Alteration of Feldspathoids Changes pH of Late-Magmatic Fluids: A Case Study from the Lovozero Peralkaline Massif, Russia
Abstract
1. Introduction
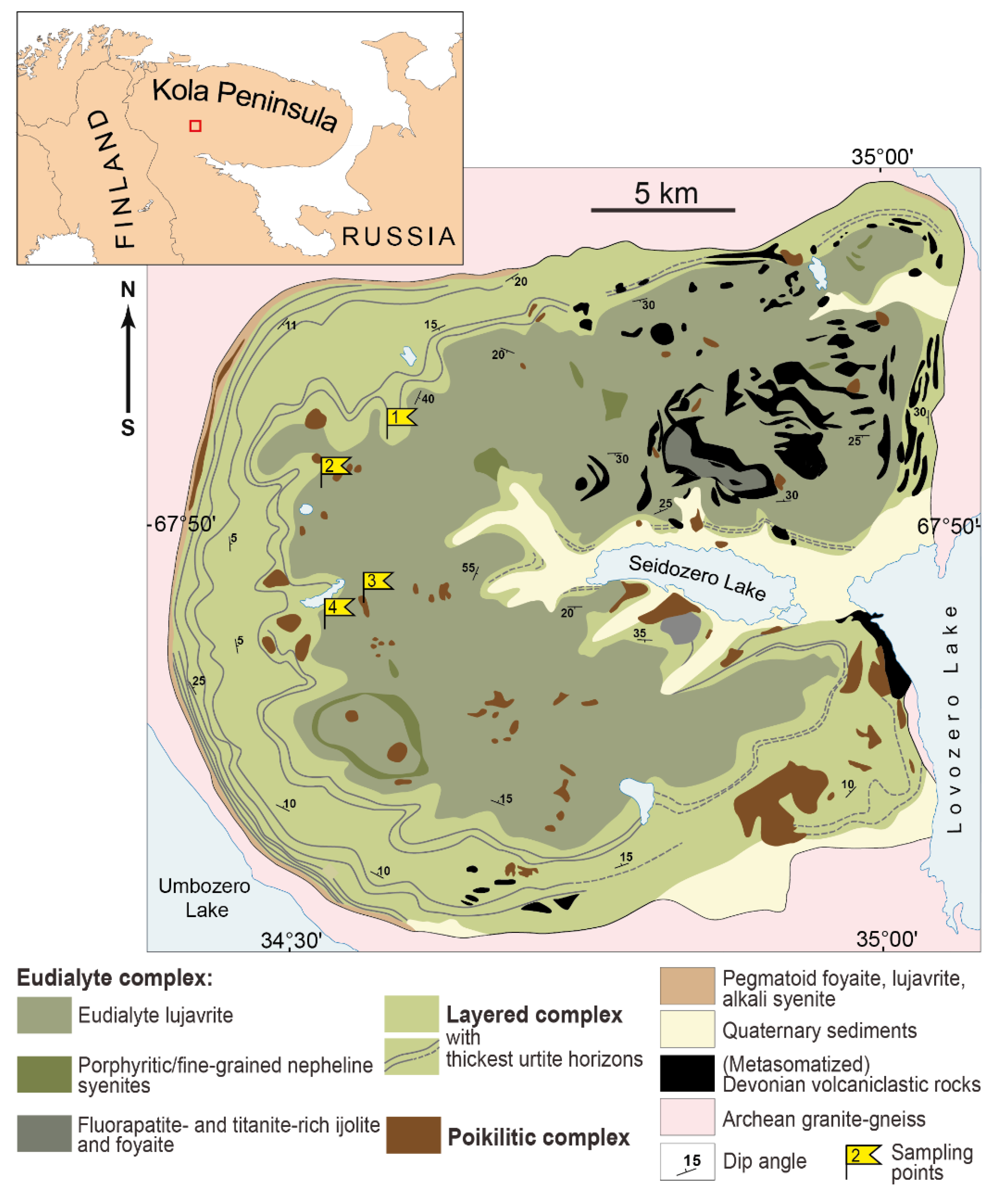
2. Geological Background and Short Petrography Description
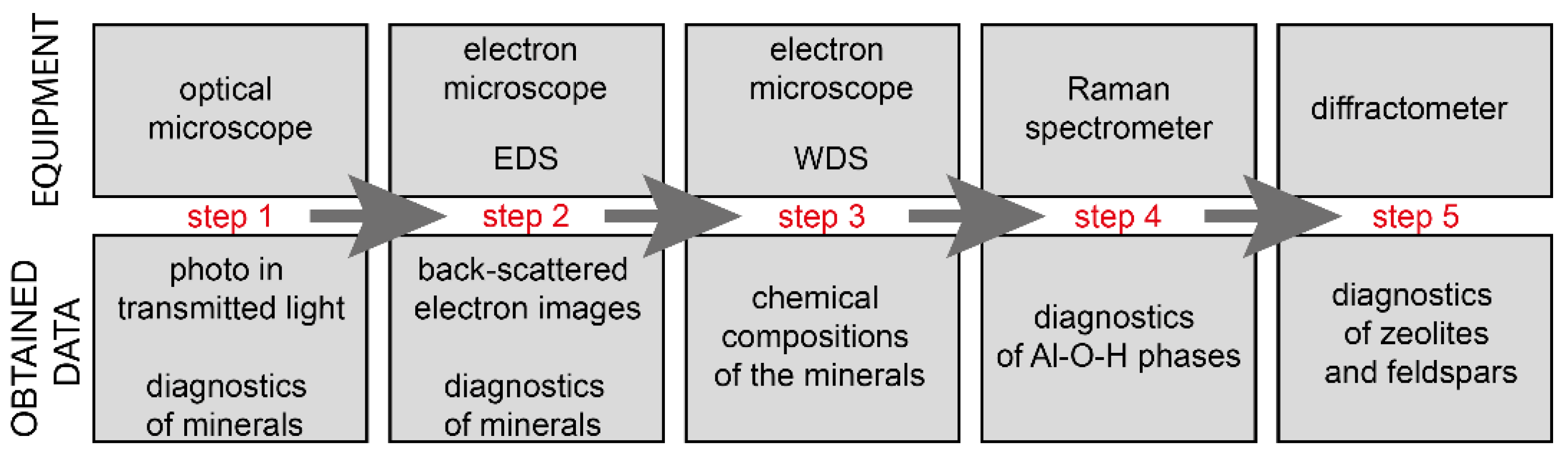
3. Materials and Methods
4. Results
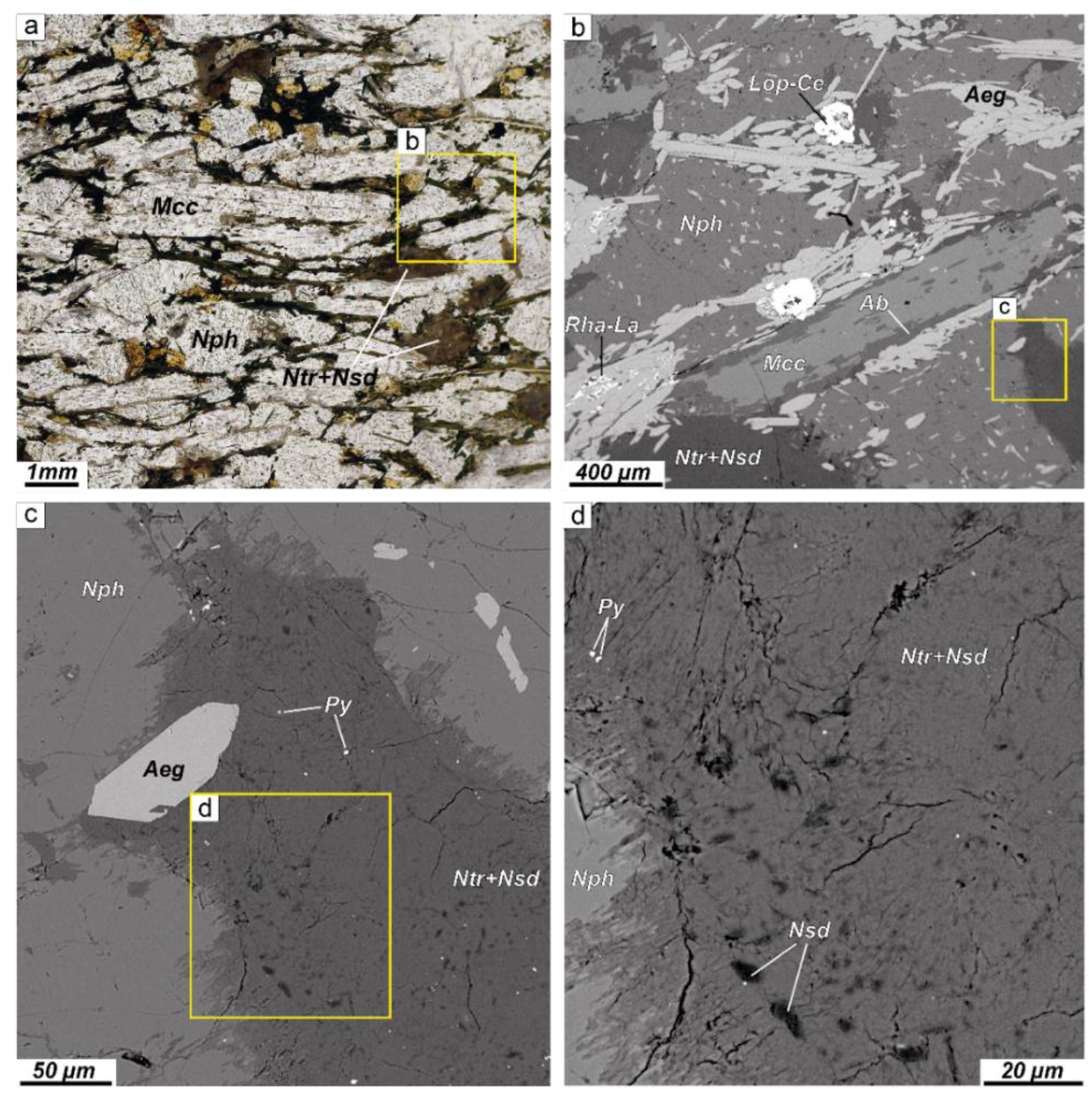
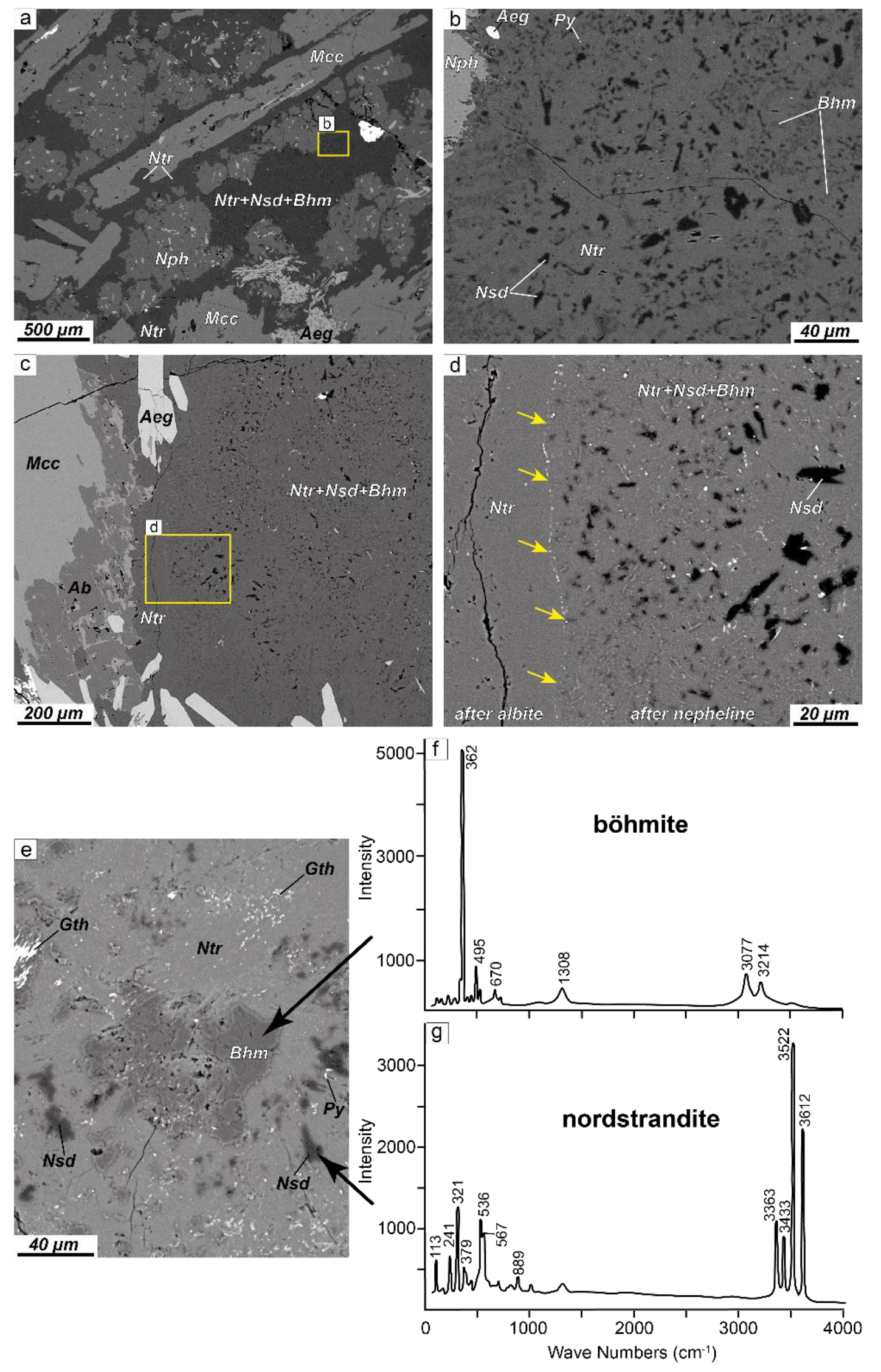
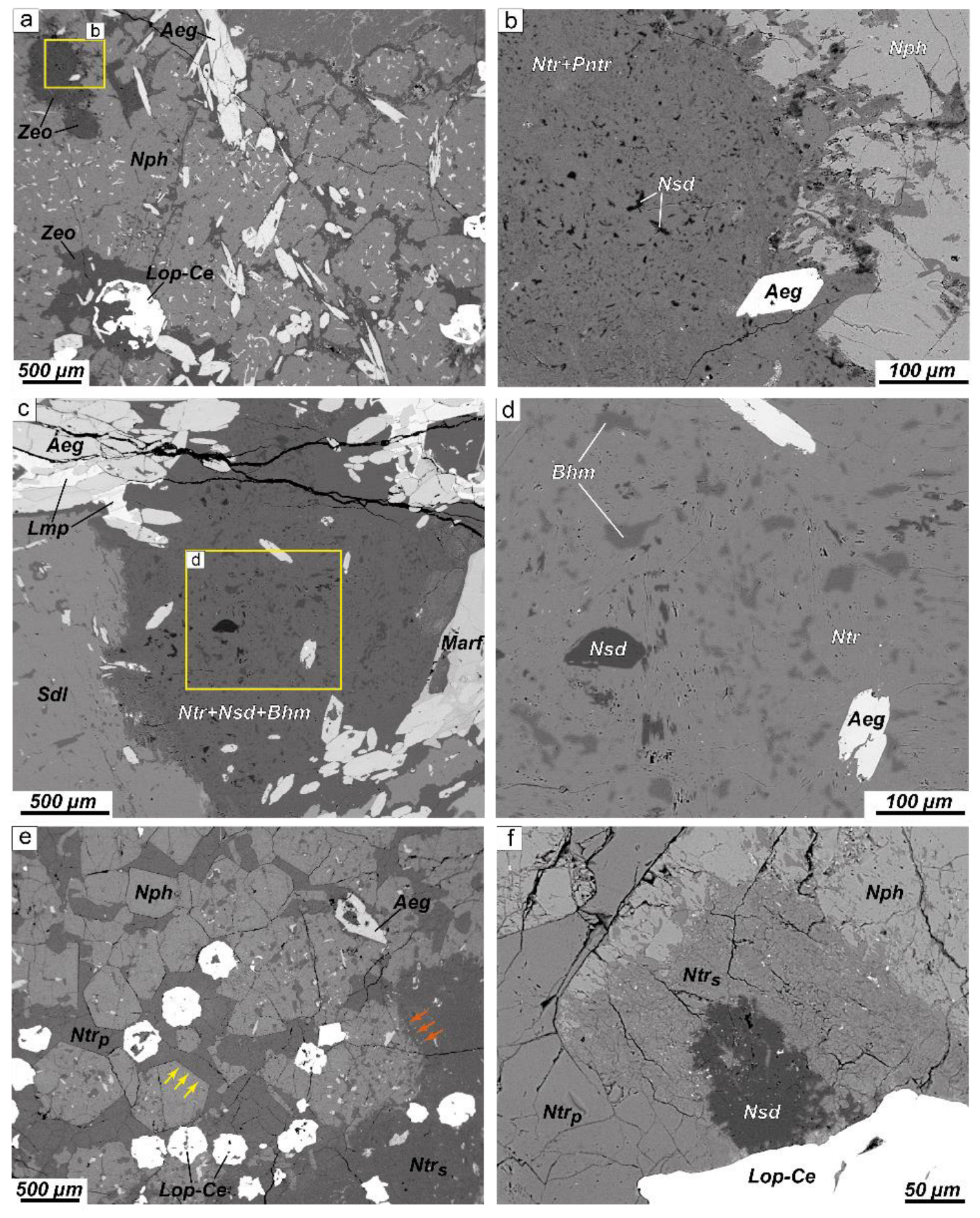
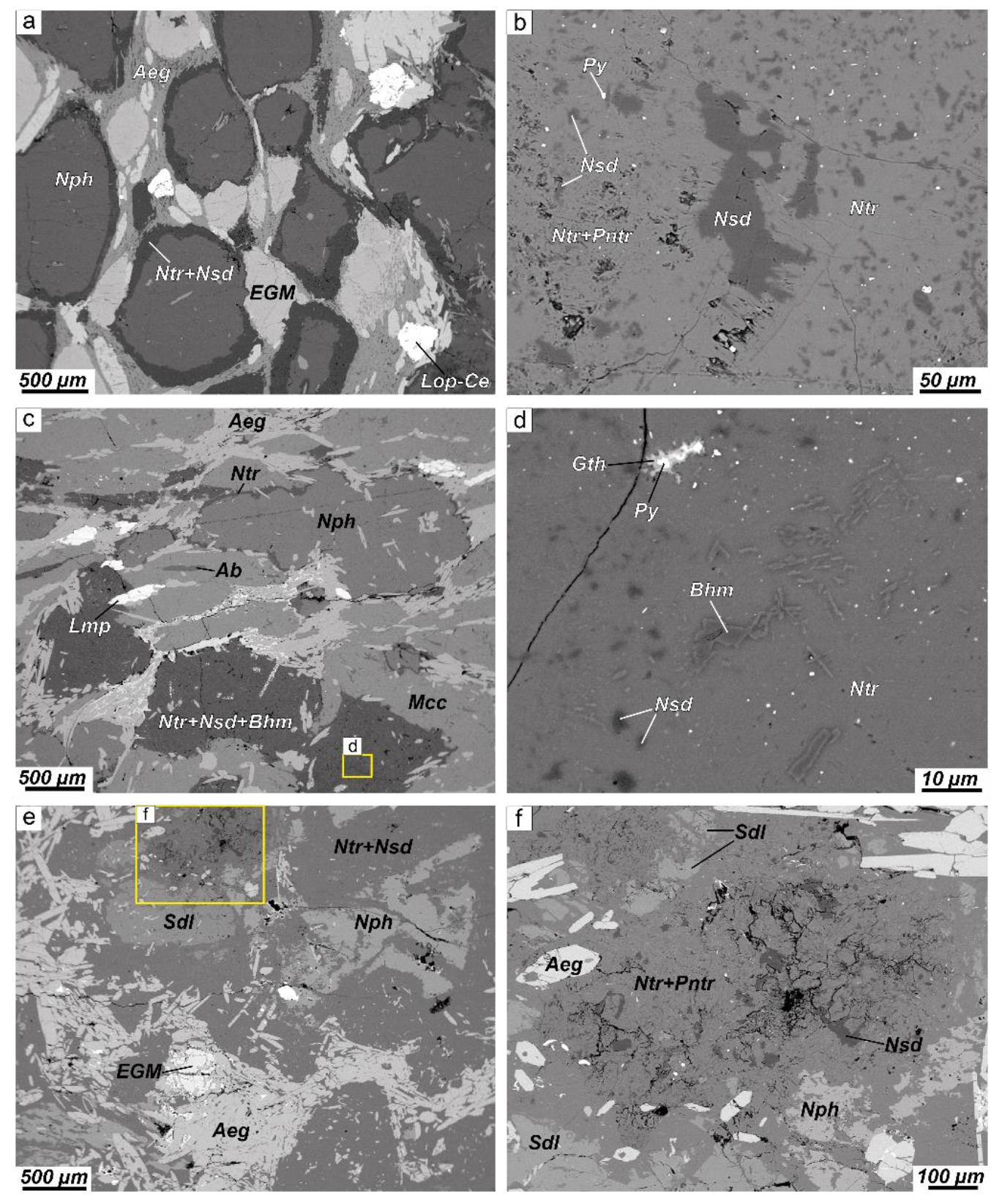
4.1. Petrography
4.1.1. Alteration of Nepheline and Sodalite in the Layered Complex
4.1.2. Alteration of Nepheline and Sodalite in the Eudialyte Complex
4.1.3. Alteration of Nepheline and Sodalite in the Poikilitic Complex
4.2. Mineral Chemistry
4.3. The Ratio of the Volumes of Nepheline Substitution Products
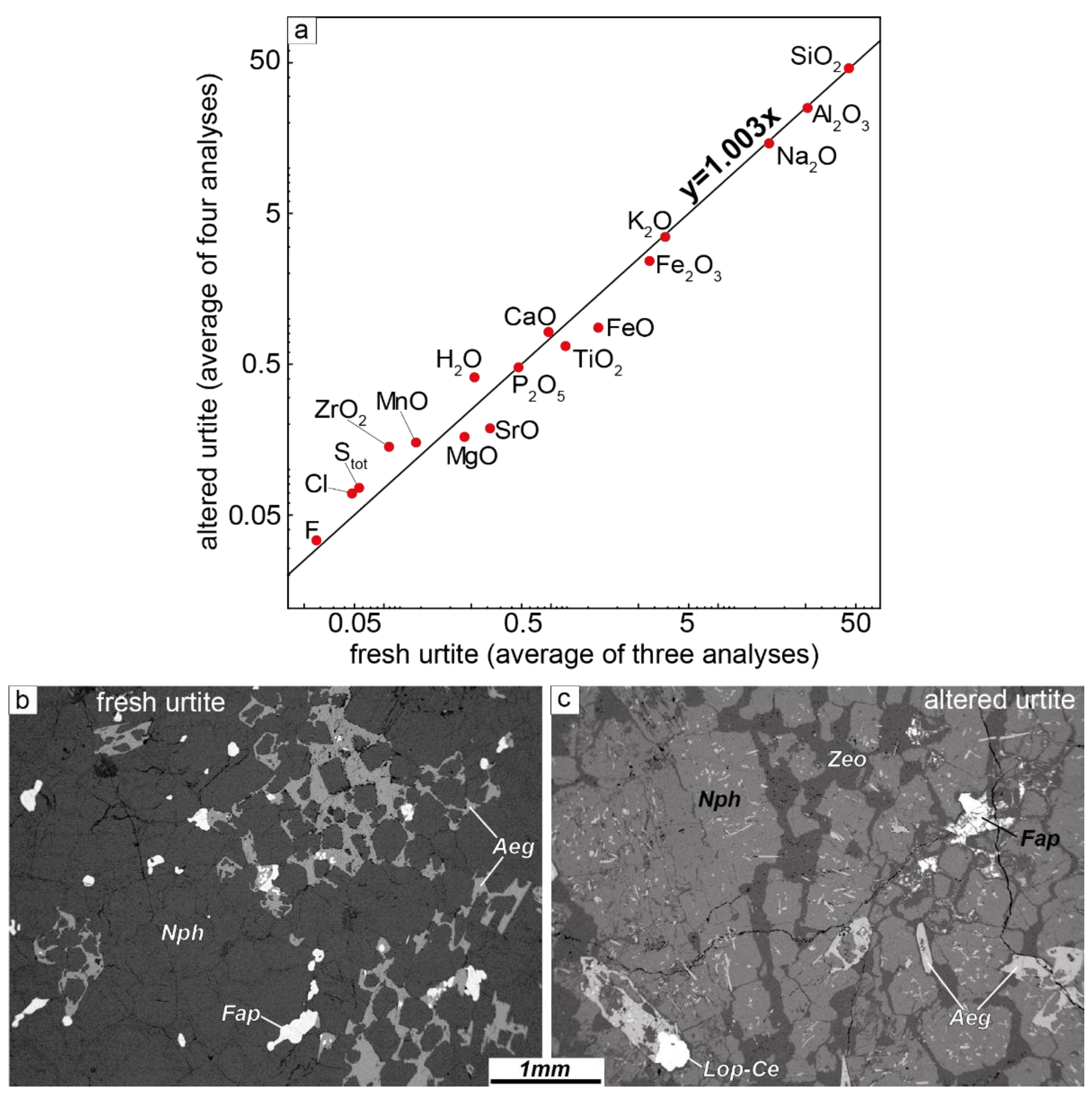
4.4. Changes in the Chemical Compositions of Rocks during the Secondary Alteration of Feldspathoids
5. Discussion
- -
- -
- -
- -
- -
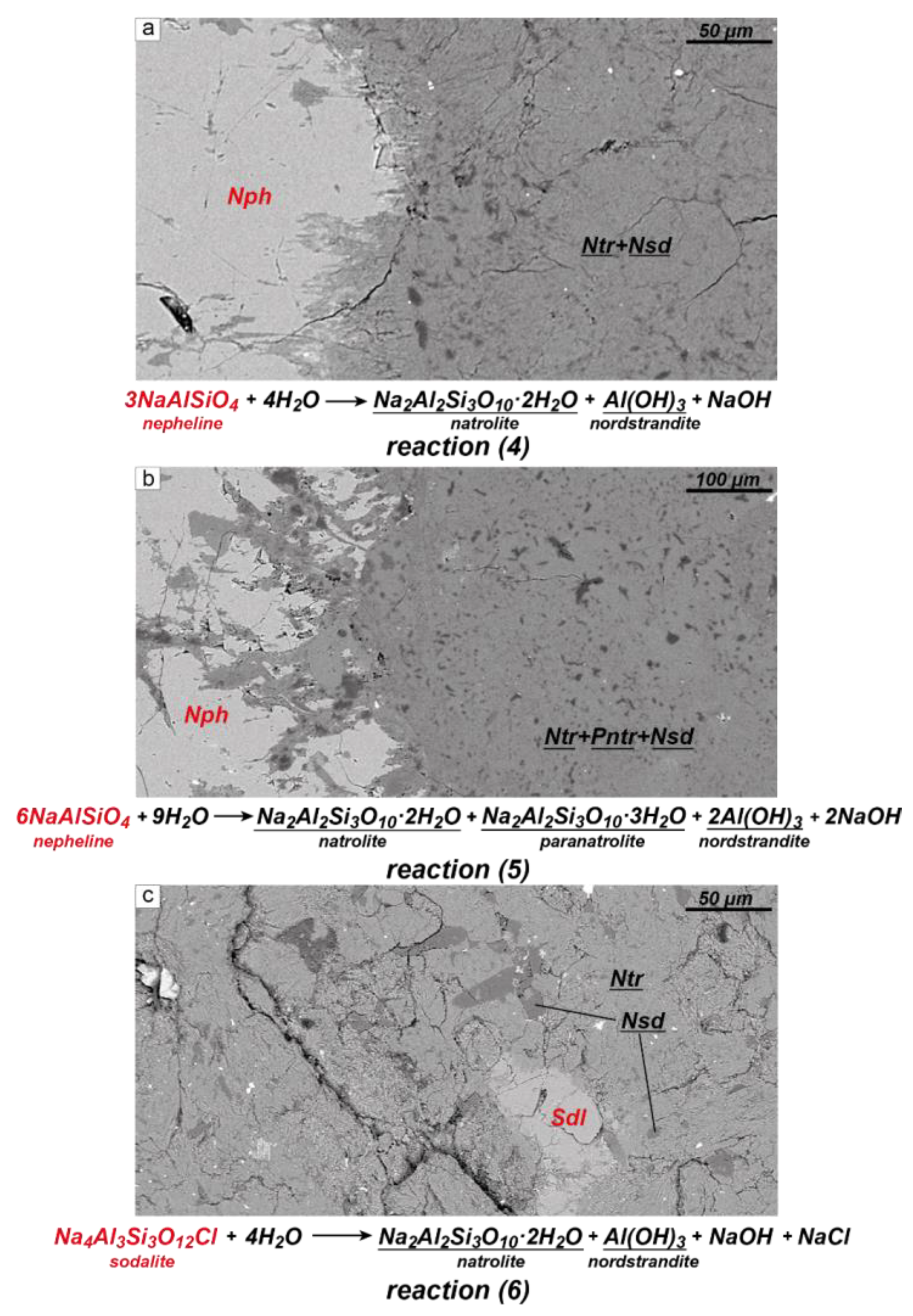
- In cases where the product phase has a different crystal structure to the parent, the product may be polycrystalline. In all of the studied samples, secondary natrolite and paranatrolite are represented by a fine-grained aggregate.
6. Conclusions
- During the late-stage (auto)metasomatic alterations of peralkaline rocks, nepheline and sodalite are replaced by the association between natrolite + nordstrandite ± paranatrolite ± böhmite. This process occurs in all main rock types of the Lovozero massif.
- The substitutions of nepheline and sodalite occur according to the following reactions: 3Nph + 4H2O → Ntr + Nsd + NaOH; 6Nph + 9H2O → Ntr + Pntr + 2Nsd + 2NaOH; Sdl + 4H2O → Ntr + Nsd + NaOH + NaCl.
- In these reactions, about one-third of the sodium from nepheline (and sodalite) is set free and passes into the fluid. This leads to an increase in the Na/Cl ratio and, hence, the pH of the fluid.
- An increase in pH stabilizes hyperagpaitic minerals, which can crystallize in close proximity to pseudomorphized nepheline and sodalite.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- LeMaitre, R.W. Igneous Rocks. A Classification and Glossary of Terms. Recommendations of the International Union of Geological Sciences Subcommission on the Systematics of Igneous Rocks; LeMaitre, R.W., Ed.; Cambridge University Press: Cambridge, MA, USA, 2005. [Google Scholar]
- Marks, M.A.; Markl, G. A Global Review on Agpaitic Rocks. Earth Sci. Rev. 2017, 173, 229–258. [Google Scholar] [CrossRef]
- Kramm, U.; Kogarko, L.N. Nd and Sr Isotope Signatures of the Khibina and Lovozero Agpaitic Centres, Kola Alkaline Province, Russia. Lithos 1994, 32, 225–242. [Google Scholar] [CrossRef]
- Marks, M.; Vennemann, T.; Siebel, W.; Markl, G. Nd-, O-, and H-Isotopic Evidence for Complex, Closed-System Fluid Evolution of the Peralkaline Ilímaussaq Intrusion, South Greenland. Geochim. Cosmochim. Acta 2004, 68, 3379–3395. [Google Scholar] [CrossRef]
- Nielsen, T.F.D. Alkaline Dyke Swarms of the Gardiner Complex and the Origin of Ultramafic Alkaline Complexes. Geochem. Int. 1994, 31, 37–56. [Google Scholar]
- Kogarko, L.N. Ore-Forming Potential of Alkaline Magmas. Lithos 1990, 26, 167–175. [Google Scholar] [CrossRef]
- Sørrensen, H. Agpaitic Nepheline Syenites: A Potential Source of Rare Elements. Appl. Geochem. 1992, 7, 417–427. [Google Scholar] [CrossRef]
- Sorensen, H. The Agpaitic Rocks—An Overview. Miner. Mag 1997, 61, 485–498. [Google Scholar] [CrossRef]
- Markl, G.; Baumgartner, L. PH Changes in Peralkaline Late-Magmatic Fluids. Contrib. Mineral. Petrol. 2002, 144, 331–346. [Google Scholar] [CrossRef]
- Weisenberger, T.; Spürgin, S.; Lahaye, Y. Hydrothermal Alteration and Zeolitization of the Fohberg Phonolite, Kaiserstuhl Volcanic Complex, Germany. Int. J. Earth Sci. 2014, 103, 2273–2300. [Google Scholar] [CrossRef]
- Schilling, J.; Marks, M.A.W.; Wenzel, T.; Vennemann, T.; Horváth, L.; Tarassoff, P.; Jacob, D.E.; Markl, G. The Magmatic to Hydrothermal Evolution of the Intrusive Mont Saint-Hilaire Complex: Insights into the Late-Stage Evolution of Peralkaline Rocks. J. Petrol. 2011, 52, 2147–2185. [Google Scholar] [CrossRef]
- Mitchell, R.H.; Wu, F.Y.; Yang, Y.H. In Situ U-Pb, Sr and Nd Isotopic Analysis of Loparite by LA-(MC)-ICP-MS. Chem. Geol. 2011, 280, 191–199. [Google Scholar] [CrossRef]
- Wu, F.Y.; Yang, Y.H.; Marks, M.A.W.; Liu, Z.C.; Zhou, Q.; Ge, W.C.; Yang, J.S.; Zhao, Z.F.; Mitchell, R.H.; Markl, G. In Situ U-Pb, Sr, Nd and Hf Isotopic Analysis of Eudialyte by LA-(MC)-ICP-MS. Chem. Geol. 2010, 273, 8–34. [Google Scholar] [CrossRef]
- Korchak, Y.A.; Men’shikov, Y.P.; Pakhomovskii, Y.A.; Yakovenchuk, V.N.; Ivanyuk, G.Y. Trap Formation of the Kola Peninsula. Petrology 2011, 19, 87–101. [Google Scholar] [CrossRef]
- Gerasimovsky, V.I.; Volkov, V.P.; Kogarko, L.N.; Polyakov, A.I.; Saprykina, T.V.; Balashov, Y.A. Geochemistry of the Lovozero Alkaline Massif; Nauka: Moscow, Russia, 1966. [Google Scholar]
- Bussen, I.V.; Sakharov, A.S. Petrology of the Lovozero Alkaline Massif; Nauka: Leningrad, Russia, 1972. [Google Scholar]
- Vlasov, K.A.; Kuzmenko, M.V.; Eskova, E.M. Lovozero Alkaline Massif; Academy of Sciences SSSR: Moskow, Russia, 1959. [Google Scholar]
- Lafuente, B.; Downs, R.T.; Yang, H.; Stone, N. The Power of Databases: The RRUFF Project. In Highlights in Mineralogical Crystallography; Armbruster, T., Danisi, R.M., Eds.; W. De Gruyter: Berlin, Germany, 2015; pp. 1–30. [Google Scholar]
- Warr, L.N. IMA-CNMNC Approved Mineral Symbols. Miner. Mag. 2021, 85, 291–320. [Google Scholar] [CrossRef]
- Johnsen, O.; Grice, J.D. The Crystal Chemistry of the Eudialyte Group. Can. Mineral. 1999, 37, 865–891. [Google Scholar]
- Pekov, I.V.; Turchkova, A.G.; Lovskaya, E.V.; Chukanov, N.V. Zeolites of Alkaline Massifs; Association “Ekost’’: Moskow, Russia, 2004. [Google Scholar]
- Chao, G.Y. Paranatrolite, a New Zeolite from Mount St-Hilaire, Quebec. Can Miner. 1980, 18, 85–88. [Google Scholar]
- Seryotkin, Y.V.; Bakakin, V.V.; Belitsky, I.A. The Crystal Structure of Paranatrolite. Eur. J. Mineral. 2004, 16, 545–550. [Google Scholar] [CrossRef]
- Grant, J.A. The Isocon Diagram-a Simple Solution to Gresens’ Equation for Metasomatic Alteration. Econ. Geol. 1986, 81, 1976–1982. [Google Scholar] [CrossRef]
- Grant, J.A. Isocon Analysis: A Brief Review of the Method and Applications. Phys. Chem. Earth 2005, 30, 997–1004. [Google Scholar] [CrossRef]
- Fall, A.; Bodnar, R.J.; Szabó, C.; Pál-Molnár, E. Fluid Evolution in the Nepheline Syenites of the Ditrǎu Alkaline Massif, Transylvania, Romania. Lithos 2007, 95, 331–345. [Google Scholar] [CrossRef]
- Putnis, A. Mineral Replacement Reactions. Rev. Miner. Geochem. 2009, 70, 87–124. [Google Scholar] [CrossRef]
- Khomyakov, A.P. Mineralogy of Hyperagpaitic Alkaline Rocks; Oxford University Press: Oxford, UK, 1995. [Google Scholar]
- Khomyakov, A.P. Salt Minerals in Ultra-Agpaitic Rocks and Ore Potential of Alkaline Massifs. Int. Geol. Rev. 1987, 29, 1446–1456. [Google Scholar] [CrossRef]


| Complex | Rock | Rock-Forming Minerals (Average Content, mod. %) | Characteristic Accessory Minerals 1 | Texture |
|---|---|---|---|---|
| Layered | Lujavrite | Microcline-perthite (45), nepheline + secondary natrolite (20), aegirine (25), magnesio-arfvedsonite (5) | EGM 2, lamprophyllite, sodalite, loparite-(Ce), barytolamprophyllite, sphalerite, fluorapatite, lorenzenite | Trachytoid; coarse- to medium-grained |
| Foyaite | Microcline-perthite (50), nepheline + secondary natrolite (35), aegirine (7) | Magnesio-arfvedsonite, EGM, sodalite, lamprophyllite, sphalerite, loparite-(Ce), murmanite, lomonosovite | Massif to weakly trachytoid; coarse- tomedium-grained | |
| Urtite | Nepheline + secondary natrolite (70), microcline-perthite (12), sodalite (7), aegirine (6) | Loparite-(Ce), fluorapatite, EGM, magnesio-arfvedsonite, titanite, murmanite, sphalerite | Massif; coarse- tomedium-grained | |
| Eudialyte | Eudialyte lujavrite | Microcline-perthite (35), EGM (25), aegirine (20), nepheline + secondary natrolite (15), magnesio-arfvedsonite (5) | Sodalite, loparite-(Ce), lamprophyllite, barytolamprophyllite, sphalerite | Trachytoid; coarse- to medium-grained |
| Foyaite | Microcline-perthite (55), nepheline + secondary natrolite (35), aegirine (7) | Magnesio-arfvedsonite, fluorapatite, sodalite, lovozerite-group minerals, EGM, murmanite, lomonosovite | Massif to weakly trachytoid; coarse- tomedium-grained | |
| Porphyritic/ fine-grained nepheline syenites | Fine-grained mass: albite (25), microcline (25), nepheline (20), aegirine (20), magnesio-arfvedsonite (5) Phenocrysts: nepheline (0–20), microcline-perthite (0–35) | EGM, lovozerite-group minerals, sodalite, murmanite, lamprophyllite | Porphyritic/ fine-grained | |
| Poikilitic | Uneven-grained nepheline syenite | Microcline-perthite, nepheline, magnesio-arfvedsonite, aegirine, orthoclase | Titanite, fluorapatite, ilmenite, diopside, EGM | Metasomatic, poikilitic |
| Poikilitic foid syenite | Orthoclase, sodalite, vishnevite, nepheline, cancrinite, katophorite, aegirine | Titanite, fluorapatite, ilmenite, diopside, phlogopite, EGM, zircon | Metasomatic, poikilitic |
| Sampling Point | Sample | Rock | Complex |
|---|---|---|---|
| 1 | LV-319A | lujavrite | Layered |
| 1 | LV-335/4 | foyaite | Layered |
| 1 | LV-319G | urtite | Layered |
| 1 | LV-335/6 | urtite | Layered |
| 1 | LV-335/2 | urtite | Layered |
| 1 | LV-315/1 | urtite | Layered |
| 1 | LV-335/3 | urtite | Layered |
| 1 | LV-306 | urtite | Layered |
| 1 | LV-303/2 | urtite | Layered |
| 1 | LV-319/3 | urtite | Layered |
| 1 | LV-319/2 | urtite | Layered |
| 1 | LV-420 | foyaite | Eudialyte |
| 2 | LV-366A-2 | eudialyte lujavrite | Eudialyte |
| 2 | LV-335E-1 | eudialyte lujavrite | Eudialyte |
| 2 | LV-350/1 | eudialyte lujavrite | Eudialyte |
| 2 | LV-354/2 | poikilitic foid syenite | Poikilitic |
| 3 | LV-356/3 | poikilitic foid syenite | Poikilitic |
| 3 | LV-382/4 | poikilitic foid syenite | Poikilitic |
| 3 | LV-384/1 | eudialyte lujavrite | Eudialyte |
| 3 | LV-377/2 | eudialyte lujavrite | Eudialyte |
| 4 | LV-363/17 | urtite | Layered |
| 4 | LV-363/6 | foyaite | Eudialyte |
| 4 | LV-363/18 | foyaite | Eudialyte |
| Abbreviation [19] | Mineral | Formula 1 |
|---|---|---|
| Ab | albite | Na(AlSi3O8) |
| Aeg | aegirine | NaFe3+Si2O6 |
| Anl | analcime | Na(AlSi2O6)·H2O |
| Bhm | böhmite | AlO(OH) |
| EGM | eudialyte-group mineral | [20] |
| Fap | fluorapatite | Ca5(PO4)3F |
| Gon | gonnardite | (Na,Ca)2(Si,Al)5O10·3H2O |
| Gth | goethite | FeO(OH) |
| Lmp | lamprophyllite | (SrNa)Ti2Na3Ti(Si2O7)2O2(OH)2 |
| Lop-Ce | loparite-(Ce) | (Na,Ce,Sr)(Ce,Th)(Ti,Nb)2O6 |
| Lvz | lovozerite | Na3CaZrSi6O15(OH)3 |
| Marf | magnesio-arfvedsonite | NaNa2(Mg4Fe3+)Si8O22(OH)2 |
| Mcc | microcline | K(AlSi3O8) |
| Mes | mesolite | Na2Ca2(Si9Al6)O30·8H2O |
| Mmn | murmanite | Na2Ti2Na2Ti2(Si2O7)2O4(H2O)4 |
| Nj | naujakasite | Na6Fe2+Al4Si8O26 |
| Nph | nepheline | Na3K(Al4Si4O16) |
| Ns | natrosilite | Na2Si2O5 |
| Nsd | nordstrandite | Al(OH)3 |
| Ntr | natrolite | Na2(Si3Al2)O10·2H2O |
| Pntr | paranatrolite | Na2(Si3Al2)O10·3H2O |
| Py | pyrite | FeS2 |
| Rha-La | rhabdophane-(La) | La(PO4)·H2O |
| Sdl | sodalite | Na4(Si3Al3)O12Cl |
| Ssp-Ce | steenstrupine-(Ce) | Na14Ce6Mn2+2Fe3+2Zr(PO4)7Si12O36(OH)2·3H2O |
| Thm-Ca | thomsonite-Ca | NaCa2(Al5Si5)O20·6H2O |
| Tn | trona | Na3(HCO3)(CO3)·2H2O |
| Tnat | thermonatrite | Na2(CO3)·H2O |
| Ttp | tugtupite | Na4BeAlSi4O12Cl |
| Usg | ussingite | Na2AlSi3O8(OH) |
| Vll | villiaumite | NaF |
| Complex | Layered | Eudialyte | Poikilitic | |||
|---|---|---|---|---|---|---|
| Rock | Lujavrite | Foyaite | Urtite | Urtite | Eudialyte Lujavrite | Poikilitic Sodalite Syenite |
| Sample | LV-319A | LV-335/4 | LV-335/6 | LV-319G | LV-350/1 | LV-356/3 |
| SiO2 | 42.88 | 41.42 | 44.95 | 41.57 | 44.80 | 45.34 |
| Al2O3 | 31.99 | 33.53 | 30.79 | 33.28 | 31.11 | 30.11 |
| Fe2O3 | 1.11 | 0.05 | 1.65 | 0.08 | 1.29 | 1.70 |
| Na2O | 15.69 | 15.48 | 16.27 | 16.11 | 16.13 | 15.88 |
| K2O | 6.63 | 6.96 | 5.32 | 6.97 | 5.69 | 5.64 |
| Sum | 98.30 | 97.44 | 98.98 | 98.01 | 99.02 | 98.67 |
| Formula based on 16 oxygens, apfu | ||||||
| Si | 4.21 | 4.10 | 4.35 | 4.10 | 4.34 | 4.40 |
| Al | 3.70 | 3.91 | 3.51 | 3.87 | 3.55 | 3.45 |
| Fe3+ | 0.08 | – | 0.12 | 0.01 | 0.09 | 0.12 |
| Na | 2.99 | 2.97 | 3.05 | 3.08 | 3.03 | 2.99 |
| K | 0.83 | 0.88 | 0.66 | 0.88 | 0.70 | 0.70 |
| Sum | 11.81 | 11.87 | 11.69 | 11.94 | 11.71 | 11.66 |
| Complex | Layered | Eudialyte | Poikilitic | ||
|---|---|---|---|---|---|
| Rock | Urtite | Urtite | Eudialyte Lujavrite | Poikilitic Sodalite Syenite | |
| Sample | LV-335/6 | LV-319G | LV-350/2 | LV-356/3 | LV-356/3 |
| SiO2 | 36.66 | 37.06 | 37.06 | 37.27 | 37.34 |
| Al2O3 | 31.66 | 31.12 | 31.22 | 30.03 | 29.87 |
| Fe2O3 | 0.05 | 0.07 | 0.15 | 0.86 | 0.36 |
| Na2O | 26.24 | 23.92 | 25.36 | 25.96 | 25.14 |
| Cl | 6.17 | 5.34 | 4.55 | 6.25 | 5.56 |
| S | 1.04 | 0.93 | 1.78 | 0.43 | 0.90 |
| –O=Cl | 1.39 | 1.21 | 1.41 | 1.03 | 1.26 |
| –O=S | 0.52 | 0.47 | 0.21 | 0.89 | 0.45 |
| Sum | 99.90 | 96.77 | 99.18 | 98.20 | 97.47 |
| Formula based on Si + Al + Fe3+ = 6, apfu | |||||
| Si | 2.97 | 3.01 | 3.01 | 3.05 | 3.08 |
| Al | 3.03 | 2.98 | 2.98 | 2.90 | 2.90 |
| Fe3+ | – | – | 0.01 | 0.05 | 0.02 |
| Na | 4.12 | 3.77 | 3.99 | 4.12 | 4.02 |
| Cl | 0.85 | 0.74 | 0.63 | 0.87 | 0.78 |
| S | 0.16 | 0.14 | 0.27 | 0.07 | 0.14 |
| Complex | Layered | Layered | Eudialyte | Poikilitic | |
|---|---|---|---|---|---|
| Rock | Foyaite | Lujavrite | Eudialyte Lujavrite | Poikilitic Sodalite Syenite | |
| Sample | LV-335/4 | LV-319A | LV-350/1 | LV-356/3 | LV-356/3 |
| Mineral | Ntr | Ntr | Pntr | Pntr | Pntr |
| SiO2 | 47.15 | 47.73 | 42.92 | 41.80 | 41.57 |
| Al2O3 | 27.20 | 26.58 | 28.48 | 31.23 | 27.90 |
| Na2O | 14.37 | 14.51 | 15.17 | 11.91 | 13.39 |
| K2O | bdl | bdl | 1.99 | 3.00 | 1.98 |
| Sum | 88.72 | 88.82 | 88.56 | 87.94 | 84.84 |
| Formula based on Si + Al = 5, apfu | |||||
| Si | 2.98 | 3.02 | 2.81 | 2.66 | 2.79 |
| Al | 2.02 | 1.98 | 2.19 | 2.34 | 2.21 |
| Na | 1.76 | 1.78 | 1.92 | 1.47 | 1.74 |
| K | – | – | 0.17 | 0.24 | 0.17 |
| H2O 1 | 2.37 | 2.36 | 2.51 | 2.57 | 3.39 |
| Sample | Rock | Complex | Number of Analyzed BSE-Images | Al-O-H Phases, % of Secondary Minerals Area (Min–Max) | Zeolites, % of Secondary Minerals Area (Min–Max) |
|---|---|---|---|---|---|
| LV-319A | lujavrite | Layered | 4 | 10.8–17.5 | 82.5–89.2 |
| LV-335/4 | foyaite | Layered | 3 | 16.8–19.2 | 80.8–83.2 |
| LV-319G | urtite | Layered | 3 | 15.0–18.2 | 81.8–85 |
| LV-335/2 | urtite | Layered | 4 | 14.9–18.5 | 81.5–85.1 |
| LV-350/1 | eudialyte lujavrite | Eudialyte | 3 | 11.0–15.4 | 84.6–89 |
| LV-377/2 | eudialyte lujavrite | Eudialyte | 5 | 16.2–21.1 | 78.9–83.8 |
| LV-363/18 | foyaite | Eudialyte | 2 | 15.8–16.1 | 83.9–84.2 |
| LV-363/6 | foyaite | Eudialyte | 3 | 14.5–18.2 | 81.8–85.5 |
| LV-356/3 | poikilitic foid syenite | Poikilitic | 1 | 17.6 | 82.4 |
| LV-382/4 | poikilitic foid syenite | Poikilitic | 2 | 16.4–16.7 | 83.3–83.6 |
| Fresh Urtite | Altered Urtite | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample | LV-303/2 | LV-319/3 | LV-319/2 | Average of Three Analyses | LV-335/2 | LV-335/3 | LV-315/1 | LV-306 | Average of Four Analyses |
| SiO2 | 45.18 | 45.15 | 45.03 | 45.12 | 47.07 | 50.12 | 43.71 | 43.56 | 46.12 |
| TiO2 | 0.46 | 0.27 | 2.03 | 0.92 | 0.48 | 0.39 | 1.24 | 0.52 | 0.66 |
| ZrO2 | 0.07 | 0.04 | 0.13 | 0.08 | 0.06 | 0.36 | 0.07 | 0.08 | 0.14 |
| Al2O3 | 25.03 | 28.26 | 24.61 | 25.97 | 23.60 | 22.87 | 27.34 | 24.57 | 24.60 |
| Fe2O3 | 3.44 | 1.45 | 3.87 | 2.92 | 2.97 | 1.84 | 2.37 | 2.48 | 2.42 |
| FeO | 1.71 | 1.10 | 1.53 | 1.45 | 0.65 | 0.78 | 0.62 | 1.41 | 0.87 |
| MnO | 0.12 | 0.09 | 0.14 | 0.12 | 0.11 | 0.19 | 0.12 | 0.18 | 0.15 |
| MgO | 0.22 | 0.09 | 0.37 | 0.23 | 0.14 | 0.13 | 0.19 | 0.20 | 0.17 |
| CaO | 1.67 | 0.10 | 0.42 | 0.73 | 0.27 | 0.37 | 0.16 | 2.48 | 0.82 |
| SrO | 0.44 | 0.40 | 0.14 | 0.33 | 0.02 | 0.05 | 0.12 | 0.56 | 0.19 |
| Na2O | 14.82 | 16.56 | 14.37 | 15.25 | 14.37 | 12.89 | 15.63 | 14.89 | 14.45 |
| K2O | 3.32 | 3.56 | 3.92 | 3.60 | 3.07 | 4.32 | 3.47 | 3.17 | 3.51 |
| P2O5 | 1.35 | 0.04 | 0.06 | 0.48 | 0.04 | 0.09 | 0.04 | 1.72 | 0.47 |
| H2O | 0.19 | 0.31 | 0.28 | 0.26 | 0.35 | 0.32 | 0.49 | 0.49 | 0.41 |
| CO2 | bdl | bdl | bdl | - | bdl | bdl | bdl | bdl | - |
| F | 0.06 | 0.02 | 0.01 | 0.03 | 0.01 | 0.01 | 0.03 | 0.08 | 0.03 |
| Cl | 0.10 | 0.03 | 0.02 | 0.05 | 0.06 | 0.04 | 0.11 | 0.07 | 0.07 |
| Stot | 0.08 | 0.03 | 0.05 | 0.05 | 0.10 | 0.01 | 0.07 | 0.12 | 0.08 |
| Loi | 1.25 | 2.57 | 2.23 | 2.02 | 6.96 | 5.56 | 3.97 | 2.92 | 4.85 |
| Sum | 99.51 | 100.06 | 99.21 | 100.33 | 100.34 | 99.85 | 99.50 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikhailova, J.A.; Pakhomovsky, Y.A.; Lyalina, L.M.; Selivanova, E.A. Alteration of Feldspathoids Changes pH of Late-Magmatic Fluids: A Case Study from the Lovozero Peralkaline Massif, Russia. Minerals 2023, 13, 39. https://doi.org/10.3390/min13010039
Mikhailova JA, Pakhomovsky YA, Lyalina LM, Selivanova EA. Alteration of Feldspathoids Changes pH of Late-Magmatic Fluids: A Case Study from the Lovozero Peralkaline Massif, Russia. Minerals. 2023; 13(1):39. https://doi.org/10.3390/min13010039
Chicago/Turabian StyleMikhailova, Julia A., Yakov A. Pakhomovsky, Lyudmila M. Lyalina, and Ekaterina A. Selivanova. 2023. "Alteration of Feldspathoids Changes pH of Late-Magmatic Fluids: A Case Study from the Lovozero Peralkaline Massif, Russia" Minerals 13, no. 1: 39. https://doi.org/10.3390/min13010039
APA StyleMikhailova, J. A., Pakhomovsky, Y. A., Lyalina, L. M., & Selivanova, E. A. (2023). Alteration of Feldspathoids Changes pH of Late-Magmatic Fluids: A Case Study from the Lovozero Peralkaline Massif, Russia. Minerals, 13(1), 39. https://doi.org/10.3390/min13010039








