Abstract
As an important strategic non-metallic mineral resource, fluorite has been widely used in various industrial fields, such as metallurgy, optics and semiconductor manufacturing, as well as fluorine-related chemical engineering. Since the major gangue minerals of fluorite ore are silicate and carbonate ones, flotation is the main beneficiation method for the concentration. Compared with the relatively easy operation for silicate-type fluorite ore, fluorite concentration from calcite has always been the most difficult challenge in the field of mineral processing. In this review, analyses of the fundamental reasons for the difficulties of flotation separation of fluorite from calcite are performed, from the similar surface properties of both calcium minerals to the deterioration by the interference of dissolved ions in the pulp during grinding and flotation. Recent achievements in the flotation separation of fluorite from calcite as the main contents are comprehensively summarized, covering all aspects of flotation reagents of collectors, depressants and modifiers. Finally, successful examples of industrial practices for fluorite and calcite flotation separation are introduced. This overview provides a detailed and comprehensive reference source for the current research status of fluorite and calcite flotation separation, and some suggestions for future research are provided.
1. Introduction
Fluorite is the primary raw material for industrial fluorine products and is widely used in industrial fields such as metallurgy, chemical engineering, optics and semiconductor manufacturing [1,2,3]. It also has great application potential in fields such as new energy, atomic energy and aerospace. Therefore, it has been listed as the critical mineral or strategic mineral resource by the United States [4], the European Union [5], China and many other countries.
1.1. Fluorite Resources
The world total reserve of fluorite in 2021 is 320 million tons, mainly distributed in Mexico, China, South Africa, Mongolia and Spain [6], exceeding 57% in these five countries. In 2021, the world fluorite product is 8.6 million tons; therefore, the static guarantee period of global fluorite resource is 37.2 years. As the largest producer, China produced 5.4 million tons of fluorite concentrate in 2021.
According to the different associated minerals, fluorite deposits in the world can be mainly assorted into four types: quartz-type, calcite-type, barite-type and polymetallic symbiosis-type [1]. It is worth mentioning that with the continuous decrease of high-quality fluorite resources, low-grade fluorite resources with complex gangue minerals have also been put on the development agenda, posing new challenges to fluorite beneficiation. From the genesis of fluorite deposits, almost all fluorite ores contain some amount of calcite [7,8]. In addition, practices showed that when the ratio of fluorite content to calcite content in raw ore is less than 5, the separation difficulty of fluorite and calciteincreases greatly, and it is difficult to obtain a high-quality fluorite concentrate product (CaF2 > 97%, CaCO3 < 1%). Therefore, it is particularly important to summarize recent achievements in the flotation separation of fluorite and calcite and propose suggestions for future research.
1.2. Calcium-Containing Minerals
Fluorite (CaF2), calcite (CaCO3), scheelite (CaWO4), apatite (Ca5(PO4)3(F, Cl, OH)) and wollastonite (Ca3Si3O9), etc., are common calcium-containing minerals. Fluorite is mainly formed by the interaction of fluoride ions in volcanic magma and calcium ions in rocks [7,8]; therefore, calcite and apatite are commonly associated gangue minerals in fluorite ores, and fluorite is also often present in scheelite ores as an associated mineral. Due to the mineral’s similar surface properties and complex dissemination relationships, the flotation separation of calcium-containing minerals has always been a difficult problem in the field of mineral processing [9,10,11], and it is also an important research focus of flotation separation.
1.3. Flotation Separation of Fluorite from Calcium-Containing Minerals
The concentrating methods used for fluorite include flotation [12,13,14], photoelectric separation (for coarse-grained or bulk fluorite beneficiation), and gravity separation (for coarse-grained fluorite and barite separation) [15]. Practices have shown that flotation can treat various types of fluorite ores, serving as the primary beneficiation approach to fluorite.
Calcite and apatite are calcium-containing gangue minerals in fluorite ores, and the flotation separation of fluorite from calcite and apatite can be achieved by using selective collectors and selective depressants [16,17]. In the beneficiation process of scheelite ores, fluorite is usually removed by reverse flotation as the gangue mineral; therefore, the use of selective depressants is crucial [18,19,20]. Despite extensive research, the problem of flotation separation of calcium-containing minerals has not been completely solved. The article entitled “Froth flotation of fluorite: A review” [1], published by Gao et al. (18 February 2021), is the newest review article about fluorite flotation. The article introduced the crystalline structure, surface properties and floatability of fluorite, and covered the research progress of various fluorite flotation reagents. In addition, the article summarizes laboratory flotation research cases of different types of fluorite ore. In contrast, the goal of our article was focused on the flotation separation of fluorite, particularly from calcite, by providing a comprehensive summary of the recent achievements (37 of the references are from 2021 and 2022) in flotation separation of fluorite and calcite, including various efforts by researchers to enlarge the difference in floatability between fluorite and calcite, selective flotation reagents and successful industrial practice cases.
In this paper, the surface properties of fluorite and calcite are first analyzed to reveal the difficulties in enlarging the floatability difference for separation purposes. The root cause of the difficulty of flotation separation of fluorite and calcite is that both fluorite and calcite use calcium ions as the active sites for adsorption of flotation reagents. The existence of lattice impurities caused huge differences in the surface properties of fluorite and calcite from different origins, resulting in the poor adaptability of flotation reagents. Moreover, the recent reagents involved in the flotation separation of fluorite and calcite and their action mechanisms are systematically summarized. The difference in adsorption of selective collectors and depressants on fluorite and calcite surfaces could be attributed to the difference in the state of calcium atoms, including density state, reactivity, lattice matching, etc. Finally, industrial practice cases of fluorite and calcite flotation separation are introduced, and suggestions for future research are provided.
2. Surface Properties of Fluorite and Calcite
As is known to all, floatability is closely related to the quantity and type of surface-exposed elements after mineral liberation, which, in turn, depends on the crystal structure and dissociation characteristics of the mineral. Fluorite has a cubic crystal structure with a space group of Fm−3 m, a = b = c = 0.5463 nm and α = β = γ = 90°, Z = 4 [21]. The calcite crystal structure belongs to the trigonal system, with a space group of R-3c, ah = bh = 0.4988 nm, ch = 1.7061 nm, α = β = 90°, γ = 120°, Z = 6 [22].The unit cell structures of fluorite and calcite are shown in Figure 1. Although different in crystal structures, fluorite and calcite have some similar surface properties, such as calcium ion broken bonds and adsorption characteristics, which significantly affect the flotation separation of fluorite and calcite.
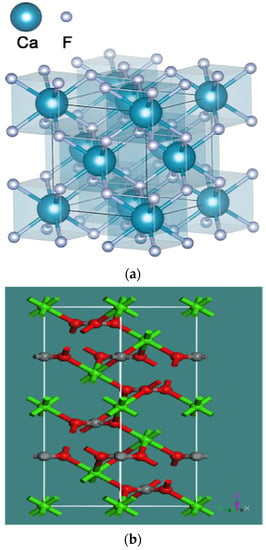
Figure 1.
Unit cell structure of (a) fluorite [21] and (b) calcite (Ca = light green, O = red, C = grey) [22].
2.1. Solubility
The solubility of the mineral surface has an important effect on its surface charge and flotation behavior, especially for semi-soluble minerals such as fluorite and calcite [23,24,25,26,27]. The pKθsp value of fluorite varies from 8.27 to 11.23 in the relevant literature, around 10.5 in most cases [28,29]. In addition, depending on the atomic arrangements of the fluorite crystal planes, the dissolution rate may vary: faster from planes {110} and {310}, and slower from planes {111} and {100} [30].
Beginning with Engel in 1889, many researchers have studied the solubility of calcite under different conditions [31,32]. The pKsp (298.15 K) values of calcite are reported to be around 8.40 [33,34].The saturated solution compositions of fluorite and calcite are shown in Figure 2 [35].
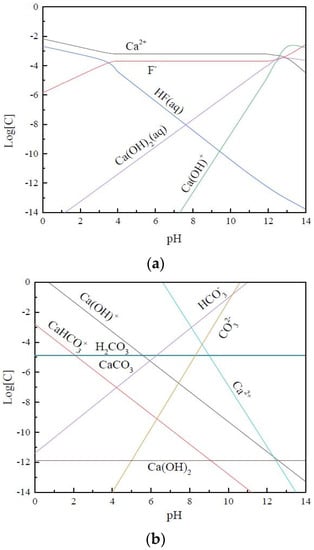
Figure 2.
Traditional distribution diagram of fluorite-solution (a) and calcite-solution (b) chemistry.
As illustrated in the diagrams, the main components of fluorite within the pH 8–10 (conventional fluorite flotation pH range) are Ca2+ and F−, and the main components of calcite are Ca2+, CO32− and HCO3−. The results of solution chemistry calculations and XPS spectral analysis in several previous studies [36,37] showed that the dissolved F− and CO32− can adsorb on the surface of calcite and fluorite, respectively and form the corresponding calcium-containing compounds, which homogenizes the chemical properties of fluorite and calcite surfaces. The surface properties homogenization phenomenon of fluorite and calcite can occur not only in solution but also in the solid-phase grinding process. In addition, Sun et al. found that the ions generated from the surface of fluorite are easier to migrate to the surface of calcite, while the ions generated from the surface of calcite have a poor adsorption capacity on the fluorite surface [37]. This phenomenon is also confirmed by the replacement reaction of a carbonate rock with fluorite [38], probably resulting from the smaller solubility product of fluorite.
2.2. Zeta Potential
Due to surface dissolution, lattice substitution and preferential adsorption of ions, the mineral surfaces may carry charges in the slurries [39]. The electrical double layer at interfaces plays a broad role in many mineral processing operations and is of primary importance in flotation [40]. The floatability of minerals can be enhanced or prevented by changing the surface charge properties of minerals, usually modified by adjusting slurry pH values. When the pH is in the range less than the point of zero charge (PZC) of minerals, the surface tends to be positively charged; on the contrary, the surface is negatively charged with increasing pH above PZC.
The Ca element in fluorite is often replaced by rare earth elements, such as cerium (Ce), yttrium (Y) [41,42], and other metal elements, such as manganese (Mn) [43]. The PZC of fluorite samples from different origins has been reported in wide ranges, from pH 6.2 to 10.6, resulting from the different lattice impurities [44]. In addition, Miller found that fluorite is very sensitive to the presence of carbon dioxide, and F− may be replaced with carbonate anion [36], which changes the PZC of fluorite.
Similar to fluorite, impurities in the calcite lattice also have a significant influence on PZC, resulting in a large change in the ranges from pH 5.8 to 10 [45,46]. The zeta potential of calcite in aqueous solution depends on both pH value and calcium concentration, and several studies [47,48,49] have shown that the zeta potential of calcite is independent of pH if the concentration of calcium is kept constant.
In conclusion, the PZCs of fluorite and calcite from different origins vary widely, and they are also affected by H+, Ca2+ ions in the solution, which increases the difficulty of flotation separation of fluorite and calcite.
2.3. Floatability
In the absence of flotation reagents, the flotation performance of minerals is mainly determined by their wettability [50], and the wettability of minerals is generally expressed by the water contact angle [51].
Fluorite can be considered either a hydrophilic or a hydrophobic mineral, which is determined by its crystal plane and lattice purity [52,53]. Several studies revealed that the contact angle of fluorite is significantly affected by origins, sample preparation and testing methods. The wettability of calcite also shows a phenomenon similar to that of fluorite [53,54]. The compilation of fluorite and calcite contact angles can be seen in Table 1 and Table 2.

Table 1.
A summary of the fluorite contact angle reported in the literature.

Table 2.
A summary of the calcite contact angle reported in the literature.
The contact angles of fluorite and calcite vary widely due to factors such as origin, testing method, crystal plane, etc. Therefore, the contact angles of fluorite and calcite should be examined case by case when studying their influence on floatability. From the perspective of chemical bonds on the mineral surface, the more broken bonds (the number of unsaturated bonds) of the calcium proton on the surfaces of fluorite and calcite, the stronger the interaction between calcium proton and water molecule, and the surface is more hydrophilic [55]. Overall, fluorite is more hydrophobic than calcite.
2.4. Difficulties in Flotation Separation of Fluorite and Calcite
In recent years, with the increasing demand for fluorite products and the continuous decrease in high-quality fluorite resources, flotation research on refractory calcite-type fluorite ore has received increasing attention [62,63].
Based on the surface properties of fluorite and calcite, the reasons for the difficulty in the flotation separation of the fluorite and calcite can be summarized as follows:
- (1)
- In the flotation of the fluorite ore, the collector is generally adsorbed on the surface of fluorite by interacting with calcium ions. However, both fluorite and calcite are calcium-containing minerals with the same flotation active sites, so it is difficult for collectors to achieve selective adsorption. For instance, when sodium oleate is mixed with dodecylamine as a collector, the flotation recovery of both fluorite and calcite is more than 70% without using a depressant [12]. For similar reasons, the depressants used for calcite also inhibit the flotation of fluorite [55,64]. For example, the flotation results showed a small amount (4 × 10−4 mol/L) of citric acid can greatly improve the depression effect of sodium silicate on calcite; the recovery of calcite was only 0.9%. However, fluorite was also depressed, and the recovery rate was only 1.1% [64].
- (2)
- In grinding and flotation, the dissolved F− and CO32− can adsorb on the surface of calcite and fluorite, respectively, and form corresponding calcium-containing compounds, which homogenizes the chemical properties of fluorite and calcite surfaces [65,66]. The analysis results of the To F-SIMS data and PCA model showed that the ions generated on the surface of fluorite migrate to the surface of calcite, which makes the surface composition of calcite closer to that of fluorite, while the ions generated on the surface of calcite have a poor adsorption capacity on the fluorite surface [37].The homogenization of surface properties further increases the difficulty of selective adsorption of flotation reagents on the surface of fluorite and calcite.
- (3)
- The PZC of fluorite and calcite from different origins varies widely, which increases the difficulty of selective adsorption of cationic collectors that adsorption on mineral surfaces by electrostatic interaction.
In addition, practices showed that when the ratio of fluorite content to calcite content in raw ore is less than 5, it is difficult to obtain a high-quality fluorite concentrate product (CaF2 > 97%,CaCO3 < 1%). In the fluorite beneficiation plant of Russia’s Yaroslavl Mining Company, ores with a ratio of fluorite content to calcite content below 2.5–3 were rejected as waste products. To solve the conundrum of the flotation separation of fluorite and calcite, scholars have made elaborate efforts. On one hand, to enlarge the difference in the floatability of fluorite and calcite by using modifiers [67,68], on the other hand, to develop selective collectors and depressants based on the difference in the density and reactivity of calcium atoms on the surface of fluorite and calcite [69,70,71]. Therefore, it is necessary to analyze and summarize recent achievements and extract valuable research results.
3. Flotation Reagents for Separating Fluorite and Calcite
Due to the similar floatability of fluorite and calcite, the research focus of flotation reagents has been put on selective collectors, selective depressants, and modifiers, which can enlarge the difference in the floatability between fluorite and calcite [67,72,73]. In this section, the types and their action mechanisms of the selective collectors, selective depressants and modifiers for fluorite and calcite flotation separation are listed and discussed.
3.1. Selective Collectors
Due to the similar surface properties and the same flotation active sites of fluorite and calcite, it is crucial to develop highly selective collectors for fluorite and calcite flotation separation. With the reviewed research results, the selective collectors can be roughly divided into three categories: (i) organic acids, (ii) combined collectors and (iii) other organic compounds.
3.1.1. Organic Acids
The benzenoid/hydroxamic type organic acids can form a three-dimensional bicyclic or polycyclic ring when chelated with Ca2+, and the difference in the calcium density state between fluorite and calcite surfaces can provide the basis for the selective adsorption of organic acids.
Styrene phosphonic acid (SPA) is a common organic acid with low toxicity that is very easy to synthesize. As a flotation collector, SPA shows good selectivity. When SPA is used as the collector for separation of fluorite and calcite, the greatest difference in recovery of fluorite (98.79%) and calcite (12.55%) was achieved with a SPA dosage of 20 mg/L and pH 6.0 [74]. The AFM 3D images(Figure 3, Figure 4 and Figure 5) [74] of fluorite and calcite in the presence of SPA revealed that the amount of hill-shaped materials on the calcite surface was much less than that on the fluorite surface, which indicated that the amount of SPA adsorbed on the calcite surface was less than that on the fluorite surface. FT-IR analysis [74] and XPS analysis [74] indicated that SPA was adsorbed on the fluorite surface by chemical interaction, and the calcium atom on fluorite was the main active site for reacting with SPA. Furthermore, the first-principles calculation demonstrated that the difference in calcium density state between fluorite and calcite surfaces may be the reason for the selective adsorption of SPA.
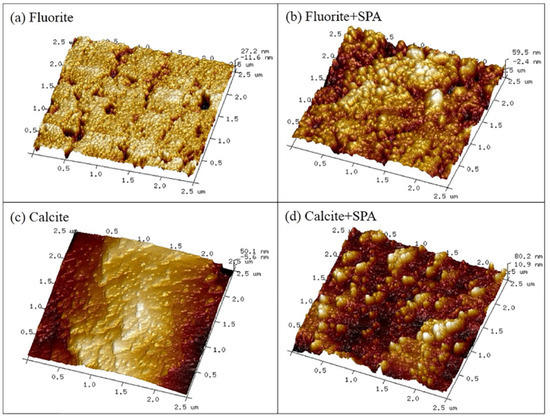
Figure 3.
AFM 3D images of fluorite (a), fluorite treated with SPA (b), calcite (c) and calcite treated with SPA (d) [74].
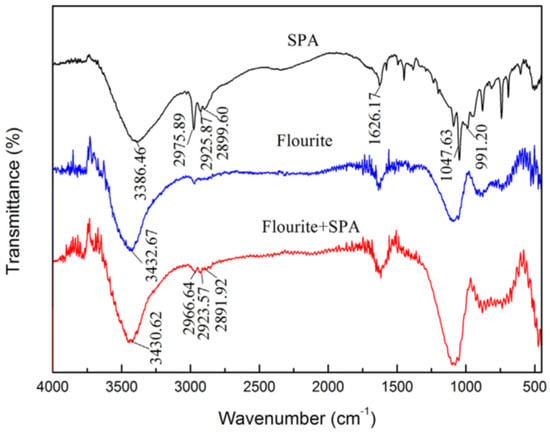
Figure 4.
FT-IR spectrum of SPA, fluorite and fluorite treated with SPA [74].
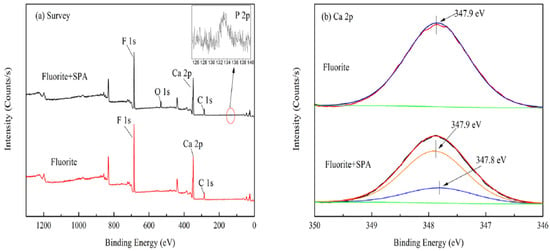
Figure 5.
Survey spectra (a) and Ca 2p high-resolution spectra (b) of fluorite and fluorite treated with SPA.
With α-benzol amino benzyl phosphoric acid (BABP) as a collector, the flotation separation of calcium-containing minerals can be accomplished by carefully controlling the slurry pH [75]. The single mineral flotation results showed that fluorite can float over a pH range from 6 to 10, while calcite is floatable at pH between 8 and 10; therefore, the selective collection of fluorite from calcite by BABP can be achieved with pH in the range from 6 to 8. Chemisorption is the main adsorption mechanism of amino phosphoric acids on calcium-containing minerals, with monovalent collector anions being the active binding species. In addition, the strength of collector adsorption appears to be determined by surface calcium site density.
Compared with sodium oleate, dioctyl di-hydroxamic acid (DODHA) shows highly selective adsorption on the fluorite surface. Results from flotation tests confirmed the effective enrichment of fluorite from calcite without the addition of depressants [76]. When the DODHA concentration was 3 × 10−4 mol/L, the recovery of fluorite reached 93.01%, whereas that for calcite was only 5.80%. The DFT calculation and XPS analysis results showed that the calcium atom density and percentage of fluorite surface were higher than that of calcite surface, which caused the difference in the adsorption capacity of DODHA on fluorite and calcite surfaces. In addition, the molecular structure analyses found that the large steric hindrance and lattice mismatch between DODHA and calcite impeded the interaction of DODHA with the calcite surface, whereas the lattice matching between DODHA and the fluorite surface favored the adsorption of DODHA to achieve the separation of fluorite and calcite.
Benzhydroxamic acid (BHA) can be used as a collector for the selective separation of fluorite from calcite without using any depressants. The optimized geometries of BHA and BHA− are shown in Figure 6 [77]. As shown in the flotation test, the recovery of fluorite promptly increases as the pH value increases from 6 to 8.5 and then remains constant at about 95% at pH 8.5–10. However, the calcite recovery did not exceed 20% in the total pH range. A higher Ca density and Ca activity on the fluorite surface mainly accounted for the selective adsorption of BHA on fluorite, hence rendering the selective separation of fluorite from calcite. In addition, the hydrogen bond plays a role in the adsorption process.
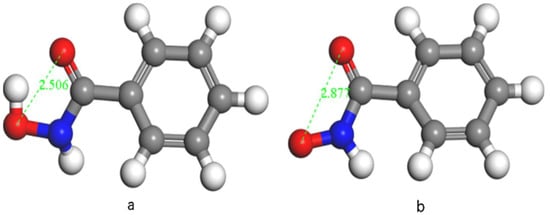
Figure 6.
Optimized geometries of (a) BHA and (b) BHA− (O = red, H = white, N = blue) [77].
3.1.2. Combined Collectors
Several studies have found that the combination of collectors can improve both the collecting ability and selectivity of reagents by leveraging the advantages of different collectors [12,78,79].
The sodium oleate and oleamide combination collector showed good selectivity for the flotation separation of fluorite and calcite. The flotation results indicated that the calcite recovery decreased to 45% when the molar ratio of sodium oleate and oleamide was 8:2, while the fluorite recovery was maintained at 85%. XPS and AFM analyses found that NaOL combined with oleamide formed mixed micelles through hydrophobic association and reacted with calcium atoms on the fluorite and calcite surfaces. The Ca-F chemical bond energy (550 kJ/mol) in the fluorite crystal was greater than that of the Ca-O chemical bond energy (173.33 kJ/mol) in the calcite crystal, suggesting that the calcium atom activity on the fluorite (111) plane was stronger than that on the calcite (104) plane. The reactivity difference of oleic acid ions in the mixed micelles with calcium atoms may be the possible reason for the difference in the flotation performance of fluorite and calcite [13].
Unlike the combined collectors from sodium oleate and oleamide (anionic/nonionic), sodium oleate and dodecylamine (DDA) is an anionic/cationic combination collector. The flotation separation of fluorite from calcite can be achieved using a mixed collector (NaOL:DDA = 9:1) and salted water glass as a depressant. At pH 11, with 1 × 10−4 mol/L combination collector and 2 × 10−3 mol/L of salted water glass, the recovery of calcite is less than 20%, and the recovery of fluorite is kept at about 85%. The FTIR measurement results showed that NaOL and DDA were co-adsorbed on the surface of fluorite, whereas only NaOL was adsorbed on the surface of calcite. The wettability analysis results showed that salted water glass hinders the adsorption of mixed collectors on the surface of calcite and selectively inhibits flotation [12]. The adsorption model of the mixed collector NaOL/DDA on the mineral surface is shown in Figure 7 [12].
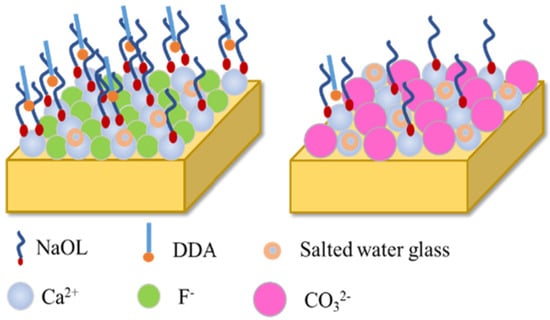
Figure 7.
Schematic of the fluorite/calcite surface with reagents [12].
3.1.3. Other Organic Compounds
In addition to organic acids and combined collectors, some special organic compounds are also used as selective collectors for the separation of fluorite and calcite. The compilation of these organic compounds can be seen in Table 3.

Table 3.
Organic compound collectors for selective separation of fluorite from calcite.
According to above research achievements, the difference in adsorption of selective collectors on the fluorite and calcite surfaces could be attributed to the difference in the state of calcium atoms, including density state, reactivity, lattice matching, etc. These differences could provide the basis for developing new, highly selective collectors for fluorite.
3.2. Selective Depressants
Compared with selective collectors, the selective depressants for the flotation separation of fluorite and calcite have been studied more deeply. Selective depressants in the flotation separation of fluorite and calcite can be roughly divided into two categories: (i) inorganic and (ii) organic.
3.2.1. Inorganic Depressants
Water glass and modified water glass are the most commonly used inorganic depressants; they have good suppression for calcite in fluorite flotation. H4SiO4, H3SiO4− and H2SiO42− are the main hydrolysates of water glass. The adsorption mechanism investigation indicated that electrostatic interactions occurred between the electronegative oxygen atom of the hydrolysates and the calcium atom on the mineral surface; hydrogen bonds were observed between the hydrogen atom of the hydrolysates and the oxygen atom or fluorine atom of the calcium-containing mineral surface [75,84]. It can be found from the inhibition mechanism that water glass has suppression effects on both calcite and fluorite, so it is necessary to increase the selectivity of water glass by modification. After acidification, the water glass was more selective between fluorite and calcite in flotation [85]. A mixture of hydrochloric acid (1.5 g/L) and water glass (50 mg/L) was used as a calcite depressant. The flotation results indicated that the addition of inorganic acid can significantly improve the depressing efficiency of water glass on calcite. This phenomenon can be explained by the exposure of the new calcite surface due to surface dissolution, which facilitates the reaction of calcite with the depressant [86]. On the other hand, the presence of acid increases the formation of colloidal silica, which is selectively adsorbed on the surface of calcite [67]. Pb-water glass is another type of modified water glass that can be used as a depressant to improve fluorite-calcite separation efficiency. The solution chemistry calculation, XPS and zeta potential analyses showed that lead ions have a strong adsorption capacity on the surface of calcite, which provides more adsorption sites for water glass. In contrast, the amount of lead ions adsorbed on the fluorite surface is small; thus, the addition of lead ions has little effect on the adsorption of water glass on the surface of fluorite [87,88].
The combination of inorganic ions and water glass is a common combined depressant of calcite during fluorite flotation. Micro-flotation experiments indicated that with acidified water glass as a depressant, the flotation recovery of fluorite is 88.72% after fluoride ion treatment, that is, approximately fourfold with respect to that without fluoride ion modification. The selective suppression mechanism can be explained by fluoride ions improving the floatability of fluorite by converting CaSiO3 on the surface of fluorite into CaF2, but the conversion ability of Si(OH)4 on the surface of calcite is weak, which increases the difference in floatability between fluorite and calcite [89]. In the flotation separation of fluorite from calcite and celestite, the Al2(SO4)3 and acidified water glass mixed depressant increased the grade and recovery of CaF2 in the fluorite concentrate by 13.10% and 3.58%, respectively, compared with using acidified water glass alone. Both Al-containing species and Si-containing species are adsorbed on the surface of the minerals, and they are more likely to be adsorbed on the calcite surface and the celestite surface, respectively, to achieve selective suppression of calcite and celestite [90].
Phosphates such as sodium hexametaphosphate (SHMP, (NaPO3)6), sodium pyrophosphate (SP, Na4P2O7) and trisodium phosphate (TSP, Na3PO4) are also used as depressants for calcite and barite in fluorite flotation. However, phosphates also suppress the flotation of fluorite, so it is not easy to accurately control a proper dosage during flotation [91,92].
In conclusion, it is difficult to achieve high-efficiency separation of fluorite and calcite by using an inorganic depressant alone, so it is necessary to modify the inorganic depressant or add inorganic acids and inorganic ions to improve the selective suppression ability for calcite.
3.2.2. Organic Depressants
Organic depressants for calcite have been intensively studied due to their advantages, such as rich sources, biodegradable nature and relatively low price. Organic depressants usually have a hydrophobic hydrocarbon chain at one endpoint and a highly hydrated polar group at another; therefore, they have better selective suppression abilities. Commonly used organic depressants for calcite are plant gum, organic acid, starch, dextrin, etc.
Plant gum is a kind of polysaccharide macromolecular organic that has been extracted from plant fruits or seeds. Commonly used plant gums include tamarind seed gum, psyllium seed gum, flaxseed gum and sesbania gum [69,91,93,94]. The selective suppression mechanism can be summarized as the adsorption effect and capacity of plant gums being stronger and higher on the calcite surface than on the fluorite surface, so it is not conducive to the adsorption of NaOL and depressed the flotation of calcite. Plant gums are adsorbed not only on the surface of calcite through hydrogen bonds but also chemically adsorbed on calcium ion sites on the surface of calcite through hydroxyl and carboxyl groups. In contrast, plant gums barely affected NaOL adsorption on the fluorite surface.
Tannic acid as a calcite flotation depressant is widely used in the beneficiation of fluorite [95,96]. Adsorption tests indicated that tannic acid adsorption onto calcite was highly dependent on solution pH, and the optimal adsorption pH value was 7–8. At this pH value, tannic acid can selectively adsorb on the calcite surface and prevent the adsorption of sodium oleate, whereas this phenomenon does not exist on the fluorite surface. The chemical shifts of tannic acid adsorbed on the calcite surface were more similar to those of a Ca(OH)2 surface than those of a fluorite surface, suggesting that tannic acid selectively adsorbs on the calcite surface via chemical interactions with Ca(OH)+. The proposed adsorption process and model for tannic acid are shown in Figure 8 [96].
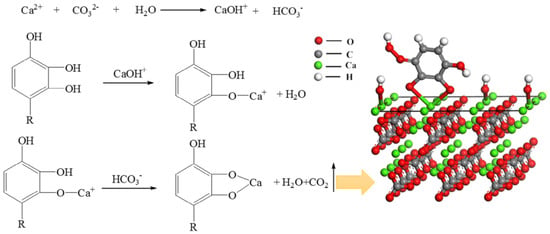
Figure 8.
Proposed adsorption process and model for tannic acid.
Polyaspartate (PASP) is another efficient selective depressant for flotation of fluorite from calcite [97,98]. The addition of PASP can hinder the adsorption of NaOL on the calcite surface but exert little influence on its adsorption on fluorite surface. Similar to PASP, polyacrylic acid (PAA) is also a highly selective depressant of calcite [70]. The flotation tests indicated that the preferential adsorption of PAA onto calcite, rather than sodium oleate, could selectively depress the flotation of calcite, allowing its separation from fluorite at a pH of 7. The mechanism of selective adsorption can be explained by the chemical bonding between the carboxyl group of PAA and the hydroxyl groups of the Ca species on the calcite surface, modifying the structure of the adsorbed layer. The proposed models for the adsorption of PAA on the calcite surface are shown in Figure 9 [70].
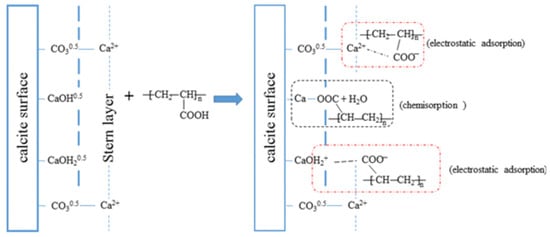
Figure 9.
Proposed models for the adsorption of PAA on calcite surfaces.
Starch and dextrin are a kind of macromolecular organic compound with the advantages of a comprehensive source, low cost, and strong depression effects on calcite [73,99]. The adsorption mechanism indicated that the -OH group of starch/dextrin as shown in Figure 10 [1], might adsorb on Ca sites at the surface of calcite through chemical bonds. The inner electron binding energy of Ca2+ in fluorite is larger than the inner electron binding energy of Ca2+ in calcite, which makes it more difficult to form chemical bonds. Therefore, the chemical adsorption of starch/dextrin on the surface of calcite is stronger than that on the surface of fluorite.
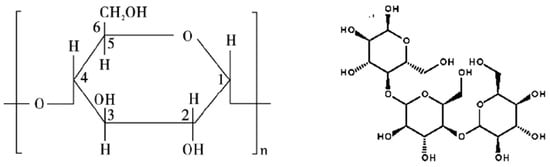
Figure 10.
Starch (left) and dextrin (right) [1].
3.3. Modifiers
3.3.1. pH Regulator
Slurry pH can directly change the surface dissolution of fluorite and calcite, thereby affecting flotation behavior. Sodium hydroxide, sodium carbonate and sulfuric acid are commonly used as pH regulators in flotation separation of fluorite and calcite [83,100]. The flotation behavior of fluorite at a high pH (>12) is distinctly different from that at a conventional flotation pH range of 8–9 [35], and efficient calcite/fluorite separation could be achieved by using a low collector dosage at pH 13 (sodium hydroxide as the pH regulator). Wang et al. [101] found that a green and low-cost 1-hydroxyethylidene-1,1-diphosphonicacid (HEDP) can be used as a pH-dependent switch to initially depress fluorite for calcite pre-removal at pH 6.0 and then activate fluorite for further upgrading fluorite concentrate at pH 8.0.
3.3.2. Anions
Anions such as carbonate, fluoride and sulfate ions can affect the flotation behavior of fluorite and calcite. Jin et al. found that when the pulp pH is greater than the pH values of the saturated solutions (pHsat) of fluorite or calcite, the flotation of fluorite or calcite can be facilitated by the addition of the anions; when the pulp pH is less than the pHsat, the flotation is inhibited [102]. The pHsat of fluorite is 6.8, while calcite is 8.2; therefore, the activation of fluorite and the inhibition of calcite can be achieved by adding these anions and controlling the pulp pH in the range of 6.8–8.2.
3.3.3. Pb2+
Lead ions are widely used in mineral flotation as modifiers [103,104]. In a novel flotation reagent system for the reverse flotation separation of fluorite and calcite, the lead ion is used as an activator for calcite. The lead ion shows strong adsorption ability on the surface of calcite, but poor on the surface of fluorite. Therefore, lead ion pretreatment can provide new adsorption sites on the surface of calcite, which allows potassium isoamyl xanthate to adsorb on the newly developed active sites on the surface of calcite and generate hydrophobic leadxanthate. The grade of the fluorite concentrate obtained after lead ion pretreatment was greatly improved, from 72.42% to 81.92%,compared with that prior to pretreatment [105].
4. Industrial Practice Cases of Flotation Separation of Fluorite and Calcite
The flotation separation of fluorite and calcite poses a long-standing challenge in the mineral processing industry. The elaborate efforts by many researchers have allowed certain successes to serve as a good foundation for the efficient separation of fluorite from calcite in practical operations. To better understand the flotation separation of fluorite and calcite, two cases of industrial beneficiation of fluorite mines with high calcite content (CaF2/CaCO3 < 3) have been introduced in this section.
(1) The Yaroslavl Mining Company processes carbonate-fluorite ores from Russia’s largest Voznesensky deposits in Primorski Krai as a basic raw mineral resource [106]. The ores are rather complex and difficult to float [107]. In practice, the technical problems are as follows:
- (1)
- Typically, when fatty acids are used as fluorite flotation collectors, the ore pulps commonly need to be heated to 35–85 °C due to the poor dispersibility of fatty acids at low temperatures [79]. There are similar problems in this beneficiation plant. The ground ore and the froth product of the second recleaning need to be steamed to 50–65 °C and 65–85 °C, respectively, then reacted with flotation reagents.
- (2)
- Selectivity for minerals will worsen with the reduction in the ratio of fluorite content to calcite content in the feed ore; raw ores with a ratio of fluorite content to calcite content below 2.5–3 were rejected as waste products.
Modification, emulsification and synergistic dosing are the conventional methods [108,109] to improve the dispersibility and collection performance of fatty acids under low temperature conditions. Therefore, improving the dispersity and selectivity of the collector at low temperatures by optimizing the flotation reagent scheme is the key to improving the flotation index. Researchers further developed a new process for selective fluorite flotation from finely disseminated carbonate-fluorite ores without high-temperature pulp treatment, while allowing the treatment of high-carbonate-fluorite ore with a ratio of fluorite content to calcite content below 2.5. The flotation reagent scheme: NaF as modifier, industrial soap as collector, oxyethylized compounds as dispersant to the collector. The mechanism for improved selectivity when collectors are used in combination with sodium fluoride can be explained by the formation of fluorine-bearing associates, characterized by a higher affinity to fluorite rather than to calcite. Moreover, the addition of oxyethylized compounds can improve the dispersibility of the collector, and the flotation separation of fluorine and calcite can be achieved at low temperatures.
The updated flotation flowsheet of the beneficiation plant of the Yaroslavl Mining Company is shown in Figure 11 [106]. For fluorite raw ore, the content of CaF2 and CaCO3 is 26.56% and 25.30%, respectively. The fluorite concentrate with CaF2 content 92.75%, recovery rate 65.16% and the harmful components CaCO3 content 2.08% could be obtained through the updated beneficiation process [106].

Figure 11.
Updated flotation flowsheet.
(2) The fluorite in Qinglong (Guizhou Province, China) is a low-grade carbonate type fluorite ore [110], with the content of CaF2 and CaCO3 is 32.25% and 11.78%, respectively. The beneficiation plant flotation reagent scheme: sodium carbonate as pH regulator, sodium hexametaphosphate and water glass salified with aluminum sulfate as depressants and oleic acid as collector. The application of a combined depressant of sodium hexametaphosphate and modified water glass can greatly improve the depression effect of calcite. The fluorite concentrate with CaF2 content 98.10%, recovery rate 83.68% and the harmful components CaCO3 content 0.83% could be obtained. The flotation flowsheet of the beneficiation plant is shown in Figure 12 [110].
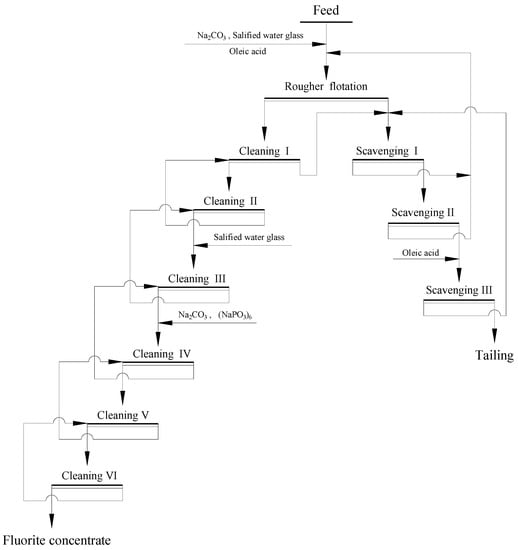
Figure 12.
The beneficiation plant flotation flowsheet [110].
5. Conclusions and Future Perspectives
This review summarizes the current available literature on the flotation separation of fluorite and calcite. With the increasing attention paid to low-grade fluorite resources with complex gangue minerals, tremendous efforts have been made to overcome the problem of fluorite and calcite flotation separation.
Besides the very similar flotation active sites, the dissolved ions during grinding and flotation will affect the surface properties to narrow the difference with each other, which is the fundamental reason for the difficulty in separating fluorite and calcite. In addition, due to the lattice impurities in fluorite and calcite, the PZC and surface wettability of fluorite and calcite from different origins vary widely, which increases the difficulty of flotation separation of fluorite and calcite.
Before the optimization of the beneficiation processes, fundamental research on the flotation separation of fluorite and calcite has focused on the development of high-selectivity flotation reagents. The modifiers, such as pH regulators, anions (CO32−, F− and SO42−) and lead ions, are used to enlarge the difference in flotation characteristics between fluorite and calcite. Furthermore, selective depressants such as acidified water glass, plant gum, tannic, polyaspartate and starch are used to further expand the difference in floatability between fluorite and calcite. With the aid of these operations, fluorite and calcite can be separated by using selective collectors, including the benzenoid/hydroxamic type organic acids, combined collectors and some special organic compounds.
By analyzing the successful industrial practice cases, it was found that the efficient separation of fluorite and calcite can be realized only by a suitable reagent scheme and reasonable flotation process.
Further research work should focus on the following aspects:
- (1)
- For complex and low-grade calcite-containing fluorite deposits [111,112], the application of pre-concentration technology is particularly important. Therefore, to minimize the content of calcite in the fluorite flotation feed to improve the efficiency of flotation separation, pre-concentration technologies, such as photoelectric separation and dense medium separation, are worth further researching.
- (2)
- Metal ions such as Pb2+ pretreatment have been found to be able to enlarge the difference in surface properties between fluorite and calcite and provide the new active sites for selective adsorption of collectors and depressants; however, the metal ion specie sand their action mechanism still need to be further studied.
- (3)
- As mentioned above, the surface properties of fluorite and calcite from different origins vary widely, so further screening of selective collectors and depressants is required, together with investigations of the action mechanism.
- (4)
- To avoid the conundrum caused by calcium ions as active sites for flotation separation, the development of selective flotation reagents reacted with CO32− in calcite and F− in fluorite requires further research.
- (5)
- In the literature, monominerals and artificially mixed samples are used as research objects for investigating the separation effect of selective flotation reagents, which have few interference factors. The actual ores and scale tests need further research to investigate the adaptability and stability of flotation reagents. Overall, this paper presents the current research status in the flotation separation of fluorite and calcite. The analysis of the difficulties in the flotation separation of fluorite and calcite and the review of flotation reagents can provide a basis for the design and development of an efficient reagent scheme for calcite-containing fluorite flotation.
Author Contributions
Investigation, J.H. and H.L.; writing-original draft preparation, J.H. and C.W.; writing-review and editing, Q.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the China Geological Survey project (DD20221698).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gao, Z.; Wang, C.; Sun, W.; Gao, Y.; Kowalczuk, P. Froth flotation of fluorite: A review. Adv. Colloid Interface Sci. 2021, 290, 102382. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Xie, L.; Cui, X.; Hu, Y.; Sun, W.; Zeng, H. Probing anisotropic surface properties and surface forces of fluorite crystals. Langmuir 2018, 34, 2511–2521. [Google Scholar] [CrossRef] [PubMed]
- Nazabal, V.; Poulain, M.; Olivier, M.; Pirasteh, P.; Camy, P.; Doualan, J.; Guy, S.; Djouama, T.; Boutarfaia, A.; Adam, J. Fluoride and oxyfluoride glasses for optical applications. J. Fluor. Chem. 2012, 134, 18–23. [Google Scholar] [CrossRef]
- Burton, J.U.S. Geological Survey Releases 2022 List Critical Minerals. Available online: https://www.usgs.gov/news/national-news-release/us-geological-survey-releases-2022-list-critical-minerals (accessed on 22 February 2022).
- Bobba, S.; Carrara, S.; Huisman, J.; Mathieux, F.; Pavel, C. Critical Raw Materials for Strategic Technologies and Sectors in the EU-A Foresight Study, European Commission: Brussels Belgium 2020. Available online: https://www.researchgate.net/publication/344237416 (accessed on 14 September 2020).
- U.S. Geological Survey. Mineral Commodity Summaries 2022. Available online: https://doi.org/10.3133/mcs2022 (accessed on 31 January 2022).
- Sotnikova, I.; Prokofiev, V.; Vladykin, N. Genesis of apatite-fluorite rock in the Burpala pluton. Dokl. Earth Sci. 2011, 441, 1703–1705. [Google Scholar] [CrossRef]
- Pei, Q.; Zhang, S.; Hayashi, K.; Wang, L.; Cao, H.; Zhao, Y.; Hu, X.; Song, K.; Chao, W. Nature and genesis of the Xiaobeigou fluorite deposit, Inner Mongolia, Northeast China: Evidence from Fluid Inclusions and Stable Isotopes. Resour. Geol. 2019, 69, 148–166. [Google Scholar] [CrossRef]
- Zhao, C.; Sun, C.; Yin, W.; Zhu, Y.; Luo, B. Effects of the crystal structures of three calcium-containing minerals on their surface characteristics in flotation. Sci. Adv. Mater. 2019, 11, 68–73. [Google Scholar] [CrossRef]
- Zhong, C.; Feng, B.; Zhang, L.; Zhang, W.; Wang, H.; Gao, Z. Flotation separation of apatite and calcite using gum arabic as a depressant. Colloids Surf. Asp. 2022, 632, 127723. [Google Scholar] [CrossRef]
- Li, B.; Shi, Q.; Liu, D.; Jin, S.; Luo, Q.; Wang, Z. The effect of nascent calcium carbonate inhibiting the flotation behavior of calcite. Miner. Eng. 2022, 180, 107478. [Google Scholar] [CrossRef]
- Li, M.; Liu, Z.; Wang, B.; Teng, X.; Jiang, H. Selective flotation separation of fluorite from calcite using mixed anionic/cationic collectors. Miner. Eng. 2022, 178, 107423. [Google Scholar] [CrossRef]
- Zhang, D.; Kang, J.; Zhu, W. Selective flotation separation of fluorite and calcite by utilising a novel anionic/nonionic collector. Colloids Surf. A Physicochem. Eng. Asp. 2022, 642, 128687. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, X.; Zheng, Y.; Ren, Z.; Fu, W.; Yang, S. Utilization of water glass as a dispersant to improve the separation performance of fluorite from barite slimes. Colloids Surf. A Physicochem. Eng. Asp. 2022, 635, 128036. [Google Scholar] [CrossRef]
- Ran, J.; Liu, Q.; Xiao, H.; Zhang, Z. Separation test of fluorite ore with barite in Hunan. Appl. Mech. Mater. 2014, 543–547, 3781–3784. [Google Scholar] [CrossRef]
- Wang, R.; Lu, Q.; Sun, W.; Lin, S.; Han, H.; Sun, W.; Wei, Z. Flotation separation of apatite from calcite based on the surface transformation by fluorite particles. Miner. Eng. 2022, 176, 107320. [Google Scholar] [CrossRef]
- Patra, A.; Taner, H.; Bordes, R.; Holmberg, K.; Larsson, A. Selective flotation of calcium minerals using double-headed collectors. J. Dispers. Sci. Technol. 2019, 40, 1205–1216. [Google Scholar] [CrossRef] [Green Version]
- Foucaud, Y.; Filippova, I.; Filippov, L. Investigation of the depressants involved in the selective flotation of scheelite from apatite, fluorite, and calcium silicates: Focus on the sodium silicate/sodium carbonate system. Powder Technol. 2019, 352, 501–512. [Google Scholar]
- Liu, J.; Wang, X.; Zhu, Y.; Han, Y. Flotation separation of scheelite from fluorite by using DTPA asa depressant. Miner. Eng. 2022, 175, 107311. [Google Scholar] [CrossRef]
- Chen, W.; Feng, Q.; Zhang, G.; Yang, Q.; Zhang., C.; Xu, F. The flotation separation of scheelite from calcite and fluorite usingdextran sulfate sodium as depressant. Int. J. Miner. Process. 2017, 169, 53–59. [Google Scholar]
- Gao, Z.; Fan, R.; Ralston, J.; Sun, W.; Hu, Y. Surface broken bonds: An efficient way to assess the surface behaviour of fluorite. Miner. Eng. 2019, 130, 15–23. [Google Scholar]
- Gao, Z.; Li, C.; Sun, W.; Hu, Y. Anisotropic surface properties of calcite: A consideration of surface broken bonds. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 53–61. [Google Scholar]
- Miller, J.; Fa, K.; Calara, J.; Paruchuri, V. The surface charge of fluorite in the absence of surface carbonation. Colloids Surf. A Physicochem. Eng. Asp. 2004, 238, 91–97. [Google Scholar]
- Fedorov, P.; Luginina, A.; Alexandrov, A.; Chernova, E. Transformation of calcite CaCO3 to fluorite CaF2 by action of KF solution. J. Fluor. Chem. 2021, 251, 109898. [Google Scholar]
- Eriksson, R.; Merta, J.; Rosenholm, J. The calcite/water interface: I. Surface charge in different electrolyte media and the influence of low-molecular-weight polyelectrolyte. J. Colloid Interface Sci. 2007, 313, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, R.; Merta, J.; Rosenholm, J. The calcite/water interface II. Effect of added lattice ions on the charge properties and adsorption of sodium polyacrylate. J. Colloid Interface Sci. 2008, 326, 396–402. [Google Scholar] [PubMed]
- Hu, Y.; Chi, R.; Xu, Z. Solution chemistry study of salt-type mineral flotation systems: Role of inorganic dispersants. Ind. Eng. Chem. Res. 2003, 42, 1641–1647. [Google Scholar]
- Garand, A.; Mucci, A. The solubility of fluorite as a function of ionic strength and solution composition at 25 ℃ and 1 atm total pressure. Mar. Chem. 2004, 91, 27–35. [Google Scholar]
- Zhang, W.; Zhou, L.; Tang, H.; Li, H.; Song, W.; Xie, G. The solubility of fluorite in Na-K-Cl solutions at temperatures up to 260 °C and ionic strengths up to 4 mol/kg H2O. Appl. Geochem. 2017, 82, 79–88. [Google Scholar]
- Godinho, J.; Piazolo, S.; Evins, L. Effect of surface orientation on dissolution rates and topography of CaF2. Geochim. Cosmochim. Acta. 2012, 86, 392–403. [Google Scholar]
- Bychkov, A.; Bénézeth, P.; Pokrovsky, O.; Shvarov, Y.; Castillo, A.; Schott, J. Experimental determination of calcitesolubility and the stability of aqueous Ca-and Na-carbonate and-bicarbonate complexes at 100–160 °C and 1–50 bar pCO2 using in situ pH measurements. Geochim. Cosmochim. Acta. 2020, 290, 352–365. [Google Scholar] [CrossRef]
- Yoshino, T.; Kagi, H. Effects of aspartic acid on calcite dissolution rate and solubility. Chem. Lett. 2008, 37, 508–509. [Google Scholar]
- Zhu, Y.; Nong, P.; Mo, N.; Zhu, Z.; Deng, H.; Tang, S.; Yang, H.; Zhang, L.; Wang, X. Dissolution and solubility of calcite-rhodochrosite solid solutions [(Ca 1−x Mn x)CO3] at 25 °C. Geochem. Trans. 2021, 22, 1. [Google Scholar]
- Jensen, D.; Boddum, J.; Tjell, J.; Christensen, T. The solubility of rhodochrosite (MnCO3) and siderite (FeCO3) in anaerobic aquatic environments. Appl. Geochem. 2002, 17, 503–511. [Google Scholar] [CrossRef]
- Wang, R.; Han, H.; Sun, W.; Nguyen, A.; Sun, W.; Wei, Z. Hydrophobic behavior of fluorite surface in strongly alkaline solution and its application in flotation. Colloids Surf. A Physicochem. Eng. Asp. 2021, 609, 125661. [Google Scholar] [CrossRef]
- Wang, R.; Han, H.; Sun, W.; Sun, W.J.; Wei, Z.; Fu, J. Surface transformation of calcium minerals and its application in flotation. Met. Mine. 2021, 6, 52–59. (In Chinese) [Google Scholar]
- Sun, R.; Liu, D.; Zhang, B.; Lai, H.; Wen, S. Homogenization phenomena of surface components of fluorite and calcite. Physicochem. Probl. Miner. Process. 2020, 57, 250–258. [Google Scholar] [CrossRef]
- Pedrosa, E.; Boeck, L.; Putnis, C.; Putnis, A. The replacement of a carbonate rock by fluorite: Kinetics and microstructure. Am. Miner. 2017, 102, 126–134. [Google Scholar] [CrossRef]
- Isah, M.; Al-Shehri, D.; Mahmoud, M.; Kamal, M.; Alade, O. Surface charge investigation of reservoir rock minerals. Energy Fuels 2021, 35, 6003–6021. [Google Scholar]
- Fuerstenau, D.; Pradip. Zeta potentials in the flotation of oxide and silicate minerals. Adv. Colloid Interface Sci. 2005, 114, 9–26. [Google Scholar] [CrossRef]
- Jiang, W.; Gao, Z.; Sun, W.; Gao, J.; Hu, Y. A density functional theory study on the effect of lattice impurities on the electronic structures and reactivity of fluorite. Minerals 2017, 7, 160. [Google Scholar] [CrossRef] [Green Version]
- Chernyshev, V.; Nikiforov, A.; Volodin, V.; Slepukhin, G. Electronic structure of Yb3+ impurity centers in fluorites. Phys. Solid State 2010, 52, 1874–1879. [Google Scholar] [CrossRef]
- Faiziev, A. Manganese in fluorites from different genetic types of deposits in Tajikistan. Geochem. Int. 2003, 41, 827–829. [Google Scholar]
- Uçar, A.; Özdag, H. Mechanism of collector adsorption in fluorite flotation. Miner. Process. Extr. Metall. 2002, 111, 100–105. [Google Scholar] [CrossRef]
- Heberling, F.; Trainor, T.; Lützenkirchen, J.; Denecke, M.; Bosbach, D. Structure and reactivity of the calcite-water interface. J. Colloid Interface Sci. 2011, 354, 843–857. [Google Scholar] [CrossRef] [PubMed]
- Mahrouqi, D.; Vinogradov, J.; Jackson, M. Zeta potential of artificial and natural calcite in aqueous solution. Adv. Colloid Interface Sci. 2017, 240, 60–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moulin, P.; Roques, H. Zeta potential measurement of calcium carbonate. J. Colloid Interface Sci. 2003, 261, 115–126. [Google Scholar] [CrossRef]
- Alroudhan, A.; Vinogradov, J.; Jackson, M. Zeta potential of intact natural limestone: Impact of potential-determining ions Ca, Mg and SO4. Colloids Surf. A Physicochem. Eng. Asp. 2016, 493, 83–98. [Google Scholar] [CrossRef] [Green Version]
- Kasha, A.; Al-Hashim, H.; Abdallah, W.; Taherian, R.; Sauerer, B. Effect of Ca2+, Mg2+ and SO42− ions on the zeta potential of calcite and dolomite particles aged with stearic acid. Colloids Surf. A Physicochem. Eng. Asp. 2015, 482, 290–299. [Google Scholar] [CrossRef]
- Ozkan, A.; Yekeler, M. A new microcolumn flotation cell for determining the wettability and floatability of minerals. J. Colloid Interface Sci. 2003, 261, 476–480. [Google Scholar] [CrossRef]
- Lourenço, S.; Woche, S.; Bachmann, Y.; Saulick, Y. Wettability of crushed air-dried minerals. Geotech. Lett. 2015, 5, 173–177. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Wang, X.; Miller, J. Wetting of selected fluorite surfaces by water. Surf. Innov. 2015, 3, 39–48. [Google Scholar] [CrossRef]
- Gao, Z.; Sun, W.; Hu, Y.; Liu, X. Anisotropic surface broken bond properties and wettability ofcalcite and fluorite crystals. Trans. Nonferrous Met. Soc. China 2012, 22, 1203–1208. [Google Scholar] [CrossRef]
- Kowalczuk, P.; Akkaya, C.; Ergun, M.; Janicki, M.; Sahbaz, O.; Drzymala, J. Water contact angle on corresponding surfaces of freshly fractured fluorite, calcite and mica. Physicochem. Probl. Miner. Process. 2017, 53, 192–201. [Google Scholar]
- Gao, Y.; Gao, Z.; Sun, W. A review of anisotropic surface properties of fluorite. Chin. J. Nonferrous Met. 2016, 26, 415–422. (In Chinese) [Google Scholar]
- Jańczuk, B.; Bruque, J.; González-Martín, M.; Moreno del Pozo, J. Wettability and surface tension of fluorite. Colloids Surf. A Physicochem. Eng. Asp. 1993, 75, 163–168. [Google Scholar] [CrossRef]
- Zawala, J.; Drzymala, J.; Malysa, K. Natural hydrophobicity and flotation of fluorite. Physicochem. Probl. Miner. Process. 2007, 41, 5–11. [Google Scholar]
- Cao, Q.; Zou, H.; Liu, D.; Wen, S.; Chen, X. Flotation separation of smithsonite from calcite using an amino-acid collector. Sep. Purif. Technol. 2022, 281, 119980. [Google Scholar] [CrossRef]
- Chen, C.; Sun, W.; Zhu, H.; Liu, R. A novel green depressant for flotation separation of scheelite from calcite. Trans. Nonferrous Met. Soc. China 2021, 31, 2493–2500. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.; Zhu, Y.; Li, Y. Selective adsorption of Na2ATP as an eco-friendly depressant on the calcite surface for effective flotation separation of cassiterite from calcite. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126899. [Google Scholar] [CrossRef]
- Kangal, M.; Bulut, G.; Guven, O. Physicochemical characterization of natural wollastonite and calcite. Minerals 2020, 10, 228. [Google Scholar] [CrossRef] [Green Version]
- Kienko, L.; Voronova, O. Selective flotation of fine-ingrained carbonate-fluorite ore in pulp of increased dispersion uniformity. J. Min. Sci. 2014, 50, 176–181. [Google Scholar] [CrossRef]
- Kienko, L.; Voronova, O.; Kondrat’ev, S. Prospects for re-processing of carbonate-fluorite ore mill tailings at Yaroslavsky mining company. J. Min. Sci. 2017, 53, 155–160. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Zhang, J.; Zhao, W. Utilization of citric acid to improve the depressive efficiency of sodium silicate on the flotation of calcite and fluorite. Min. Met. Explor. 2022, 39, 855–862. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, L.; Wu, H.; Zhou, H.; Meng, J.; Huo, X.; Huang, L. Adsorption of octanohydroxamic acid at fluorite surface in presence of calcite species. Trans. Nonferrous Met. Soc. China 2021, 31, 3891–3904. [Google Scholar] [CrossRef]
- Wang, R.; Wei, Z.; Han, H.; Sun, W.; Hu, Y.; Wang, J.; Wang, L.; Liu, H.; Yang, Y.; Zhang, C. Fluorite particles as a novel calcite recovery depressant in scheelite flotation using Pb-BHA complexes as collectors. Miner. Eng. 2019, 132, 84–91. [Google Scholar] [CrossRef]
- Zhou, Q.; Lu, S. Acidized sodium silicate-an effective modifier in fluorite flotation. Miner. Eng. 1992, 5, 435–444. [Google Scholar] [CrossRef]
- Pomazov, V.; Kondrat’ev, S.; Rostovtsev, V. Improving the finely disseminated carbonate-fluorite ore flotation with FLOTOL-7,9 agent. J. Min. Sci. 2012, 48, 920–927. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Y.; Tang, X.; Cao, Y.; Luo, X. Flotation separation of fluorite from calcite by using psyllium seed gum as depressant. Miner. Eng. 2020, 159, 106514. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, Z.; Hu, Y.; Sun, W.; Tang, H.; Yin, Z.; He, J.; Guan, Q.; Zhu, Y. The effect of polyacrylic acid on the surface properties of calcite and fluorite aiming at their selective flotation. Physicochem. Probl. Miner. Process. 2018, 54, 868–877. [Google Scholar]
- Zhou, H.; Yang, Z.; Zhang, Y.; Sun, W.; Gao, Z.; Lei, M. Effect of artemisia sphaerocephalakrasch. Gum on the flotation separation of fluorite from calcite. Miner. Eng. 2021, 174, 107249. [Google Scholar] [CrossRef]
- Karlkvist, T.; Patra, A.; Romain, B.; Holmberg, K.; Rao, K.H. Flotation selectivity of novel alkyl dicarboxylate reagents for calcite-fluorite separation. Tenside Surfactants Deterg. 2016, 53, 516–523. [Google Scholar] [CrossRef]
- Zhu, W.; Pan, J.; Yu, X.; He, G.; Liu, C.; Yang, S.; Zeng, Y.; Zeng, A.; Liu, T. The flotation separation of fluorite from calcite using hydroxypropyl starch as a depressant. Colloids Surf. A Physicochem. Eng. Asp. 2021, 616, 126168. [Google Scholar] [CrossRef]
- Liu, J.; Xie, R.; Zhu, Y.; Li, Y.; Liu, C. Flotation behavior and mechanism of styrene phosphonic acid as collector on the flotation separation of fluorite from calcite. J. Mol. Liq. 2021, 326, 115261. [Google Scholar] [CrossRef]
- Hu, Y.; Xu, Z. Interactions of amphoteric amino phosphoric acids with calcium-containing minerals and selective flotation. Int. J. Miner. Process. 2003, 72, 87–94. [Google Scholar]
- Duan, H.; Liu, W.; Liu, W.; Shen, Y.; Gu, X.; Qiu, J.; Zhou, S. Selective adsorption of a novel X-shaped surfactant dioctyldi -hydroxamic acid on fluorite surface leading the effective flotation separation of fluorite from calcite and barite. J. Mol. Liq. 2021, 344, 117941. [Google Scholar] [CrossRef]
- Jiang, W.; Gao, Z.; Khoso, S.; Gao, J.; Sun, W.; Pu, W.; Hu, Y. Selective adsorption of benzhydroxamic acid on fluorite rendering selective separation of fluorite/calcite. Appl. Surf. Sci. 2018, 435, 752–758. [Google Scholar]
- Zhang, C.; Zhou, Q.; An, B.; Yue, T.; Chen, S.; Liu, M.; He, J.; Zhu, J.; Chen, D.; Hu, B.; et al. Flotation behavior and synergistic mechanism of benzohydroxamic acid and sodium butyl-xanthate as combined collectors for malachite beneficiation. Minerals 2021, 11, 59. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, S. Beneficiation of fluorite by flotation in a new chemical scheme. Miner. Eng. 2003, 16, 597–600. [Google Scholar] [CrossRef]
- Fa, K.; Tao, J.; Nalaskowski, J.; Miller, J. Interaction forces between a calcium dioleate sphere and calcite/fluorite surfaces and their significance in flotation. Langmuir 2003, 19, 10523–10530. [Google Scholar] [CrossRef]
- Fa, K.; Nguyen, A.; Miller, J. Interaction of calcium dioleate collector colloids with calcite and fluorite surfaces as revealed by AFM force measurements and molecular dynamics simulation. Int. J. Miner. Process. 2006, 81, 166–177. [Google Scholar]
- Sun, W.; Han, H.; Sun, W.; Wang, R.; Wei, Z. Novel insights into the role of colloidal calcium dioleate in the flotation of calcium minerals. Miner. Eng. 2022, 175, 107274. [Google Scholar] [CrossRef]
- Gao, J.; Sun, W.; Hu, Y.; Wang, L.; Liu, R.; Gao, Z.; Chen, P.; Tang, H.; Jiang, W.; Lyu, F. Propyl gallate: A novel collector for flotation separation of fluorite from calcite. Chem. Eng. Sci. 2019, 193, 255–263. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, Y. Adsorption of H4SiO4 as a hydrolysate of sodium silicate on surfaces of fluorite (111), calcite (104), and scheelite (112): A density functional theory approach. Int. J. Electrochem. Sci. 2019, 14, 10807–10818. [Google Scholar] [CrossRef]
- Zhou, W.; Moreno, J.; Torres, R.; Valle, H.; Song, S. Flotation of fluorite from ores by using acidized water glass as depressant. Miner. Eng. 2013, 45, 142–145. [Google Scholar] [CrossRef]
- Gao, J.; Hu, Y.; Sun, W.; Liu, R.; Gao, Z.; Han, H.; Lyu, F.; Jiang, W. Enhanced separation of fluorite from calcite in acidic condition. Miner. Eng. 2019, 133, 103–105. [Google Scholar] [CrossRef]
- Sun, R.; Liu, D.; Liu, Y.; Wang, D.; Wen, S. Pb-water glass as a depressant in the flotation separation of fluorite from calcite. Colloids Surf. A Physicochem. Eng. Asp. 2021, 629, 127447. [Google Scholar] [CrossRef]
- Yao, W.; Li, M.; Zhang, M.; Cui, R.; Shi, J.; Ning, J. Effects of Pb2+ ions on the flotation behavior of scheelite, calcite, and fluorite in the presence of water glass. Colloids Surf. A Physicochem. Eng. Asp. 2022, 632, 127826. [Google Scholar] [CrossRef]
- Wang, D.; Liu, D.; Mao, Y.; Sun, R.; Liu, R.; Wen, S. Effect of fluoride ion on the separation of fluorite from calcite using flotation with acidified water glass. Minerals 2021, 11, 1203. [Google Scholar] [CrossRef]
- Tian, J.; Xu, L.; Sun, W.; Zeng, X.; Fang, S.; Han, H.; Hong, K.; Hu, Y. Use of Al2(SO4)3 and acidified water glass as mixture depressants in flotation separation of fluorite from calcite and celestite. Miner. Eng. 2019, 137, 160–170. [Google Scholar] [CrossRef]
- Wang, M.; Huang, G.; Zhang, G.; Chen, Y.; Liu, D.; Li, C. Selective flotation separation of fluorite from calcite by application of flaxseed gum as depressant. Miner. Eng. 2021, 168, 106938. [Google Scholar] [CrossRef]
- Liu, C.; Song, S.; Li, H. Selective flotation of fluorite from barite using trisodium phosphate as a depressant. Miner. Eng. 2019, 134, 390–393. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, Z.; Tang, X.; Sun, W.; Gao, Z.; Luo, X. Enhancing flotation separation effect of fluorite and calcite with polysaccharide depressant tamarind seed gum. Colloids Surf. A Physicochem. Eng. Asp. 2021, 624, 126784. [Google Scholar] [CrossRef]
- Qian, W.; Dong, L.; Jiao, F.; Qin, W. Selective flotation separation of fluorite from calcite by using sesbania gum as depressant. Miner. Eng. 2021, 174, 107239. [Google Scholar]
- Tangarfa, M.; Hassani, N.; Alaoui, A. Behavior and mechanism of tannic acid adsorption on the calcite surface: Isothermal, kinetic, and thermodynamic studies. ACS Omega 2019, 4, 19647–19654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Sun, W.; Hu, Y.; Tang, H.; Gao, J.; Yin, Z.; Guan, Q. Selective adsorption of tannic acid on calcite and implications for separation of fluorite minerals. J. Colloid Interface Sci. 2018, 512, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Qin, W.; Chen, C.; Chai, L.; Jiao, F.; Jia, W. Flotation separation of fluorite from calcite using polyaspartate as depressant. Miner. Eng. 2018, 120, 80–86. [Google Scholar] [CrossRef]
- Chen, C.; Hu, Y.; Zhu, H.; Sun, W.; Qin, W.; Liu, R.; Gao, Z. Inhibition performance and adsorption of polycarboxylic acids in calcite flotation. Miner. Eng. 2019, 133, 60–68. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, B.; Yan, H.; Zhang, L.; Zhong, C.; Wang, T.; Wang, H.; Xu, L. Adsorption and depression mechanism of an eco-friendly depressant dextrin onto fluorite and calcite for the efficiency flotation separation. Colloids Surf. A Physicochem. Eng. Asp. 2022, 635, 127987. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, D.; Li, H.; Feng, Q.; Xu, G.; Yuan, J.; Jia, X. Influence of pulp pH on flotation of fluorite and calcite. Adv. Mat. Res. 2012, 616–618, 614–618. [Google Scholar] [CrossRef]
- Wang, J.; Li, W.; Zhou, Z.; Gao, Z.; Hu, Y.; Sun, W. 1-Hydroxyethylidene-1,1-diphosphonic acid used aspH-dependent switch to depress and activate fluorite flotation I: Depressing behavior and mechanism. Chem. Eng. Sci. 2020, 214, 115369. [Google Scholar] [CrossRef]
- Jin, S.; Ou, L.; Shi, Q. Effect of negative ions in solution on flotation behavior of fluorite and calcite. Chin. J. Nonferrous Met. 2019, 29, 1324–1329. (In Chinese) [Google Scholar]
- Feng, B.; Guo, W.; Xu, H.; Peng, J.; Luo, X.; Zhu, X. The combined effect of lead ion and sodium silicate in the flotation separation of scheelite from calcite. Sep. Sci. Technol. 2017, 52, 567–573. [Google Scholar] [CrossRef]
- Cao, Q.; Chen, X.; Feng, Q.; Wen, S. Activation mechanism of lead ion in the flotation of stibnite. Miner. Eng. 2018, 119, 173–182. [Google Scholar] [CrossRef]
- Sun, R.; Liu, D.; Li, Y.; Wang, D.; Wen, S. Influence of lead ion pretreatment surface modification on reverse flotation separation of fluorite and calcite. Miner. Eng. 2021, 171, 107077. [Google Scholar] [CrossRef]
- Kienko, L.; Samatova, L.; Voronova, O.; Kondrat’ev, S. Lower temperature flotation of carbonate-fluorite ores. J. Min. Sci. 2010, 46, 317–323. [Google Scholar] [CrossRef]
- Kienko, L.; Voronova, O.; Kondrat’ev, S. Effect of composition of grouped collectors on flotation of mining waste at Yaroslavskaya Mining Company. J. Min. Sci. 2021, 57, 674–680. [Google Scholar] [CrossRef]
- Atrafi, A.; Gomez, C.; Finch, J.; Pawlik, M. Frothing behavior of aqueous solutions of oleic acid. Miner. Eng. 2012, 36–38, 138–144. [Google Scholar] [CrossRef]
- Atrafi, A.; Pawlik, M. Surface tension and gas dispersion properties of fatty acid solutions. Miner. Eng. 2016, 85, 138–147. [Google Scholar] [CrossRef]
- Niu, Y.; Huang, M. Beneficiafion production practice of carbonate-type fluorite ore in Qinglong. Conserv. Util. Miner. Resour. 2010, 3, 16–19. (In Chinese) [Google Scholar]
- Wang, J.; Zu, P.; Cao, Z. Preconcentration of iron, rare earth, and fluorite from Bayan Obo ore using superconducting magnetic separation. Min. Met. Explor. 2021, 38, 701–712. [Google Scholar] [CrossRef]
- Nguyen, T.; Tran, L.; Mueller, K.; Coudert, L.; Mercier, G.; Blais, G. Pre-concentration of fluorite from a rare earth element carbonatite deposit through the combination of magnetic separation and leaching. Miner. Eng. 2021, 174, 106998. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).