The REE-Zr-U-Th Minerals of the Maronia Monzodiorite, N. Greece: Implications on the Saturation and Segregation Mechanisms of Critical Metals in Intermediate–Mafic Compositions
Abstract
1. Introduction
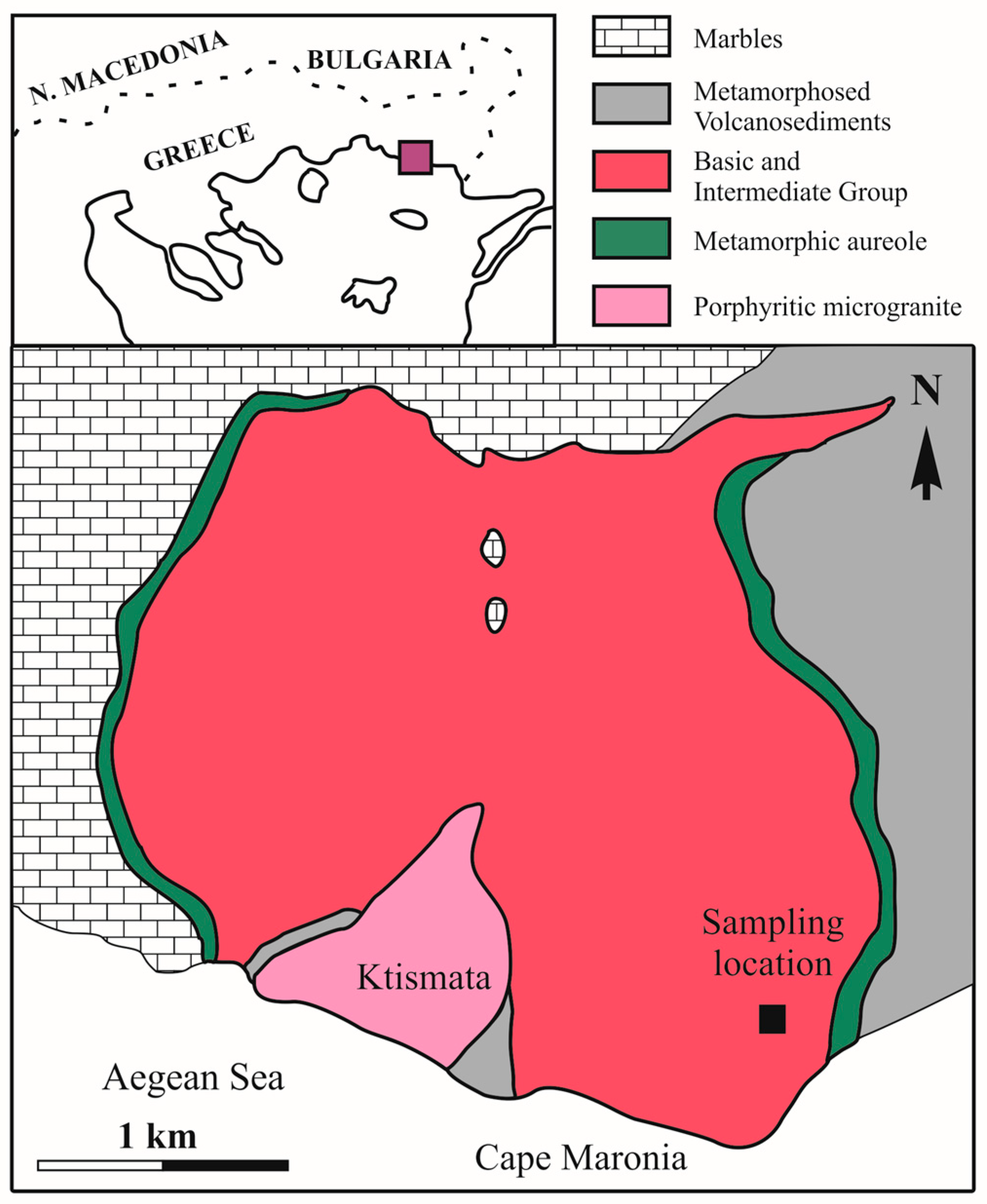
2. Geological Setting
2.1. Regional Geology
2.2. Geology of Maronia Pluton
3. Materials and Methods
4. Results
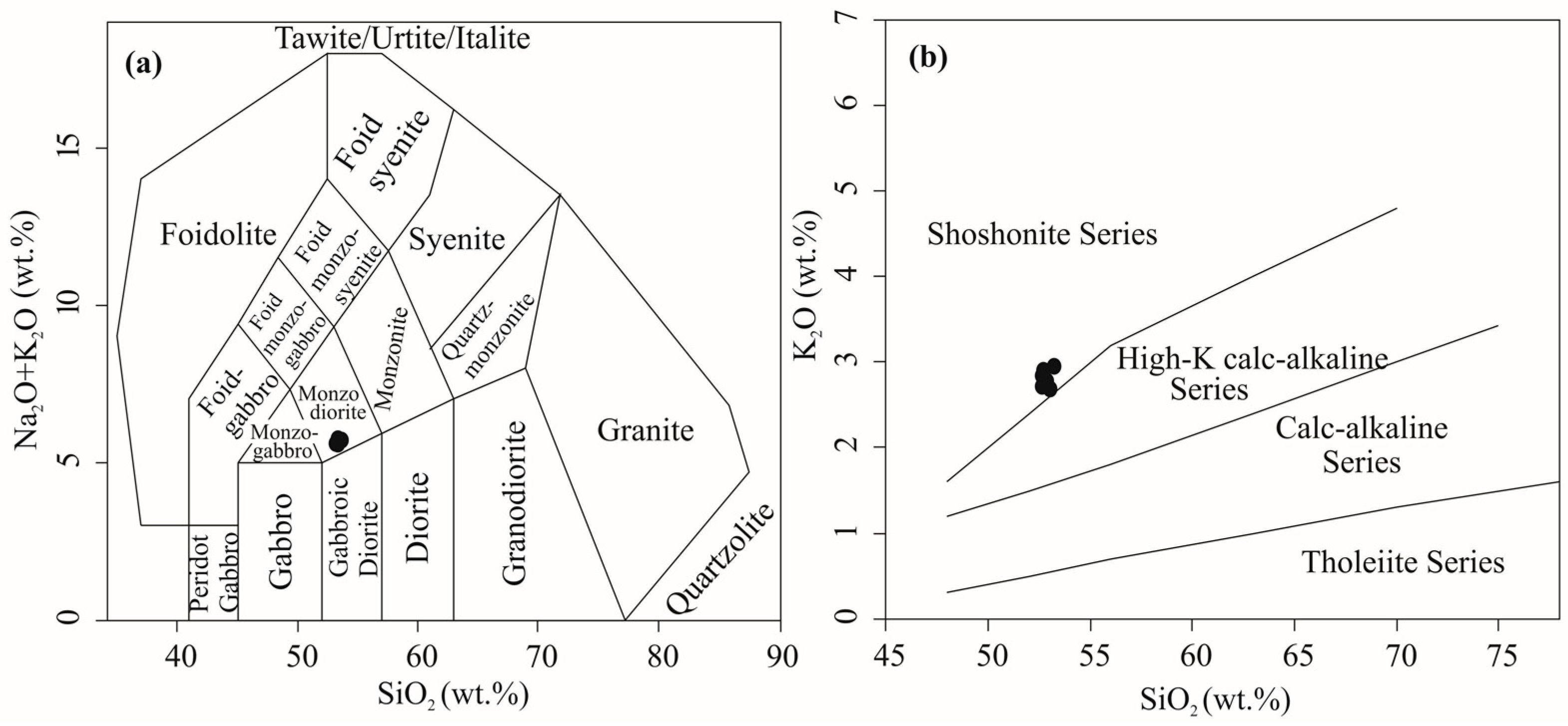
4.1. Whole-Rock Geochemistry
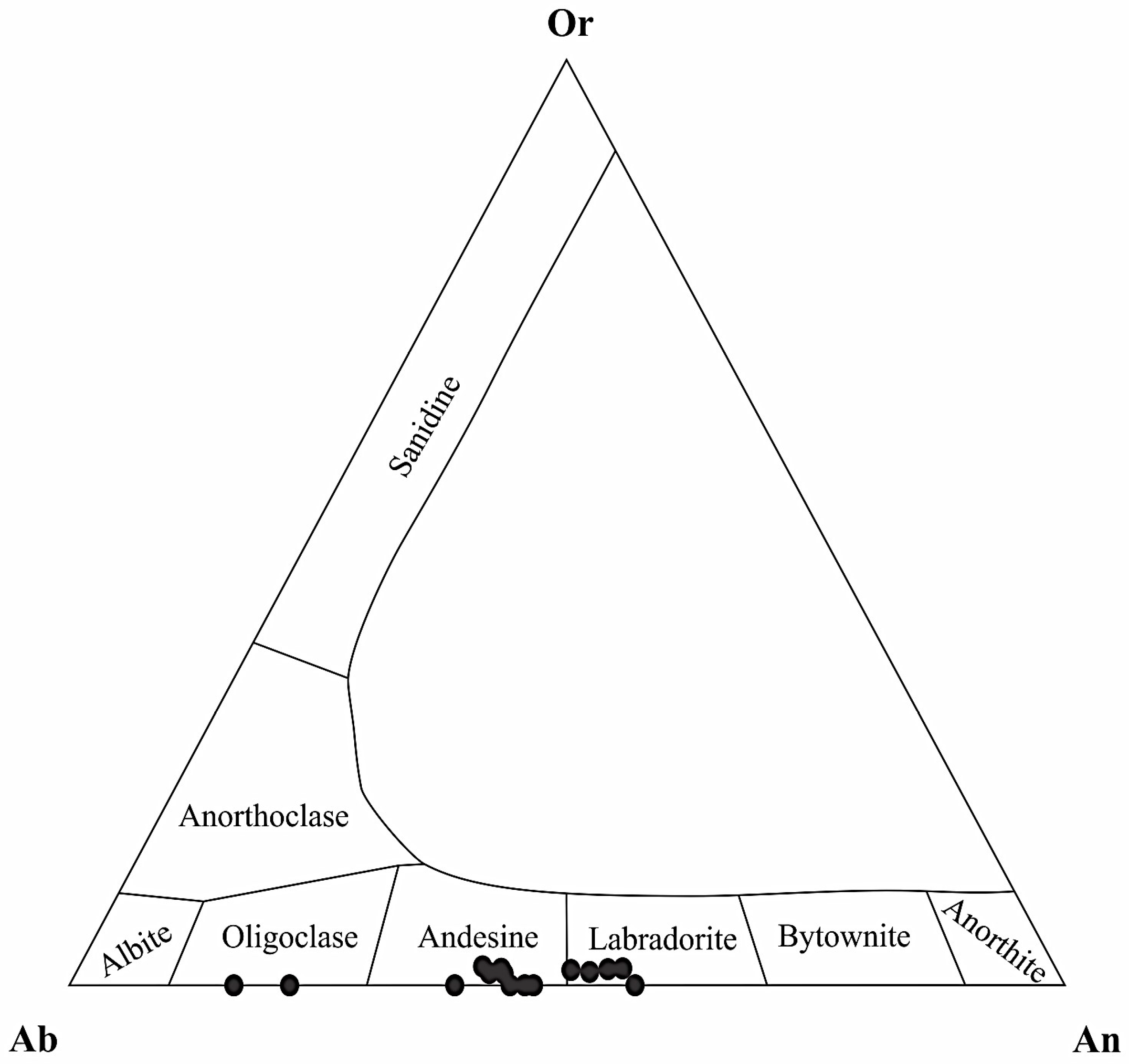
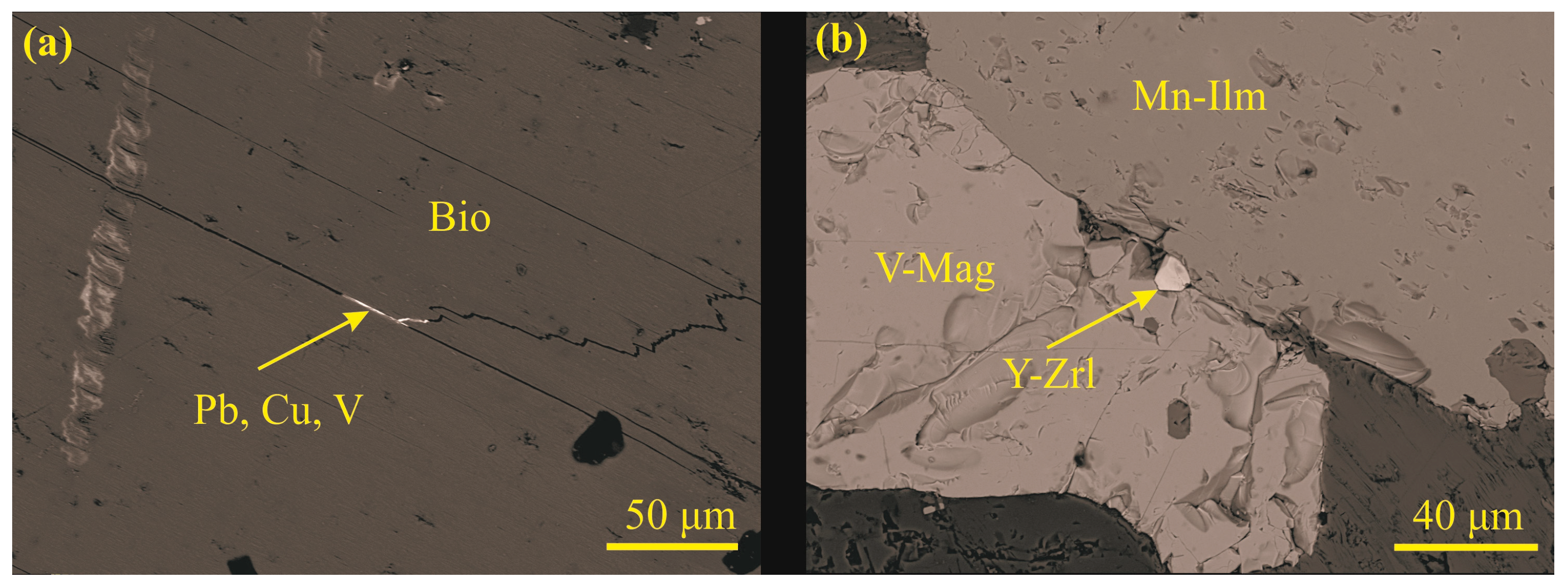
4.2. Main Mineralogy of Maronia Monzodiorite
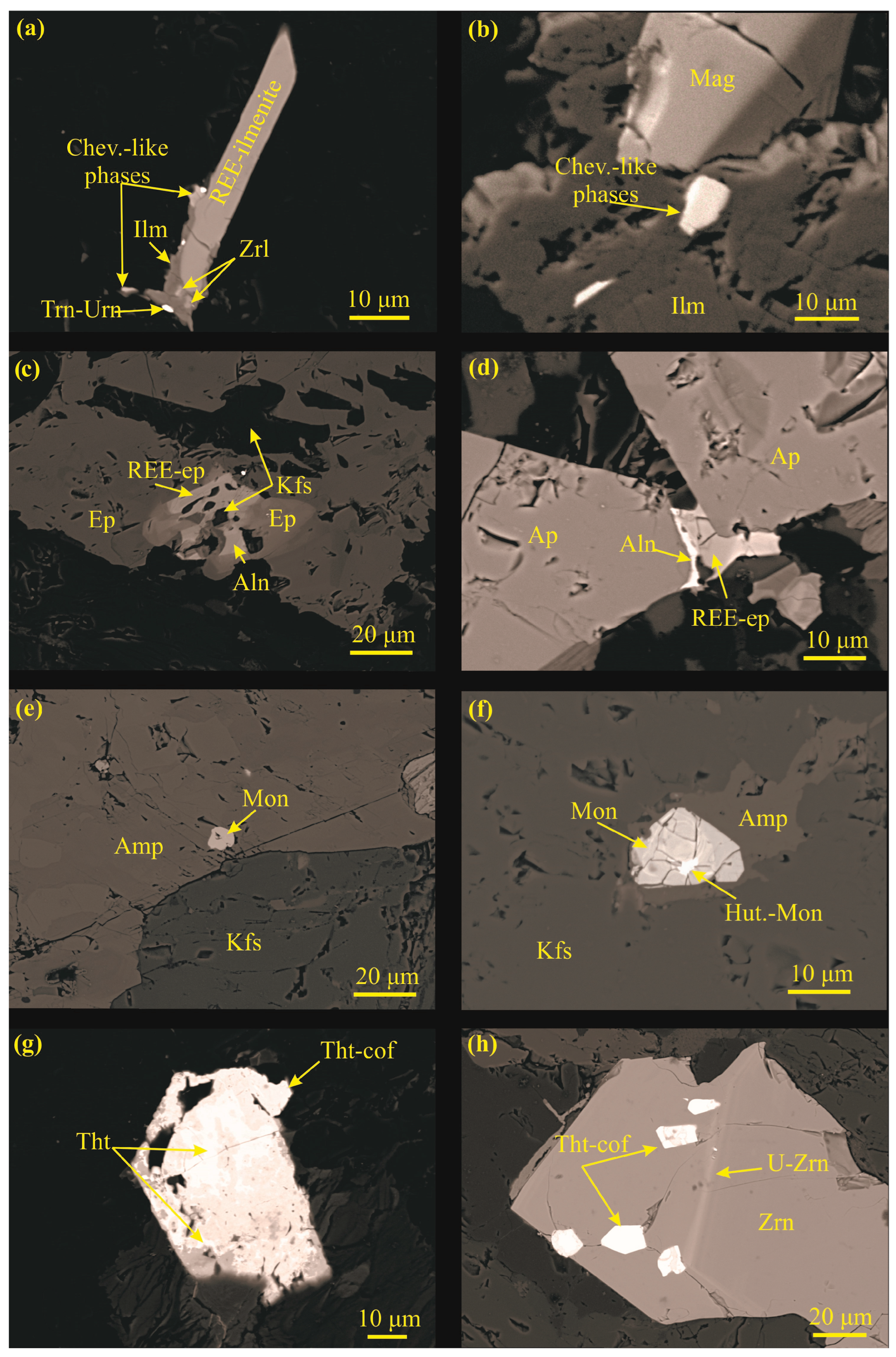
4.3. The REE-Ti-Zr-U-Th Minerals of Maronia Monzodiorite
4.3.1. Chevkinite-(Ce)-Like Phase
4.3.2. Allanite-(Ce)
4.3.3. Monazite-(Ce) and Huttonitic Monazite
4.3.4. Thorite–Coffinite
4.3.5. Baddeleyite
4.3.6. Zirconolite
4.3.7. Uraninite–Thorianite
5. Discussion
5.1. Formation Conditions and Paragenetic Sequence of the REE-Zr-U-Th Minerals
5.1.1. Formation of the REE-Ti-Zr Mineral Assemblage
5.1.2. Formation of the REE-Ca-P Mineral Assemblage
5.1.3. Formation of the Th-U Mineral Assemblage
5.2. The Relationship between the REE-Ti-Zr-U-Th Minerals and the Evolution of Maronia Pluton
5.3. Implications on the Mineral Exploration of Critical Metals
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- David, R. Wilburn Byproduct Metals and Rare-Earth Elements Used in the Production of Light-Emitting Diodes—Overview of Principal Sources of Supply and Material Requirements for Selected Markets; U.S. Geological Survey: Reston, VA, USA, 2012; p. 15.
- Ippolito, N.M.; Innocenzi, V.; De Michelis, I.; Medici, F.; Vegliò, F. Rare Earth Elements Recovery from Fluorescent Lamps: A New Thermal Pretreatment to Improve the Efficiency of the Hydrometallurgical Process. J. Clean. Prod. 2017, 153, 287–298. [Google Scholar] [CrossRef]
- Chakhmouradian, A.R.; Wall, F. Rare Earth Elements: Minerals, Mines, Magnets (and More). Elements 2012, 8, 333–340. [Google Scholar] [CrossRef]
- Moss, R.L.; Tzimas, E.; Kara, H.; Willis, P.; Kooroshy, J. Critical Metals in Strategic Energy Technologies; European Commission: Luxembourg, 2011; ISBN 9789279206986.
- Charalampides, G.; Vatalis, K.I.; Apostoplos, B.; Ploutarch-Nikolas, B. Rare Earth Elements: Industrial Applications and Economic Dependency of Europe. Procedia Econ. Financ. 2015, 24, 126–135. [Google Scholar] [CrossRef]
- Jonsson, E.; Törmänen, T.; Keiding, J.K.; Bjerkgård, T.; Eilu, P.; Pokki, J.; Gautneb, H.; Reginiussen, H.; Rosa, D.; Sadeghi, M.; et al. Critical Metals and Minerals in the Nordic Countries of Europe: Diversity of Mineralization and Green Energy Potential. Geol. Soc. Lond. Spec. Publ. 2023, 526, 95–152. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Fan, H.R.; Zhou, L.; Yang, K.F.; She, H.D. Carbonatite-Related REE Deposits: An Overview. Minerals 2020, 10, 965. [Google Scholar] [CrossRef]
- Gysi, A.P.; Williams-Jones, A.E. Hydrothermal Mobilization of Pegmatite-Hosted REE and Zr at Strange Lake, Canada: A Reaction Path Model. Geochim. Cosmochim. Acta 2013, 122, 324–352. [Google Scholar] [CrossRef]
- Groves, D.I.; Bierlein, F.P.; Meinert, L.D.; Hitzman, M.W. Iron Oxide Copper-Gold (IOCG) Deposits through Earth History: Implications for Origin, Lithospheric Setting, and Distinction from Other Epigenetic Iron Oxide Deposits. Econ. Geol. 2010, 105, 641–654. [Google Scholar] [CrossRef]
- Goodenough, K.M.; Schilling, J.; Jonsson, E.; Kalvig, P.; Charles, N.; Tuduri, J.; Deady, E.A.; Sadeghi, M.; Schiellerup, H.; Müller, A.; et al. Europe’s Rare Earth Element Resource Potential: An Overview of REE Metallogenetic Provinces and Their Geodynamic Setting. Ore Geol. Rev. 2016, 72, 838–856. [Google Scholar] [CrossRef]
- Sørensen, H. The Llimaussaq Alkaline Complex, South Greenland: Status of Mineralogical Research with New Results Anniversary Volume with List of Minerals Geological Survey of Denmark and Greenland Ministry of the Environment; Geological Survey of Denmark and Greenland: Copenhagen, Denmark, 2001. [Google Scholar]
- Savel’eva, V.B.; Karmanov, N.S. REE Minerals of Alkaline Metasomatic Rocks in the Main Sayan Fault. Geol. Ore Depos. 2008, 50, 681–696. [Google Scholar] [CrossRef]
- Bodeving, S.; Williams-Jones, A.E.; Swinden, S. Carbonate–Silicate Melt Immiscibility, REE Mineralising Fluids, and the Evolution of the Lofdal Intrusive Suite, Namibia. Lithos 2017, 268–271, 383–398. [Google Scholar] [CrossRef]
- Schmitt, A.K.; Trumbull, R.B.; Dulski, P.; Emmermann, R. Zr-Nb-REE Mineralization in Peralkaline Granites from the Amis Complex, Brandberg (Namibia): Evidence for Magmatic Pre-Enrichment from Melt Inclusions. Econ. Geol. 2002, 97, 399–413. [Google Scholar] [CrossRef]
- Walters, A.S.; Goodenough, K.M.; Hughes, H.S.R.; Roberts, N.M.W.; Gunn, A.G.; Rushton, J.; Lacinska, A. Enrichment of Rare Earth Elements during Magmatic and Post-Magmatic Processes: A Case Study from the Loch Loyal Syenite Complex, Northern Scotland. Contrib. Mineral. Petrol. 2013, 166, 1177–1202. [Google Scholar] [CrossRef]
- Poitrasson, F.; Pin, C.; Duthou, J.L. Hydrothermal Remobilization of Rare Earth Elements and Its Effect on Nd Isotopes in Rhyolite and Granite. Earth Planet. Sci. Lett. 1995, 130, 1–11. [Google Scholar] [CrossRef]
- Williams-Jones, A.E.; Migdisov, A.A.; Samson, I.M. Hydrothermal Mobilisation of the Rare Earth Elements-a Tale of “Ceria” and “Yttria”. Elements 2012, 8, 355–360. [Google Scholar] [CrossRef]
- Honour, V.C.; Goodenough, K.M.; Shaw, R.A.; Gabudianu, I.; Hirtopanu, P. REE Mineralisation within the Ditrău Alkaline Complex, Romania: Interplay of Magmatic and Hydrothermal Processes. Lithos 2018, 314–315, 360–381. [Google Scholar] [CrossRef]
- Stergiou, C.L.; Melfos, V.; Voudouris, P.; Spry, P.G.; Papadopoulou, L.; Chatzipetros, A.; Giouri, K.; Mavrogonatos, C.; Filippidis, A. The Geology, Geochemistry, and Origin of the Porphyry Cu-Au-(Mo) System at Vathi, Serbo-Macedonian Massif, Greece. Appl. Sci. 2021, 11, 479. [Google Scholar] [CrossRef]
- Jolivet, L.; Brun, J.P. Cenozoic Geodynamic Evolution of the Aegean. Int. J. Earth Sci. 2010, 99, 109–138. [Google Scholar] [CrossRef]
- Stampfli, G.M. Plate Tectonics of the Apulia-Adria Microcontinents. In CROP Project-Deep Seismic Explorations of the Central Mediterranean and Italy; Elsevier: Amsterdam, The Netherlands, 2005; pp. 747–766. [Google Scholar]
- Himmerkus, F.; Ander, B.; Reischmann, T.; Kostopoulos, D. Gondwana-Derived Terranes in the Northern Hellenides. Mem. Geol. Soc. Am. 2007, 200, 379–390. [Google Scholar] [CrossRef]
- Papanikolaou, D. Tectonostratigraphic Models of the Alpine Terranes and Subduction History of the Hellenides. Tectonophysics 2013, 595–596, 1–24. [Google Scholar] [CrossRef]
- Jolivet, L.; Faccenna, C.; Huet, B.; Labrousse, L.; Le Pourhiet, L.; Lacombe, O.; Lecomte, E.; Burov, E.; Denèle, Y.; Brun, J.P.; et al. Aegean Tectonics: Strain Localisation, Slab Tearing and Trench Retreat. Tectonophysics 2013, 597–598, 1–33. [Google Scholar] [CrossRef]
- Georgiev, N.; Pleuger, J.; Froitzheim, N.; Sarov, S.; Jahn-Awe, S.; Nagel, T.J. Separate Eocene-Early Oligocene and Miocene Stages of Extension and Core Complex Formation in the Western Rhodopes, Mesta Basin, and Pirin Mountains (Bulgaria). Tectonophysics 2010, 487, 59–84. [Google Scholar] [CrossRef]
- Wawrzenitz, N.; Krohe, A. Exhumation and Doming of the Thasos Metamorphic Core Complex (S Rhodope, Greece): Structural and Geochronological Constraints. Tectonophysics 1998, 285, 301–332. [Google Scholar] [CrossRef]
- Brun, J.P.; Sokoutis, D. 45 m.y. of Aegean Crust and Mantle Flow Driven by Trench Retreat. Geology 2010, 38, 815–818. [Google Scholar] [CrossRef]
- Rohrmeier, M.K.; Von Quadt, A.; Driesner, T.; Heinrich, C.A.; Handler, R.; Ovtcharova, M.; Ivanov, Z.; Petrov, P.; Sarov, S.; Peytcheva, I. Post-Orogenic Extension and Hydrothermal Ore Formation: High-Precision Geochronology of the Central Rhodopian Metamorphic Core Complex (Bulgaria-Greece). Econ. Geol. 2013, 108, 691–718. [Google Scholar] [CrossRef]
- Stübner, K.; Drost, K.; Schoenberg, R.; Böhme, M.; Starke, J.; Ehlers, T.A. Asynchronous Timing of Extension and Basin Formation in the South Rhodope Core Complex, SW Bulgaria, and Northern Greece. Tectonics 2016, 35, 136–159. [Google Scholar] [CrossRef]
- Moulas, E.; Schenker, F.L.; Burg, J.P.; Kostopoulos, D. Metamorphic Conditions and Structural Evolution of the Kesebir-Kardamos Dome: Rhodope Metamorphic Complex (Greece-Bulgaria). Int. J. Earth Sci. 2017, 106, 2667–2685. [Google Scholar] [CrossRef]
- Brun, J.P.; Sokoutis, D. Kinematics of the Southern Rhodope Core Complex (North Greece). Int. J. Earth Sci. 2007, 96, 1079–1099. [Google Scholar] [CrossRef]
- Mposkos, E.; Krohe, A.; Baziotis, I. Deep Tectonics in the Eastern Hellenides Uncovered: The Record of Variscan Continental Amalgamation, Permo-Triassic Rifting, and Early Alpine Collision in Pre-Variscan Continental Crust in the W-Rhodope (Vertiscos-Ograzden Complex, N-Greece). Tectonics 2021, 40, e2019TC005557. [Google Scholar] [CrossRef]
- Krohe, A.; Mposkos, E. Multiple Generations of Extensional Detachments in the Rhodope Mountains (Northern Greece): Evidence of Episodic Exhumation of High-Pressure Rocks. Geol. Soc. Spec. Publ. 2002, 204, 151–178. [Google Scholar] [CrossRef]
- Bonev, N.; Filipov, P.; Raicheva, R.; Chiaradia, M.; Moritz, R. Detrital Zircon Age and Sr Isotopic Constraints for a Late Palaeozoic Carbonate Platform in the Lower Rhodope Thrust System, Pirin, SW Bulgaria. Geol. Mag. 2019, 156, 2117–2124. [Google Scholar] [CrossRef]
- Bauer, C.; Rubatto, D.; Krenn, K.; Proyer, A.; Hoinkes, G. A Zircon Study from the Rhodope Metamorphic Complex, N-Greece: Time Record of a Multistage Evolution. Lithos 2007, 99, 207–228. [Google Scholar] [CrossRef]
- Bonev, N.; Moritz, R.; Marton, I.; Chiaradia, M.; Marchev, P. Geochemistry, Tectonics, and Crustal Evolution of Basement Rocks in the Eastern Rhodope Massif, Bulgaria. Int. Geol. Rev. 2010, 52, 269–297. [Google Scholar] [CrossRef]
- Bonev, N.G.; Stampfli, G.M. New Structural and Petrologic Data on Mesozoic Schists in the Rhodope (Bulgaria): Geodynamic Implications. Comptes Rendus—Geosci. 2003, 335, 691–699. [Google Scholar] [CrossRef]
- Kounov, A.; Seward, D.; Burg, J.P.; Stockli, D.; Wüthrich, E. Cenozoic Thermal Evolution of the Central Rhodope Metamorphic Complex (Southern Bulgaria). Int. J. Earth Sci. 2020, 109, 1589–1611. [Google Scholar] [CrossRef]
- Brun, J.P.; Sokoutis, D. Core Complex Segmentation in North Aegean, A Dynamic View. Tectonics 2018, 37, 1797–1830. [Google Scholar] [CrossRef]
- Kydonakis, K.; Brun, J.P.; Sokoutis, D. North Aegean Core Complexes, the Gravity Spreading of a Thrust Wedge. J. Geophys. Res. Solid Earth 2015, 120, 595–616. [Google Scholar] [CrossRef]
- Kounov, A.; Wüthrich, E.; Seward, D.; Burg, J.P.; Stockli, D. Low-Temperature Constraints on the Cenozoic Thermal Evolution of the Southern Rhodope Core Complex (Northern Greece). Int. J. Earth Sci. 2015, 104, 1337–1352. [Google Scholar] [CrossRef]
- Ricou, L.; Burg, J.; Godfriaux, I.; Ivanov, Z. Rhodope and Vardar: The Metamorphic and the Olistostromic Paired Belts Related to the Cretaceous Subduction under Europe. Geodin. Acta 1998, 11, 285–309. [Google Scholar] [CrossRef]
- Abbo, A.; Avigad, D.; Gerdes, A. Crustal Evolution of Peri-Gondwana Crust into Present Day Europe: The Serbo-Macedonian and Rhodope Massifs as a Case Study. Lithos 2020, 356–357, 105295. [Google Scholar] [CrossRef]
- Meinhold, G.; Kostopoulos, D.K. The Circum-Rhodope Belt, Northern Greece: Age, Provenance, and Tectonic Setting. Tectonophysics 2013, 595–596, 55–68. [Google Scholar] [CrossRef]
- Bonev, N.; Stampfli, G. Alpine Tectonic Evolution of a Jurassic Subduction-Accretionary Complex: Deformation, Kinematics and 40Ar/39Ar Age Constraints on the Mesozoic Low-Grade Schists of the Circum-Rhodope Belt in the Eastern Rhodope-Thrace Region, Bulgaria-Greece. J. Geodyn. 2011, 52, 143–167. [Google Scholar] [CrossRef]
- Papadopoulos, P.; Arvanitides, N.D.; Zanas, I. Some Preliminary Geological Aspects on the Makri Unit (Phyllite Series); Peri-Rhodopian Zone. Geol. Rhodopica 1989, 1, 34–42. [Google Scholar]
- Kolokotroni, C. The Emplacement and Petrogenesis of the Vrondou Granitoid Pluton, Rhodope Massif, NE Greece. Doctoral Dissertation, University of Edinburgh, Edinburgh, UK, 1992. [Google Scholar]
- Dinter, D.A.; Macfarlane, A.; Hames, W.; Isachsen, C.; Bowring, S.; Royden, L. U-Pb and 40Ar/39Ar Geochronology of the Symvolon Granodiorite: Implications for the Thermal and Structural Evolution of the Rhodope Metamorphic Core Complex, Northeastern Greece. Tectonics 1995, 14, 886–908. [Google Scholar] [CrossRef]
- Castorina, F.; Koroneos, A.; Masi, U.; Eleftheriadis, G. Geochemical and Sr-Nd Isotopic Evidence for Origin and Evolution of the Miocene Pangeon Granitoids, Southern Rhodope, Greece. Int. Geol. Rev. 2014, 56, 622–652. [Google Scholar] [CrossRef]
- Soldatos, T.; Koroneos, A.; Kamenov, B.K. New U-Pb and Ar-Ar Mineral Ages for the Barutin-Buynovo-Elatia-Skaloti-Paranesti Batholith (Bulgaria and Greece): Refinement of Its Debatable Age. Geochem. Mineral. Petrol. 2008, 46, 85–102. [Google Scholar]
- Papadopoulou, L.; Christofides, G.; Koroneos, A.; Bröcker, M.; Soldatos, T.; Eleftheriadis, G. Evolution and Origin of the Maronia Pluton, Thrace, Greece. Bull. Geol. Soc. Greece 2004, 36, 568. [Google Scholar] [CrossRef]
- Del Moro, A.; Innocenti, F.; Kyriakopoulos, C.; Manetti, P.; Papadopoulos, P. Tertiary Granitoids from Thrace (Northern Greece): Sr Isotopic and Petrochemical Data. Neues Jahrb. Mineral. Abh. 1988, 159, 113–115. [Google Scholar]
- Perkins, R.J.; Cooper, F.J.; Condon, D.J.; Tattitch, B.; Naden, J. Post-Collisional Cenozoic Extension in the Northern Aegean: The High-K to Shoshonitic Intrusive Rocks of the Maronia Magmatic Corridor, Northeastern Greece. Lithosphere 2018, 10, 582–601. [Google Scholar] [CrossRef]
- Papadopoulou, L. Mineral Phase Equilibria, Crystallization Conditions and Evolution of the Maroneia Plutonite, Thrace. Ph.D. Thesis, Aristotelian University of Thessaloniki, Thessaloniki, Greece, 2003. [Google Scholar]
- Melfos, V.; Vavelidis, M.; Christofides, G.; Seidel, E. Origin and Evolution of the Tertiary Maronia Porphyry Copper-Molybdenum Deposit, Thrace, Greece. Miner. Depos. 2002, 37, 648–668. [Google Scholar] [CrossRef]
- Biggazzi, G.; Del Moro, A.; Innocenti, F.; Kyriakopoulos, K.; Manetti, P.; Papadopoulos, P.; Norelli, P.; Magganas, A. The Magmatic Intrusive Complex of Petrota, West Thrace: Age and Geodynamic Significance. Geol. Rhodopica 1989, 1, 290–297. [Google Scholar]
- Melfos, V.; Voudouris, P.; Melfou, M.; Sanchez, M.G.; Papadopoulou, L.; Filippidis, A.; Spry, P.G.; Schaarschmidt, A.; Klemd, R.; Haase, K.M.; et al. Mineralization of the Maronia Porphyry Cu-Mo ± Re. Minerals 2020, 10, 182. [Google Scholar] [CrossRef]
- Schaarschmidt, A.; Klemd, R.; Regelous, M.; Voudouris, P.C.; Melfos, V.; Haase, K.M. The Formation of Shoshonitic Magma and Its Relationship to Porphyry-Type Mineralisation: The Maronia Pluton in NE Greece. Lithos 2021, 380–381, 105911. [Google Scholar] [CrossRef]
- Skentzou, D. Mineralogical and Geochemical Study of Lanthanide (REE) and Actinide Mineralization in Maroneia Plutonite. Bachelor’s Thesis, Thrace National and Kapodistrian University of Athens, Athens, Greece, 2021. [Google Scholar]
- Vasilatos, C.; Economou-Eliopoulos, M. Fossilized Bacteria in Fe-Mn-Mineralization: Evidence from the Legrena Valley, W. Lavrion Mine (Greece). Minerals 2018, 8, 107. [Google Scholar] [CrossRef]
- Janoušek, V.; Farrow, C.M.; Erban, V. Interpretation of Whole-Rock Geochemical Data in Igneous Geochemistry: Introducing Geochemical Data Toolkit (GCDkit). J. Petrol. 2006, 47, 1255–1259. [Google Scholar] [CrossRef]
- Middlemost, E.A.K. Naming Materials in the Magma/Igneous Rock System. Earth Sci. Rev. 1994, 37, 215–224. [Google Scholar] [CrossRef]
- Peccerillo, A.; Taylor, S.R. Geochemistry of Eocene Calc-Alkaline Volcanic Rocks from the Kastamonu Area, Northern Turkey. Contrib. Mineral. Petrol. 1976, 58, 63–81. [Google Scholar] [CrossRef]
- MacDonald, R.; Belkin, H.E. Compositional Variation in Minerals of the Chevkinite Group. Mineral. Mag. 2002, 66, 1075–1098. [Google Scholar] [CrossRef]
- Macdonald, R.; Bagiński, B.; Belkin, H.E.; Stachowicz, M. Composition, Paragenesis, and Alteration of the Chevkinite Group of Minerals. Am. Mineral. 2019, 104, 348–369. [Google Scholar] [CrossRef]
- Dessimoz, M.; Müntener, O.; Ulmer, P. A Case for Hornblende Dominated Fractionation of Arc Magmas: The Chelan Complex (Washington Cascades). Contrib. Mineral. Petrol. 2012, 163, 567–589. [Google Scholar] [CrossRef]
- Lesnov, F.P. Rare Earth Elements in Ultramafic and Mafic Rocks and Their Minerals-Minor and Accessory Minerals; Anoshin, G.N., Ed.; CRC Press: Boca Raton, FL, USA, 2010; ISBN 9780203119679. [Google Scholar]
- Linthout, K. Tripartite Division of the System 2REEPO4-CaTh (PO4)2-2ThSiO4, Discreditation of Brabantite, and Recognition of Cheralite as the Name for Members Dominated by CaTh(PO4)2. Can. Mineral. 2007, 45, 503–508. [Google Scholar] [CrossRef]
- Bayliss, P.; Mazzi, F.; Munno, R.; White, T.J. Mineral Nomenclature: Zirconolite. Mineral. Mag. 1989, 53, 565–569. [Google Scholar] [CrossRef]
- Gualda, G.A.R.R.; Vlach, S.R.F.F. The Serra Da Graciosa A-Type Granites and Syenites, Southern Brazil. Part 1: Regional Setting and Geological Characterization. An. Acad. Bras. Cienc. 2007, 79, 405–430. [Google Scholar] [CrossRef]
- Xie, L.; Wang, R.C.; Wang, D.Z.; Qiu, J.S. A Survey of Accessory Mineral Assemblages in Peralkaline and More Aluminous A-Type Granites of the Southeast Coastal Area of China. Mineral. Mag. 2006, 70, 709–729. [Google Scholar] [CrossRef]
- MacDonald, R.; Marshall, A.S.; Dawson, J.B.; Hinton, R.W.; Hill, P.G. Chevkinite-Group Minerals from Salic Volcanic Rocks of the East African Rift. Mineral. Mag. 2002, 66, 287–299. [Google Scholar] [CrossRef]
- Jiang, N. Hydrothermal Alteration of Chevkinite-(Ce) in the Shuiquangou Syenitic Intrusion, Northern China. Chem. Geol. 2006, 227, 100–112. [Google Scholar] [CrossRef]
- Papoutsa, A.D.; Pe-Piper, G. The Relationship between REE-Y-Nb-Th Minerals and the Evolution of an A-Type Granite, Wentworth Pluton, Nova Scotia. Am. Mineral. 2013, 98, 444–462. [Google Scholar] [CrossRef]
- Muhling, J.R.; Suvorova, A.A.; Rasmussen, B. The Occurrence and Composition of Chevkinite-(Ce) and Perrierite-(Ce) in Tholeiitic Intrusive Rocks and Lunar Mare Basalt. Am. Mineral. 2014, 99, 1911–1921. [Google Scholar] [CrossRef]
- Nejbert, K.; Bagiński, B.; Kotowski, J.; Jokubauskas, P.; Jurewicz, E.; Macdonald, R. Chevkinite-Group Minerals in Poland. Acta Geol. Pol. 2020, 70, 97–106. [Google Scholar] [CrossRef]
- Belkin, H.E.; Macdonald, R.; Grew, E.S. Chevkinite-Group Minerals from Granulite-Facies Metamorphic Rocks and Associated Pegmatites of East Antarctica and South India. Mineral. Mag. 2009, 73, 149–164. [Google Scholar] [CrossRef]
- Scaillet, B.; MacDonald, R. Phase Relations of Peralkaline Silicic Magmas and Petrogenetic Implications. J. Petrol. 2001, 42, 825–845. [Google Scholar] [CrossRef]
- Green, T.H.; Pearson, N.J. Experimental Crystallization of Chevkinite/Perrierite from REE-Enriched Silicate Liquids at High Pressure and Temperature. Mineral. Mag. 1988, 52, 113–120. [Google Scholar] [CrossRef][Green Version]
- Bagiński, B.; Macdonald, R. The Chevkinite Group: Underestimated Accessory Phases from a Wide Range of Parageneses. Mineralogia 2013, 44, 99–114. [Google Scholar] [CrossRef]
- Domańska-Siuda, J.; Nejbert, K.; Bagiński, B.; Macdonald, R.; Kotowski, J.; Stachowicz, M. Chevkinite-Group Minerals in Selected Intrusions of the Mazury Complex, North-Eastern Poland: Insights into the Formation of a Titanite-like Phase by Hydrothermal Alteration. Mineral. Petrol. 2022, 116, 105–119. [Google Scholar] [CrossRef]
- Gieré, R.; Williams, C.T.; Lumpkin, G.R. Chemical Characteristics of Natural Zirconolite. Schweiz. Mineral. Petrogr. Mitt. 1998, 78, 433–459. [Google Scholar]
- Wilke, M.; Schmidt, C.; Dubrail, J.; Appel, K.; Borchert, M.; Kvashnina, K.; Manning, C.E. Zircon Solubility and Zirconium Complexation in H2O + Na2O + SiO2 ± Al2O3 Fluids at High Pressure and Temperature. Earth Planet. Sci. Lett. 2012, 349–350, 15–25. [Google Scholar] [CrossRef]
- Watson, E.B.; Harrison, T.M. Zircon Saturation Revisited: Temperature and Composition Effects in a Variety of Crustal Magma Types. Earth Planet. Sci. Lett. 1983, 64, 295–304. [Google Scholar] [CrossRef]
- Boehnke, P.; Watson, E.B.; Trail, D.; Harrison, T.M.; Schmitt, A.K. Zircon Saturation Re-Revisited. Chem. Geol. 2013, 351, 324–334. [Google Scholar] [CrossRef]
- Borisov, A.; Aranovich, L. Zircon Solubility in Silicate Melts: New Experiments and Probability of Zircon Crystallization in Deeply Evolved Basic Melts. Chem. Geol. 2019, 510, 103–112. [Google Scholar] [CrossRef]
- Hermann, J. Allanite: Thorium and Light Rare Earth Element Carrier in Subducted Crust. Chem. Geol. 2002, 192, 289–306. [Google Scholar] [CrossRef]
- Smith, M.P.; Henderson, P.; Jeffries, T. The Formation and Alteration of Allanite in Skarn from the Beinn an Dubhaich Granite Aureole, Skye. Eur. J. Mineral. 2002, 14, 471–486. [Google Scholar] [CrossRef]
- Papoutsa, A.; Pe-Piper, G. Variation of REE-Hydrothermal Circulation in Complex Shear Zones: The Cobequid Highlands, Nova Scotia. Can. Mineral. 2014, 52, 943–968. [Google Scholar] [CrossRef]
- Ishihara, S.; Hua, R.; Hoshino, M.; Murakami, H. REE Abundance and Ree Minerals in Granitic Rocks in the Nanling Range, Jiangxi Province, Southern China, and Generation of the REE-Rich Weathered Crust Deposits. Resour. Geol. 2008, 58, 355–372. [Google Scholar] [CrossRef]
- Randive, K.R.; Vijaya Kumar, J.; Korakoppa, M.M.; Sahu, M.K. Occurrence of REE Mineralization in the Layered Gabbros of Phenai Mata Igneous Complex, Gujarat, India. Curr. Sci. 2017, 112, 231–235. [Google Scholar]
- Berger, A.; Rosenberg, C.; Schaltegger, U. Stability and Isotopic Dating of Monazite and Allanite in Partially Molten Rocks: Examples from the Central Alps. Swiss J. Geosci. 2009, 102, 15–29. [Google Scholar] [CrossRef]
- Peristeridou, E.; Melfos, V.; Papadopoulou, L.; Kantiranis, N.; Voudouris, P. Mineralogy and Mineral Chemistry of the REE-Rich Black Sands in Beaches of the Kavala District, Northern Greece. Geosciences 2022, 12, 277. [Google Scholar] [CrossRef]
- Harlov, D.E.; Wirth, R.; Hetherington, C.J. The Relative Stability of Monazite and Huttonite at 300–900 °C and 200–1000 MPa: Metasomatism and the Propagation of Metastable Mineral Phases. Am. Mineral. 2007, 92, 1652–1664. [Google Scholar] [CrossRef]
- Wang, X.; Griffin, W.L.; Chen, J.; Huang, P.; Li, X. U and Th Contents and Th/U Ratios of Zircon in Felsic and Mafic Magmatic Rocks: Improved Zircon-Melt Distribution Coefficients. Acta Geol. Sin. 2011, 85, 164–174. [Google Scholar]
- Fowler, A.; Prokoph, A.; Stern, R.; Dupuis, C. Organization of Oscillatory Zoning in Zircon: Analysis, Scaling, Geochemistry, and Model of a Zircon from Kipawa, Quebec, Canada. Geochim. Cosmochim. Acta 2002, 66, 311–328. [Google Scholar] [CrossRef]
- Min, M.; Fang, C.; Fayek, M. Petrography and Genetic History of Coffinite and Uraninite from the Liueryiqi Granite-Hosted Uranium Deposit, SE China. Ore Geol. Rev. 2005, 26, 187–197. [Google Scholar] [CrossRef]
- Seydoux-Guillaume, A.M.; Montel, J.M.; Bingen, B.; Bosse, V.; de Parseval, P.; Paquette, J.L.; Janots, E.; Wirth, R. Low-Temperature Alteration of Monazite: Fluid Mediated Coupled Dissolution-Precipitation, Irradiation Damage, and Disturbance of the U-Pb and Th-Pb Chronometers. Chem. Geol. 2012, 330–331, 140–158. [Google Scholar] [CrossRef]
- Mercadier, J.; Annesley, I.R.; Mckechnie, C.L.; Bogdan, T.S.; Creighton, S. Magmatic and Metamorphic Uraninite Mineralization in the Western Margin of the Trans-Hudson Orogen (Saskatchewan, Canada): A Uranium Source for Unconformity-Related Uranium Deposits? Econ. Geol. 2013, 108, 1037–1065. [Google Scholar] [CrossRef]
- Zaccarini, F.; Stumpfl, E.F.; Garuti, G. Zirconolite and Zr-Th-U Minerals in Chromitites of the Finero Complex, Western Alps, Italy: Evidence for Carbonatite-Type Metasomatism in a Subcontinental Mantle Plume. Can. Mineral. 2004, 42, 1825–1845. [Google Scholar] [CrossRef]
- Pohjolainen, E. Uranium Deposits of Finland; Elsevier Inc.: Amsterdam, The Netherlands, 2015; ISBN 9780124104761. [Google Scholar]
- Pipera, K.; Koroneos, A.; Soldatos, T.; Poli, G.; Christofides, G. Origin of the High-K Tertiary Magmatism in Northern Greece: Implications for Mantle Geochemistry and Geotectonic Setting. Bull. Geol. Soc. Greece 2013, 47, 416. [Google Scholar] [CrossRef][Green Version]
- Pe-Piper, G.; Piper, D.J.W. Late Cenozoic, Post-Collisional Aegean Igneous Rocks: Nd, Pb and Sr Isotopic Constraints on Petrogenetic and Tectonic Models. Geol. Mag. 2001, 138, 653–668. [Google Scholar] [CrossRef]
- Rajesh, V.J.; Yokoyama, K.; Santosh, M.; Arai, S.; Oh, C.W.; Kim, S.W. Zirconolite and Baddeleyite in an Ultramafic Suite from Southern India: Early Ordovician Carbonatite-Type Melts Associated with Extensional Collapse of the Gondwana Crust. J. Geol. 2006, 114, 171–188. [Google Scholar] [CrossRef]
- Dini, A.; Rocchi, S.; Westerman, D.S. Reaction Microtextures of REE-Y-Th-U Accessory Minerals in the Monte Capanne Pluton (Elba Island, Italy): A Possible Indicator of Hybridization Processes. Lithos 2004, 78, 101–118. [Google Scholar] [CrossRef]
- Broska, I.; Petrík, I.; Williams, C.T. Coexisting Monazite and Allanite in Peraluminous Granitoids of the Tribec Mountains, Western Carpathians. Am. Mineral. 2000, 85, 22–32. [Google Scholar] [CrossRef]
- Carlier, G.; Lorand, J.P. Zr-Rich Accessory Minerals (Titanite, Perrierite, Zirconolite, Baddeleyite) Record Strong Oxidation Associated with Magma Mixing in the South Peruvian Potassic Province. Lithos 2008, 104, 54–70. [Google Scholar] [CrossRef]
- Mathieu, L. Origin of the Vanadiferous Serpentine–Magnetite Rocks of the Mt. Sorcerer Area, Lac Doré Layered Intrusion, Chibougamau, Québec. Geosciences 2019, 9, 110. [Google Scholar] [CrossRef]
- Devaraju, T.C.; Jayaraj, K.R.; Sudhakara, T.L.; Alapieti, T.T.; Spiering, B.; Kaukonen, R.J. Mineralogy, Geochemistry and Petrogenesis of the V-Ti-Bearing and Chromiferous Magnetite Deposits Hosted by Neoarchaean Channagiri Mafic-Ultramafic Complex, Western Dharwar Craton, India: Implications for Emplacement in Differentiated Pulses. Cent. Eur. J. Geosci. 2014, 6, 518–548. [Google Scholar] [CrossRef]
- Czamanske, G.K.; Mihálik, P. Oxidation during Magmatic Differentiation, Finnmarka Complex, Oslo Area, Norway: Part 1, the Opaque Oxides. J. Petrol. 1972, 13, 493–509. [Google Scholar] [CrossRef]
- Tarassova, E.; Tarassov, M.; Tacheva, E.; Nedialkov, R. Indicative Properties of Accessory Magnetite and Ilmenite from Mixed Magmas of Petrohan Pluton, Western Balkan, Bulgaria. In Proceedings of the National Conference “Geosciences 2009”, Sofia, Bugaria, 3–4 December 2009; pp. 13–14. [Google Scholar]
- Pe-Piper, G.; Piper, D.J.W.; Papoutsa, A. Mid Carboniferous Lamprophyres, Cobequid Fault Zone, Eastern Canada, Linked to Sodic Granites, Voluminous Gabbro, and Albitization. Lithos 2018, 296–299, 316–331. [Google Scholar] [CrossRef]

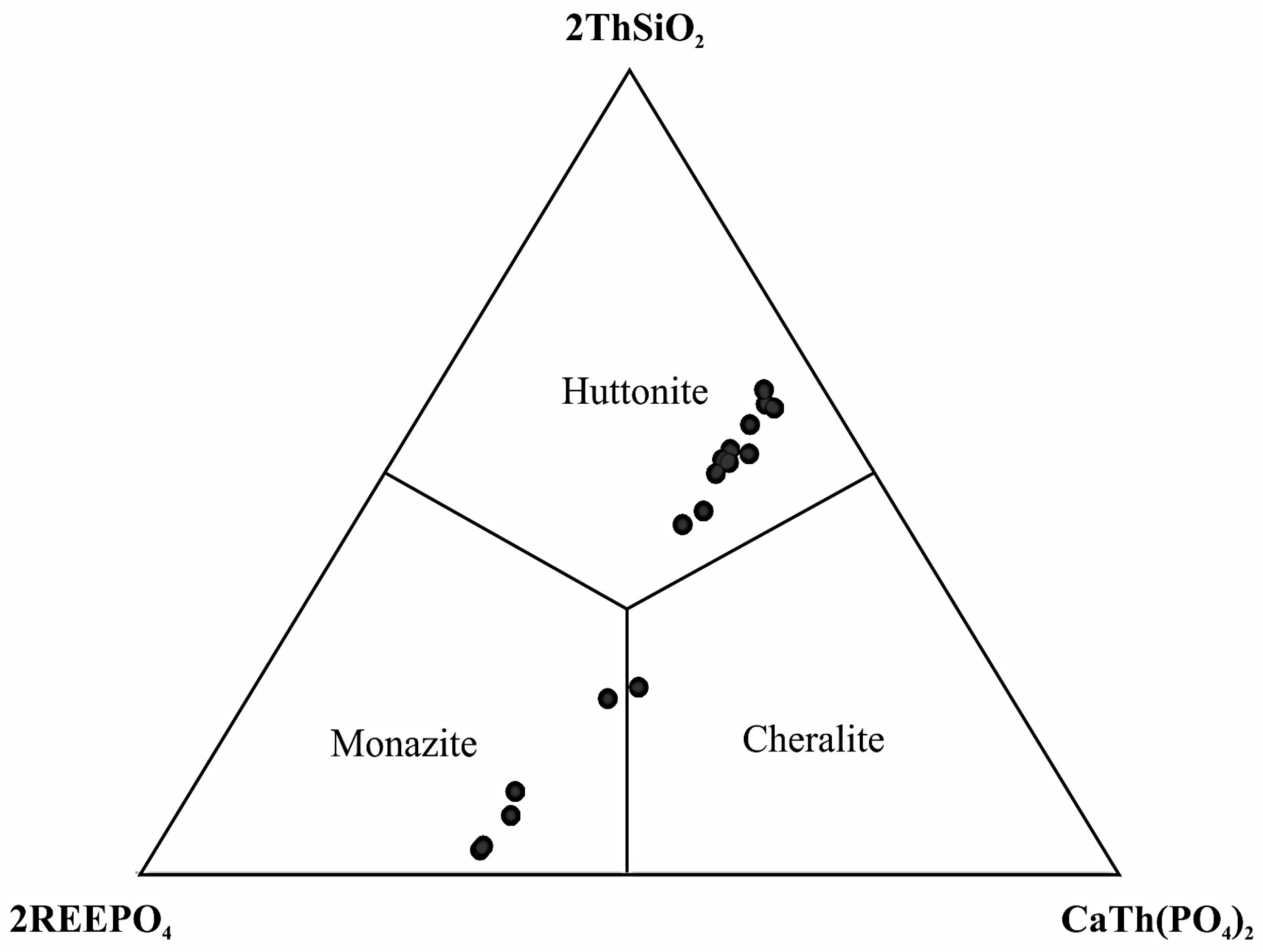
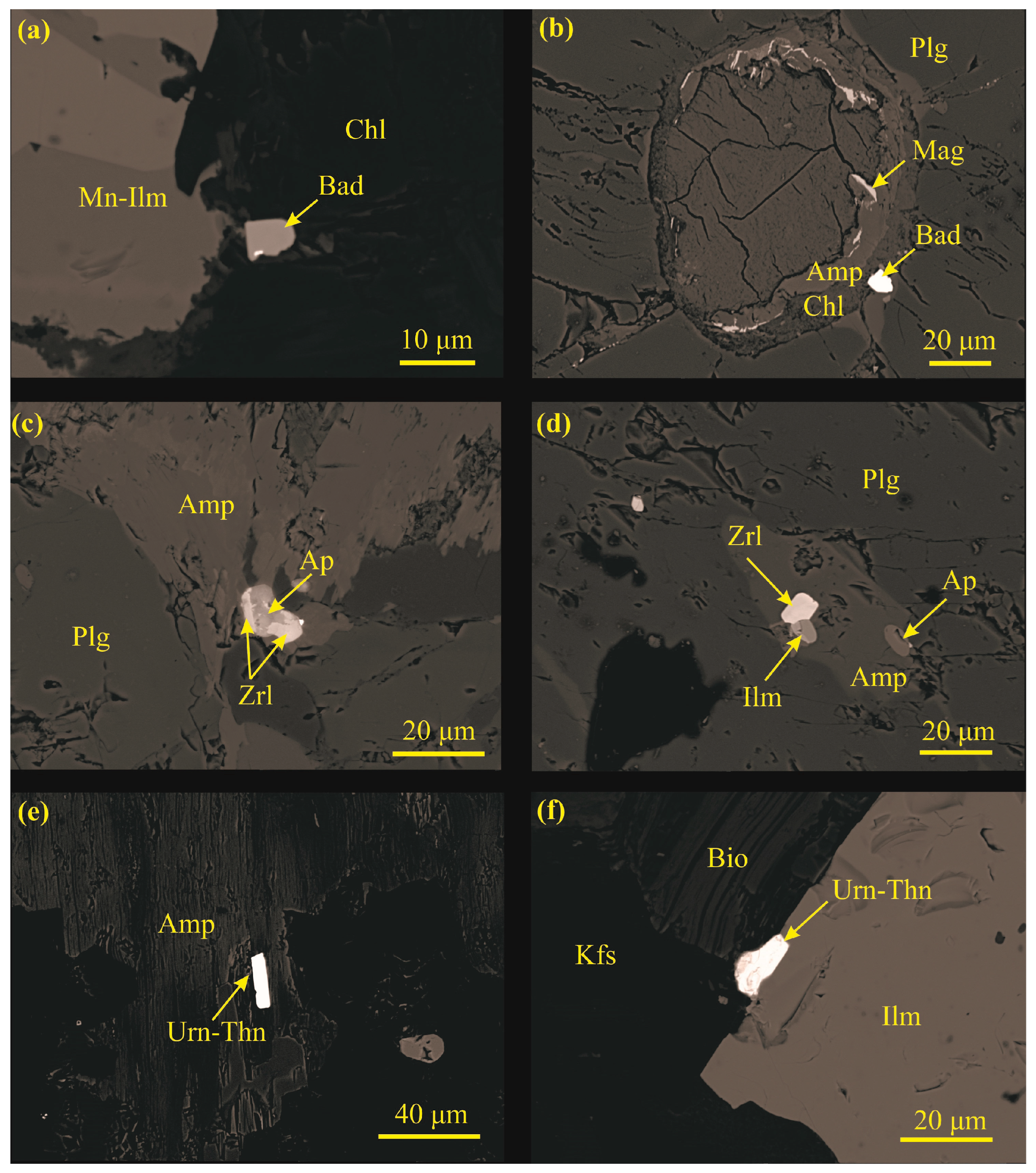

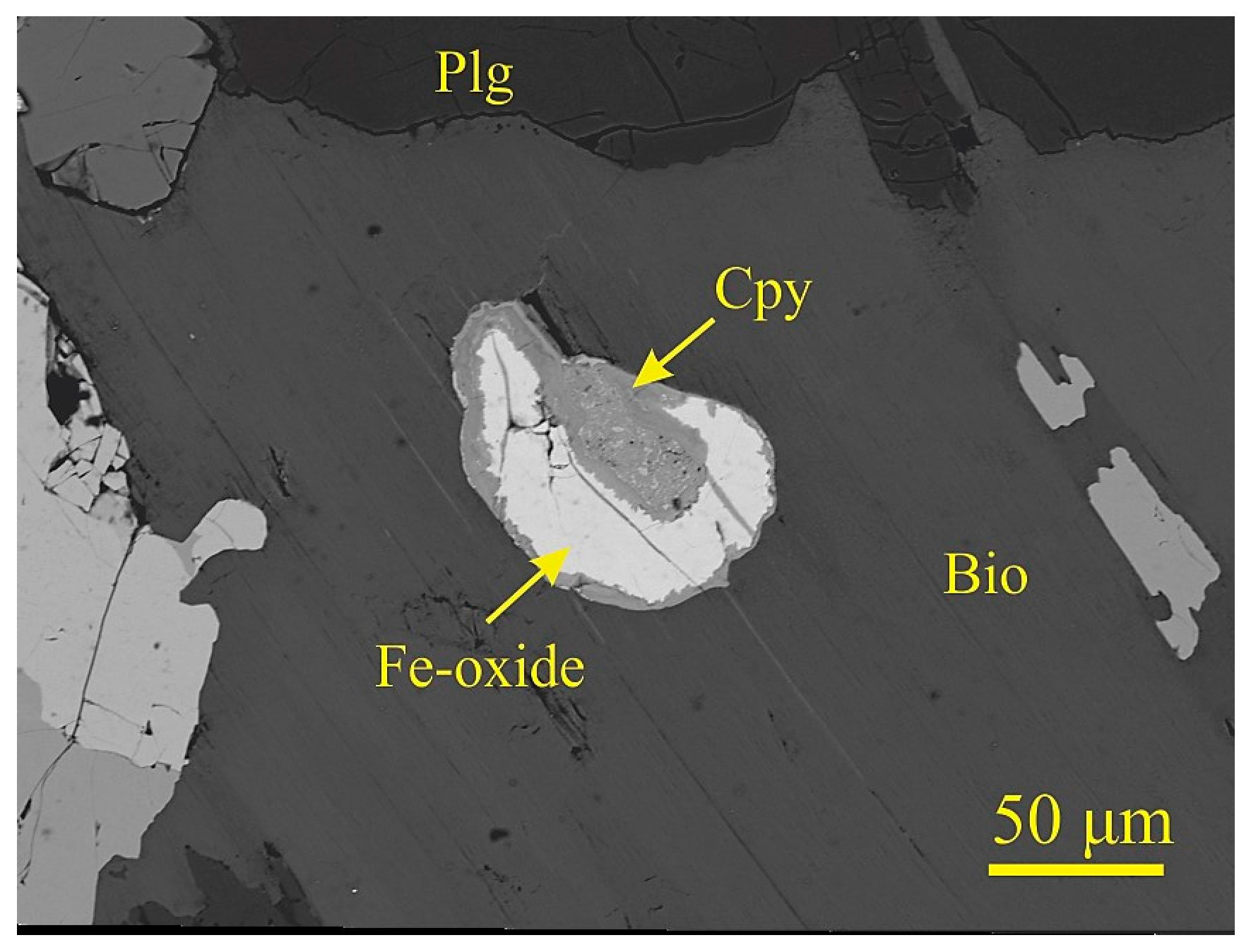
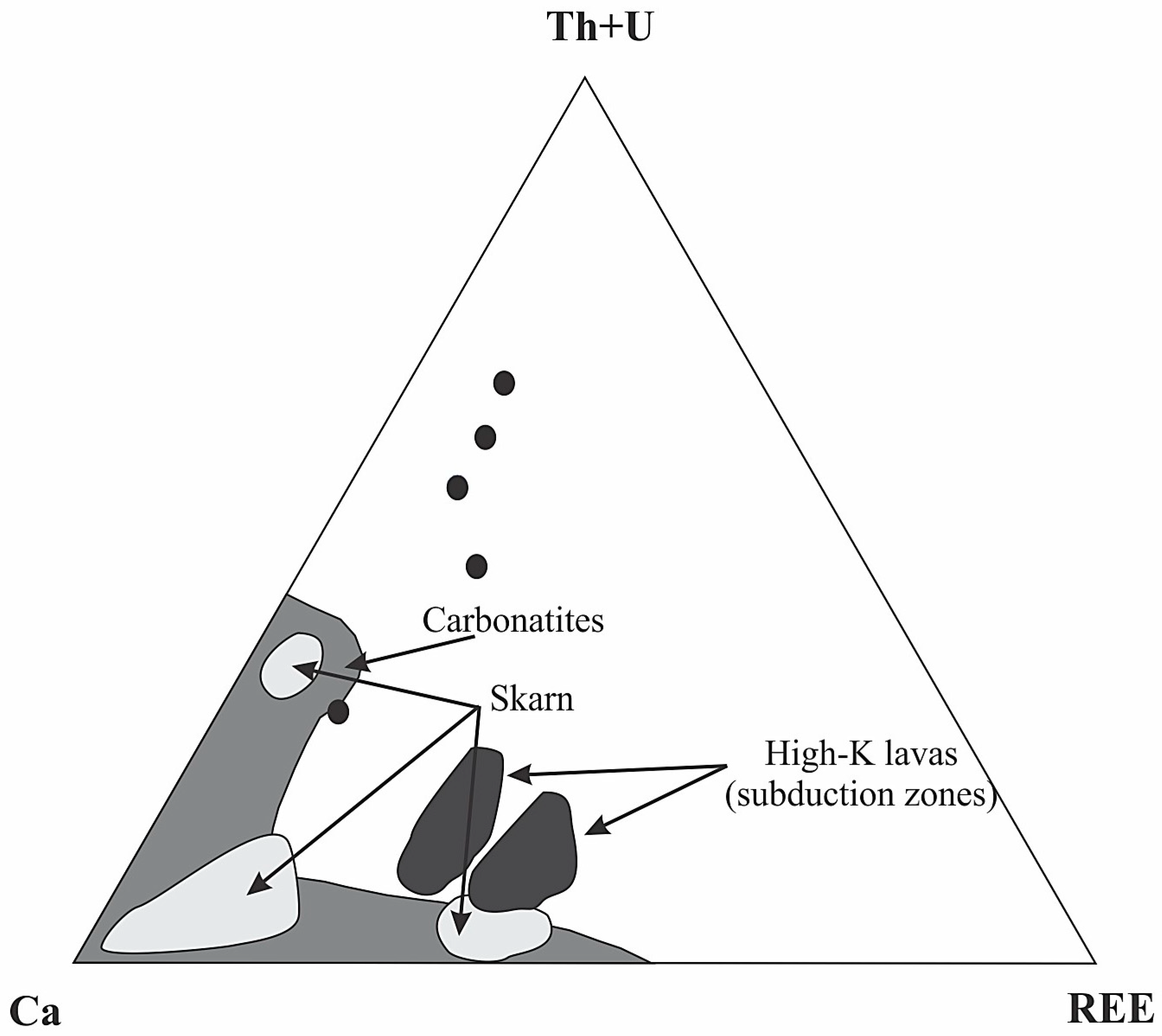
| Sample | MAR-1 | MAR-2 | MAR-3 | MAR-4 | MAR-5 | MAR-6 |
|---|---|---|---|---|---|---|
| Major oxides (wt.%) | ||||||
| SiO2 | 52.9 | 52.6 | 52.7 | 52.6 | 53 | 53.2 |
| TiO2 | 0.89 | 0.91 | 0.94 | 0.96 | 0.81 | 0.95 |
| Al2O3 | 16.62 | 16.5 | 16.43 | 16.49 | 16.9 | 16.33 |
| MnO | 0.16 | 0.16 | 0.15 | 0.16 | 0.16 | 0.16 |
| MgO | 4.96 | 4.99 | 4.94 | 4.97 | 4.95 | 4.96 |
| Fe2O3 | 9.37 | 9.23 | 9.26 | 9.22 | 9.12 | 9.28 |
| CaO | 8.49 | 8.28 | 8.17 | 8.25 | 8.45 | 8.16 |
| Na2O | 2.84 | 2.82 | 2.83 | 2.84 | 2.91 | 2.8 |
| K2O | 2.77 | 2.73 | 2.91 | 2.85 | 2.69 | 2.96 |
| P2O5 | 0.48 | 0.47 | 0.46 | 0.47 | 0.48 | 0.47 |
| LOI | 0.81 | 0.92 | 0.95 | 0.83 | 0.75 | 0.72 |
| Total | 100.30 | 99.62 | 99.75 | 99.65 | 100.23 | 99.99 |
| Trace elements (ppm) | ||||||
| Ba | 1072 | 1086 | 1045 | 855 | 1106 | 891 |
| Co | 37.5 | 38.9 | 39.8 | 42.4 | 37.5 | 40.3 |
| Ga | 17.4 | 17.3 | 17.6 | 18 | 17.6 | 18.3 |
| Hf | 4.6 | 4.5 | 5.3 | 5.2 | 5.3 | 6 |
| Nb | 10.4 | 10.9 | 10 | 11.1 | 8.8 | 11.6 |
| Rb | 122 | 125 | 130 | 135 | 122 | 139 |
| Sr | 725 | 756 | 724 | 710 | 778 | 710 |
| Ta | 0.9 | 1 | 1.3 | 1.1 | 1.1 | 1.1 |
| Th | 23.1 | 26.6 | 25.2 | 21.7 | 23 | 23.8 |
| U | 6.4 | 7.8 | 6.5 | 5.9 | 6.1 | 6.3 |
| V | 258 | 271 | 260 | 266 | 254 | 257 |
| Zr | 147 | 138 | 163 | 180 | 179 | 213 |
| Y | 22.5 | 25 | 23.2 | 24.2 | 24.3 | 24.7 |
| Mo | 1 | 1.3 | 1.2 | 1.2 | 0.8 | 0.9 |
| Cu | 96.3 | 89.8 | 87.5 | 84.2 | 71.5 | 83.5 |
| Pb | 14.2 | 9.6 | 12.7 | 10.8 | 7.6 | 9.8 |
| Zn | 41 | 40 | 43 | 42 | 41 | 39 |
| Ni | 19.4 | 19.5 | 19.8 | 18.6 | 17 | 17.4 |
| As | 1.4 | 1.6 | 1.7 | 1.5 | 1.6 | 1.4 |
| Cr | 37.6 | 30.8 | 34.2 | 20.5 | 34.2 | 23.9 |
| La | 33.6 | 36.3 | 36.4 | 37.5 | 37.1 | 37.5 |
| Ce | 70.2 | 74.8 | 73.9 | 78 | 77.3 | 77.4 |
| Pr | 8.91 | 9.49 | 9.26 | 9.86 | 9.65 | 10 |
| Nd | 35.6 | 39 | 37.8 | 39.2 | 38.7 | 41.3 |
| Sm | 7.18 | 7.19 | 7.51 | 7.51 | 7.44 | 7.69 |
| Eu | 1.71 | 1.85 | 1.73 | 1.71 | 1.76 | 1.72 |
| Gd | 6.07 | 6.5 | 6.33 | 6.53 | 6.57 | 6.53 |
| Tb | 0.78 | 0.77 | 0.78 | 0.8 | 0.79 | 0.82 |
| Dy | 4.73 | 4.88 | 4.75 | 4.73 | 4.38 | 4.99 |
| Ho | 0.72 | 0.78 | 0.86 | 0.86 | 0.77 | 0.9 |
| Er | 2.33 | 2.28 | 2.28 | 2.42 | 2.46 | 2.52 |
| Tm | 0.34 | 0.37 | 0.35 | 0.39 | 0.36 | 0.38 |
| Yb | 2.02 | 2.38 | 2.18 | 2.43 | 2.25 | 2.39 |
| Lu | 0.31 | 0.36 | 0.34 | 0.36 | 0.35 | 0.36 |
| ΣREE | 174 | 187 | 184 | 192 | 190 | 194 |
| Sample | MAR-1 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mineral | REE-ilm | Chv. phase | Aln | Mon | Hut.-Mon | Tht | Tht-cof | Bad | Zrl | Y-Zrl | Urn-Thn | Mn-Ilm | V-Mag |
| SiO2 | n.d. | 22.5 | 34.6 | 1.60 | 14.1 | 18.2 | 17.9 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| TiO2 | 51.6 | 19.3 | n.d. | n.d. | n.d. | n.d. | n.d. | 1.28 | 34.3 | 34.9 | n.d. | 49.1 | n.d. |
| Al2O3 | n.d. | 3.17 | 19.2 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| MnO | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| MgO | n.d. | n.d. | 0.74 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| FeO | 27.7 | 8.82 | 14.7 | n.d. | n.d. | n.d. | n.d. | 1.74 | 12.5 | 14.3 | 1.49 | 47.9 | 98.0 |
| V2O3 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 3.05 | 2.00 |
| CaO | 5.92 | 6.04 | 10.9 | n.d. | 1.13 | n.d. | n.d. | 0.52 | 7.41 | 5.99 | n.d. | n.d. | n.d. |
| Na2O | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| K2O | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| P2O5 | n.d. | n.d. | n.d. | 30.8 | 8.57 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| La2O3 | 4.20 | 11.3 | 6.11 | 25.0 | 6.30 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Ce2O3 | 3.35 | 21.4 | 11.1 | 34.0 | 9.44 | n.d. | n.d. | n.d. | 4.70 | n.d. | n.d. | n.d. | n.d. |
| Nd2O3 | n.d. | 7.46 | 2.61 | 6.10 | 2.88 | n.d. | n.d. | n.d. | 2.90 | n.d. | n.d. | n.d. | n.d. |
| Y2O3 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 12.3 | n.d. | n.d. | n.d. |
| ZrO2 | n.d. | 0.00 | n.d. | n.d. | n.d. | n.d. | n.d. | 96.5 | 23.4 | 32.4 | n.d. | n.d. | n.d. |
| ThO2 | 1.25 | 0.00 | n.d. | 2.56 | 57.1 | 81.8 | 61.2 | n.d. | 2.38 | n.d. | 20.6 | n.d. | n.d. |
| UO2 | 5.98 | n.d. | n.d. | n.d. | n.d. | n.d. | 20.9 | n.d. | 13.8 | n.d. | 77.9 | n.d. | n.d. |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasilatos, C.; Papoutsa, A. The REE-Zr-U-Th Minerals of the Maronia Monzodiorite, N. Greece: Implications on the Saturation and Segregation Mechanisms of Critical Metals in Intermediate–Mafic Compositions. Minerals 2023, 13, 1256. https://doi.org/10.3390/min13101256
Vasilatos C, Papoutsa A. The REE-Zr-U-Th Minerals of the Maronia Monzodiorite, N. Greece: Implications on the Saturation and Segregation Mechanisms of Critical Metals in Intermediate–Mafic Compositions. Minerals. 2023; 13(10):1256. https://doi.org/10.3390/min13101256
Chicago/Turabian StyleVasilatos, Charalampos, and Angeliki Papoutsa. 2023. "The REE-Zr-U-Th Minerals of the Maronia Monzodiorite, N. Greece: Implications on the Saturation and Segregation Mechanisms of Critical Metals in Intermediate–Mafic Compositions" Minerals 13, no. 10: 1256. https://doi.org/10.3390/min13101256
APA StyleVasilatos, C., & Papoutsa, A. (2023). The REE-Zr-U-Th Minerals of the Maronia Monzodiorite, N. Greece: Implications on the Saturation and Segregation Mechanisms of Critical Metals in Intermediate–Mafic Compositions. Minerals, 13(10), 1256. https://doi.org/10.3390/min13101256







