Metal Lability and Mass Transfer Response to Direct-Planting Phytostabilization of Pyritic Mine Tailings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Site Experimental Setup
2.2. Sample Collection and Storage
2.3. Chemical Characterization
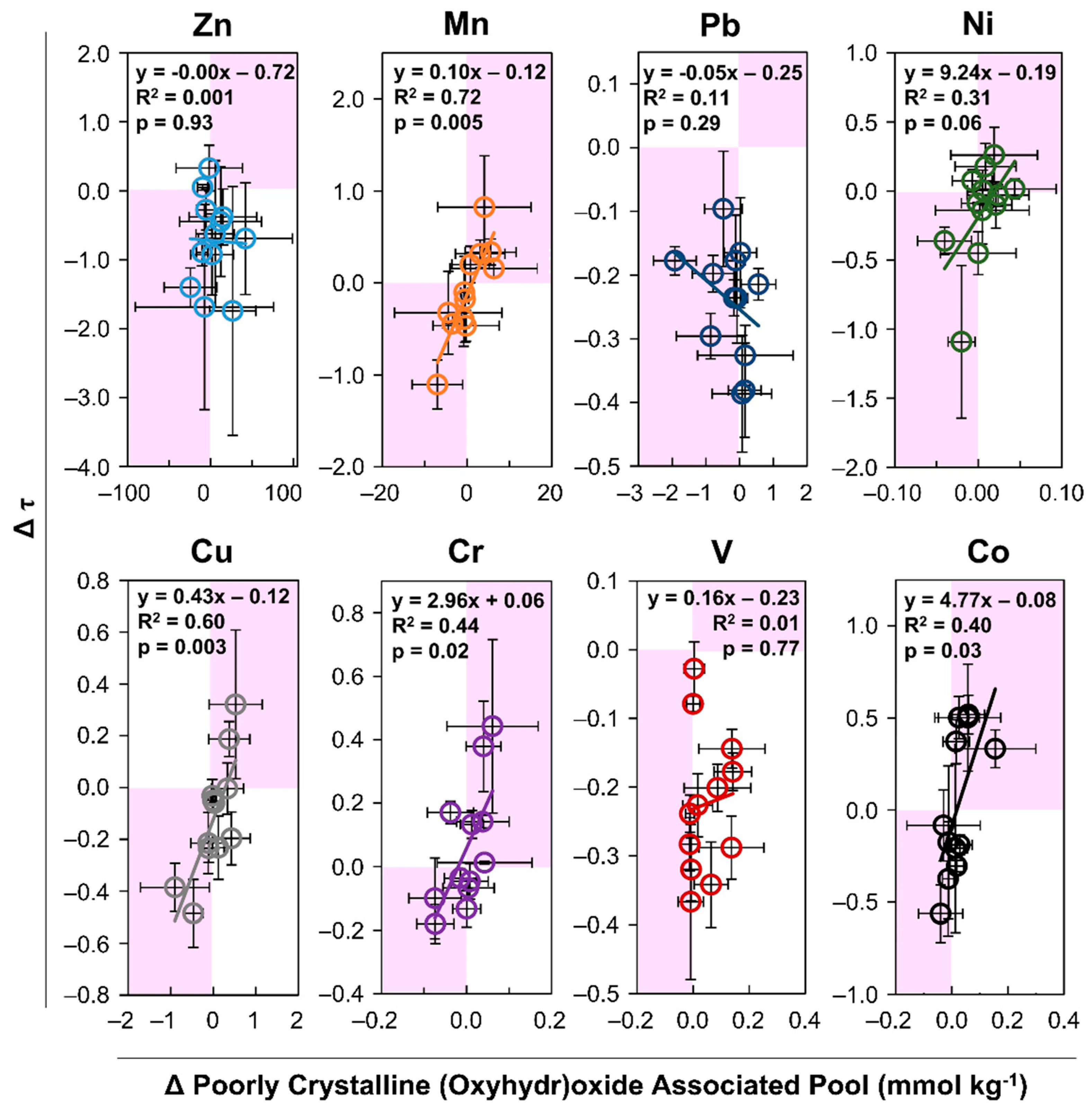
3. Results
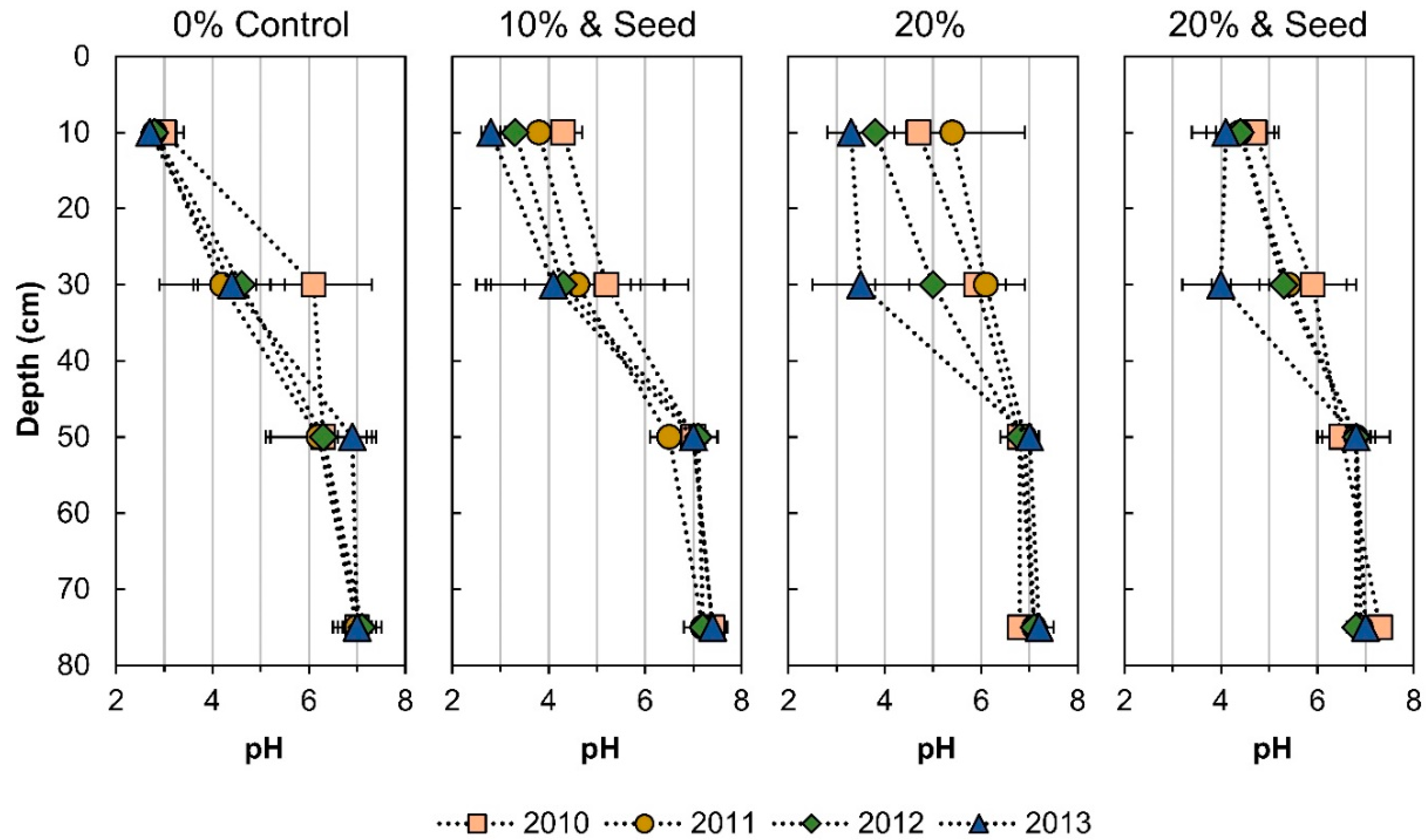
3.1. Phytostablization Effect on Acidification and Buffering
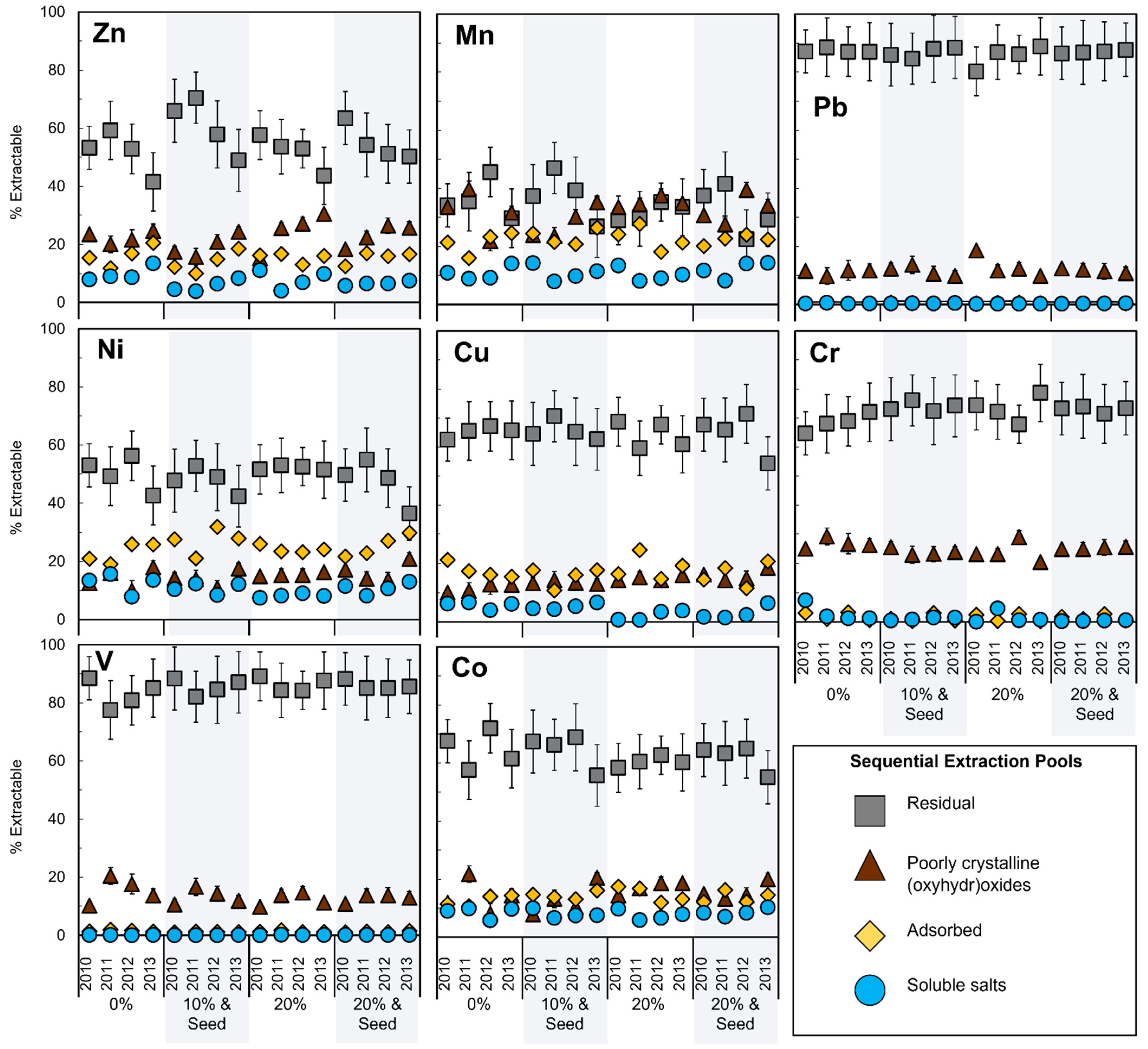
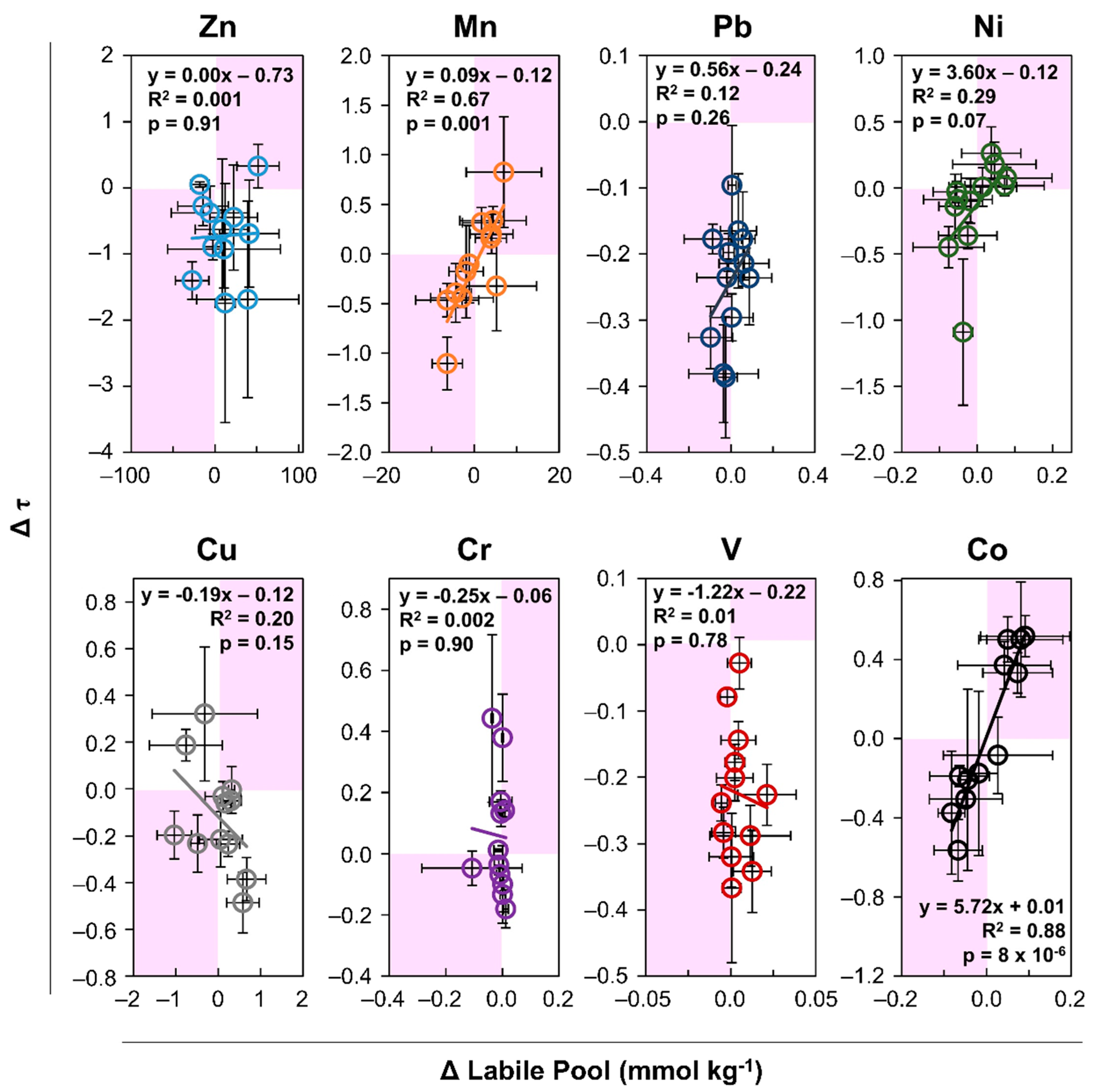
3.2. Metal Lability
3.2.1. Depth Effect
3.2.2. Organic Matter Effect
3.2.3. Temporal Effect
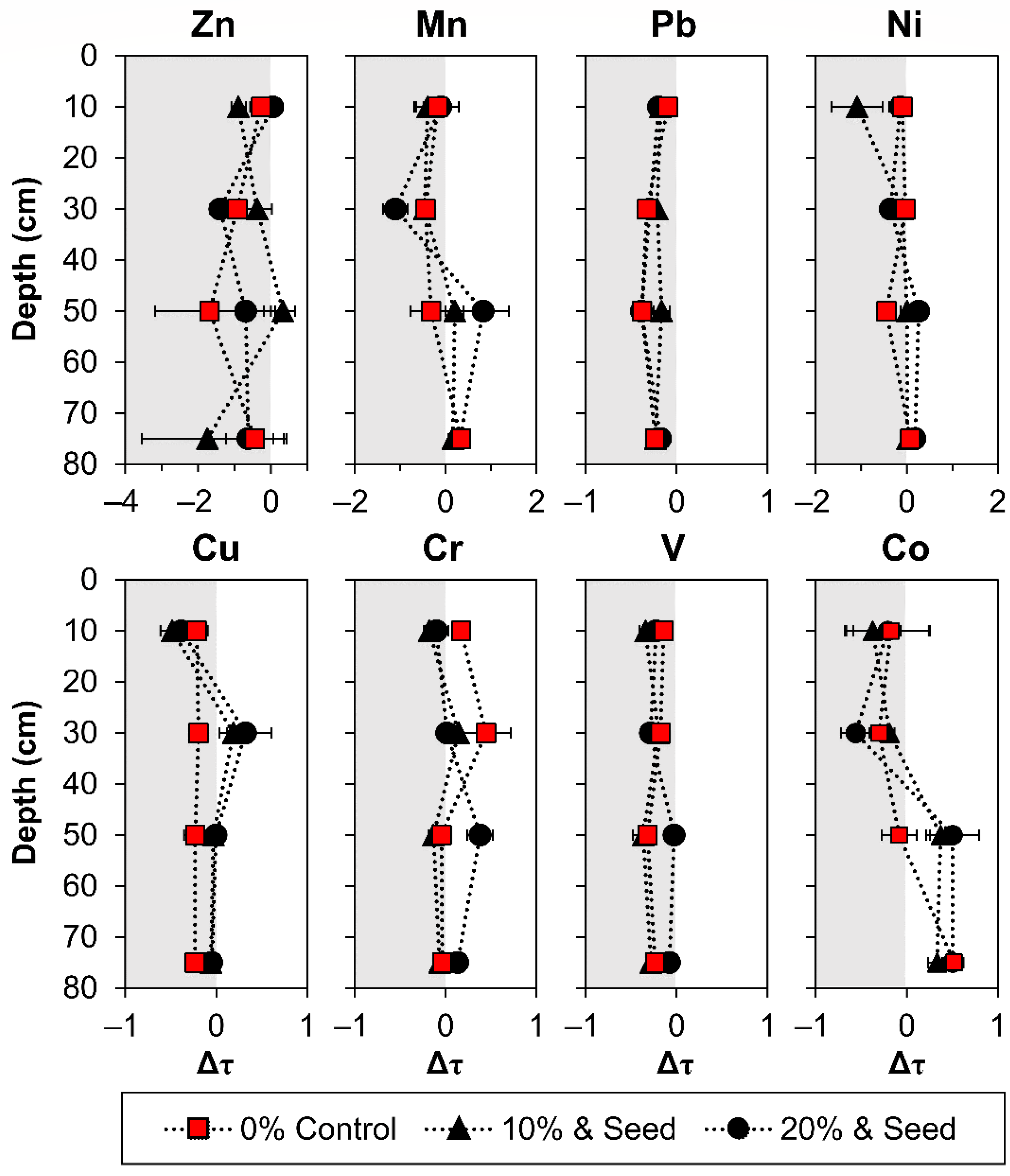
3.3. Phytostabilization Effect on Mass Transfer
4. Discussion
4.1. Acidification
4.2. Metal Lability
4.3. Recalcitrant Metals
5. Conclusions
- The acidification of oxidizing surface tailings largely controlled the subsurface fate of Mn, Co, Ni, Cu, and Zn.
- Mass transfer was observed for phytostabilization treatments and the control for Mn, Co, Cu, and Zn translocation from the surface.
- Phytostabilization promoted Cr mobility from the surface and enrichment at depths relative to the unamended control.
- Phytostabilization reduced zinc mobility in the oxic zone.
- Pb was mostly recalcitrant as plumbojarosite and was not mobilized by weathering or compost-assisted phytostabilization.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El Berkaoui, M.; El Adnani, M.; Hakkou, R.; Ouhammou, A.; Bendaou, N.; Smouni, A. Assessment of the Transfer of Trace Metals to Spontaneous Plants on Abandoned Pyrrhotite Mine: Potential Application for Phytostabilization of Phosphate Wastes. Plants 2022, 11, 179. [Google Scholar] [CrossRef] [PubMed]
- Hammarstrom, J.M.; Seal, R.R.; Meier, A.L.; Kornfeld, J.M. Secondary sulfate minerals associated with acid drainage in the eastern US: Recycling of metals and acidity in surficial environments. Chem. Geol. 2005, 215, 407–431. [Google Scholar] [CrossRef] [Green Version]
- Mendez, M.O.; Maier, R.M. Phytoremediation of mine tailings in temperate and arid environments. Rev. Environ. Sci. Bio/Technol. 2008, 7, 47–59. [Google Scholar] [CrossRef]
- Hammond, C.M.; Root, R.A.; Maier, R.M.; Chorover, J. Arsenic and iron speciation and mobilization during phytostabilization of pyritic mine tailings. Geochim. Cosmochim. Acta 2020, 286, 306–323. [Google Scholar] [CrossRef]
- Csavina, J.; Field, J.; Taylor, M.P.; Gao, S.; Landazuri, A.; Betterton, E.A.; Saez, A.E. A review on the importance of metals and metalloids in atmospheric dust and aerosol from mining operations. Sci. Total Environ. 2012, 433, 58–73. [Google Scholar] [CrossRef] [Green Version]
- Gil-Loaiza, J.; White, S.A.; Root, R.A.; Solis-Dominguez, F.A.; Hammond, C.M.; Chorover, J.; Maier, R.M. Phytostabilization of mine tailings using compost-assisted direct planting: Translating greenhouse results to the field. Sci. Total Environ. 2016, 565, 451–461. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.B.; Baumgartl, T.; Mulligan, D. Is rhizosphere remediation sufficient for sustainable revegetation of mine tailings? Ann. Bot. 2012, 110, 223–238. [Google Scholar] [CrossRef] [Green Version]
- Mendez, M.O.; Maier, R.M. Phytostabilization of mine tailings in arid and semiarid environments-An emerging remediation technology. Environ. Health Perspect. 2008, 116, 278–283. [Google Scholar] [CrossRef] [Green Version]
- Navarro, M.C.; Pérez-Sirvent, C.; Martínez-Sánchez, M.J.; Vidal, J.; Tovar, P.J.; Bech, J. Abandoned mine sites as a source of contamination by heavy metals: A case study in a semi-arid zone. J. Geochem. Explor. 2008, 96, 183–193. [Google Scholar] [CrossRef]
- Ramirez-Andreotta, M.D.; Brusseau, M.L.; Artiola, J.F.; Maier, R.M. A greenhouse and field-based study to determine the accumulation of arsenic in common homegrown vegetables grown in mining-affected soils. Sci. Total Environ. 2013, 443, 299–306. [Google Scholar] [CrossRef] [Green Version]
- Ramirez-Andreotta, M.D.; Brusseau, M.L.; Beamer, P.; Maier, R.M. Home gardening near a mining site in an arsenic-endemic region of Arizona: Assessing arsenic exposure dose and risk via ingestion of home garden vegetables, soils, and water. Sci. Total Environ. 2013, 454–455, 373–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammond, C.M.; Root, R.A.; Maier, R.M.; Chorover, J. Mechanisms of arsenic sequestration by Prosopis juliflora during phytostabilization of metalliferous mine tailings. Environ. Sci. Technol. 2018, 52, 1156–1164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques, A.P.G.C.; Rangel, A.O.S.S.; Castro, P.M.L. Remediation of Heavy Metal Contaminated Soils: Phytoremediation as a Potentially Promising Clean-Up Technology. Crit. Rev. Environ. Sci. Technol. 2009, 39, 622–654. [Google Scholar] [CrossRef]
- Vangronsveld, J.; Herzig, R.; Weyens, N.; Boulet, J.; Adriaensen, K.; Ruttens, A.; Thewys, T.; Vassilev, A.; Meers, E.; Nehnevajova, E.; et al. Phytoremediation of contaminated soils and groundwater: Lessons from the field. Environ. Sci. Pollut. Res. 2009, 16, 765–794. [Google Scholar] [CrossRef]
- Tordoff, G.M.; Baker, A.J.M.; Willis, A.J. Current approaches to the revegetation and reclamation of metalliferous mine wastes. Chemosphere 2000, 41, 219–228. [Google Scholar] [CrossRef]
- Venkateswarlu, K.; Nirola, R.; Kuppusamy, S.; Thavamani, P.; Naidu, R.; Megharaj, M. Abandoned metalliferous mines: Ecological impacts and potential approaches for reclamation. Rev. Environ. Sci. Bio-Technol. 2016, 15, 327–354. [Google Scholar] [CrossRef]
- Root, R.A.; Hayes, S.M.; Hammond, C.M.; Maier, R.M.; Chorover, J. Toxic metal(loid) speciation during weathering of iron sulfide mine tailings under semi-arid climate. Appl. Geochem. 2015, 62, 131–149. [Google Scholar] [CrossRef] [Green Version]
- Levy, D.B.; Custis, K.H.; Casey, W.H.; Rock, P.A. A comparison of metal attenuation in mine residue and overburden material from an abandoned copper mine. Appl. Geochem. 1997, 12, 203–211. [Google Scholar] [CrossRef]
- Parker, S.R.; Gammons, C.H.; Jones, C.A.; Nimick, D.A. Role of hydrous iron oxide formation in attenuation and diel cycling of dissolved trace metals in a stream affected by acid rock drainage. Water Air Soil Pollut. 2007, 181, 247–263. [Google Scholar] [CrossRef]
- Romero, F.M.; Canet, C.; Alfonso, P.; Zambrana, R.N.; Soto, N. The role of cassiterite controlling arsenic mobility in an abandoned stanniferous tailings impoundment at Llallagua, Bolivia. Sci. Total Environ. 2014, 481, 100–107. [Google Scholar] [CrossRef]
- Romero, F.M.; Prol-Ledesma, R.M.; Canet, C.; Alvares, L.N.; Perez-Vazquez, R. Acid drainage at the inactive Santa Lucia mine, western Cuba: Natural attenuation of arsenic, barium and lead, and geochemical behavior of rare earth elements. Appl. Geochem. 2010, 25, 716–727. [Google Scholar] [CrossRef]
- Wilkin, R.T. Contaminant Attenuation Processes at Mine Sites. Mine Water Environ. 2008, 27, 251–258. [Google Scholar] [CrossRef]
- Brown, S.; Chaney, R.; Hallfrisch, J.; Ryan, J.A.; Berti, W.R. In situ soil treatments to reduce the phyto- and bioavailability of lead, zinc, and cadmium. J. Environ. Qual. 2004, 33, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Conesa, H.M.; Robinson, B.H.; Schulin, R.; Nowack, B. Growth of Lygeum spartum in acid mine tailings: Response of plants developed from seedlings, rhizomes and at field conditions. Environ. Pollut. 2007, 145, 700–707. [Google Scholar] [CrossRef]
- Lee, S.H.; Ji, W.; Lee, W.S.; Koo, N.; Koh, I.H.; Kim, M.S.; Park, J.S. Influence of amendments and aided phytostabilization on metal availability and mobility in Pb/Zn mine tailings. J. Environ. Manag. 2014, 139, 15–21. [Google Scholar] [CrossRef]
- Madejon, P.; Perez-de-Mora, A.; Burgos, P.; Cabrera, F.; Lepp, N.W.; Madejon, E. Do amended, polluted soils require re-treatment for sustainable risk reduction?-Evidence from field experiments. Geoderma 2010, 159, 174–181. [Google Scholar] [CrossRef]
- Pardo, T.; Clemente, R.; Epelde, L.; Garbisu, C.; Bernal, M.P. Evaluation of the phytostabilisation efficiency in a trace elements contaminated soil using soil health indicators. J. Hazard. Mater. 2014, 268, 68–76. [Google Scholar] [CrossRef]
- Brown, S.; Svendsen, A.; Henry, C. Restoration of High Zinc and Lead Tailings with Municipal Biosolids and Lime: A Field Study. J. Environ. Qual. 2009, 38, 2189–2197. [Google Scholar] [CrossRef]
- Clemente, R.; Walker, D.J.; Pardo, T.; Martinez-Fernandez, D.; Bernal, M.P. The use of a halophytic plant species and organic amendments for the remediation of a trace elements-contaminated soil under semi-arid conditions. J. Hazard. Mater. 2012, 223, 63–71. [Google Scholar] [CrossRef]
- Lottermoser, B.G.; Ashley, P.M.; Costelloe, M.T. Contaminant dispersion at the rehabilitated Mary Kathleen uranium mine, Australia. Environ. Geol. 2005, 48, 748–761. [Google Scholar] [CrossRef]
- Santibañez, C.; De La Fuente, L.M.; Bustamante, E.; Silva, S.; León-Lobos, P.; Ginocchio, R. Potential use of organic- and hard-rock mine wastes on aided phytostabilization of large-scale mine tailings under semiarid Mediterranean climatic conditions: Short-term field study. Appl. Environ. Soil Sci. 2012, 2012, 895817. [Google Scholar] [CrossRef] [Green Version]
- Zornoza, R.; Faz, A.; Carmona, D.M.; Kabas, S.; Martinez-Martinez, S.; Acosta, J.A. Plant Cover and Soil Biochemical Properties in a Mine Tailing Pond Five Years After Application of Marble Wastes and Organic Amendments. Pedosphere 2012, 22, 22–32. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency Superfund ID # AZ0000309013 in Dewey-Humboldt, Arizona, U.S.A. Available online: https://www.azdeq.gov/superfund/IronKingMine (accessed on 1 May 2022).
- Hayes, S.M.; Root, R.A.; Perdrial, N.; Maier, R.M.; Chorover, J. Surficial weathering of iron sulfide mine tailings under semi-arid climate. Geochim. Cosmochim. Acta 2014, 141, 240–257. [Google Scholar] [CrossRef] [Green Version]
- Stovern, M.; Felix, O.; Csavina, J.; Rine, K.P.; Russell, M.R.; Jones, R.M.; King, M.; Betterton, E.A.; Saez, A.E. Simulation of windblown dust transport from a mine tailings impoundment using a computational fluid dynamics model. Aeolian Res. 2014, 14, 75–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youn, J.S.; Csavina, J.; Rine, K.P.; Shingler, T.; Taylor, M.P.; Saez, A.E.; Betterton, E.A.; Sorooshian, A. Hygroscopic Properties and Respiratory System Deposition Behavior of Particulate Matter Emitted by Mining and Smelting Operations. Environ. Sci. Technol. 2016, 50, 11706–11713. [Google Scholar] [CrossRef] [Green Version]
- Arizona Administrative Code § R18-7-210-Remediation Standards; Title 18. Environmental Quality. AZDEQ: Phoenix, AZ, USA, 2009; Chapter 7. Available online: https://apps.azsos.gov/public_services/Title_18/18-07.pdf (accessed on 1 May 2022).
- Gil-Loaiza, J.; Field, J.P.; White, S.A.; Csavina, J.; Felix, O.; Betterton, E.A.; Sáez, A.E.; Maier, R.M. Phytoremediation Reduces Dust Emissions from Metal(loid)-Contaminated Mine Tailings. Environ. Sci. Technol. 2018, 52, 5851–5858. [Google Scholar] [CrossRef]
- Creasey, S.C. Geology of the Iron King Mine, Yavapai County, Arizona. Econ. Geol. 1952, 47, 24–56. [Google Scholar] [CrossRef]
- Solis-Dominguez, F.A.; White, S.A.; Hutter, T.B.; Amistadi, M.K.; Root, R.A.; Chorover, J.; Maier, R.M. Response of Key Soil Parameters during Compost-Assisted Phytostabilization in Extremely Acidic Tailings: Effect of Plant Species. Environ. Sci. Technol. 2012, 46, 1019–1027. [Google Scholar] [CrossRef] [Green Version]
- Dold, B. Speciation of the most soluble phases in a sequential extraction procedure adapted for geochemical studies of copper sulfide mine waste. J. Geochem. Explor. 2003, 80, 55–68. [Google Scholar] [CrossRef]
- Keon, N.E.; Swartz, C.H.; Brabander, D.J.; Harvey, C.F.; Hemond, H.F. Validation of an arsenic sequential extraction method for evaluating mobility in sediments. Environ. Sci. Technol. 2001, 35, 2778–2784. [Google Scholar] [CrossRef]
- Brimhall, G.H.; Dietrich, W.E. Constitutive mass balance relations between chemical-composition, volume, density, porosity, and strain in metasomatic hydrochemical systems-Results on weathering and pedogenesis. Geochim. Cosmochim. Acta 1987, 51, 567–587. [Google Scholar] [CrossRef]
- Smuda, J.; Dold, B.; Spangenberg, J.E.; Friese, K.; Kobek, M.R.; Bustos, C.A.; Pfeifer, H.R. Element cycling during the transition from alkaline to acidic environment in an active porphyry copper tailings impoundment, Chuquicamata, Chile. J. Geochem. Explor. 2014, 140, 23–40. [Google Scholar] [CrossRef]
- Gunsinger, M.R.; Ptacek, C.J.; Blowes, D.W.; Jambor, J.L.; Moncur, M.C. Mechanisms controlling acid neutralization and metal mobility within a Ni-rich tailings impoundment. Appl. Geochem. 2006, 21, 1301–1321. [Google Scholar] [CrossRef]
- Johnson, R.H.; Blowes, D.W.; Robertson, W.D.; Jambor, J.L. The hydrogeochemistry of the Nickel Rim mine tailings impoundment, Sudbury, Ontario. J. Contam. Hydrol. 2000, 41, 49–80. [Google Scholar] [CrossRef]
- Bradl, H.B. Adsorption of heavy metal ions on soils and soils constituents. J. Colloid Interface Sci. 2004, 277, 1–18. [Google Scholar] [CrossRef]
- Kerndorff, H.; Schnitzer, M. Sorption of metals on humic-acid. Geochim. Cosmochim. Acta 1980, 44, 1701–1708. [Google Scholar] [CrossRef]
- Romero, F.M.; Armienta, M.A.; Gonzalez-Hernandez, G. Solid-phase control on the mobility of potentially toxic elements in an abandoned lead/zinc mine tailings impoundment. Appl. Geochem. 2007, 22, 109–127. [Google Scholar] [CrossRef]
- Karna, R.R.; Noerpel, M.R.; Nelson, C.; Elek, B.; Herbin-Davis, K.; Diamond, G.; Bradham, K.; Thomas, D.J.; Scheckel, K.G. Bioavailable soil Pb minimized by in situ transformation to plumbojarosite. Proc. Natl. Acad. Sci. USA 2021, 118, e2020315117. [Google Scholar] [CrossRef]
- Hakkou, R.; Benzaazoua, M.; Bussiere, B. Acid Mine Drainage at the Abandoned Kettara Mine (Morocco): 1. Environmental Characterization. Mine Water Environ. 2008, 27, 145–159. [Google Scholar] [CrossRef]
| Step | Extractant | Conc. (M) | Time, Temp. | Target Phase | References |
|---|---|---|---|---|---|
| 1 | DI H2O, Milli-Q water | – | 1 h, 25 °C | Soluble salts | [41] |
| (18.2 MΩ-cm) | |||||
| 2 | NaH2PO4, monosodium phosphate, pH 5 | 1 | 24 h, 25 °C | Exchangeable and adsorbed ions | [42] |
| 3 | Tamm’s reagent, ammonium oxalate, pH 3, dark | 0.2 | 2 h, 25 °C | Poorly crystalline Al, Mn, and Fe (hydr)oxides | [41,42] |
| Element | V | Cr | Mn | Co | Ni | Cu | Zn | Pb | pH |
|---|---|---|---|---|---|---|---|---|---|
| V | |||||||||
| Cr | 0.82 | ||||||||
| Mn | 0.13 | 0.16 | |||||||
| Co | 0.21 | 0.20 | 0.76 | ||||||
| Ni | 0.35 | 0.42 | 0.64 | 0.81 | |||||
| Cu | 0.07 | 0.28 | 0.58 | 0.55 | 0.66 | ||||
| Zn | 0.12 | 0.25 | 0.73 | 0.68 | 0.62 | 0.78 | |||
| Pb | 0.18 | 0.15 | 0.15 | 0.05 | 0.26 | 0.27 | 0.08 | ||
| pH | −0.17 | −0.24 | −0.73 | −0.76 | −0.79 | −0.80 | −0.85 | −0.12 | |
| Inverse Correlation | No Correlation | Direct Correlation | |||||||
 | |||||||||
| −1 | 0 | +1 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammond, C.M.; Root, R.A.; Maier, R.M.; Chorover, J. Metal Lability and Mass Transfer Response to Direct-Planting Phytostabilization of Pyritic Mine Tailings. Minerals 2022, 12, 757. https://doi.org/10.3390/min12060757
Hammond CM, Root RA, Maier RM, Chorover J. Metal Lability and Mass Transfer Response to Direct-Planting Phytostabilization of Pyritic Mine Tailings. Minerals. 2022; 12(6):757. https://doi.org/10.3390/min12060757
Chicago/Turabian StyleHammond, Corin M., Robert A. Root, Raina M. Maier, and Jon Chorover. 2022. "Metal Lability and Mass Transfer Response to Direct-Planting Phytostabilization of Pyritic Mine Tailings" Minerals 12, no. 6: 757. https://doi.org/10.3390/min12060757
APA StyleHammond, C. M., Root, R. A., Maier, R. M., & Chorover, J. (2022). Metal Lability and Mass Transfer Response to Direct-Planting Phytostabilization of Pyritic Mine Tailings. Minerals, 12(6), 757. https://doi.org/10.3390/min12060757







