [40,46]. Consequently, the effects of NaCl on water structure are primarily governed by interactions between Cl− ions and water molecules.
While dissolved Na+ and Cl− ions are non-Raman-active, they influence water structure. In aqueous NaCl solutions, the spectral changes observed in Raman OH stretching bands are primarily attributed to the effects of Cl− ions on water structure. To analyze these spectral changes, the Raman OH stretching bands are fitted with two sub-bands, denoted as (HB)s and (HB)w, representing strong (around 3220 cm−1) and weak hydrogen-bonded (around 3450 cm−1) structural components, respectively (Figure 2). Although a larger number of fitted Gaussian sub-bands may provide better fits, they do not significantly improve the accuracy of calculated salinity. Furthermore, they may also increase uncertainty when Raman intensity is weak. Therefore, the intensity ratio between the two sub-bands is calculated and used to determine the Cl− molarity of aqueous NaCl solutions (Figure 4).
A linear CO2 molecule exhibits four primary vibrational modes. These modes are ν1 (∑g+), the symmetrical stretch at 1339.5 cm−1, ν2 (∑u), the doubly degenerate deformation mode at 667.3 cm−1, and ν3 (∑u+), the antisymmetric stretch at 2349.1 cm−1. While ν2 and ν3 are infrared active, ν1 is Raman-active. Fermi resonance between 2ν2 and ν1 results in a pair of bands with nearly equal intensity often denoted as ν− and ν+ (Figure 5). Under ambient conditions, the Fermi diad of 12CO2 is observed at 1282 cm−1 (ν−) and 1386 cm−1 (ν+). As pressure increases, both bands shift slightly towards lower wavenumbers [47].
Gas CO2 or two CO2 phases (gas phase and liquid phase) can be found in synthetic fluid inclusions. In comparison with pure CO2, the Fermi doublet of dissolved CO2 shifts to a lower wavenumber and becomes much broader (Figure 5). This is in accordance with other studies on dissolved CO2 in water [48,49]. In addition, the frequency shift of ν− of dissolved CO2 is more obvious than that of ν+, and the intensity ratio of ν+/ν− becomes larger than that in pure CO2.
Most of the dissolved CO2 molecules exist in the form of weakly hydrated CO2 molecules. In Leung et al. work [50], they studied the hydration structure of dissolved CO2 in liquid water using ab initio MD, which means that CO2 behaves like a hydrophobic species. Additionally, based on the spatial integration of OCO2-Hw g(r) to r = 2.5 Å, it yields 0.3 coordinating water molecules per oxygen [50]. This means that the interactions between dissolved CO2 and water molecules may be weak. Prasetyo and Hofer’s hybrid quantum mechanical/molecular mechanical (QM/MM) simulations indicate an average C-Owater distance of 3.9 Å. Coupled with the relatively low intensity of the first shell peak (1.5), this suggests that interactions between solvent molecules and CO2 molecules are weak [50,51,52].
CO2 is one of the most important compounds that exist in many geological environments [9,10,11,12]. The gas CO2 is soluble in water in which more than 99% exists as the dissolved gas. A small fraction of CO2 (about 1%) may be hydrolyzed to form carbonic acid (H2CO3) molecules, then break down into bicarbonate (HCO3−) and carbonate (CO32−) ions through proton transfer reactions [51,53]. In theory, the chemical equilibrium can be expressed as CO2 + H2O = H2CO3, H2CO3 = HCO3− + H+, HCO3− = CO32− + H+, and equilibrium constant at 298 K can be determined to be 1.70 × 10−3, 2.5 × 10−4, and 4.69 × 10−11, respectively. Due to the weak reaction between CO2 and water, the concentrations of HCO3− and CO32− ions are low. In this work, no HCO3− and CO32− ions can be found in the measured Raman spectra of H2O-CO2 or H2O-NaCl-CO2 systems. This is also in accordance with Davis and Oliver’s [54] study on the H2O-CO2 system. It is reasonable to ignore the effects of HCO3− and CO32− ions on Raman OH stretching bands.
The preceding discussion indicates that dissolved CO2 has a minimal impact on water structure. Compared to the effects of NaCl on Raman OH stretching bands, the effects of CO2 may be reasonably ignored. In other words, regarding the spectral changes in Raman OH stretching bands of the H2O-NaCl-CO2 system, it may be reasonably ascribed to the effects arising from dissolved Cl− ions. Therefore, Equation (3) may be reasonably extended to determine the Cl− concentrations in the H2O-CO2-NaCl system. After the Raman OH stretching bands are fitted into two sub-bands, the intensity ratio between them may be applied to calculate the Cl− molarity of the H2O-NaCl-CO2 system (Table 2). It is observed that the computed findings closely approximate the Cl− concentrations in the synthetic H2O-NaCl-CO2 system (Figure 7).
To demonstrate the reliability of the above method, it is also used to determine the salinity of natural fluid inclusion of the H2O-NaCl-CO2 system. Natural fluid inclusions are commonly preserved within host birefringent crystals, such as quartz. The Raman OH stretching bands may be influenced by the birefringence of host minerals [18,20]. This is also demonstrated by our previous work [21]. In combination with a previous study [21], it is suitable to choose the host quartz mineral to be perpendicular to the c-axis to determine the salinity of inclusion.
Based on Equation (3), Raman spectroscopy is applied to calculate the Cl− molarity of natural fluid inclusions, which is close to the measurement results by microthermometry (Table 3). From the study, Raman spectroscopy may reasonably be used to determine the salinity of inclusions, especially for small diameter (<5 μm) or CO2-rich fluid inclusions, which cannot be accurately measured by microthermometry.
In natural fluid inclusions, the most common anion is Cl− ion. Of course, other anions may also be expected in fluid inclusions, such as CO32−, SO42−, HCO3−, and NO3− [20]. During Raman OH stretching bands are used to determine the Cl− concentrations of fluid inclusions containing the above anions, it is necessary to correctly evaluate the effects of these anions on Raman OH stretching bands of water.
To understand the effects of Na2CO3 and Na2SO4 on water structure, Raman spectra of Na2CO3 and Na2SO4 solutions are also recorded (Figure 9). Based on the calculated normalized intensities, the obvious effects of Na2CO3 and Na2SO4 may be found on the Raman OH stretching bands (Figure 9). Therefore, before Raman OH stretching bands are used to determine the Cl− concentrations of solutions containing CO32− and SO42− ions, it is necessary to take into account the effects of CO32− and SO42− ions on the ratio of I(HB)w/I(HB)s.
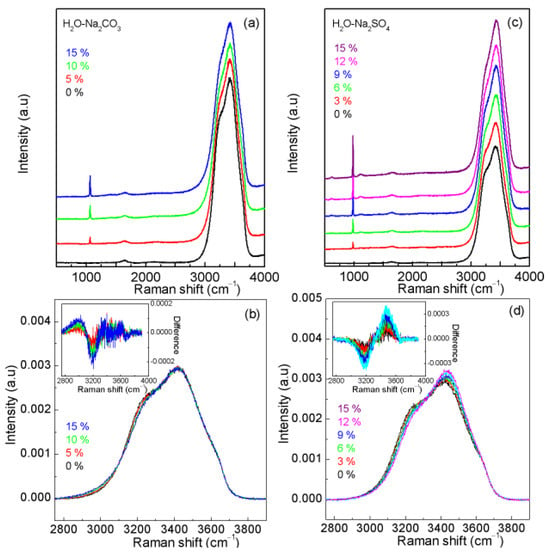
Figure 9.
The normalized intensities of H2O-Na2CO3 (a,b) and H2O-Na2SO4 (c,d) systems. In comparison with pure water, obvious spectral changes are found for Na2CO3 (a,b) and Na2SO4 (c,d). Solution spectral changes are mostly associated with the interactions between Cl− and water, as indicated by the structural analysis. The intensity ratio of the two fitted sub-bands can be utilized to determine the Cl− concentrations in NaCl solutions.
5. Conclusions
In this work, Raman spectroscopy is applied to determine the salinity of fluid inclusions in the H2O-NaCl-CO2 system. From the study, the following conclusions are derived,
- (1)
- For the H2O-NaCl system, the addition of NaCl salts decreases the intensity of the sub-band below 3330 cm−1 but increases the intensity of the sub-band above 3330 cm−1. The spectral changes are mostly associated with the interactions between Cl− and water, as indicated by the structural analysis. The intensity ratio of the two fitted sub-bands can be utilized to determine the Cl− concentrations in NaCl solutions.
- (2)
- According to the Raman spectroscopic study, the effects of CO2 on water structure may be weak. It is reasonable to ignore the effects of CO2 on Raman OH stretching bands during they are utilized to measure the salinity of the H2O-NaCl-CO2 system. Therefore, the above method is reasonably extended to quantitatively measure the salinity (Cl− molarity scale) of the H2O-NaCl-CO2 system.
Author Contributions
Writing—review and editing, X.H.; conceptualization, formal analysis, investigation, data curation, writing—original draft preparation, and writing—review and editing, W.-Q.W.; writing—review and editing, Y.-Z.L.; conceptualization, formal analysis, investigation, resources, data curation, writing—original draft preparation, writing—review and editing, and supervision, Q.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (grant number 8200908846).
Data Availability Statement
Data are contained within the article.
Acknowledgments
The editor and reviewers are greatly appreciated for providing good suggestions to revise the paper.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hezarkhani, A.; Willims-Jones, A.E. Controls of alteration and mineralization in the Sungun porphyry copper deposit, Iran: Evidence from fluid inclusions and stable isotopes. Econ. Geol. 1998, 93, 651–670. [Google Scholar] [CrossRef]
- Mollai, H.; Sharma, R.; Pe-Piper, G. Copper mineralization around the Ahar batholith, north of Ahar (NW Iran): Evidence for fluid evolution and the origin of the skarn ore deposit. Ore Geol. Rev. 2009, 35, 401–414. [Google Scholar] [CrossRef]
- Ping, H.W.; Chen, H.H.; Song, G.Q.; Liu, H.M. Oil cracking of deep petroleum in Minfeng Sag in North Dongying depression, Bohai Bay basin, China: Evidence from natural fluid inclusions. J. Earth Sci. 2010, 21, 455–470. [Google Scholar] [CrossRef]
- Tian, Z.; Zhang, X.; Liu, C.; Meng, Q.; Du, Z.; Yan, J. Characterization of the influence of hydrated ions on the oxygen-hydrogen stretching vibration of water by Raman spectroscopy. Anal. Lett. 2020, 53, 2034–2046. [Google Scholar] [CrossRef]
- Wang, K.; Chi, G.; Bethune, K.M.; Li, Z.; Blamey, N.; Card, C.; Potter, E.G.; Liu, Y. Fluid P-T-X characteristics and evidence for boiling in the formation of the Phoenix uranium deposit (Athabasca Basin, Canada): Implications for unconformity-related uranium mineralization mechanisms. Ore Geol. Rev. 2018, 101, 122–142. [Google Scholar] [CrossRef]
- Xie, X.; Fan, Z.; Liu, X.; Lu, Y. Geochemistry of formation water and its implication on overpressured fluid flow in the Dongying Depression of the Bohaiwan Basin, China. J. Geochem. Explor. 2006, 89, 432–435. [Google Scholar] [CrossRef]
- Yang, T.; Zhu, L.; Zhang, G.; Wang, F.; Lu, R.; Xia, J.; Zhang, Y. Geological and geochemical constraints on genesis of the Liziyuan gold-dominated polymetal deposit, Western Qinling orogen, central China. Int. Geol. Rev. 2012, 54, 1944–1966. [Google Scholar] [CrossRef]
- Bodnar, R.J. Revised equation and table for determining the freezing point depression of H2O-NaCl solutions. Geochim. Cosmochim. Acta 1993, 57, 683–684. [Google Scholar] [CrossRef]
- Roedder, E. Fluid inclusions. In Reviews in Mineralogy; Ribbe, H.P., Ed.; Mineralogical Society of America: Washington, DC, USA, 1984; Volume 12, pp. 1–644. [Google Scholar]
- Bakker, R.J.; Diamond, L.W. Determination of the composition and molar volume of H2O-CO2 fluid inclusions by microthermometry. Geochim. Cosmochim. Acta 2000, 64, 1753–1764. [Google Scholar] [CrossRef]
- Diamond, L.W. Review of the systematics of CO2-H2O fluid inclusions. Lithos 2001, 55, 69–99. [Google Scholar] [CrossRef]
- Tsunogae, T.; Santosh, M.; Dubessy, J. Fluid characteristics of high to ultrahigh-temperature metamorphism in Southern India: A quantitative Raman spectroscopic study. Precambrian Res. 2008, 162, 198–211. [Google Scholar] [CrossRef]
- Fall, A.; Tattitch, B.; Bodnar, R.J. Combined microthermometric and Raman spectroscopic technique to determine the salinity of H2O-CO2-NaCl fluid inclusions based on clathrate melting. Geochim. Cosmochim. Acta 2011, 75, 951–964. [Google Scholar] [CrossRef]
- Bakker, R.J. Raman spectra of fluid and crystal mixtures in the systems H2O, H2O-NaCl and H2O-MgCl2 at low temperatures: Applications to fluid-inclusion research. Can. Mineral. 2004, 42, 1283–1314. [Google Scholar] [CrossRef]
- Bakker, R.J.; Baumgartner, M. Unexpected phase assemblages in inclusions with ternary H2O-salt fluids at low temperatures. Cent. Eur. J. Geosci. 2012, 4, 225–237. [Google Scholar] [CrossRef]
- Davis, D.W.; Lowenstein, T.K.; Spencer, R.J. Melting behavior of fluid inclusions in laboratory-grown halite crystals in the systems NaCl-H2O, NaCl-KCl-H2O, NaCl-MgCl2-H2O, and NaCl-CaCl2-H2O. Geochim. Cosmochim. Acta 1990, 54, 591–601. [Google Scholar] [CrossRef]
- Mernagh, T.P.; Wilde, A.R. The use of the laser Raman microprobe for the determination of salinity in fluid inclusions. Geochim. Cosmochim. Acta 1989, 53, 765–771. [Google Scholar] [CrossRef]
- Baumgartner, M.; Bakker, R.J. Raman spectroscopy of pure H2O and NaCl-H2O containing synthetic fluid inclusions in quartz-A study of polarization effects. Miner. Petrol. 2009, 95, 1–15. [Google Scholar] [CrossRef]
- Georgiev, G.; Kalkanjiev, T.; Petrov, V.; Nickolov, Z. Determination of salts in water solutions by a skewing parameter of the water Raman band. Appl. Spectrosc. 1984, 38, 593–595. [Google Scholar] [CrossRef]
- Dubessy, J.; Lhomme, T.; Boiron, M.C.; Rull, F. Determination of chlorinity in aqueous fluids using Raman spectroscopy of the stretching band of water at room temperature: Application to fluid inclusions. Appl. Spectrosc. 2002, 56, 99–106. [Google Scholar] [CrossRef]
- Sun, Q.; Zhao, L.; Li, N.; Liu, J. Raman spectroscopic study for the determination of Cl− concentration (molarity scale) in aqueous solutions: Application to fluid inclusions. Chem. Geol. 2010, 272, 55–61. [Google Scholar] [CrossRef]
- Wang, X.; Hu, W.; Chou, I.-M. Raman spectroscopic characterization on the OH stretching bands in NaCl-Na2CO3-Na2SO4-CO2-H2O systems: Implications for the measurement of chloride concentrations in fluid inclusions. J. Geochem. Explor. 2013, 132, 111–119. [Google Scholar] [CrossRef]
- Chou, I.-M.; Song, Y.; Burruss, R.C. A new method for synthesizing fluid inclusions in fused silica capillaries containing organic and inorganic material. Geochim. Cosmochim. Acta 2008, 72, 5217–5231. [Google Scholar] [CrossRef]
- Lu, W.; Chou, I.; Burruss, R.C.; Song, Y. Unified equation for calculating methane vapor pressures in the CH4-H2O system with measured Raman shifts. Geochem. Cosmochim. Acta 2007, 71, 3969–3978. [Google Scholar] [CrossRef]
- Aarnoutse, P.; Westerhuis, J. Quantitative Raman reaction monitoring using the solvent as internal standard. Anal. Chem. 2005, 77, 1228–1236. [Google Scholar] [CrossRef] [PubMed]
- Caillet, A.; Puel, F.; Fevotte, G. In-line monitoring of partial and overall solid concentration during solvent-mediated phase transition using Raman spectroscopy. Int. J. Pharm. 2006, 307, 201–208. [Google Scholar] [CrossRef]
- Sun, Q.; Qin, C. Raman OH stretching band of water as an internal standard to determine carbonate concentrations. Chem. Geol. 2011, 283, 274–278. [Google Scholar] [CrossRef]
- Collins, P.L.F. Gas hydrates in CO2-bearing fluid inclusions and the use of freezing data for estimation of salinity. Econ. Geol. 1979, 74, 1435–1444. [Google Scholar] [CrossRef]
- Nilsson, A.; Pettersson, L.G.M. Perspective on the structure of liquid water. Chem. Phys. 2011, 389, 1–34. [Google Scholar] [CrossRef]
- Stanley, H.E.; Teixeira, J. Interpretation of the unusual behavior of H2O and D2O at low temperatures. Test of a percolation model. J. Chem. Phys. 1980, 73, 3404–3422. [Google Scholar] [CrossRef]
- Fraley, P.E.; Rao, K.N. High resolution infrared spectra of water vapor, ν1 and ν3 band of H2O. J. Mol. Spectrosc. 1969, 29, 348–364. [Google Scholar] [CrossRef]
- Ludwig, R. The effect of hydrogen bonding on the thermodynamic and spectroscopic properties of molecular clusters and liquids. Phys. Chem. Chem. Phys. 2002, 4, 5481–5487. [Google Scholar] [CrossRef]
- Sun, Q. The Raman OH stretching bands of liquid water. Vib. Spectrosc. 2009, 51, 213–217. [Google Scholar] [CrossRef]
- Sun, Q. Raman spectroscopic study of the effects of dissolved NaCl on water structure. Vib. Spectrosc. 2012, 62, 110–114. [Google Scholar] [CrossRef]
- Sun, Q. Local statistical interpretation for water structure. Chem. Phys. Lett. 2013, 568, 90–94. [Google Scholar] [CrossRef]
- Bouazizi, S.; Nasr, S.; Jaîdane, N.; Bellissent-Funel, M.C. Local order in aqueous NaCl solutions and pure water: X-ray scattering and molecular dynamics simulations study. J. Phys. Chem. B 2006, 110, 23515–23523. [Google Scholar] [CrossRef]
- Nag; Chakraborty, D.; Chandra, A. Effects of ion concentration on the hydrogen bonded structure of water in the vicinity of ions in aqueous NaCl solutions. J. Chem. Sci. 2008, 120, 71–77. [Google Scholar] [CrossRef]
- Soper, A.K.; Weckström, K. Ion solvation and water structure in potassium halide aqueous solutions. Biophys. Chem. 2006, 124, 180–191. [Google Scholar] [CrossRef]
- Collins, K.D.; Neilson, G.W.; Enderby, J.E. Ions in water: Characterizing the forces that control chemical processes and biological structure. Biophys. Chem. 2007, 128, 95–104. [Google Scholar] [CrossRef]
- Cappa, C.D.; Smith, J.D.; Messer, B.M.; Cohen, R.C.; Saykally, R.J. Effects of cations on the hydrogen bond network of liquid water: New results from X-ray absorption spectroscopy of liquid microjets. J. Phys. Chem. B 2006, 110, 5301–5309. [Google Scholar] [CrossRef]
- Moilanen, D.E.; Fenn, E.E.; Wong, D.; Fayer, M.D. Water dynamics in large and small reverse micelles: From two ensembles to collective behavior. J. Chem. Phys. 2009, 131, 014704. [Google Scholar] [CrossRef]
- Omta, A.W.; Kropman, M.F.; Woutersen, S.; Bakker, H.J. Influence of ions on the hydrogen-bond structure in liquid water. J. Chem. Phys. 2003, 119, 12457–12461. [Google Scholar] [CrossRef]
- Turton, D.A.; Hunger, J.; Hefter, G.; Buchner, R.; Wynne, K. Glasslike behavior in aqueous electrolyte solutions. J. Chem. Phys. 2008, 128, 161102. [Google Scholar] [CrossRef] [PubMed]
- White, J.A.; Schwegler, E.; Galli, G.; Gygi, F. The solvation of Na+ in water: First-principles simulations. J. Chem. Phys. 2000, 113, 4668–4673. [Google Scholar] [CrossRef]
- Aziz, E.F.; Zimina, A.; Freiwald, M.; Eisebitt, S.; Eberhardt, W. Molecular and electronic structure in NaCl electrolytes of varying concentration: Identification of spectral fingerprints. J. Chem. Phys. 2006, 124, 114502. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, Z.; Sun, X.; Zhang, L.; Xu, M.; Xu, X. Insight into the liquid structure of water and sodium chloride solutions using stimulated Raman scattering. Phys. Rev. Appl. 2020, 13, 024030. [Google Scholar] [CrossRef]
- Wang, X.; Chou, I.-M.; Hu, W.; Burruss, R.C.; Sun, Q.; Song, Y. Raman spectroscopic measurements of CO2 density: Experimental calibration with high-pressure optical cell (HPOC) and fused silica capillary capsule (FSCC) with application to fluid inclusion observations. Geochim. Cosmochim. Acta 2011, 75, 4080–4093. [Google Scholar] [CrossRef]
- Anderson, G.R. The Raman-spectra of carbon dioxide in liquid H2O and D2O. J. Phys. Chem. 1977, 81, 273–276. [Google Scholar] [CrossRef]
- Rudolph, W.W.; Fische, D.; Irmer, G. Vibrational spectroscopic studies and density functional theory calculations of speciation in the CO2-water system. Appl. Spectrosc. 2006, 60, 130–144. [Google Scholar] [CrossRef]
- Leung, K.; Nielsen, I.M.; Kurtz, I. Ab initio molecular dynamics study of carbon dioxide and bicarbonate hydration and the nucleophilic attack of hydroxide on CO2. J. Phys. Chem. B 2007, 111, 4453–4459. [Google Scholar] [CrossRef]
- Lam, R.K.; England, A.H.; Smith, J.W.; Rizzuto, A.M.; Shih, O.; Prendergast, D.; Saykally, R.J. The hydration structure of dissolved carbon dioxide from X-ray absorption spectroscopy. Chem. Phys. Lett. 2015, 633, 214–217. [Google Scholar] [CrossRef]
- Prasetyo, N.; Hofer, T.S. Structure, dynamics, and hydration free energy of carbon dioxide in aqueous solution: A quantum mechanical/molecular mechanics molecular dynamics thermodynamic integration (QM/MM MD TI) simulation study. J. Chem. Theory Comput. 2018, 14, 6472–6483. [Google Scholar] [CrossRef] [PubMed]
- Soli, A.L.; Byrne, R.H. CO2 system hydration and dehydration kinetics and the equilibrium CO2/H2CO3 ratio in aqueous NaCl solution. Mar. Chem. 2002, 78, 65–73. [Google Scholar] [CrossRef]
- Davis, A.R.; Oliver, B.G. A vibrational-spectroscopic study of the species present in the CO2-H2O system. J. Solut. Chem. 1972, 1, 329–339. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).