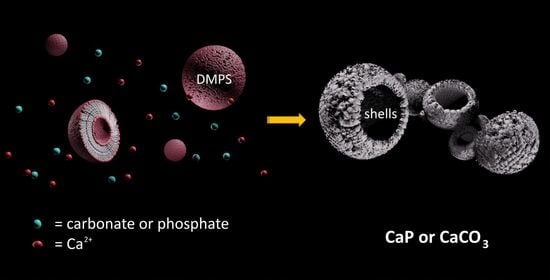
Precipitation of Calcium Phosphates and Calcium Carbonates in the Presence of Differently Charged Liposomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Liposome Preparation
2.3. Precipitation Systems
2.4. Characterization
2.4.1. Characterization of Liposomes
2.4.2. Characterization of Precipitates
3. Results and Discussion
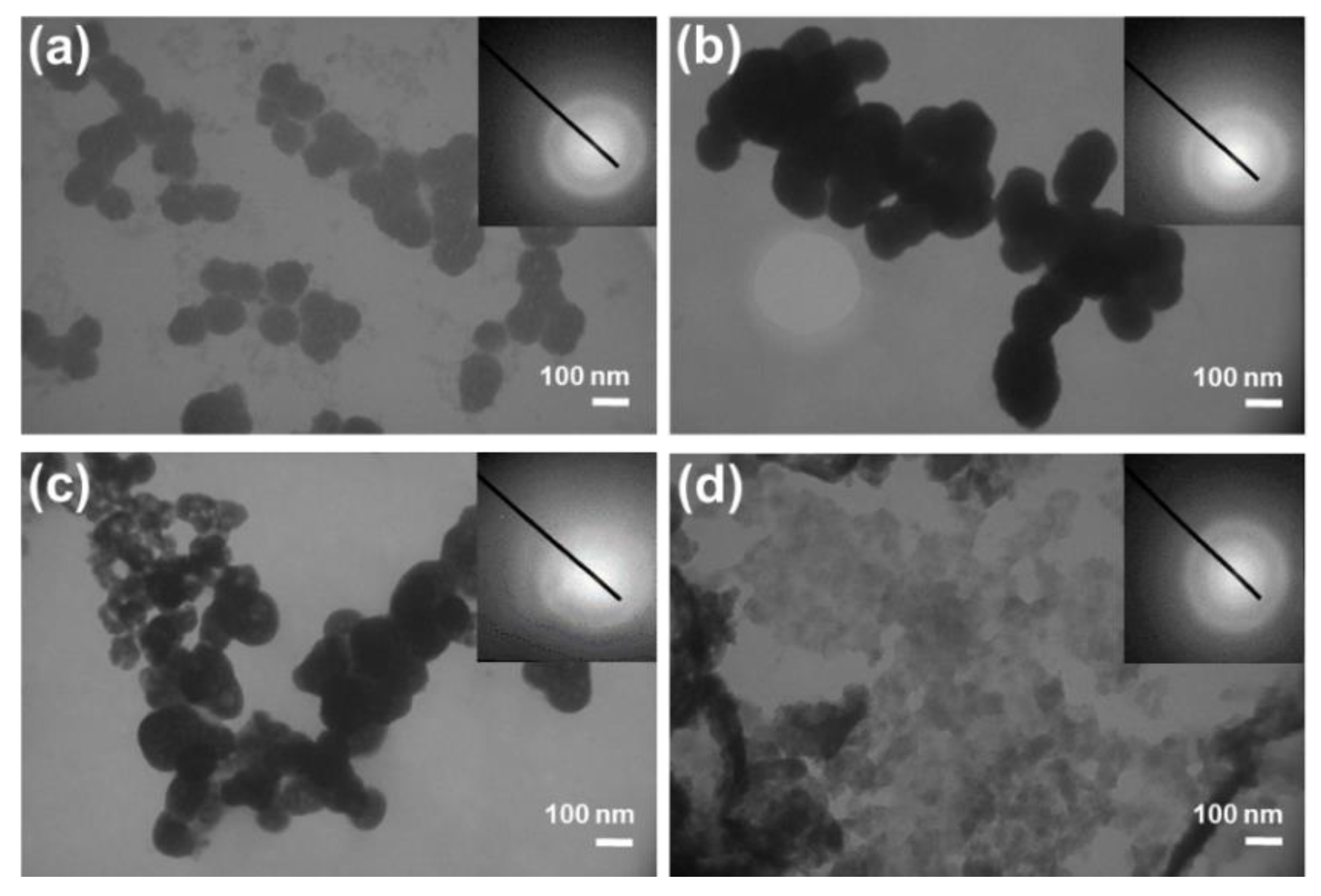
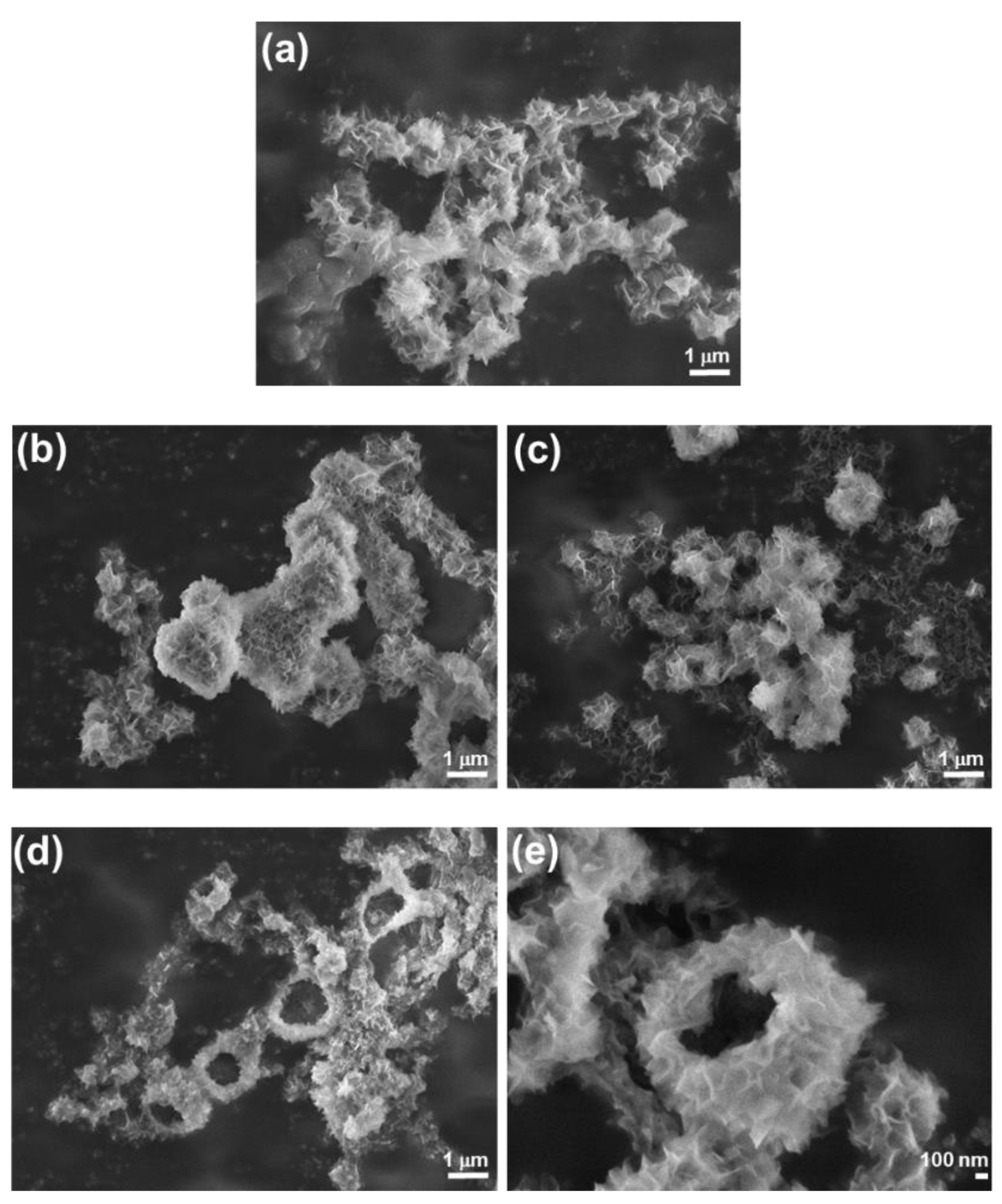
3.1. Influence of the Liposomes on Precipitation of Calcium Phosphates
3.2. Influence of the Liposomes on Precipitation of Calcium Carbonates
3.3. Comparison of the Liposomes’ Influence on CaP and CaCO3 Precipitation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Falini, G.; Fermani, S. The Strategic Role of Adsorption Phenomena in Biomineralization: Biomineralization. Cryst. Res. Technol. 2013, 48, 864–876. [Google Scholar] [CrossRef]
- Lowenstam, H.A.; Weiner, S. On Biomineralization; Oxford University Press: New York, NY, USA, 1989; ISBN 978-0-19-504977-0. [Google Scholar]
- Collier, J.H.; Messersmith, P.B. Phospholipid Strategies in Biomineralization and Biomaterials Research. Annu. Rev. Mater. Res. 2001, 31, 237–263. [Google Scholar] [CrossRef]
- Boonrungsiman, S.; Gentleman, E.; Carzaniga, R.; Evans, N.D.; McComb, D.W.; Porter, A.E.; Stevens, M.M. The Role of Intracellular Calcium Phosphate in Osteoblast-Mediated Bone Apatite Formation. Proc. Natl. Acad. Sci. USA 2012, 109, 14170–14175. [Google Scholar] [CrossRef] [PubMed]
- Weiner, S.; Addadi, L. Crystallization Pathways in Biomineralization. Annu. Rev. Mater. Res. 2011, 41, 21–40. [Google Scholar] [CrossRef]
- Ansari, S.; de Wildt, B.W.M.; Vis, M.A.M.; de Korte, C.E.; Ito, K.; Hofmann, S.; Yuana, Y. Matrix Vesicles: Role in Bone Mineralization and Potential Use as Therapeutics. Pharmaceuticals 2021, 14, 289. [Google Scholar] [CrossRef]
- Guibert, C.; Landoulsi, J. Enzymatic Approach in Calcium Phosphate Biomineralization: A Contribution to Reconcile the Physicochemical with the Physiological View. Int. J. Mol. Sci. 2021, 22, 12957. [Google Scholar] [CrossRef]
- Young, J.R.; Didymus, J.M.; Brown, P.R.; Prins, B.; Mann, S. Crystal Assembly and Phylogenetic Evolution in Heterococcoliths. Nature 1992, 356, 516. [Google Scholar] [CrossRef]
- Golub, E.E. Role of Matrix Vesicles in Biomineralization. Biochim. Biophys. Acta-Gen. Subj. 2009, 1790, 1592–1598. [Google Scholar] [CrossRef]
- Komeili, A. Molecular Mechanisms of Compartmentalization and Biomineralization in Magnetotactic Bacteria. FEMS Microbiol. Rev. 2012, 36, 232–255. [Google Scholar] [CrossRef]
- Tester, C.C.; Joester, D. Precipitation in Liposomes as a Model for Intracellular Biomineralization. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2013; Volume 532, pp. 257–276. ISBN 978-0-12-416617-2. [Google Scholar]
- Favarin, B.Z.; Bolean, M.; Ramos, A.P.; Magrini, A.; Rosato, N.; Millan, J.L.; Bottini, M.; Costa-Filho, A.J.; Ciancaglini, P. Lipid Composition Modulates ATP Hydrolysis and Calcium Phosphate Mineral Propagation by TNAP-Harboring Proteoliposomes. Arch. Biochem. Biophys. 2020, 691, 108482. [Google Scholar] [CrossRef]
- Qi, C.; Musetti, S.; Fu, L.-H.; Zhu, Y.-J.; Huang, L. Biomolecule-Assisted Green Synthesis of Nanostructured Calcium Phosphates and Their Biomedical Applications. Chem. Soc. Rev. 2019, 48, 2698–2737. [Google Scholar] [CrossRef] [PubMed]
- Bewernitz, M.A.; Lovett, A.C.; Gower, L.B. Liquid–Solid Core-Shell Microcapsules of Calcium Carbonate Coated Emulsions and Liposomes. Appl. Sci. 2020, 10, 8551. [Google Scholar] [CrossRef]
- Šegota, S.; Vojta, D.; Pletikapić, G.; Baranović, G. Ionic Strength and Composition Govern the Elasticity of Biological Membranes. A Study of Model DMPC Bilayers by Force- and Transmission IR Spectroscopy. Chem. Phys. Lipids 2015, 186, 17–29. [Google Scholar] [CrossRef]
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of Liposomes as Drug Delivery System for Therapeutic Applications. Int. J. Pharm. 2021, 601, 120571. [Google Scholar] [CrossRef]
- Song, J.; Wang, R.; Liu, Z.; Zhang, H. Preparation and Characterization of Calcium Carbonate Microspheres and Their Potential Application as Drug Carriers. Mol. Med. Rep. 2018, 17, 8403–8408. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Nancollas, G.H. Calcium Orthophosphates: Crystallization and Dissolution. Chem. Rev. 2008, 108, 4628–4669. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium Orthophosphates: Applications in Nature, Biology, and Medicine; CRC Press: Boca Raton, FL, USA, 2012; ISBN 978-981-4364-17-1. [Google Scholar]
- Dorozhkin, S.V.; Epple, M. Biological and Medical Significance of Calcium Phosphates. Angew. Chem. Int. Ed. 2002, 41, 3130–3146. [Google Scholar] [CrossRef]
- Erdogan, N.; Eken, H.A. Precipitated Calcium Carbonate Production, Synthesis and Properties. Physicochem. Probl. Miner. Process. 2017, 57, 57–68. [Google Scholar] [CrossRef]
- Amjad, Z. (Ed.) Calcium Phosphates in Biological and Industrial Systems; Springer: New York, NY, USA, 1998; ISBN 978-0-7923-8046-7. [Google Scholar]
- Sikirić, M.D.; Füredi-Milhofer, H. The Influence of Surface Active Molecules on the Crystallization of Biominerals in Solution. Adv. Colloid Interface Sci. 2006, 128–130, 135–158. [Google Scholar] [CrossRef]
- Omelon, S.J.; Grynpas, M.D. Relationships between Polyphosphate Chemistry, Biochemistry and Apatite Biomineralization. Chem. Rev. 2008, 108, 4694–4715. [Google Scholar] [CrossRef]
- Rey, C.; Combes, C.; Drouet, C.; Sfihi, H. Chemical Diversity of Apatites. Adv. Sci. Technol. 2006, 49, 27–36. [Google Scholar] [CrossRef]
- Falini, G.; Albeck, S.; Weiner, S.; Addadi, L. Control of Aragonite or Calcite Polymorphism by Mollusk Shell Macromolecules. Science 1996, 271, 67–69. [Google Scholar] [CrossRef]
- Njegić-Džakula, B.; Brečević, L.; Falini, G.; Kralj, D. Calcite Crystal Growth Kinetics in the Presence of Charged Synthetic Polypeptides. Cryst. Growth Des. 2009, 9, 2425–2434. [Google Scholar] [CrossRef]
- Štajner, L.; Kontrec, J.; Njegić Džakula, B.; Maltar-Strmečki, N.; Plodinec, M.; Lyons, D.M.; Kralj, D. The Effect of Different Amino Acids on Spontaneous Precipitation of Calcium Carbonate Polymorphs. J. Cryst. Growth 2018, 486, 71–81. [Google Scholar] [CrossRef]
- Mann, S. Biomineralization: Principles and Concepts in Bioinorganic Materials Chemistry; Oxford University Press: New York, NY, USA, 2001; ISBN 978-0-19-850882-3. [Google Scholar]
- Chai, Y.; Yamaguchi, T.; Tagaya, M. Fabrication of Phospholipid Vesicle-Interacted Calcium Phosphate Films with Sterilization Stability. Cryst. Growth Des. 2017, 17, 4977–4983. [Google Scholar] [CrossRef]
- Yeo, C.-H.; Zein, S.H.S.; Ahmad, A.L.; McPhail, D.S. Comparison of DOPA and DPPA Liposome Templates for the Synthesis of Calcium Phosphate Nanoshells. Ceram. Int. 2012, 38, 561–570. [Google Scholar] [CrossRef]
- Schmidt, H.T.; Ostafin, A.E. Liposome Directed Growth of Calcium Phosphate Nanoshells. Adv. Mater. 2002, 14, 532–535. [Google Scholar] [CrossRef]
- Szcześ, A.; Sternik, D. Properties of Calcium Carbonate Precipitated in the Presence of DPPC Liposomes Modified with the Phospholipase A2. J. Therm. Anal. Calorim. 2016, 123, 2357–2365. [Google Scholar] [CrossRef]
- Gong, J.; Zhou, Z.; Hu, X.; Wong, M.; Wong, K.; Du, Z. Self-Assembled Chitosan Nanotemplates for Biomineralization of Controlled Calcite Nanoarchitectures. ACS Appl. Mater. Interfaces 2009, 1, 26–29. [Google Scholar] [CrossRef]
- Szcześ, A. Effects of DPPC/Cholesterol Liposomes on the Properties of Freshly Precipitated Calcium Carbonate. Colloids Surf. B Biointerfaces 2013, 101, 44–48. [Google Scholar] [CrossRef]
- Wan, P.; Zhao, Y.; Tong, H.; Yang, Z.; Zhu, Z.; Shen, X.; Hu, J. The Inducing Effect of Lecithin Liposome Organic Template on the Nucleation and Crystal Growth of Calcium Carbonate. Mater. Sci. Eng. C 2009, 29, 222–227. [Google Scholar] [CrossRef]
- Eanes, E.D.; Hailer, A.W.; Costa, J.L. Calcium Phosphate Formation in Aqueous Suspensions of Multilamellar Liposomes. Calcif. Tissue Int. 1984, 36, 421–430. [Google Scholar] [CrossRef]
- Heywood, B.R.; Eanes, E.D. An Ultrastructural Study of the Effects of Acidic Phospholipid Substitutions on Calcium Phosphate Precipitation in Anionic Liposomes. Calcif. Tissue Int. 1992, 50, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.L.; Chen, Q.H.; Wang, H.; Cui, F.Z. Influence of Concentration of Calcium Ion on Controlled Precipitation of Calcium Phosphate within Unilamellar Lipid Vesicles. J. Cryst. Growth 1998, 186, 245–250. [Google Scholar] [CrossRef]
- Messersmith, P.B.; Vallabhaneni, S.; Nguyen, V. Preparation of Calcium-Loaded Liposomes and Their Use in Calcium Phosphate Formation. Chem. Mater. 1998, 10, 109–116. [Google Scholar] [CrossRef]
- Ramedani, A.; Yazdanpanah, A.; Moztarzadeh, F.; Mozafari, M. On the Use of Nanoliposomes as Soft Templates for Controlled Nucleation and Growth of Hydroxyapatite Nanocrystals under Hydrothermal Conditions. Ceram. Int. 2014, 40, 9377–9381. [Google Scholar] [CrossRef]
- Chen, W.-Y.; Yang, C.-I.; Lin, C.-J.; Huang, S.-J.; Chan, J.C.C. Characterization of the Crystallization Pathway of Calcium Phosphate in Liposomes. J. Phys. Chem. C 2014, 118, 12022–12027. [Google Scholar] [CrossRef]
- Tester, C.C.; Brock, R.E.; Wu, C.-H.; Krejci, M.R.; Weigand, S.; Joester, D. In Vitro Synthesis and Stabilization of Amorphous Calcium Carbonate (ACC) Nanoparticles within Liposomes. CrystEngComm 2011, 13, 3975. [Google Scholar] [CrossRef]
- Tester, C.C.; Whittaker, M.L.; Joester, D. Controlling Nucleation in Giant Liposomes. Chem. Commun. 2014, 50, 5619–5622. [Google Scholar] [CrossRef]
- Gopal, K.; Lu, Z.; de Villiers, M.M.; Lvov, Y. Composite Phospholipid−Calcium Carbonate Microparticles: Influence of Anionic Phospholipids on the Crystallization of Calcium Carbonate. J. Phys. Chem. B 2006, 110, 2471–2474. [Google Scholar] [CrossRef]
- Rivero Berti, I.; Dell’ Arciprete, M.L.; Dittler, M.L.; Miñan, A.; de Mele, M.F.L.; Gonzalez, M. Delivery of Fluorophores by Calcium Phosphate-Coated Nanoliposomes and Interaction with Staphylococcus Aureus Biofilms. Colloids Surf. B Biointerfaces 2016, 142, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Tagaya, M.; Yamaguchi, T.; Shiba, K. Preparation of Phospholipid Vesicle-Templated Calcium Phosphate Nanostructures and Their Cytocompatibility. Cryst. Growth Des. 2016, 16, 2843–2849. [Google Scholar] [CrossRef]
- Fukui, Y.; Fujimoto, K. Control in Mineralization by the Polysaccharide-Coated Liposome via the Counter-Diffusion of Ions. Chem. Mater. 2011, 23, 4701–4708. [Google Scholar] [CrossRef]
- Nguyen, S.; Solheim, L.; Bye, R.; Rykke, M.; Hiorth, M.; Smistad, G. The Influence of Liposomal Formulation Factors on the Interactions between Liposomes and Hydroxyapatite. Colloids Surf. B Biointerfaces 2010, 76, 354–361. [Google Scholar] [CrossRef][Green Version]
- Skrtic, D.; Eanes, E.D. Membrane-Mediated Precipitation of Calcium Phosphate in Model Liposomes with Matrix Vesicle-like Lipid Composition. Bone Miner. 1992, 16, 109–119. [Google Scholar] [CrossRef]
- Beato, C.; Fernández, M.S.; Fermani, S.; Reggi, M.; Neira-Carrillo, A.; Rao, A.; Falini, G.; Arias, J.L. Calcium Carbonate Crystallization in Tailored Constrained Environments. CrystEngComm 2015, 17, 5953–5961. [Google Scholar] [CrossRef]
- Buljan Meić, I.; Kontrec, J.; Domazet Jurašin, D.; Selmani, A.; Njegić Džakula, B.; Maltar-Strmečki, N.; Lyons, D.M.; Plodinec, M.; Čeh, M.; Gajović, A.; et al. How Similar Are Amorphous Calcium Carbonate and Calcium Phosphate? A Comparative Study of Amorphous Phase Formation Conditions. CrystEngComm 2018, 20, 35–50. [Google Scholar] [CrossRef]
- Buljan Meić, I.; Kontrec, J.; Domazet Jurašin, D.; Njegić Džakula, B.; Štajner, L.; Lyons, D.M.; Dutour Sikirić, M.; Kralj, D. Comparative Study of Calcium Carbonates and Calcium Phosphates Precipitation in Model Systems Mimicking the Inorganic Environment for Biomineralization. Cryst. Growth Des. 2017, 17, 1103–1117. [Google Scholar] [CrossRef]
- Addadi, L.; Joester, D.; Nudelman, F.; Weiner, S. Mollusk Shell Formation: A Source of New Concepts for Understanding Biomineralization Processes. Chem.-A Eur. J. 2006, 12, 980–987. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium Orthophosphates. Application in Nature, Biology and Medicine. Materials 2012, 2, 399–498. [Google Scholar] [CrossRef]
- Kahil, K.; Weiner, S.; Addadi, L.; Gal, A. Ion Pathways in Biomineralization: Perspectives on Uptake, Transport, and Deposition of Calcium, Carbonate, and Phosphate. J. Am. Chem. Soc. 2021, 143, 21100–21112. [Google Scholar] [CrossRef] [PubMed]
- Bentov, S.; Weil, S.; Glazer, L.; Sagi, A.; Berman, A. Stabilization of Amorphous Calcium Carbonate by Phosphate Rich Organic Matrix Proteins and by Single Phosphoamino Acids. J. Struct. Biol. 2010, 171, 207–215. [Google Scholar] [CrossRef]
- Zou, Z.; Polishchuk, I.; Bertinetti, L.; Pokroy, B.; Politi, Y.; Fratzl, P.; Habraken, W.J.E.M. Additives Influence the Phase Behavior of Calcium Carbonate Solution by a Cooperative Ion-Association Process. J. Mater. Chem. B 2018, 6, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Onoda, H.; Yamazaki, S. Homogenous Hydrothermal Synthesis of Calcium Phosphate with Calcium Carbonate and Corbicula Shells. J. Asian Ceram. Soc. 2016, 4, 403–406. [Google Scholar] [CrossRef]
- Cestari, F.; Agostinacchio, F.; Galotta, A.; Chemello, G.; Motta, A.; Sglavo, V.M. Nano-Hydroxyapatite Derived from Biogenic and Bioinspired Calcium Carbonates: Synthesis and In Vitro Bioactivity. Nanomaterials 2021, 11, 264. [Google Scholar] [CrossRef] [PubMed]
- Bleek, K.; Taubert, A. New Developments in Polymer-Controlled, Bioinspired Calcium Phosphate Mineralization from Aqueous Solution. Acta Biomater. 2013, 9, 6283–6321. [Google Scholar] [CrossRef]
- Bar-Yosef Ofir, P.; Govrin-Lippman, R.; Garti, N.; Füredi-Milhofer, H. The Influence of Polyelectrolytes on the Formation and Phase Transformation of Amorphous Calcium Phosphate. Cryst. Growth Des. 2004, 4, 177–183. [Google Scholar] [CrossRef]
- Brečević, L.; Füredi-Milhofer, H. Precipitation of Calcium Phosphates from Electrolyte Solutions: II. The Formation and Transformation of the Precipitates. Calcif. Tissue Res. 1972, 10, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Eans, E.D.; Gillessen, I.H.; Posner, A.S. Intermediate States in the Precipitation of Hydroxyapatite. Nature 1965, 208, 365–367. [Google Scholar] [CrossRef]
- Selmani, A.; Coha, I.; Magdić, K.; Čolović, B.; Jokanović, V.; Šegota, S.; Gajović, S.; Gajović, A.; Jurašin, D.; Dutour Sikirić, M. Multiscale Study of the Influence of Cationic Surfactants on Amorphous Calcium Phosphate Precipitation. CrystEngComm 2015, 17, 8529–8548. [Google Scholar] [CrossRef]
- Ding, H.; Pan, H.; Xu, X.; Tang, R. Toward a Detailed Understanding of Magnesium Ions on Hydroxyapatite Crystallization Inhibition. Cryst. Growth Des. 2014, 14, 763–769. [Google Scholar] [CrossRef]
- Despotović, R.; Filipović-Vinceković, N.; Füredi-Milhofer, H. Precipitation of Calcium Phosphates from Electrolyte Solutions. Calcif. Tissue Res. 1975, 18, 13–26. [Google Scholar] [CrossRef]
- Wang, C.-G.; Liao, J.-W.; Gou, B.-D.; Huang, J.; Tang, R.-K.; Tao, J.-H.; Zhang, T.-L.; Wang, K. Crystallization at Multiple Sites inside Particles of Amorphous Calcium Phosphate. Cryst. Growth Des. 2009, 9, 2620–2626. [Google Scholar] [CrossRef]
- Li, S.; Wang, L. Phosphorylated Osteopontin Peptides Inhibit Crystallization by Resisting the Aggregation of Calcium Phosphate Nanoparticles. CrystEngComm 2012, 14, 8037. [Google Scholar] [CrossRef]
- Chen, Y.; Gu, W.; Pan, H.; Jiang, S.; Tang, R. Stabilizing Amorphous Calcium Phosphate Phase by Citrate Adsorption. CrystEngComm 2014, 16, 1864–1867. [Google Scholar] [CrossRef]
- Wuthier, R.E.; Eanes, E.D. Effect of Phospholipids on the Transformation of Amorphous Calcium Phosphate to Hydroxyapatitein Vitro. Calcif. Tissue Res. 1975, 19, 197–210. [Google Scholar] [CrossRef]
- Pokhrel, R.; Gerstman, B.S.; Hutcheson, J.D.; Chapagain, P.P. In Silico Investigations of Calcium Phosphate Mineralization in Extracellular Vesicles. J. Phys. Chem. B 2018, 122, 3782–3789. [Google Scholar] [CrossRef]
- Brečević, L.; Hlady, V.; Füredi-Milhofer, H. Influence of Gelatin on the Precipitation of Amorphous Calcium Phosphate. Colloids Surf. 1987, 28, 301–313. [Google Scholar] [CrossRef]
- Sugiura, Y.; Onuma, K.; Kimura, Y.; Miura, H.; Tsukamoto, K. Morphological Evolution of Precipitates during Transformation of Amorphous Calcium Phosphate into Octacalcium Phosphate in Relation to Role of Intermediate Phase. J. Cryst. Growth 2011, 332, 58–67. [Google Scholar] [CrossRef]
- Koutsopoulos, S. Synthesis and Characterization of Hydroxyapatite Crystals: A Review Study on the Analytical Methods. J. Biomed. Mater. Res. 2002, 62, 600–612. [Google Scholar] [CrossRef]
- Mochales, C.; Wilson, R.M.; Dowker, S.E.P.; Ginebra, M.-P. Dry Mechanosynthesis of Nanocrystalline Calcium Deficient Hydroxyapatite: Structural Characterisation. J. Alloy. Compd. 2011, 509, 7389–7394. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Amorphous Calcium (Ortho)Phosphates. Acta Biomater. 2010, 6, 4457–4475. [Google Scholar] [CrossRef]
- Liou, S.-C.; Chen, S.-Y.; Lee, H.-Y.; Bow, J.-S. Structural Characterization of Nano-Sized Calcium Deficient Apatite Powders. Biomaterials 2004, 25, 189–196. [Google Scholar] [CrossRef]
- Merolli, A.; Santin, M. Role of Phosphatidyl-Serine in Bone Repair and Its Technological Exploitation. Molecules 2009, 14, 5367–5381. [Google Scholar] [CrossRef]
- Hendrickson, H.S.; Fullington, J.G. Stabilities of Metal Complexes of Phospholipids: Ca(II), Mg(II), and Ni(II) Complexes of Phosphatidylserine and Triphosphoinositide. Biochemistry 1965, 4, 1599–1605. [Google Scholar] [CrossRef]
- Andersen, F.A.; Brečević, L.; Beuter, G.; Dell’Amico, D.B.; Calderazzo, F.; Bjerrum, N.J.; Underhill, A.E. Infrared Spectra of Amorphous and Crystalline Calcium Carbonate. Acta Chem. Scand. 1991, 45, 1018–1024. [Google Scholar] [CrossRef]
- Andersen, F.A.; Kralj, D. Determination of the Composition of Calcite-Vaterite Mixtures by Infrared Spectrophotometry. Appl. Spectrosc. 1991, 45, 1748–1751. [Google Scholar] [CrossRef]
- Larkin, P. Infrared and Raman Spectroscopy: Principles and Spectral Interpretation; Elsevier: Amsterdam, The Netherlands; Boston, MA, USA, 2011; ISBN 978-0-12-386984-5. [Google Scholar]
- Grassmann, O.; Müller, G.; Löbmann, P. Organic−Inorganic Hybrid Structure of Calcite Crystalline Assemblies Grown in a Gelatin Hydrogel Matrix: Relevance to Biomineralization. Chem. Mater. 2002, 14, 4530–4535. [Google Scholar] [CrossRef]
- Wang, J.; Xu, Y.; Zhao, Y.; Huang, Y.; Wang, D.; Jiang, L.; Wu, J.; Xu, D. Morphology and Crystalline Characterization of Abalone Shell and Mimetic Mineralization. J. Cryst. Growth 2003, 252, 367–371. [Google Scholar] [CrossRef]
- Shen, Q.; Chen, Y.; Wei, H.; Zhao, Y.; Wang, D.; Xu, D. Suspension Effect of Poly(Styrene-Ran-Methacrylic Acid) Latex Particles on Crystal Growth of Calcium Carbonate. Cryst. Growth Des. 2005, 5, 1387–1391. [Google Scholar] [CrossRef]
- Ihli, J.; Wong, W.C.; Noel, E.H.; Kim, Y.-Y.; Kulak, A.N.; Christenson, H.K.; Duer, M.J.; Meldrum, F.C. Dehydration and Crystallization of Amorphous Calcium Carbonate in Solution and in Air. Nat. Commun. 2014, 5, 3169. [Google Scholar] [CrossRef]
- Brečević, L.; Nöthig-Laslo, V.; Kralj, D.; Popović, S. Effect of Divalent Cations on the Formation and Structure of Calcium Carbonate Polymorphs. J. Chem. Soc. Faraday Trans. 1996, 92, 1017–1022. [Google Scholar] [CrossRef]
- Picker, A.; Nuss, H.; Guenoun, P.; Chevallard, C. Polymer Vesicles as Microreactors for Bioinspired Calcium Carbonate Precipitation. Langmuir 2011, 27, 3213–3218. [Google Scholar] [CrossRef]
- Messersmith, P.B.; Starke, S. Thermally Triggered Calcium Phosphate Formation from Calcium-Loaded Liposomes. Chem. Mater. 1998, 10, 117–124. [Google Scholar] [CrossRef]
- Eanes, E.D.; Hailer, A.W. Liposome-Mediated Calcium Phosphate Formation in Metastable Solutions. Calcif. Tissue Int. 1985, 37, 390–394. [Google Scholar] [CrossRef]
- Erceg, I.; Selmani, A.; Gajović, A.; Radatović, B.; Šegota, S.; Ćurlin, M.; Strasser, V.; Kontrec, J.; Kralj, D.; Maltar-Strmečki, N.; et al. Precipitation at Room Temperature as a Fast and Versatile Method for Calcium Phosphate/TiO2 Nanocomposites Synthesis. Nanomaterials 2021, 11, 1523. [Google Scholar] [CrossRef]
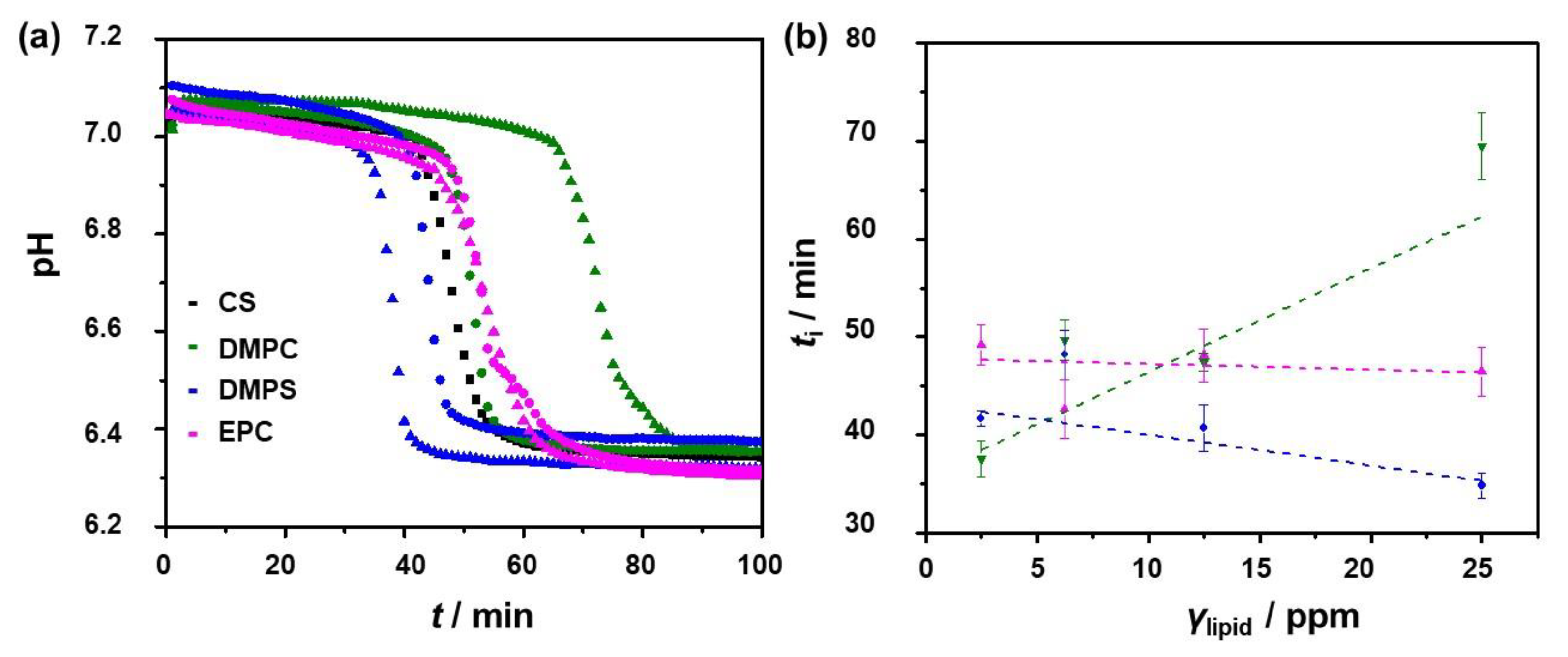

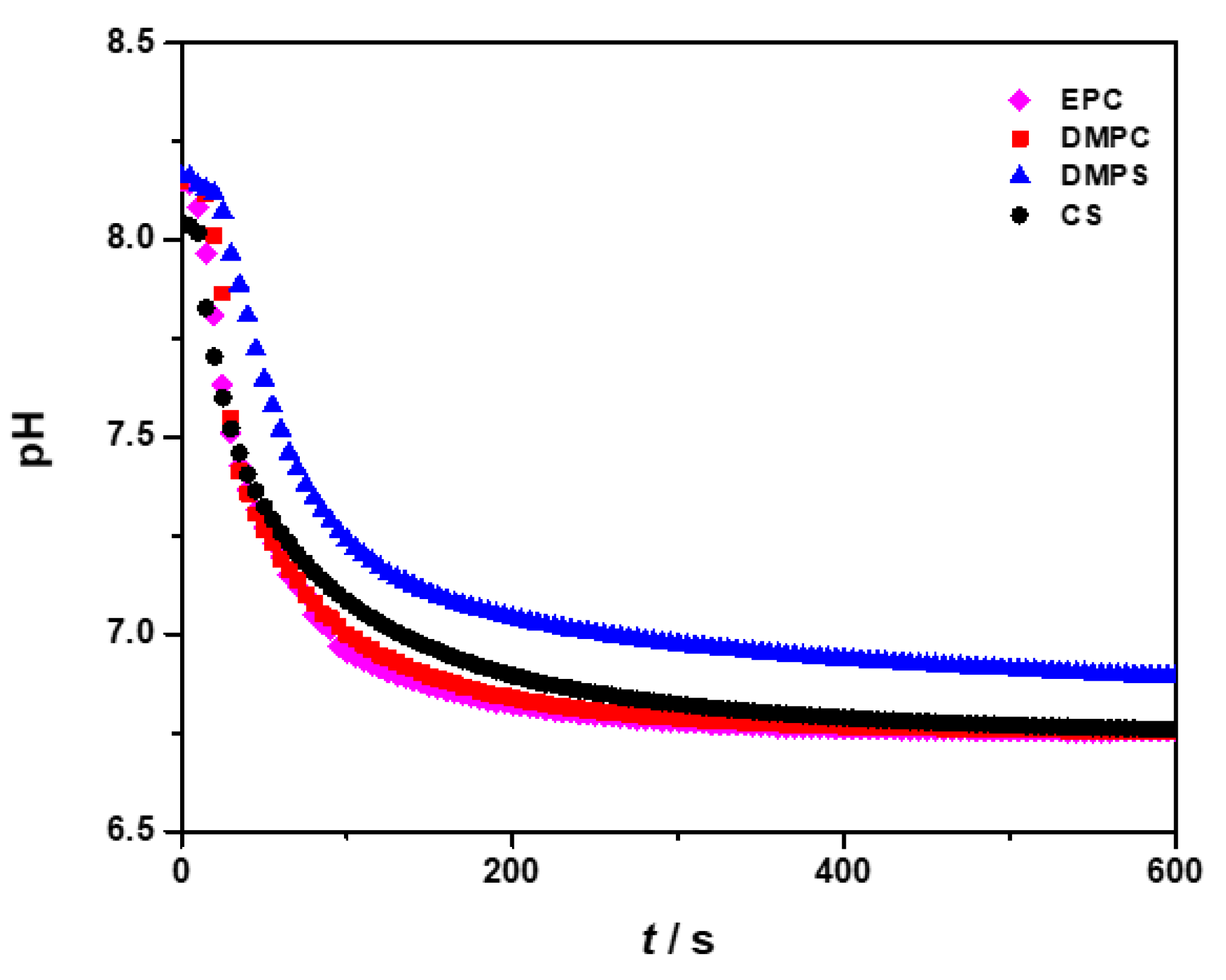
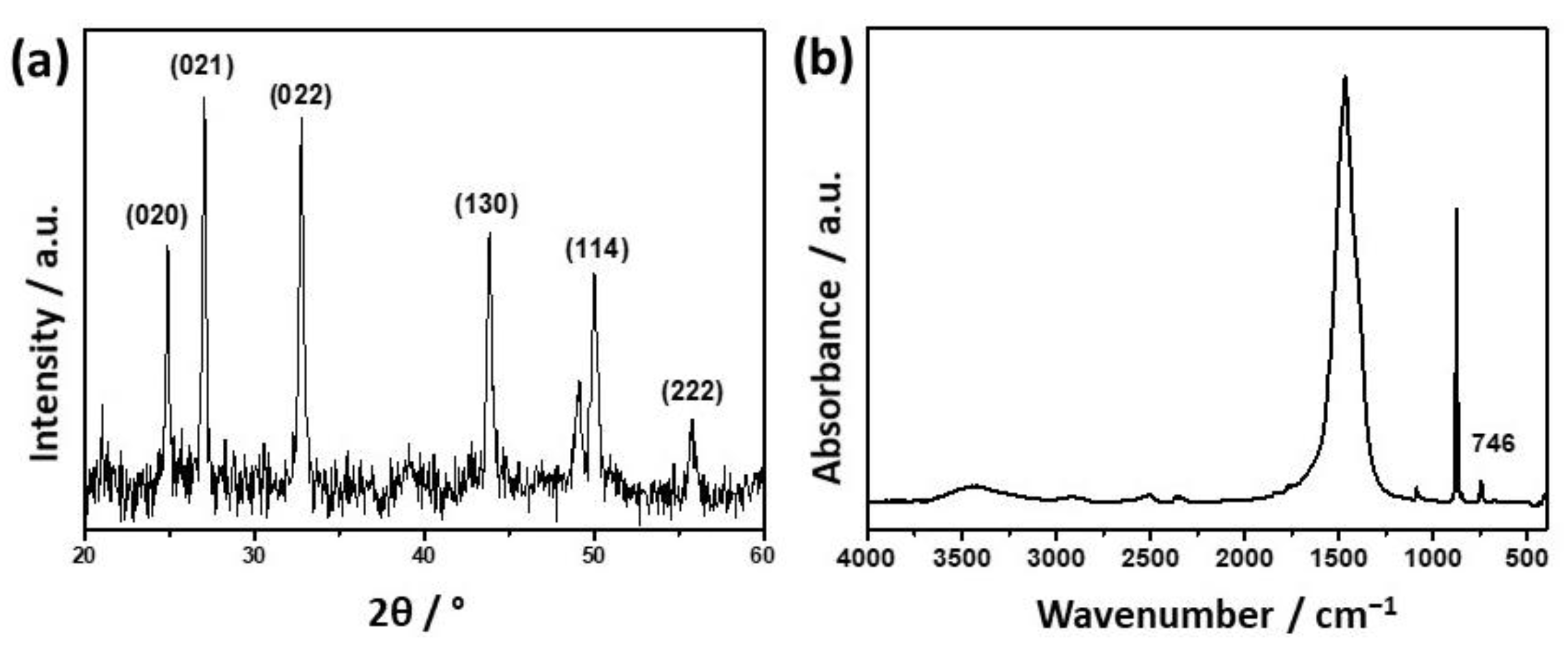


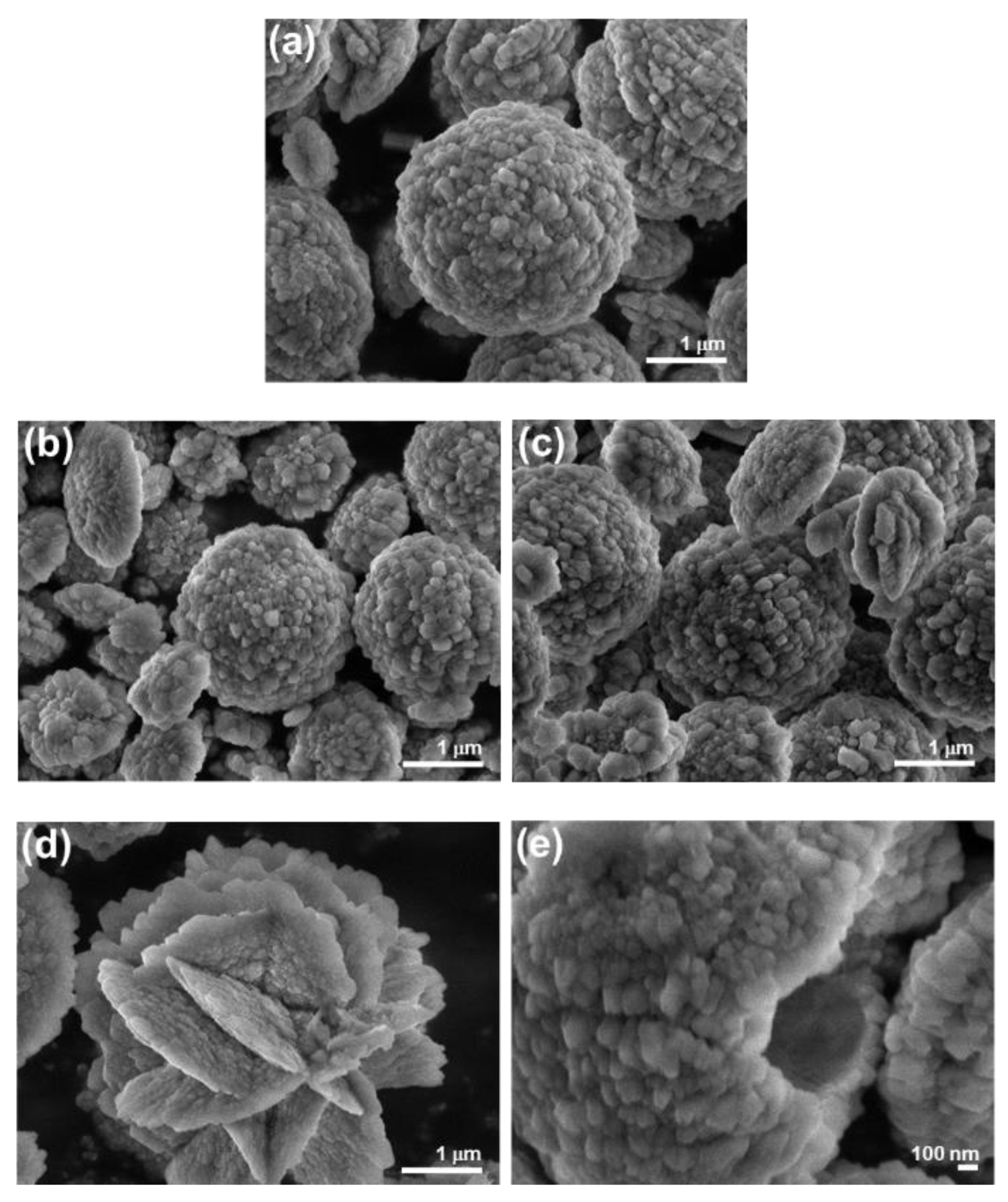
| Precipitation System | γ (Lipid)/ppm | ti/min |
|---|---|---|
| CS | 0 | 42.6 ± 2.3 |
| DMPC | 2.5 | 37.6 ± 1.8 |
| DMPC | 6.25 | 49.7 ± 2.1 |
| DMPC | 12.5 | 47.4 ± 0.9 |
| DMPC | 25.0 | 69.5 ± 3.4 |
| DMPS | 2.5 | 41.7 ± 0.8 |
| DMPS | 6.25 | 48.2 ± 2.5 |
| DMPS | 12.5 | 40.7 ± 2.4 |
| DMPS | 25.0 | 34.8 ± 1.3 |
| EPC | 2.5 | 49.2 ± 2.1 |
| EPC | 6.25 | 42.7 ± 3.0 |
| EPC | 12.5 | 48.1 ± 2.7 |
| EPC | 25.0 | 46.5 ± 2.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erceg, I.; Kontrec, J.; Strasser, V.; Selmani, A.; Domazet Jurašin, D.; Ćurlin, M.; Džakula, B.N.; Matijaković Mlinarić, N.; Šegota, S.; Lyons, D.M.; et al. Precipitation of Calcium Phosphates and Calcium Carbonates in the Presence of Differently Charged Liposomes. Minerals 2022, 12, 208. https://doi.org/10.3390/min12020208
Erceg I, Kontrec J, Strasser V, Selmani A, Domazet Jurašin D, Ćurlin M, Džakula BN, Matijaković Mlinarić N, Šegota S, Lyons DM, et al. Precipitation of Calcium Phosphates and Calcium Carbonates in the Presence of Differently Charged Liposomes. Minerals. 2022; 12(2):208. https://doi.org/10.3390/min12020208
Chicago/Turabian StyleErceg, Ina, Jasminka Kontrec, Vida Strasser, Atiđa Selmani, Darija Domazet Jurašin, Marija Ćurlin, Branka Njegić Džakula, Nives Matijaković Mlinarić, Suzana Šegota, Daniel M. Lyons, and et al. 2022. "Precipitation of Calcium Phosphates and Calcium Carbonates in the Presence of Differently Charged Liposomes" Minerals 12, no. 2: 208. https://doi.org/10.3390/min12020208
APA StyleErceg, I., Kontrec, J., Strasser, V., Selmani, A., Domazet Jurašin, D., Ćurlin, M., Džakula, B. N., Matijaković Mlinarić, N., Šegota, S., Lyons, D. M., Kralj, D., & Dutour Sikirić, M. (2022). Precipitation of Calcium Phosphates and Calcium Carbonates in the Presence of Differently Charged Liposomes. Minerals, 12(2), 208. https://doi.org/10.3390/min12020208