Infrared and Raman Spectroscopy of Mullite Ceramics Synthesized from Fly Ash and Kaolin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Method (IR and Raman Spectroscopy)
3. Results and Discussion
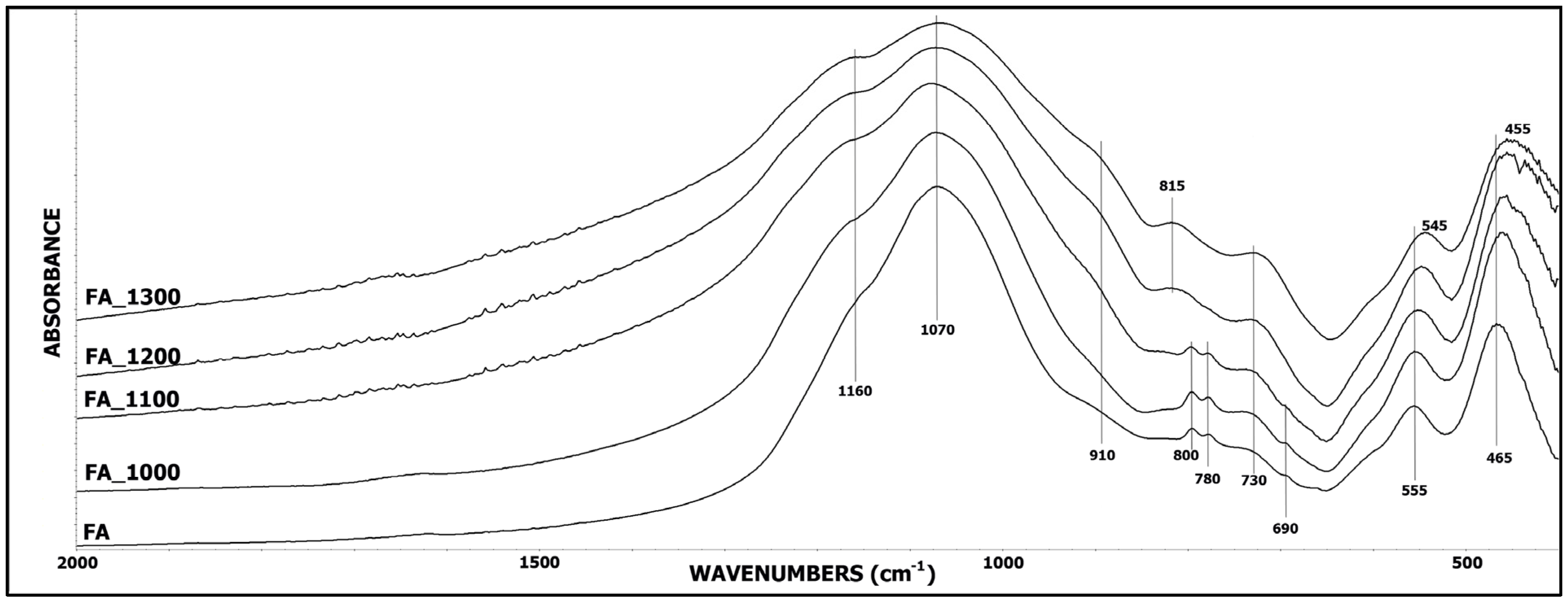
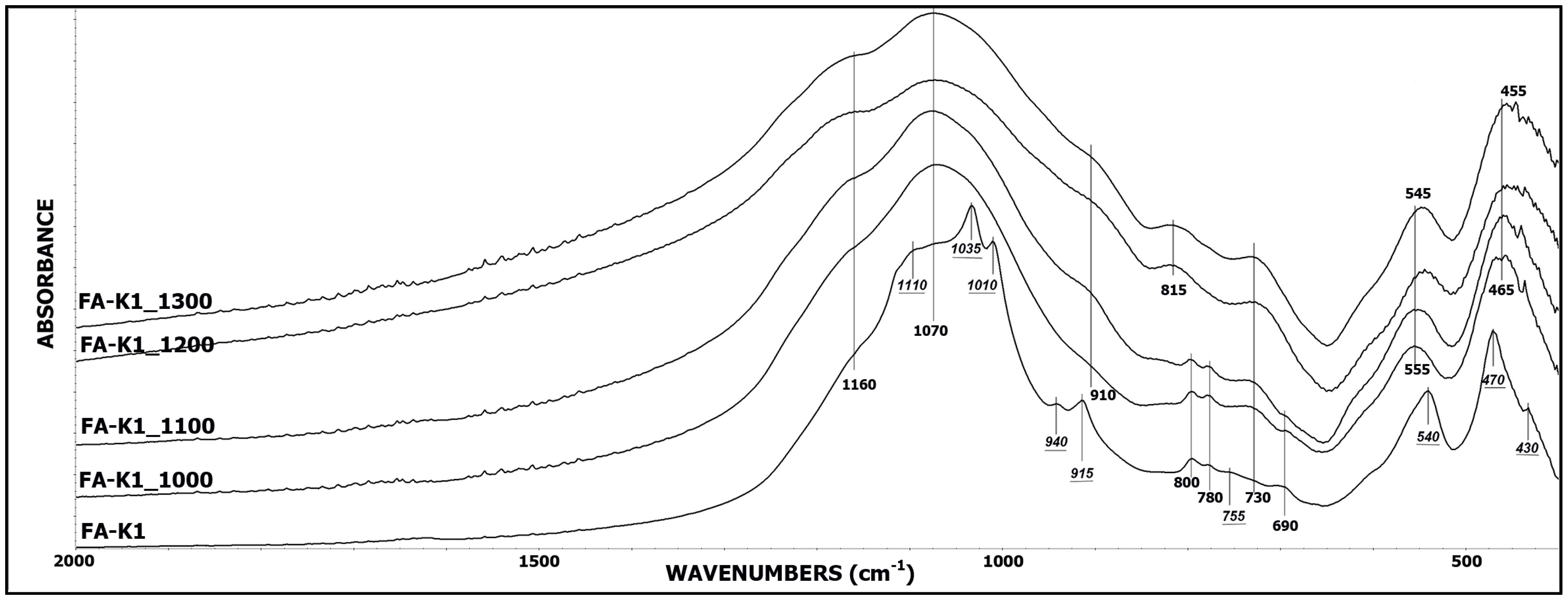
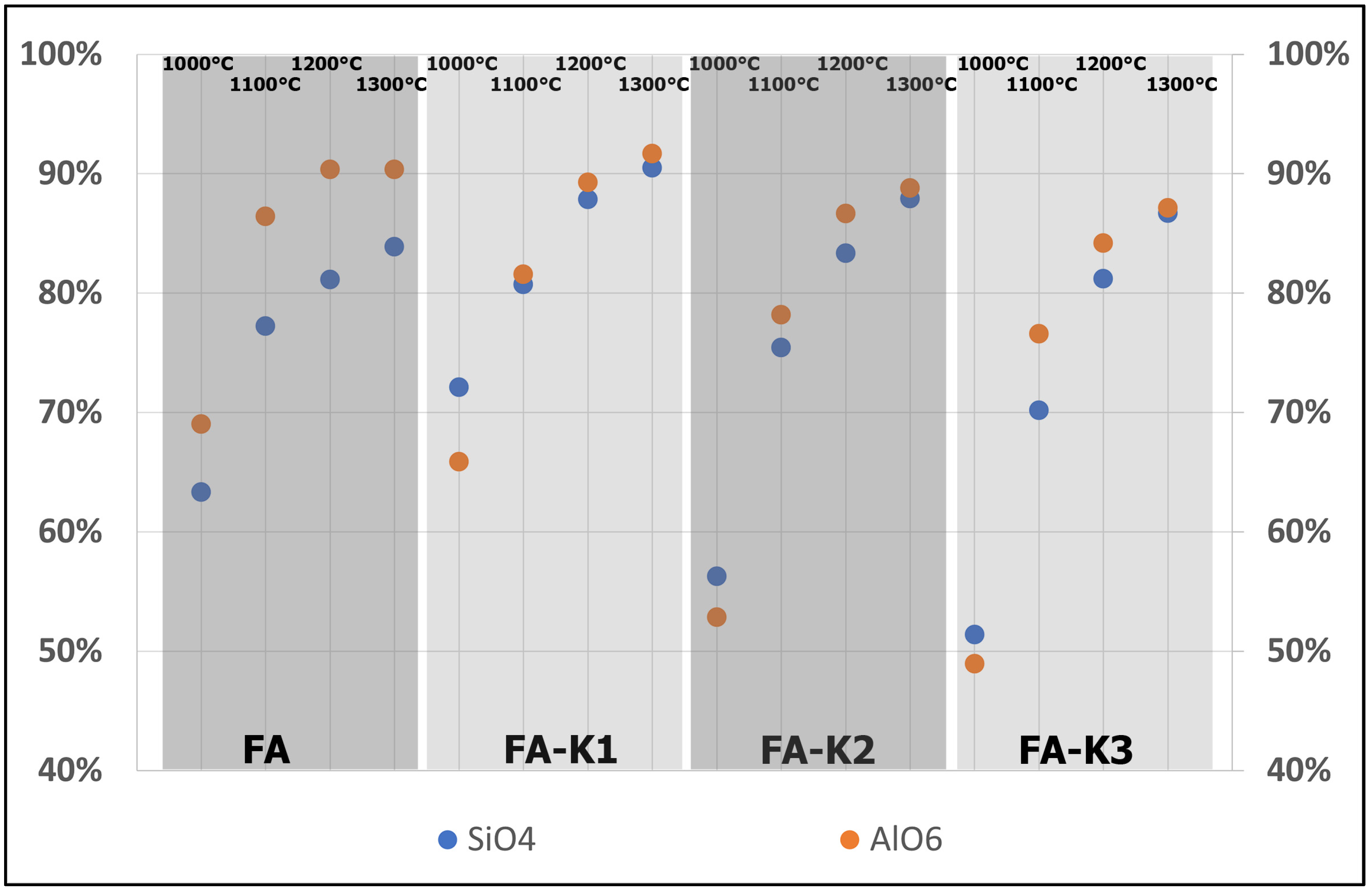
3.1. IR Spectroscopy
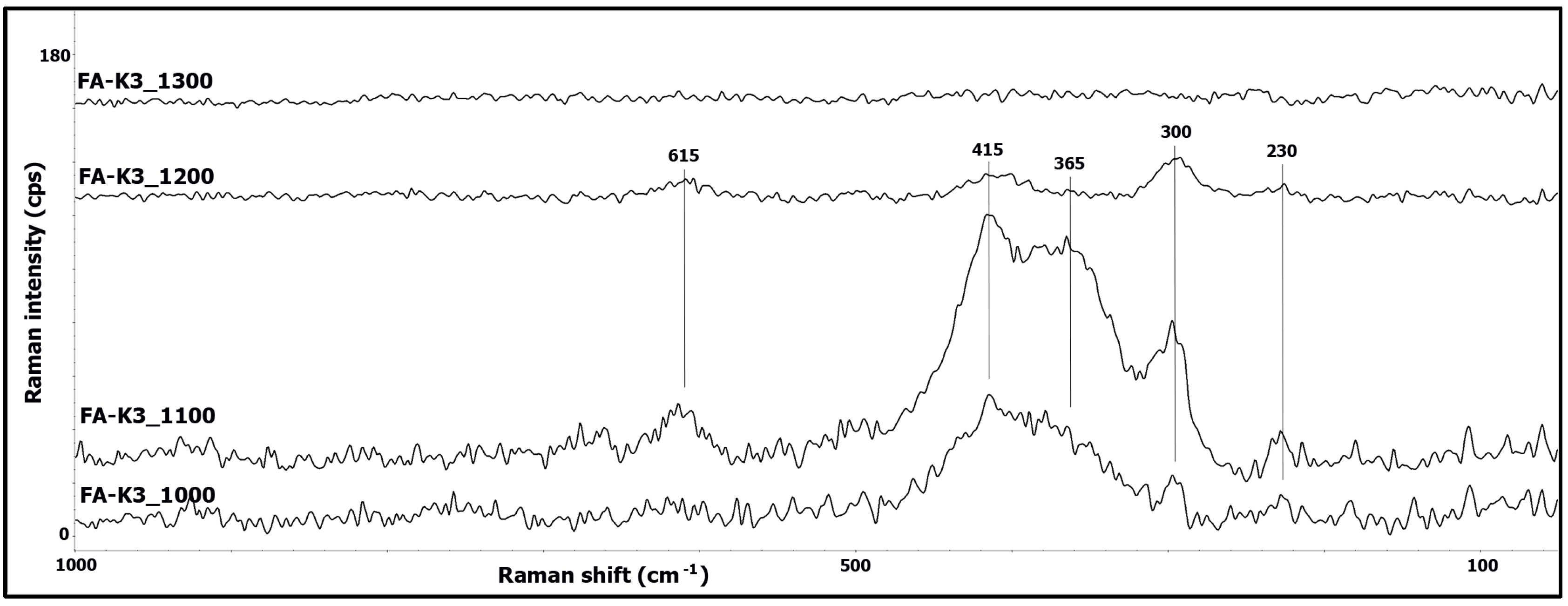
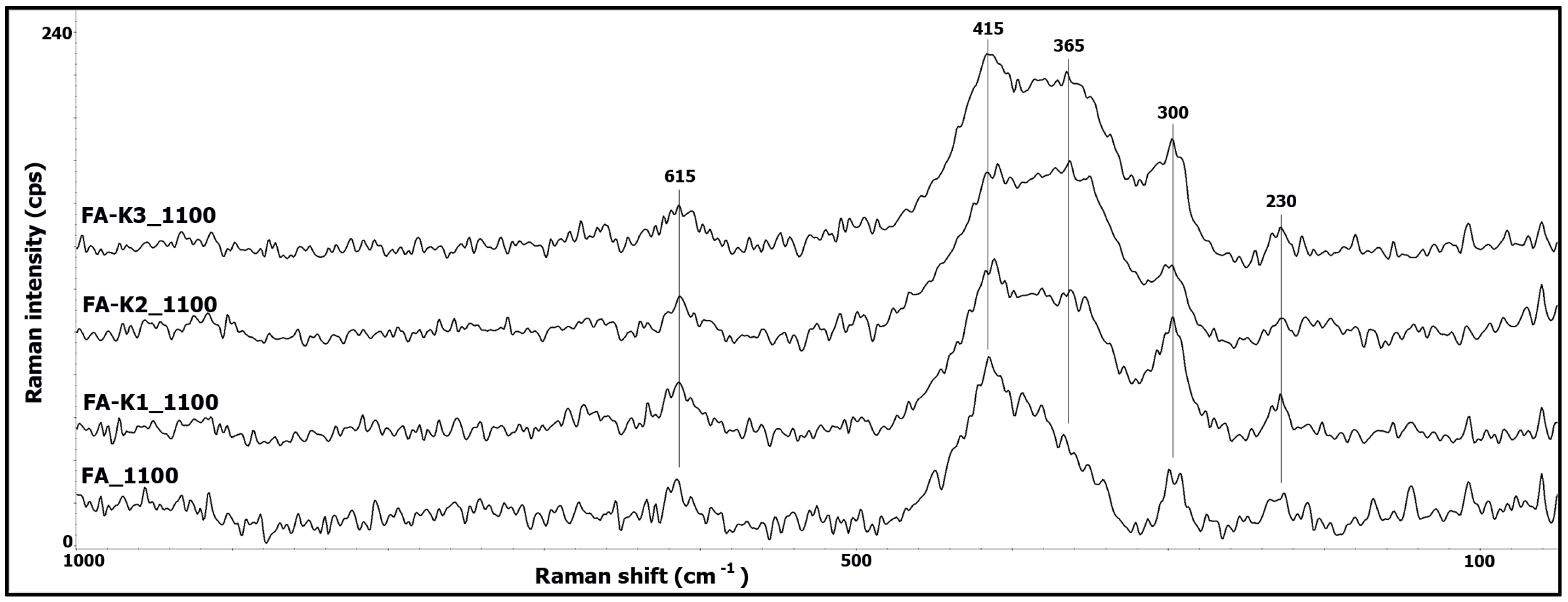
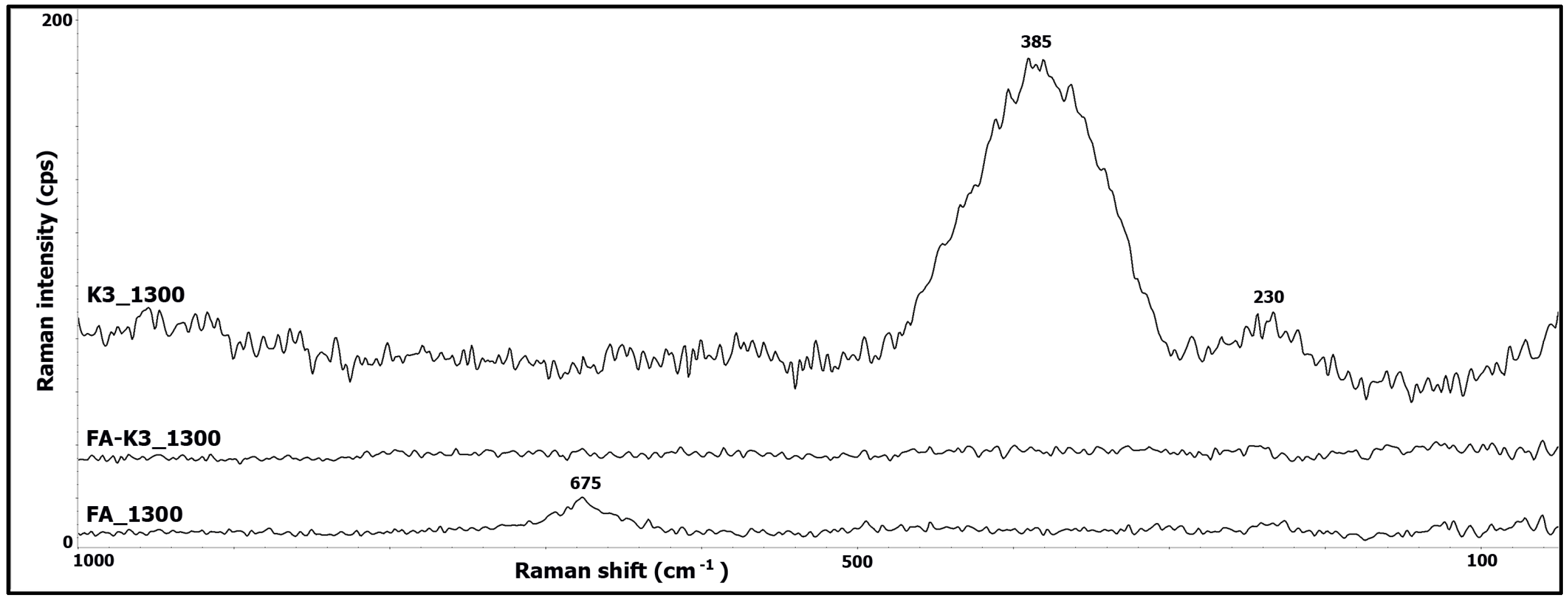
3.2. Raman Spectroscopy
4. Conclusions
Supplementary Materials
Funding
Conflicts of Interest
References
- Panda, L.; Dash, S. Characterization and utilization of coal fly ash: A review. Emerg. Mater. Res. 2020, w9, 921–934. [Google Scholar] [CrossRef]
- Potgiered-Vermaak, S.S.; Potgieter, J.H.; Belleil, M.; DeWeerdt, F.; Van Grieken, R. The application of Raman spectrometry to the investigation of cement Part II: A micro-Raman study of OPC, slag and fly ash. Cem. Concr. Res. 2006, 26, 663–670. [Google Scholar] [CrossRef]
- Hossain, S.S.; Roy, P. Sustainable ceramics derived from solid wastes: A review. J. Asian Ceram. Soc. 2020, 8, 984–1009. [Google Scholar] [CrossRef]
- Kamseu, E.; Leonelli, C.; Boccaccini, D.N.; Veronesi, P.; Miselli, P.; Pellacani, G.; Melo, U.C. Characterization of porcelain composition using two China clays from Cameroon. Ceram. Int. 2007, 33, 851–857. [Google Scholar] [CrossRef]
- Luo, Y.; Zheng, S.; Ma, S.; Liu, C.; Wang, X. Ceramics tiles derived from coal fly ash: Preparation and mechanical characterization. Ceram. Int. 2017, 43, 11935–11966. [Google Scholar] [CrossRef]
- Valášková, M.; Klika, Z.; Novosad, B.; Smetana, B. Crystallization and quantification of crystalline and non-crystalline phases in kaolin-based cordierites. Materials 2019, 12, 3104. [Google Scholar] [CrossRef]
- Schneider, H.; Komarneni, S. (Eds.) Mullite; Wiley VCH: Weinheim, Germany, 2005. [Google Scholar]
- Zacco, A.; Borgese, L.; Giaconcelli, A.; Struis, R.P.W.J.; Depero, L.E.; Bontempi, E. Review of fly ash inertisation treatments and recycling. Environ. Chem. Lett. 2014, 12, 153–175. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G. A new approach for the classification of coal fly ashes based on their orogin, composition, properties, and behavior. Fuel 2007, 86, 1490–1512. [Google Scholar] [CrossRef]
- Painter, P.C.; Coleman, M.M.; Jenkins, R.G.; Walker, P.L. Fourier transform infrared study of acid-demineralization coal. Fuel 1978, 57, 125–126. [Google Scholar] [CrossRef]
- Madejová, J.; Komadel, P. Baseline study of the clay minerals society source clays: Infrared methods. Clays Clay Miner. 2001, 49, 410–432. [Google Scholar] [CrossRef]
- Mukherjee, S.; Srivastava, S. Minerals transformations in northeastern region coals of India on heat treatment. Energy Fuels 2006, 20, 1089–1096. [Google Scholar] [CrossRef]
- Bai, J.; Li, W.; Li, B. Characterization of low-temperature coal ash behaviors at high temperatures under reducing atmosphere. Fuel 2008, 87, 583–591. [Google Scholar] [CrossRef]
- D’Alessio, A.; Vergamini, P.; Benedetti, E. FT-IR investigation of the structural changes of Sulcis and South Africa coals under progressive heating in vacuum. Fuel 2000, 79, 1215–1220. [Google Scholar] [CrossRef]
- Cooke, N.E.; Fuller, O.M.; Gaikwad, R.P. FI-IR spectroscopic analysis of coals and coal extracts. Fuel 1986, 65, 1254–1260. [Google Scholar] [CrossRef]
- Fysil, S.A.; Swinkels, D.A.J.; Fredericks, P.M. Near-Infrared diffuse reflectance spectroscopy of coal. Appl. Spectrosc. 1985, 39, 354–357. [Google Scholar]
- Fredericks, P.M.; Le, J.B.; Osborn, P.R.; Swinkels, D.A.J. Materials characterization using factor analysis of FT-IR spectra. Patr 1: Results. Appl. Spectrosc. 1985, 39, 303–310. [Google Scholar] [CrossRef]
- Alciaturi, C.E.; Escobar, M.E.; Vallejo, R. Predisction of coal properties by derivate DRIFT spectroscopy. Fuel 1996, 74, 491–499. [Google Scholar] [CrossRef]
- Cloke, M.; Gilfillan, A.; Lester, E. The characterization of coals and density separated coal fractions using FTIR and manual and automated petrographic analysis. Fuel 1997, 76, 1289–1296. [Google Scholar] [CrossRef]
- Ritz, M.; Vaculíková, L.; Kupková, J.; Plevová, E.; Bartoňová, L. Different level of fluorescence in Raman spectra of montmorillonites. Vib. Spectrosc. 2016, 84, 7–15. [Google Scholar] [CrossRef]
- Žyrkowski, M.; Costa Neto, R.; Santos, L.F.; Witkowski, K. Characterization of fly-ash cenospheres from coal-fired power plant unit. Fuel 2016, 174, 49–53. [Google Scholar] [CrossRef]
- Tai, F.C.; Wei, C.; Chang, S.H.; Chen, W.S. Raman and X-ray diffraction analysis on unburned carbon powder refined from fly ash. J. Raman Spectrosc. 2010, 41, 933–937. [Google Scholar] [CrossRef]
- Yin, Y.; Yin, J.; Zhang, W.; Tian, H.; Hu, Z.; Ruan, M.; Xu, H.; Liu, L.; Yan, X.; Chen, D. FT-IR and micro-Raman spectroscopic characterization of minerals in high-calcium coal ashes. J. Energy Inst. 2018, 91, 389–396. [Google Scholar] [CrossRef]
- Valášková, M.; Blahůšková, V.; Vlček, J. Effects of kaolin additives in fly ash on sintering and properties of mullite ceramics. Minerals 2021, 11, 887. [Google Scholar] [CrossRef]
- Agarwal, A.; Davis, K.M.; Tomozawa, M. A simple IR spectroscopic method for determining fictive temperature of silica glasses. J. Non-Cryst. Solids 1995, 185, 191–198. [Google Scholar] [CrossRef]
- Hemmati, M.; Austen Angell, C. IR absorption of silicate glasses studies by ion dynamics computer simulation. I. IR spectra of SiO2 glass in the rigid ion model approximation. J. Non-Cryst. Solids 1997, 217, 239–249. [Google Scholar] [CrossRef]
- Morioka, T.; Kimura, S.; Tsuda, N.; Kaito, C.; Saito, Y.; Koike, C. Study of the structure of silica film by infrared spectroscopy and electron diffraction analyses. Mon. Not. R. Astron. Soc. 1998, 299, 78–82. [Google Scholar] [CrossRef]
- Jin, X.H.; Gao, J.; Guo, J.K. The structural change of diphasic mullite gel studies by XRD an IR spectrum analysis. J. Eur. Ceram. 2002, 22, 1307–1311. [Google Scholar] [CrossRef]
- Tomozawa, M.; Hong, J.W.; Ryu, S.R. Infrared (IR) investigation of the structural changes of silica glasses with fictive temperature. J. Non-Cryst. Solids 2005, 351, 1054–1060. [Google Scholar] [CrossRef]
- Isahykyan, A.R.; Beglaryan, H.A.; Pirumyan, P.A.; Papakhchyan, L.R.; Zulumyan, N.H. An IR spectroscopic study of amorpous silicas. Russ. J. Phys. Chem. 2011, 85, 72–75. [Google Scholar] [CrossRef]
- Musić, S.; Filipović-Vinceković, N.; Sekanović, L. Precipitation of amorphous SiO2 particles and their properties. Braz. J. Chem. Eng. 2011, 28, 89–94. [Google Scholar] [CrossRef]
- Mozgawa, W.; Król, M.; Dyczek, J.; Deja, J. Investigation of the coal ashes using IR spectroscopy. Spectrochim. Acta A 2014, 132, 889–894. [Google Scholar] [CrossRef]
- Mozgawa, W.; Sirarz, M.; Król, M. Spectroscopic Characterization of Silicate Amorphous Materials. In Molecular Spectroscopy-Experiment and Theory: From Molecules to Functional Materials; Kolezynski, A., Król, M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; Volume 26, pp. 457–481. [Google Scholar]
- Yin, Y.; Yin, H.; Wu, Z.; Qi, C.; Tian, H.; Zhang, W.; Hu, Z.; Feng, L. Characterization of coals and coal ashes with high Si content using combined second-derivative infrared spectroscopy and Raman spectroscopy. Crystals 2019, 9, 513. [Google Scholar] [CrossRef]
- Ellerbrock, R.; Stein, M.; Schaller, J. Comparing amorphous silica, short-range-ordered silicates and silicic acid species by FTIR. Sci. Rep. 2022, 12, 11708. [Google Scholar] [CrossRef]
- Vaculíková, L.; Plevová, E. Identification of clay minerals and micas in sedimentary rocks. Acta Geodyn. Geomater. 2005, 2, 167–175. [Google Scholar]
- Cuadros, J.; Vega, R.; Toscano, A. Mid-infrared features of kaolinite-dickite. Clays Clay Miner. 2015, 63, 73–84. [Google Scholar] [CrossRef]
- Colomban, P. Structure of oxide gels and glasses by infrared and Raman scattering. J. Mater. Sci. 1989, 24, 3011–3020. [Google Scholar] [CrossRef]
- Shoval, S.; Boudeulle, M.; Panczer, G. Identification of the thermal phases in firing kaolinite to mullite by using micro-Raman spectroscopy and curve fittiing. Opt. Mater. 2011, 34, 404–409. [Google Scholar] [CrossRef]
- Bost, N.; Duraipandian, S.; Gumbretiere, G.; Poirier, J. Raman spectra of synthetic and natural mullite. Vib. Spectrosc. 2016, 82, 50–52. [Google Scholar] [CrossRef]
- Murshed, M.M.; Šehović, M.; Fischer, M.; Senyshyn, A.; Schneider, H.; Gesing, T.M. Thermal behavior of mullite between 4 K and 1320 K. J. Am. Ceram. 2017, 100, 5259–5273. [Google Scholar] [CrossRef]
- Biswal, B.; Mishra, D.K.; Das, S.N.; Bhuyan, S. Structural, micro-structural, optical and dielectric behavior of mullite ceramics. Ceram. Int. 2021, 47, 32252–32263. [Google Scholar] [CrossRef]
- Jorio, A.; Saito, R.; Dresselhaus, G.; Dresselhaus, M.S. Raman Spectroscopy in Graphene Related System, 1st ed.; Wiley-VCH.: Weinheim, Germany, 2011; pp. 299–325. [Google Scholar]
| Sample | SiO2 | TiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | Na2O | SO3 | P2O5 |
|---|---|---|---|---|---|---|---|---|---|---|
| FA | 44.83 | 1.26 | 23.93 | 7.16 | 2.83 | 1.81 | 3.03 | 0.68 | 0.72 | 0.69 |
| K1 | 49.52 | 1.01 | 30.57 | 0.76 | 0.23 | 0.23 | 1.48 | 3.76 | <0.1 | <0.1 |
| K2 | 49.63 | 0.75 | 29.70 | 0.71 | 0.26 | 0.28 | 2.36 | 3.96 | <0.1 | <0.1 |
| K3 | 48.51 | 0.87 | 34.03 | 0.80 | 0.30 | 0.35 | 2.74 | <0.1 | <0.1 | <0.1 |
| Description of Sample | Annealing Temperature | Sample Name |
|---|---|---|
| Fly ash | - | FA |
| 1000 °C | FA_1000 | |
| 1100 °C | FA_1100 | |
| 1200 °C | FA_1200 | |
| 1300 °C | FA_1300 | |
| Fly ash with kaolin K1 | - | FA-K1 |
| 1000 °C | FA-K1_1000 | |
| 1100 °C | FA-K1_1100 | |
| 1200 °C | FA-K1_1200 | |
| 1300 °C | FA-K1_1300 | |
| Fly ash with kaolin K2 | - | FA-K2 |
| 1000 °C | FA-K2_1000 | |
| 1100 °C | FA-K2_1100 | |
| 1200 °C | FA-K2_1200 | |
| 1300 °C | FA-K2_1300 | |
| Fly ash with kaolin K3 | - | FA-K3 |
| 1000 °C | FA-K3_1000 | |
| 1100 °C | FA-K3_1100 | |
| 1200 °C | FA-K3_1200 | |
| 1300 °C | FA-K3_1300 |
| Sample Name | Band at about 550 cm−1 | Band at about 450 cm−1 | ||
|---|---|---|---|---|
| Absorbance | % Decrease | Absorbance | % Decrease | |
| FA | 0.180 | - | 0.581 | - |
| FA_1000 | 0.066 | 63% | 0.180 | 69% |
| FA_1100 | 0.041 | 77% | 0.079 | 86% |
| FA_1200 | 0.034 | 81% | 0.056 | 90% |
| FA_1300 | 0.029 | 84% | 0.056 | 90% |
| FA-K1 | 0.337 | - | 0.624 | - |
| FA-K1_1000 | 0.094 | 72% | 0.213 | 66% |
| FA-K1_1100 | 0.065 | 81% | 0.115 | 82% |
| FA-K1_1200 | 0.041 | 88% | 0.067 | 89% |
| FA-K1_1300 | 0.032 | 91% | 0.052 | 92% |
| FA-K2 | 0.240 | - | 0.509 | - |
| FA-K2_1000 | 0.105 | 56% | 0.240 | 53% |
| FA-K2_1100 | 0.059 | 75% | 0.111 | 78% |
| FA-K2_1200 | 0.040 | 83% | 0.068 | 87% |
| FA-K2_1300 | 0.029 | 88% | 0.057 | 89% |
| FA-K3 | 0.218 | - | 0.474 | - |
| FA-K3_1000 | 0.106 | 51% | 0.242 | 49% |
| FA-K3_1100 | 0.065 | 70% | 0.111 | 77% |
| FA-K3_1200 | 0.041 | 81% | 0.075 | 84% |
| FA-K3_1300 | 0.029 | 87% | 0.061 | 87% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ritz, M. Infrared and Raman Spectroscopy of Mullite Ceramics Synthesized from Fly Ash and Kaolin. Minerals 2023, 13, 864. https://doi.org/10.3390/min13070864
Ritz M. Infrared and Raman Spectroscopy of Mullite Ceramics Synthesized from Fly Ash and Kaolin. Minerals. 2023; 13(7):864. https://doi.org/10.3390/min13070864
Chicago/Turabian StyleRitz, Michal. 2023. "Infrared and Raman Spectroscopy of Mullite Ceramics Synthesized from Fly Ash and Kaolin" Minerals 13, no. 7: 864. https://doi.org/10.3390/min13070864
APA StyleRitz, M. (2023). Infrared and Raman Spectroscopy of Mullite Ceramics Synthesized from Fly Ash and Kaolin. Minerals, 13(7), 864. https://doi.org/10.3390/min13070864






