Iron in Nepheline: Crystal Chemical Features and Petrological Applications
Abstract
1. Introduction
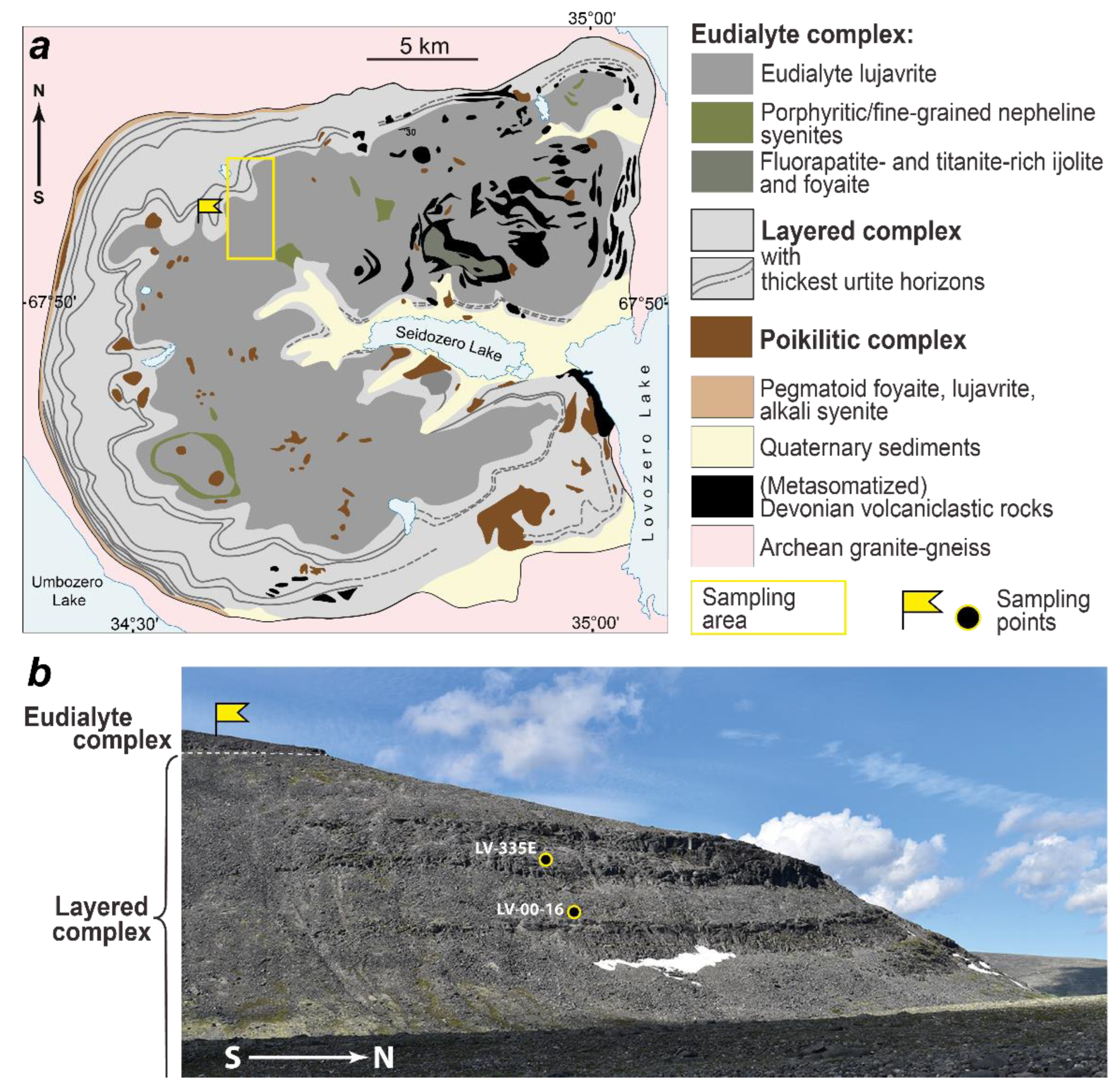
2. Short Geological and Petrography Backgrounds
3. Materials and Methods
4. Results
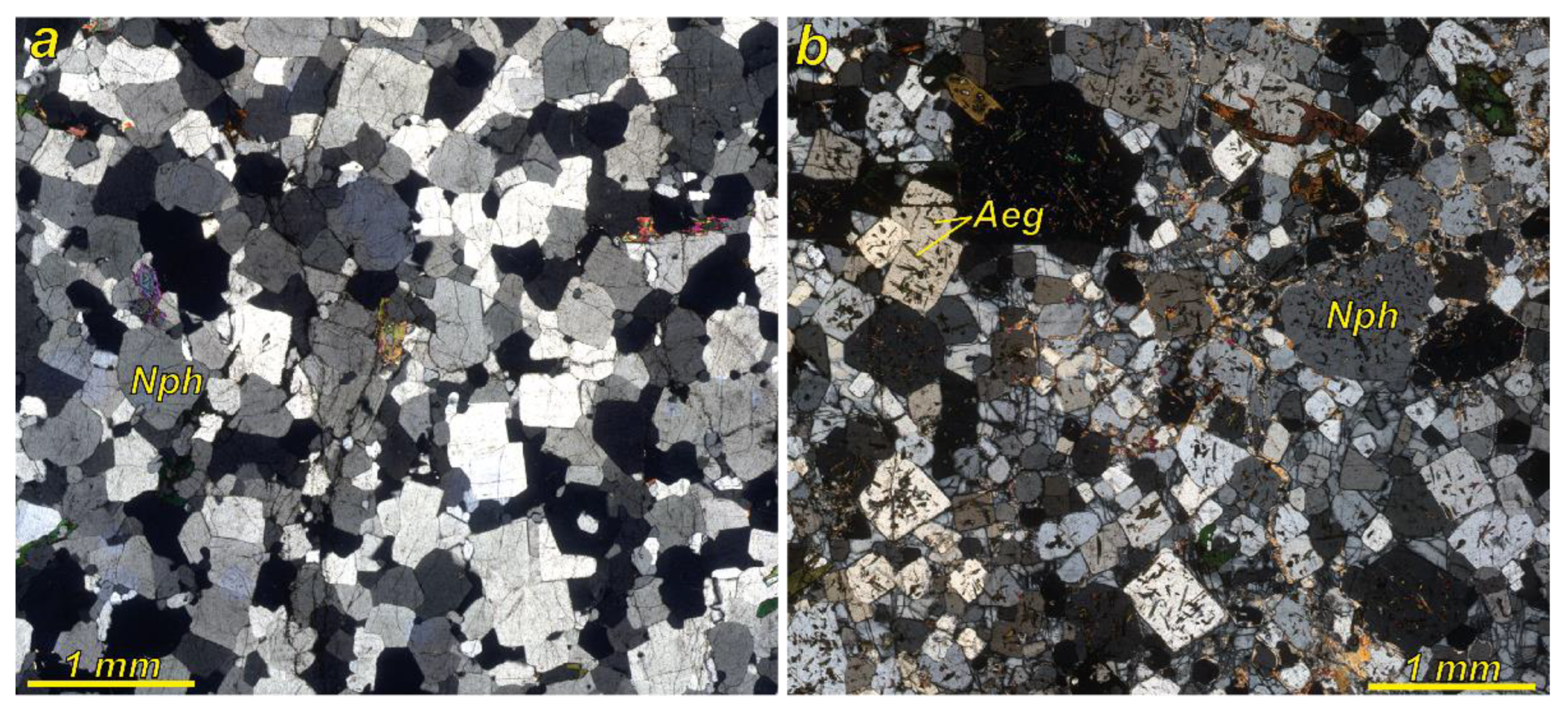
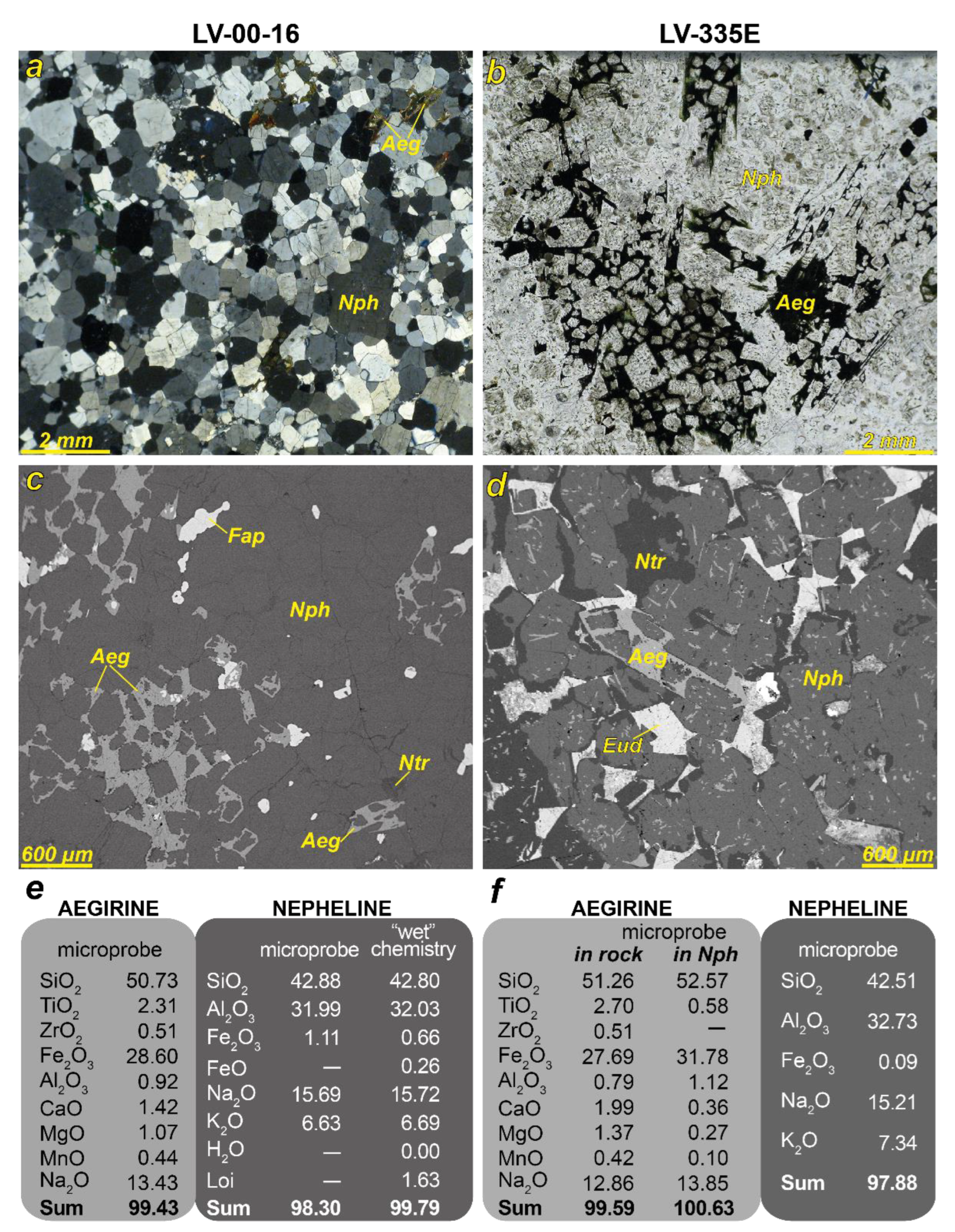
4.1. Chemical Composition of Nepheline and Aegirine (Samples LV-00-16 and LV-335E)
- aegirine in rock (LV-00-16): Na1.00 Ca0.06 Mg0.06 Mn0.02 Fe3+0.83 Al0.04 Ti0.07 Si1.95 O6;
- aegirine in rock (LV-335E): Na0.96 Ca0.08 Mg0.08 Mn0.01 Fe3+0.80 Al0.04 Ti0.08 Si1.96 O6;
- aegirine inside nepheline (LV-335E): Na1.02 Ca0.01 Mg0.01 Fe3+0.91 Al0.05 Ti0.02 Si1.99 O6.
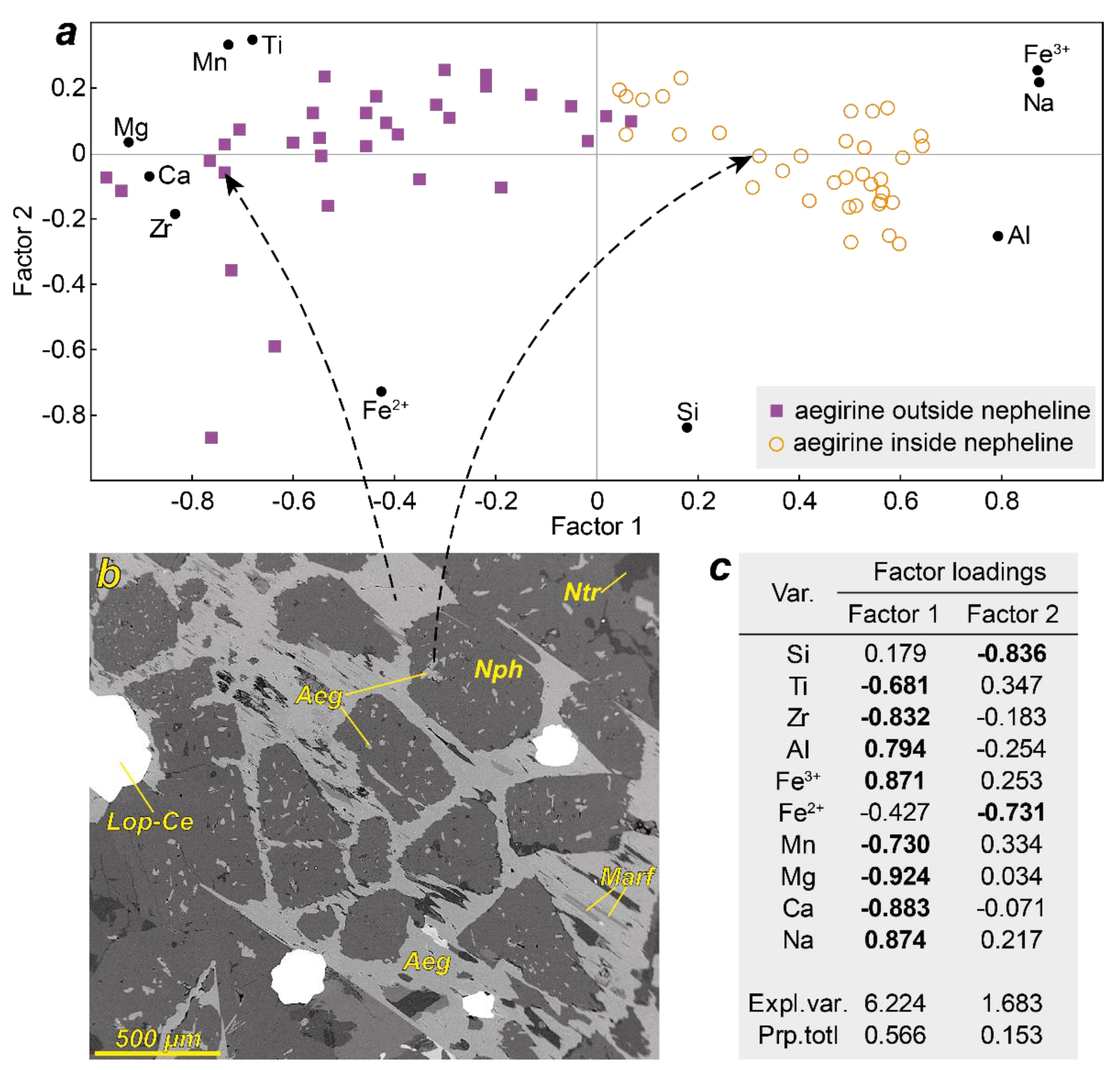
4.2. Chemical Composition of the Nepheline and Aegirine (Samples from Kedykvyrpakh Loparite Deposit)
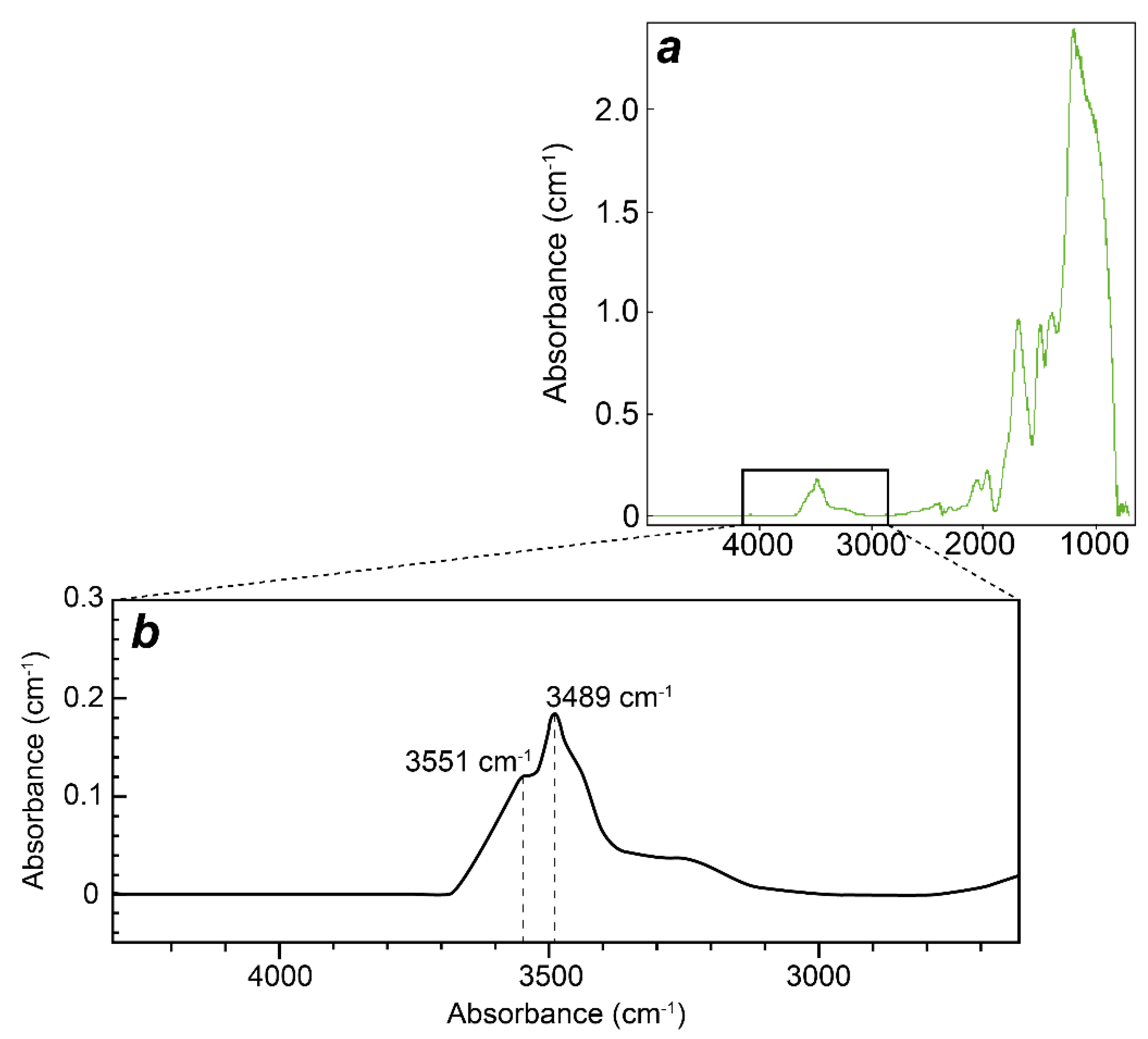
4.3. FTIR Spectroscopy
4.4. Crystal Structure of the Nepheline
5. Discussion
6. Conclusions
- Nepheline from the rocks of the Lovozero alkaline massif constantly contains ferrous iron in addition to ferric iron. The presence of molecular water in the composition of nepheline was also evidenced.
- The incorporation of ferrous iron into the nepheline crystal structure is associated with an increase in the coordination number from four to five (or six), due to the inclusion of additional water molecules that form point [FeO4(H2O)n]-defects (where n = 1, 2).
- The iron content in nepheline is closely related to the presence of small needle-like aegirine inclusions. The total iron content in nepheline saturated with aegirine needles is approximately an order of magnitude lower than in nepheline free from aegirine inclusions.
- It is probable that aegirine inclusions in nepheline formed as a result of the decomposition of the nepheline–“iron nepheline” solid solution. This process is triggered by the oxidation of ferrous iron in the crystal structure of nepheline.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Markl, G.; Marks, M.; Schwinn, G.; Sommer, H. Phase Equilibrium Constraints on Intensive Crystallization Parameters of the Ilímaussaq Complex, South Greenland. J. Petrol. 2001, 42, 2231–2258. [Google Scholar] [CrossRef]
- Gerasimovsky, V.I.; Volkov, V.P.; Kogarko, L.N.; Polyakov, A.I.; Saprykina, T.V.; Balashov, Y.A. Geochemistry of the Lovozero Alkaline Massif; Nauka: Moscow, Russia, 1966. [Google Scholar]
- Sorensen, H. The Agpaitic Rocks—An Overview. Mineral. Mag. 1997, 61, 485–498. [Google Scholar] [CrossRef]
- Larsen, L.M.; Sørensen, H. The Ilímaussaq Intrusion-Progressive Crystallization and Formation of Layering in an Agpaitic Magma. Geol. Soc. Spec. Publ. 1987, 30, 473–488. [Google Scholar] [CrossRef]
- Sørensen, H. Brief Introduction to the Geology of the Ilímaussaq Alkaline Complex, South Greenland, and Its Exploration History. Geol. Greenl. Surv. Bull. 2001, 190, 7–23. [Google Scholar] [CrossRef]
- Kostyleva-Labuntsova, E.E.; Borutskii, B.E.; Sokolova, M.N.; Shlykova, Z.V. Mineralogy of the Khibiny Massif: Magmatism and Postmagmatic Transformations; Nauka: Moscow, Russia, 1978. [Google Scholar]
- Semenov, E.I. Mineralogy of the Lovozero Alkaline Massif; Nauka: Moscow, Russia, 1972. [Google Scholar]
- Balassone, G.; Beran, A. Variable Water Content of Nepheline from Somma-Vesuvio, Italy. Mineral. Petrol. 1995, 52, 75–83. [Google Scholar] [CrossRef]
- Balassone, G.; Kahlenberg, V.; Altomare, A.; Mormone, A.; Rizzi, R.; Saviano, M.; Mondillo, N. Nephelines from the Somma-Vesuvius Volcanic Complex (Southern Italy): Crystal-Chemical, Structural and Genetic Investigations. Mineral. Petrol. 2014, 108, 71–90. [Google Scholar] [CrossRef]
- Dill, H.G. The “Chessboard” Classification Scheme of Mineral Deposits: Mineralogy and Geology from Aluminum to Zirconium. Earth Sci. Rev. 2010, 100, 1–420. [Google Scholar] [CrossRef]
- Sizyakov, V.M.; Bazhin, V.Y.; Sizyakova, E.V. Feasibility Study of the Use of Nepheline-Limestone Charges Instead of Bauxite. Metallurgist 2016, 59, 1135–1141. [Google Scholar] [CrossRef]
- Bagani, M.; Balomenos, E.; Panias, D. Nepheline Syenite as an Alternative Source for Aluminum Production. Minerals 2021, 11, 734. [Google Scholar] [CrossRef]
- Jena, S.K.; Dhawan, N.; Rao, D.S.; Misra, P.K.; Mishra, B.K.; Das, B. Studies on Extraction of Potassium Values from Nepheline Syenite. Int. J. Miner. Process. 2014, 133, 13–22. [Google Scholar] [CrossRef]
- Buerger, M.J. The Stuffed Derivates of the Silica Structures. Am. Mineral. 1954, 39, 600–614. [Google Scholar]
- Buerger, M.J. Derivative Crystal Structures. J. Chem. Phys. 1947, 15, 17–27. [Google Scholar] [CrossRef]
- Hahn, T.; Buerger, M.J. The Detailed Structure of Nepheline, KNa3Al4Si4O16. Z. Für Krist. Cryst. Mater. 1954, 106, 308–338. [Google Scholar] [CrossRef]
- Tait, K.T.; Sokolova, E.V.; Hawthorne, F.C.; Khomyakov, A.P. The Crystal Chemistry of Nepheline. Can. Mineral. 2003, 41, 61–70. [Google Scholar] [CrossRef]
- Vulić, P.; Balić-Žunić, T.; Belmonte, L.J.; Kahlenberg, V. Crystal Chemistry of Nephelines from Ijolites and Nepheline-Rich Pegmatites: Influence of Composition and Genesis on the Crystal Structure Investigated by X-ray Diffraction. Mineral. Petrol. 2011, 101, 185–194. [Google Scholar] [CrossRef]
- Antao, S.M.; Hassan, I. Nepheline: Structure of Three Samples from the Bancroft Area, Ontario, Obtained Using Synchrotron High-Resolution Powder X-Ray Diffraction. Can. Mineral. 2010, 48, 69–80. [Google Scholar] [CrossRef]
- Angel, R.J.; Gatta, G.D.; Ballaran, T.B.; Carpenter, M.A. The Mechanism of Coupling in the Modulated Structure of Nepheline. Can. Mineral. 2008, 46, 1465–1476. [Google Scholar] [CrossRef]
- Hassan, I.; Antao, S.M.; Hersi, A.A.M. Single-Crystal XRD, TEM, and Thermal Studies of the Satellite Reflections in Nepheline. Can. Mineral. 2003, 41, 759–783. [Google Scholar] [CrossRef]
- Hovis, G.L.; Crelling, J.; Wattles, D.; Dreibelbis, B.; Dennison, A.; Keohane, M.; Brennan, S. Thermal Expansion of Nepheline-Kalsilite Crystalline Solutions. Mineral. Mag. 2003, 67, 535–546. [Google Scholar] [CrossRef]
- Sahama, T.G. Order-Disorder in Natural Nepheline Solid Solutions. J. Petrol. 1962, 3, 65–81. [Google Scholar] [CrossRef]
- Rastsvetaeva, R.K.; Aksenov, S.M.; Chukanov, N.V. Disordering of Al and Si in Nepheline from Grauliai (Germany). Rep. Acad. Sci. 2010, 435, 760–763. [Google Scholar]
- Dollase, W.A.; Peacor, D.R. Si-Al Ordering in Nephelines. Contrib. Mineral. Petrol. 1971, 30, 129–134. [Google Scholar] [CrossRef]
- McConnell, J.D.C. Time-Temperature Study of the Intensity of Satellite Reflections in Nepheline. Am. Mineral. 1981, 66, 990–996. [Google Scholar]
- Gatta, G.D.; Angel, R.J. Elastic Behavior and Pressure-Induced Structural Evolution of Nepheline: Implications for the Nature of the Modulated Superstructure. Am. Mineral. 2007, 92, 1446–1455. [Google Scholar] [CrossRef]
- Merlino, S. Feldspathoids: Their Average and Real Structures. In Feldspars and Feldspathoids; Brown, W.L., Ed.; Springer: Dordrecht, The Netherlands, 1984; Volume 137. [Google Scholar]
- McConnell, J.D.C. Electron-Diffraction Study of Subsidiary Maxima of Scattered Intensity in Nepheline. Mineral. Mag. J. Mineral. Soc. 1961, 33, 114–124. [Google Scholar] [CrossRef][Green Version]
- Hayward, S.A.; Pryde, A.K.A.; de Dombal, R.F.; Carpenter, M.A.; Dove, M.T. Rigid Unit Modes in Disordered Nepheline: A Study of a Displacive Incommensurate Phase Transition. Phys. Chem. Miner. 2000, 27, 285–290. [Google Scholar] [CrossRef]
- Hamilton, D.L. Nephelines as Crystallization Temperature Indicators. J. Geol. 1961, 69, 321–329. [Google Scholar] [CrossRef]
- Hamilton, D.L.; MacKenzie, W.S. Nepheline Solid Solution in the System NaAlSiO4-KAlSiO4-SiO2. J. Petrol. 1960, 1, 56–72. [Google Scholar] [CrossRef]
- Henderson, C.M.B. Nepheline Solid Solution Compositions: Stoichiometry Revisited, Reviewed, Clarified and Rationalised. Mineral. Mag. 2020, 84, 813–838. [Google Scholar] [CrossRef]
- Henderson, C.M.B.; de Oliveira, Í.L. Substitution of ‘Small’ Divalent Cations (e.g., Mg) for Si and Al in the Nepheline Tetrahedral Framework: 1. Calculation of Atomic Formulae and Stoichiometry Parameters. Mineral. Mag. 2022, 86, 243–253. [Google Scholar] [CrossRef]
- De Oliveira, Í.L.; Henderson, C.M.B. Substitution of ‘Small’ Divalent Cations (e.g., Mg) for Si and Al in the Nepheline Tetrahedral Framework: 2. The Occurrence of Mg-Rich Nepheline and Kalsilite. Mineral. Mag. 2022, 86, 254–262. [Google Scholar] [CrossRef]
- Simakin, A.G.; Salova, T.P.; Zavel’skii, V.O. Incorporation of Water in the Structure of Nepheline from the Data of NMR and IR Spectrometry. Geochem. Int. 2008, 46, 622–626. [Google Scholar] [CrossRef]
- Onuma, K.; Iwai, T.; Yagi, K. Nepheline-“iron Nepheline” Solid Solutions. J. Fac. Sci. Hokkaido Univ. Ser. 4 Geol. Mineral. 1984, 15, 179–190. [Google Scholar]
- Edgar, A.D. Chemistry, Occurrence and Paragenesis of Feldspathoids: A Review. In Feldspars and Feldspathoids; Brown, W.L., Ed.; Springer: Dordrecht, The Netherlands, 1984; Volume 137, pp. 501–532. [Google Scholar]
- Bussen, I.V.; Sakharov, A.S.; Uspenskaya, E.I. Rock-Forming Nepheline of the Lovozero Massif. In Geochemistry, Petrology and Mineralogy of Alkaline Rocks; Nauka: Moskow, Russia, 1971; pp. 184–200. [Google Scholar]
- Mikhailova, J.A.; Ivanyuk, G.Y.; Kalashnikov, A.O.; Pakhomovsky, Y.A.; Bazai, A.V.; Yakovenchuk, V.N. Petrogenesis of the Eudialyte Complex of the Lovozero Alkaline Massif (Kola Peninsula, Russia). Minerals 2019, 9, 581. [Google Scholar] [CrossRef]
- Ikorsky, S.V. Organic Matter in Minerals of Igneous Rocks: Case Study of the Khibiny Alkaline Massif; Nauka: Leningrad, Russia, 1967. [Google Scholar]
- Bussen, I.V.; Sakharov, A.S. Petrology of the Lovozero Alkaline Massif; Nauka: Leningrad, Russia, 1972. [Google Scholar]
- Vlasov, K.A.; Kuzmenko, M.V.; Eskova, E.M. Lovozero Alkaline Massif; Academy of Sciences SSSR: Moskow, Russia, 1959. [Google Scholar]
- Dorfman, M.D.; Ikorsky, S.V.; Samoilovich, M.I.; Lebedev, V.S. On the Nature of Aegirine Inclusions in Nepheline of the Khibiny Alkaline Massif. Proc. Fersman Mineral. Mus. 1973, 22, 70–80. [Google Scholar]
- Oxford Diffraction. CrysAlisPro; Oxford Diffraction Ltd.: Abingdon, UK, 2009. [Google Scholar]
- Petricek, V.; Dusek, M.; Palatinus, L. Crystallographic Computing System JANA2006: General Features. Z. Fuer. Krist. 2014, 229, 345–352. [Google Scholar]
- Brandenburg, K.; Putz, H. DIAMOND, Version 3; Crystal Impact GbR: Bonn, Germany, 2005. [Google Scholar]
- Prince, E. International Tables for Crystallography, 3rd ed.; Mathematical, Physical and Chemical Tables, Volume C; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Warr, L.N. IMA-CNMNC Approved Mineral Symbols. Mineral. Mag. 2021, 85, 291–320. [Google Scholar] [CrossRef]
- Johnsen, O.; Grice, J.D. The Crystal Chemistry of the Eudialyte Group. Can. Mineral. 1999, 37, 865–891. [Google Scholar]
- Brown, I.D.; Altermatt, D. Bond-Valence Parameters Obtained from a Systematic Analysis of the Inorganic Crystal Structure Database. Acta Crystallogr. Sect. B 1985, 41, 244–247. [Google Scholar] [CrossRef]
- Brese, N.E.; O’Keeffe, M. Bond-Valence Parameters for Solids. Acta Cryst. B 1991, 47, 192–197. [Google Scholar] [CrossRef]
- Friese, K.; Grzechnik, A.; Petříček, V.; Schönleber, A.; van Smaalen, S.; Morgenroth, W. Modulated Structure of Nepheline. Acta Cryst. B 2011, 67, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Antao, S.M.; Hovis, G.L. Structural Variations across the Nepheline (NaAlSiO4)–Kalsilite (KAlSiO4) Series. Am. Mineral. 2021, 106, 801–811. [Google Scholar] [CrossRef]
- Terry, R.J.; McMillen, C.D.; Kolis, J.W. Hydrothermal Single Crystal Growth and Structural Investigation of the Nepheline and Kalsilite Stuffed Tridymite Species. J. Chem. Crystallogr. 2022, 1–13. [Google Scholar] [CrossRef]
- Antao, S.M.; Nicholls, J.W. Crystal Chemistry of Three Volcanic K-Rich Nepheline Samples From Oldoinyo Lengai, Tanzania and Mount Nyiragongo, Eastern Congo, Africa. Front. Earth Sci. 2018, 6, 155. [Google Scholar] [CrossRef]
- Hamada, M.; Akasaka, M.; Ohfuji, H. Crystal Chemistry of K-Rich Nepheline in Nephelinite from Hamada, Shimane Prefecture, Japan. Mineral. Mag. 2019, 83, 239–247. [Google Scholar] [CrossRef]
- Jones, J.B. Al–O and Si–O Tetrahedral Distances in Aluminosilicate Framework Structures. Acta Cryst. B 1968, 24, 355–358. [Google Scholar] [CrossRef]
- Goldfarb, D.; Strohmaier, K.G.; Vaughan, D.E.W.; Thomann, H.; Poluektov, O.G.; Schmidt, J. Studies of Framework Iron in Zeolites by Pulsed ENDOR at 95 GHz. J. Am. Chem. Soc. 1996, 118, 4665–4671. [Google Scholar] [CrossRef]
- Van Bokhoven, J.A.; Lamberti, C. Structure of Aluminum, Iron, and Other Heteroatoms in Zeolites by X-ray Absorption Spectroscopy. Coord. Chem. Rev. 2014, 277–278, 275–290. [Google Scholar] [CrossRef]
- Goldfarb, D.; Bernardo, M.; Strohmaier, K.G.; Vaughan, D.E.W.; Thomann, H. Characterization of Iron in Zeolites by X-Band and Q-Band ESR, Pulsed ESR, and UV-Visible Spectroscopies. J. Am. Chem. Soc. 1994, 116, 6344–6353. [Google Scholar] [CrossRef]
- Zaki, M.T.M.; El-Atrash, A.M.; Mahmoud, W.H.; El-Sayed, A.Y. Determination of Iron in Aluminium and Nickel Metals, Non-Ferrous Alloys, Feldspar and Portland Cement Using Morin and Triton X-100. Analyst 1988, 113, 937. [Google Scholar] [CrossRef]
- Hofmeister, A.M.; Rossman, G.R. Determination of Fe3+ and Fe2+ Concentrations in Feldspar by Optical Absorption and EPR Spectroscopy. Phys. Chem. Min. 1984, 11, 213–224. [Google Scholar] [CrossRef]
- Ershova, V.A.; Sandulyak, A.V.; Sandulyak, A.A.; Mirsaitov, S.F. Results of Magnetic Control of Iron Impurities in Feldspar. Glass Ceram. 2014, 70, 457–458. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Felspar Polymorphs: Diversity, Complexity, Stability. Zap. RMO (Proc. Russ. Mineral. Soc.) 2020, 149, 16–66. [Google Scholar] [CrossRef]
- Shchipalkina, N.; Pekov, I.; Britvin, S.; Koshlyakova, N.; Vigasina, M.; Sidorov, E. A New Mineral Ferrisanidine, K[Fe3+Si3O8], the First Natural Feldspar with Species-Defining Iron. Minerals 2019, 9, 770. [Google Scholar] [CrossRef]
- Taroev, V.; Göttlicher, J.; Kroll, H.; Kashaev, A.; Suvorova, L.; Pentinghaus, H.; Bernotat-Wulf, H.; Breit, U.; Tauson, V.; Lashkevich, V. Synthesis and Structural State of K-Feldspars in the System K[AlSi3O8]-K[FeSi3O8]. Eur. J. Mineral. 2008, 20, 635–651. [Google Scholar] [CrossRef]
- Henry, P.F.; Weller, M.T. CsFeSiO4: A Maximum Iron Content Zeotype. Chem. Commun. 1998, 24, 2723–2724. [Google Scholar] [CrossRef]
- Groeneveld, J.D.; Burianek, M.; Birkenstock, J.; Fischer, L.A.; Shannon, R.D.; Fischer, R.X. Synthesis, Revised Crystal Structures, and Refractive Indices of ABW-Type CsMTiO4 (M=Al, Fe, Ga) and ANA-Type CsTi1.10Si1.90O6.50, and the Determination of the Electronic Polarizability of 4-Coordinated Ti4+. Z. Krist. Cryst. Mater. 2020, 235, 533–551. [Google Scholar] [CrossRef]
- Dawson, J.B.; Hill, P.G. Mineral Chemistry of a Peralkaline Combeite-Lamprophyllite Nephelinite from Oldoinyo Lengai, Tanzania. Mineral. Mag. 2018, 62, 179–196. [Google Scholar] [CrossRef]
- Blancher, S.B.; D’Arco, P.; Fonteilles, M.; Pascal, M.-L. Evolution of Nepheline from Mafic to Highly Differentiated Members of the Alkaline Series: The Messum Complex, Namibia. Mineral. Mag. 2010, 74, 415–432. [Google Scholar] [CrossRef]
- Mitchell, R.H.; Platt, R.G. Mineralogy and Petrology of Nepheline Syenites from the Coldwell Alkaline Complex, Ontario, Canada. J. Petrol. 1982, 23, 186–214. [Google Scholar] [CrossRef]
- Jackson, W.E.; Farges, F.; Yeager, M.; Mabrouk, P.A.; Rossano, S.; Waychunas, G.A.; Solomon, E.I.; Brown, G.E. Multi-Spectroscopic Study of Fe(II) in Silicate Glasses: Implications for the Coordination Environment of Fe(II) in Silicate Melts. Geochim. Cosmochim. Acta 2005, 69, 4315–4332. [Google Scholar] [CrossRef]
- Mysen, B.O. The Structural Behavior of Ferric and Ferrous Iron in Aluminosilicate Glass near Meta-Aluminosilicate Joins. Geochim. Cosmochim. Acta 2006, 70, 2337–2353. [Google Scholar] [CrossRef]
- Beran, A. UR-Spektroskopischer Nachweis von H2O in Nephelin. Tschermaks Mineral. Petrogr. Mitt. 1974, 21, 299–304. [Google Scholar] [CrossRef]
- Yesinowski, J.P.; Eckert, H.; Rossmant, G.R. Chaiacterization of Hydrous Species in Minerals by High-Speed 1H MAS-NMR. J. Am. Chem. Soc. 1988, 110, 1367–1375. [Google Scholar] [CrossRef]
- Beran, A.; Rossman, G.R. The Water Content of Nepheline. Mineral. Petrol. 1989, 40, 235–240. [Google Scholar] [CrossRef]
- Bakakin, V.V. Quasipollucites and Their Mixed Frameworks, Block Isomorphism, and Superstructures. Crystallogr. Rep. 2009, 54, 763–769. [Google Scholar] [CrossRef]
- Baerlocher, C.; McCusker, L.B. Atlas of Zeolite Framework Types; Elsevier: Amsterdam, The Netherlands, 2007; ISBN 9780444530646. [Google Scholar]
- Rastsvetaeva, R.K.; Chukanov, N.V. New Data on the Isomorphism in Eudialyte-Group Minerals. 2. Crystal-Chemical Mechanisms of Blocky Isomorphism at the Key Sites. Minerals 2020, 10, 720. [Google Scholar] [CrossRef]
- Aksenov, S.M.; Bykova, E.A.; Rastsvetaeva, R.K.; Chukanov, N.V.; Makarova, I.P.; Hanfland, M.; Dubrovinsky, L. Microporous Crystal Structure of Labuntsovite-Fe and High-Pressure Behavior up to 23 GPa. Acta Cryst. B Struct. Sci. Cryst. Eng. Mater. 2018, 74, 1–11. [Google Scholar] [CrossRef]
- Aksenov, S.M.; Kabanova, N.A.; Chukanov, N.V.; Panikorovskii, T.L.; Blatov, V.A.; Krivovichev, S.V. The Role of Local Heteropolyhedral Substitutions in the Stoichiometry, Topological Characteristics and Ion-Migration Paths in the Eudialyte-Related Structures: A Quantitative Analysis. Acta Cryst. B Struct. Sci. Cryst. Eng. Mater. 2022, 78, 80–90. [Google Scholar] [CrossRef]
- Rastsvetaeva, R.K.; Chukanov, N.V. Crystal Chemistry of Labuntsovite-Group Minerals. Crystallogr. Rep. 2022, 67, 471–493. [Google Scholar] [CrossRef]
- Balmer, M.L.; Huang, Q.; Wong-Ng, W.; Roth, R.S.; Santoro, A. Neutron and X-Ray Diffraction Study of the Crystal Structure of CsTiSi2O6.5. J. Solid State Chem. 1997, 130, 97–102. [Google Scholar] [CrossRef]
- Kopp, O.C.; Harris, L.A.; Clark, G.W.; Yakel, H.L. A Hydrothermally Synthesized Iron Analog of Pollucite—Its Structure and Significance. Am. Mineral. 1963, 48, 100–109. [Google Scholar]
- Belyanchikov, M.A.; Bedran, Z.V.; Savinov, M.; Bednyakov, P.; Proschek, P.; Prokleska, J.; Abalmasov, V.A.; Zhukova, E.S.; Thomas, V.G.; Dudka, A.; et al. Single-Particle and Collective Excitations of Polar Water Molecules Confined in Nano-Pores within a Cordierite Crystal Lattice. Phys. Chem. Chem. Phys. 2022, 24, 6890–6904. [Google Scholar] [CrossRef] [PubMed]
- Kolesnikov, A.I.; Reiter, G.F.; Choudhury, N.; Prisk, T.R.; Mamontov, E.; Podlesnyak, A.; Ehlers, G.; Seel, A.G.; Wesolowski, D.J.; Anovitz, L.M. Quantum Tunneling of Water in Beryl: A New State of the Water Molecule. Phys. Rev. Lett. 2016, 116, 167802. [Google Scholar] [CrossRef]
- Belyanchikov, M.A.; Savinov, M.; Bedran, Z.V.; Bednyakov, P.; Proschek, P.; Prokleska, J.; Abalmasov, V.A.; Petzelt, J.; Zhukova, E.S.; Thomas, V.G. Dielectric Ordering of Water Molecules Arranged in a Dipolar Lattice. Nat. Commun. 2020, 11, 3927. [Google Scholar] [CrossRef]
- Nivin, V.A.; Treloar, P.J.; Konopleva, N.G.; Ikorsky, S.V. A Review of the Occurrence, Form and Origin of C-Bearing Species in the Khibiny Alkaline Igneous Complex, Kola Peninsula, NW Russia. Lithos 2005, 85, 93–112. [Google Scholar] [CrossRef]
- Ikorsky, S.V.; Nivin, V.A.; Pripachkin, V.A. Geochemistry of Gases of Endogenous Formations; Nauka: Saint-Peterburg, Russia, 1992. [Google Scholar]
- Yagi, K. A Reconnaissance of the Systems Acmite-Diopside and Acmite-Nepheline. Rep. Dir. Geophys. Lab. Carnegie. Instn. 1962, 61, 98–109. [Google Scholar]
- Bailey, D.K.; Schairer, J.F. The System Na2O-Al2O3-Fe2O3-SiO2 at 1 Atmosphere, and the Petrogenesis of Alkaline Rocks. J. Petrol. 1966, 7, 114–170. [Google Scholar] [CrossRef]
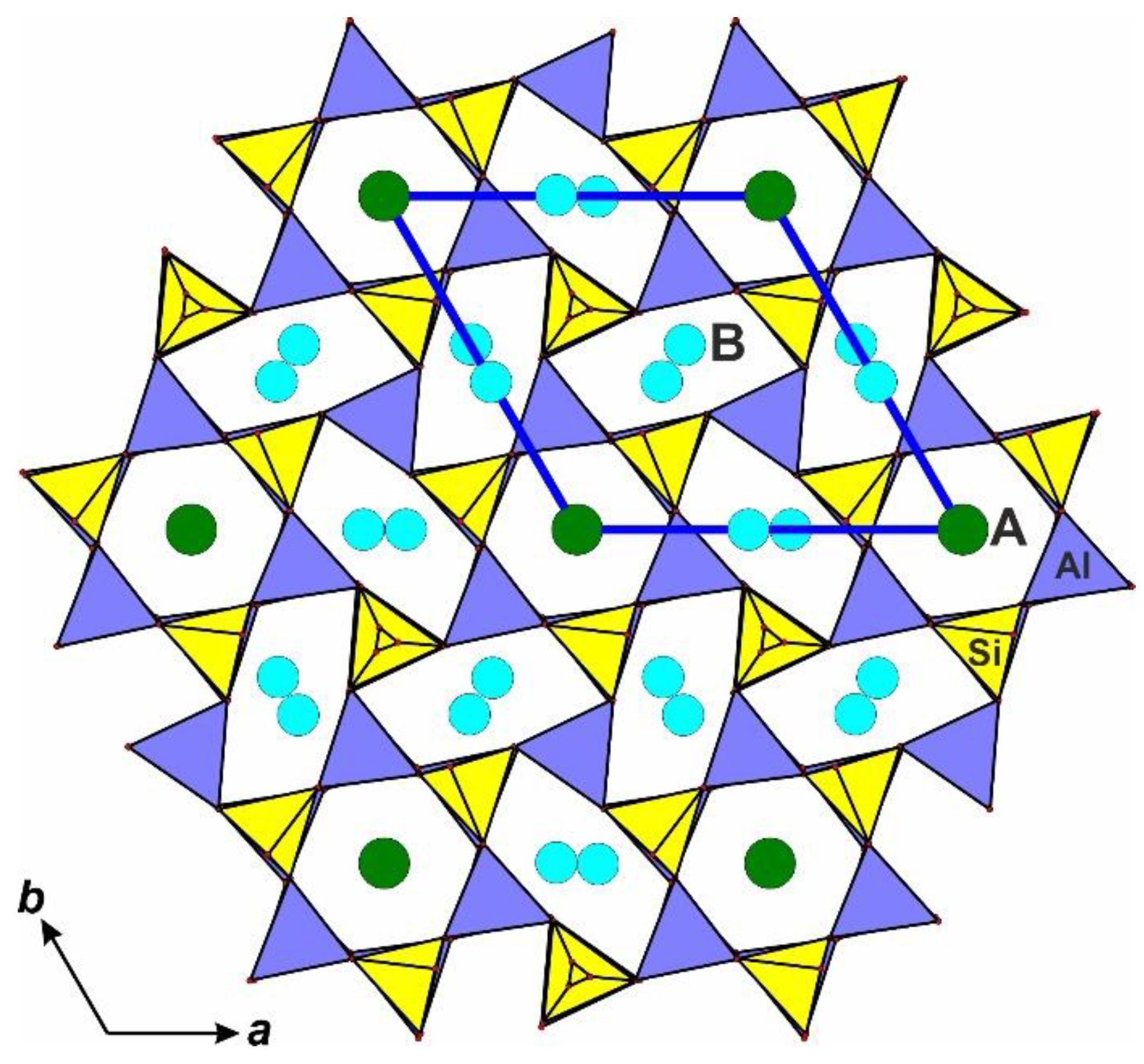

| Abbreviation | Mineral | Formula * |
|---|---|---|
| Aeg | aegirine | NaFe3+Si2O6 |
| Eud | eudialyte-group mineral | [50] |
| Fap | fluorapatite | Ca5(PO4)3F |
| Lop-Ce | loparite-(Ce) | (Na,Ce,Sr)(Ce,Th)(Ti,Nb)2O6 |
| Marf | magnesio-arfvedsonite | NaNa2(Fe2+4Fe3+)Si8O22(OH)2 |
| Nph | nepheline | Na3K(Al4Si4O16) |
| Ntr | natrolite | Na2(Si3Al2)O10·2H2O |
| Sample | LV-III-5-6 | LV-III-5-2 | LV-III-4-5 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mineral | Nph | Aeg outside Nph | Aeg inside Nph | Nph | Aeg outside Nph | Aeg inside Nph | Nph | Aeg inside Nph | Aeg outside Nph |
| SiO2 | 42.45 | 52.83 | 52.64 | 40.95 | 52.17 | 53.05 | 42.89 | 53.60 | 53.72 |
| ZrO2 | – | 0.59 | b.d.l. | – | 0.57 | b.d.l. | – | b.d.l. | b.d.l. |
| TiO2 | – | 4.16 | 2.96 | – | 2.31 | 0.44 | – | 2.67 | 3.44 |
| Al2O3 | 33.53 | 0.96 | 1.24 | 33.51 | 0.99 | 2.32 | 32.95 | 1.18 | 1.29 |
| FeO | 0.13 | 24.42 | 24.99 | 0.12 | 23.01 | 27.04 | 0.13 | 26.08 | 24.65 |
| MgO | – | 1.22 | 0.93 | – | 2.88 | 0.45 | – | 0.83 | 0.82 |
| CaO | – | 2.09 | 0.90 | – | 5.15 | 0.13 | – | 0.54 | 0.48 |
| MnO | – | 0.56 | 0.42 | – | 0.60 | 0.05 | – | 0.47 | 0.88 |
| Na2O | 16.21 | 13.18 | 14.81 | 15.89 | 11.17 | 14.11 | 16.68 | 14.45 | 14.58 |
| K2O | 7.21 | – | – | 7.22 | – | – | 6.61 | – | – |
| Sum | 99.52 | 100.06 | 99.02 | 97.69 | 98.84 | 97.59 | 99.25 | 99.83 | 99.94 |
| Formula based on 16 oxygens for nepheline and on 4 cations and 6 oxygens for aegirine, apfu | |||||||||
| Si | 4.13 | 1.96 | 1.94 | 4.06 | 1.96 | 1.98 | 4.17 | 1.97 | 1.97 |
| Ti | – | 0.12 | 0.08 | – | 0.07 | 0.01 | – | 0.07 | 0.09 |
| Zr | – | 0.01 | 0.00 | – | 0.01 | 0.00 | – | 0.00 | 0.00 |
| Al | 3.84 | 0.04 | 0.05 | 3.92 | 0.04 | 0.10 | 3.78 | 0.05 | 0.06 |
| Fe3+ | 0.01 | 0.74 | 0.77 | 0.01 | 0.70 | 0.85 | 0.01 | 0.80 | 0.75 |
| Fe2+ | – | 0.02 | 0.00 | – | 0.02 | 0.00 | – | 0.00 | 0.00 |
| Mn | – | 0.02 | 0.01 | – | 0.02 | 0.00 | – | 0.01 | 0.03 |
| Mg | – | 0.07 | 0.05 | – | 0.16 | 0.03 | – | 0.05 | 0.04 |
| Ca | – | 0.08 | 0.04 | – | 0.21 | 0.01 | – | 0.02 | 0.02 |
| Na | 3.05 | 0.95 | 1.06 | 3.06 | 0.81 | 1.02 | 3.15 | 1.03 | 1.03 |
| K | 0.89 | – | – | 0.91 | – | – | 0.82 | – | – |
| Sum | 11.92 | 4.00 | 4.00 | 11.96 | 4.00 | 4.00 | 11.92 | 4.00 | 4.00 |
| Sample Number | LV-00-16 | LV-355E |
|---|---|---|
| Mineral name | Nepheline | Nepheline |
| Formula weight (g) | 570.6 | 575.4 |
| Temperature (K) | 293 | |
| Cell setting | Hexagonal | |
| Space group | P63 | |
| a (Å) | 9.9965(7) | 10.0020(3) |
| b (Å) | 9.9965(7) | 10.0020(3) |
| c (Å) | 8.3796(17) | 8.3831(2) |
| V (Å3) | 725.19(16) | 726.29(4) |
| Z | 2 | |
| Calculated density, Dx (g cm−3) | 2.613 | 2.6504 |
| Crystal size (mm) | 0.02 × 0.06 × 0.08 | 0.01 × 0.03 × 0.06 |
| Crystal form | Irregular grain | Irregular grain |
| Data collection | ||
| Diffractometer | Rigaku XtaLAB Synergy, HyPix detector | |
| Radiation; λ | MoKα; 0.71073 | |
| Absorption coefficient, μ (mm−1) | 1.045 | 1.109 |
| F (000) | 561 | 561 |
| Data range θ(º); h, k, l | 3.38–33.55; −12 <h < 0, 0 <k < 15, −12 <l < 12 | 3.38–33.15; −15 <h < 15, −11 <k < 15, −11 <l < 12 |
| No. of measured reflections | 1692 | 8368 |
| Total reflections (N2)/observed(N1) | 1623/1551 | 1602/1510 |
| Criterion for observed reflections | I > 2σ(I) | |
| Rint (%) | 0.015 | 0.016 |
| Refinement | ||
| Refinement on | Full-matrix least squares on F | |
| Weight scheme | 1/(σ2|F|+ 0.0004F2) | 1/(σ2|F|+ 0.0004F2) |
| R1, wR1 [I > 2σ(I)] | 0.0191, 0.0282 | 0.0211, 0.0328 |
| R1, wR1 [all] | 0.0204, 0.0286 | 0.0233, 0.0334 |
| GooF (Goodness of fit) | 1.12 | 1.27 |
| Max./min. residual e density, (eÅ−3) | 0.27/–0.27 | 0.24/–0.30 |
| Site | LV-00-16 | LV-355E | ||||
|---|---|---|---|---|---|---|
| ecalc | Mean Distance | Composition | ecalc | Mean Distance | Composition | |
| A | 13.49 | 3.011 | K0.72□0.28 | 16.00 | 3.007 | K0.842□0.158 |
| B | 11.54 | 2.620 | Na2.898□0.102 | 10.52 | 2.624 | Na2.868□0.132 |
| T(1) | 13.0 | 1.721 | Al0.72Si0.26Fe0.02 | 12.42 | 1.701 | Al0.66Si0.34 |
| T(2) | 13.48 | 1.618 | Si0.89Al0.11 | 14.00 | 1.637 | Si0.78Al0.22 |
| T(3) | 13.64 | 1.617 | Si2.662Al0.338 | 13.45 | 1.621 | Si2.53Al0.37 |
| T(4) | 12.75 | 1.727 | Al2.37Si0.57Fe0.06 | 12.81 | 1.730 | Al2.46Si0.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikhailova, J.A.; Aksenov, S.M.; Pakhomovsky, Y.A.; Moine, B.N.; Dusséaux, C.; Vaitieva, Y.A.; Voronin, M. Iron in Nepheline: Crystal Chemical Features and Petrological Applications. Minerals 2022, 12, 1257. https://doi.org/10.3390/min12101257
Mikhailova JA, Aksenov SM, Pakhomovsky YA, Moine BN, Dusséaux C, Vaitieva YA, Voronin M. Iron in Nepheline: Crystal Chemical Features and Petrological Applications. Minerals. 2022; 12(10):1257. https://doi.org/10.3390/min12101257
Chicago/Turabian StyleMikhailova, Julia A., Sergey M. Aksenov, Yakov A. Pakhomovsky, Bertrand N. Moine, Camille Dusséaux, Yulia A. Vaitieva, and Mikhail Voronin. 2022. "Iron in Nepheline: Crystal Chemical Features and Petrological Applications" Minerals 12, no. 10: 1257. https://doi.org/10.3390/min12101257
APA StyleMikhailova, J. A., Aksenov, S. M., Pakhomovsky, Y. A., Moine, B. N., Dusséaux, C., Vaitieva, Y. A., & Voronin, M. (2022). Iron in Nepheline: Crystal Chemical Features and Petrological Applications. Minerals, 12(10), 1257. https://doi.org/10.3390/min12101257







