Abstract
Water–rock interactions and scouring actions are recognized key factors that significantly influence the disintegration of rock on the surface of slopes. However, the research on rock disintegration, specifically under the action of scouring, is limited, which makes it difficult to understand the characteristics of rock disintegration. Therefore, in this study disintegration tests were performed on the gypsum karst breccia collected from the Zhoukoudian site in Beijing, using a self-made disintegration test device. Further, this study investigated the impact of solution pH, flow velocity, and the number of cycles on the characteristics of rock disintegration. The changes in pore structure, microstructure, and mineral composition of the rock were analyzed using nuclear magnetic resonance (NMR), scanning electron microscopy (SEM), X-ray diffraction (XRD), and inductively coupled plasma optical emission spectrometer (ICP-OES) methods. The findings reveal that the cumulative relative disintegration amount of the gypsum karst breccia experiences an increase as the pH value of the soaking solution decreases and the number of cycles increases. Once a specific flow rate is attained, the cumulative relative disintegration amount stabilizes (about 73%) and no longer exhibits significant changes. This phenomenon signifies the presence of a stabilizing flow rate for disintegration. The stable flow rate concerning rock disintegration is influenced by both the solution’s pH and the number of cycles. Following acid contamination, the rock sample’s particle morphology undergoes disruption, leading to the dissolution of cement. This, in turn, leads to an augmented release of Ca2+, Al3+, and Ma2+ ions in the solution, intensifying the disintegration of the rock samples. Conversely, alkali contamination prompts secondary cementation, mitigating localized damage. This results in a marginal increase in the calcite content within the rock samples (from 15.3% to 19.2%), while the release of Ca2+ in the solution experiences a decrease. Additionally, there is a slight increase in the release of Al3+ (a maximum increase of 1.71 mg/L), which minimally inhibits the disintegration of the rock samples. Notably, the rock disintegration predominantly occurs around macropores, and the effect of solution pH on the disintegration characteristics and stable flow rate is primarily due to the changes in the relative proportion of macropore volume in the rock samples. The findings of this study have significant implications for the prediction and control of slope-related issues.
1. Introduction
The softening and disintegration of rocks is the major cause of geologic hazards such as landslides, mudslides, and erosion [1]. Economic development has increased the concentration of acid and alkali pollutants in industrial and domestic wastewater, as well as an increase in the frequency of acid rain [2,3]. This has led to a more complex environment for rock endowment. In addition, during rainfall, the occurrence of surface-breaking runoff with varying flow rates at different locations on the slope surface results in different degrees of disintegration of the acid- and alkaline-contaminated rocks [4]. Therefore, investigating the effects and underlying mechanism of hydro-chemical damages on the disintegration characteristics of rocks subjected to the scouring effect can provide a scientific foundation for the prevention and prediction of geologic disasters.
The softening and disintegration of rocks occur due to the influence of various external factors such as freeze–thaw cycles [5,6], dry and wet cycles [7,8,9,10], and high temperatures [11]. These factors interact in a complex manner. Additionally, the internal mineral composition of the rock and the macro-performance of microstructural changes play crucial roles in this process [12,13,14,15,16,17,18,19,20,21,22,23]. The effects of variations in the water chemistry on the deterioration and disintegration properties of rocks have attracted the attention of several researchers [24]. Vergara et al. [8] investigated the effect of aqueous solution chemistry on the physical and mechanical properties of rocks and demonstrated a strong influence of aqueous solution chemistry on the swelling and shrinkage of shales. Wakim et al. [25] also suggested that the primary mechanisms responsible for the structural damage, structural decay, and weakening of rocks are the swelling and dissolution processes of clay minerals. Zhao et al. [26] examined the relationship between rock disintegration and cation release in acidic environments through indoor experiments and observed a strong correlation between cation release and particles <20 mm. Li et al. [27] documented that the primary cause behind the heightened disintegration of mudstones under the influence of acid rain hydrochemistry is the provision of an extra pathway for rock disintegration due to the damage inflicted by acid rain hydrochemistry. Moreover, Deng et al. [28], and Huang and Zhan [29] quantitatively characterized the disintegration properties of rocks treated with different pH solutions. Based on the fractal theory and energy dissipation principle, good results were achieved in their study. Zhang et al. [9,10,30,31,32] provide an overview of indoor testing techniques for assessing rock disintegration. The study also investigates the impact of various testing methods on the properties of rock disintegration. However, the above-mentioned studies did not consider the effect of water scouring action on the disintegration characteristics of the rock and could not accurately simulate and reveal the effect of rainfall on the damage to the rock slope surfaces.
Zhao et al. [33] demonstrated that the geotechnical particles under the action of water scouring were primarily subjected to the uplift force of water, the drag force of water, the underwater gravity force of particles, and the inter-particle bonding force. The equation for the estimation of the critical flow rate of geotechnical particles in the water flow was formulated in accordance with the principle of mechanical equilibrium. To investigate the initial phenomenon of slope geotechnical particles under different water flow conditions, Wang et al. [34] designed a scour starting test device for experimental study and concluded that the initial flow rate of geotechnical particles is mainly affected by dry density, clay content, and slope. Wu et al. [35] studied the scour damage process of slopes under different rainfall intensities through indoor slope scour experiments. In addition, few researchers [33,36] have evaluated the damage to geotechnical bodies on riverbeds and slopes when subjected to water scouring. The prior investigations concerning scour damage in geotechnical formations omitted the inclusion of the wetting caused by rainfall over various durations before the scouring process. Additionally, they did not address the consequences of the cementation weakening in geotechnical particles resulting from numerous wet and dry cycles. Consequently, an accurate simulation and examination of the influence of rainfall on the surface layer deterioration of geotechnical slopes could not be achieved.
The unique gypsum karst breccia at the Zhoukoudian site in Beijing contains a large number of mineral components, such as mud and clay minerals, which are easy to disintegrate after interacting with water, resulting in a decrease in rock integrity. This provides geological conditions for the occurrence of geological disasters under extreme climatic conditions. For example, in the “Beijing 7.21 heavy rain”, many landslides and collapses occurred at the Zhoukoudian site. This has led to several negative impacts on the protection of sites. Therefore, a comprehensive study of the disintegration characteristics of the gypsum karst breccia under different pH solutions and scouring conditions is crucial for predicting and preventing geologic hazards at the Zhoukoudian site.
In this research, we focus on the gypsum karst breccia as the subject of investigation. A bespoke disintegration test apparatus with a controlled flow rate was developed for this study. Subsequently, disintegration experiments were conducted on the gypsum karst breccia samples. These samples were soaked in solutions of varying pH levels, subjected to different flow rates, and exposed to varying cycle counts. The aim was to analyze how the pH of the soaking solution, flow rate, and number of cycles influence the disintegration characteristics of the rock samples. Through the utilization of NMR, SEM, XRD, and ICP-OES techniques, we delved into the investigation of several aspects. This included exploring the pore structure of the disintegrated rock samples, examining the microstructure of the immersed rock specimens, studying mineral compositions, and analyzing the release of cations into the solution. These analytical methods were employed to unveil the disintegration pattern of the gypsum karst breccia subjected to scouring and to elucidate the underlying mechanism of its formation. This comprehensive analysis reveals the disintegration patterns of gypsum karst breccias and illuminates their formation mechanisms.
2. Methodology
2.1. Material Survey and Sample Preparation
Samples of gypsum karst breccia collected from the surface layer of the slope of the Peking Man site (site 2) at Zhoukoudian (latitude: 39°41′ N and longitude: 115°51′ E) were selected as the study materials (Figure 1). The physical properties of the rock samples were determined according to the standardized RTP methods, and the results are presented in Table 1. The mineral composition of the rock samples was identified using XRD (Table 2).

Figure 1.
Sampling site and sample of gypsum karst breccia.

Table 1.
Physical properties of the rock used in the experiments.

Table 2.
The main mineral composition of the gypsum karst breccia.
For experimental testing, the acid–base solution-contaminated samples were prepared by immersing the rock samples in acid–base solutions. Initially, the gypsum karst breccia samples were shaped into spherical samples with a diameter of 40 mm [37]. Each group of 10 samples was assigned a unique number, as illustrated in Figure 2. The acidic solution was prepared using concentrated sulfuric acid, while the basic solution was prepared using high-purity sodium hydroxide. Varying concentrations of acid and base were used to prepare solutions with pH values of 2, 5, 7, 9, and 11. The pH values of 2 and 11 were selected to simulate the extremely acidic and alkaline conditions.

Figure 2.
Rock samples.
2.2. Experimental Equipment
The slake durability apparatus is generally used for testing the disintegration of rocks. However, in this study, the fixed speed of the screen drum does not reveal the impact of varying scouring flow rates on rock disintegration. Furthermore, the continuous collisions between the rock samples and the screen drum during its rotation lead to rock sample damage, flaking, and deviation in test results. Therefore, a flow-rate-controlled experimental apparatus for scour disintegration (Figure 3) was designed to evaluate the disintegration characteristics of rocks under the influence of scouring. The experimental apparatus comprises a water reservoir, a flow rate controller, a disintegration tank, and a disintegrating particle collection box.
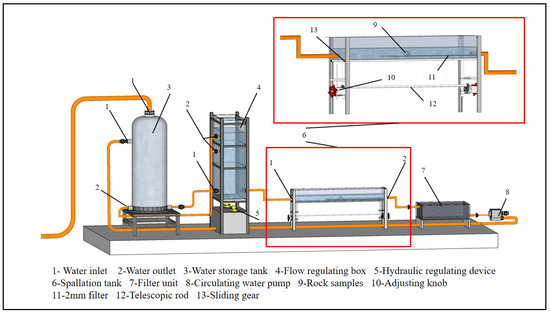
Figure 3.
Flow-rate-controlled scouring and disintegration test device.
The details of the experimental apparatus are as follows:
For an accurate simulation of the disintegration phenomenon of rock and soil masses on the slope surfaces, a disintegration test tank (Figure 3) was designed. The test tank is an open-top glass tank constructed using tempered glass, measuring 1920 mm in length, 240 mm in width, and 240 mm in height. The rock sample was positioned at the center of the test tank, maintaining a distance of 960 mm from the outlet as well as the inlet, which is over three times greater than the equivalent pipe diameter (i.e., 270.9 mm). This arrangement can prevent the rapid flow caused by the abrupt changes in the outlet and inlet cross-sections. Additionally, the rock sample was prepared in the form of a sphere 40 mm in diameter and placed at the center of the test tank’s cross-section. This placement helps to minimize the impact of boundary effects and water turbulence resulting from the angles on the surface of the rock sample.
The average flow velocity of the section located 100 mm in front of the rock sample was measured using an LS-300A flow velocity instrument. The measured flow velocity is multiplied by K (a flow-rate-increasing factor) to account for the reduction in the cross-sectional area caused by the scouring velocity of the rock sample. The expression for K is given by Equation (1):
where A1 is the cross-sectional area of water flow in the disintegration test tank and Ar is the cross-sectional area of the rock sample.
The water reservoir continuously supplies the water to the flow rate adjustment box until the end of the experimental run. This ensures a steady overflow of water from the top of the box to maintain a stable flow rate in the disintegration tank. By adjusting the height of the adjustment box, flow rates in the disintegration tank can be varied. The disintegrating particle collection box was employed to collect the particles resulting from the scouring and disintegration of rock samples.
2.3. Experimental Procedures
The test flow chart and test scheme used in this study are shown in Figure 4 and Table 3, respectively. After soaking the rock in the solution with different pH values, the experiment was carried out 10 times at different flow rates. In order to save space, only the test schemes with pH 2 and pH 11 are listed in Table 3, which is similar in other cases.
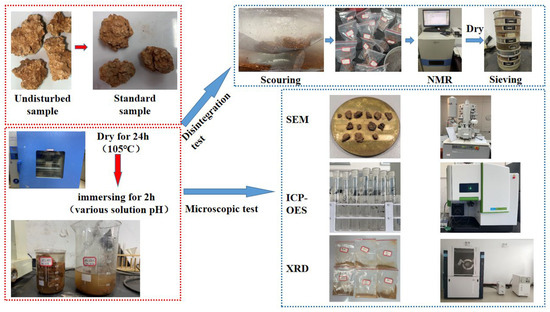
Figure 4.
The test procedure.

Table 3.
Experimental plan.
2.3.1. Disintegration Test
Adhering to the guidelines outlined in the “Rock Test Procedure for Water Conservancy and Hydropower Engineering” (SL/T264-2020), the individual test steps unfold as follows:
Firstly, the prepared rock samples were analyzed using the NMR method, and those with similar relaxation spectra and porosity were chosen for further testing. To minimize the impact of initial moisture content, each set of specimens was dried at 105 °C for 24 h before testing. Subsequently, the mass of each dried specimen was recorded after it cooled to a constant temperature. Next, specimens from each group were submerged in specific pH solutions for 2 h. Then, the specimen was placed in the disintegration tank and subjected to scouring at different flow rates for 2 h. Later, the disintegration residue samples and disintegrated particles were placed in an oven at 105 °C for 24 h. To minimize the test errors caused by mechanical disturbances, the specimen was cooled down to room temperature and manually sieved using standard sieves of sizes 60, 40, 20, 10, 5, and 2 mm. Finally, the weights of the sieved particles were measured, and the mass conservation method was applied to determine the weight of particles <2 mm.
The above steps were repeated for 10 cycles of tests for each group of specimens.
2.3.2. Microscopic Test
SEM analysis was performed on the gypsum karst breccia samples immersed in varying pH solutions for 2 h. Firstly, small pieces were extracted from the samples. Further, the relatively flat natural section was chosen as the observation surface, while the remaining surfaces were polished using sandpaper to obtain the desired size (approximately 2 mm in thickness). The surface debris was eliminated using a suction ball. To enhance the conductivity of the rock samples, a layer of gold film was applied to the surface of each test block before SEM analysis.
After the rock sample was dried for 24 h after soaking, a small piece was ground into particles using a 200-mesh particle size, and then XRD analysis was performed. The test equipment used was D8 Advance, and major configurations were as follows: the maximum power at 3 KW, maximum tube flow at 60 KV, and maximum tube pressure of the X-ray emitter at 80 mA.
The washed disintegration residue samples were saturated using a vacuum saturation device for 12 h. Subsequently, the disintegration residue samples were prepared for NMR analysis. This provides insights into the potential changes that may occur in T2 distribution and porosity. The AniMR-150 rock MRI imaging analysis system was used for NMR analysis.
The concentrations of metal ions leaching from the rock samples during the test were determined using an inductively coupled plasma optical emission spectrometer (ICP-OES). The procedure was as follows: 10 mL sample of the rock sample solution was collected for testing. The sample was then filtered using a 0.45 µm membrane. The test conditions were as follows: RF generator power of 1.3 kW, injection flow rate of 1.0 L/min, flushing time of 60 s, plasma gas flow rate of 10 L/min, atomizer flow rate of 1.0 L/min, auxiliary gas flow rate of 0.2 L/min, and axial observation direction.
3. Results and Discussion
3.1. Cumulative Relative Disintegration Volume
The disintegration of rocks leads to the evolution of gradation. To facilitate a comprehensive understanding of the disintegration characteristics of rocks, the particles with sizes less than 2 mm were considered disintegrated particles in rock disintegration tests. The disintegrated particles were collected at the end of each testing cycle, and their masses were measured and divided by the total mass of the specimen to calculate the relative amount of disintegration per cycle. Next, the relative disintegration amount per cycle obtained during the multi-cycle test was combined to determine the cumulative relative disintegration amount.
Figure 5 shows the cumulative relative disintegration amount of the gypsum karst breccia under varying flow rates and pH values. As shown in Figure 5, the cumulative relative disintegration amount increases with the increase in the flow rate and number of cycles, while it decreases with an increase in pH. Under the action of 1 m/s flow rate, when the pH value of the immersion solution was reduced from 7 to 2, the cumulative relative disintegration amount increased by 12.93%, more than twice that of pH 7, and when pH 7 increased to 11, the cumulative relative disintegration decreased by 16.99%. This observation indicates that the disintegration of the gypsum karst breccia is heightened following leaching with acidic solutions. Conversely, the disintegration of the gypsum karst breccia is subdued following leaching with alkaline solutions, both under the influence of the same flow rate. In this study, it was observed that when the flow rate reached a specific threshold, the variation in cumulative relative disintegration amount was negligible, thereby establishing a stable flow rate of disintegration. Additionally, it was also revealed that the stable flow rate of disintegration was strongly influenced by both the pH of the solution and the number of cycles.
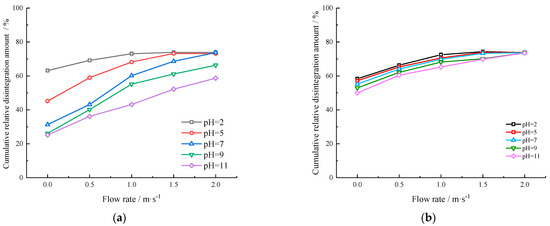
Figure 5.
Cumulative relative disintegration amount of the species in different pH of solution over different flow rates: (a) 1 cycle; (b) 10 cycles.
3.2. Stable Flow Rate of Disintegration
Figure 6 illustrates the relationship between the stable flow rates of disintegration and the pH values of the solution at various cycles, using the data from Figure 5. Figure 6 demonstrates that the stable flow rate of disintegration has a good correlation with the solution pH, indicating a decreasing trend with the increase in solution pH. In the first cycle, the disintegration stable flow rate at pH 11 is more than twice that at pH 7. The stable flow rate of disintegration was less sensitive to changes in the acidic conditions compared to the alkaline conditions. During the first cycle, when the pH value of the soaking solution was reduced from 7 to 2, the disintegration stable flow rate was only reduced by 54.26%. Combined with the change in cumulative relative disintegration amount, the above phenomenon can be interpreted as the change in pore structure and mineral composition in rock samples after acid corrosion, which leads to a decrease in the ability of particles to resist water erosion. On the contrary, after immersion in alkaline solution, the internal deterioration effect of rock samples was improved, and the ability of particles to resist water erosion was increased. In addition, the influence of solution pH on the stable flow rate of disintegration decreased with the increase in the number of cycles.
where A1, A2, x0, and dx are fitting parameters related to the pH values of the solution.
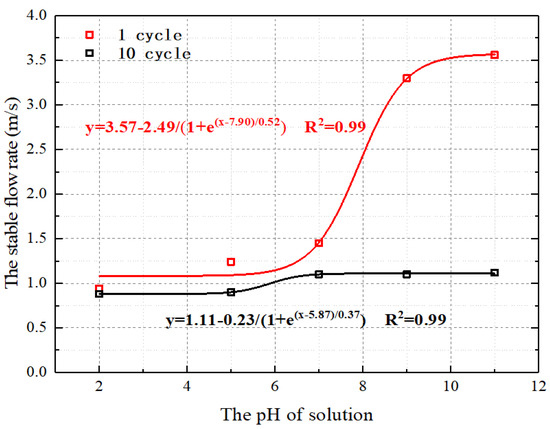
Figure 6.
Stable flow rate of disintegration in different pH values of the solution and different cycles.
3.3. NMR T2 Spectrum Distribution and Porosity
The presence of pores in rocks has an impact on rock disintegration characteristics. The variations in the solution pH could lead to changes in the rock pore structure and the strength of particle cementation. This phenomenon could explain the variations in the stable flow rate of disintegration observed at different pH values of the solution. Additionally, NMR analysis can provide insights into the changes in rock pore structure. The relaxation time and intensity of the NMR T2 relaxation spectrum are directly proportional to the pore size and the number of rock samples, respectively [38,39,40,41,42]. Therefore, the T2 spectrum distribution can effectively depict the rock pore structure. The relaxation times [0.01, 3], [3, 50], and [50, 10,000] correspond to small, medium, and large pores within the rock sample, respectively.
NMR analysis was conducted on the gypsum karst breccia following the disintegration test to obtain the T2 relaxation spectra (Figure 7), which revealed the following: first, in the acidic (or alkaline) environment, the T2 spectrum distribution of the samples transitions from three peaks to two peaks (or one peak) with the decrease (or increase) in pH of the solution. Second, at a constant flow rate in an acidic (or alkaline) environment, the intensity of the small pore peak increases from 58.3214 n/a to 84.6321 n/a (0 m/s), 61.3217 n/a to 93.3214 n/a (0.5 m/s), 76.3214 n/a to 96.8523 n/a (1 m/s), 48.6321 n/a to 87.5634 n/a (1.5 m/s), and 68.6354 n/a to 97.6325 n/a (2 m/s), respectively, while the peak strength of the medium and large pores decrease, and the relaxation time corresponding to the peak strength decreases. In contrast, the peak intensities of the medium and large pores decrease with the decrease (or increase) in the solution pH. Among them, the peak intensity in the middle pore region decreases by 32.86 n/a at most, while the peak intensity in the large pore region decreases to 0. In addition, the relaxation time corresponding to the peak intensity decreases. The reasons for the above phenomenon are as follows: due to the scouring effect of water flow, the particles around the macropores have low cementation strength and disintegrated the parent body, resulting in a significant reduction in the number of macropores in the rock sample, which is manifested as a decrease in signal strength in the relaxation spectrum. For medium pores, in addition to large pores, a small number of small pores develop into medium pores. Therefore, although the signal strength of medium pores decreases, the reduction is smaller than that of large pores. In an acidic environment, the corrosion effect of the solution further promotes the development of pores, and the number of medium and large pores in the interior decreases further after the same flow rate. In the alkaline environment, the secondary minerals generated by the chemical reaction fill the larger pores, so the signal strength of the medium and large pores is also reduced. Third, when the flow rate of the rock samples tends to be stable, no significant changes in the peak intensities, relaxation times, and peak patterns of the breccia specimens are observed. This indicates that the internal pores of the rock samples undergo minimal changes at stable flow rates.
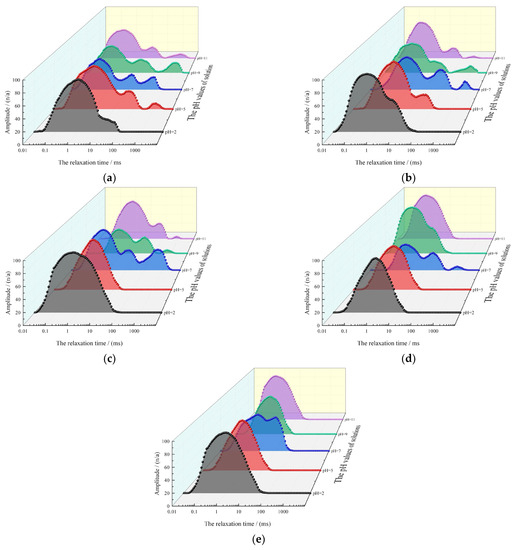
Figure 7.
The T2 relaxation spectrum of the gypsum karst breccia under different pH values of solutions and different flow rate: (a) 0 m/s; (b) 0.5 m/s; (c) 1 m/s; (d) 1.5 m/s; (e) 2 m/s.
As shown in Figure 8, NMR analysis reveals changes in the porosity of samples subjected to various chemical erosion processes and different scouring flow rates. The following observations can be drawn from Figure 8: in acidic conditions, the cumulative relative disintegration amount of rock samples increases, and porosity decreases with the decrease in solution pH. On the other hand, in alkaline conditions, the cumulative relative disintegration amount and porosity decrease with the increase in solution pH. When the flow rate of the rock samples tends to be stable, no significant changes in the cumulative relative disintegration amount and porosity are observed. These observations also indicate a strong correlation between the cumulative relative disintegration amount, the stable flow rate of rock samples, and the solution pH during scouring. Further, the mechanisms underlying rock disintegration in acidic and alkaline conditions are different.
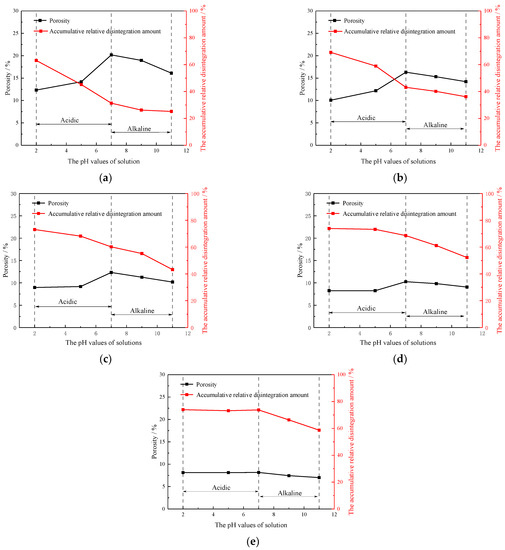
Figure 8.
The porosity and cumulative relative disintegration amount of the rocks under different the pH values of solution and different flow rates: (a) 0 m/s; (b) 0.5 m/s; (c) 1 m/s; (d) 1.5 m/s; (e) 2 m/s.
To delve deeper into understanding the correlation between the pH value of the soaking solution and the alteration in distinct pore sizes subsequent to rock sample disintegration due to scouring effects, the proportions of large, medium, and small pore volumes (Dmac, Dmes, and Dmic) in rock samples following disintegration under varying flow rates and solution pH are calculated using the following equations:
where Dmic, Dmes, and Dmac represent the proportions of small, medium, and large pore volumes within the rock samples; Vtoal, Vmic, Vmes, and Vmac indicate the total volume and the volumes of small, medium, and large pores within the rock samples, respectively.
Figure 9 shows the proportions of different size pore volumes within the rock samples under varying solution pH and flow rates, as calculated by Equations (3) and (4) and depicted in Figure 7, following the disintegration tests. It is revealed that firstly, at the same flow rate, Dmic increases; Dmes increases to a maximum value, and then decreases; and Dmac rapidly decreases to 0, when the solution pH decreases (or increases) in acidic (or alkaline) environments. Combined with the cumulative relative disintegration of the rock samples, this indicates that the disintegration primarily takes place in the macropores surrounding the particles. Secondly, when the flow rate of sample disintegration tends to be stable. Dmic and Dmes exhibit no significant changes and no large pores are present within the rock samples. The reason for the above phenomenon is consistent with the change in the relaxation spectrum of rock samples under the action of different flow rates and pH values.
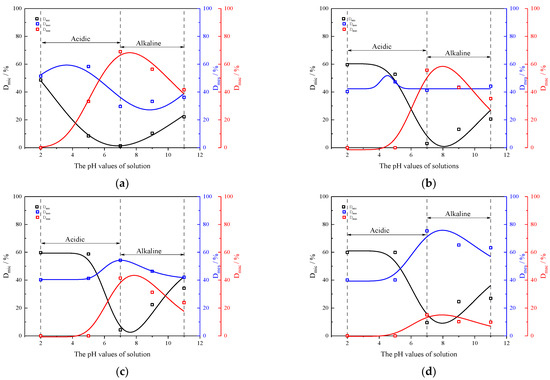
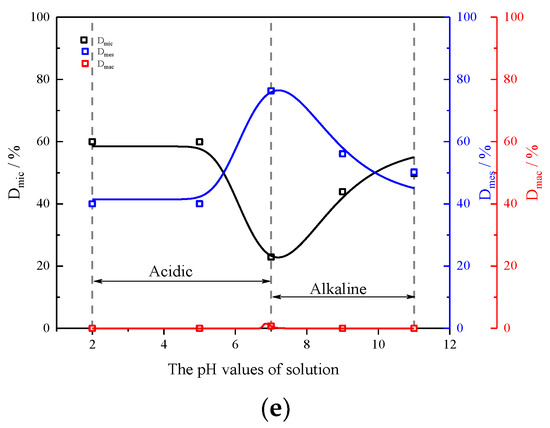
Figure 9.
The proportion of different pore volume inside rock samples with different pH values of solutions and different flow rates: (a) 0 m/s; (b) 0.5 m/s; (c) 1 m/s; (d) 1.5 m/s; (e) 2 m/s.
4. Microscopic Test
Previous studies show that changes in the microstructure of porous media such as rocks are an important cause of changes in their macroscopic mechanical properties [43,44]. The pH values of the solutions and the flow rates have a significant influence on the pore structure as well as the mineral composition and content of the rock samples. To elucidate the formation mechanisms behind the disintegration characteristics and the variations in the stable flow rate of disintegration of the gypsum karst breccia, multiple tests, including XRD, SEM, and ICP analyses, were performed on rock samples exposed to solutions of varying pH.
4.1. Characterization of Particle Structure
SEM analysis helps to observe the distinct changes in the structural characteristics of gypsum karst breccia particles following treatment with various pH solutions. Due to space constraints, only SEM images of the gypsum karst breccia particles treated with solutions of pH 2, 7, and 11 were included in this study (Figure 10).
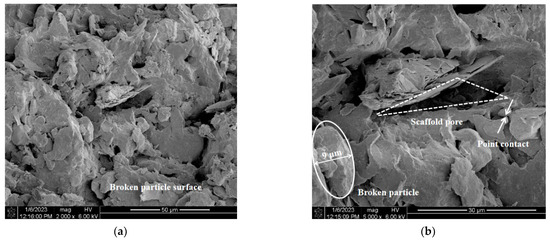
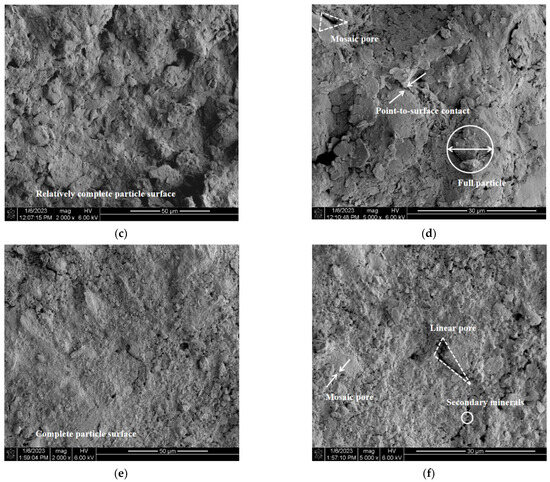
Figure 10.
Microstructure characteristics of mineral surface with different pH values of solutions: (a) pH 2, ×2000; (b) pH 2, ×5000; (c) pH 7, ×2000 ×; (d) pH 9, ×5000; (e) pH 11, ×2000; (f) pH 11, ×5000.
This study analyzed the particle structural characteristics of the rock samples from four perspectives: particle morphology, cementing material, intergranular contact mode, and pore structure. Following treatment with a pH 7 solution, the particle morphology of the rock specimens appeared to be well-preserved, with complete and smooth surfaces, as shown in the figure. The grains contained a small amount of cementing material, primarily exhibiting point-to-face bonding, while the pore structure exhibited a combination of scaffolding and mosaic structures. The particles in the bracket structure exhibit low cementation strength, making them susceptible to peeling off under the influence of scouring, thereby intensifying the disintegration of the rock samples. After the rock samples were immersed in pH 2 solution, a large amount of cementing material dissolved, the original dense structure in the visual area became loose, and the proportion of point–plane contact between grains increased. At the same time, the corrosion effect of soil structure appears, which is intuitively manifested as the boundary between pores and particles in the visual field is no longer clear, the surface of skeleton particles becomes broken and disorderly, and the proportion of scaffold pores increases significantly, leading to the disintegration of rock samples. After treatment with pH 11 solution, the microstructure of the rock samples appeared relatively intact, with a higher density when compared with the rock samples treated with pH 2 and pH 7 solutions. There was a decrease in the proportion of the support structure alongside the loss of cementing material. In addition, a significant number of suspended particles were visible on the surface of the rock samples, while the pores within the support structure were filled with secondary cementing materials. This helps in minimizing the localized damage to the rock samples, thereby enhancing their integrity and bonding strength.
Furthermore, it can be inferred that the treatment of the rock samples with an acidic solution leads to an increase in the number of internal support pores and a decrease in the bonding strength between particles. These changes can be attributed to the corrosion and deterioration of the rock structure. As a result, at the same flow rates, there was an intensification of the rock sample disintegration, resulting in a reduced stable flow rate of disintegration. However, there was a decrease in the proportion of internal support pores and mosaic pores due to the enhancement of the secondary structure. This resulted in an increased cementation strength between the particles. As a result, the disintegration of rock samples caused by alkali contamination is reduced, and the stable flow rate of disintegration increases.
4.2. XRD and ICP Test
It is imperative to consider the impact of variations in mineral composition and content within the gypsum karst breccia on the disintegration characteristics, stable flow rate of disintegration, and microstructure. Therefore, the rock samples subjected to different pH treatments were analyzed using XRD analysis, and the corresponding results are presented in Figure 11. Examination of data in Figure 11 reveals that the treatment with an acidic solution leads to a significant decrease in the content of calcite and clay minerals (illite and kaolinite), while the quartz content shows a significant increase. When the pH value of the soaking solution is reduced from 7 to 2, the content of calcite decreases from 15.3% to 5.4%, the content of clay mineral decreases from 44.3% to 24.1%, and the content of quartz increases from 28.9% to 41.2%. Further, this trend became more pronounced with the decrease in the pH of the solution. Subsequent treatment of the rock samples with an alkaline solution resulted in a slight increase in the calcite content, while the content of quartz and clay minerals exhibited a slight decrease. When the pH value of soaking solution increased from 7 to 11, calcite content increased from 15.3% to 19.2%, the content of quartz decreased from 28.9% to 18%, and clay minerals decreased from 44.3% to 35.5%. Furthermore, this trend became more apparent at higher pH values of the solution.
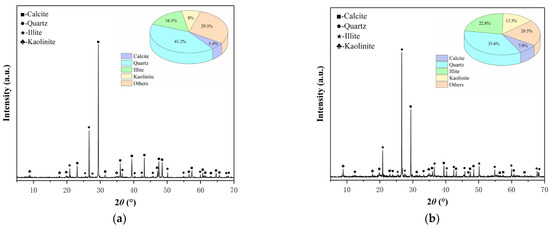
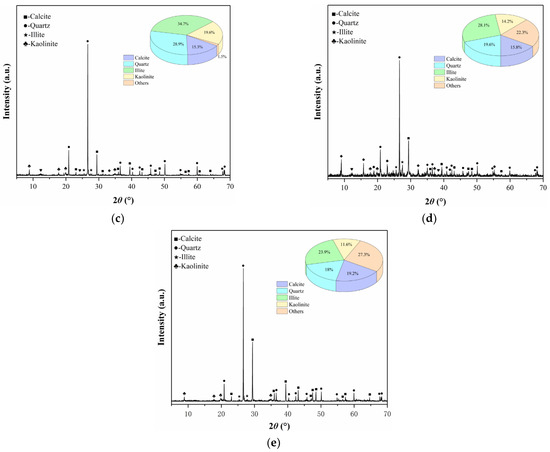
Figure 11.
X-ray diffraction spectrum of the rock samples with different pH values of solutions: (a) pH 2; (b) pH 5; (c) pH 7; (d) pH 9; (e) pH 11.
To gain better insights into the variations in mineral composition in the gypsum karst breccia subjected to different conditions, ICP analysis of solutions obtained after immersing the rock samples was performed in this study. The results are presented in Figure 12. The data in Figure 12 reveal the following observations: the treatment of rock samples with an acidic solution leads to a decrease in the release of Na+ ions in the solution, while the release of Al3+ and Mg2+ ions slightly increases. When the pH value of the soaking solution was reduced from 7 to 2, the release of the above ions ranged from 9.24 mg/L to 0.09 mg/L, 6.25 mg/L to 16.35 mg/L, and 0.813 mg/L to 3.79 mg/L, respectively. In addition, there was a significant increase in the release of Ca2+; in a pH 2 solution, Ca2+ is released about seven times as much as in a pH 7 solution. These trends became more pronounced with the decrease in the solution pH. On the other hand, following the treatment of the specimens in an alkaline solution, there was a slight decrease in the release of Na+ and Mg2+ ions in the solution, a significant decrease in the release of Ca2+ ions, and a slight increase in the content of Al3+ ions. When the pH value of the soaking solution increased from 7 to 11, Ca2+ decreased by 14.602 mg/L, and Al3+ ion increased by 1.71 mg/L. Furthermore, these trends became significant with the increase in the solution pH. The results of cation release from the immersion solutions at different pH levels were consistent with the changes observed in the mineral content in the rock samples.
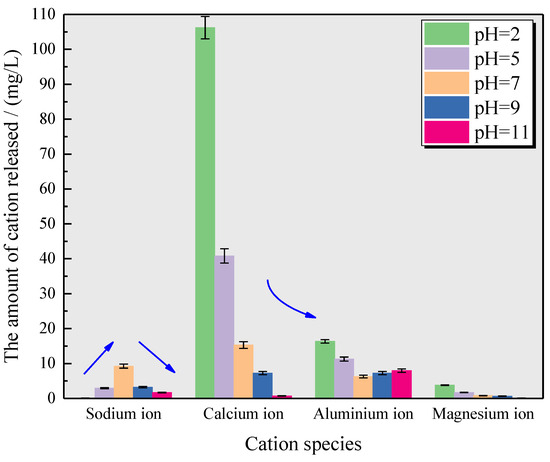
Figure 12.
Cations released in the immersing solution with different pH values of solutions.
The major reasons for the above-mentioned phenomenon can be explained as follows: in an acidic solution, a series of hydrolysis reactions occur within the gypsum karst breccia when it comes in contact with H+ ions, resulting in the dissolution of calcite, illite, and kaolinite present in the rock samples. This leads to the displacement of cations (Ca2+, Al3+, and Mg2+), thereby increasing the release of these ions into the solution. In alkaline solution, Ca2+ and Mg3+ inside the rock samples react chemically with OH– ions in the solution to form Ca(OH)2 and Mg(OH)2 precipitates, leading to a decrease in the release of Ca2+ and Mg2+ in the solution, and these newborn mucilaginous gels or flocs flow together with fine particles wrapped around them due to the Brown effect and fill the surrounding pores, and then form a stronger linkage with stronger contact points after embedded hardening. After embedding and hardening, a stronger linkage is formed, which directly strengthens the inter-particle cohesion and inter-particle friction. In addition, Ca2+ reacts chemically with OH– and CO2 to form CaCO3, which fills the internal pores of the rock samples, leading to an increase in calcite content and an improvement in the microstructure. At the same time, quartz is hydrolyzed under strong alkaline environment, which is the main reason for the decrease in quartz content and the increase in Al3+ release in the solution. Not only that, NaOH meeting CO2 produces Na2CO3, and with a slight change in temperature and humidity, it forms high water compounds and bring a certain expansion pressure, therefore, after soaking the NaOH solution, the sample, after coming into contact with the air for a few moments, develops a few white crystals on the surface. This shows that the potential reason for the disintegration inhibition and the increase in disintegration stabilization flow rate of the rock samples under alkaline environment should be that the improvement effect of the nascent structure inside the rock samples is stronger than the damage effect of the initial structure.
5. Mechanisms Underlying the Formation of Scouring and Disintegration Properties of Rock Samples
The pores within the rock samples facilitate the passage of the solution through the interior of the rock, where it undergoes physicochemical reactions that lead to changes in the mechanical parameters, including intergranular cementation.
Table 2 shows a significant presence of hydrophilic clay minerals and water-soluble mud cement within the interior of the gypsum karst breccia. The treatment of the rock samples with an acid solution resulted in the dissolution of calcite and clay minerals in the rock samples (Figure 11 and Figure 12), resulting in the deterioration of microstructural integrity (Figure 10) as well as a decrease in particle cohesion. Macroscopically, it was evident that the cumulative relative disintegration of the rock samples increased with a decrease in the pH of the acidic solution while the flow rate remained constant.
Upon treating the gypsum karst breccia in an alkaline solution, two key reactions were observed. Firstly, the dissolution effect led to the dissolution of clay minerals and quartz particles in the rock samples. Secondly, the reaction of Ca2+, Mg2+, and other ions with OH– led to the formation of precipitates that occupied the pores surrounding the rock, leading to a secondary structural improvement effect and the reduction in localized damages in the rock samples (Figure 11 and Figure 12). The SEM analysis of the rock samples after treatment with an alkaline solution reveals several findings: firstly, the improvement in the nascent structure outweighs the deterioration of the rock structure, resulting in enhanced microstructural integrity (Figure 10). Secondly, the cohesion between particles is enhanced. Macroscopically, the cumulative relative disintegration of the rock decreases with an increase in the pH of the alkaline solution at the same flow rate. Additionally, the reaction between NaOH and CO2 produces Na2CO3. Due to a slight variation in temperature and humidity, the highly hydrous compound Na2CO3·10H2O is formed, which adheres to the surface layer of the rock sample, as shown in Figure 10.
The process of rock disintegration due to scouring is significantly affected by the shear strength among constituent particles and the shear force exerted by water. The particle-induced shear force resulting from water scouring can be estimated using Equation (6):
In this study, the following parameters were considered as constants under constant conditions of rock samples and the scouring test environment: KS indicates particle surface roughness, χ is a function associated with the particle surface roughness, and C2 represents a parameter associated with resistance to the water flow in the opposite direction. The disintegrated particles collected and analyzed in this study have a diameter (yd) smaller than 2 mm. Therefore, for subsequent analysis, these parameters were considered as constants.
The shear strength between rock particles is estimated using the M-C criterion as follows:
where and indicate the normal stress and cementation force between particles, respectively, and s the friction coefficient between the particles. In the surface geotechnical body of the slope, where there are no additional stresses, the normal stress Qp between particles can be assumed as negligible and excluded from calculations. Therefore, the shear strength responsible for resistance to particle disintegration can be primarily attributed to the inter-particle cementation strength Cp. When the cement type remains consistent, the cementation strength Cp remains constant, and the inter-particle cementation force is determined by the cementation area.
Given the above, the disintegration characteristics of gypsum karst breccias under scouring, the effects of immersion treatment with solutions of different pH values, and the underlying mechanisms that influence the stable flow rate of disintegration are provided below.
(1) The particles surrounding the large pores of the rock samples exhibit minimal cementation area and the lowest resistance to shear disintegration. Therefore, the disintegration of the gypsum karst breccia predominantly occurs in these particle regions. As shown in Figure 8, the cumulative relative disintegration of the rock samples decreases rapidly with a decrease in the volume of the large pores;
(2) As disintegration progresses, the macropores undergo a significant reduction, while the cementation area between residual particles and the surrounding particles on the rock sample surface significantly increases when compared to the particles surrounding the macropores. Hence, the cementation force of particles increases correspondingly. The process of disintegration ceases when this force becomes equivalent to the shear force exerted by water flow, indicating the attainment of the stable flow rate of disintegration;
(3) In acidic solutions, a decrease in solution pH increases the corrosion effect, leading to the pore space expansion and facilitating the penetration of the solution into the interior regions of the rock sample. This intensifies the water–rock interactions. Therefore, the decrease in pH of the soaking solution leads to an increase in the pores within the rock samples, a decrease in the cement strength between particles, and a weakening of the resistance to disintegration and water scouring. As evident in Figure 5 and Figure 6, a decrease in solution pH under acidic conditions is associated with an increase in cumulative relative disintegration as well as a decrease in the stable flow rate of disintegration;
(4) In alkaline solutions, with the increase in the solution pH, the positive impact of the nascent structure becomes more significant than the deteriorating effect. This results in an improved microstructure of the rock samples, a reduction in the pore volume, an increase in the cementation strength between particles, and enhanced resistance to water-flushing shear forces. As shown in Figure 5 and Figure 6, the cumulative relative disintegration decreases, and the stable flow rate of disintegration increases with an increase in solution pH under alkaline conditions.
In the design of practical engineering disaster prevention measures, to consider the influence of different influencing factors on the strength of the gypsum karst breccia layer, we should multiply the corresponding reduction coefficient based on obtaining the strength index of rock. However, at present, the specific value of the reduction coefficient cannot be determined because the relationship between the pH value and the flow rate of the solution and the disintegration degree of gypsum karst breccia is only obtained in this paper, and the strength value of the rock with different disintegration degrees is not studied. This paper is an exploratory experiment aimed at highlighting the effect of acid rain on rock disintegration behavior. Later, the author will establish the corresponding relationship between the disintegration degree of rock and its strength. Combined with the results of this paper, the strength reduction coefficient of rock under different pH acid rain is proposed to provide a theoretical reference for engineering design. At the same time, due to the limitation of test space, the test device in this paper can provide a maximum flow rate of 3 m/s, and it is necessary to change the test device when studying the influence of higher flow rates on rock disintegration.
6. Conclusions
In this paper, a self-designed disintegration test device was used to carry out disintegration tests at different flow rates for different numbers of cycles on the gypsum karst breccia soaked in different pH solutions, and the pore changes of the disintegrated samples and the changes in the microstructure and mineral composition of the soaked samples were investigated with the help of NMR, SEM, XRD, and ICP-OES, which led to the following main conclusions:
(1) The cumulative relative disintegration of the gypsum karst breccia increases with the decrease in solution pH. In addition, it increases with an increase in the number of cycles and the flow rate. When the flow rate reaches a certain threshold, the amount of cumulative relative disintegration does not exhibit a significant change (about 73%), indicating the attainment of a stable flow rate of disintegration. This stable flow rate of disintegration demonstrates a good correlation with the pH of the solution (or the number of cycles);
(2) Under acidic conditions (or alkaline conditions), the decrease (or increase) in the solution pH leads to a significant increase in the proportion of small pore volume within the gypsum karst breccia (maximum increase of 61.33%). The proportion of medium pore volume increases to a maximum value and then decreases, while the proportion of large pore volume decreases rapidly to zero. Upon reaching the flow rate associated with disintegration stabilization, the ratios of small and medium pore volumes reach a state of equilibrium. However, the proportion of large pore volume remains consistently at 0. Therefore, disintegration of gypsum karst breccia predominantly occurs in proximity to large pores;
(3) Under acidic conditions, a decrease in solution pH results in the dissolution of clay minerals and calcite within the rock samples. This leads to a decrease in the release of Na+, a slight increase in the release of Al3+ and Mg2+, and a significant increase in the release of Ca2+ in the solution (maximum increase of 90.89 mg/L). The rock samples exhibit noticeable deterioration due to corrosion, resulting in surface damage and increased pore space of the scaffolds. Therefore, the corrosion effect in an acidic environment is the main reason for the deterioration of rock disintegration and the decrease in the stable flow rate of disintegration;
(4) Under alkaline conditions, the increase in the solution pH decreases the content of clay minerals and quartz, while increasing the calcite content. Moreover, the release of Na+ and Ma2+ from the solution slightly decreases, the release of Ca2+ significantly decreases (reduced by 95.4%), and the release of Al3+ slightly increases. In addition, the surface of the rock samples remains intact, and there is a decrease in the pore space of the scaffold. Therefore, in alkaline environment, the improvement effect of the new structure is stronger than the corrosion effect, which is the main reason to restrain the disintegration of rock sample and increase the stable flow rate of disintegration;
(5) The influence of pH value of solution and flow velocity on rock disintegration characteristics is essentially caused by the dynamic change of relative proportion of large pore volume in rock samples.
Author Contributions
Conceptualization, X.H., C.L. and C.W.; methodology, X.H. and C.L.; formal analysis, X.H.; resources, C.L.; data curation, X.H.; writing—original draft preparation, X.H.; writing—review and editing, C.L., X.Z. and Z.W.; project administration, C.L. and X.Z.; funding acquisition, C.L. and X.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China (41272300), the Major Projects of Scientific and Technological Innovation of CRTG (2018-53), and Natural Science Foundation of Fujian Province (2020J05133).
Data Availability Statement
The original contributions proposed in the study are included in the article; any further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare that they have no conflict of interest to report regarding the present study.
References
- Lee, Y.F.; Chi, Y.Y. Rainfall-induced landslide risk at Lushan, Taiwan. Eng. Geol. 2011, 123, 113–121. [Google Scholar] [CrossRef]
- Li, P.; Qian, H.; Wu, J. Environment: Accelerate research on land creation. Nature 2014, 510, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Pu, W.; Zhang, X.; Xu, J.; Zhao, X.; Xu, X.; Dong, F.; Yu, B. Characteristics and influencing factors of acid rain in Beijing. J. Appl. Meteorol. 2010, 21, 464–472. [Google Scholar] [CrossRef]
- Pan, H.; Li, T.; Wu, B.; Ren, Y.; Song, T. Centrifugal model test of large-scale landslide stability of curved sliding surface under rainfall conditions. Chin. J. Geotech. Eng. 2016, 38, 696–704. [Google Scholar] [CrossRef]
- Yavuz, H. Effect of freeze–thaw and thermal shock weathering on the physical and mechanical properties of an andesite stone. Bull. Eng. Geol. Environ. 2011, 70, 187–192. [Google Scholar] [CrossRef]
- Liang, B.; Tan, X.; Jiang, L.; Jiao, B. Experimental study on the influence of freeze-thaw and dry-wet cycles on the disintegration characteristics of argillous rock. J. Geotech. Eng. 2016, 38, 705–711. [Google Scholar] [CrossRef]
- Ghobadi, M.H.; Mousavi, S. The effect of pH and salty solutions on durability of sandstones of the Aghajari Formation in Khouzestan province, southwest of Iran. Arab. J. Geosci. 2014, 7, 641–653. [Google Scholar] [CrossRef]
- Vergara, M.R.; Triantafyllidis, T. Swelling behavior of volcanic rocks under cyclic wetting and drying. Int. J. Rock Mech. Min. Sci. 2015, 80, 231–240. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, Q.; Hu, Z. Effects of rainwater softening on red mudstone of deep-seated landslide, Southwest China. Eng. Geol. 2016, 204, 1–13. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, W.; Huang, J.; Ouyang, S.; Zhang, Z. Swelling characteristics of red sandstone under cyclic wetting and drying. Geotech. Geol. Eng. 2020, 38, 4289–4306. [Google Scholar] [CrossRef]
- Yan, L.; Liu, P.; Peng, H.; Kašanin-Grubin, M.; Lin, K. Laboratory study of the effect of temperature difference on the disintegration of redbed softrock. Phys. Geogr. 2019, 40, 149–163. [Google Scholar] [CrossRef]
- Erguler, Z.A.; Shakoor, A. Relative contribution of various climatic processes in disintegration of clay-bearing rocks. Eng. Geol. 2009, 108, 36–42. [Google Scholar] [CrossRef]
- Sarman, R.; Shakoor, A.; Palmer, D.F. A multiple regression approach to predict swelling in mudrocks. Bull. Assoc. Eng. Geol. 1994, 31, 107–121. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, A.; Liu, G. Laboratory investigation of disintegration characteristics of purple mudstone under different hydrothermal conditions. J. Mt. Sci. 2012, 9, 127–136. [Google Scholar] [CrossRef]
- Zhu, J.; Deng, H. Durability classification of red beds rocks in central Yunnan based on particle size distribution and slaking procedure. J. Mt. Sci. 2019, 16, 714–724. [Google Scholar] [CrossRef]
- Wu, D.; Liu, H.; Wang, G. Laboratory experimental study on collapsibility of red bed soft rock. Chin. J. Rock Mech. Eng. 2010, 29, 173–4179. [Google Scholar]
- Zeng, L.; Zhang, H.; Qi, S.; Liu, J. Experimental study of calving characteristics of carbonaceous mudstone based on energy dissipation principle. J. Cent. South Univ. Nat. Sci. Ed. 2021, 52, 4181–4189. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, L.; Yang, Q.; Wang, Z.; Cheng, A.; Mo, L.; Yan, J. Permeability and disintegration characteristics of composite improved phyllite soil by red clay and cement. Minerals 2022, 13, 32. [Google Scholar] [CrossRef]
- Flegner, P.; Kačur, J.; Durdán, M.; Leššo, I.; Laciak, M. Measurement and processing of vibro-acoustic signal from the process of rock disintegration by rotary drilling. Measurement 2014, 56, 178–193. [Google Scholar] [CrossRef]
- Flegner, P.; Kačur, J.; Frančáková, R.; Durdán, M.; Laciak, M. Application of Cluster Analysis for Classification of Vibration Signals from Drilling Stand Aggregates. Appl. Sci. 2023, 13, 6337. [Google Scholar] [CrossRef]
- Flegner, P.; Kačur, J.; Durdán, M.; Laciak, M. Statistical Process Control Charts Applied to Rock Disintegration Quality Improvement. Appl. Sci. 2020, 10, 8343. [Google Scholar] [CrossRef]
- Erguler, Z.A.; Ulusay, R. Assessment of physical disintegration characteristics of clay-bearing rocks: Disintegration index test and a new durability classification chart. Eng. Geol. 2009, 105, 11–19. [Google Scholar] [CrossRef]
- Knapp, S.; Krautblatter, M. Conceptual framework of energy dissipation during disintegration in rock avalanches. Front. Earth Sci. 2020, 8, 263. [Google Scholar] [CrossRef]
- Hu, Q.; Xu, Y.; Deng, X.; Hu, S.; Xu, J.; Zhou, F.; Chi, R.A. Effect of potassium salt on swelling of halloysite clay mineral during leaching process of ionic rare earth ore. Minerals 2023, 13, 906. [Google Scholar] [CrossRef]
- Wakim, J.; Hadj-Hassen, F.; De Windt, L. Effect of aqueous solution chemistry on the swelling and shrinkage of the Tournemire shale. Int. J. Rock Mech. Min. Sci. 2009, 46, 1378–1382. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, C.; Deng, L.; Liu, G. Impacts of simulated acid solution on the disintegration and cation release of purple rock (mudstone) in Southwest China. Geomorphology 2018, 316, 35–43. [Google Scholar] [CrossRef]
- Li, K.; Zhao, X.; Xiao, D.; Li, J. Study on disintegration mechanism of silty mudstone aggravated by chemical damage of acid rain. Rock Soil Mech. 2020, 41, 2693–2702. [Google Scholar] [CrossRef]
- Deng, T.; Huang, M.; Zhan, J. Fractal evolution of clay-type rock collapse process under different pH environments. J. Tongji Univ. Nat. Sci. Ed. 2014, 42, 1480–1485. [Google Scholar] [CrossRef]
- Huang, M.; Zhan, J. Disintegration test and energy dissipation characteristics of soft rock in acid-base solution environment. Rock Soil Mech. 2015, 36, 2607–2612. [Google Scholar] [CrossRef]
- Zhang, Z.T.; Gao, W.H. Effect of different test methods on the disintegration behaviour of soft rock and the evolution model of disintegration breakage under cyclic wetting and drying. Eng. Geol. 2020, 279, 105888. [Google Scholar] [CrossRef]
- Zhang, Z.T.; Gao, W.H.; Wang, X.; Zhang, J.Q.; Tang, X.Y. Degradation-induced evolution of particle roundness and its effect on the shear behaviour of railway ballast. Transp. Geotech. 2020, 24, 100388. [Google Scholar] [CrossRef]
- Zhang, Z.T.; Gao, W.H.; Zeng, C.F.; Tang, X.Y.; Wu, J. Evolution of the disintegration breakage of red-bed soft rock using a logistic regression model. Transp. Geotech. 2020, 24, 100382. [Google Scholar] [CrossRef]
- Zhao, G.; Visser, P.J.; Peeters, P.; Vrijling, J.K. Headcut migration prediction of the cohesive embankment breach. Eng. Geol. 2013, 164, 18–25. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Y.; Zhan, Q.; Wang, S. Critical starting of slope soil under thin layer water erosion condition. J. Civil Environ. Eng. 2023, 1–11. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, C.; Song, P.; Zhu, H.; Ma, D. Rain erosion test and three-dimensional particle flow fluid-solid coupling simulation on steep slope of loess. Rock Soil Mech. 2014, 35, 977–985. [Google Scholar] [CrossRef]
- Zuo, L.; Roelvink, D.; Lu, Y.; Li, S. On incipient motion of silt-sand under combined action of waves and currents. Appl. Ocean Res. 2017, 69, 116–125. [Google Scholar] [CrossRef]
- ASTM D4644-87; Standard Test Method for Slake Durability of Shales and Similar Weak Rocks. ASTM: West Conshohocken, PA, USA, 1998.
- Li, X.; Liu, Z.; Feng, X.; Zhang, H.; Feng, J. Effects of acid sulfate and chloride ion on the pore structure and mechanical properties of sandstone under dynamic loading. Rock Mech. Rock Eng. 2021, 54, 6105–6121. [Google Scholar] [CrossRef]
- Roy, D.G.; Singh, T.N.; Kodikara, J.; Das, R. Effect of water saturation on the fracture and mechanical properties of sedimentary rocks. Rock Mech. Rock Eng. 2017, 50, 2585–2600. [Google Scholar] [CrossRef]
- Li, H.; Zhong, Z.; Liu, X.; Sheng, Y.; Yang, D. Micro-damage evolution and macro-mechanical property degradation of limestone due to chemical effects. Int. J. Rock Mech. Min. Sci. 2018, 110, 257–265. [Google Scholar] [CrossRef]
- Li, J.; Zhou, K.; Liu, W.; Deng, H. NMR research on deterioration characteristics of microscopic structure of sandstones in freeze–thaw cycles. Trans. Nonferr. Metals Soc. China 2016, 26, 2997–3003. [Google Scholar] [CrossRef]
- Xu, J.; Zhai, C.; Qin, L.; Wu, S.; Sun, Y.; Dong, R. Characteristics of pores under the influence of cyclic cryogenic liquid carbon dioxide using low-field nuclear magnetic resonance. Geofluids 2018, 2018, 1682125. [Google Scholar] [CrossRef]
- Abd, S.M.; Mhaimeed, I.S.; Tayeh, B.A.; Najm, H.M.; Qaidi, S. Investigation of the use of textile carbon yarns as sustainable shear reinforcement in concrete beams. Case Stud. Constr. Mater. 2023, 18, e01765. [Google Scholar] [CrossRef]
- Tayeh, B.A.; Akeed, M.H.; Qaidi, S.; Bakar, B.H.A. Ultra-high-performance concrete: Impacts of steel fibre shape and content on flowability, compressive strength and modulus of rupture. Case Stud. Constr. Mater. 2022, 17, e01615. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).