The Solid Solution between NaClO3 and NaBrO3 Revisited
Abstract
1. Introduction
1.1. General Concept of Solid Solutions and Motivations for the Choice of NaClO3-NaBrO3 System
1.2. Crystal Structure of NaClO3 and NaBrO3 Pure Compounds and Chirality in the Solid State
1.3. NaClO3(x)BrO3(1−x) Mixed Crystals: Kinetic Ordering and Deviation from Ideal Cubic Structure
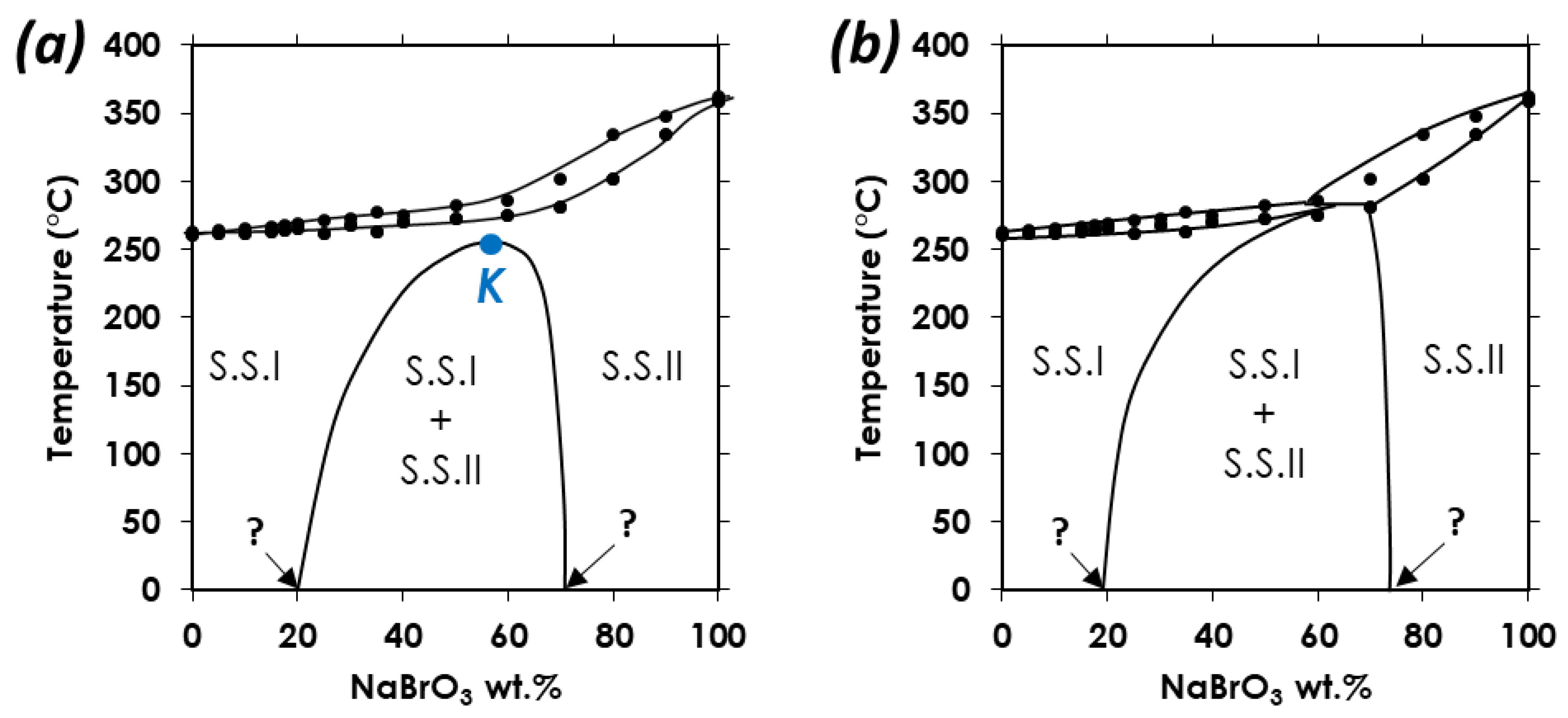
1.4. Heterogeneous Equilibria
1.5. Summary of the Literature Review and Aim of That Study
2. Material and Methods
2.1. Commercial Material
2.2. Crystallization by Solvent Evaporation
2.3. Crystallization by Spray-Drying
2.4. XRPD
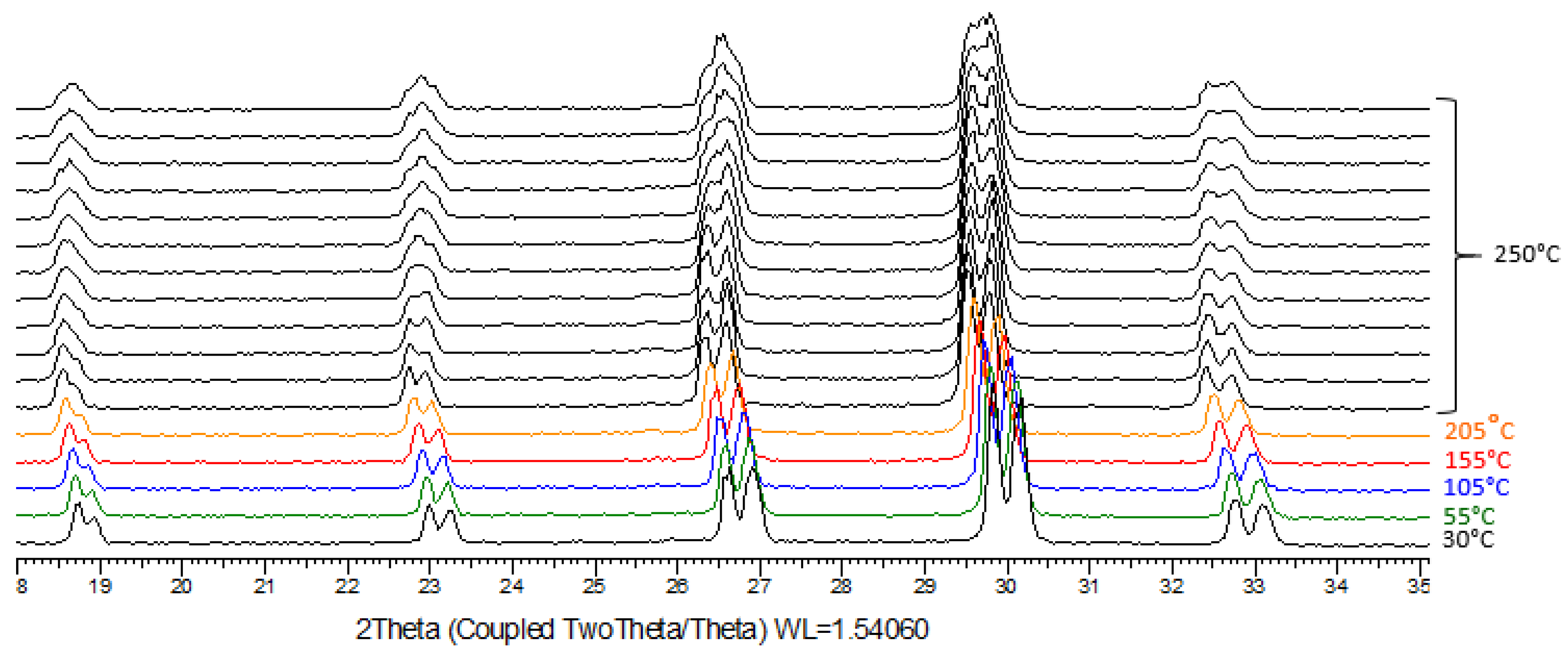
2.5. TR-XRPD
2.6. DSC
2.7. SEM
2.8. SEM-EDX
2.9. Optical Microscope
3. Results and Discussion
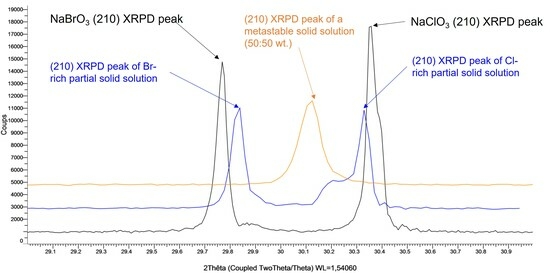
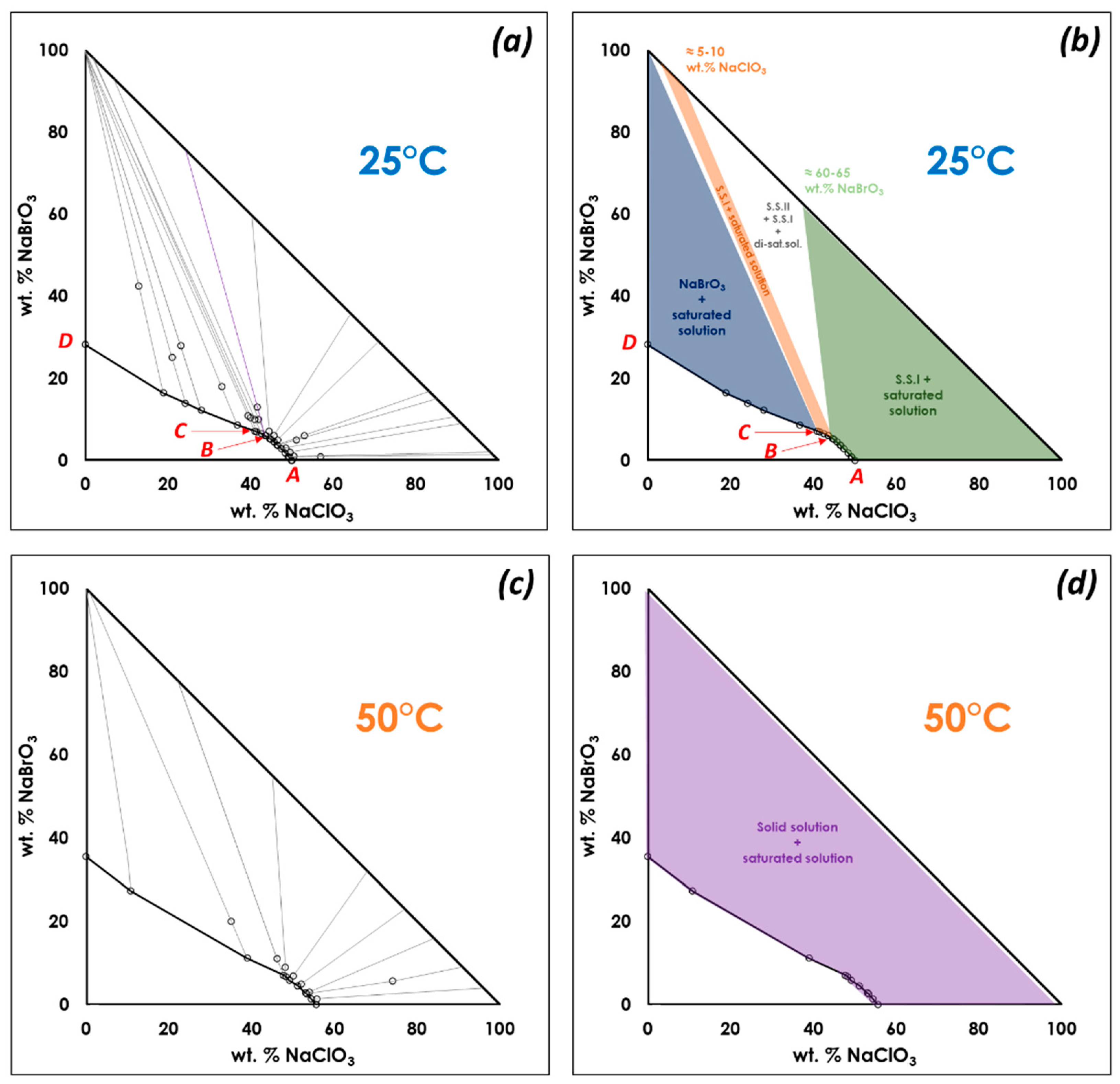
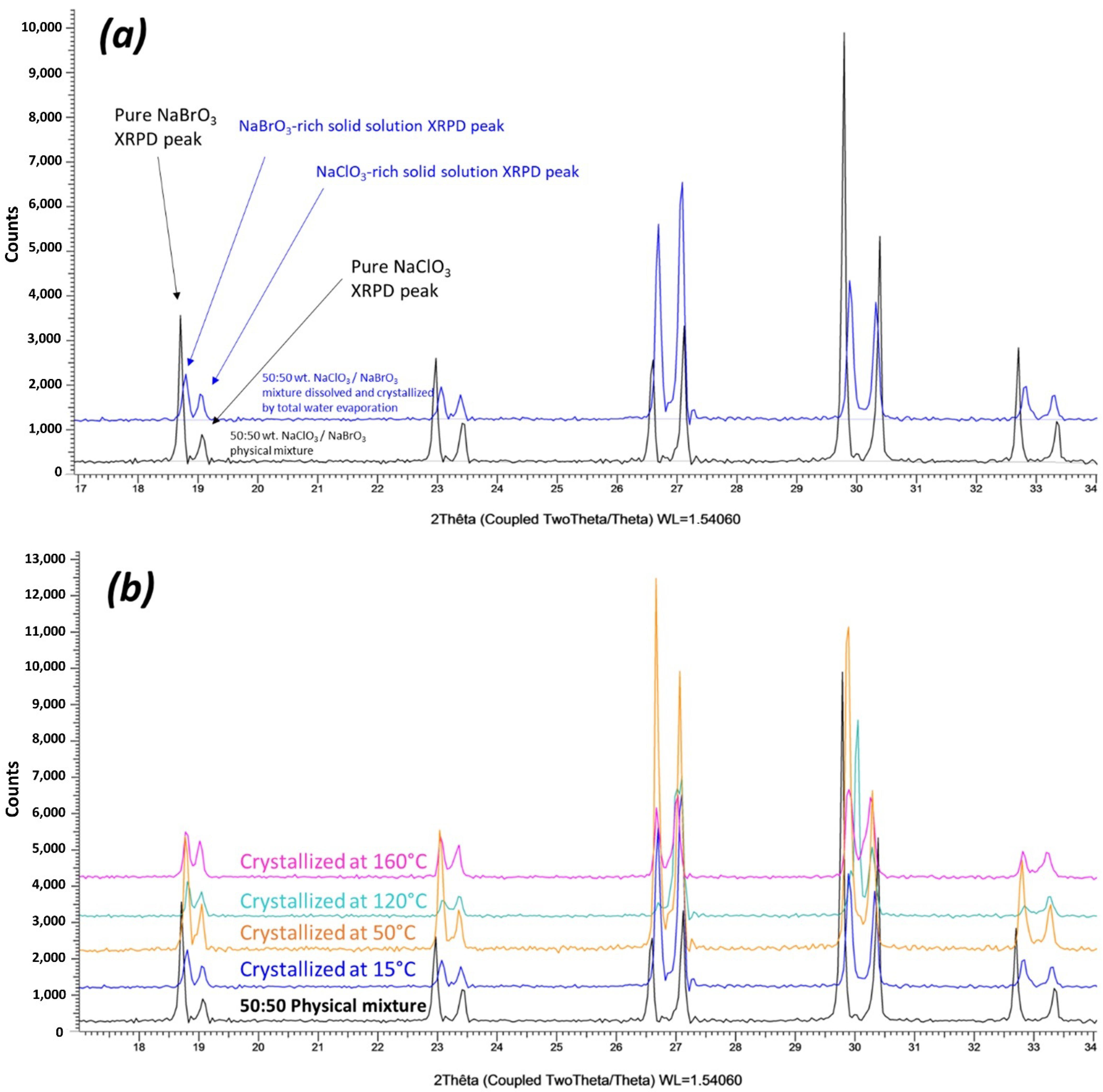
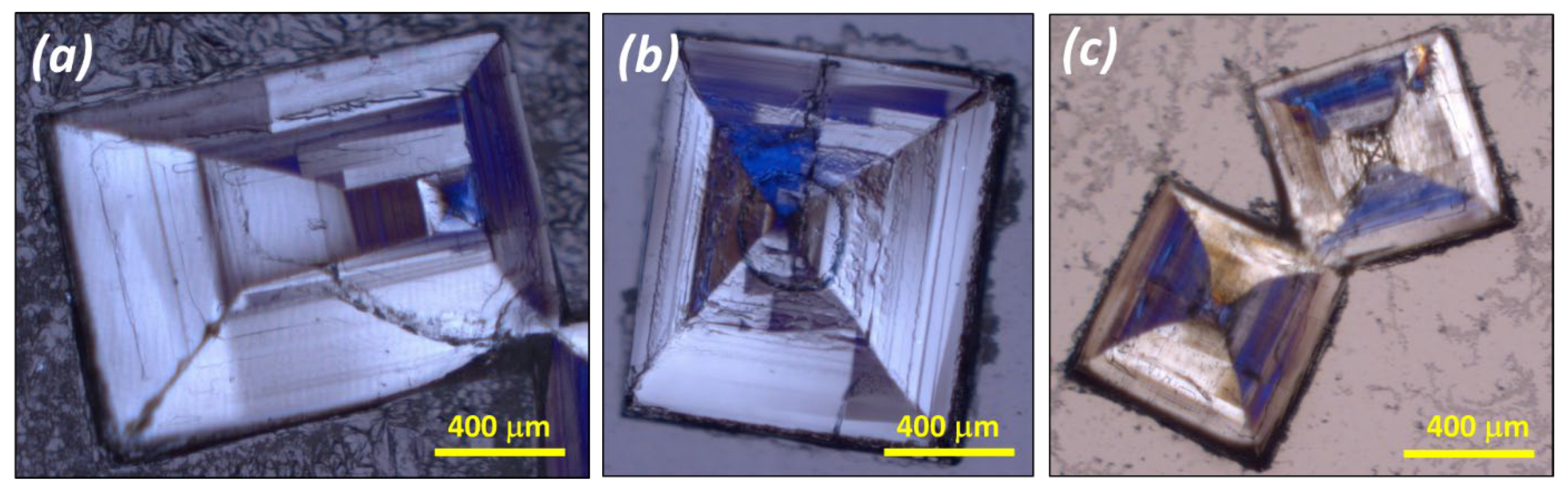
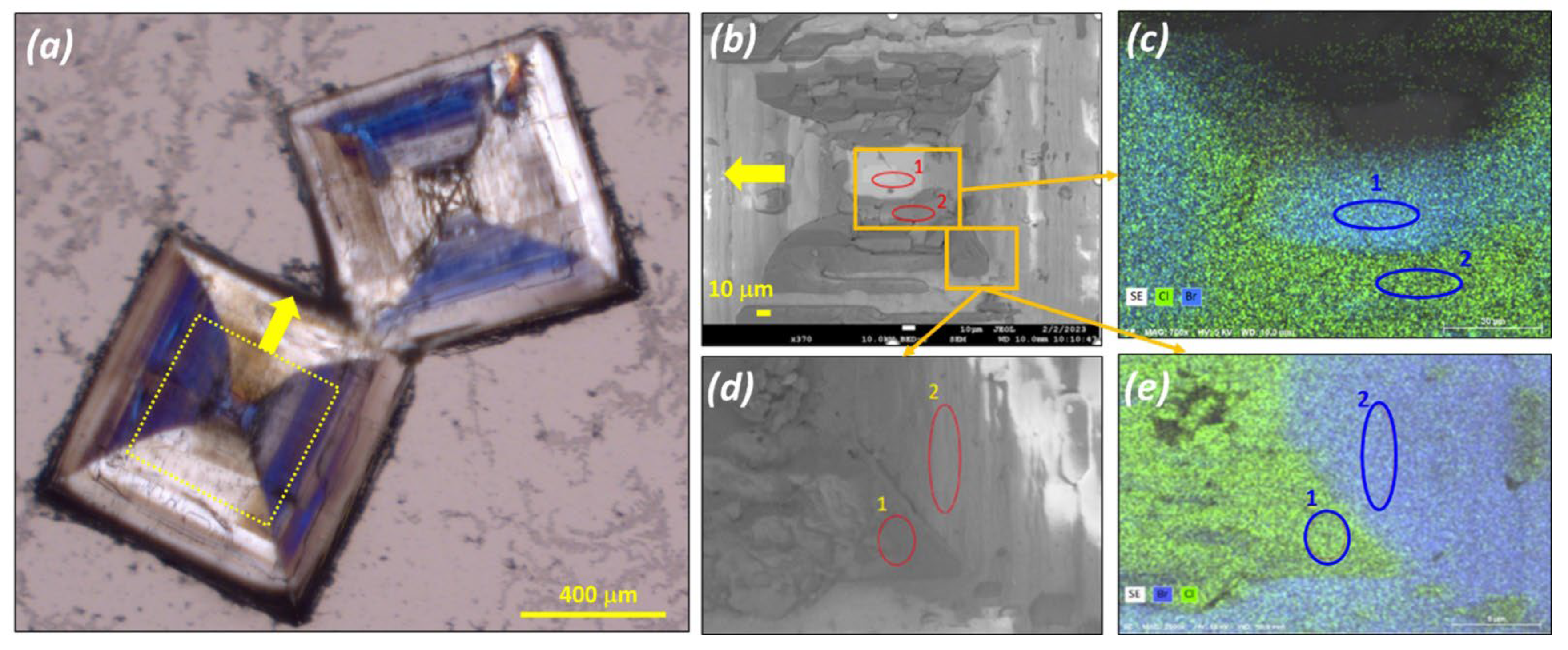
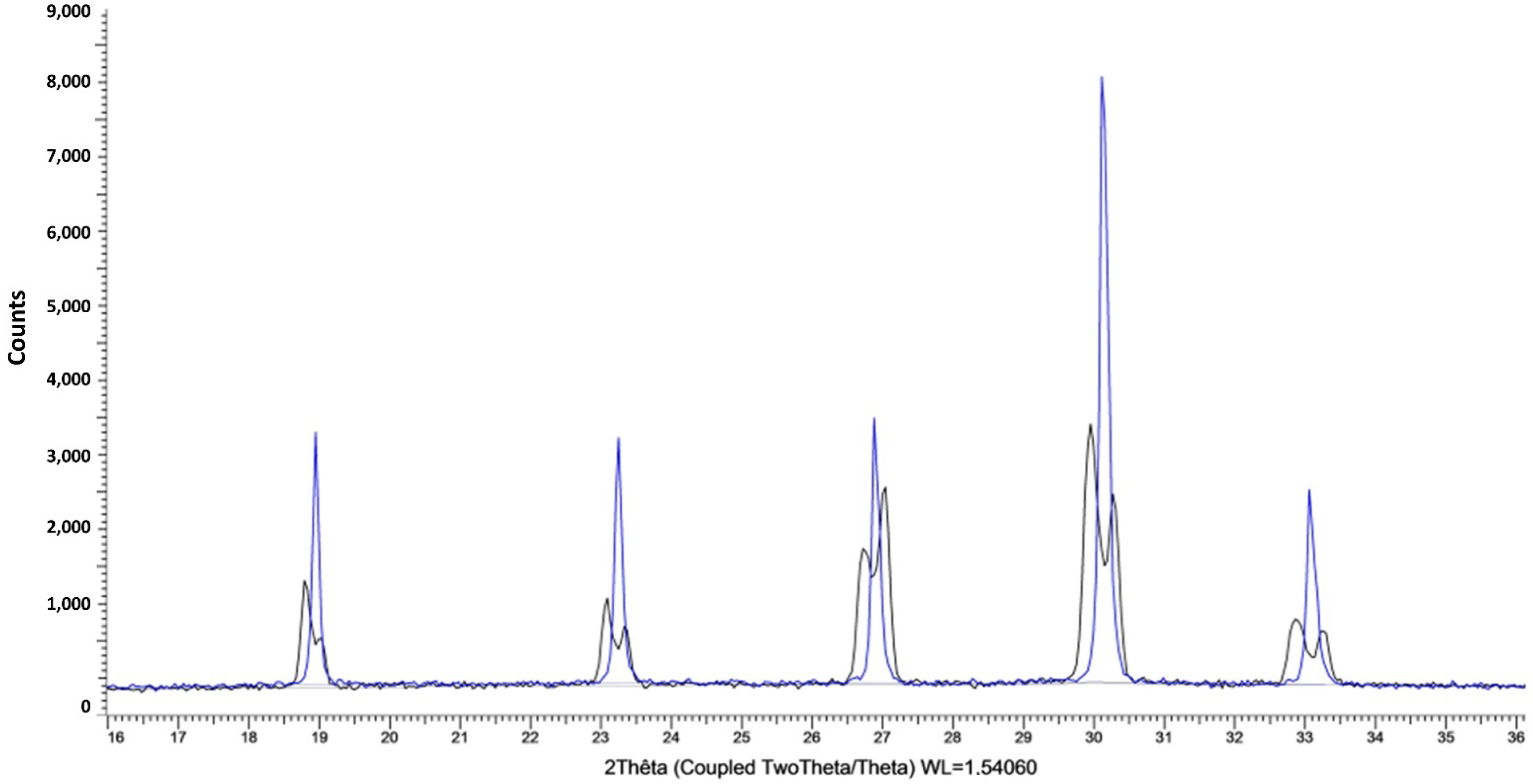
3.1. Confirmation of Kinetic Ordering through Classical (Slow) Water-Evaporation Crystallization Process Evidence of a Miscibility Gap at 20 °C
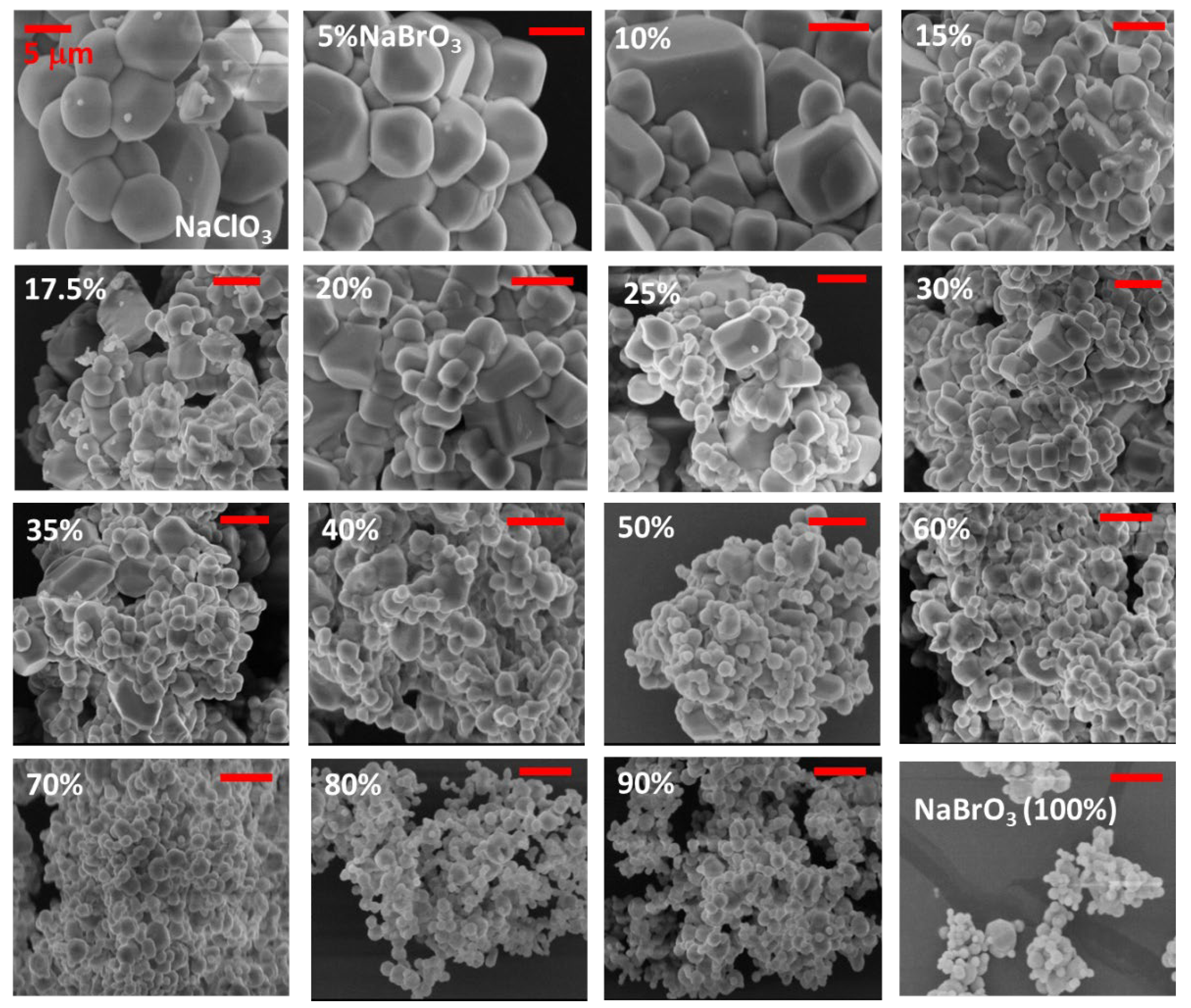
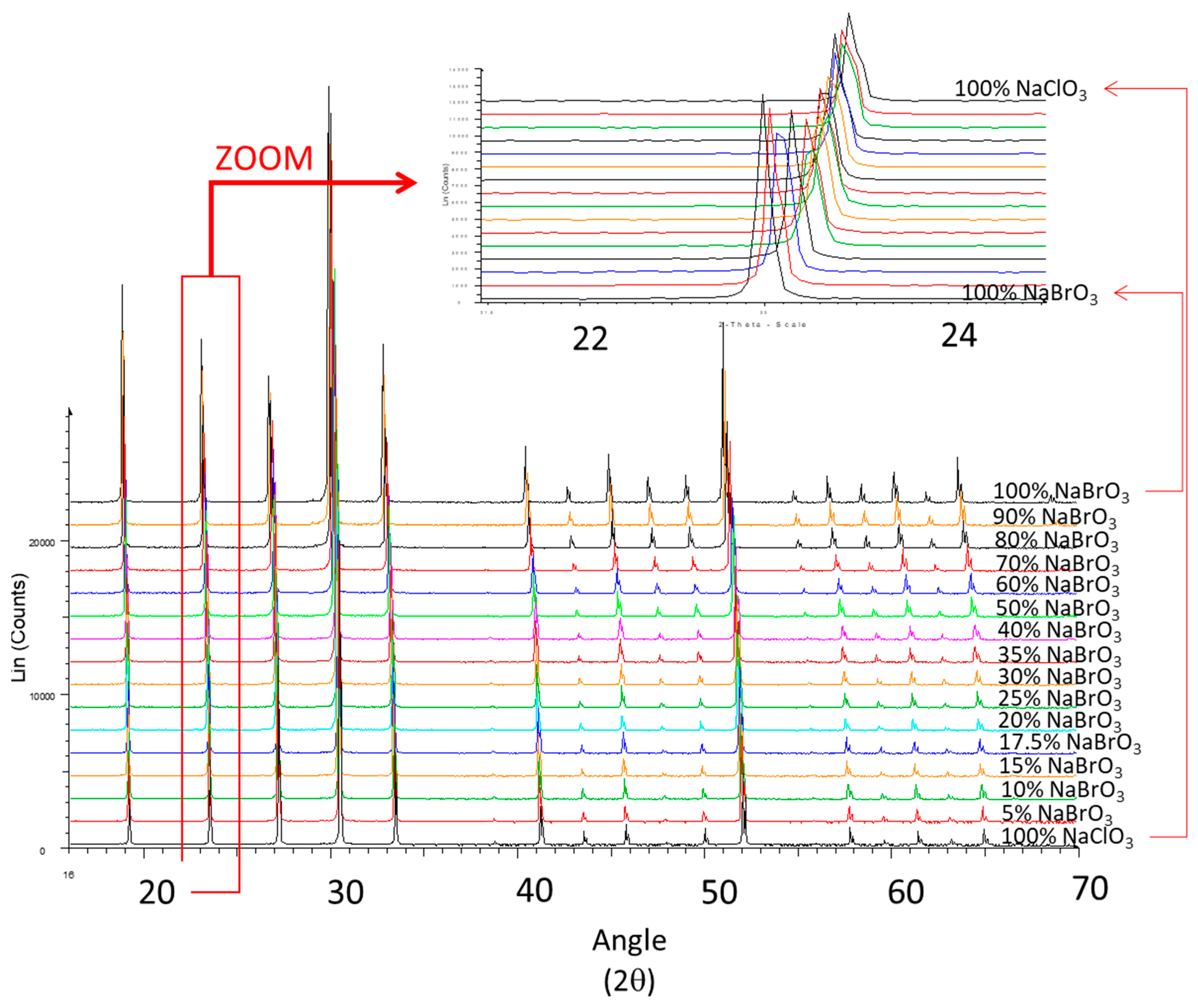
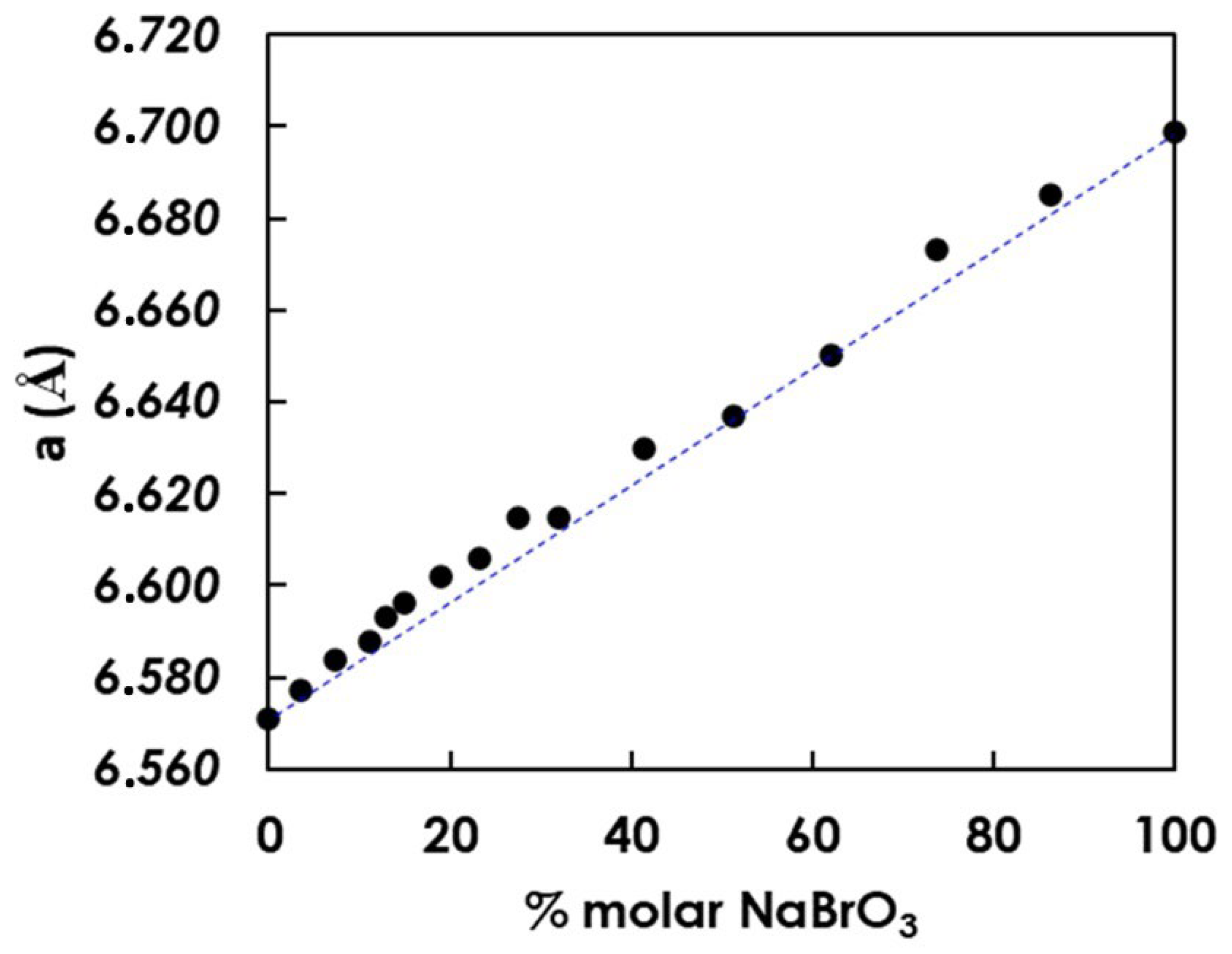
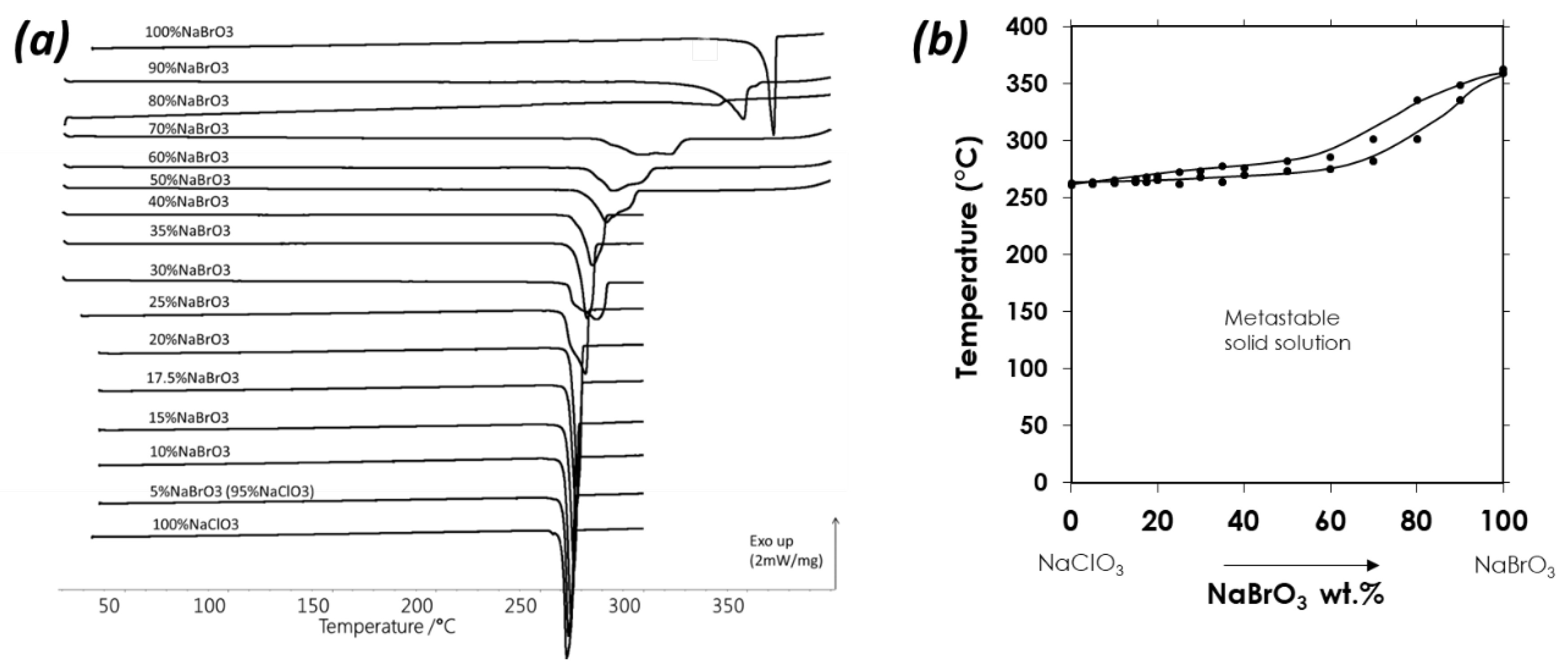
3.2. Crystallization of NaClO3/NaBrO3 Mixtures by Spray-Drying. Evidence of a Metastable Complete Solid Solution
4. Conclusions & Perspectives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vegard, L. The constitution of the solid solution and the space filling of the atoms. Z. Phys. 1921, 5, 17–26. [Google Scholar] [CrossRef]
- King, H.W. Qualitative size-factors for metallic solid solutions. J. Mater. Sci. 1966, 1, 79–90. [Google Scholar] [CrossRef]
- Denton, A.R.; Ashcroft, N.W. Vegard’s law. Phys. Rev. A 1991, 43, 3161–3164. [Google Scholar] [CrossRef]
- Kipping, F.S.; Pope, W.J. Enantiomorphism. J. Chem. Soc. Trans. 1898, 73, 606–617. [Google Scholar] [CrossRef]
- Kondepudi, D.K.; Kaufman, R.J.; Singh, N. Chiral symmetry breaking in sodium chlorate crystallization. Science 1990, 250, 975–976. [Google Scholar] [CrossRef] [PubMed]
- Kondepudi, D.K.; Prigogine, I. Sensitivity of nonequilibrium systems. Phys. A Stat. Mech. Appl. 1981, 107, 1–24. [Google Scholar] [CrossRef]
- Kondepudi, D.K.; Nelson, G.W. Chiral symmetry breaking in nonequilibrium systems. Phys. Rev. Lett. 1983, 50, 1023–1026. [Google Scholar] [CrossRef]
- Kondepudi, D.K.; Nelson, G.W. Weak neutral currents and the origin of biomolecular chirality. Nature 1985, 314, 438–441. [Google Scholar] [CrossRef]
- Buhse, T.; Durand, D.; Kondepudi, D.; Laudadio, J.; Spilker, S. Chiral symmetry breaking in crystallization: The role of convection. Phys. Rev. Lett. 2000, 84, 4405–4408. [Google Scholar] [CrossRef]
- Viedma, C. Chiral symmetry breaking during crystallization: Complete chiral purity induced by nonlinear autocatalysis and recycling. Phys. Rev. Lett. 2005, 94, 065504. [Google Scholar] [CrossRef]
- Suwannasang, K.; Flood, A.; Rougeot, C.; Coquerel, G. Using Programmed Heating-Cooling with Racemization in Solution for Complete Symmetry Breaking of a Conglomerate Forming System. Cryst. Growth Des. 2013, 13, 3498–3504. [Google Scholar] [CrossRef]
- Rougeot, C.; Guillen, F.; Plaquevent, J.-C.; Coquerel, G. Ultrasound-Enhanced Deracemization: Towards the Existence of Agonist Effects in the Interpretation of Spontaneous Symmetry Breaking. Cryst. Growth Des. 2015, 15, 2151–2155. [Google Scholar] [CrossRef]
- Viedma, C.; Cintas, P. Homochiraliry beyond grinding: Deracemizing chrial crystals by temperature gradient under boiling. Chem. Commun. 2011, 47, 12786–12788. [Google Scholar] [CrossRef] [PubMed]
- Viedma, C.; McBride, J.M.; Kahr, B.; Cintas, P. Enantiomeric-Specific Oriented Attachment: Formation of Macroscopic Homochiral Crystal Aggregates from a Racemic System. Angew. Chem. Int. Ed. 2013, 52, 10545–10548. [Google Scholar] [CrossRef] [PubMed]
- Schindler, M.; Brandel, C.; Kim, W.-S.; Coquerel, G. Temperature Cycling Induced Deracemization of NaClO3 under the Influence of Na2S2O6. Cryst. Growth Des. 2020, 20, 414–421. [Google Scholar] [CrossRef]
- Buckley, H.E. 2. The Influence of RO4 and Related Ions on the Crystalline Form of Sodium Chlorate. Z. Krist. 1930, 75, 15–31. [Google Scholar]
- Ristic, R.; Sherwood, J.N.; Wojciechowskf, K. Morphology and growth kinetics of large sodium chlorate crystals grown in the presence and absence of dithionate impurity. J. Phys. Chem. 1993, 97, 10774–10782. [Google Scholar] [CrossRef]
- Gopalan, P.; Peterson, M.L.; Crundwell, G.; Kahr, B. Reevaluating Structures for Mixed Crystals of Simple Isomorphous Salts: NaClxBr1-xO3. J. Am. Chem. Soc. 1993, 115, 3366–3367. [Google Scholar] [CrossRef]
- Ristic, R.; Shekunov, B.Y.; Sherwood, J.N. Growth of the tetrahedral faces of sodium chlorate crystals in the presence of dithionate impurity. J. Cryst. Growth 1994, 139, 336–343. [Google Scholar] [CrossRef]
- Crundwell, G.Y.; Gopalan, P.; Bakulin, A.; Peterson, M.L.; Kahr, B. Effect of Habit Modification on Optical and X-ray Structures of Sodium Halate Mixed Crystals: The Etiology of Anomalous Double Refraction. Acta Cryst. 1997, B53, 189–202. [Google Scholar] [CrossRef]
- Kahr, B.; Gurney, R.W. Dyeing crystals. Chem. Rev. 2001, 101, 893–951. [Google Scholar] [CrossRef] [PubMed]
- Kaminsky, W.; Claborn, K.; Kahr, B. Polarimetric imaging of crystals. Chem. Soc. Rev. 2004, 33, 514–525. [Google Scholar] [CrossRef]
- Lan, Z.-P.; Lai, X.; Roberts, K.; Klapper, H. X-ray Topographic and Polarized Optical Microscopy Studies of Inversion Twinning in Sodium Chlorate Crystals Grown in the Presence of Sodium Dithionate Impurities. Cryst. Growth Des. 2014, 14, 6084–6092. [Google Scholar] [CrossRef]
- Chandrasekaran, K.S.; Monhanlal, S.K. The X-ray anomalous dispersion and optical rotation in the crystalline solid solution NaClO3: NaBrO3. Pramana 1976, 7, 152–159. [Google Scholar] [CrossRef]
- Subhadra, K.G.; Hussain, K.A. Melting Temperatures of NaClO3-NaBrO3 Mixed Crystals. Cryst. Res. Technol. 1987, 22, 827–833. [Google Scholar] [CrossRef]
- Swenson, T.; Ricci, J.E. The Ternary Systems KBrO3-KClO3-H2O at 25 °C and NaBrO3-NaClO3-H2O at 25 and 50 °C. J. Am. Chem. Soc. 1939, 61, 1974–1977. [Google Scholar] [CrossRef]
- Dickinson, R.G.; Goodhue, E.A. The Crystal Structure of Sodium Chlorate and Sodium Bromate. J. Am. Chem. Soc. 1921, 43, 2045–2055. [Google Scholar] [CrossRef]
- Abrahams, S.C.; Bernstein, J.L. Remeasurement of Optically Active NaClO3 and NaBrO3. Acta Cryst. 1977, B33, 3601–3604. [Google Scholar] [CrossRef]
- Savage, A.; Miller, R.C. Measurements of Second Harmonic Generation of Ruby Laser Line in Piezoelectric Crystals. Appl. Opt. 1962, 1, 661–664. [Google Scholar] [CrossRef]
- Simon, H.J.; Bloembergen, N. Second-Harmonic Light Generation in Crystals with Natural Optical Activity. Phys. Rev. 1968, 171, 1104–1114. [Google Scholar] [CrossRef]
- Matsuura, T.; Koshima, H. Review: Introduction to chiral crystallization of achiral compounds. Spontaneous generation of chirality. J. Photochem. Photobiol. 2005, 6, 7–24. [Google Scholar] [CrossRef]
- Ramachandran, G.N.; Chandrasekaran, K.S. The Absolute Configuration of Sodium Chlorate. Acta Crystallogr. 1957, 10, 671–675. [Google Scholar] [CrossRef]
- Beurskens-Kerssen, G.; Kroon, J.; Endeman, H.J.; Van Laar, J.; Bijvoet, J.M. Absolute Configuration and Rotatory Power of the Crystals of NaBrO3 and NaClO3. In Crystallography and Crystal Perfection; Academic Press Inc.: Cambridge, MA, USA, 1963; pp. 225–236. [Google Scholar]
- Chandrasekhar, S.; Madhava, M.S. Optical roratory dispersion of crystals of sodium chlorate and sodium bromate. Acta Cryst. 1967, 23, 911. [Google Scholar] [CrossRef]
- Sanjeevi Raja, C.; Mohanlal, S.K.; Chandrasekaran, K.S. An X-ray diffraction study of the size effect on a crystalline solid solution of sodium halates. Z. Krist. 1984, 166, 121–127. [Google Scholar]
- Shtukenberg, A.G.; Rozhdestvenskaya, I.V.; Popov, D.Y.; Punin, Y.O. Kinetic ordering of atoms in sodium chlorate-bromate solid solutions. J. Solid State Chem. 2004, 177, 4732–4742. [Google Scholar] [CrossRef]
- Su, J.; Song, Y.; Zhang, D.; Chang, X. Characterization of unidirectionally grown NaCl1−xBrxO3 crystals. Powder Diffr. 2009, 24, 234–238. [Google Scholar] [CrossRef]
- Hulliger, J.; Wüst, T.; Brahimi, K.; Burgener, M.; Aboufadl, H. A stochastic principle behind polar properties of condensed molecular matter. New J. Chem. 2013, 37, 2229–2235. [Google Scholar] [CrossRef]
- Coquerel, G.; Sanselme, M.; Lafontaine, A. Method and Measuring Scattering of X-rays, Its Applications and Implementation. Device. Patent EP2694953A1, 12 February 2012. [Google Scholar]
- Bak, S.Y.; Coquerel, G.; Kim, W.-S.; Park, B.J. Solution Volume Effects on Spontaneous Chiral Symmetry Breaking of Sodium Chlorate Crystals. J. Phys. Chem. Lett. 2023, 14, 785–790. [Google Scholar] [CrossRef]

| Parameters | Protocol 1 | Protocol 2 |
|---|---|---|
| Concentration of the NaClO3(1−x)-NaBrO3(x) aqueous solutions | 2 g/150 mL water | 6 g/50 mL water |
| The inlet temperature of the drying nitrogen | 220 °C | 220 °C |
| Feed flow rate (peristaltic pump) | 360 mL/h | 300 mL/h |
| The temperature of the feed solution | Room temperature | Ca. 60 °C |
| Atomizing airflow rate | 473 L/h | 500 L/h |
| Aspiration | 40 m3/h | 40 m3/h |
| Location of the powder at the end of the process | Mainly in the bowl collector | In the drying chamber, the cyclone and bowl collector |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simon, F.; Couvrat, N.; Bilot, C.; Marinel, S.; Malo, S.; Coquerel, G. The Solid Solution between NaClO3 and NaBrO3 Revisited. Minerals 2023, 13, 1006. https://doi.org/10.3390/min13081006
Simon F, Couvrat N, Bilot C, Marinel S, Malo S, Coquerel G. The Solid Solution between NaClO3 and NaBrO3 Revisited. Minerals. 2023; 13(8):1006. https://doi.org/10.3390/min13081006
Chicago/Turabian StyleSimon, Florent, Nicolas Couvrat, Christelle Bilot, Sylvain Marinel, Sylvie Malo, and Gérard Coquerel. 2023. "The Solid Solution between NaClO3 and NaBrO3 Revisited" Minerals 13, no. 8: 1006. https://doi.org/10.3390/min13081006
APA StyleSimon, F., Couvrat, N., Bilot, C., Marinel, S., Malo, S., & Coquerel, G. (2023). The Solid Solution between NaClO3 and NaBrO3 Revisited. Minerals, 13(8), 1006. https://doi.org/10.3390/min13081006