Laser-Induced Breakdown Spectroscopy Applied to the Quantification of K, Ca, Mg and Mn Nutrients in Organo-Mineral, Mineral P Fertilizers and Rock Fertilizers
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. LIBS Instrument Set-Up and FAAS Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Population Projected to Reach 9.8 Billion in 2050, and 11.2 Billion in 2100. Available online: https://www.un.org/en/desa/world-population-projected-reach-98-billion-2050-and-112-billion-2100 (accessed on 25 July 2024).
- Lu, C.; Tian, H. Global nitrogen and phosphorus fertilizer use for agriculture production in the past half century: Shifted hot spots and nutrient imbalance. Earth Syst. Sci. Data 2017, 9, 181–192. [Google Scholar] [CrossRef]
- Pavinato, P.S.; Cherubin, M.R.; Soltangheisi, A.; Rocha, G.C.; Chadwick, D.R.; Jones, D.L. Revealing soil legacy phosphorus to promote sustainable agriculture in Brazil. Sci. Rep. 2020, 10, 15615. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Yan, G.; Hu, J.; Liu, X.; Zou, X.; Tie, L.; Yuan, R.; Yang, Y.; Xiao, L.; Cui, X.; et al. The Responses of Leaf Litter Calcium, Magnesium, and Manganese Dynamics to Simulated Nitrogen Deposition and Reduced Precipitation Vary with Different Decomposition Stages. Forests 2021, 12, 1473. [Google Scholar] [CrossRef]
- Capră, L.; Stoica, R.; Ivan, G.-R.; Șuică-Bunghez, I.-R.; Oancea, F. The Optimization and Validation of the Method for the Determination of Micronutrients in Organic Fertilizers by Inductively Coupled Plasma Optical Emission Spectrometry. Chem. Proc. 2023, 13, 10. [Google Scholar] [CrossRef]
- Ullah, R.; Abbas, Z.; Bilal, M.; Habib, F.; Iqbal, J.; Bashir, F.; Noor, S.; Qazi, M.A.; Niaz, A.; Baig, K.S.; et al. Method development and validation for the determination of potassium (K2O) in fertilizer samples by flame photometry technique. J. King Saud Univ. Sci. 2022, 34, 102070. [Google Scholar] [CrossRef]
- Farooq, W.A.; Al-Mutairi, F.N.; Khater, A.E.M.; Al-Dwayyan, A.S.; AlSalhi, M.S.; Atif, M. Elemental analysis of fertilizer using laser induced breakdown spectroscopy. Opt. Spectrosc. 2012, 112, 874–880. [Google Scholar] [CrossRef]
- Marangoni, B.S.; Silva, K.S.; Nicolodelli, G.; Senesi, G.S.; Cabral, J.S.; Villas-Boas, P.R.; Silva, C.S.; Teixeira, P.C.; Nogueira, A.R.A.; Benites, V.M.; et al. Phosphorus quantification in fertilizers using laser induced breakdown spectroscopy (LIBS): A methodology of analysis to correct physical matrix effects. Anal. Methods 2016, 8, 78–82. [Google Scholar] [CrossRef]
- Yao, S.; Lu, J.; Li, J.; Chen, K.; Li, J.; Dong, M. Multi-elemental analysis of fertilizer using laser-induced breakdown spectroscopy coupled with partial least squares regression. J. Anal. At Spectrom. 2010, 25, 1733. [Google Scholar] [CrossRef]
- Groisman, Y.; Gaft, M. Online analysis of potassium fertilizers by Laser-Induced Breakdown Spectroscopy. Spectrochim. Acta Part B At. Spectrosc. 2010, 65, 744–749. [Google Scholar] [CrossRef]
- Senesi, G.S.; Romano, R.A.; Marangoni, B.S.; Nicolodelli, G.; Villas-Boas, P.R.; Benites, V.M.; Milori, D.M.B.P. Laser-Induced Breakdown Spectroscopy Associated with Multivariate Analysis Applied to Discriminate Fertilizers of Different Nature. J. Appl. Spectrosc. 2017, 84, 923–928. [Google Scholar] [CrossRef]
- Nicolodelli, G.; Cabral, J.; Menegatti, C.R.; Marangoni, B.; Senesi, G.S. Recent advances and future trends in LIBS applications to agricultural materials and their food derivatives: An overview of developments in the last decade (2010–2019). Part I. Soils and fertilizers. TrAC Trends Anal. Chem. 2019, 115, 70–82. [Google Scholar] [CrossRef]
- Wei, L.; Ding, Y.; Chen, J.; Yang, L.; Wei, J.; Shi, Y.; Ma, Z.; Wang, Z.; Chen, W.; Zhao, X. Quantitative analysis of fertilizer using laser-induced breakdown spectroscopy combined with random forest algorithm. Front. Chem. 2023, 11, 1123003. [Google Scholar] [CrossRef] [PubMed]
- Babos, D.V.; Ramos, J.F.K.; Francisco, G.C.; de Melo Benites, V.; Milori, D.M.B.P. Laser-induced breakdown spectroscopy and digital image data fusion for determination of the Al, Ca, Fe, Mg, and P in mineral fertilizer: Overcome matrix effects in solid direct analysis. J. Opt. Soc. Am. B 2023, 40, 654. [Google Scholar] [CrossRef]
- Zhang, B.; Sha, W.; Jiang, Y.; Cui, Z. Quantitative analysis of nitrogen in ammonium phosphate fertilizers using laser-induced breakdown spectroscopy. Appl. Opt. 2019, 58, 3277. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Zhao, Y.; Yu, K. LIBS in agriculture: A review focusing on revealing nutritional and toxic elements in soil, water, and crops. Comput. Electron. Agric. 2022, 197, 106986. [Google Scholar] [CrossRef]
- Cervantes, C. Laser Induced Breakdown Spectroscopy (LIBS) Applied to the Quantification of Elements in Fertilizers [Internet]. 2017. Available online: http://www.teses.usp.br/teses/disponiveis/75/75135/tde-20042017-101150/ (accessed on 2 October 2024).
- Aguilera, J.A.; Aragón, C.; Madurga, V.; Manrique, J. Study of matrix effects in laser induced breakdown spectroscopy on metallic samples using plasma characterization by emission spectroscopy. Spectrochim. Acta Part B At. Spectrosc. 2009, 64, 993–998. [Google Scholar] [CrossRef]
- Singh, V.K.; Tripathi, D.K.; Deguchi, Y.; Wang, Z. (Eds.) Laser Induced Breakdown Spectroscopy (LIBS); Wiley: Hoboken, NJ, USA, 2023. [Google Scholar] [CrossRef]
- NIST Database. Available online: http://physics.nist.gov/PhysRefData/ASD/lines_form.html (accessed on 23 May 2023).
- Yang, L.; Zhang, Y.; Li, Y.; Qin, L.; Dong, J.; Ji, F.; Wei, Y.; Chang, S.; Lu, R. Enhancement of LIBS plasma and efficient collection of emitted light by open smooth triangular cavities. J. Anal. At. Spectrom. 2023, 38, 1182–1191. [Google Scholar] [CrossRef]
- Lin, J.; Yang, J.; Huang, Y.; Lin, X. A study of the temperature variation effect in a steel sample for rapid analysis using LIBS. Opt. Laser Technol. 2022, 147, 107707. [Google Scholar] [CrossRef]
- Hooker, A.; Greene, C.H.; Clark, W. Classical examination of the Stark effect in hydrogen. Phys. Rev. A 1997, 55, 4609–4612. [Google Scholar] [CrossRef]
- Currie, L.A. Limits for qualitative detection and quantitative determination. Application to radiochemistry. Anal. Chem. 1968, 40, 586–593. [Google Scholar] [CrossRef]

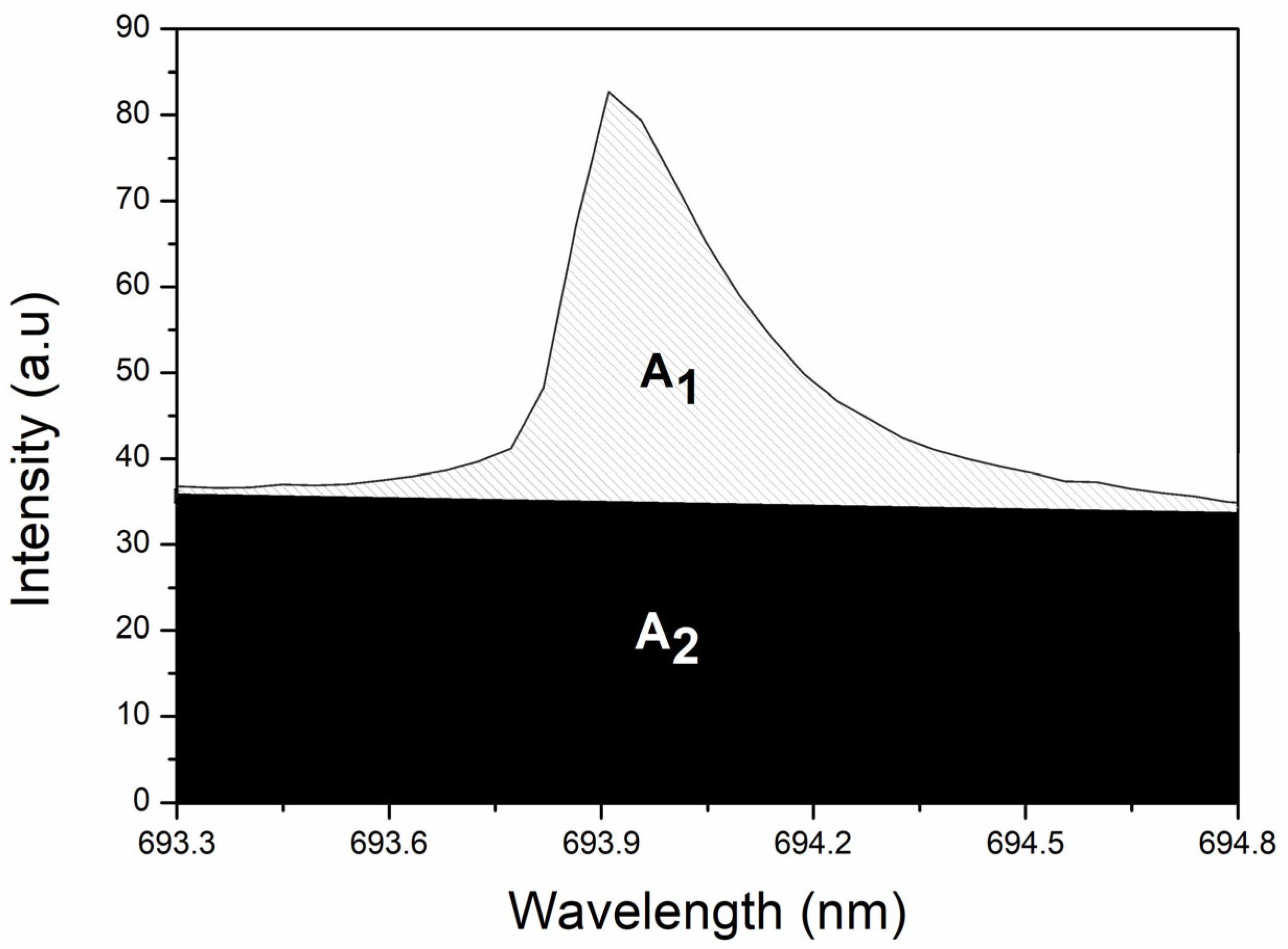
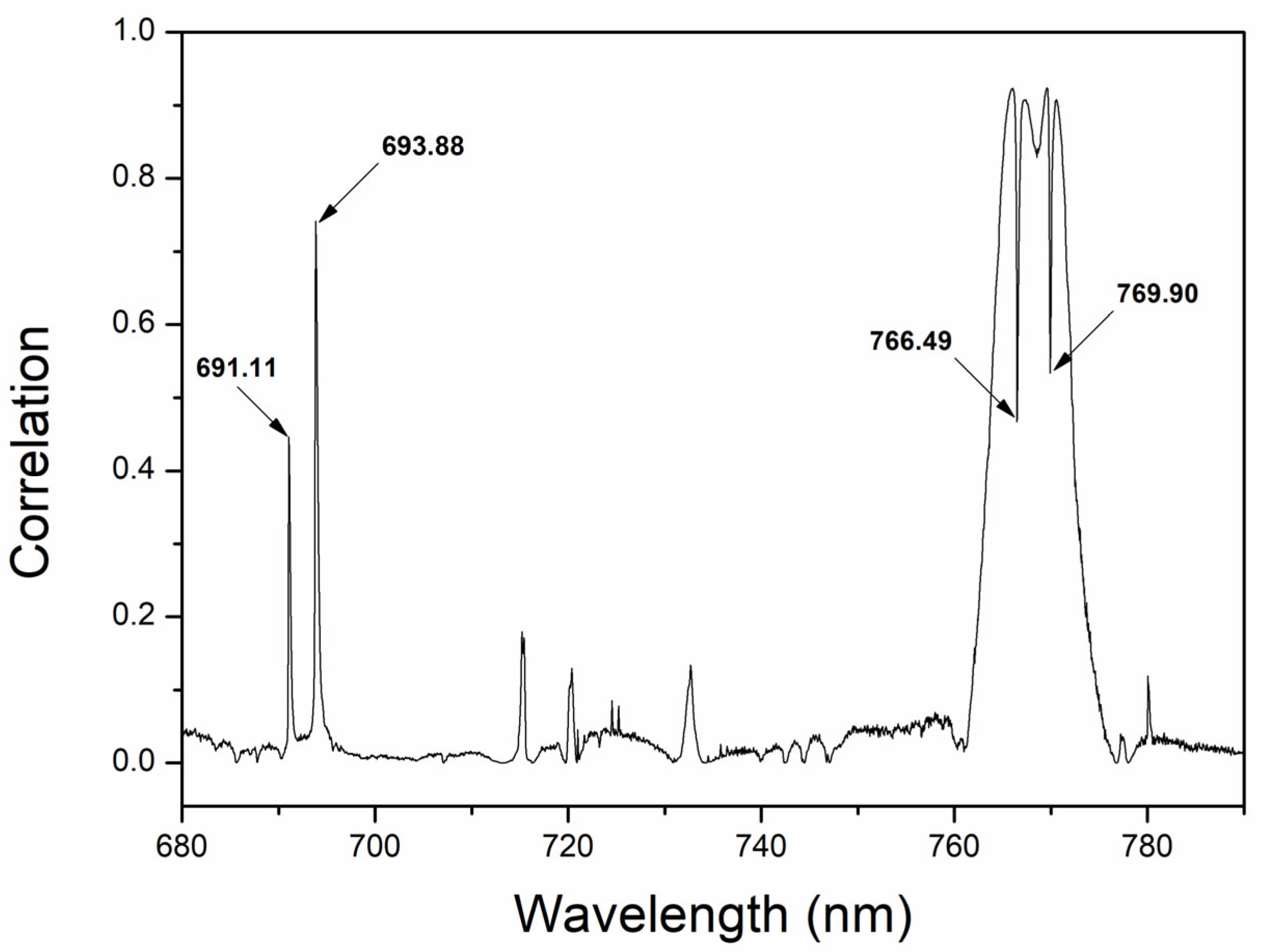



| Elements/Ion | Emission Line (nm) | Atomic Transition Probabilities (s−1) | Upper Level Energy (eV) |
|---|---|---|---|
| K I | 693.88 | 4.956 × 106 | 3.403 |
| Ca I | 610.27 | 9.600 × 106 | 3.910 |
| Mn II | 256.30 | 2.170 × 108 | 8.250 |
| Mg I | 285.20 | 4.910 × 108 | 4.346 |
| Elements | LIBS-FAAS LOD (mg/kg) |
|---|---|
| K | 66 |
| Ca | 35 |
| Mg | 5.4 |
| Mn | 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cervantes, C.; Marangoni, B.S.; Nicolodelli, G.; Senesi, G.S.; Villas-Boas, P.R.; Silva, C.S.; Nogueira, A.R.A.; Benites, V.M.; Milori, D.M.B.P. Laser-Induced Breakdown Spectroscopy Applied to the Quantification of K, Ca, Mg and Mn Nutrients in Organo-Mineral, Mineral P Fertilizers and Rock Fertilizers. Minerals 2024, 14, 1109. https://doi.org/10.3390/min14111109
Cervantes C, Marangoni BS, Nicolodelli G, Senesi GS, Villas-Boas PR, Silva CS, Nogueira ARA, Benites VM, Milori DMBP. Laser-Induced Breakdown Spectroscopy Applied to the Quantification of K, Ca, Mg and Mn Nutrients in Organo-Mineral, Mineral P Fertilizers and Rock Fertilizers. Minerals. 2024; 14(11):1109. https://doi.org/10.3390/min14111109
Chicago/Turabian StyleCervantes, Cesar, Bruno S. Marangoni, Gustavo Nicolodelli, Giorgio S. Senesi, Paulino R. Villas-Boas, Caroline S. Silva, Ana Rita A. Nogueira, Vinicius M. Benites, and Débora M. B. P. Milori. 2024. "Laser-Induced Breakdown Spectroscopy Applied to the Quantification of K, Ca, Mg and Mn Nutrients in Organo-Mineral, Mineral P Fertilizers and Rock Fertilizers" Minerals 14, no. 11: 1109. https://doi.org/10.3390/min14111109
APA StyleCervantes, C., Marangoni, B. S., Nicolodelli, G., Senesi, G. S., Villas-Boas, P. R., Silva, C. S., Nogueira, A. R. A., Benites, V. M., & Milori, D. M. B. P. (2024). Laser-Induced Breakdown Spectroscopy Applied to the Quantification of K, Ca, Mg and Mn Nutrients in Organo-Mineral, Mineral P Fertilizers and Rock Fertilizers. Minerals, 14(11), 1109. https://doi.org/10.3390/min14111109









