Research on the Preparation of Sinter for COREX Reduction Process by Varying Basicity and MgO Content
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
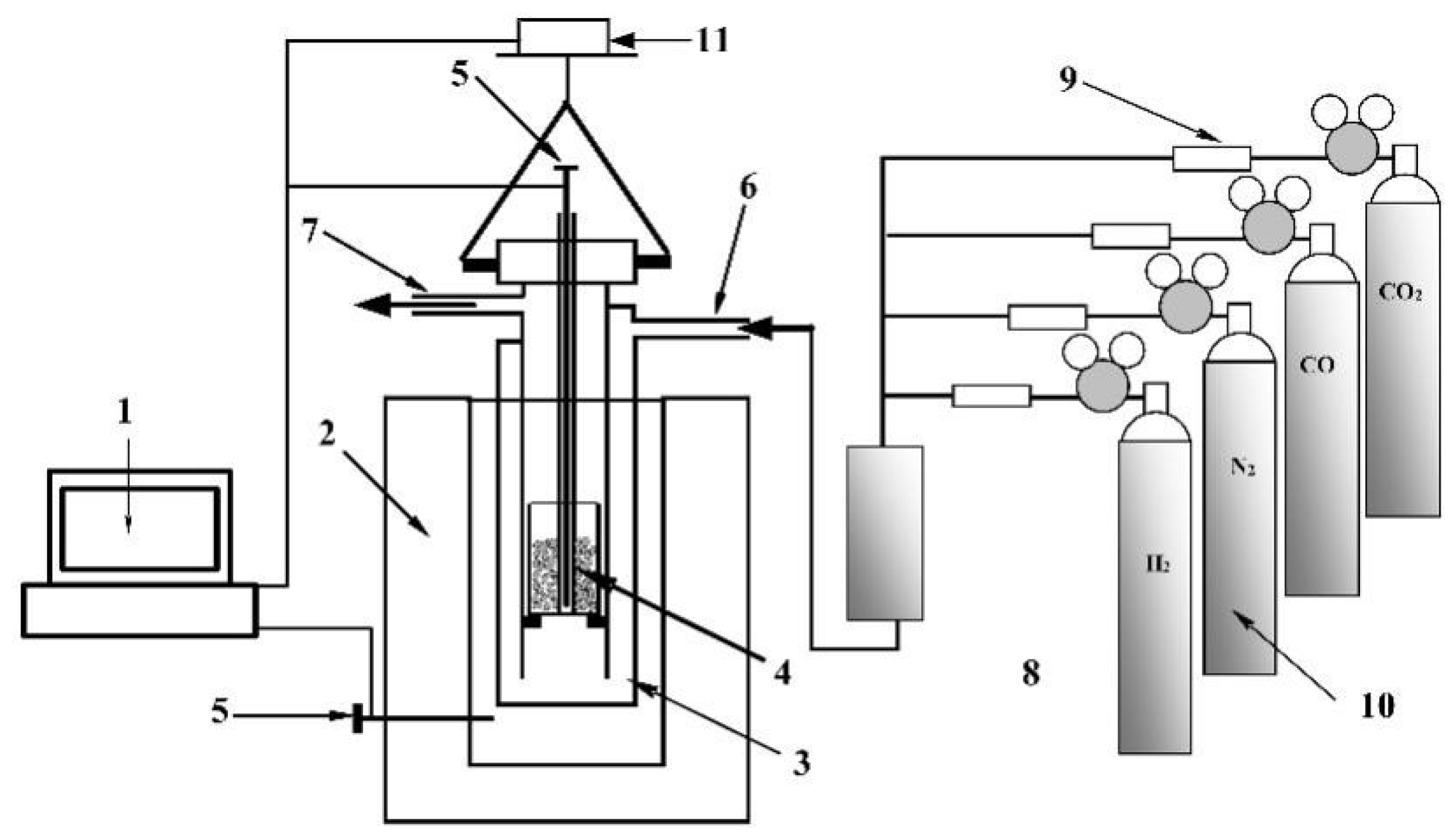
2.2. Methods
3. Results and Discussion
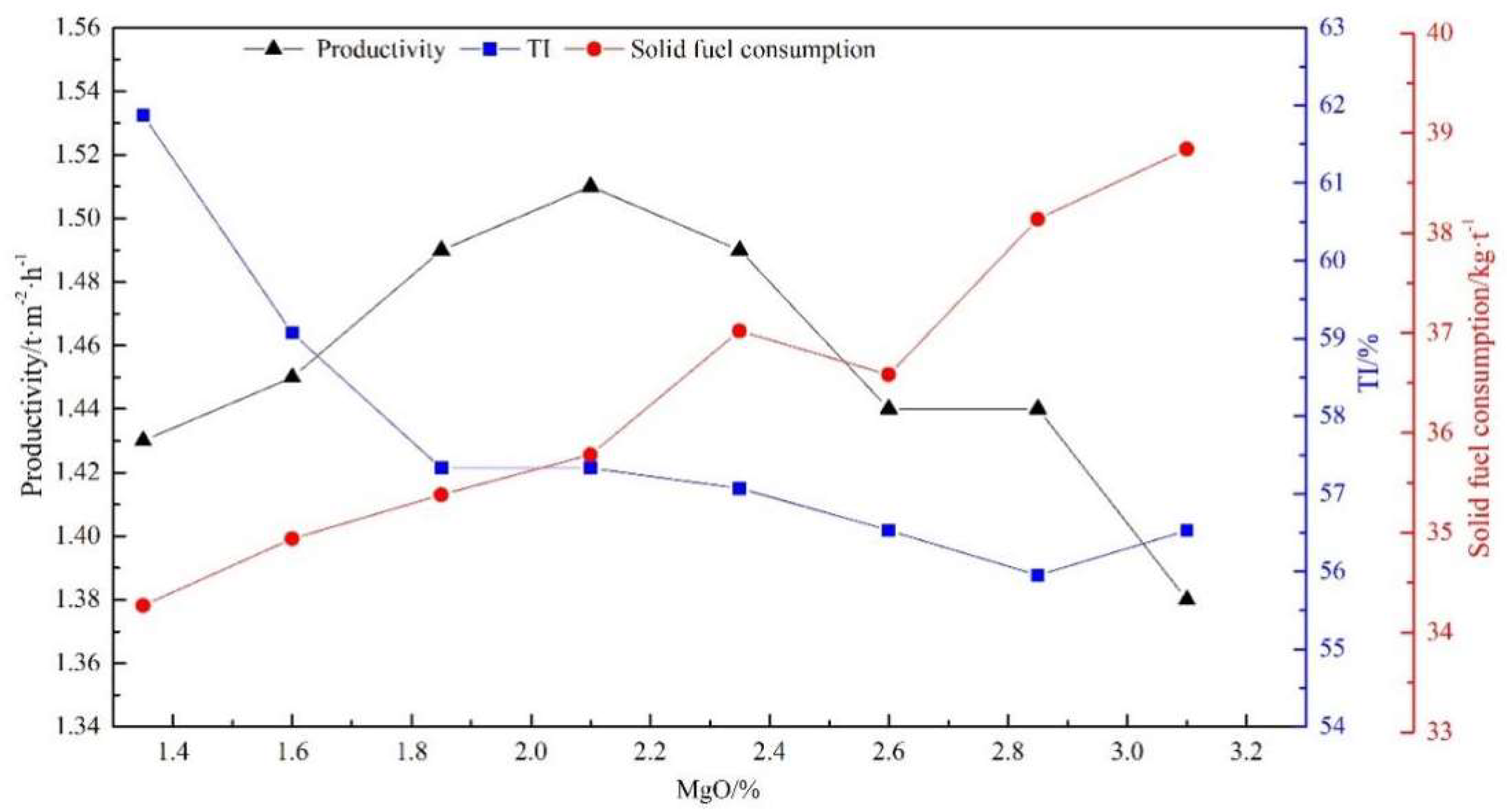
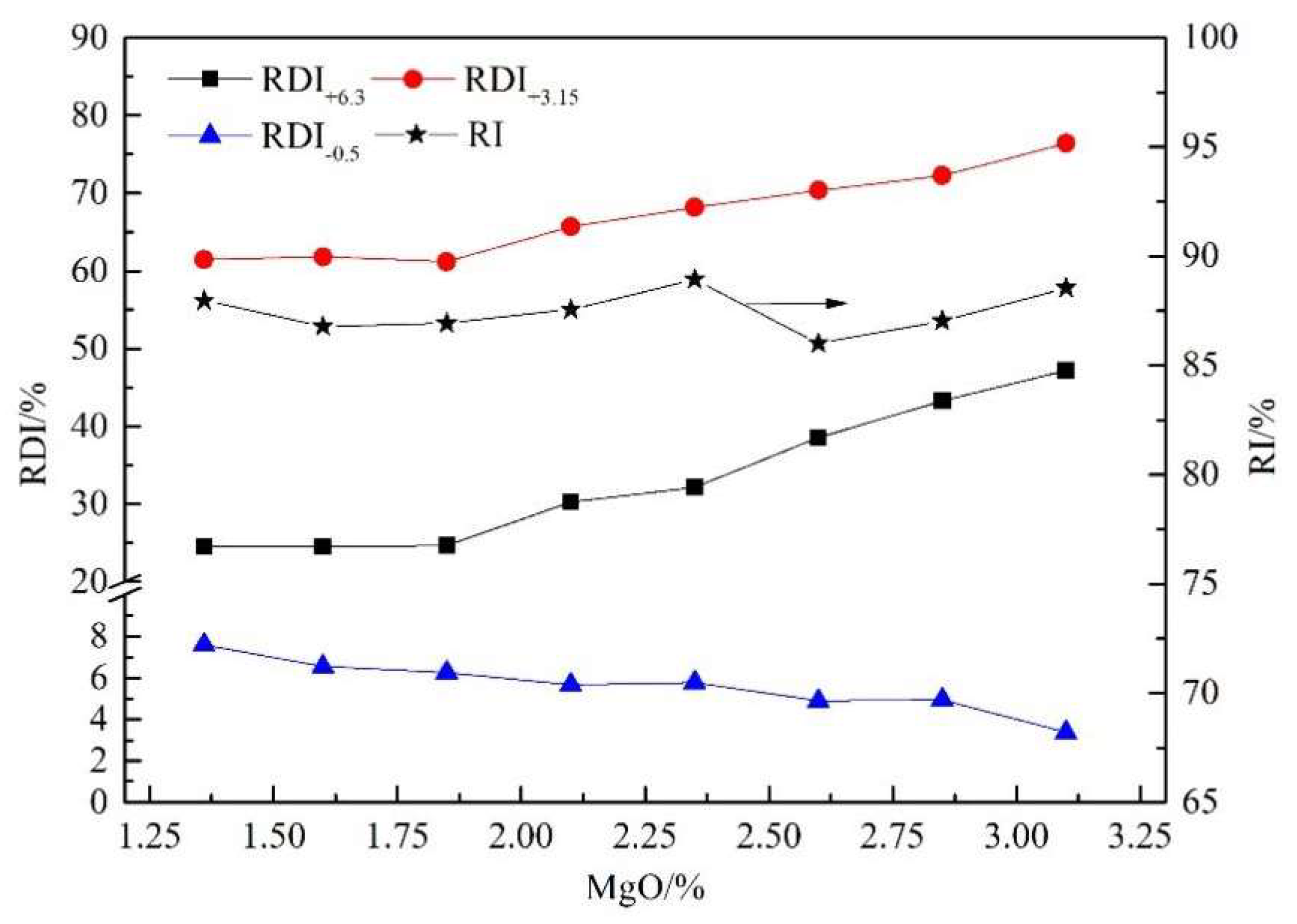
3.1. Sintering Performance and Reduction Properties of Sinter with Different MgO Content
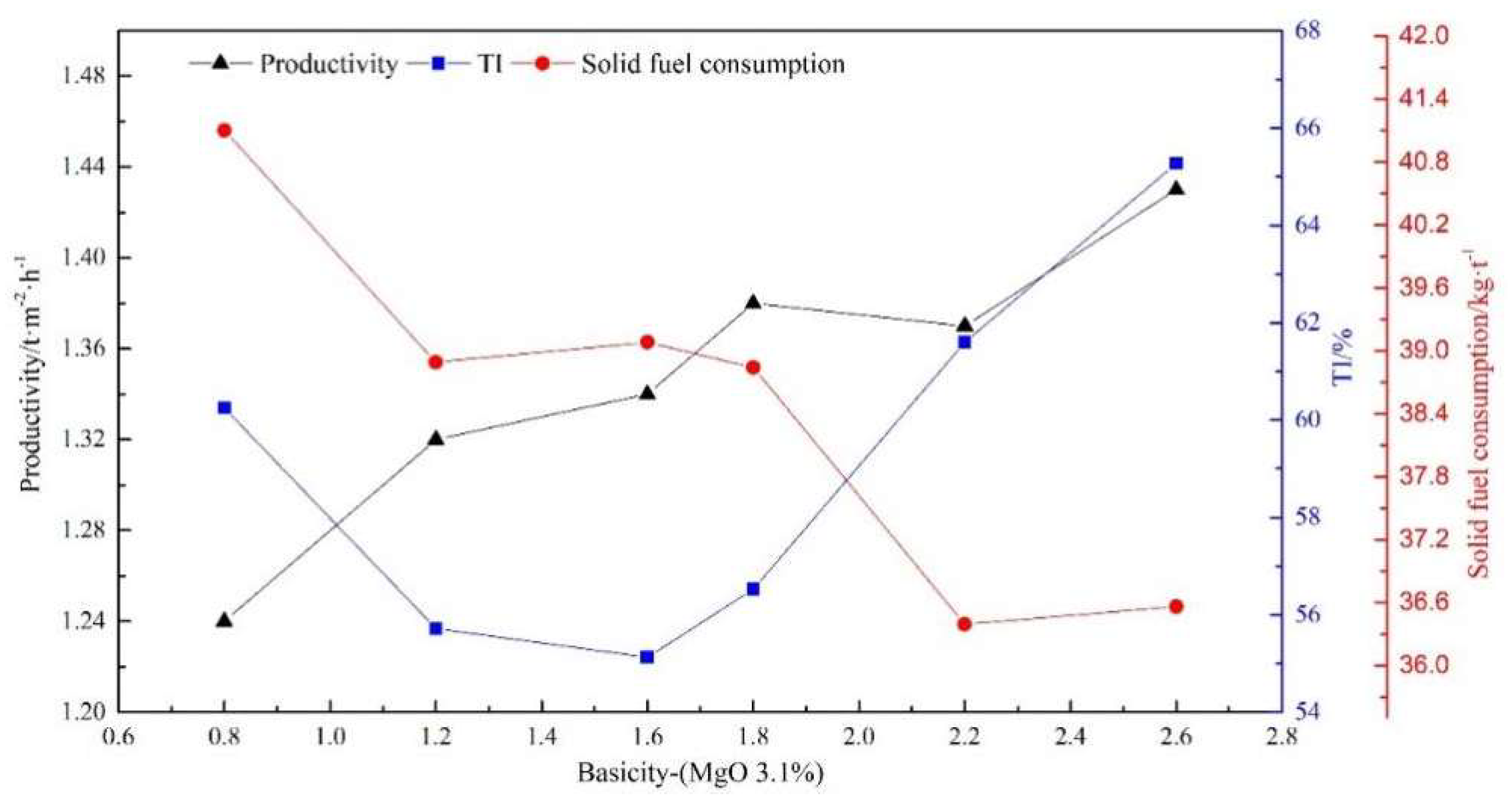
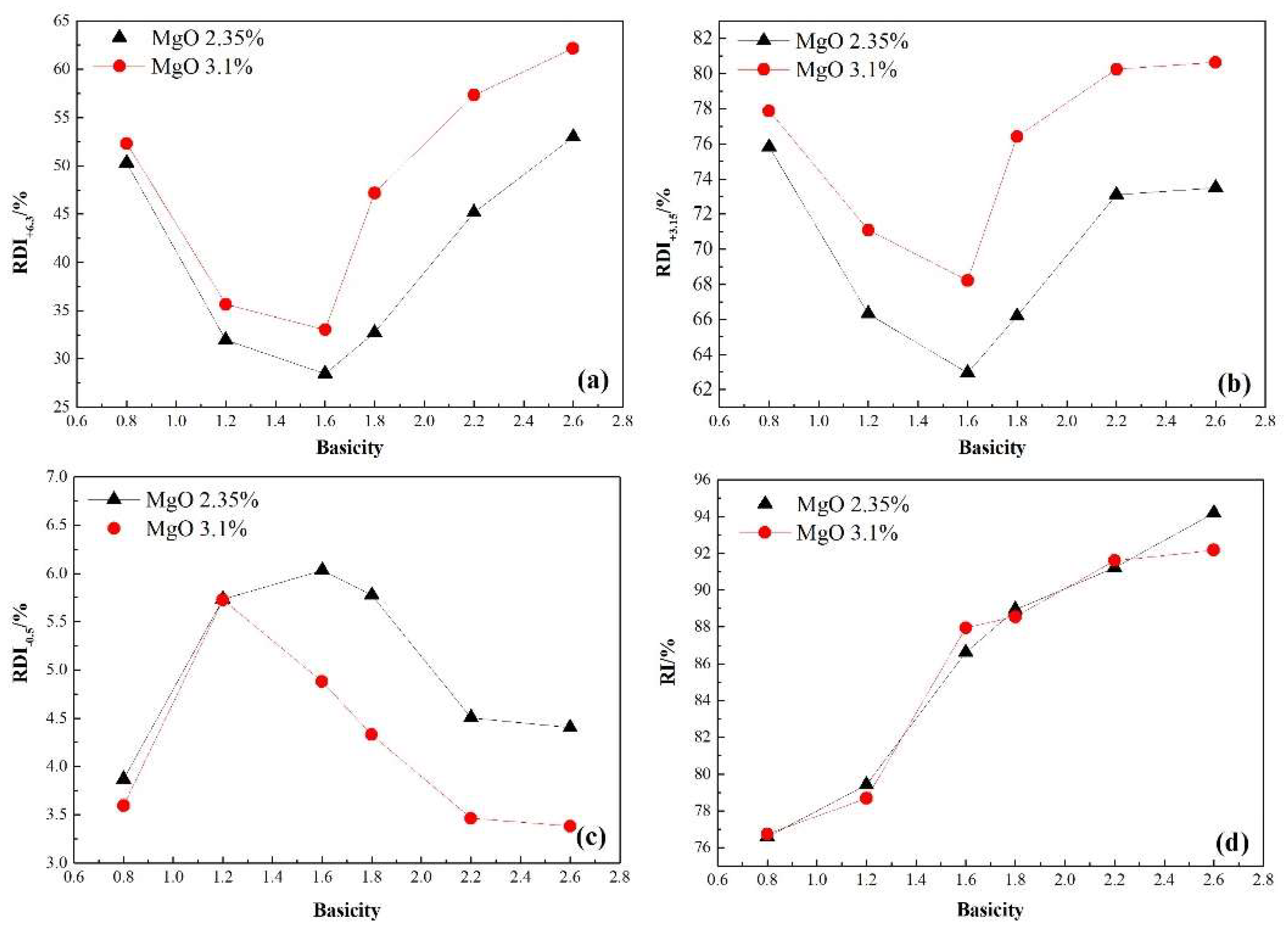
3.2. Effect of Sinter Basicity on the Sintering Performance and Reduction Properties of Sinter with Various MgO Content
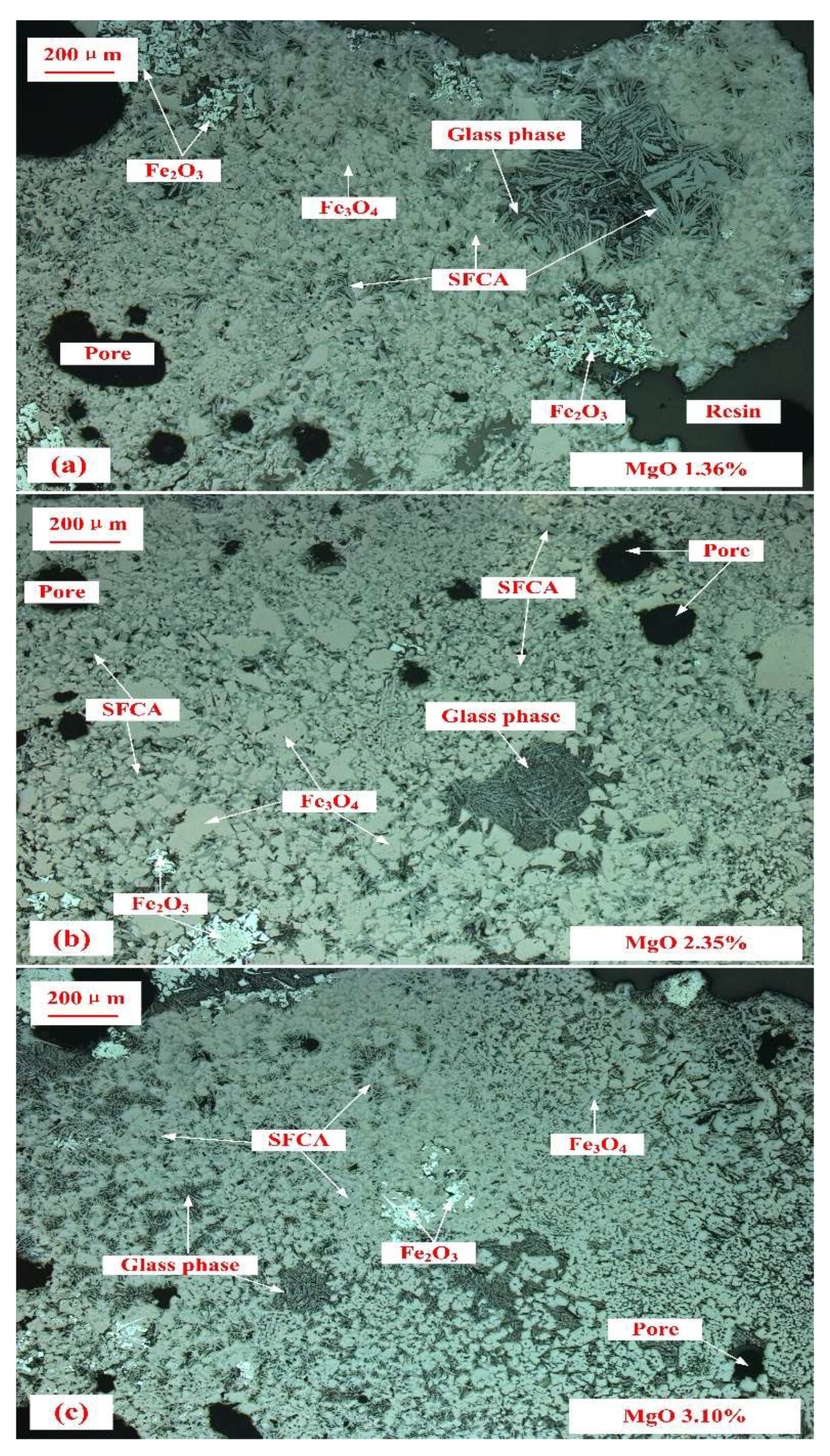
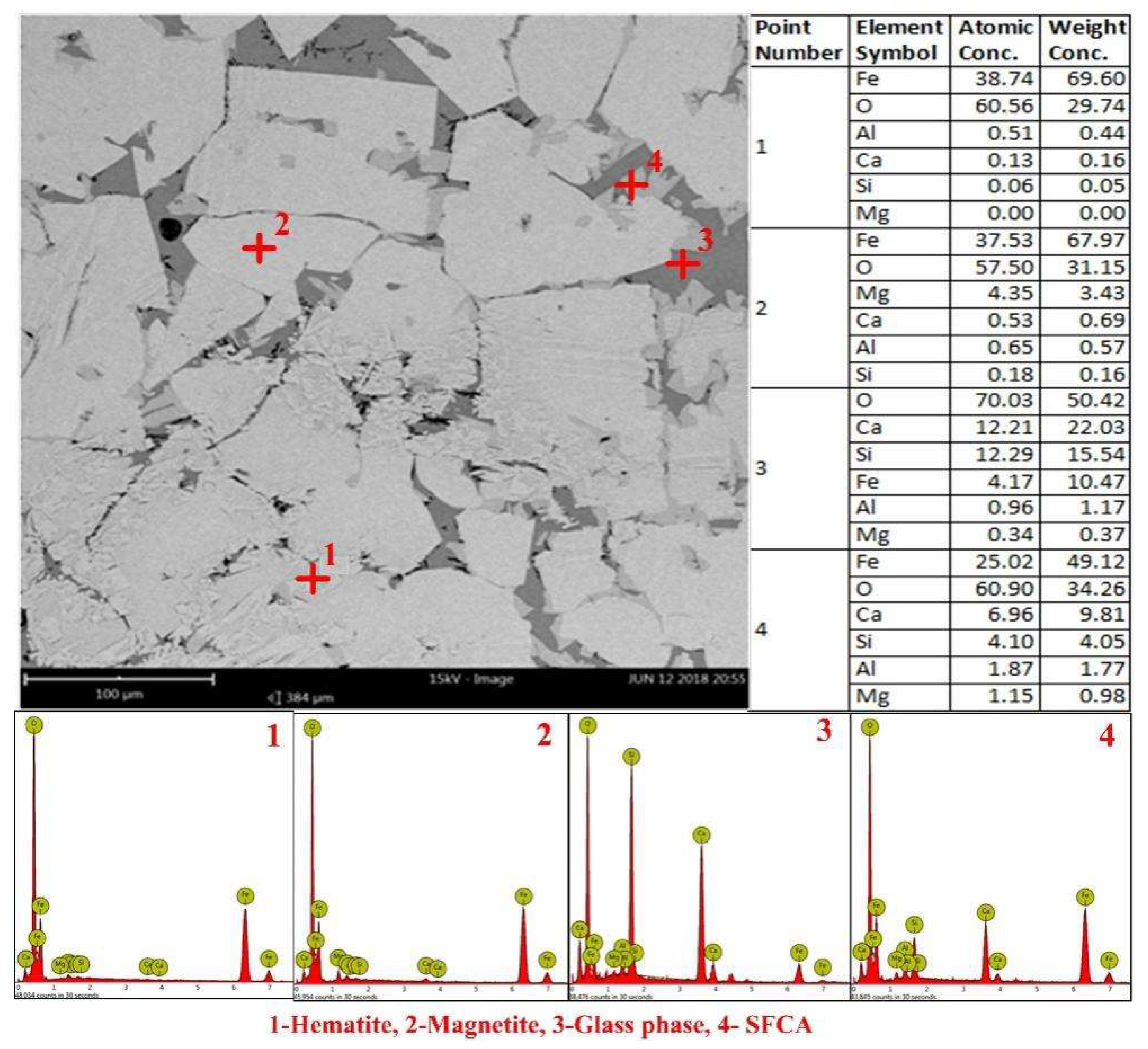
3.3. Mineralogy of Sinter with Different Basicity and MgO Content
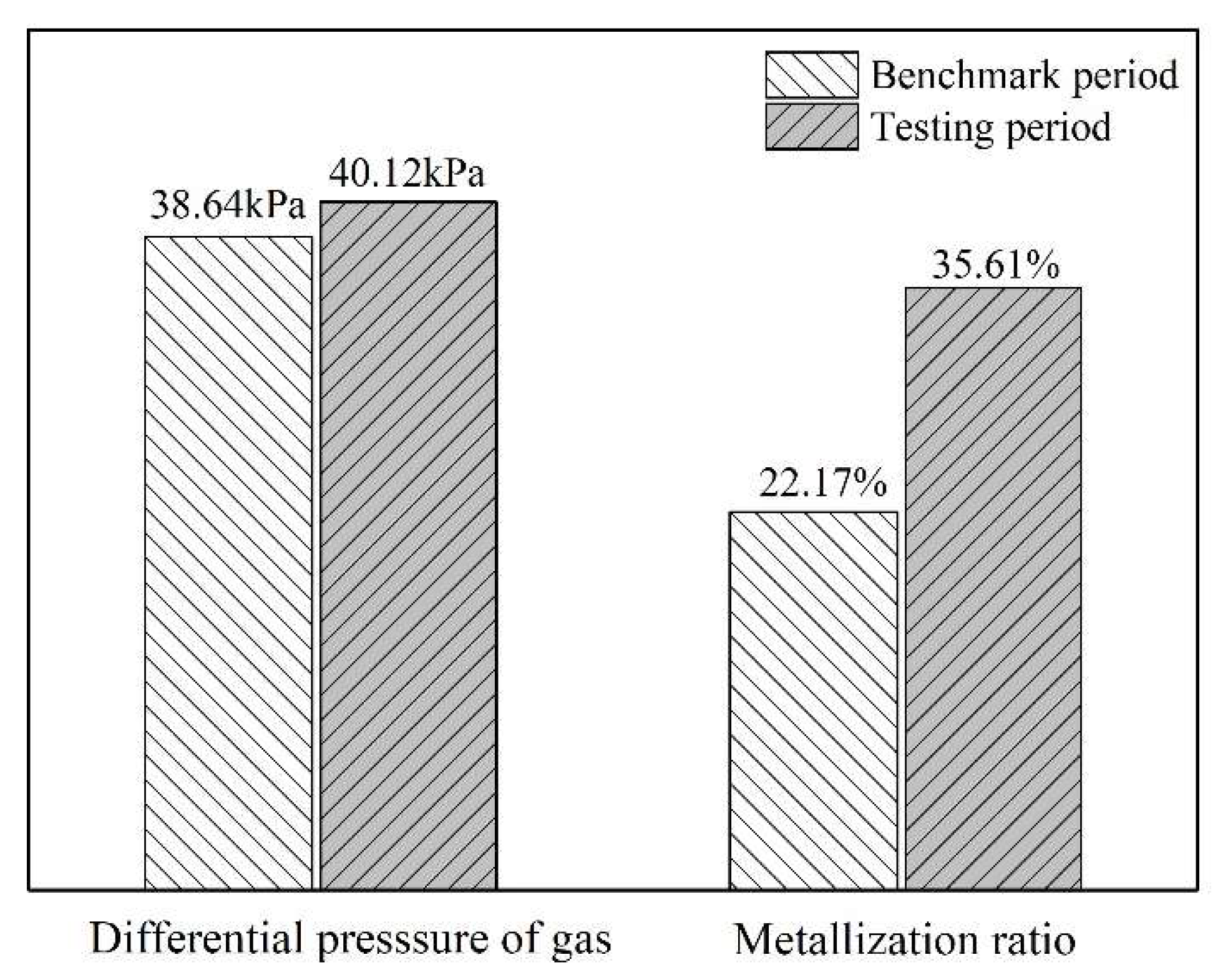
3.4. Industrial Tests of Increasing the Sinter Proportion in the Composite Burden for the COREX Process
4. Conclusions
- (1)
- For sintering with a basicity of 1.8, increasing the MgO content from natural (1.36%) to 3.1% decreased the sinter strength by 5.44 percentage points and increased solid fuel consumption by 4.57 kg/t. In the meantime, the RDI+6.3 and RDI+3.15 of sinter were both greatly improved, while the RI changed slightly under conditions simulating the COREX shaft furnace. However, only increasing the MgO content of sinter could not completely meet the requirement of composite burden for the reduction degradation performance of sinter.
- (2)
- As the sinter basicity increased from 0.8 to 2.6 regardless of MgO content, the productivity increased gradually while the solid fuel consumption decreased, the sinter strength varied as a V-shaped curve, and the minimum value occurred at a basicity of approximately 1.6. Simultaneously, the RDI+6.3 and RDI+3.15 of sinter behaved nearly the same as the sinter strength, while the RI increased significantly under conditions simulating the COREX shaft furnace. Taking comprehensive consideration of sintering and reduction performance, the combination of basicity 2.2+ and MgO content 2.35%+ of sinter was recommended for preparing sinter for the COREX process.
- (3)
- Fewer SFCA and hematite were observed in the sinter with a higher MgO content, which were related to the decrease in sinter strength and reduction of inner stress respectively. The binding phase in sinter with low basicity (0.8) and high basicity (2.6) was glass and SFCA, respectively. They were associated with the relatively higher sinter strength. The changes in the mineralogy of sinter determined the variations in the RDI of sinter with different MgO content and basicity, by affecting the sinter strength and probable reduction of inner stress.
- (4)
- After increasing the MgO content and basicity of sinter to 2.4% and 2.4 respectively in industrial tests, the sinter proportion in the composite burden was further raised to 40%, and the COREX shaft furnace maintained stable running conditions.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Song, J.Y.; Jiang, Z.Y.; Cheng, B.; Xu, A.J. Comparison of energy consumption and CO2 emission for three steel production routes—integrated steel plant equipped with blast furnace, oxygen blast furnace or COREX. Metals 2019, 9, 364. [Google Scholar] [CrossRef]
- Kumar, P.P.; Gupta, D.; Naha, T.K.; Gupta, S.S. Factors affecting fuel rate in COREX process. Ironmak. Steelmak. 2013, 33, 293–298. [Google Scholar] [CrossRef]
- Qiu, Z.L.; Luo, Z.G.; Wang, F.; Nie, H.Q.; Chen, R.; Zou, Z.S. The effect of screw design on solid behaviors and the interaction between solid and screws in COREX shaft furnace. Steel Res. Int. 2017, 88, 1600259. [Google Scholar] [CrossRef]
- Eberle, A.; Siuka, D.; Bohm, C. New COREX C-3000 plant for Baosteel and status of the COREX technology. Stahl Eisen 2006, 126, 31. [Google Scholar]
- Zhu, R.L.; Zhu, J.M.; Song, W.G. Present operation situation and development prospect of Baosteel COREX-3000. Baosteel Technol. 2011, 6, 12–17. (In Chinese) [Google Scholar]
- Chen, R.P.; Tan, P. Analysis the production performance of Baosteel COREX-3000 to guide the COREX production of Bayi steel. Xinjiang Iron Steel 2014, 2, 19–21. (In Chinese) [Google Scholar]
- Tian, B.S.; Li, W.H. Production practice of blowing-in OY furnace of Bayi-steel. Xinjiang Iron Steel 2015, 4, 1–4. (In Chinese) [Google Scholar]
- Zou, Q.F.; Chen, R.L. Optimization of production process control of OY furnace of Bayi-steel. Ironmaking 2017, 36, 60–62. (In Chinese) [Google Scholar]
- Shi, B.J.; Zhu, D.Q.; Pan, J.; Xue, Y.X. Reduction behaviors of sinter made from magnetite concentrates in reducing process simulated COREX shaft furnace. In Proceedings of the 8th International Symposium on High-Temperature Metallurgical Processing, San Diego, CA, USA, 26 February–2 March 2017; Springer International Publishing AG: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Bai, M.H.; Han, S.F.; Zhang, W.Y.; Xu, K.; Long, H. Influence of bed conditions on gas flow in the COREX shaft furnace by DEM–CFD modelling. Ironmak. Steelmak. 2017, 44, 685–691. [Google Scholar] [CrossRef]
- Hou, Q.F.; Samman, M.; Li, J.; Yu, A.B. Modeling the gas-solid flow in the reduction shaft of COREX. ISIJ Int. 2014, 54, 1772–1780. [Google Scholar] [CrossRef]
- Shi, B.J.; Zhu, D.Q.; Pan, J.; Hu, B.; Wang, Z.C. Reducing process of sinter in COREX shaft furnace and influence of sinter proportion on reduction properties of composite burden. J. Cent. South Univ. 2021, 28, 690–698. [Google Scholar] [CrossRef]
- Asada, M.; Shima, M.; Omori, Y. Measurement of macro strain in the course of reduction of the skeletal hematite in sinter. Tetsu-To-Hagane 1987, 73, 1901–1908. [Google Scholar] [CrossRef][Green Version]
- Loo, C.E.; Bristow, N.J. Mechanism of low-temperature reduction degradation of iron ore sinters. Trans. Inst. Min. Metall. Sect. C 1994, 103, 126–135. [Google Scholar]
- Nakajima, R.; Sumigama, T.; Wakimoto, K.; Nagano, S.; Kawata, H.; Sakurai, M. Reduction degradation behavior of sinter in the blast furnace shaft(blast furnace phenomena). Tetsu-To-Hagane 1987, 73, 1964–1971. [Google Scholar] [CrossRef][Green Version]
- Umadevi, T.; Sah, R.; Mahapatra, P.C. Influence of sinter basicity (CaO/SiO2) on low and high alumina iron ore sinter quality. Miner. Process. Extr. Metall. 2014, 123, 75–85. [Google Scholar] [CrossRef]
- Tang, W.D.; Yang, S.T.; Zhang, L.H.; Huang, Z.; Yang, H.; Xue, X.X. Effects of basicity and temperature on mineralogy and reduction behaviors of high-chromium vanadium-titanium magnetite sinters. J. Cent. South Univ. 2019, 26, 132–145. [Google Scholar] [CrossRef]
- Murakami, T.; Kamiya, Y.; Kodaira, T.; Kasai, E. Reduction disintegration behavior of iron ore sinter under high H2 and H2O conditions. ISIJ Int. 2012, 52, 1447–1453. [Google Scholar] [CrossRef]
- Shi, B.J.; Zhu, D.Q.; Pan, J.; Liu, X.Q.; Li, S.W. Combined effect of MgO and basicity varied by different dolomite and burnt lime addition on sintering performance of magnetite concentrates. Ironmak. Steelmak. 2020, 47, 567–573. [Google Scholar] [CrossRef]
- Zhu, D.Q.; Chou, J.L.; Shi, B.J.; Pan, J. Influence of MgO on low temperature reduction and mineralogical changes of sinter in simulated COREX shaft furnace reducing conditions. Minerals 2019, 9, 272. [Google Scholar] [CrossRef]
- Hisao, K.; Tsutomu, O.; Mitsuru, K.; Matsumoto, A.; Hamano, T.; Tsukihashi, F. Effect of Al2O3 and MgO additions on liquidus for the CaO-SiO2-FeOx system at 1573K. ISIJ Int. 2005, 45, 506–512. [Google Scholar]
- Pei, Y.D.; Wu, S.L.; Zhao, Z.X.; An, G.; Su, B.; Pan, W. In-situ observation of influence of MgO on high temperature properties of sinter ore. Ironmak. Steelmak. 2014, 49, 14–19. (In Chinese) [Google Scholar]
- Jiang, X.; Wu, G.S.; Wei, G.; Li, X.G.; Shen, F.M. Effect of MgO on sintering process and metallurgical properties of sinter. Ironmak. Steelmak. 2006, 41, 1–5. (In Chinese) [Google Scholar]
- Guo, Y.F.; Guo, X.M. Effect of MgO on low temperature reduction process of hematite fines sinter. J. Iron Steel Res. 2017, 29, 697–703. (In Chinese) [Google Scholar] [CrossRef]
- Yang, S.T.; Zhou, M.; Jiang, T.; Xue, X.X. Study on Sintering Characteristics of Ultra-Poor Vanadium-Titanium Magnetite. Minerals 2021, 11, 515. [Google Scholar] [CrossRef]
- Bobylev, G.S.; Kovalenko, A.G.; Padalka, V.P.; Kochura, V.V.; Khaibulaev, A.S. Full-scale experimental test production of high-basicity sinter in the Enakievo metallurgical plant sinter shop. Metallurgist 2020, 64, 741–749. [Google Scholar] [CrossRef]
- Fan, X.H.; Li, W.Q.; Gan, M.; Chen, X.L.; Yuan, L.S.; Ji, Z.Y.; Yu, Z.Y.; Huang, X.X.; Su, D. Influence and mechanism of MgO on strength of high basicity sinter. J. Cent. South Univ. (Sci. Technol.) 2012, 43, 3325–3330. (In Chinese) [Google Scholar] [CrossRef]
- Yadav, U.S.; Pandey, B.D.; Das, B.K.; Jena, D.N. Influence of magnesia on sintering characteristics of iron ore. Ironmak. Steelmak. 2002, 29, 91–95. [Google Scholar] [CrossRef]


| Materials | TFe | FeO | SiO2 | CaO | MgO | Al2O3 | P | S | LOI * |
|---|---|---|---|---|---|---|---|---|---|
| Blending ore | 61.44 | 23.66 | 5.38 | 2.10 | 1.27 | 1.14 | 0.024 | 0.269 | 2.56 |
| Burnt lime | 0.27 | - | 4.51 | 82.28 | 1.65 | 0.26 | 0.010 | 0.080 | 10.08 |
| Dolomite | 0.15 | - | 0.39 | 31.76 | 20.06 | 0.18 | 0.001 | 0.005 | 46.31 |
| Coke breeze | 2.51 | - | 6.97 | 2.03 | 0.41 | 3.75 | 0.034 | 0.110 | 82.91 |
| Basicity | MgO Content | Burnt Lime | Dolomite |
|---|---|---|---|
| 1.8 | 1.36% | 2.30 kg | 0.0 kg |
| 1.8 | 1.60% | 2.13 kg | 0.29 kg |
| 1.8 | 1.85% | 1.99 kg | 0.59 kg |
| 1.8 | 2.10% | 1.86 kg | 0.88 kg |
| 1.8 | 2.35% | 1.72 kg | 1.18 kg |
| 1.8 | 2.60% | 1.59 kg | 1.45 kg |
| 1.8 | 2.85% | 1.47 kg | 1.74 kg |
| 1.8 | 3.10% | 1.32 kg | 2.01 kg |
| MgO Content | Basicity | Hematite | Magnetite | Glass Phase | SFCA | Porosity |
|---|---|---|---|---|---|---|
| 1.36% | 1.8 | 15.37 | 32.02 | 11.73 | 25.56 | 14.96 |
| 2.35% | 1.8 | 13.58 | 34.45 | 14.83 | 21.06 | 16.08 |
| 3.10% | 1.8 | 7.25 | 40.27 | 15.96 | 19.63 | 16.89 |
| 2.35% | 0.8 | 12.49 | 57.79 | 17.54 | - | 12.18 |
| 2.35% | 1.6 | 11.93 | 44.68 | 13.86 | 13.52 | 16.01 |
| 2.35% | 2.6 | 6.62 | 25.02 | 9.02 | 40.20 | 19.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, B.; Zhu, D.; Pan, J.; Wang, Z. Research on the Preparation of Sinter for COREX Reduction Process by Varying Basicity and MgO Content. Minerals 2022, 12, 207. https://doi.org/10.3390/min12020207
Shi B, Zhu D, Pan J, Wang Z. Research on the Preparation of Sinter for COREX Reduction Process by Varying Basicity and MgO Content. Minerals. 2022; 12(2):207. https://doi.org/10.3390/min12020207
Chicago/Turabian StyleShi, Benjing, Deqing Zhu, Jian Pan, and Zhaocai Wang. 2022. "Research on the Preparation of Sinter for COREX Reduction Process by Varying Basicity and MgO Content" Minerals 12, no. 2: 207. https://doi.org/10.3390/min12020207
APA StyleShi, B., Zhu, D., Pan, J., & Wang, Z. (2022). Research on the Preparation of Sinter for COREX Reduction Process by Varying Basicity and MgO Content. Minerals, 12(2), 207. https://doi.org/10.3390/min12020207






