3.1. LIBS Spectrum and Line Assignment
Figure 3a and
Figure 3b show representative LIBS spectra of the spodumene sample without any added analyte elements, covering the wavelength regions of 302–407 nm and 755–830 nm, respectively. The spectral intensity values shown in
Figure 3a,b are available as raw data in the
Supplementary Materials. The spectrum in the shorter wavelength region in
Figure 3a was recorded by the high-resolution spectrometer. It reveals emission lines of the matrix elements Li (323.27 nm), Al (308.22, 309.27, 394.40, and 396.15 nm), and Si (390.55 nm) of the spodumene sample. Along the emission lines of the matrix elements, those of the impurity elements, Fe, Be, Ca, Ti, Na, Mg, and Mn, could be assigned in this wavelength region.
Figure 3b shows the LIBS spectrum of the spodumene sample in the longer-wavelength region. It is a part of the spectrum recorded by the low-resolution spectrometer. The wavelength region between 750 and 840 nm is of particular interest in this work because it includes the emission lines of K I (766.49 and 769.90 nm) and Na I (818.33 and 819.48 nm). As well as the emission lines of K I and Na I, those of O I, Rb I, and Li I could be assigned.
Among the thirteen elements of which emission lines were observed in the LIBS spectra, Be and Na were chosen as the analytes to demonstrate the feasibility of LIBS for analyzing light elements that are inaccessible via XRF. In the high-resolution spectrum, two discernible Be emissions at 313.06 and 332.12 nm and the Na I emission at 330.25 nm were identified and marked with an asterisk (*) (
Figure 3a). In the low-resolution spectrum, the Na I emission at 819.40 nm was identified and marked with an asterisk (*) (
Figure 3b). All of these four emission peaks are composed of two or more closely spaced lines. Their corresponding species and spectroscopic parameters are listed in
Table 1. The spectroscopic parameters
λ,
Aki,
Ei,
Ek, and
gk are the wavelength, the spontaneous emission coefficient, the lower-level energy, the upper-level energy, and the upper-level statistical weight, respectively.
Other than the light elements, K was also chosen as one of the analytes in this work. In typical LIBS analysis, K I shows two strong emission lines at 766.49 and 769.90 nm, as observed in the low-resolution spectrum (
Figure 3b), and two weak emission lines at 404.41 and 404.72 nm. The weak emission lines around 404 nm were actually observed in the high-resolution spectrum as shown in
Figure 3c. Spectroscopic parameters of the four K I emission lines are also listed in
Table 1. However, the Fe I emission line at 404.58 nm overlaps both of them (see the assignments in
Figure 3c). Due to this interference, the weak emission lines, which are better in obtaining reliable sensitivity critical to accuracy in the standard addition method, could not be utilized. Thus, there was no choice other than the stronger K I emission lines as the analyte signal for determining the concentration of K. The K I emission line at 766.49 nm marked with an asterisk (*) in
Figure 3b was chosen for the analysis of K. In order to obtain an accurate analysis result of the K concentration, the feasibility of weak intensities at the edge of the strong peak profile was investigated in the following. All of the assignments presented herein were based on the NIST Atomic Spectra Database [
28].
Figure 4 shows the variations in the selected emission line intensities for impurity elements (Be, Na, and K) with relative concentrations. The spectra presented are the averages of the corresponding nine measurements, with each measurement being the accumulation of 100 laser shots. This averaging was intended to compensate for fluctuations in laser energy and the inhomogeneity of the prepared samples. The signal intensities of the emission lines increase as the concentrations of Be, Na, and K rise. The samples corresponding to the spectra shown in
Figure 4a were prepared by adding different amounts of BeO to the pristine spodumene sample. The Be concentration was increased from 0 to 0.8 wt.% as noted in
Figure 4a. It should be mentioned that the five LIBS spectra show relatively small variation in the Na I emission line at 330.24 nm as the Be concentration increases (
Figure 4a). The five corresponding spodumene samples were prepared adding the varied amount of Be, not Na. Thus, this observation indicates the homogeneity of the prepared samples. The spectra shown in
Figure 4b were recorded with the samples prepared by adding Na
2O to the pristine spodumene sample. Consistently, the spectra show the clear intensity correlation of the Na I emission peaks at 819.40 nm with rather constant emission intensities of K I lines at 766.49 and 769.90 nm. Considering that the five spodumene samples were prepared by varying the added amounts of Na, not K, this also demonstrates that the sample preparation process in this work provided enough homogeneity. The relative standard deviation (RSD) of the emission intensities of Na I at 330.24 nm (
Figure 4a) and those of K I emission intensities at 766.49 and 769.90 nm (
Figure 4b) were estimated to be 8.44%, 5.06%, and 5.55%, respectively. In comparison with the RSD values of the K I emission line intensities, the larger RSD of the Na I peak intensity can be attributed to the weaker intensity of the Na I emission than those of the K I emission intensities. Although any spectral intensity normalization process was not applied in this analysis, those RSDs of 5%–8% are at the acceptable level of typical LIBS measurements with homogeneous samples.
Figure 4c and
Figure 4d show the expanded spectra around 330 and 768 nm where the Na I and K I emission peaks were observed, respectively. The corresponding samples were prepared by adding Na
2O and KO
2, respectively. Thus, the observed peaks revealed significant intensity variations correlated to the relative concentrations of Na and K. Overall, the results are consistent and can be used to generate calibration curves for Be, Na, and K, enabling the determination of their concentrations in the pristine spodumene sample.
3.2. Quantification of Be
To determine the concentration of Be in the pristine spodumene sample, the two Be II and Be I emission intensities at 313.06 and 332.12 nm, respectively, were employed for the analyte signals. The Be II and Be I emission intensities were plotted with respect to the relative concentrations of Be and are shown in
Figure 5a and
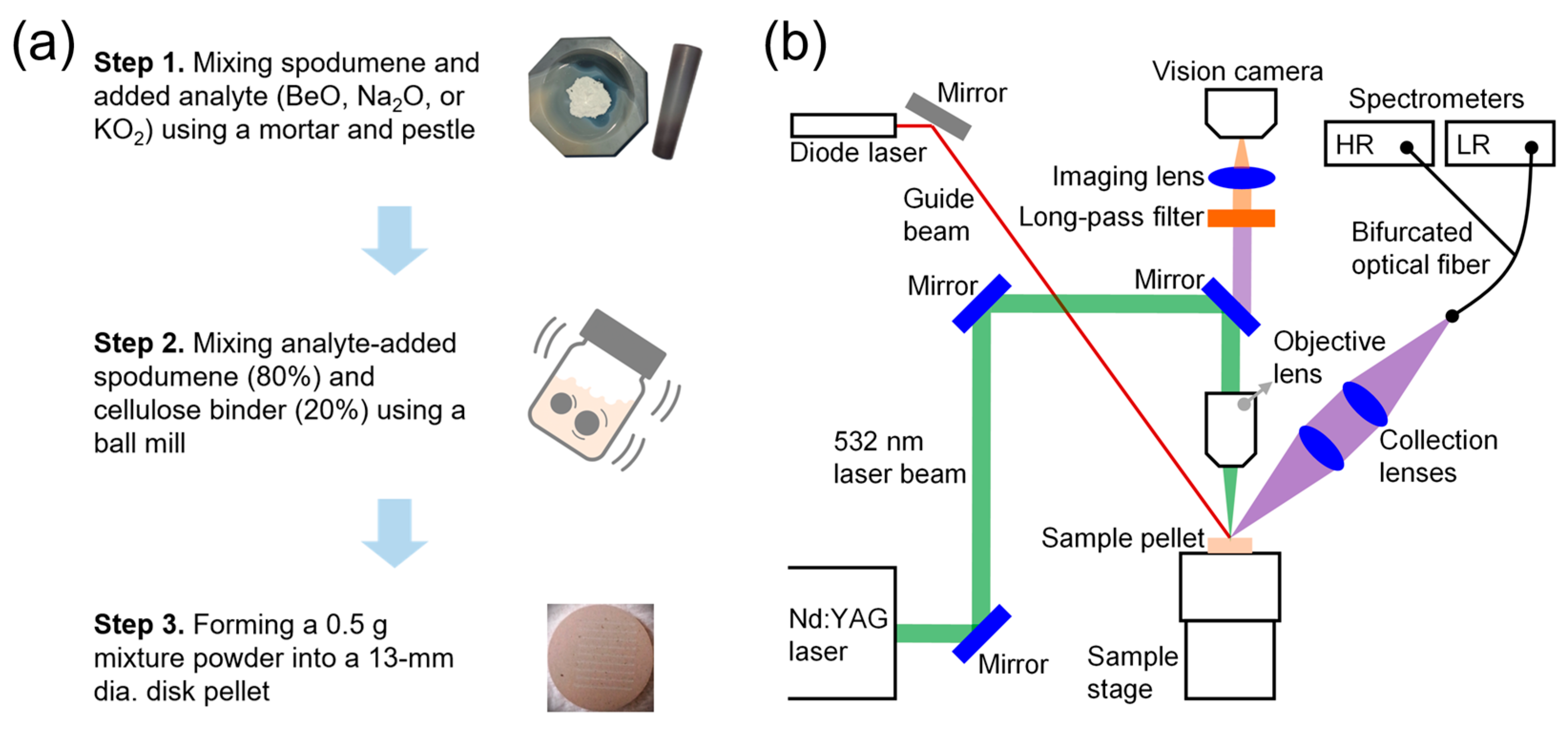
Figure 5b, respectively. The relative concentration of 0 wt.% corresponds to the pristine spodumene sample. It should be noted that the dominant factor affecting sample uniformity lies in how well the spodumene and the added analyte compounds were mixed, which manifests as compositional inhomogeneity at the millimeter scale on the sample pellet surface. This effect is clearly illustrated in the calibration curves based on the Be II (313.06 nm) and Be I (332.12) nm emission peak intensities. Notably, the standard deviation of emission intensities in the unspiked spodumene sample (0 wt.% Be) is significantly smaller than those in the spiked samples. This observation indicates that the initial mixing step (Step 1) of spodumene with analyte compounds in the LIBS sample preparation process (
Figure 1b) plays the most critical role in determining sample uniformity. In our LIBS analysis, nine line-scans were performed over a 7 mm × 9 mm area on the surface of a 13 mm-diameter pellet, and the averaged data were used for quantification. This averaging process helps mitigate the effect of millimeter-scale inhomogeneity caused by incomplete mixing, allowing us to obtain representative bulk composition values, although the incomplete mixing definitely decreased the precision performance. The measured intensities were fitted well to a linear function,
y =
a +
bx, where
y and
x represent the emission intensity and the relative concentration, respectively. The coefficients of determination,
R2, were 0.990 and 0.989 for the linear fits of the Be II and Be I emission intensities, respectively. Following the principle of the standard addition method, the concentration of Be in the pristine spodumene samples was determined by calculating the concentration value corresponding to the zero intensity,
y = 0 [
27]. Thus, the concentration of Be in the pristine sample was calculated using the following equation.
In the above equation,
a and
b are the intercept and the slope determined by the linear fits. Fitting the two Be emission peaks, however, led to remarkably different concentrations of Be in the pristine spodumene. The concentrations, 1390 ± 130 and 215 ± 30 mg/kg, were obtained from fitting the Be II and Be I emission intensities, respectively. As mentioned in the Materials and Methods Section, the concentrations of Be, Na, and K in the pristine spodumene were determined by ICP-OES prior to LIBS analysis. From the ICP-OES analysis, the Be concentration in the pristine spodumene was determined to be 253 ± 13 mg/kg. The analyte concentrations determined by LIBS and ICP-OES are listed in
Table 2.
Comparing the two LIBS results with those from ICP-OES, the Be I emission peak was found to provide a more accurate result with a relative error of 15%. However, the stronger Be II emission peak showed remarkable deviation from the ICP-OES result. The failure of the stronger Be II emission peak to determine the Be concentration in the pristine spodumene using the standard addition method can be understood by investigating the spectroscopic parameters listed in
Table 1. One of the drawbacks in the application of the standard addition method to LIBS analysis is the varying sensitivity of LIBS signal intensities to the analyte concentration. Generally, the sensitivity in LIBS analysis shows linearity in the concentration region much narrower than those of other techniques such as ICP-OES, ICP-MS, and AAS. This is mainly due to self-absorption that is the re-absorption of the light emitted from the hot plasma core passing through the cold periphery of the plasma [
29]. It should be noted that the lower energy level of the Be II emission is the ground state, whereas, for the Be I emission, it is an excited state that can further relax to lower or ground states (see the parameters listed in
Table 1). The emission lines with zero lower-level energy like that of Be II at 313.06 nm, called “resonance lines”, are much more labile to the self-absorption because they have no other way than re-absorption after emission. On the other hand, the non-resonance lines like that of Be I at 332.12 can suppress the self-absorption effect on their emission intensities by relaxing further down to the lower levels [
29]. In addition to the lower-level energy, the spontaneous emission coefficients should be compared between the Be II and Be I emissions. There is a proportional relationship between the spontaneous emission and absorption coefficients,
Aki and
Bik, respectively, as shown below.
In the above equation,
h,
ν,
c, and
gi indicate Planck’s constant, the frequency of the emitted light, the speed of light, and the statistical weight of the lower level, respectively. This equation indicates that the stronger emission line is more sensitive to self-absorption due to the larger absorption coefficient proportional to the spontaneous emission coefficient [
30]. Although the Be II and Be I emission peaks are composed of a few closely spaced lines, the Be II peak contains the lines with larger spontaneous emission coefficients than those of the lines in the Be I peak. This indicates that the Be II emission peak intensity is more strongly influenced by the self-absorption than that of Be I. Also, the self-absorption effect becomes stronger when the analyte concentration is higher [
29]. As a result, the emission intensity is decreased more by the re-absorption as the analyte concentration goes higher. This makes the sensitivity of the emission intensity to the analyte concentration lower in the higher concentrations. Considering these factors related to the self-absorption effect, the low accuracy of fitting the Be II emission intensities in determining the Be concentration can be attributed to the slope,
b, that is too small due to the self-absorption.
It should be noted that larger fluctuations in the intensities of Be compared to Na are observed, although the experiment is performed under similar conditions (see the following
Figure 6). This shows that Be is more sensitive to the plasma conditions (plasma temperatures and densities) that vary with the shot-to-shot variations in the laser energy. This is supported by the fact that the ionization potential of Be (9.32 eV) is much higher compared to the ionization potential of Na (5.1 eV). Thus, small variations in laser energies may result in a large difference in the number of electrons detached from the Be atom. To improve measurement precision, we also investigated the effect of internal standardization by normalizing the Be I emission intensity at 332.13 nm with the Al I emission at 308.22 nm. Although this approach significantly reduced the RSD, it compromised sensitivity due to potential self-absorption in the reference line. As the standard addition method prioritizes sensitivity to ensure accurate calibration slopes, the raw Be I intensity yielded a Be concentration closer to the ICP-OES result. Therefore, in the following, we focused on selecting analyte lines that provide both high sensitivity and minimal self-absorption, rather than applying internal standardization.
3.3. Quantification of Na
To determine the concentration of Na in the pristine spodumene sample, the two Na I emission intensities at 330.24 and 819.48.12 nm were employed for the analyte signals. The Na I emission intensities were plotted with respect to the relative concentrations of Na and are shown in
Figure 6a and
Figure 6b, respectively. The intensities of both Na I emission peaks were fitted well using a linear function, and the R
2 values are noted in the corresponding panels of
Figure 6. The concentration of Na in the pristine spodumene was calculated as the ratio of the fitted parameters,
a/
b. Fitting the intensities of the Na I emission peak at 330.24 nm resulted in the Na concentration of 4340 ± 570 mg/kg. Comparing this result with the Na concentration determined by ICP-OES, 2660 ± 130 mg/kg, the relative error was estimated to be 63%. On the other hand, using the Na I emission peak at 819.48 nm, the Na concentration was determined to be 3000 ± 140 mg/kg, which agreed with the result from ICP-OES within 13% relative error. To rationalize the better accuracy of the Na I emission peak at 819.48 nm, it should be noted that the peak at 819.48 nm is composed of non-resonance lines (see the spectroscopic parameters listed in
Table 1). Thus, the emission intensities could not be affected by the self-absorption. However, the peak intensity at 330.24 nm is contributed by two resonance lines. The larger Na concentration value from fitting the Na I peak intensities at 330.24 nm than that from ICP-OES can be attributed to the decreased sensitivity by the self-absorption effect on the measured intensities.
Both of the Na I emission peak intensities at 330.24 nm and 819.48 nm showed comparable RSDs of 3.8% and 4.2%, respectively. These RSD values are the average of the RSDs of the five measurement groups with the spodumene samples spiked with 0, 0.2, 0.4, 0.6, and 0.8 wt.% of added Na. It is important to note that, in principle, RSD and signal-to-noise ratio (SNR) are not significantly influenced by whether the corresponding emission line is a resonance or a non-resonance line. Rather, it is the sensitivity—not the precision—that is affected by the resonance characteristics of the emission line. Accordingly, the measurement precision, as reflected by the RSD, was similar between the resonance Na I line at 330.24 nm and the non-resonance Na I line at 819.48 nm. In contrast, the Be II emission at 313.04 nm exhibited a higher RSD of 47%, while the Be I emission at 332.13 nm showed an RSD of 39%. This discrepancy can be attributed to the particularly high ionization potential of Be (9.32 eV), as mentioned earlier. The formation of the ionic species Be II requires higher plasma temperatures, making its signal more susceptible to plasma temperature fluctuations. This results in greater uncertainty in intensity measurements compared to the atomic species Be I.
3.4. Quantification of K
As mentioned above, K I emission lines were observed around 404.5 and 768 nm. The K I emission lines around 404.5 nm may be more appropriate for the standard addition method because their intensities are much weaker than those around 768 nm. However, they were interfered with by the Fe I emission line at 404.58 nm (see
Figure 3c). The spectral interference would make the standard addition method inaccurate due to uncertainty in setting the baseline of the analyte signal. The analyte concentration is determined from the parameters
a and
b, fitted using a linear function as
a/
b. The parameter
a is the analyte signal corresponding to the pristine sample. However,
a can be overestimated if the analyte signal is interfered with by other nearby emissions and if the baseline is not properly subtracted. This would lead to overestimation of the analyte concentration even though the sensitivity,
b, has been measured accurately. The strong K I emission line at 766.49 nm was thus chosen for the analyte signal. This resulted in the ratio,
a/
b, corresponding to 16,800 ± 1400 mg/kg, which is too large to be accepted as the concentration of K in the pristine spodumene sample (3240 ± 160 mg/kg). The cause of the large deviation in the determined K concentration can be attributed to the decreased sensitivity due to the self-absorption of the strong and resonant K I emission line (see
Aki and
Ei values listed in
Table 1). Whether the K I emission line intensities at 766.49 and 769.90 nm were affected by the self-absorption or not can be checked by investigating the intensity ratio of them. When their intensities are free from the self-absorption, the ratio of the K I line intensity at 766.49 nm to that at 769.90 nm is estimated to be ~2:1 considering their spectroscopic parameters listed in
Table 1. The two emission lines have very similar spectroscopic parameters other than
gk. The
gk value of the K I emission line at 766.49 nm is 4, twice of that at 769.90 nm. The observed ratio of the K I emission line intensity at 766.49 nm to that at 769.90 nm is 1.6:1. This definitely indicates that the K I emission line intensities were affected by the self-absorption. The stronger one of the two lines was affected by the self-absorption more severely. Thus, the intensity ratio decreased from 2:1 to 1.6:1.
Inspired by Equation (2), which indicates that weaker emissions are less re-absorbed, the accuracy of fitting the edge intensities of the K I peak emission profile was investigated in the following. This approach is supported by the mechanism of the self-absorption that becomes more severe in the center of the spectral line profile than in the wings [
29]. The expanded spectra around 765.5 nm in
Figure 7 shows the profile of the K I emission line centered at 766.49 nm for the spodumene samples to which different amounts of KO
2 were added. The relative concentrations of K were noted in the panel of
Figure 6. The emission intensities measured at 766.48, 765.92, 765.35, 764.78, 764.21, 763.65, and 763.08 nm are represented by
I1,
I2,
I3,
I4,
I5,
I6, and
IB. Among them, the intensity at 763.08 nm,
IB, was taken as the baseline intensity.
Thus, the six calibration curves shown in
Figure 8 were constructed using the baseline-subtracted intensities—
Figure 8a
I1 −
IB,
Figure 8b
I2 −
IB,
Figure 8c
I3 −
IB,
Figure 8d
I4 −
IB,
Figure 8e
I5 −
IB, and
Figure 8f
I6 −
IB. The baseline subtraction is also crucial to obtain accurate results from the standard addition method because the analyte concentration is calculated as
a/
b. This indicates that the analyte concentration can be overestimated when the baseline is not completely subtracted from the measured intensities. Herein, any possible spectral interference to the K I emission line at 766.49 nm was not identified, and thus the baseline,
IB, seems to be due to continuum background emission. The wavelength shift from the center of the K I emission line at 766.49 nm to the blue side, ∆
λ, increases from
I1 to
I6 and simultaneously the emission intensity value also decreases. Thus, the self-absorption effect becomes weaker as ∆
λ increases. In
Figure 8, the red solid lines are the linear fits of the baseline-subtracted intensity values, and the red dashed lines are the extrapolation to the relative concentration values corresponding to the zero intensities. From the fitted parameters,
a and
b, the ratio values of
a/
b were calculated and noted in the corresponding panels. As expected from considering the self-absorption effect, the intensity values,
I6 −
IB, at the furthest edge of the K I line profile provided the most accurate K concentration values, (
a/
b) × 10,000 = 3060 ± 480 mg/kg, leading to the error of 5.5% from the ICP-OES result, 3240 ± 160 mg/kg.
The predicted K concentrations, obtained by fitting
I1 −
IB,
I2 −
IB,
I3 −
IB,
I4 −
IB,
I5 −
IB, and
I6 −
IB, show a systematic trend with Δλ.
Figure 9a shows the (
a/
b) × 10,000 values from the six calibration curves from LIBS, corresponding to the K concentrations in mg/kg, with respect to Δλ along with the ICP-OES result. As the wavelength at which the intensities were taken for the calibration curve of the standard addition method was shifted toward the blue-side edge, the K concentration approached more closely to the ICP-OES result. This can be rationalized by the decreased self-absorption effect on the weaker edge emission intensities and justified by the increased sensitivity of the emission intensity to the K concentration. The six calibration curves shown in
Figure 8 exhibit a decrease in the slope of the linear fit from
I1 −
IB to
I6 −
IB. The fitted parameter
b, corresponding to the slope, cannot be directly compared among the six calibration curves because the baseline-subtracted intensities differ significantly among them. To compare the sensitivities obtained from signals measured at the different intensity levels, it is necessary to introduce the concept of “% sensitivity”. Sensitivity refers to the ability of a method or instrument to detect small changes in analyte concentration. It is generally defined as the slope of the calibration curve, Δ
I/Δ
c, where Δ
I and Δ
c are changes in the signal intensity and the analyte concentration, respectively. In addition, % sensitivity uses the % change in the signal intensity, Δ%
I, in place of Δ
I. The % change in the signal intensity can be estimated as below.
In the above equation,
Imax and
Imin represent the measured intensities for the standards with the maximum and minimum analyte concentrations. Herein,
Imax and
Imin represent the K I emission intensities for the spodumene samples to which KO
2 compounds were added to achieve the relative concentration of K 0.8 and 0 wt.%, respectively. Then, the % sensitivity values were calculated as Δ%
I/Δ
c with Δ
c = (0.8 − 0.0) wt.% and noted in the corresponding panels of
Figure 8. As expected due to the self-absorption effect, which is more pronounced for stronger emissions and higher analyte concentrations, the % sensitivity increases for the weaker edge emission intensities. The weakest emission intensity,
I6 −
IB, exhibits a 72.3% decrease as the K concentration decreases from 0.8 to 0.0 wt.%. This is the most sensitive change in the emission intensity among the six analyte signals
I1 −
IB,
I2 −
IB,
I3 −
IB,
I4 −
IB,
I5 −
IB, and
I6 −
IB. The strongest analyte signal,
I1 −
IB, showed a % sensitivity of 35.5%, which is much smaller than that of the weakest analyte signal,
I6 −
IB.
It should be noted that while a weaker signal is preferred to achieve accurate results in the standard addition method, it is disadvantageous for precision. This is well demonstrated in
Figure 9b. The blue-filled squares in
Figure 9b represent the relative standard deviation of the K concentration determined by fitting the baseline-subtracted K I emission intensities,
I1 −
IB,
I2 −
IB,
I3 −
IB,
I4 −
IB,
I5 −
IB, and
I6 −
IB, that were taken at the wavelengths shifted toward the blue side by Δ
λ = 0.10, 0.57, 1.14, 1.71, 2.28, and 2.84 nm, respectively. Although the relative standard deviation decreases from Δ
λ = 0.10 to 1.14 nm, it increases afterward and the weakest analyte signal at Δ
λ = 2.84 nm shows the largest standard deviation. However, the relative error, indicated by the red-filled squares in
Figure 9b, decreases as Δ
λ increases. This observation suggests that accuracy and precision do not always improve concurrently, particularly when weak emission signals are used. As Δλ increases, the edge intensity becomes weaker, which leads to a higher sensitivity in the calibration curve and thus improves the accuracy of the standard addition method, as reflected in the decreasing relative error. However, the relative standard deviation increases for these weaker signals because it is calculated as the ratio of the standard deviation to the mean intensity. While the absolute fluctuation in signal intensity may remain similar, the average intensity becomes smaller as weaker edges are used, resulting in a larger RSD. Therefore, although the use of edge intensities effectively enhances analytical accuracy by mitigating self-absorption effects, it imposes a trade-off in terms of decreased precision. This explains the diverging trends of relative error and relative standard deviation in
Figure 9b, even though both metrics were derived from the same measurement data.
Finally, we compared our results with previously reported concentrations of Be, Na, and K in various spodumene samples. These concentrations vary depending on the spodumene type and the geological setting of the deposits [
8,
31,
32,
33]. Göd et al. analyzed spodumene from Weinebene, Austria and reported Na
2O (2.07–4.74 wt.%), K
2O (1.20–5.58 wt.%), and Be (11–110 ppm) in amphibolite-hosted pegmatites, and Na
2O (1.20–5.58 wt.%), K
2O (1.99–2.71 wt.%), and Be (41–68 ppm) in micaschist-hosted pegmatites [
31]. Fosu et al. used Mineral Liberation Analysis to assess spodumene concentrates from Li-Cs-Ta-type pegmatites, reporting K and Na concentrations of 1.34 wt.% and ~0.76 wt.%, respectively, and compared results with energy-dispersive X-ray spectroscopy (EDS), XRF, and ICP-OES [
32]. Wang et al. found Na (818–3139 ppm), K (12.2–1682 ppm), and Be (4.11–15.4 ppm) in spodumene pegmatites from Lhozhag, eastern Himalaya [
8]. Skublov et al. examined impurity element zoning in colorless beryl from spodumene pegmatites in the Pashki deposit, Afghanistan, and reported Na (6591–8879 ppm) and K (511–910 ppm) [
33]. In line with these findings, our data also show relatively high concentrations of Na and K (3000 ± 140 mg/kg and 3040 ± 480 mg/kg, respectively) and significantly lower concentrations of Be (253 ± 13 mg/kg).