The Effect of Silicon-Containing Minerals on Coal Evolution at High-Temperature Pre-Graphitization Stage
Abstract
1. Introduction
2. Samples and Methods
2.1. Sample Selection and Preparation
2.2. High-Temperature Treatment
2.3. X-ray Diffraction
2.4. Raman Spectroscopy
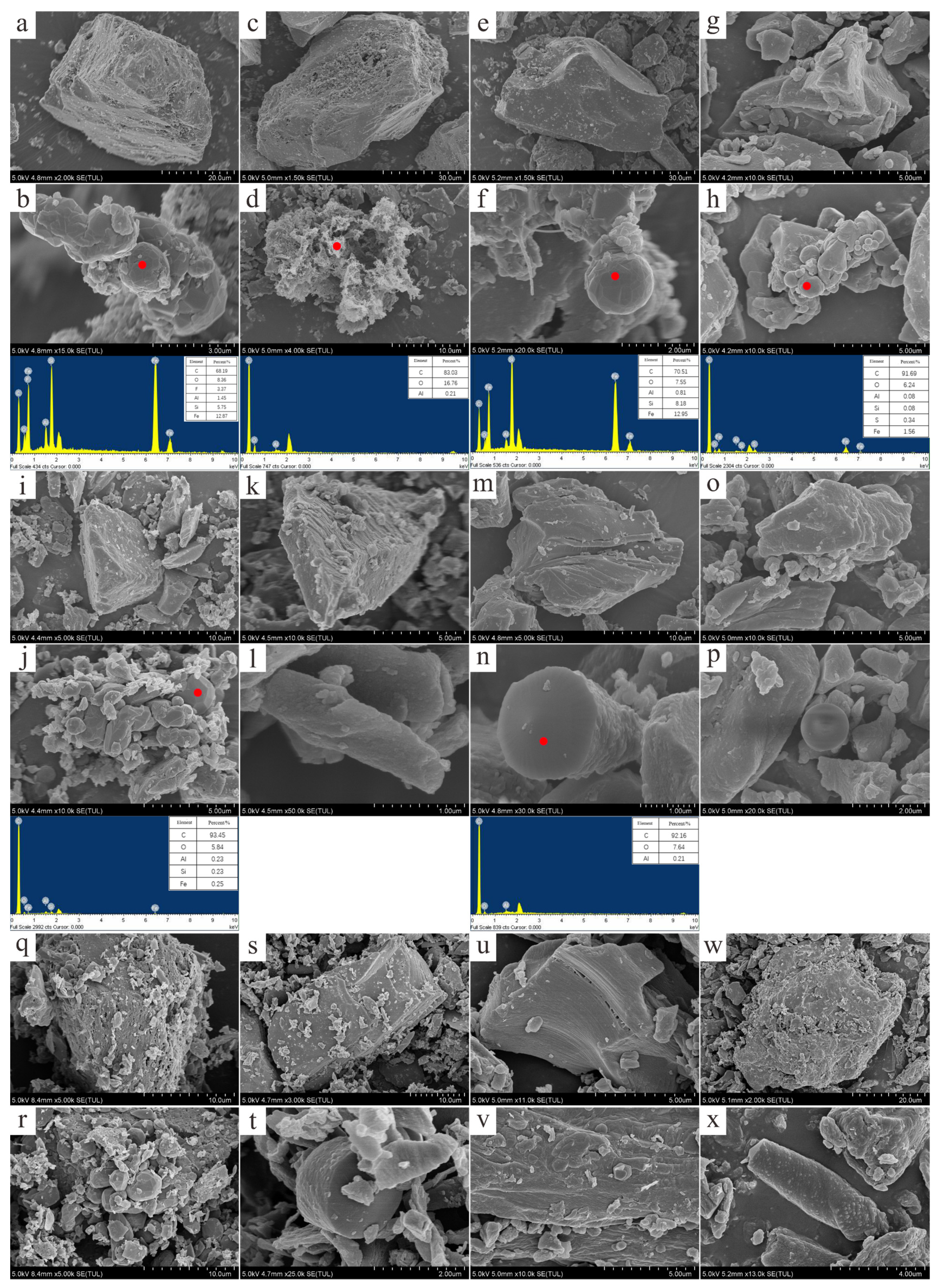
2.5. SEM/EDS
3. Results and Discussion
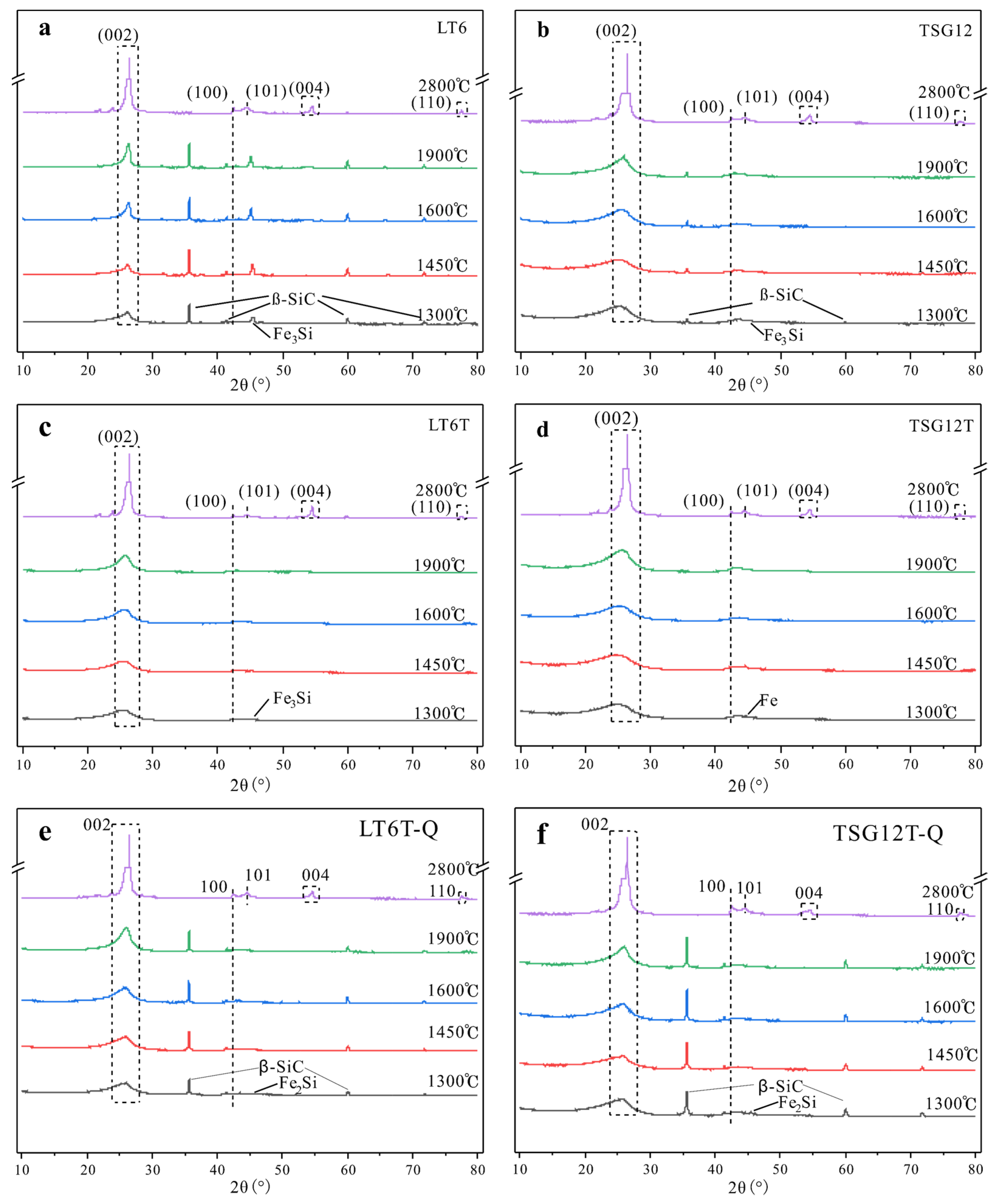
3.1. Mineral Features at the Pre-Graphitization Stage
3.2. Chemical Structural Evolution during the Pre-Graphitization Stage
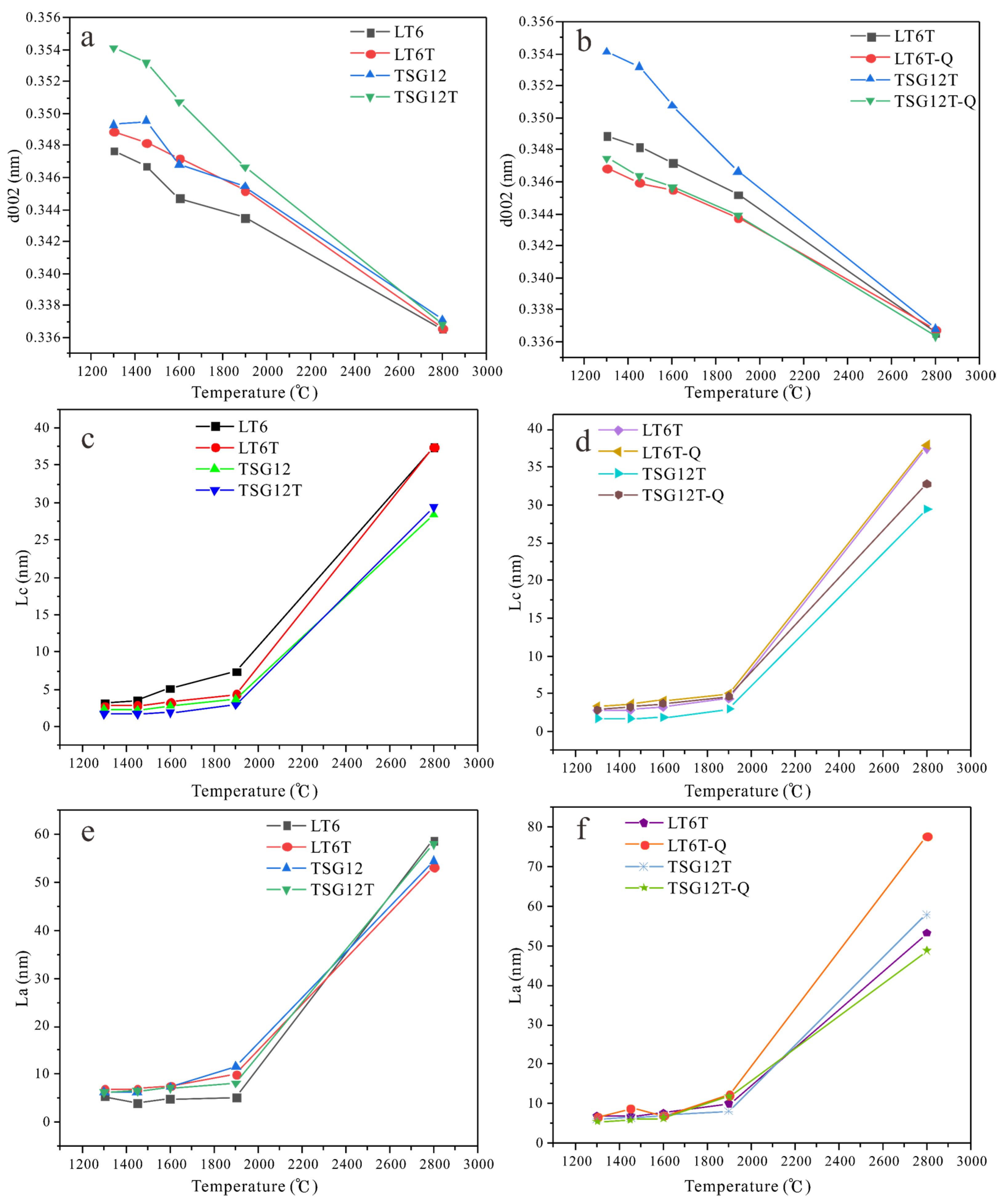
3.2.1. XRD Analysis
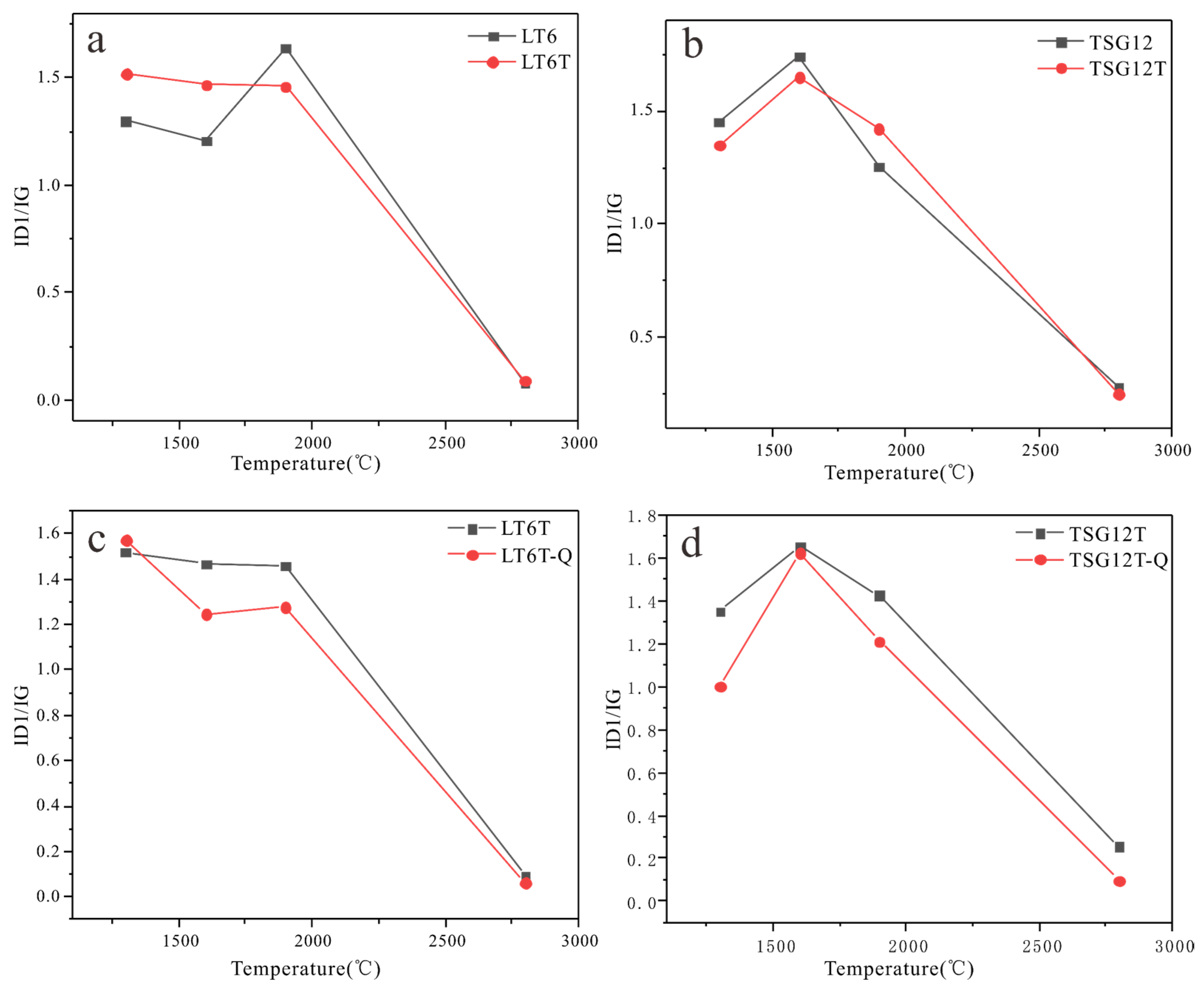
3.2.2. Raman Spectroscopy Analysis
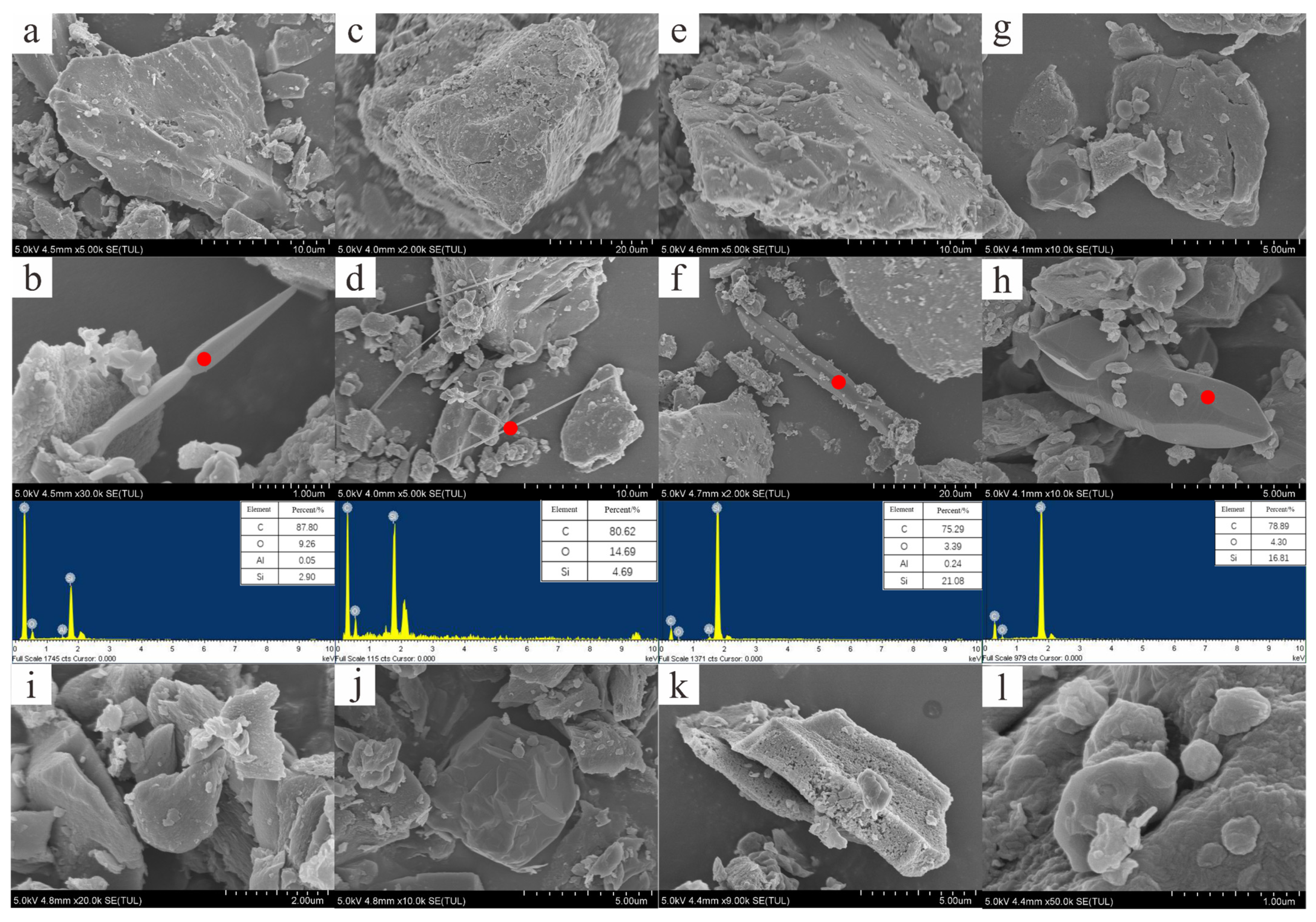
3.3. Characteristics of Minerals and Morphology
3.4. Effect of Silicon-Containing Minerals on the Pre-Graphitization Stage
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klemens, P.G.; Pedraza, D.F. Thermal conductivity of graphite in the basal plane. Carbon 1994, 32, 735–741. [Google Scholar] [CrossRef]
- Ōya, A.; Marsh, H. Phenomena of catalytic graphitization. J. Mater. Sci. 1982, 17, 309–322. [Google Scholar] [CrossRef]
- Gao, T.M.; Chen, Q.S.; Yu, W.J.; Shen, L. Projection of China’s graphite demand and development prospects. Resour. Sci. 2015, 37, 1059–1067. [Google Scholar]
- Kwiecińska, B.; Petersen, H.I. Graphite, semi-graphite, natural coke, and natural char classification—ICCP system. Int. J. Coal Geol. 2004, 57, 99–116. [Google Scholar] [CrossRef]
- Zhang, X.M.; Wang, S.Q.; Chen, H.; Li, X.Q.; Zhang, Y.X. An investigation on the graphitization of low-rank coal: Evolutional characteristics of aromatic structure. J. China Coal Soc. 2022, 47, 2768–2778. [Google Scholar]
- Liu, X.Y.; Tao, H.C.; Tang, C.Y.; Yang, X.L. Anthracite-derived carbon as superior anode for lithium/potassium-ion batteries. Chem. Eng. Sci. 2022, 248, 117200. [Google Scholar] [CrossRef]
- Wang, Q.L.; Yu, M.Q.; Gong, J.N.; Zhang, F.T. Study on the properties of coal-based high-purity graphite. Mater. Express 2019, 9, 668–674. [Google Scholar] [CrossRef]
- Guo, R.N.; Li, W.B.; Han, Y.X. Progress on the separation, purification and application of natural graphite. Chem. Ind. Eng. Prog. 2021, 40, 6155–6172. [Google Scholar]
- Li, H.T.; Cao, D.Y.; Zhang, W.G.; Wang, L. XRD and Raman spectroscopy characterization of graphitization trajectories of high-rank coal. Spectrosc. Spectr. Anal. 2021, 41, 2491–2498. [Google Scholar]
- Tang, Y.G.; Xu, J.J.; Huan, X.; Wang, S.Q.; Chen, P.X. Preparation and spectroscopic characterization of coal-based graphene from anthracite in Xiaofalu, Yunan, China. J. China Coal Soc. 2020, 45, 740–748. [Google Scholar]
- Gong, L.B. Research on the Influence of Mineralizers on Coal Graphitization and Its Control Mechanism; Hebei University of Engineering: Handan, China, 2021. [Google Scholar]
- Oberlin, A.; Terriere, G. Graphitization studies of anthracites by high resolution electron microscopy. Carbon 1975, 13, 367–376. [Google Scholar] [CrossRef]
- Atria, J.V.; Rusinko, F.; Schobert, H.H. Structural ordering of Pennsylvania anthracites on heat treatment to 2000~2900 °C. Energy Fuels 2002, 16, 1343–1347. [Google Scholar] [CrossRef]
- Rodrigues, S.; Suárez-Ruiz, I.; Marques, M.; Flores, D.; Camean, I.; García, A.B. Development of graphite-like particles from the high temperature treatment of carbonized anthracites. Int. J. Coal Geol. 2011, 85, 219–226. [Google Scholar] [CrossRef]
- Rodrigues, S.; Suárez-Ruiz, I.; Marques, M.; Camean, I.; Flores, D. Microstructural evolution of high temperature treated anthracites of different rank. Int. J. Coal Geol. 2011, 87, 204–211. [Google Scholar] [CrossRef]
- Borunova, A.B.; Streletskii, A.N.; Permenov, D.G.; Leonov, A.V. The effect of the mechanical activation dose on the defective structure of artificial graphite. Colloid J. 2015, 77, 125–134. [Google Scholar] [CrossRef]
- Qiu, T. Research on the Graphitization Process of Coal and the Migration Rule of Coal-Derived Minerals; China University of Mining and Technology: Xuzhou, China, 2019. [Google Scholar]
- Tian, Y. The Influence of Mineralizer on Coal Graphitization and Its Mechanism; Hebei University of Engineering: Handan, China, 2021. [Google Scholar]
- Nyathi, M.S.; Clifford, C.B.; Schobert, H.H. Characterization of graphitic materials prepared from different rank Pennsylvania anthracites. Fuel 2013, 114, 244–250. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhao, Z.B.; Zhang, Y.T.; Meng, B.; Zhou, A.N.; Qiu, J.S. Graphene sheets from graphitized anthracite coal: Preparation, decoration, and application. Energy Fuels 2012, 26, 5186–5192. [Google Scholar] [CrossRef]
- Franklin, R.E. Crystallite growth in graphitizing and non-graphitizing carbons. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1951, 209, 196–218. [Google Scholar]
- Bustin, R.M.; Ross, J.V.; Rouzaud, J.-N. Mechanisms of graphite formation from kerogen: Experimental evidence. Int. J. Coal Geol. 1995, 28, 1–36. [Google Scholar] [CrossRef]
- Cao, D.Y.; Li, X.M.; Zhang, S.R. Influence of tectonic stress on coalification-stress degradation mechanism and stress polycondensation mechanism. Sci. China (Ser. D Earth Sci.) 2006, 1, 59–68. [Google Scholar] [CrossRef]
- Zhang, X.M.; Wang, S.Q.; Chen, H.; Wang, X.X.; Deng, J.S.; Li, X.Q.; Zhang, Y.X. Observation of carbon nanostructure and evolution of chemical structure from coal to graphite by high temperature treatment, using componential determination, X-ray diffraction and high-resolution transmission electron microscope. Fuel 2023, 332, 126145. [Google Scholar] [CrossRef]
- Li, J.Q.; Qin, Y.; Chen, Y.L. Occurrence and origin of graphite microcrystal in meta-anthracite. Coal Geol. Explor. 2020, 48, 27–33. [Google Scholar]
- Franklin, R.E. The structure of graphitic carbons. Acta Crystallogr. 1951, 4, 253. [Google Scholar] [CrossRef]
- González, D.; Montes-Morán, M.A.; Garcia, A.B. Influence of inherent coal mineral matter on the structural characteristics of graphite materials prepared from Anthracites. Energy Fuels 2005, 19, 263–269. [Google Scholar] [CrossRef]
- Pappano, P.J.; Schobert, H.H. Effect of natural mineral inclusions on the graphitizability of a Pennsylvania anthracite. Energy Fuels 2009, 23, 422–428. [Google Scholar] [CrossRef]
- Rodrigues, S.; Suárez-Ruiz, I.; Marques, M.; Flores, D. Catalytic role of mineral matter in structural transformation of anthracites during high temperature treatment. Int. J. Coal Geol. 2012, 93, 49–55. [Google Scholar] [CrossRef]
- Liu, B.; He, Q.H.; Jiang, Z.H.; Xu, R.F.; Hu, B.X. Relationship between coal ash composition and ash fusion temperatures. Fuel 2013, 105, 293–300. [Google Scholar] [CrossRef]
- Wang, L.P.; Yao, Z.X.; Guo, Z.M.; Shen, X.F.; Li, Z.A.; Zhou, Z.Q.; Wang, Y.L.; Yang, J.G. Effects of solvent extraction on the microstructure of bituminous coal-based graphite. Carbon Lett. 2022, 32, 741–749. [Google Scholar] [CrossRef]
- Baraniecki, C.; Pinchbeck, P.H.; Pickering, F.B. Some aspects of graphitization induced by iron and ferro-silicon additions. Carbon 1969, 7, 213–224. [Google Scholar] [CrossRef]
- Pappano, P.J. A Mechanism of Pennsylvania Anthracite Graphitization Involoving Carbide Formation and Decomposition; The Pennsylvania State University: State College, PA, USA, 2003. [Google Scholar]
- Lin, Q.Y.; Feng, Z.H.; Liu, Z.J.; Guo, Q.G.; Hu, Z.J.; He, L.L.; Ye, H.Q. Atomic scale investigations of catalyst and catalytic graphitization in a silicon and titanium doped graphite. Carbon 2015, 88, 252–261. [Google Scholar] [CrossRef]
- Liu, H.Y.; Xu, L.; Jin, Y.; Fan, B.G.; Qiao, X.L.; Yang, Y.X. Effect of mineral matter on structure and dielectric properties of chars. Fuel 2018, 222, 370–374. [Google Scholar] [CrossRef]
- Li, H.T.; Zhang, H.; Li, K.J.; Zhang, J.J.; Sun, M.M.; Su, B.X. Catalytic graphitization of coke carbon by iron: Understanding the evolution of carbon Structure, morphology and lattice fringes. Fuel 2020, 279, 118531. [Google Scholar] [CrossRef]
- Lu, L.; Sahajwalla, V.; Kong, C.; Harris, D. Quantitative X-ray diffraction analysis and its application to various coals. Carbon 2001, 39, 1821–1833. [Google Scholar] [CrossRef]
- Li, M.F.; Zeng, F.G.; Chang, H.Z.; Xu, B.S.; Wang, W. Aggregate structure evolution of low-rank coals during pyrolysis by in-situ X-ray diffraction. Int. J. Coal Geol. 2013, 116–117, 262–269. [Google Scholar] [CrossRef]
- Jiang, J.Y.; Yang, W.H.; Cheng, Y.P.; Liu, Z.D.; Zhang, Q. Molecular structure characterization of middle-high rank coal via XRD, Raman and FTIR spectroscopy: Implications for coalification. Fuel 2019, 239, 559–572. [Google Scholar] [CrossRef]
- Takagi, H.; Maruyama, K.; Yoshizawa, N.; Yamada, Y.; Sato, Y. XRD analysis of carbon stacking structure in coal during heat treatment. Fuel 2004, 83, 2427–2433. [Google Scholar] [CrossRef]
- Li, J.Q.; Oin, Y.; Chen, Y.L.; Shen, J.; Song, Y.; Wang, Z.W. Structural characteristics and evolution of meta-anthracite to coaly graphite: A quantitative investigation using X-ray diffraction, Raman spectroscopy, and high-resolution transmission electron microscopy. Fuel 2023, 333, 126334. [Google Scholar] [CrossRef]
- Lin, Q.; Guet, J.M. Characterization of coals and macerals by X-ray diffraction. Fuel 1990, 69, 821–825. [Google Scholar] [CrossRef]
- Wang, S.Q.; Sun, Y.B.; Shu, K.K.; Jiang, D.; Ma, W. Structural characterization of coal components of Late Permian coals from Southern China by X-ray diffraction and Raman spectroscopy. J. Biochem. Anal. Stud. 2017, 2, 1–5. [Google Scholar]
- Seehra, M.S.; Pavlovic, A.S. X-ray diffraction, thermal expansion, electrical conductivity, and optical microscopy studies of coal-based graphites. Carbon 1993, 31, 557–564. [Google Scholar] [CrossRef]
- Keown, D.M.; Li, X.J.; Hayashi, J.; Li, C.Z. Evolution of biomass char structure during oxidation in O2 as revealed with FT-Raman spectroscopy. Fuel Process. Technol. 2008, 89, 1429–1435. [Google Scholar] [CrossRef]
- Morga, R. Micro-Raman spectroscopy of carbonized semifusinite and fusinite. Int. J. Coal Geol. 2011, 87, 253–267. [Google Scholar] [CrossRef]
- Baysal, M.; Yürüm, A.; Yıldız, B.; Yürüm, Y. Structure of some western Anatolia coals investigated by FTIR, Raman, 13C solid state NMR spectroscopy and X-ray diffraction. Int. J. Coal Geol. 2016, 163, 166–176. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Li, Z.S. Raman spectroscopic study of chemical structure and thermal maturity of vitrinite from a suite of Australia coals. Fuel 2019, 241, 188–198. [Google Scholar] [CrossRef]
- Vranjes-Wessely, S.; Misch, D.; Issa, I.; Kiener, D.; Fink, R.; Seemann, T.; Liu, B.; Rantitsch, G.; Sachsenhofer, R.F. Nanoscale pore structure of Carboniferous coals from the Ukrainian Donets Basin: A combined HRTEM and gas sorption study. Int. J. Coal Geol. 2020, 224, 103484. [Google Scholar] [CrossRef]
- Xu, J.; Tang, H.; Su, S.; Liu, J.W.; Han, H.D.; Zhang, L.P.; Xu, K.; Wang, Y.; Hu, S.; Zhou, Y.B.; et al. Micro-Raman spectroscopy study of 32 kinds of Chinese coals: Second-order Raman spectrum and its correlations with coal properties. Energy Fuels 2017, 31, 7884–7893. [Google Scholar] [CrossRef]
- Xu, J.; Xiang, X.R.; Xu, K.; He, L.M.; Han, H.D.; Su, S.; Wang, Y.; Hu, S.; Xiang, J. Developing micro-Raman spectroscopy for char structure characterization in the scale of micro- and bulk: A case study of Zhundong coal pyrolysis. Fuel 2021, 291, 120168. [Google Scholar] [CrossRef]
- Sheng, C.D. Char structure characterised by Raman spectroscopy and its correlations with combustion reactivity. Fuel 2007, 86, 2316–2324. [Google Scholar] [CrossRef]
- Hinrichs, R.; Brown, M.T.; Vasconcellos, M.A.Z.; Abrashev, M.V.; Kalkreuth, W. Simple procedure for an estimation of the coal rank using micro-Raman spectroscopy. Int. J. Coal Geol. 2014, 136, 52–58. [Google Scholar] [CrossRef]
- Sadezky, A.; Muckenhuber, H.; Grothe, H.; Niessner, R.; Pöschl, U. Raman microspectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information. Carbon 2005, 43, 1731–1742. [Google Scholar] [CrossRef]
- Li, K.; Rimmer, S.M.; Liu, Q.; Zhang, Y.M. Micro-Raman spectroscopy of microscopically distinguishable components of naturally graphitized coals from Central Hunan province, China. Energy Fuels 2019, 33, 1037–1048. [Google Scholar] [CrossRef]
- Li, K.; Rimmer, S.M.; Liu, Q. Geochemical and petrographic analysis of graphitized coals from Central Hunan, China. Int. J. Coal Geol. 2018, 195, 267–279. [Google Scholar] [CrossRef]
- Li, X.J.; Hayashi, J.I.; Li, C.Z. FT-Raman spectroscopic study of the evolution of char structure during the pyrolysis of a Victorian brown coal. Fuel 2006, 85, 1700–1707. [Google Scholar] [CrossRef]
- Tai, F.C.; Lee, S.C.; Wei, C.H.; Tyan, S.L. Correlation between ID/IG ratio from visible Raman spectra and sp2/sp3 ratio from XPS spectra of annealed hydrogenated DLC film. Mater. Trans. 2006, 47, 1847–1852. [Google Scholar] [CrossRef]
- Wang, X.L.; Wang, S.Q.; Hao, C.; Zhao, Y.G.; Song, X.X. Quantifying orientation and curvature in HRTEM lattice fringe micrographs of naturally thermally altered coals: New insights from a structural evolution perspective. Fuel 2022, 309, 122180. [Google Scholar] [CrossRef]
- Wang, J.; Morishita, K.; Takarada, T. High-temperature interactions between coal char and mixtures of calcium oxide, quartz, and kaolinite. Energy Fuels 2001, 15, 1145–1152. [Google Scholar] [CrossRef]

| Sample | Ro | Proximate Analysis (%) | Ultimate Analysis (wt.%, daf) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mad | Ad | Vdaf | C | H | O * | N | S | ||
| LT | 5.67 | 3.64 | 19.76 | 4.96 | 94.90 | 1.18 | 2.79 | 0.56 | 0.56 |
| TSG | 1.02 | 2.37 | 18.23 | 32.08 | 83.65 | 3.91 | 10.93 | 0.90 | 0.60 |
| Type | Shift/cm−1 | Assignment |
|---|---|---|
| D1 | 1350 | The ring breathing vibration in the graphite subunit or polycyclic aromatic hydrocarbon compounds (PAHs) or to aromatics with 6 or more rings |
| D2 | 1620 | Disordered graphite lattice |
| D3 | 1500 | Caromatic-Calkyl aromatic (aliphatic) ethers, C-C on hydro-aromatic rings, C-C on aliphatic structures or olefin-like structures |
| D4 | 1200 | Caromatic-Calkyl; aromatic (aliphatic) ethers; C-C on hydro-aromatic rings; hexagonal diamond carbon sp3; C-H on aromatic rings |
| G | 1580 | The aromatic ring breathing in the graphene sheets, aromatic ring quadrant breathing, alkene C=C |
| 2D1 | 2560 | Overtone of the D1 band, C-C between aromatic rings, large aromatic rings system |
| D1+G | 2860 | Combination of D1 band and G band, large aromatic rings system |
| 2G | 3180 | Overtone of the G band, the ring breathing vibration |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, Y.; Wang, S.; Li, X. The Effect of Silicon-Containing Minerals on Coal Evolution at High-Temperature Pre-Graphitization Stage. Minerals 2023, 13, 20. https://doi.org/10.3390/min13010020
Shao Y, Wang S, Li X. The Effect of Silicon-Containing Minerals on Coal Evolution at High-Temperature Pre-Graphitization Stage. Minerals. 2023; 13(1):20. https://doi.org/10.3390/min13010020
Chicago/Turabian StyleShao, Yan, Shaoqing Wang, and Xueqi Li. 2023. "The Effect of Silicon-Containing Minerals on Coal Evolution at High-Temperature Pre-Graphitization Stage" Minerals 13, no. 1: 20. https://doi.org/10.3390/min13010020
APA StyleShao, Y., Wang, S., & Li, X. (2023). The Effect of Silicon-Containing Minerals on Coal Evolution at High-Temperature Pre-Graphitization Stage. Minerals, 13(1), 20. https://doi.org/10.3390/min13010020





