Study of Dendromass Ashes Fusibility with the Addition of Magnesite, Limestone and Alumina
Abstract
1. Introduction
2. Experimental
2.1. Ashes and Modifying Additives
2.2. Indexes Used to Predict Behaviour of Ash at Higher Temperature
2.3. Determination of Ash Fusibility according to STN ISO 540
3. Results
Composition and Fusibility Temperatures of Bio-Ash
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Niu, Y.; Tan, H.; Hui, S. Ash-related Issues during Biomass Combustion: Alkali-induced Slagging. Silicate Melt-induced Slagging (Ash Fusion). Agglomeration. Corrosion. Ash Utilization and Related Countermeasures. Prog. Energy Combust. Sci. 2016, 52, 1–61. [Google Scholar] [CrossRef]
- Plešingerová, B.; Vadász, P.; Medveď, D.; Jablonský, G.; Sučik, G.; Dzurňák, R. Characterisation of Dendromass Ash Fractions Captured in Power Plant with a View to their Further Utilization. Acta Montan. Slovaca. 2023; 28, in press. [Google Scholar]
- Garcia-Maraver, A.; Mata-Sanchez, J.; Carpi, M.; Perez-Jimenez, J. Critical Review of Predictive Coefficients for Biomass Ash Deposition Tendency. J. Energy Inst. 2017, 90, 214–228. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G.; Song, Y.C.; Li, W.Y.; Feng, J. Ash Contents and Ash–forming Elements of Biomass and their Significance for Solid Biofuel Combustion. Fuel 2017, 208, 377–409. [Google Scholar] [CrossRef]
- Tortosa Masiá, A.A.; Buhre, B.J.P.; Gupta, R.P.; Wall, T.F. Characterising Ash of Biomass and Waste. Fuel Process. Technol. 2007, 88, 1071–1081. [Google Scholar] [CrossRef]
- Agraniotis, M.; Grammelis, P.; Papapavlou, C.; Kakaras, E. Experimental Investigation on the Combustion Behaviour of Pre-dried Greek Lignite. Fuel Process. Technol. 2009, 90, 1071–1079. [Google Scholar] [CrossRef]
- Miles, P.E.; Miles, T.R., Jr.; Baxter, L.L.; Bryers, R.W.; Jenkins, B.M.; Oden, L.L. Alkali Deposits Found in Biomass Power Plants. A Preliminary Investigation of Their Extent and Nature; NREL/TP-4338142; National Renewable Energy Laboratory: Golden, CO, USA, 1995; Volume 2, p. 122.
- Berlanga, C.; Ruiz, J.A. Study of Corrosion in a Biomass Boiler. J. Chem. 2013, 8, 272090. [Google Scholar] [CrossRef]
- Lindberg, D.K.; Tesfaye, F. The Thermodynamics of Slag Forming Inorganic Phases in Biomass Combustion Process. Eng. Technol. 2017, 27–41. [Google Scholar] [CrossRef]
- Vamvuka, D.; Kakaras, E. Ash Properties and Environmental Impact of Various Biomass and Coal Fuels and their Blends. Fuel Process. Technol. 2011, 92, 570–581. [Google Scholar] [CrossRef]
- Couch, G. Understanding Slagging and Fouling in PF Combustion; IEACR/72; IEA Coal Research: London, UK, 1994; ISBN 92-9029-240-7. [Google Scholar]
- Skorupska, N.M. Coal Specifications-Impact on Power Station Performance; IEACR/52; IEA Coal Research: London, UK, 1993; p. 120. [Google Scholar]
- Barroso, J.; Ballester, J.; Pina, A. Study of Coal Ash Deposition in an Entrained Flow Reactor: Assessment of Traditional and Alternative Slagging Indices. Fuel Process. Technol. 2007, 88, 865–876. [Google Scholar] [CrossRef]
- Pintana, P.; Tippayawong, N.; Nuntaphun, A.; Thongchiew, P. Characterization of Slag from Combustion of Pulverized Lignite with High Calcium Content in Utility Boiler. Energy Explor. Exploit. 2014, 32, 471–482. [Google Scholar] [CrossRef]
- Yu, L.Y.; Wang, L.W.; Li, P.S. Study on Prediction Models of Biomass Ash Softening Temperature Based on Ash Composition. J. Energy Inst. 2014, 87, 215–219. [Google Scholar] [CrossRef]
- Pintana, P.; Tippayawong, N. Predicting Ash Deposit Tendency in Thermal Utilization of Biomass. Eng. J. 2016, 20, 15–23. [Google Scholar] [CrossRef]
- Dunnu, G.; Maier, J.; Schffknecht, G. Ash Fusibility and Compositional Data of Solid Recovered Fuels. Fuel 2010, 89, 1534–1540. [Google Scholar] [CrossRef]
- Kim, J.R.; Lee, Y.S.; Min, D.J.; Jung, S.M.; Yi, S.H. Influence of MgO and Al2O3 Contents on Viscosity of Blast Furnace type slags containing FeO. ISIJ Int. 2004, 44, 81291. [Google Scholar] [CrossRef]
- Zelkowski, J. Kohlecharaterisierung und Kohleverbrennung; VGB PowerTech, e.V.: Essen, Germany, 2004; ISBN 978-3-86875-216-8. [Google Scholar]
- Raask, E. Mineral Impurities in Coal Combustion: Behavior, Problems and Remedial Measures, 1st ed.; Taylor & Francis: Abingdon, UK, 1985; ISBN 089116362X. [Google Scholar]
- Wenjia, S.; Tang, L.; Zhu, X.; Wu, Y.; Rong, Y.; Zhu, Z.; Koyama, S. Fusibility and Flow Properties of Coal And Slag. Fuel 2009, 88, 297–304. [Google Scholar]
- Ovčačíková, H.; Velička, M.; Vlček, J.; Topinková, M.; Klárová, M.; Burda, J. Corrosive Effect of Wood ash Produced by Biomas, Combustion on Refractory Materials in a Binary Al-Si System. Materials 2022, 15, 5796. [Google Scholar] [CrossRef]
- Vamvuka, D.; Deli, M.; Stratakis, A. Effect of Mineral Additives on Fusion Behavior of Agricultural Residue Ashes during Combustion or Co-combustion with Lignite. J. Energy Power Technol. 2021, 3, 22. [Google Scholar] [CrossRef]
- Werther, J.; Saebger, M.; Hartge, E.-U.; Ogada, T.; Siagi, Z. Combustion of Agricultural Residues. Prog. Energy Combust. Sci. 2000, 26, 1–27. [Google Scholar] [CrossRef]
- Miles, T.R.; Baxter, L.L.; Bryers, R.W.; Jenkins, B.M.; Oden, L.L. Boiler Deposits from Firing. Biomass Bioenergy 1996, 10, 125–138. [Google Scholar] [CrossRef]
- Plešingerová, B.; Vadász, P.; Medveď, D.; Sučik, G.; Macháček, J.; Popovič, Ľ.; Ivánová, D.; Bakajsová, R. The Effect of Increasing MgO Content in Dendromass on Ash Fusibility and Corrosion of Corundum Refractory Castable. Ceram. Int. 2022, 48, 21739–21747. [Google Scholar] [CrossRef]
- Nguyen, H.K.; Moon, J.H.; Jo, S.H.; Park, S.J.; Bae, D.H.; Seo, M.W.; Ra, H.W.; Yoon, S.J.; Yoon, S.M.; Lee, J.G.; et al. Ash Characteristics of Oxy-Biomass Combustion in a Circulating Fluidized Bed with Kaolin Addition. Energy 2021, 230, 120871. [Google Scholar] [CrossRef]
- Holubcik, M.; Jandacka, J. Mathematical Model for Prediction of Biomass Ash Melting Temperature Using Additives. Komunikacie 2014, 16, 48–53. [Google Scholar] [CrossRef]
- Öhman, M.; Boström, D.; Nordin, A.; Hedman, H. Effect of Kaolin and Limestone Addition on Slag Formation during Combustion of Wood Fuels. Energy Fuels 2004, 18, 1370–1376. [Google Scholar] [CrossRef]
- Batir, O.; Selçuk, N.; Kulah, G. Effect of Kaolin Addition on Alkali Capture Capability during Combustion of Olive Residue. Combust. Sci. Technol. 2019, 191, 43–53. [Google Scholar] [CrossRef]
- STN ISO 540; Uhlie a Koks. Stanovenie Taviteľnosti Popola. ÚNMS SR: Bratislava, Slovakia, 2010.
- Reynaert, C.; Sniežek, E.; Szczerba, J. Corrosion Tests for Refractory Materials Intended for the Steel Industry—A Review. Ceram.-Silikáty 2020, 64, 278–288. [Google Scholar] [CrossRef]
- Thy, P.; Jenkins, B.M.; Lesher, C.E. High-temperature Melting Behavior of Urban Wood Fuel Ash. Energy Fuels 1999, 13, 839–850. [Google Scholar] [CrossRef]
- Kingery, W.D.; Bowen, H.K.; Uhlmann, D.R. Introduction to Ceramics, 2nd ed.; John Wiley and Sons. Inc.: Hoboken, NJ, USA, 1976; p. 469. ISBN 0-471-47860-1. [Google Scholar]
- Nordgren, D.; Hedman, H.; Padban, N.; Boström, D.; Öhman, M. Ash Transformations in Pulverised Fuel Co-combustion of Straw and Woody Biomass. Fuel Process. Technol. 2013, 105, 52–58. [Google Scholar] [CrossRef]
- Plešingerová, B.; Derin, B.; Vadász, P.; Medveď, D. Analysis of Deposits from Combustion Chamber of Boiler for Dendromass. Fuel 2020, 266, 117069. [Google Scholar] [CrossRef]
- Holubčík, M.; Jandačka, J.; Malcho, M. Ash melting temperature prediction from chemical composition of biomass ash. Holist. Approach Environ. 2015, 5, 119–125. [Google Scholar]
| Characterization of Ash | Index Definition | Indices Value of Ash Inclination to Slagging, Fouling, and Ash Fusibility Temperature | |||
|---|---|---|---|---|---|
| Low | Medium | High | Extremely High | ||
| Silica content | S = | <20 | 20–25 | >25 | - |
| Basicity | ˂0.5 | 0.5–1.0 | 1.0–1.75 | >1.75 | |
| Slagging index | ˂0.6 | 0.6–2.0 | 2.0–2.6 | - | |
| Fouling index | ˂0.6 | 0.6–40 | >40 | - | |
| Slag viscosity index | >72 | 65–72 | ˂65 | - | |
| Alkalinity-Total alkali | >0.3 | 0.3–0.4 | >0.4 | - | |
| Silica-Alumina ratio | ˂0.31 and >3 | - | 0.3–3.0 | - | |
| Bed agglomeration index | - | - | ˂0.15 | - | |
| Ash fusibility index [°C] | >1342 | 1232–1342 | 1052–1232 | ˂1052 | |
| Initial softening temperature [°C] | DT (IDT)-initial deformation temperature (softening) | >1100 | 900–1100 | ˂900 | - |
| Sample | Chemical Composition [wt.%] | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Al2O3 | SiO2 | CaO | MgO | Fe2O3 | MnO | Na2O | K2O | L.O.I. | |
| S09 | 5.5 | 25 | 48.8 | 3.6 | 3.1 | 1.3 | 0.4 | 3.3 | 9 |
| S10 | 5.9 | 35.6 | 28.0 | 4.0 | 2.9 | 0.8 | 1.0 | 8.2 | 10.8 |
| T10 | 6.6 | 48.9 | 21.1 | 2.9 | 2.8 | 0.6 | 1.4 | 6.5 | 4.7 |
| C09 | 3.2 | 17.2 | 47.6 | 3.5 | 2.5 | 0.8 | 0.3 | 3.7 | 21.1 |
| C10 | 4.5 | 21.7 | 38.0 | 3.4 | 2.1 | 0.8 | 0.7 | 7.0 | 12.6 |
| F09 | 1.2 | 3.2 | 50.3 | 4.1 | 1.5 | 1.2 | 0.3 | 15.1 | 23.1 |
| F10 | 1.3 | 4.6 | 32.6 | 3.2 | 0.8 | 0.8 | 1.0 | 18.4 | 20.9 |
| S09M | 4.7 | 18.5 | 35.8 | 17.2 | 3.6 | 1 | 1 | 2.3 | 15.9 |
| C09M | 3.2 | 12.8 | 34.3 | 17.8 | 3.2 | 0.6 | 1 | 2.6 | 24.4 |
| F09M | 1.8 | 3.3 | 36 | 18.4 | 2.2 | 0.8 | 1 | 10.2 | 26 |
| S09L | 3.8 | 16.9 | 49.8 | 2.5 | 2.2 | 0.9 | 0.4 | 2.2 | 20.3 |
| C09L | 2.3 | 11.7 | 49.0 | 2.4 | 1.8 | 0.5 | 0.3 | 2.5 | 28.4 |
| F09L | 1.0 | 2.3 | 50.8 | 2.8 | 1.1 | 0.8 | 0.3 | 10.1 | 29.7 |
| S09A | 37.0 | 16.7 | 32.5 | 2.4 | 2.1 | 0.9 | 0.3 | 2.2 | 6.0 |
| S10A | 37.3 | 23.7 | 18.7 | 2.6 | 1.9 | 0.5 | 0.7 | 5.5 | 7.2 |
| T10A | 37.7 | 32.6 | 14.1 | 2.0 | 1.9 | 0.4 | 0.9 | 4.4 | 3.1 |
| C09A | 35.5 | 11.5 | 31.7 | 2.3 | 1.7 | 0.5 | 0.2 | 2.5 | 14.1 |
| C10A | 36.4 | 14.5 | 25.3 | 2.3 | 1.4 | 0.6 | 0.5 | 4.7 | 8.4 |
| F09A | 34.1 | 2.1 | 33.5 | 2.7 | 1.0 | 0.8 | 0.2 | 10.7 | 15.4 |
| F10A | 34.2 | 3.1 | 21.8 | 2.1 | 0.5 | 0.5 | 0.7 | 12.3 | 14.0 |
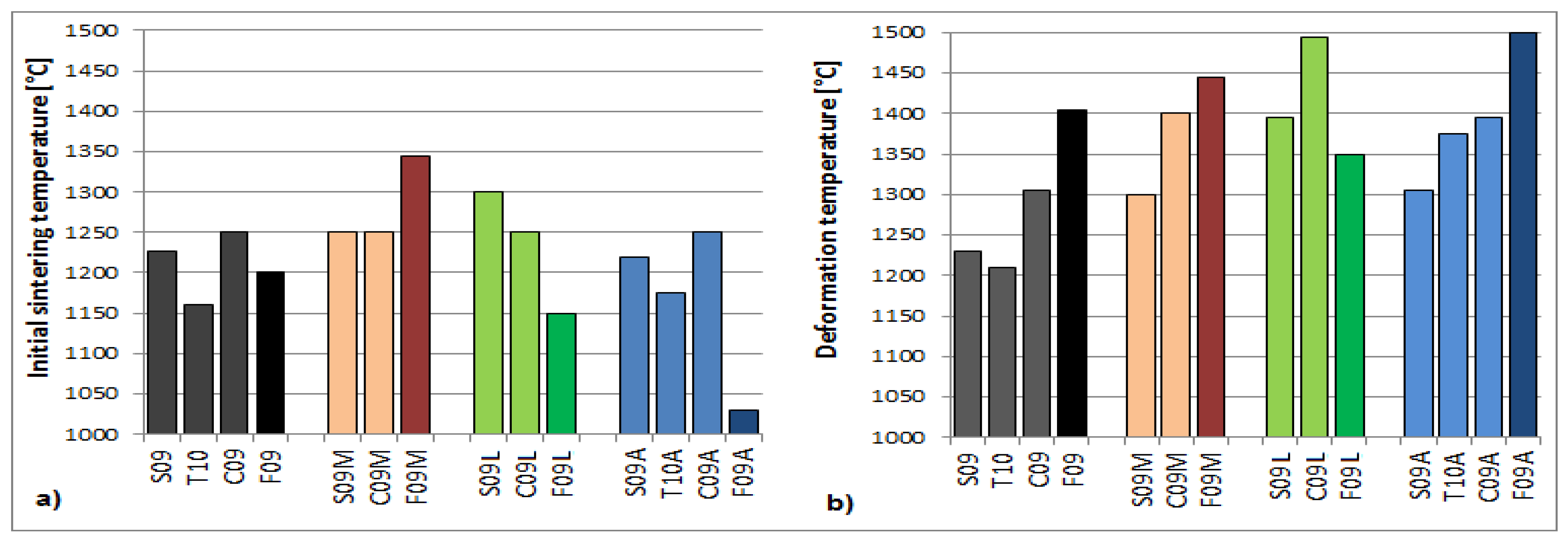
| Sample | Initial Sintering Temperature Tsin [°C] | Deformation Temperature DT [°C] | Spherical Temperature ST [°C] | Hemisphere Temperature HT [°C] | Flow Temperature FT [°C] | Ash Fusibility Index AFI [°C] |
|---|---|---|---|---|---|---|
| S09 | 1226 | 1230 | 1235 | 1238 | 1242 | 1232 |
| S10 | 1180 | 1205 | 1215 | 1215 | 1220 | 1207 |
| T10 | 1160 | 1210 | 1220 | 1225 | 1250 | 1213 |
| C09 | 1250 | 1305 | 1320 | 1345 | 1395 | 1313 |
| C10 | 1300 | 1340 | 1385 | 1430 | 1470 | 1358 |
| F09 | 1200 | 1405 | 1430 | 1432 | 1440 | 1410 |
| F10 | 1200 | 1340 | 1450 | 1455 | 1460 | 1363 |
| S09M | 1250 | 1300 | 1305 | 1310 | 1320 | 1302 |
| C09M | 1250 | 1400 | 1440 | 1450 | >1500 | 1410 |
| F09M | 1345 | 1445 | 1495 | >1500 | >1500 | 1466 |
| S09L | 1300 | 1395 | 1440 | 1460 | 1490 | 1408 |
| C09L | 1250 | >1500 | - | - | - | - |
| F09L | 1150 | 1350 | 1405 | 1410 | 1420 | 1362 |
| S09A | 1220 | 1305 | 1320 | 1325 | 1340 | 1309 |
| S10A | 1185 | 1230 | 1250 | 1255 | 1275 | 1235 |
| T10A | 1175 | 1375 | 1385 | 1390 | 1400 | 1378 |
| C09A | 1250 | 1395 | 1415 | 1415 | 1420 | 1399 |
| C10A | 1185 | 1425 | 1460 | 1460 | 1465 | 1432 |
| F09A | 1030 | >1500 | - | - | - | - |
| F10A | 1050 | >1500 | - | - | - | - |
| Sample | Indices of Slagging, Fouling and Ash Fusibility vs. DT Temperature, Index AFI, and Tsin | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | B/A | Rs | Fu | SR | TA | S/A | BAI | DT [°C] | AFI [°C] | Tsin [°C] | |
| S09 | 25 | 2.0 | 6.9 | 7.3 | 30.9 | 3.7 | 4.5 | 0.84 | 1230 | 1232 +++ | 1226 |
| +++ | ++++ | ++++ | ++ | +++ | ++++ | + | + | + | |||
| S10 | 35.6 | 1.08 | 3.13 | 9.9 | 50.5 | 9.2 | 6.0 | 0.3 | 1205 | 1207 | 1180 |
| ++++ | ++ | ++++ | ++ | +++ | ++++ | + | + | + | +++ | ||
| T10 | 48.9 | 0.64 | 1.79 | 8.5 | 64.6 | 7.9 | 7.4 | 0.35 | 1210 | 1213 | 1160 |
| ++++ | + | ++ | ++ | ++ | ++++ | + | + | + | +++ | ||
| C09 | 17.2 | 2.83 ++++ | 7.9 | 11.3 | 24.2 | 4.0 | 5.4 | 0.62 | 1305 | 1313 | 1250 |
| ++ | ++++ | ++ | +++ | ++++ | + | + | + | ++ | |||
| C10 | 21.7 | 1.99 | 4.18 | 15.3 | 33.3 | 7.7 | 4.8 | 0.27 | 1340 | 1358 | 1300 |
| +++ | ++++ | ++++ | ++ | +++ | ++++ | + | + | + | + | ||
| F09 | 3.2 | 16.25 | 27.6 | 250.2 | 5.4 | 15.4 | 2.7 | 0.1 | 1405 | 1410 | 1200 |
| + | ++++ | ++++ | ++++ | ++++ | ++++ | +++ | +++ | + | + | ||
| F10 | 4.6 | 9.63 | 7.74 | 186.8 | 11.2 | 19.4 | 3.5 | 0.04 | 1340 | 1363 | 1200 |
| + | ++++ | ++++ | ++++ | ++++ | ++++ | ++ | +++ | + | + | ||
| S09M | 18.5 | 2.64 | 10.57 | 8.7 | 24.5 | 3.3 | 3.9 | 1.09 | 1300 | 1302 | 1250 |
| ++ | ++++ | ++++ | ++ | +++ | ++++ | ++ | + | + | ++ | ||
| C09M | 12.8 | 3.7 ++++ | 13.13 ++++ | 13.3 ++ | 18.7 ++++ | 3.6 | 4.0 | 0.9 | 1400 | 1410 | 1250 |
| ++ | ++++ | ++ | + | + | + | ||||||
| F09M | 3.3 | 13.33 | 32.0 | 149.3 | 5.5 | 11.2 | 1.8 | 0.2 | 1445 | 1466 | 1345 |
| + | ++++ | ++++ | ++++ | ++++ | ++++ | +++ | ++ | + | + | ||
| S09L | 16.9 | 2.8 | 6.82 | 7.2 | 23.6 | 2.6 | 4.4 | 0.85 | 1395 | 1408 | 1300 |
| ++ | ++++ | ++++ | ++ | ++++ | ++++ | ++ | + | + | + | ||
| C09L | 11.7 | 4.04 | 8.03 | 6.8 | 18.0 | 2.8 | 5.1 | 0.64 | >1500 | - | 1250 |
| ++ | ++++ | ++++ | ++ | ++++ | ++++ | + | + | + | |||
| F09L | 2.3 | 20.0 | 22.0 | 208.0 | 4.0 | 10.4 | 2.3 | 0.1 | 1350 | 1362 | 1150 |
| + | ++++ | ++++ | ++++ | ++++ | ++++ | +++ | +++ | + | + | ||
| S09A | 16.7 | 0.74 | 1.55 | 1.8 | 31.1 | 2.5 | 0.45 | 0.84 | 1305 | 1309 | 1220 |
| ++ | ++ | ++ | ++ | ++++ | ++++ | +++ | + | + | ++ | ||
| S10A | 23.7 | 0.49 | 0.93 | 3.0 | 50.5 | 6.2 | 0.6 | 0.3 | 1230 | 1235 | 1185 |
| +++ | + | ++ | ++ | +++ | ++++ | +++ | + | + | +++ | ||
| T10A | 32.6 | 0.34 | 0.65 | 1.8 | 64.4 | 5.3 | 0.86 | 0.36 | 1375 | 1378 | 1175 |
| ++++ | + | ++ | ++ | ++ | ++++ | +++ | + | + | + | ||
| C09A | 11.5 | 0.82 | 1.4 | 2.2 | 24.3 | 2.9 | 0.3 | 0.63 | 1395 | 1399 | 1250 |
| ++ | ++ | ++ | ++ | ++++ | ++++ | +++ | + | + | + | ||
| C10A | 14.5 | 0.68 | 0.95 | 3.5 | 33.3 | 5.2 | 0.4 | 0.27 | 1425 | 1432 | 1185 |
| ++ | + | ++ | ++ | ++++ | ++++ | +++ | + | + | + | ||
| F09A | 2.1 | 1.31 | 1.31 | 13.4 | 5.4 | 10.9 | 0.06 | 0.09 | >1500 | - | 1030 |
| + | +++ | ++ | ++ | ++++ | ++++ | + | +++ | + | |||
| F10A | 3.1 | 1.02 | 0.51 | 13.3 | 11.3 | 13.0 | 0.09 | 0.04 | >1500 | - | 1050 |
| + | ++ | + | ++ | ++++ | ++++ | + | +++ | + | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vadász, P.; Plešingerová, B.; Medveď, D.; Sučik, G.; Bakajsová, R.; Petrov, V. Study of Dendromass Ashes Fusibility with the Addition of Magnesite, Limestone and Alumina. Minerals 2023, 13, 631. https://doi.org/10.3390/min13050631
Vadász P, Plešingerová B, Medveď D, Sučik G, Bakajsová R, Petrov V. Study of Dendromass Ashes Fusibility with the Addition of Magnesite, Limestone and Alumina. Minerals. 2023; 13(5):631. https://doi.org/10.3390/min13050631
Chicago/Turabian StyleVadász, Pavol, Beatrice Plešingerová, Dávid Medveď, Gabriel Sučik, Radka Bakajsová, and Vladimír Petrov. 2023. "Study of Dendromass Ashes Fusibility with the Addition of Magnesite, Limestone and Alumina" Minerals 13, no. 5: 631. https://doi.org/10.3390/min13050631
APA StyleVadász, P., Plešingerová, B., Medveď, D., Sučik, G., Bakajsová, R., & Petrov, V. (2023). Study of Dendromass Ashes Fusibility with the Addition of Magnesite, Limestone and Alumina. Minerals, 13(5), 631. https://doi.org/10.3390/min13050631






