Abstract
Black shale ore contains rich strategic metal resources such as vanadium, nickel, and molybdenum, but due to its complex composition, it is currently only used in the vanadium extraction industry. Metals such as nickel and molybdenum have not been effectively recovered, resulting in environmental pollution and resource waste. Using mineralogical features and a combination of beneficiation and metallurgy-based tests, the present work carried out feasibility studies of the combined beneficiation and metallurgy processes. The mineralogical features of the stone coal sample were studied using chemical analysis, an automatic mineral analyzer (BPMA), etc., and we identified the main phase composition, embedded characteristics, and particle size distribution of the associated strategic metals, vanadium, nickel, and molybdenum. The results showed that the grade of V2O5 in the stone coal was 1.29%, which was mainly present in carbonaceous clay and mica minerals. The nickel grade was 0.53%, mainly in the form of nickel–magnesium spinel and a small amount of nickel-containing magnesite. The stone coal contained 0.11% molybdenum; the mineral particles were fine, mostly in the form of molybdenite, and some were associated with carbonaceous matter and carbonaceous clay minerals. Based on the mineralogical feature, we proposed using the scrubbing–desliming and flotation process to enrich vanadium, nickel, and molybdenum. Our preliminary experiments obtained two products: vanadium–molybdenum-rich sludge and nickel-containing tailings. The V2O5 and molybdenum grades in the sludge were 4.10% and 0.44%, respectively, and the recovery was 41.31% and 51.40%, respectively. The nickel grade in the tailings was 1.49%. These products were roasted and leached. The vanadium, nickel, and molybdenum in the stone coal were effectively recovered through the beneficiation–metallurgy combination process, and the comprehensive utilization rate of the stone coal was improved.
1. Introduction
Vanadium (V), nickel (Ni), and molybdenum (Mo) are all important strategic metal resources and are widely used in steel, electronics, aerospace, the military, industry, and so on [1,2,3]. Key metals in black shale have received growing attention as global ore grades have continued to decline [4,5]. Stone coal is a vanadium-bearing shale (total organic carbon content > 10%) and a polymetallic symbiotic ore. More than 60 associated elements have been found in stone coal and, usually, the V content is the highest, followed by Mo, Ni, U, P, etc. [6]. China has a total of 61.88 billion tons of stone coal reserves, out of which there are 118 million tons of V2O5 reserves, which is the main raw materials used for vanadium production, apart from vanadium titanomagnetite [7]. China started researching methods of extracting vanadium from stone coal in the 1970s, and over time, a series of achievements were made regarding the research and development of metallurgical vanadium extraction processes and mechanisms [8,9]. However, the contents of the associated nickel and molybdenum found in stone coal are generally low and difficult to recover. At present, there are few studies on the recovery of nickel and molybdenum from stone coal.
The occurrence states of vanadium, nickel, and molybdenum in stone coal in different regions are complex and diverse, and the combined beneficiation and smelting processes used to extract them also vary. According to the literature, vanadium in stone coal mainly exists in the form of V3+ in minerals such as muscovite (KAl2[AlSi3AlO10](OH,F)2) and kaolinite (Al2Si2O5(OH)4), and it replaces Al3+, Fe3+, etc., which exist in the four 4-coordinate silicon–oxygen tetrahedron “multiple network layer” and the six-coordinate aluminum–oxygen octahedron “single network layer” [10,11]. A small amount of V, including V(IV) and V(V), adsorbs on the surface of organic matter, iron oxide, or clay minerals, or exists in the form of independent minerals such as calcium vanadium garnet (Ca3V2[SiO4]3) and vanadium uranite [10,12]. Nickel and molybdenum in stone coal mostly exist as sulfides. Mineralogical feature studies have shown that nickel-bearing minerals include vaesite (Ni2S), polydymite (Ni3S4), gersdorffite (NiAsS), millerite (NiS), and pyrite containing nickel ((FeNi)9S8) (colloidal pyrite). Molybdenum-bearing minerals include molybdenite and carbonaceous-sulfide–sulfur–molybdenum aggregates [13,14]. Due to the different occurrence states of vanadium and the mineral composition characteristics of stone coal in various regions, there have been many studies on the combined beneficiation–metallurgy process. For high-carbon stone coal, Wang et al. [15] adopted a combined flotation–decarburization–flotation vanadium-leaching process. The V2O5 grade was increased from 0.92% to 1.32% with a recovery of 88.38%, and the leaching efficiency of the vanadium concentrate was 85%. Carbon concentrate obtained with flotation can be used as fuel, and the leaching amount and production costs can be reduced by using this process. For calcareous stone coal, Liu [16] adopted a combined roasting–decarburization–desliming–flotation–leaching process to obtain V2O5 concentrate with a grade of 1.07% and a recovery of 83.30%. In total, 62.52% of the CaO was removed, and the leaching efficiency of the vanadium concentrate increased by 13.53% compared with the direct leaching of raw ore. The combined beneficiation and metallurgy process can effectively reduce the ore-processing volume and acid consumption of vanadium extraction from stone coal. For siliceous stone coal, Gu et al. [17] enriched vanadium-bearing mica and clay-mineral-absorbing vanadium using a preferential decarburization–desliming–flotation process. After a locked-circuit process of separation and smelting, the V2O5 grade of the mixed vanadium concentrate was 2.04% and the recovery was 83.41%. The process eliminated 64.87% of gangue minerals. In addition to vanadium recovery, some researchers have studied the comprehensive recovery of nickel, molybdenum, and other associated elements from stone coal. Liu et al. [18] proposed a new technology using flotation–roasting–alkali-leaching. Using flotation to extract molybdenum, the molybdenum concentrate grade was increased from 0.036% in the head ore to 0.112%, and the recovery was 81.3%. The molybdenum extraction tailings were treated by the calcium-roasting–alkali leaching process, and the vanadium leaching efficiency reached over 80% with the overall recovery of 78.2%. This process has proceeded to the stage of a small-scale application. At present, stone coal is still mainly used to extract vanadium, and the recovery of nickel and molybdenum is less frequently studied. Research on the occurrence states of nickel and molybdenum in stone coal is not comprehensive, and it is still difficult to effectively guide the separation and extraction of nickel and molybdenum from stone coal. A mineralogical feature study of nickel and molybdenum in stone coal may provide a foundation for the efficient utilization of nickel and molybdenum resources.
Determining the beneficiation–metallurgy process is mainly performed by finding the mineral composition of ore samples and the occurrence states of the target minerals. The key to the comprehensive utilization of vanadium, nickel, and molybdenum resources in stone coal is to systematically study the occurrence states of these three metals in the stone coal. By analyzing the chemical and mineral composition, physical properties, dissemination characteristics, and grain size distribution of the ore, one can obtain basic information to support the feasibility study of beneficiation and metallurgy processes [19]. Comprehensively recovering vanadium and the strategic metals nickel and molybdenum from stone coal not only reduces metal resources waste, but also brings certain economic benefits, and it reduces the risk of nickel and molybdenum polluting the environment.
In the present work, a systematic mineralogical feature study on stone coal from Shaanxi Province was carried out. The mineral types, occurrence states, and distribution characteristics of vanadium, nickel, and molybdenum were identified. Based on the study, we propose to use the scrubbing–desliming and flotation process to pre-enrich vanadium, nickel, and molybdenum.
2. Materials and Methods
2.1. Raw Ore and Reagents
The stone coal used in this study came from Shaanxi Province. The raw ore was crushed to ~2 mm with a jaw crusher and a double-roller crusher and then mixed. Analysis showed that the organic carbon content in the sample was 6.13%, V2O5 was 1.29%, Mo was 0.11%, and Ni was 0.53%. In the flotation process, water glass was used as a dispersant; terpineol was used as a foaming agent; kerosene, butyl xanthate, butylammonium dithiophosphate, and 3030C (cationic surfactant) were used as collectors; sulfuric acid was used as a pH regulator; sulfuric acid was used as an acid-leaching agent; calcium fluoride was used as a leaching aid.
2.2. Mineralogy Study
A chemical analysis of V, Ni, Mo, and other elements in the raw ore was carried out using acid digestion—ICP-OES (Agliet 725, Agilent Technologies Inc., Santa Clara, CA, USA) [20], and we obtained the distribution of vanadium, nickel, and molybdenum in the raw ore and its fractions. The main components of the raw ore were identified by X-ray fluorescence (XRF, PANalytical Axios, Almelo, Holland) semi-quantitative analysis. X-ray diffraction (XRD) analysis was conducted using a D/Max-IIIA X-ray diffractometer (Malvern Panalytical, Shanghai, China) with Cu Kα radiation at 40 kV and 30 mA with a scanning rate of 15 (°)/min from 5° to 70°.
We used the BGRIMM Process Mineralogy Analyzer (BPMA, BGRIMM Technology Group, Beijing, China) which consists of a TESCAN VEGA scanning electron microscope, a Bruker QUANTAX 200 dual-probe spectrometer, and automatic analysis software for processing mineralogy (BMPA V2.0) [21]. The stone coal inlay sample was prepared by mixing the ore, epoxy resin, and heated carnauba wax in a mold and then consolidating the particle specimen into blocks through slow hardening of the epoxy resin, followed by grinding and polishing. The sample analysis method was: scanning electron microscope high-vacuum mode, backscattered electron (BSE) probe, acceleration voltage (HV) 20 kV, and working distance (WD) 15.4 mm. The basic principle of the BPMA is to use the system to control the scanning electron microscope to obtain a backscattered electron image that can reflect the different characteristics of the mineral chemical components and combine with the X-ray energy spectrometer data to identify the minerals. This is a rapid and quantitative test of mineral composition and content, particle size, dissociation degree, and other mineralogical parameters in stone coal [22]. Analyses of C were conducted with a high-frequency infrared absorption spectrometer (CS-3000G, NCS Testing Technology Co., Ltd., Beijing, China).
2.3. Recovery Method of Vanadium, Nickel, and Molybdenum
(1) Beneficiation experiments
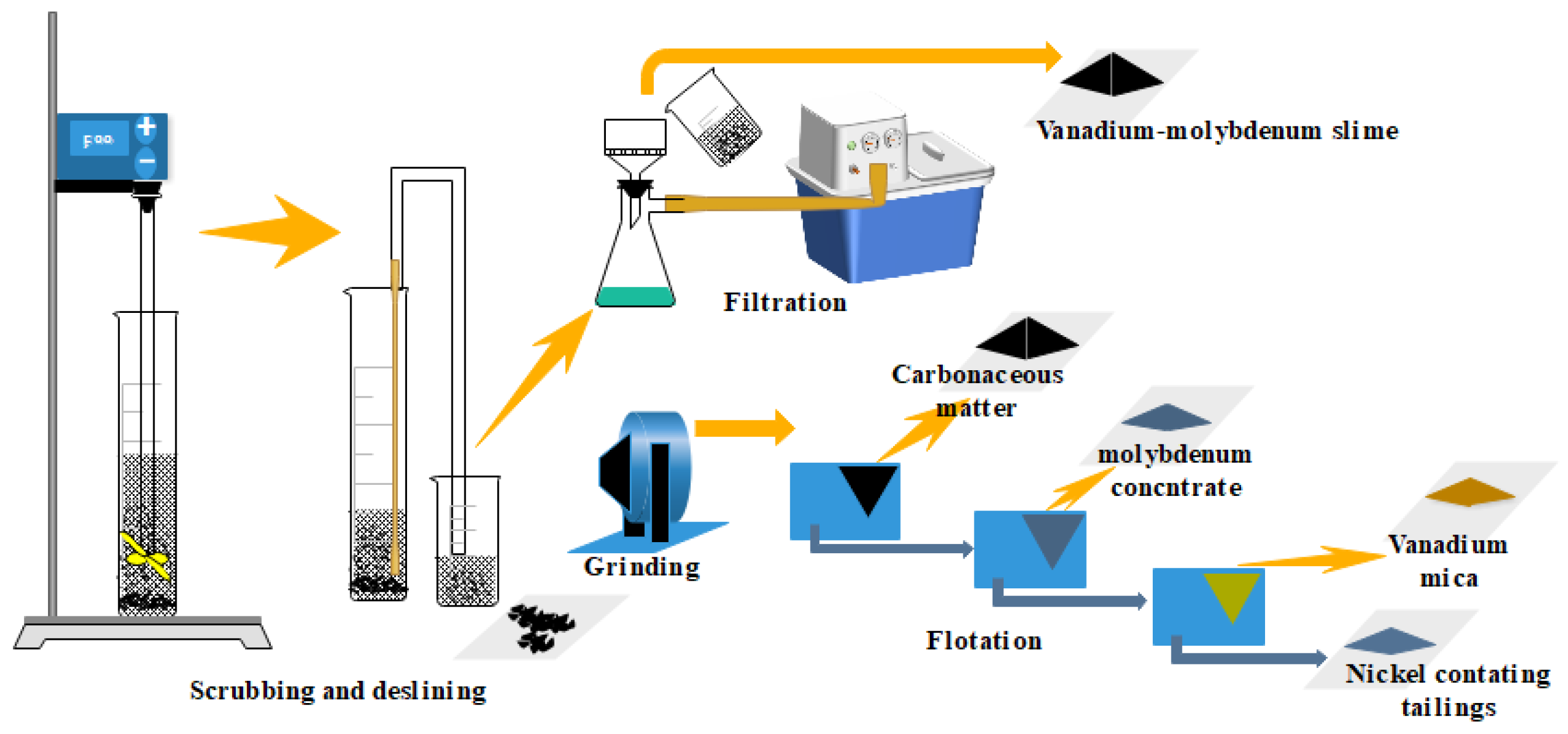
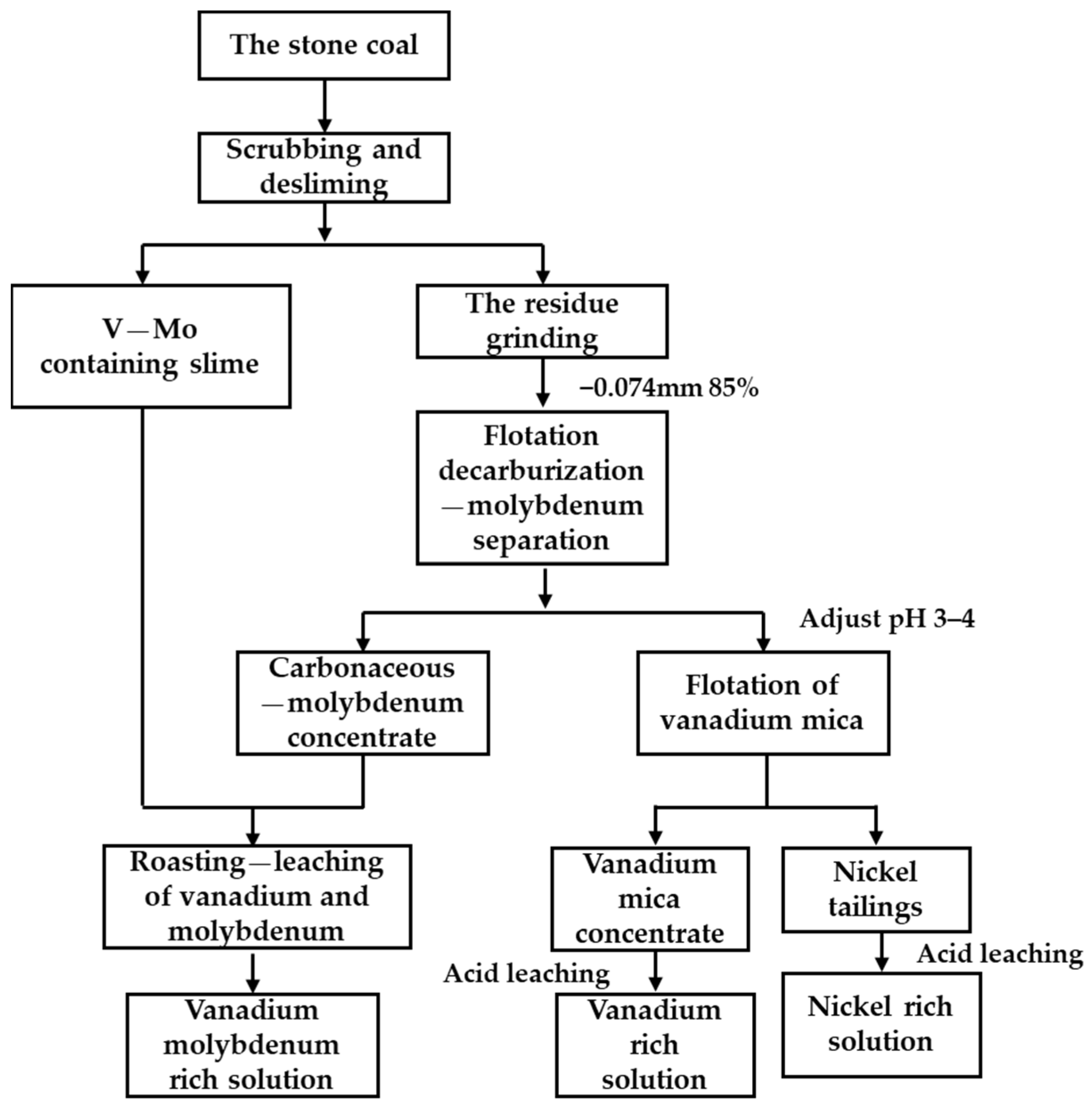
During the scrubbing and desliming test, the slurry concentration was 33%, sodium silicate was 2 kg/t, and the stirring speed was 500 rpm. After stirring for 20 min, water was added to adjust the slurry concentration to 20%. After another 5 min, stirring was stopped, and the slurry was allowed to settle for 5 min. The sludge was filtered and dried to obtain vanadium and molybdenum concentrates. The scrubbed tailings were ground to a fineness of 85%—74 µm, then added with kerosene (300 g/t) and terpenic oil (300 g/t) for decarburization. Next, butyl xanthate (60 g/t), butylammonium dithiophosphate (60 g/t), and terpenic oil (150 g/t) were added for molybdenite flotation. Based on the pH conditions of quartz and mica flotation separation, we added sulfuric acid to adjust the pH of the pulp to 3–4. 3030C (350 g/t) was used to float mica to obtain vanadium mica concentrate. In this study, beneficiation experiments were carried out three times. Average values of the results are reported here. The experimental flow chart is shown in Figure 1.
Figure 1.
The experimental flow chart of scrubbing–desliming–flotation.
(2) Metallurgy experiments
The vanadium–molybdenum slime, carbonaceous matter, and molybdenum concentrate were roasted and decarburized at 650 °C for 2 h, and then leached with 1.84 mol/L of sulfuric acid for 10 h at a liquid–solid ratio of 4:1. The vanadium-containing mica concentrate and nickel-containing tailings were directly leached under the same condition as the roasted slag above. In this study, leaching experiments were carried out three times, and the average value of the leaching efficiency is presented here.
3. Results and Discussion
3.1. Mineralogy Study
3.1.1. X-ray Fluorescence Semi-Quantitative Analysis and Chemical Analysis

Table 1.
X-ray fluorescence semi-quantitative analysis results.

Table 2.
Chemical multielement analysis.
According to the results in Table 1 and Table 2, the main chemical elements in the sample were Si, S, C, Na, Mg, Al, etc., and the content of SiO2 in the raw ore was relatively high, followed by Mg and Na. The contents of the valuable elements V, Ni, and Mo were 0.72%, 0.53%, and 0.11%, respectively, all of which reached or exceeded the mining grade, and the comprehensive recovery value was high. The grade of V2O5 was 1.29% in the stone coal.
3.1.2. XRD Results
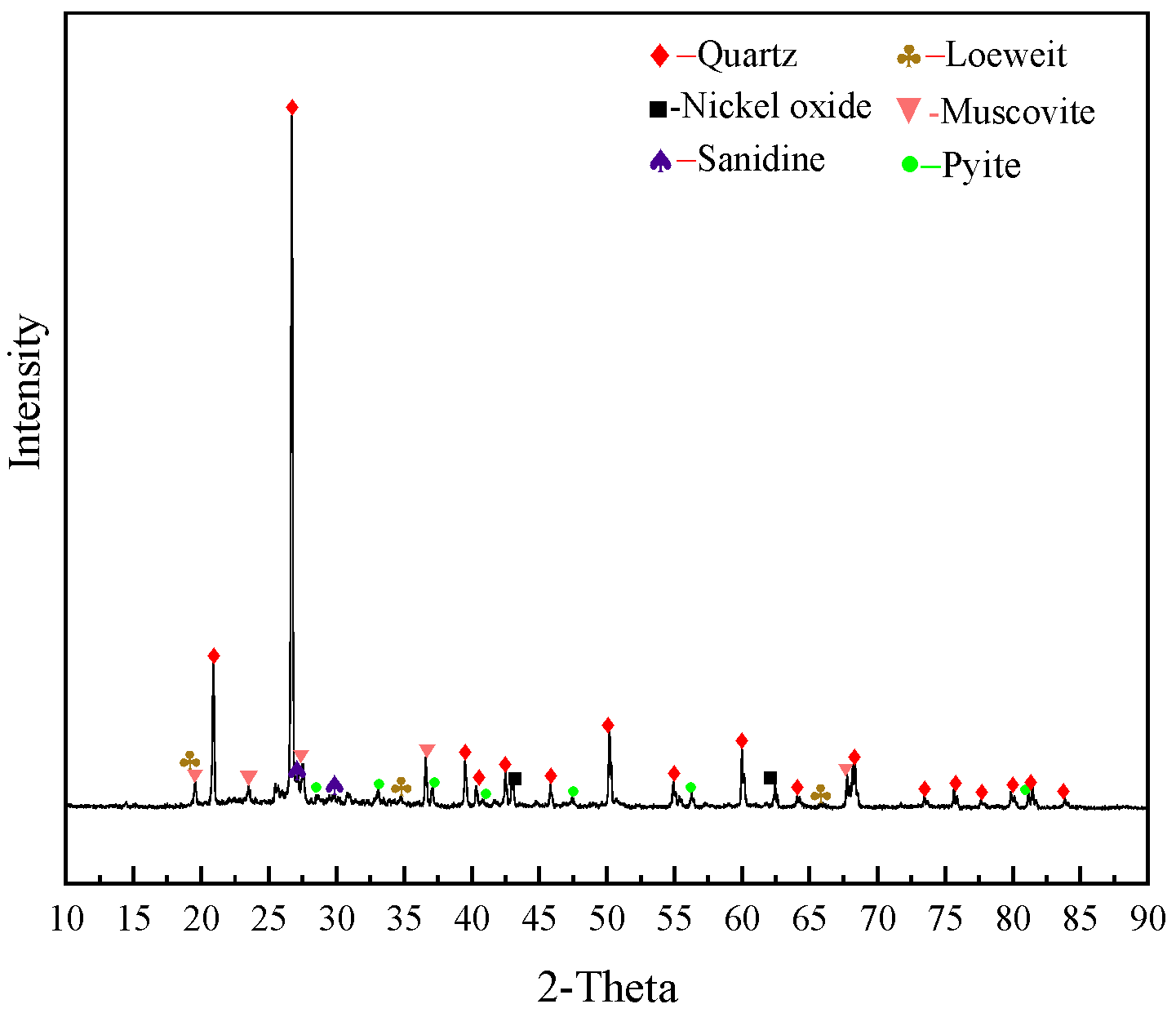
The XRD analysis result is shown in Figure 2. The main minerals of the stone coal were quartz and mica with a small amount of sanidine, nickel oxide, loewite, and pyrite. Because the carbon material was amorphous and the degree of crystallization was poor, the peak value did not appear in the spectrum. Molybdenum mineral did not either due to its low content. V(III) in the stone coal was mostly in the form of lattice substitution in muscovite [10], and no independent V mineral was found in the diffraction analysis.
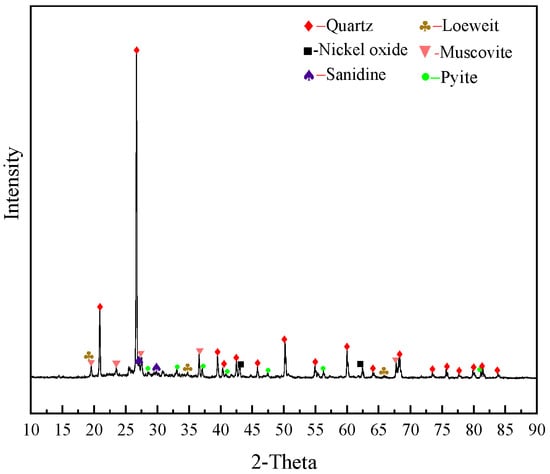
Figure 2.
XRD pattern of the stone coal raw ore.
3.1.3. Mineral Composition and Embedded Characteristics
(1) Mineral composition
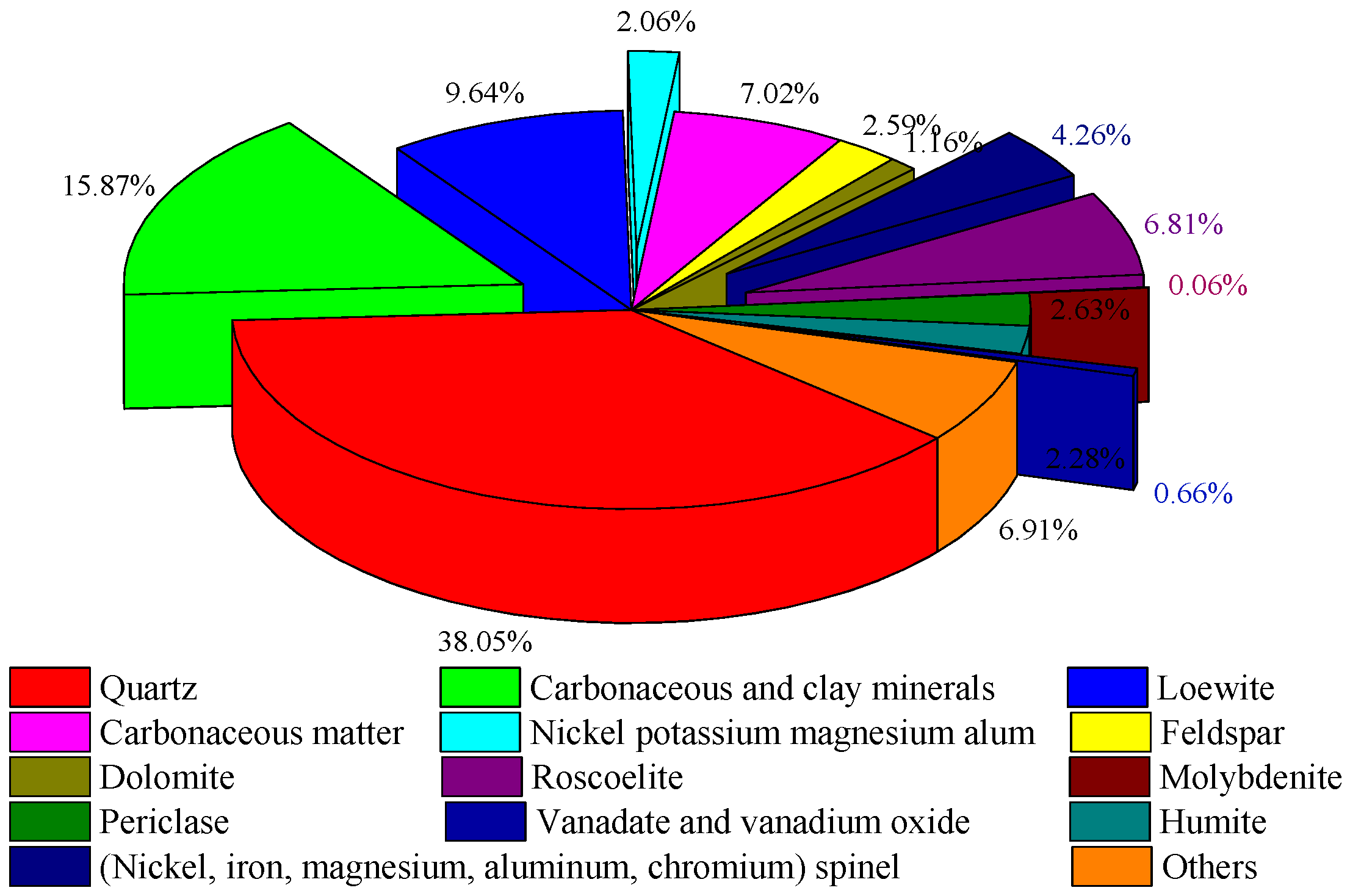
The main gangue minerals in the sample were quartzes, which made up 38.05% of the content. Sulphate minerals such as loewite and nickel–potassium–magnesium–alum accounted for about 12%. Carbonaceous matter content in the stone coal was 15.87%, some of which existed in the form of organic carbon, and some closely bound to clay minerals. Vanadium-containing minerals included vanadium mica and carbonaceous clay minerals. Nickel-containing minerals were nickel (magnesium, iron, aluminum, and chromium) spinel, and the molybdenum-containing mineral was molybdenite. The mineral composition of the stone coal is shown in Figure 3. The embedding characteristics of the vanadium, nickel, and molybdenum minerals are shown in Figure 4
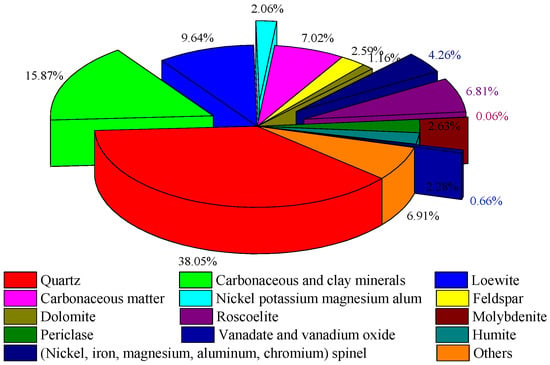
Figure 3.
Mineral composition of the stone coal.

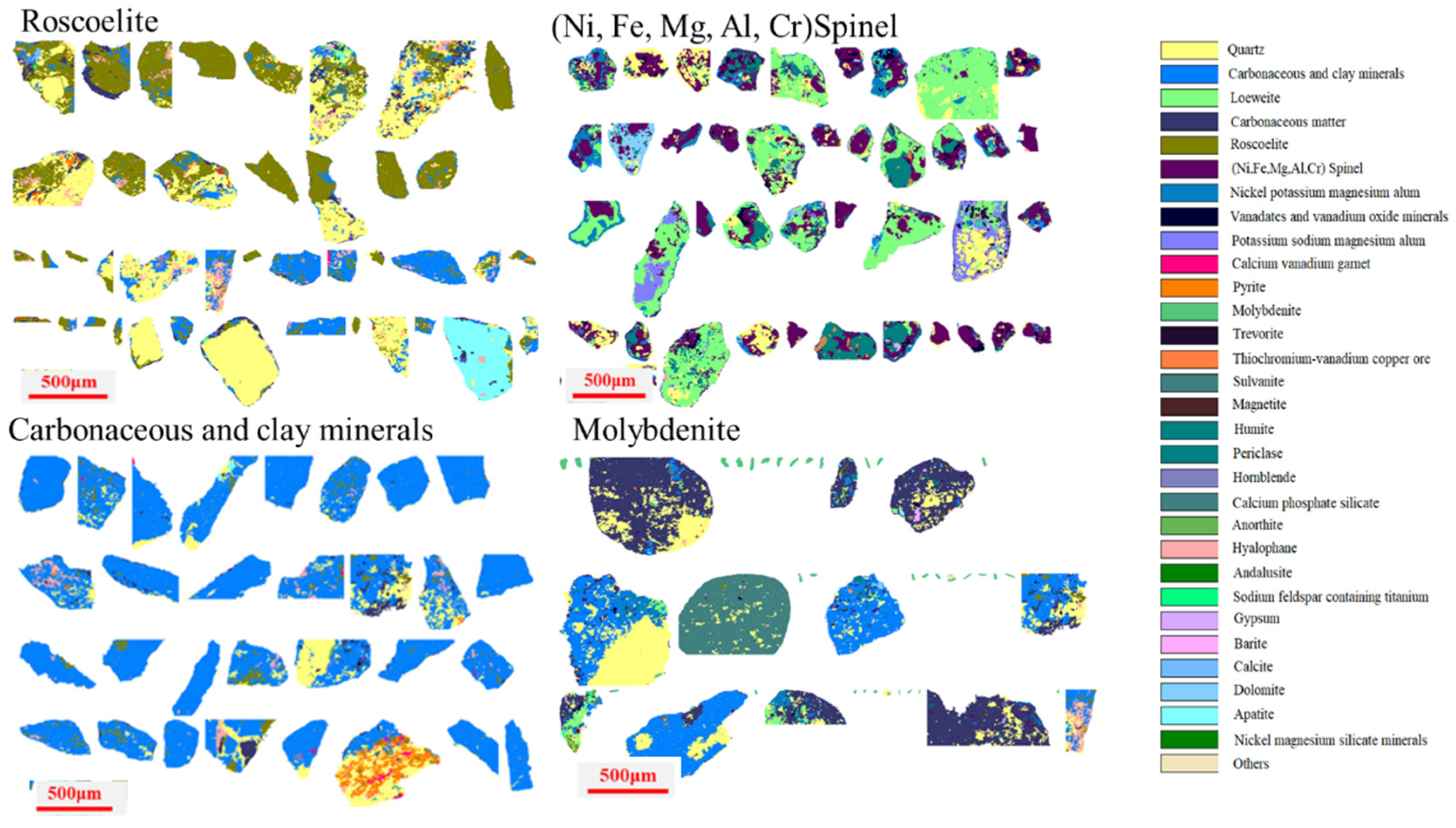
Figure 4.
Embedded characteristics of key minerals.
Results of the ore screening are shown in Table 3. The coarse particles yield was high, with +425 µm and +150 µm accounting for 39.37% and 16.39%, respectively. The intermediate particles yield was lower than that of the coarse particles with −150 + 106, −106 + 74, and −74 + 4 µm accounting for less than 10%. The fine particles yield (−45 µm) was 12.51%. From the perspective of metal distribution, vanadium and nickel were mainly distributed in coarse and fine-grained fractions, and the distribution in the intermediate particles was very small. Most of the molybdenum was distributed in micro-fine ores, and the distribution in coarse and intermediate particles was smaller. Based on the metal grades, the nickel grade was low and only in the +425 µm part, and the other grades were similar. The vanadium content in the fine particles was highest, and the grades of the coarse particles and intermediate particles were close. The molybdenum grade increased gradually with decreasing particle size. In general, the distribution rates and grades of the vanadium and molybdenum in the fine-grained ores were high. Vanadium and molybdenum were mainly distributed in fine-grained ores, while nickel was evenly distributed in each grain size. We also found that the raw ore dissolved in the wet screening process. The dissolution process was further analyzed.

Table 3.
Screening results for the raw ore.
(2) Analysis of the vanadium phase and occurrence states
The BPMA’s results show that the vanadium minerals in the stone coal included vanadium mica, carbonaceous clay minerals, calcium vanadium garnet, a small amount of vanadium oxide, and sulfur–chromium–vanadium–copper ore. The analysis results of the vanadium phase are shown in Table 4. We can see that the main minerals containing vanadium were vanadium mica and clay minerals, and a small amount was distributed in tourmaline and garnet. Vanadium in clay minerals and mica minerals accounted for 65.64% and 24.43% of the total vanadium, respectively.

Table 4.
Phase analysis of vanadium in the stone coal.
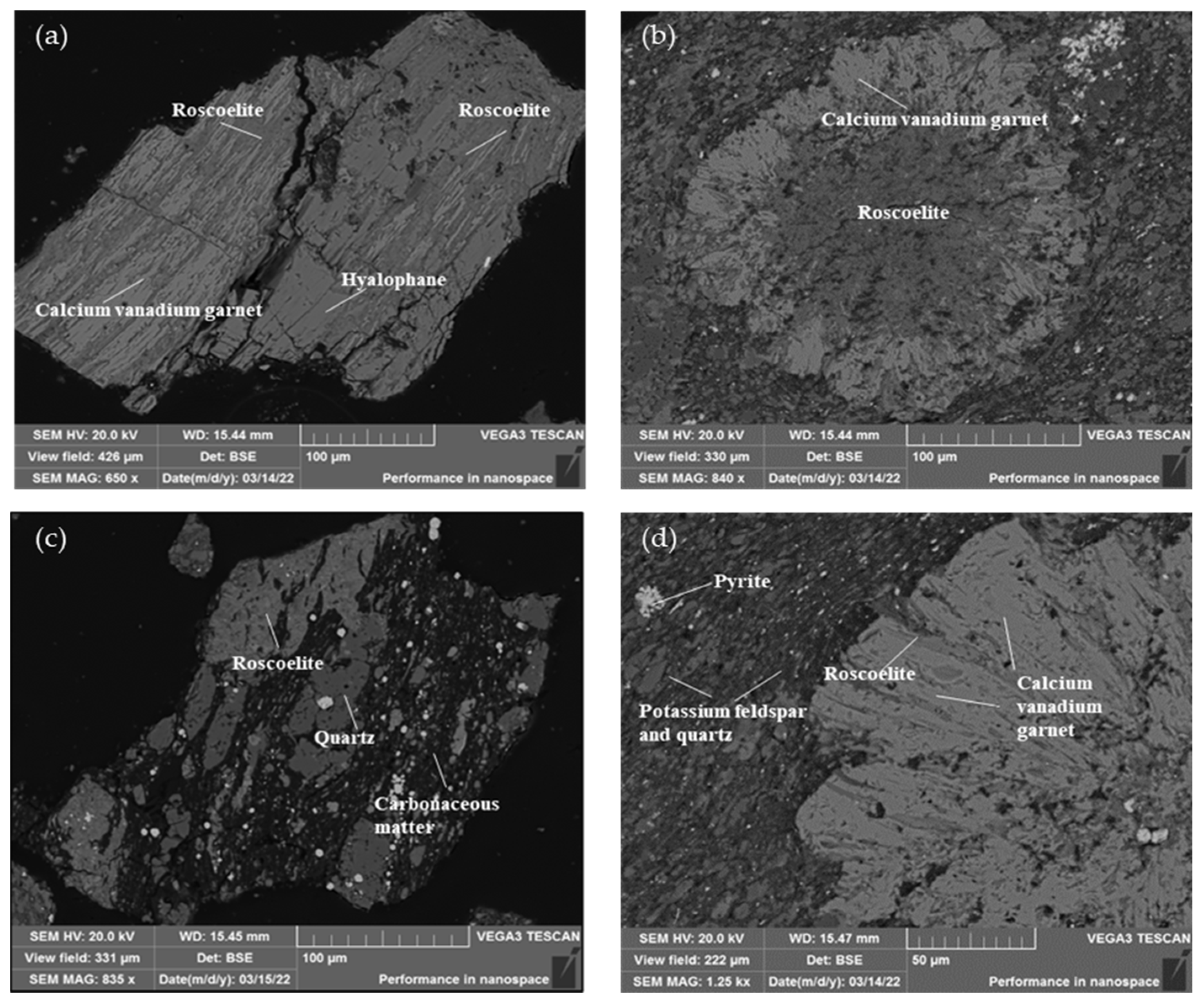
Subsequently, the dissemination characteristics of the vanadium-containing minerals were studied. Because the optical properties of vanadium-containing minerals in stone coal are similar to those of quartz, feldspar, and other major gangue minerals, it is difficult to distinguish them [23]. The content of vanadium minerals in the samples was small, and it took a long time to search for vanadium minerals using a microscope. Therefore, the automatic analysis system (BPMA) for minerals was used to quickly and intuitively analyze the mineral composition in the stone coal. The distribution of vanadium in the detection area was obtained by using a backscattered electron image combined with an X-ray energy spectrum analysis, and the area with high vanadium content was analyzed. The main vanadium minerals in the stone coal are shown in Figure 5
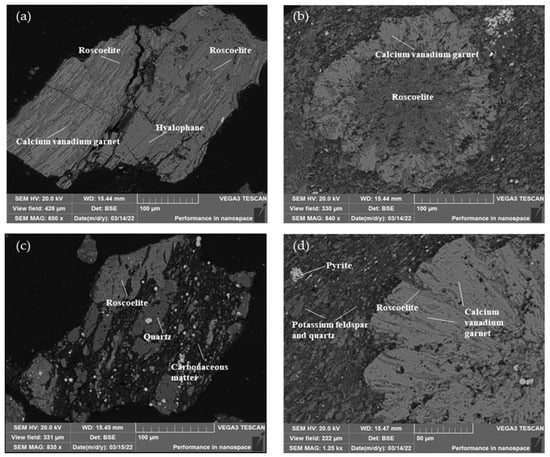
Figure 5.
Scanning electron microscope images of roscoelite (K{V2[AlSi3O10]}(OH)2) in the stone coal: (a) picture of roscoelite associated with calcium vanadium garnet and hyalophane ((K,Ba)Al(Al,Si)3O8); (b) picture of roscoelite associated with calcium vanadium garnet; (c) picture of roscoelite associated with quartz and carbonaceous matter; (d) picture of roscoelite associated with calcium–vanadium garnet and other gangue minerals.
According to the morphology and dissemination relationship in the vanadium-containing mica in the stone coal listed in Figure 4, it can be seen that the particle size of the vanadium-containing mica was not uniform: there was no regular shape, and most of them were closely associated with quartz, calcium–vanadium garnet, feldspar, carbonaceous materials, and other minerals. Some of the calcium–vanadium garnets were surrounded by vanadium mica. Carbonaceous matter filled the spaces between vanadium mica and quartz in strips. Pyrite was dispersed on the surface of the feldspar and quartz aggregates in the form of micro-fine particles. Most of the vanadium mica and calcium vanadium garnets coexisted, so they could be recovered together using flotation.
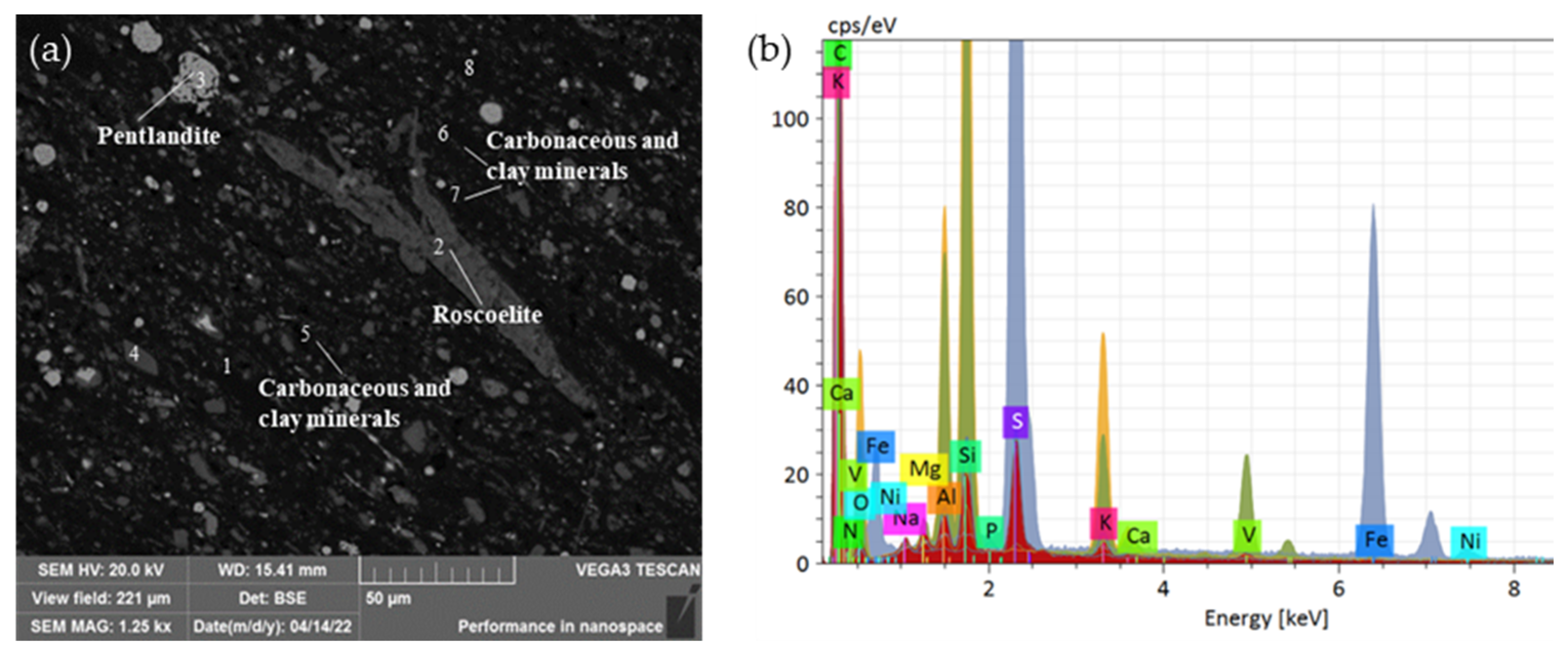
In addition to roscoelite, the carbonaceous clay minerals also contained some vanadium. Figure 6 shows the vanadium distribution in the mica and clay minerals. The carbonaceous clay minerals were mostly veined and disseminated among the vanadium-bearing mica, feldspar, and quartz. The X-ray spectrum in Table 5 shows that the mass fraction of V in the carbonaceous clay minerals was about 0.5%, and that in the vanadium mica was 13.33%.
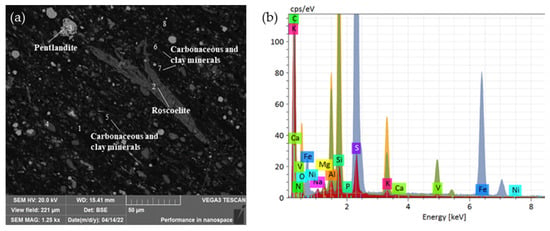
Figure 6.
Vanadium occurrence in the ore: (a) Scanning electron microscope image of roscoelite and carbonaceous clay minerals in stone coal, 1–8 were the analysis positions of the energy spectral; (b) EDS analysis of the minerals containing vanadium.

Table 5.
Spectral microdomain composition analysis of vanadium mica and carbonaceous clay minerals at positions 1–8 in Figure 6a.
In summary, the vanadium-containing minerals in stone coal were mainly vanadium mica and vanadium-containing carbonaceous clay minerals. The above vanadium-containing minerals can be pre-concentrated using a beneficiation process, and then the vanadium can be extracted using different leaching methods to reduce the amount of leaching ore and the leaching cost.
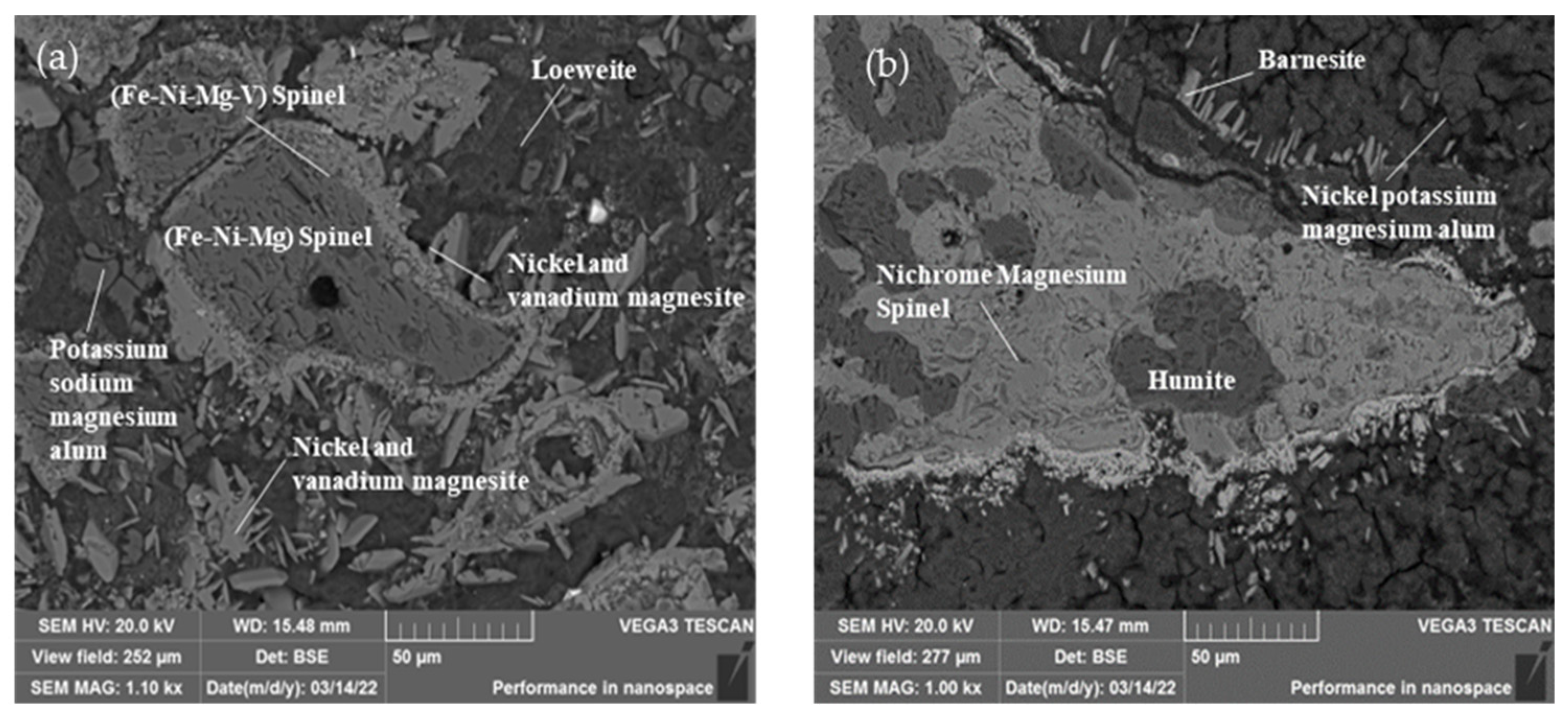
(3) Nickel-containing minerals
The nickel-bearing minerals in the stone coal are shown in Table 6. Nickel in the stone coal samples mainly existed in the form of nickel spinel. The nickel-bearing minerals were mainly nickel magnesium spinel and nickel-bearing potassium magnesium alum, accounting for 2.28% and 2.06% of the total mineral content, respectively. The mass fractions of the nickel were 13.43% and 7.05%, respectively. Figure 7 shows the main morphology and dissemination relationship in the nickel-bearing minerals. The Ni-bearing spinel was mostly short-vein aggregate, symbiotic with soluble sodium alum and nickel–vanadium–magnesium ore. The Ni-vanadium-magnesium ore was mainly slender, with a particle size of 10–50 µm, and was embedded around the nickel-bearing spinel.

Table 6.
Main nickel minerals in raw ore.
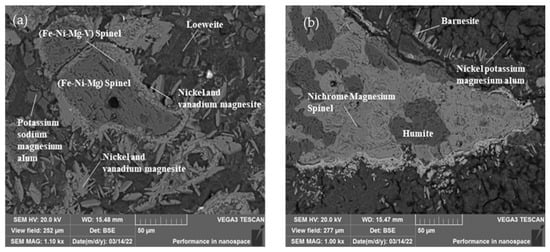
Figure 7.
Nickel occurrence in the ore: (a) Scanning electron microscope image of nickel spinel in ore; (b) Scanning electron microscope image of minerals associated with nickel spinel.
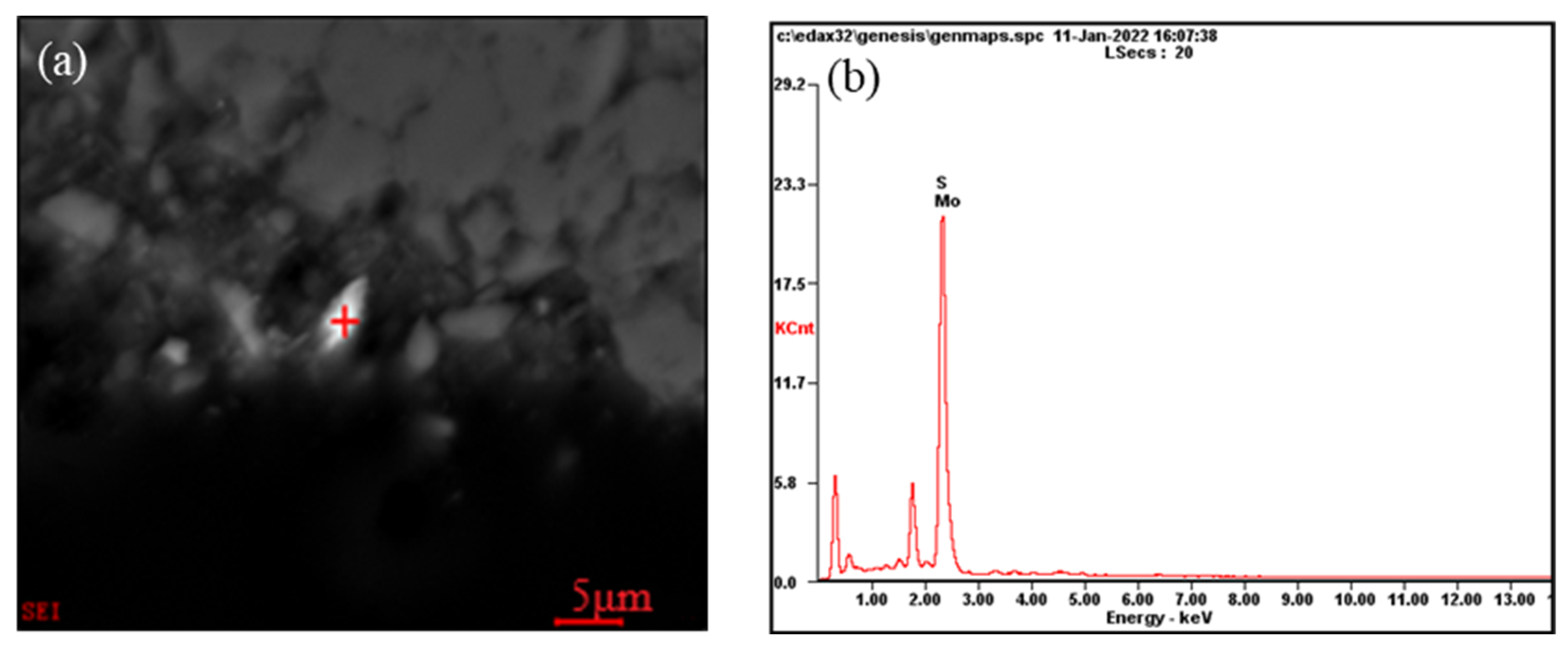
(4) Molybdenum-containing minerals
The Mo grade in the stone coal samples was 0.11%. The molybdenum minerals were mainly made up of molybdenite, and the total mineral content accounted for only 0.06%. Figure 8 shows the occurrence states of molybdenite in the stone coal. Combined with the screening results in Table 3, it can be seen that most of the molybdenum was distributed in fine particles. The particle size was generally <5 µm. From the intercalation relationship in the molybdenite listed in Figure 4, it can be seen that the molybdenite was mostly liberated in the form of monomer particles, and some were embedded in carbonaceous materials, carbonaceous clay minerals, quartz, silicon, and humite in the form of fine needles.
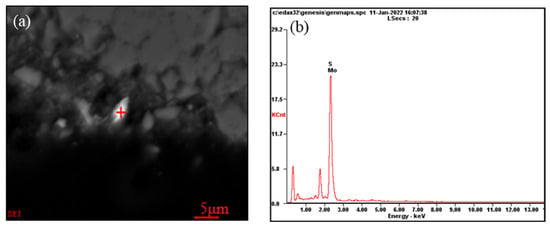
Figure 8.
Molybdenite occurrence in the ore: (a) Scanning electron microscope image of molybdenite in stone coal; (b) EDS analysis for the molybdenite at “+” position.
(5) Soluble minerals
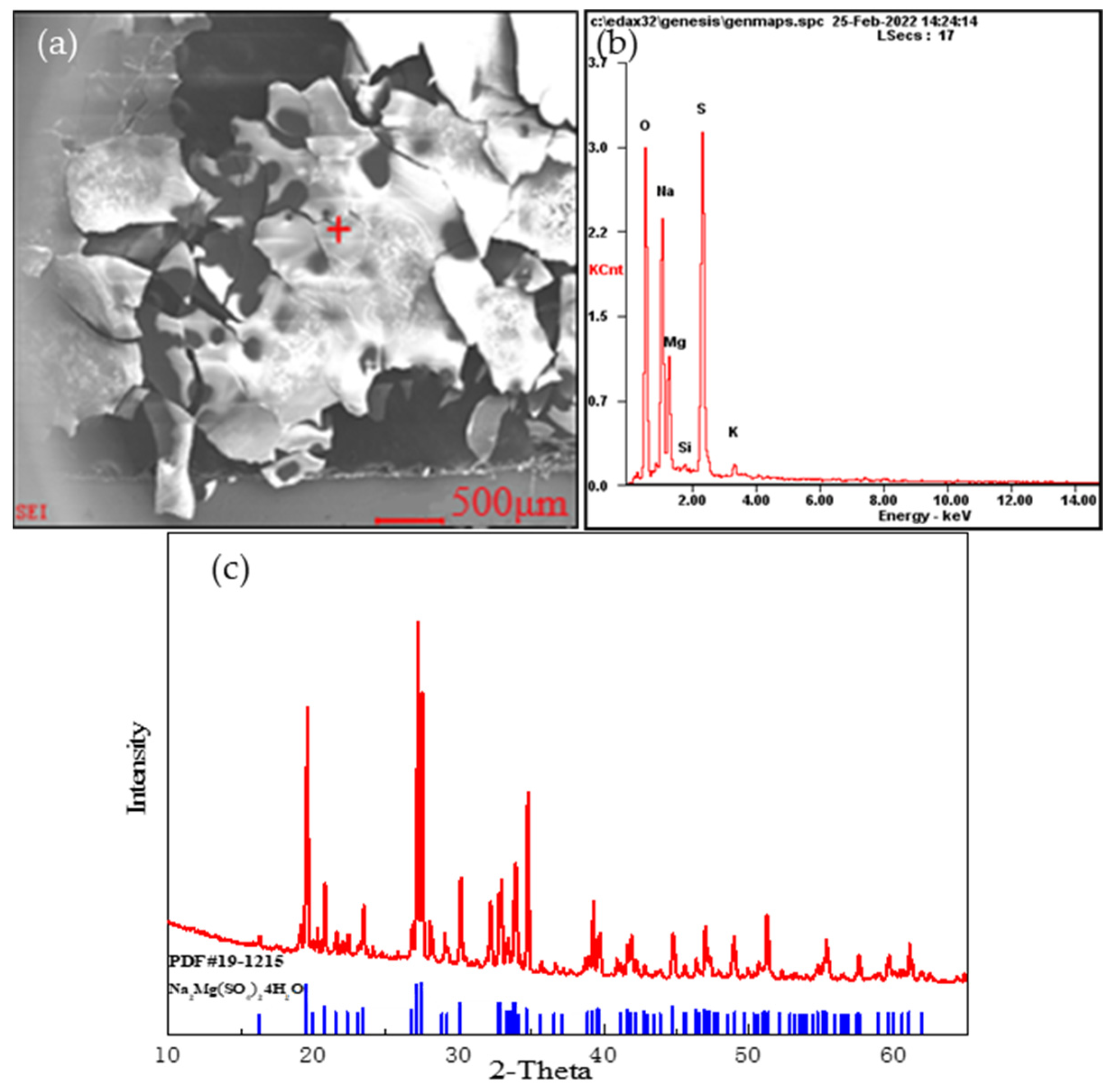
During the screening process, we found that part of the stone coal sample had dissolved in water, resulting in quality loss. Combined with the results of the BPMA, we speculated that the dissolved part was made up of soluble (sodium, magnesium, and potassium) sulfate minerals, which accounted for about 12.45% of the total mineral amount. To further verify the chemical composition of the soluble substances, a 10 g sample was stirred in 50 mL of deionized water for 1 h. In total, 8.3 g of residue was left after stirring. The main components in the solution are shown in Table 7. Part of the filtrate was evaporated and crystallized, and the crystalline sample was analyzed using SEM-EDS and XRD. The results are shown in Figure 9.

Table 7.
Content of the main elements before and after dissolution.
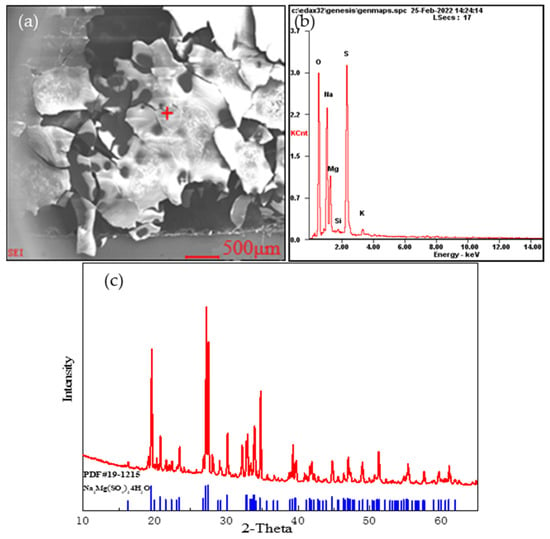
Figure 9.
Soluble minerals: (a) Scanning electron microscope image of the soluble minerals; (b) EDS analysis at “+” position; (c) X-ray diffraction analysis of the soluble minerals.
According to the changes in the main elements before and after dissolution and an analysis of the crystal spectrum, the soluble parts of the stone coal sample were found to be sodium–magnesium alum, potassium–sodium–magnesium alum, and nickel–sodium–magnesium alum. In addition, a small amount of V, Mo, and Ni was also detected in the filtrate.
3.2. Principle Process Determination
The V2O5, Ni, and Mo contents in the stone coal samples were 1.29%, 0.53%, and 0.11%, respectively, and the organic carbon content was 6.13%. Vanadium mainly occurred in the vanadium mica and carbonaceous clay minerals; nickel mostly existed in the form of spinel; molybdenum existed in the form of molybdenite with a high degree of dissociation. Vanadium in the form of carbonaceous clay minerals can be recovered using scrubbing and desliming techniques [24,25,26]. Vanadium mica can be recovered using flotation [27,28,29]. Liberated molybdenite has good floatability and can be enriched using flotation [30]. In addition, some molybdenite and carbonaceous clay minerals are associated closely, so scrubbing–desliming can simultaneously enrich molybdenum and vanadium. Because the mineral properties of nickel-bearing spinel and vanadium-bearing minerals are quite different, the beneficiation process cannot be used for simultaneous enrichment, but vanadium and nickel can be extracted with leaching.
The mud content in the stone coal samples is high, and the mud will adhere to the surface of useful minerals and gangue minerals, which will affect the flotation reagents on the surface of the value minerals. Thus, desliming is also required before flotation [31]. Crushed stone coal samples have fine ore particle sizes and can be deslimed without grinding.
The difficulty of using the flotation process for vanadium mica is separating the vanadium mica from gangue minerals such as quartz and feldspar. Under alkaline conditions, collectors such as sodium oleate [32,33], sodium petroleum sulfonate [34], and dodecyl ammonium chloride [35] can be used to separate non-metallic minerals from gangue minerals such as quartz. Studies have shown that the zero point of mica is very low, and fine mica can be recovered by using cationic collectors in an acidic medium [36,37]. However, due to the complex solution ions in the pulp and the fact that quartz, feldspar, and mica are silicate minerals, it is necessary to enrich vanadium mica with highly efficient and selective collectors [38,39].
Based on the above analysis, combined with the mineralogical characteristics of stone coal samples, we propose a combined process for vanadium, nickel, and molybdenum recovery, as shown in Figure 10.
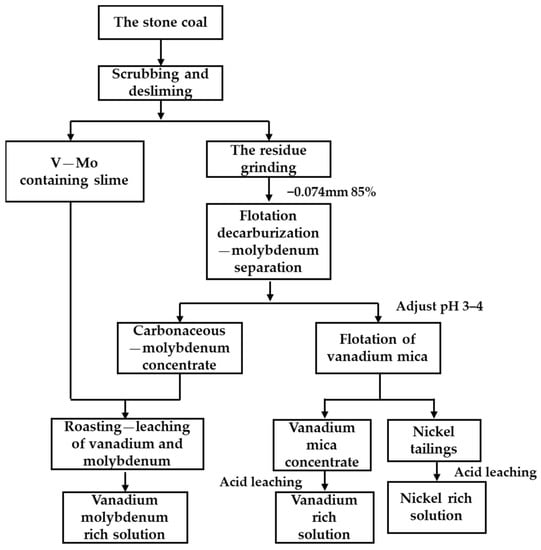
Figure 10.
Basic process flow chart of vanadium, nickel, and molybdenum recovery.
The crushed stone coal sample (−2 mm) contained many fine particles, which could be directly scrubbed and deslimed using sodium silicate as a dispersant. After desliming, the tailings were reground to 85%—0.074 mm, and the minerals containing molybdenum, vanadium, and nickel were fully liberated before flotation [40,41,42]. Carbonaceous matter in stone coal interferes with other minerals during flotation, so decarburization was required before molybdenum and vanadium flotation could proceed. The collector used for flotation–decarbonization was kerosene, and the collectors used for molybdenum flotation were butylammonium dithiophosphate and butyl xanthate. Then, the pH of the pulp was adjusted to 3~4, and the vanadium mica was collected by 3030C. Vanadium and molybdenum needed to be extracted with a roasting–leaching process due to the high carbon content in the vanadium–molybdenum slime obtained by scrubbing and the molybdenum concentrate obtained using flotation, while the vanadium mica and nickel-bearing minerals enriched by flotation could be directly acid-leached under mild conditions.
3.3. Exploratory Experiments Based on Mineralogy
3.3.1. Beneficiation Enrichment Experiments
Combined with the mineralogical features study, the scrubbing–desliming–flotation method was selected to enrich vanadium, molybdenum, and nickel.
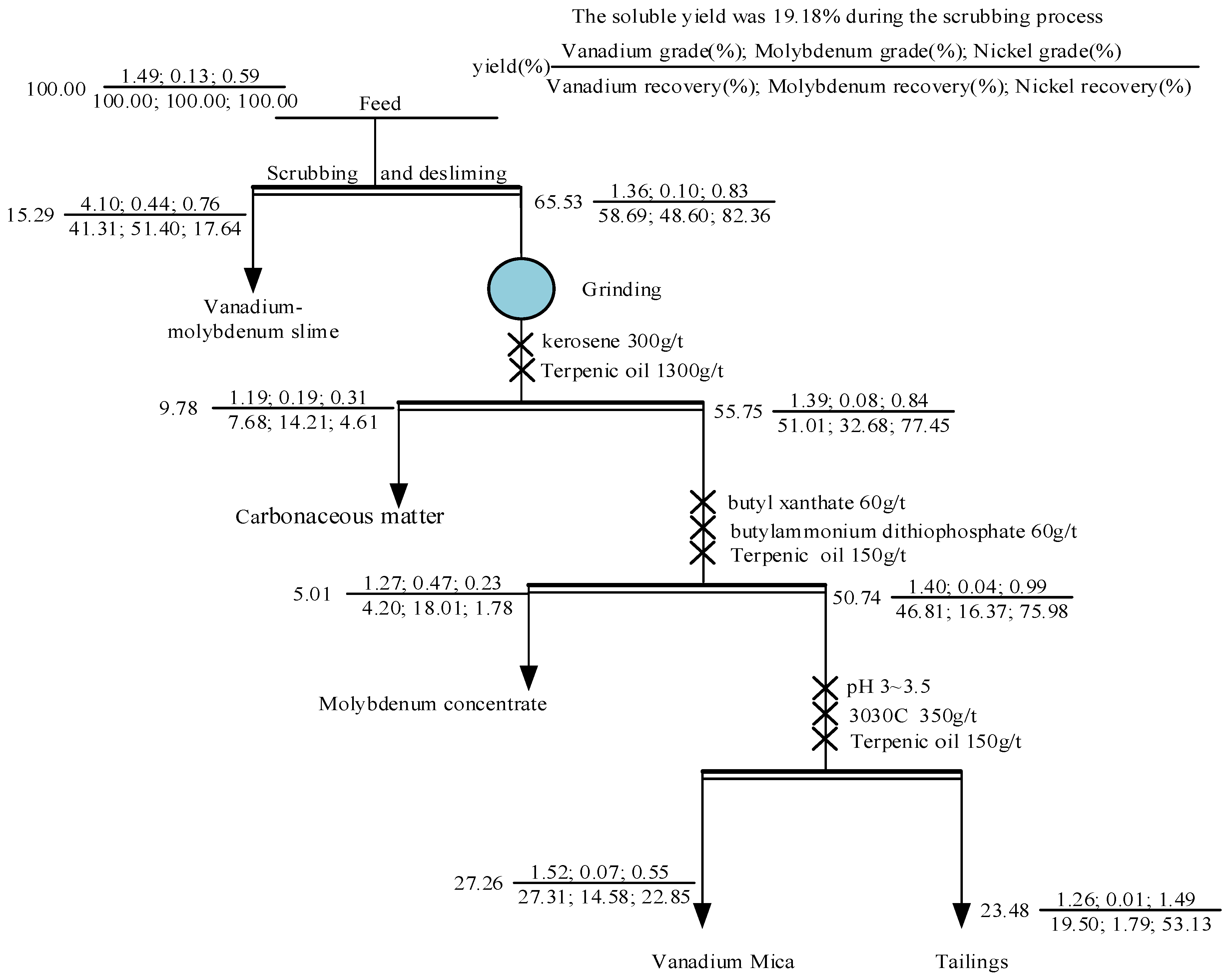
First, the raw ore was scrubbed and deslimed. At a certain pulp concentration, sodium silicate was added to disperse the mineral particles. Then, the pulp was stirred at high strength. Finally, the fine mud vanadium concentrate was obtained via sedimentation and classification. The coarse tailings were separated via grinding and flotation, and the vanadium and nickel were separated from the coarse scrubbing tailings via flotation. The test results are shown in Table 8, and the process flow with the assay of each product is shown in Figure 11.

Table 8.
Experimental results for scrubbing–desliming–flotation.
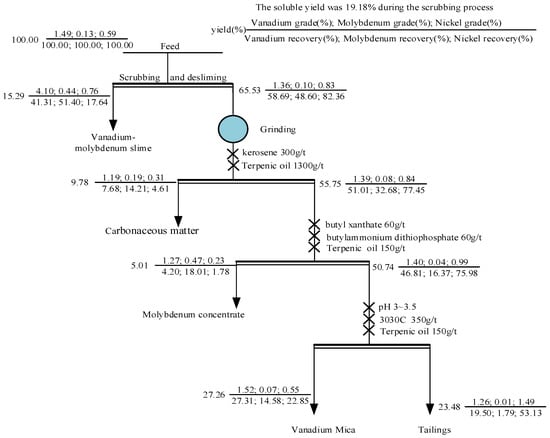
Figure 11.
Flow chart of quantity and quality.
From the results in Table 8, it can be seen that scrubbing–desliming and flotation enriched the vanadium, nickel, and molybdenum in different products. The grade of V2O5 in the vanadium-molybdenum slime was 4.10%, and the recovery was 41.31%. The Mo grade was 0.44% and the recovery was 51.40%. Based on the mineralogical characteristics of molybdenum, a small amount of molybdenum was enriched in the carbonaceous matter and molybdenum concentrate, but the recovery was not high. Based on a phase analysis of the vanadium, we found that, in addition to clay minerals, it was also partially present in mica. However, the enrichment effect of vanadium mica in the flotation test results was poor, and the V2O5 grade in the concentrate was only 1.52%. This is because the stone coal contained many gangue minerals with a similar floatability to vanadium-containing mica, such as silicate and quartz, which made the flotation separation process difficult. Furthermore, the vanadium was partially substituted in the mica, so the mica contained less vanadium, and the flotation process produced vanadium-containing mica with similar mineral properties to vanadium-free aluminosilicate, resulting in more concentrate and yielding small improvements in its grade [10,43]. Based on the occurrence states of nickel in the stone coal, it can be seen that the floatability of nickel was poor, the nickel grade in the tailings was 1.49%, and the recovery was 53.13%. Because stone coal also contained nickel–vanadium symbiotic ore, a small part of the nickel was produced in the vanadium-containing mica concentrate. The above results demonstrate that this process can effectively recover vanadium, nickel, and molybdenum from stone coal by multiple steps.
3.3.2. Vanadium, Nickel, and Molybdenum Leaching Experiments
Corresponding leaching experiments were carried out for different products obtained in the beneficiation experiments. Tang [44] and Zhao [45]’s experiments showed that carbon can be completely removed by roasting stone coal at 700 °C for 1 h. In our laboratory work, the vanadium–molybdenum slime, carbonaceous matter, and molybdenum concentrate were mixed and roasted at 650 °C for 2 h. The roasted slag was leached for 10 h at room temperature, a sulfuric acid concentration of 1.84 mol/L, and a liquid–solid ratio of 4:1. The vanadium-containing mica concentrate and nickel-containing tailings were leached under the same condition as above for the roasted slag. The results are shown in Table 9. At the same time, a fluoride leaching experiment was carried out on the raw ore. The results are shown in Table 10.

Table 9.
Leaching efficiency of different beneficiation products.

Table 10.
Experimental conditions and results of fluorination leaching in the stone coal raw ore.
It can be seen from Table 10 that the leaching efficiency of the molybdenum in the slime–carbon–molybdenum concentrate was as high as 94.10% after roasting. This was due to the oxidation of MoS2 to MoO3 at 650 °C, which is more easily leached using sulfuric acid. The leaching efficiency of the vanadium was 72.55%. As vanadium in clay minerals mostly exists in the form of V(IV) and V(V) adsorption states, it can be dissolved in acid [10]. The leaching efficiency of the vanadium in the vanadium-containing mica was low, which may be related to the crystal structure of the vanadium mica. Therefore, in the process of leaching, it is necessary to add leaching aids to strengthen the fracturing of Si-O and Al/(V)-O in mica [46,47,48]. According to the results of the direct fluorination leaching of the raw ore in Table 10, it can be seen that the vanadium, nickel, and molybdenum were first pre-concentrated by the beneficiation process, after which the roasting–sulfuric-acid-leaching or direct acid leaching processes were used for different products to reduce acid consumption without producing fluorine-containing wastewater and reducing pollution in water resources.
4. Conclusions
(1) V2O5, Ni, and Mo grades in the stone coal were 1.29%, 0.53%, and 0.11%, respectively. The vanadium-bearing minerals were mainly carbonaceous clay minerals and vanadium-bearing mica. The nickel-bearing minerals were mainly nickel magnesium spinel with a small amount of nickel-bearing potassium–magnesium alum. The molybdenum-bearing minerals were mainly molybdenite mostly in the form of molybdenite monomer and partially embedded in carbonaceous materials, carbonaceous clay, and other minerals. The stone coal sample also contained soluble minerals such as sodium magnesium alum, potassium sodium magnesium alum, and nickel–sodium–magnesium alum. The loss of vanadium, nickel, and molybdenum in soluble fractions was comparatively small.
(2) Based on the research results of the mineralogical features study, we propose enriching vanadium, nickel, and molybdenum using a scrubbing–desliming–flotation process to obtain vanadium-rich molybdenum slime and nickel-containing tailings. In our experiment, the V2O5 grade increased from 1.49% to 4.10%, and the recovery was 41.31%. The Mo grade increased from 0.13% to 0.44%, and the recovery was 52.70%. The Ni grade in the flotation tailings was 1.49%.
(3) The obtained vanadium-molybdenum slime, carbonaceous matter, and molybdenum concentrate were roasted and acid-leached; the leaching efficiency of V was 72.55% and the leaching efficiency of Mo was 94.10%. The vanadium-bearing mica concentrate and tailings were directly leached using acid. When the dosage of sulfuric acid was 1.84 mol/L, the leaching efficiency of V and Ni were 50.74% and 67.86%, respectively. Compared with the direct fluorination leaching of the raw ore, the leaching effects of vanadium and nickel were similar, but the leaching efficiency of molybdenum was higher with a lower dosage of sulfuric acid. The current test was only an exploration and needs to be optimized in the follow-up work.
(4) After a mineralogical features study of stone coal from Shaanxi Province, the phase composition and inlay characteristics of vanadium, nickel, and molybdenum were identified, and a scrubbing and sludge–flotation–leaching process was selected to realize the green and clean enrichment of vanadium, nickel, and molybdenum metals. This may provide guidance and reference for industrial applications.
Author Contributions
Conceptualization, M.W. and L.C.; methodology, M.W.; software, L.C.; validation, W.L., J.W. and H.Y.; formal analysis, M.W. and X.Y.; investigation, M.W.; resources, J.W.; writing—original draft preparation, M.W.; writing—review and editing, W.L. and J.W.; visualization, L.C.; supervision, J.W.; project administration, J.W., X.Y. and W.L.; funding acquisition, J.W. and X.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Project (2020YFC1909703).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors are grateful to the financial support from the National Key Research and Development Project (2020YFC1909703).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Santos, D.A.; Dixit, M.K.; Kumar, P.P.; Banerjee, S. Assessing the role of vanadium technologies in decarbonizing hard-to-abate sectors and enabling the energy transition. iScience 2021, 24, 103277. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, A.; Zhong, W.; Zhu, D.; Wang, C. Analysis of international nickel flow based on the industrial chain. Resour. Policy 2022, 77, 102729. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, B.; Han, G.; Wang, M.; Huang, Y.; Su, S.; Xue, Y.; Wang, Y. Clean separation and purification for strategic metals of molybdenum and rhenium from minerals and waste alloy scraps—A review. Resour. Conserv. Recy. 2022, 181, 106232. [Google Scholar] [CrossRef]
- Vind, J.; Tamm, K. Review of the extraction of key metallic values from black shales in relation to their geological and mineralogical properties. Miner. Eng. 2021, 174, 107271. [Google Scholar] [CrossRef]
- Kenzhaliyev, B.K.; Surkova, T.Y.; Azlan, M.N.; Yulusov, S.B.; Sukurov, B.M.; Yessimova, D.M. Black shale ore of Big Karatau is a raw material source of rare and rare earth elements. Hydrometallurgy 2021, 205, 105733. [Google Scholar] [CrossRef]
- Dai, S.; Zheng, X.; Wang, X.; Finkelman, R.B.; Jiang, Y.; Ren, D.; Yan, X.; Zhou, Y. Stone coal in China: A review. Int. Geol. Rev. 2018, 60, 736–753. [Google Scholar] [CrossRef]
- Dai, Z.; Zhang, E. Technical status of leaching vanadium from stone coal vanadium ore. Min. Metall. Eng. 2015, 35, 85–88. [Google Scholar]
- Bao, S.; Chen, B.; Zhang, Y. Research status and prospect of vanadium extraction technology for vanadium-bearing shale in China. Metal. Mine. 2020, 10, 20. [Google Scholar]
- Kang, X.; Ye, G.; Zhu, S.; Liang, X. Research progress on V2O5 extraction from vanadium-bearing shale. Iron. Steel Vanadium. Titan. 2022, 43, 25–34. [Google Scholar]
- Zhang, C.; Niu, M.; Chu, F. Analysis on occurrence characteristics and extraction process of vanadium in stone coal. Mod. Min. 2018, 34, 91–95. [Google Scholar]
- Wang, M.; Huang, S.; Chen, B.; Wang, X. A review of processing technologies for vanadium extraction from stone coal. Min. Proc. Ext. Met. 2020, 129, 290–298. [Google Scholar] [CrossRef]
- Li, M.; Liang, D.; He, X. Study on mineralogy of a stone-coal vanadium ore from Hubei. Metal Mine. 2015, 2, 87–91. [Google Scholar]
- Zhang, W.; Hou, E.; Yang, J.; Li, H.; Huan, B. Study on vanadium-molybdenum-selenium and other associated elements in stone coal. Rare Metals 2019, 43, 1092–1102. [Google Scholar]
- Li, Y. Geochemistry characteristics of Ni Mo Pt group elements in the lower Cambrian bone coal in Hunan Province. J. China Coal Soc. 1996, 3, 38–41. [Google Scholar]
- Wang, L.; Sun, W.; Zhang, Q. Recovery of vanadium and carbon from low-grade stone coal by flotation. T. Nonferr. Metal. Soc. 2015, 25, 3767–3773. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Y.; BAO, S. Vanadium recovery from stone coal through roasting and flotation. T. Nonferr. Metal. Soc. 2017, 27, 197–203. [Google Scholar] [CrossRef]
- Gu, X.; Sun, W.; Liu, R.; Song, S.; Chen, X. Study on mineral processing of a decarburized stone coal in Hunan. Nonferrous Met. Miner. Process. Sect. 2014, 5, 67–71. [Google Scholar]
- Liu, Q. Flotation and Vertical Roasting Process for Extracting Molybdenum from Vanadium-Bearing Molybdenum Coal Mine; Hubei Qinjiahe Mining Co., Ltd.: Hubei, China, 2014. [Google Scholar]
- Zhou, J.; Gu, Y. Chapter–6—Geometallurgical Characterization and Automated Mineralogy of Gold Ores. In Gold Ore Process, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 95–111. [Google Scholar]
- Chen, B.; Ma, L.; Chen, Y.; Ma, H. Chemical phase of vanadium in stone coal. Phys. Test. Chem. Anal. Part B Chem. Anal. 2017, 53, 1052–1056. [Google Scholar]
- Wen, L.; Jia, M.; Wang, Q.; Fu, Q.; Zhao, J. A new SEM-based automated mineralogy system: BPMA and its application prospects in mining industry. Nonferrous Met. Miner. Process. Sect. 2021, 2, 12–23. [Google Scholar]
- Wen, L.; Fu, Q.; Yu, Z.; Jia, M. Application of Automated Quantitative Mineralogy System in the Process Mineralogy Study of Low-grade Fine-grained Molybdenum Ore. Nonferrous Met. Miner. Process. Sect. 2022, 2, 31–38. [Google Scholar]
- Wang, L.; Zhang, Q.; Sun, W. Research on Occurrence of Vanadium in Stone Coal Deposit at Loufanggou Area, Shangluo City, Shanxi Province, China. Acta Mineral. Sin. 2017, 37, 29–35. [Google Scholar]
- Liu, J.; Sun, W.; Wang, L.; Liu, W.; Zhang, M. Study on a New Beneficiation Technology of a Vanadium-Bearing Stone Coal Ore in Shaanxi. Nonferrous Met. Miner. Process. Sect. 2015, 2, 58–63. [Google Scholar]
- Chen, C.; Zuo, H.; Wang, H. Research on vanadium ore dressing technology. China Met. Bull. 2018, 11, 230. [Google Scholar]
- Liu, X.; Zhang, Y.; Bao, S.; Ren, L.; Liu, C. Experimental Research on Selective Desliming of Vanadium Bearing Stone Coal Ore. Nonferrous Met. Miner. Process. Sect. 2015, 6, 41–44. [Google Scholar]
- Wang, L.; Sun, W.; Liu, R.; Gu, X. Flotation recovery of vanadium from low-grade stone coal. T. Nonferr. Metal. Soc. 2014, 24, 1145–1151. [Google Scholar] [CrossRef]
- Sun, W.; Gu, X.; Liu, R.; Ma, Y.; Zheng, B. Occurrence State of Vanadium Minerals in Carbonaceous Stone Coal and Corresponding Beneficiation Technology. Min. Metall. Eng. 2013, 33, 28–31. [Google Scholar]
- Xing, X.; Wan, H.; Ning, S.; She, Z. Experimental Research on Mineral Processing and Vanadium Extraction from a Stone Coal Vanadium Ore. Nonferrous Met. Miner. Process. Sect. 2016, 3, 43–47. [Google Scholar]
- Yi, G.; Macha, E.; Dyke, J.; Ed Macha, R.; McKay, T.; Free, M.L. Recent progress on research of molybdenite flotation: A review. Adv. Colloid Interface Sci. 2021, 295, 102466. [Google Scholar] [CrossRef]
- Chen, X.; Yang, J.; Mao, Y.; Liu, H. An Innovation Process for Comprehensive Utilization of Low-Grade Clay-Vanadium Ores. Nonferrous Met. Miner. Process. Sect. 2010, 5, 9–12. [Google Scholar]
- Jin, J.; Gao, H.; Ren, Z.; Chen, Z. The flotation of kyanite and sillimanite with sodium oleate as the collector. Minerals 2016, 6, 90. [Google Scholar] [CrossRef]
- Ren, Z.; Shen, Y.; Gao, H.; Chen, H.; Liu, C.; Chen, Z. Comparison of Sodium Oleate and Sodium Petroleum Sulfonate for Low-Temperature Flotation of Fluorite and the Collecting Mechanisms. Min. Metall. Explor. 2021, 38, 2527–2536. [Google Scholar] [CrossRef]
- Chen, Z.; Ren, Z.; Gao, H.; Zheng, R.; Jin, Y.; Niu, C. Flotation studies of fluorite and barite with sodium petroleum sulfonate and sodium hexametaphosphate. J. Mater. Res. Technol. 2019, 8, 1267–1273. [Google Scholar] [CrossRef]
- Chen, Z.; Ren, Z.; Gao, H.; Lu, J.; Jin, J.; Min, F. The effects of calcium ions on the flotation of sillimanite using dodecylammonium chloride. Minerals 2017, 7, 28. [Google Scholar] [CrossRef]
- Di, Y.; Jiang, A.; Huang, H.; Luo, Q.; Wei, W.; Wang, R.; Chen, S. Molecular dynamics simulations of adsorption behavior of DDAH, NaOL and mixed DDAH/NaOL surfactants on muscovite (001) surface in aqueous solution. J. Mol. Graphics Modell. 2022, 113, 108161. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; He, D.; Deng, B.; Hu, Y.; Wang, Q. Flotation Separation Behavior of Muscovite and Quartz. Metal. Mine. 2019, 8, 108–112. [Google Scholar]
- Ren, L.; Qiu, H.; Zhang, Y.; Nguyen, A.V.; Zhang, M.; Wei, P.; Long, Q. Effects of alkyl ether amine and calcium ions on fine quartz flotation and its guidance for upgrading vanadium from stone coal. Powder Technol. 2018, 338, 180–189. [Google Scholar] [CrossRef]
- Zou, Z.; Luo, H.; Tang, J. Effect of common Metal Ions on the Flotation Performance of Muscovite and Quartz. Conserv. Util. Miner. Resour. 2018, 1, 66–71. [Google Scholar]
- Lv, H.; Xie, X.; Tong, X.; Chen, L.; Chen, Y. Flotation of carbon from a vanadium leaching residue of unroasted stone coal. Int. J. Oil Gas Coal Technol. 2018, 19, 217–229. [Google Scholar] [CrossRef]
- Wang, C.; Yang, Y. Flotation Technology for Reducing Carbon Content in Vanadium-bearing Stone Coal. Min. Metall. Eng. 2018, 38, 64–66. [Google Scholar] [CrossRef]
- Zhang, L. Study on Mineral Processing of a Low-Grade Vanadium Stone Coal Containing High Grade Carbon. Hunan Nonferrous Met. 2015, 31, 10–14. [Google Scholar]
- Ye, G.; Zhu, S.; Chen, Z.; Hu, Y.; Dong, X.; Zhang, H. A Research Review on Beneficiation Pre-Concentration of Vanadium-Bearing Stone Coal. Chin. J. Rare Met. 2022, 46, 120–130. [Google Scholar]
- Tang, J.; Zhang, Y.; Bao, S.; Liu, C. Pre-concentration of vanadium-bearing mica from stone coal by roasting-flotation. Physicochem. Probl. Miner. Process. 2017, 53, 402–412. [Google Scholar]
- Zhang, J.; Zhang, Y.; Huang, J.; Liu, T. Process of Blank Roasting-Sulphuric Acid Leaching of Vanadium with Leaching Agent from Stone Coal. Rare Met. 2013, 37, 446–452. [Google Scholar]
- Huang, Z.; Chen, T.; Lin, H.; Zhou, Y.; Yan, B.; Tian, H. Research Progress in Vanadium Recovery by Hydrometallurgical Acid Leaching from Stone Coal. Rare Met. Cem. Carbides 2020, 3, 11–17. [Google Scholar]
- Zhang, Y.; Xue, N.; Liu, T.; Huang, J. Green extraction technology of vanadium-bearing shale via whole hydrometallurgical process. J. China U. Min. Techno. 2022, 3, 520–531. [Google Scholar]
- Zheng, Q.; Zhang, Y.; Liu, T.; Huang, J.; Xue, N. Removal Process of Structural Oxygen from Tetrahedrons in Muscovite during Acid Leaching of Vanadium-Bearing Shale. Minerals 2018, 8, 208. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).