Carbon Sources as a Factor Determining the Activity of Microbial Oxidation of Sulfide Concentrate at Elevated Temperature
Abstract
:1. Introduction
- microorganisms capable of oxidizing either ferrous iron (Fe2+) or reduced inorganic sulfur compounds (RISC), as well as ones oxidizing both Fe2+ and RISC;
- microorganisms with different type of carbon nutrition including autotrophs, which fix dissolved carbon dioxide using the energy obtained by the oxidation of ferrous iron and RISC, and mixo- and heterotrophs, which require organic carbon sources for stable growth, despite the fact that they also gain energy by the oxidation of inorganic compounds [1,24,25].
2. Materials and Methods
2.1. Concentrate
2.2. Experimental Setup and Biooxidaton
2.3. Sampling and Analysis
2.4. Cyanidation Test
2.5. Microbial Population Analysis
3. Results
3.1. Biooxidtion
3.2. Cyanidation Tests
3.3. Microbial Population Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Johnson, D.B. Biomining—Biotechnologies for extracting and recovering metals from ores and waste materials. Curr. Opin. Biotechnol. 2014, 30, 24–31. [Google Scholar] [CrossRef]
- Johnson, D.B. The Evolution, Current Status, and Future Prospects of Using Biotechnologies in the Mineral Extraction and Metal Recovery Sectors. Minerals 2018, 8, 343. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, A.; Cezac, P.; Hoadley, A.F.A.; Contaminea, F.; D’Hugues, P. A review of sulfide minerals microbially assisted leaching in stirredtank reactors. Int. Biodeterior. Biodegrad. 2017, 119, 118–146. [Google Scholar] [CrossRef]
- Kaksonen, A.H.; Boxall, N.J.; Gumulya, Y.; Khaleque, H.N.; Morris, C.; Bohu, T.; Cheng, K.Y.; Usher, K.M.; Lakaniemi, A.-M. Recent progress in biohydrometallurgy and microbial characterization. Hydrometallurgy 2018, 180, 7–25. [Google Scholar] [CrossRef]
- Morin, D.H.R.; d’Hugues, P. Bioleaching of a cobalt containing pyrite in stirred reactors: A case study from laboratory scale to industrial application. In Biomining; Rawlings, D.E., Johnson, B.D., Eds.; Springer: Berlin, Germany, 2007; pp. 35–55. [Google Scholar] [CrossRef]
- Neale, J.; Seppala, J.; Laukka, A.; van Aswegen, P.; Barnett, S.; Gericke, M. The MONDO minerals nickel sulfide bioleach project: From test work to early plant operation. Solid State Phenom. 2017, 262, 28–32. [Google Scholar] [CrossRef]
- van Aswegen, P.C.; van Niekerk, J.; Olivier, W. The BIOX process for the treatment of refractory gold concentrate. In Biomining; Rawlings, D.E., Johnson, B.D., Eds.; Springer: Berlin, Germany, 2007; pp. 1–35. [Google Scholar] [CrossRef]
- Gericke, M.; Neale, J.W.; van Staden, P.J. A Mintek perspective of the past 25 years in minerals bioleaching. J. S. Afr. Inst. Min. Metall. 2017, 109, 567–585. [Google Scholar]
- Belyi, A.V.; Chernov, D.V.; Solopova, N.V. Development of BIONORD® technology on Olimpiada deposit refractory arsenic–gold ores treatment in conditions of Extreme North. Hydrometallurgy 2018, 179, 188–191. [Google Scholar] [CrossRef]
- Rawlings, D.E.; Coram, N.J.; Gardner, M.N.; Deane, S.M. Thiobacillus caldus and Leptospirillum ferrooxidans are widely distributed in continuous flow biooxidation tanks used to treat a variety of metal containing ores and concentrates. In Biohydrometallurgy and the Environment: Toward the Mining of the 21st Century. Part A; Amils, R., Ballester, A., Eds.; Elsevier: Amsterdam, The Netherlands, 1999; pp. 777–786. [Google Scholar] [CrossRef]
- Coram, N.J.; Rawlings, D.E. Molecular relationship between two groups of the genus Leptospirillum and the finding that Leptospirillum ferriphilum sp. nov. dominates South African commercial biooxidation tanks that operate at 40 °C. Appl. Environ. Microbiol. 2002, 68, 838–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okibe, N.; Gericke, M.; Hallberg, K.B.; Johnson, D.B. Enumeration and characterization of acidophilic microorganisms isolated from a pilot plant stirred tank bioleaching operation. Appl. Environ. Microbiol. 2003, 69, 1936–1943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dopson, M.; Lindstrom, E.B. Analysis of community composition during moderately thermophilic bioleaching of pyrite, arsenical pyrite, and chalcopyrite. Microb. Ecol. 2004, 48, 19–28. [Google Scholar] [CrossRef]
- Spolaore, P.; Joulian, C.; Gouin, J.; Ibáñez, A.; Auge, T.; Morin, D.; d’ Hugues, P. Bioleaching of an organic–rich polymetallic concentrate using stirred–tank technology. Hydrometallurgy 2009, 99, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Spolaore, P.; Joulian, C.; Gouin, J.; Morin, D.; d’ Hugues, P. Relationship between bioleaching performance, bacterial community structure and mineralogy in the bioleaching of a copper concentrate in stirred–tank reactors. Appl. Microbiol. Biotechnol. 2010, 89, 441–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Su, L.; Zhang, L.; Zeng, W.; Wu, J.; Wan, L.; Qiu, G.; Chen, X.; Zhou, H. Bioleaching of chalcopyrite by defined mixed moderately thermophilic consortium including a marine acidophilic halotolerant bacterium. Bioresour. Technol. 2012, 121, 348–354. [Google Scholar] [CrossRef]
- van Hille, R.P.; van Wyk, N.; Froneman, T.; Harrison, S.T.L. Dynamic evolution of the microbial community in BIOX leaching tanks. Adv. Mater. Res. 2013, 825, 331–334. [Google Scholar] [CrossRef]
- Kondrat’eva, T.F.; Pivovarova, T.A.; Bulaev, A.G.; Moshchanetskii, P.V.; Tsaplina, I.A.; Grigor’eva, N.V.; Zhuravleva, A.E.; Melamud, V.S.; Belyi, A.V. Selection of a community of acidochemolithotrophic microorganisms with a high oxidation rate of pyrrhotite–containing sulphide ore flotation concentrate. Appl. Biochem. Microbiol. 2013, 49, 495–501. [Google Scholar] [CrossRef]
- Muravyov, M.I.; Bulaev, A.G. Two–step oxidation of a refractory gold–bearing sulfidic concentrate and the effect of organic nutrients on its biooxidation. Miner. Eng. 2013, 45, 108–114. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, W.; Qiu, G.; Chen, X.; Zhou, H. A moderately thermophilic mixed microbial culture for bioleaching of chalcopyrite concentrate at high pulp density. Appl. Environ. Microbiol. 2014, 80, 741–750. [Google Scholar] [CrossRef] [Green Version]
- Hedrich, S.; Guézennec, A.-G.; Charron, M.; Schippers, A.; Joulian, C. Quantitative monitoring of microbial species during bioleaching of a copper concentrate. Front. Microbiol. 2016, 7, 20441. [Google Scholar] [CrossRef] [PubMed]
- Bulaev, A.; Belyi, A.; Panyushkina, A.; Solopova, N.; Pivovarova, T. Microbial population of industrial biooxidation reactors. Solid State Phenom. Submitt. 2017, 262, 48–52. [Google Scholar] [CrossRef]
- Bulaev, A.; Melamud, V.; Boduen, A. Bioleaching of non-ferrous metals from arsenic-bearing sulfide concentrate. Solid State Phenom. 2020, 299, 1064–1068. [Google Scholar] [CrossRef]
- Karavaiko, G.I.; Dubinina, G.A.; Kondrateva, T.F. Lithotrophic microorganisms of the oxidative cycles of sulfur and iron. Microbiology (Mikrobiologiya) 2006, 75, 512–545. [Google Scholar] [CrossRef]
- Schippers, A. Microorganisms involved in bioleaching and nucleic acid-based molecular methods for their identification and quantification. In Microbial Processing of Metal Sulfides; Donati, E.R., Sand, W., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 3–33. [Google Scholar] [CrossRef]
- Gonzalez-Tori, E.; Llobet-Brossa, E.; Casamayor, E.O.; Amann, R.; Amils, R. Microbial ecology of an extreme acidic environment, the Tinto River. Appl. Environ. Microbiol. 2003, 69, 4853–4865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Druschel, G.K.; Baker, B.J.; Gihring, T.H.; Banfield, J.F. Acid mine drainage biogeochemistry at Iron Mountain. Calif. Geochem. Trans. 2004, 5, 13–32. [Google Scholar] [CrossRef] [Green Version]
- Schnaitman, C.; Lundgren, D.G. Organic compounds in the spent medium of Ferrobacillus ferrooxidans. Can. J. Microbiol. 1965, 11, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Borichewski, R.M. Keto acids as growth-limiting factors in autotrophic growth of Thiobacillus thiooxidans. J. Bacteriol. 1967, 93, 597–599. [Google Scholar] [CrossRef] [Green Version]
- Okibe, N.; Johnson, D.B. Biooxidation of pyrite by defined mixed cultures of moderately thermophilic acidophiles in pH-controlled bioreactors: Significance of microbial interactions. Biotechnol. Bioeng. 2004, 87, 574–583. [Google Scholar] [CrossRef]
- Nancucheo, I.; Johnson, D.B. Production of glycolic acid by chemolithotrophic iron- and sulfur-oxidizing bacteria and its role in delineating and sustaining acidophilic sulfide mineral-oxidizing consortia. Appl. Environ. Microbiol. 2010, 76, 461–467. [Google Scholar] [CrossRef] [Green Version]
- Bulaev, A.; Nechaeva, A.; Elkina, Y.; Melamud, V. Effect of carbon sources on pyrite-arsenopyrite concentrate bio-oxidation and growth of microbial population in stirred tank reactors. Microorganisms 2021, 9, 2350. [Google Scholar] [CrossRef]
- Bulaev, A.G.; Boduen, A.Y.; Ukraintsev, I.V. Biooxidation of persistent gold-bearing ore concentrate of the Bestobe deposit. Obogashchenie Rud 2019, 6, 9–14. [Google Scholar] [CrossRef]
- Bulaev, A.G.; Erofeeva, T.V.; Vorobeva, K.S.; Chelidze, G.G.; Ramonova, A.A. Effect of Organic Nutrients on the Activity of Archaea of the Ferroplasmaceae Family. Moscow Univ. Biol. Sci. Bull. 2018, 73, 146–152. [Google Scholar] [CrossRef]
- Reznikov, A.A.; Mulikovskaya, E.P.; Sokolov, I.Y. Metody Analiza Prirodnykh Vod (Methods for Analysis of Natural Waters); Nedra: Moscow, Russia, 1970; 140p. (In Russian) [Google Scholar]
- Surovskaya, I.A.; Titov, V.I.; Brodskaya, V.M.; Vasil’ev, P.I.; Lipshits, B.M.; Elentukh, B.M. Tekhnicheskii Analiz Tsvetnoi Metallurgii (Technical Analysis in Nonferrous Metallurgy); Metallurgizdat: Moscow, Russia, 1957; 567p. (In Russian) [Google Scholar]
- Filippova, N.A. Fazovyi Analiz Rud I Produktov Ikh Pererabotki (Phase Analysis of Ores and Products of Their Processing); Khimiya: Moscow, Russia, 1975; 280p. (In Russian) [Google Scholar]
- Pimenov, N.V.; Merkel, A.Y.; Samylina, O.S.; Kanapatskii, T.A.; Tikhonova, E.N.; Vlasova, M.A.; Tarnovetskii, I.Y.; Malakhova, T.V. Structure of Microbial Mats in the Mramornaya Bay (Crimea) Coastal Areas. Microbiology (Mikrobiologiya) 2018, 87, 681–691. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pẽa, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335. [Google Scholar] [CrossRef] [Green Version]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckneret, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Golyshina, O.V.; Yakimov, M.M.; Lünsdorf, H.; Ferrer, M.; Nimtz, M.; Timmis, K.N.; Wray, V.; Tindall, B.J.; Golyshin, P.N. Acidiplasma aeolicum gen. nov., sp. nov., a euryarchaeon of the family Ferroplasmaceae isolated from a hydrothermal pool, and transfer of Ferroplasma cupricumulans to Acidiplasma cupricumulans comb. nov. Int. J. Syst. Evol. Microbiol. 2009, 59, 2815–2824. [Google Scholar] [CrossRef]
- Golyshina, O.V.; Lünsdorf, H.; Kublanov, I.V.; Goldenstein, N.I.; Hinrichs, K.U.; Golyshin, P.N. The novel extremely acidophilic, cell-wall-deficient archaeon Cuniculiplasma divulgatum gen. nov., sp. nov. represents a new family, Cuniculiplasmataceae fam. nov., of the order. Int. J. Syst. Evol. Microbiol. 2016, 66, 332–340. [Google Scholar] [CrossRef]
- Bulaev, A.G. Effect of organic carbon source on pyrite biooxidation by moderately thermophilic acidophilic microorganisms. Microbiology 2020, 89, 301–308. [Google Scholar] [CrossRef]
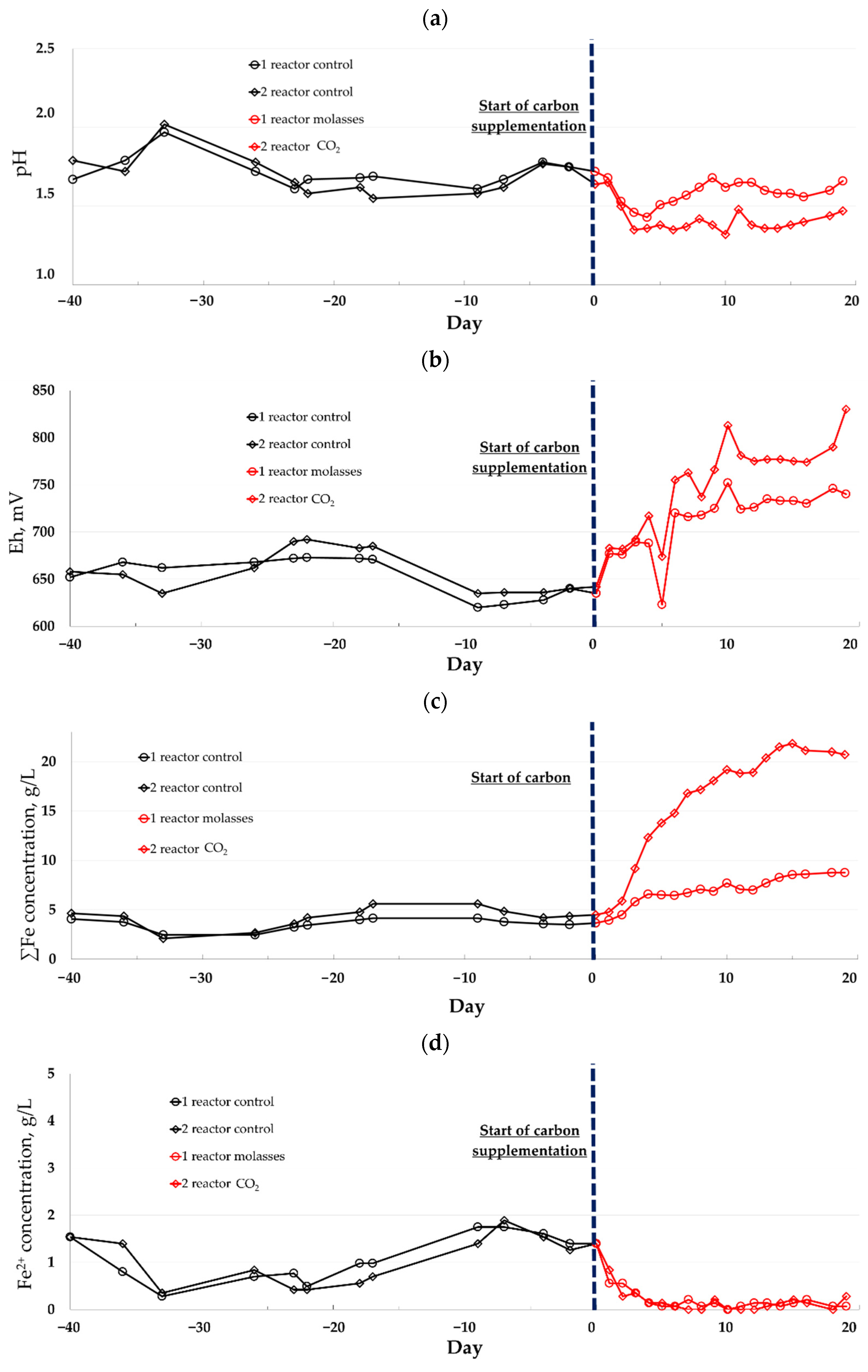

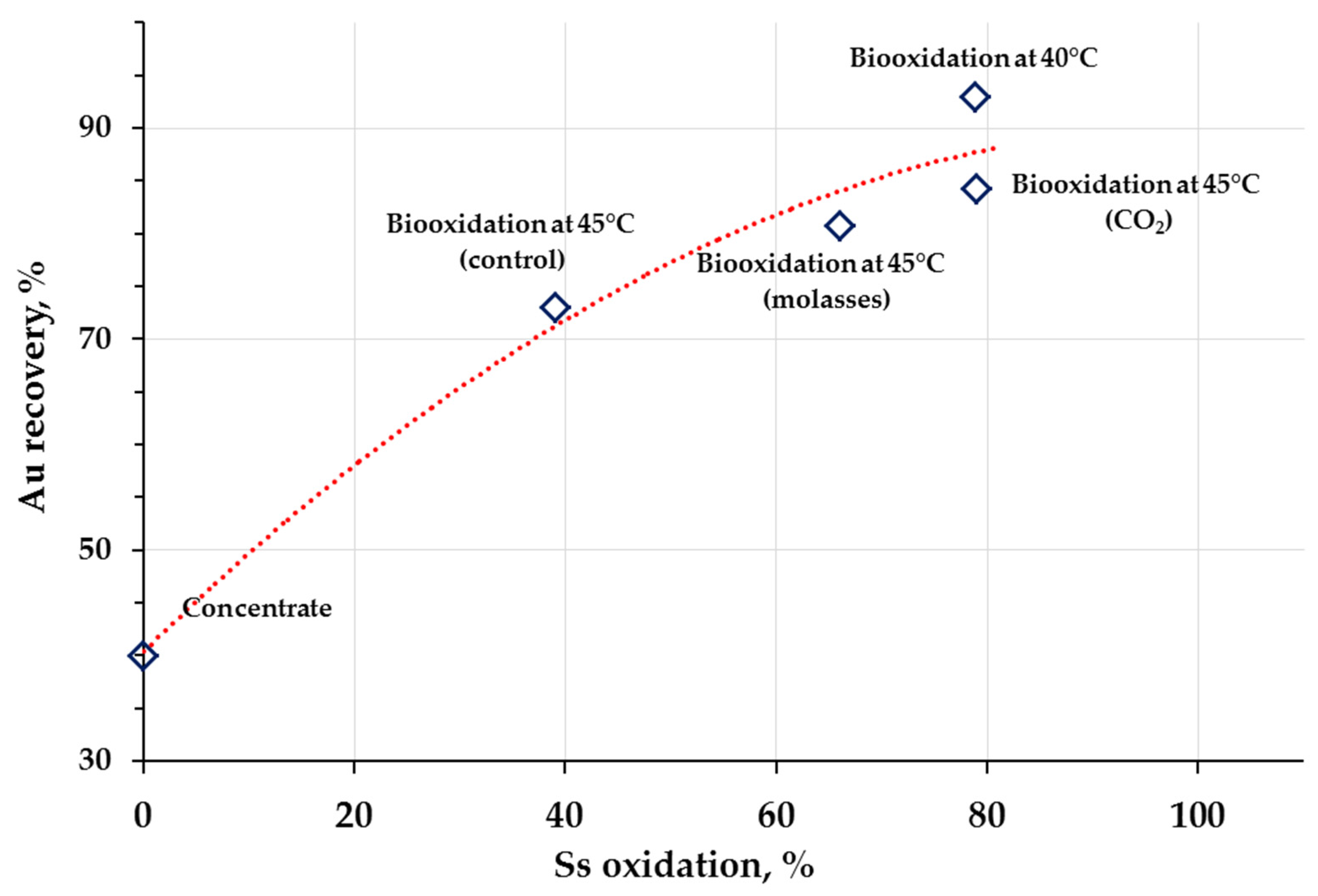
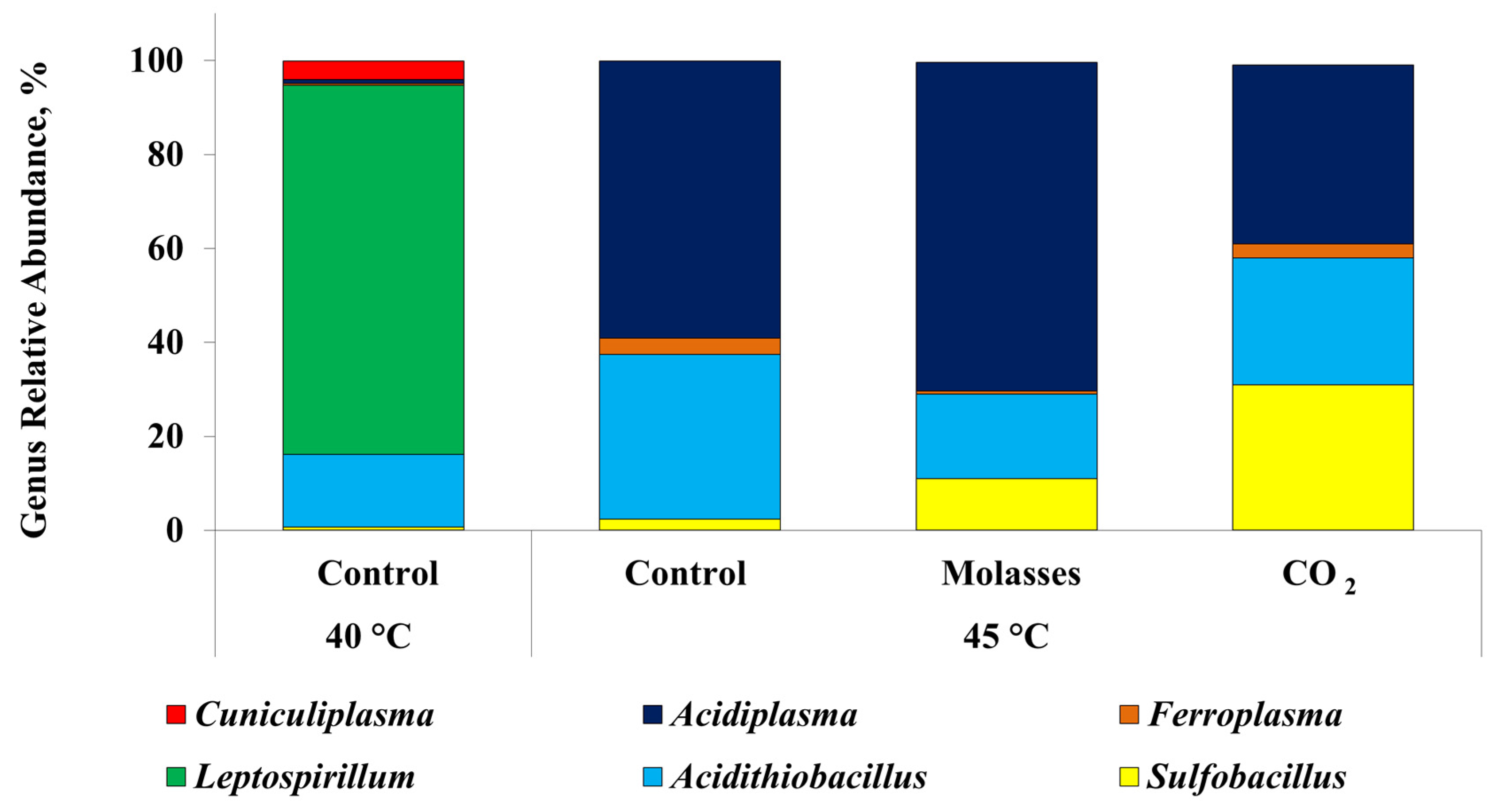
| Composition | Concentrate Composition (Main Sulfide Minerals) | t, °C | Reference |
|---|---|---|---|
| Acidithiobacilluss caldus, Leptospirillum ferriphilum | Pyrite and arsenopyrite | 40 | [10,11] |
| A. caldus, L. ferriphilum, Sulfobacillus sp., Ferroplasma sp. | Pyrrhotite, chalcopyrite, sphalerite | 45 | [12] |
| A. caldus, Sulfobacillus thermosulfidooxidans, “Sulfobacillus montserratensis” | Pyrite, arsenopyrite, chalcopyrite | 45 | [13] |
| A. caldus, L. ferriphilum, F. acidiphilum, S. benefaciens | Pyrite | 42 | [5] |
| A. caldus, L. ferriphilum, S. thermosulfidooxidans, S. benefaciens | Chalcopyrite | 42 | [14] |
| A. caldus, L. ferriphilum, S. thermosulfidooxidans, | Chalcopyrite | 42 | [15] |
| A. caldus, L. ferriphilum, Sulfobacillus sp. TPY, F. thermophilum | Chalcopyrite | 45 | [16] |
| A. caldus, L. ferriphilum, Sulfobacillus sp., Ferroplasma sp., Acidiplasma sp. | Pyrite and arsenopyrite | 40–50 | [17] |
| A. caldus, A. ferrooxidans, L. ferriphilum, S. thermosulfidooxidans, F. acidiphilum | Pyrite, arsenopyrite and pyrrhotite | 35 | [18] |
| A. caldus, Sulfobacillus sp., Acidiplasma sp. | Pyrite and arsenopyrite | 45 | [19] |
| A. caldus, S. acidophilus, F. thermophilum | Chalcopyrite | 45 | [20] |
| A. caldus, L. ferriphilum, S. thermosulfidooxidans, S. benefaciens | Chalcopyrite | 42 | [21] |
| A. thiooxidans, Acidiphilium multivorum, Acidiferrobacter thiooxidans, L. ferriphilum, F. acidiphilum | Pyrite, arsenopyrite and pyrrhotite | 39–42 | [22] |
| A. caldus, S. benefaciens, F. acidiphilum, Cuniculiplasma divulgatum | Pyrite, chalcopyrite, tennantite, sphalerite | 40 | [23] |
| Component | Content, % |
|---|---|
| SiO2 | 36.7 |
| Al2O3 | 15.9 |
| CaO | 2.9 |
| Stot | 18.3 |
| Ss | 18.3 |
| Fetot | 20.0 |
| Astot | 7.25 |
| Au, g/t | 62.35 |
| Experiment | pH | Eh, mV | Concentration, g/L | H2SO4 Consumption, kg/t | |||
|---|---|---|---|---|---|---|---|
| Carbon Source | T, °C | Fe3+ | Fetotal | As | |||
| Air (control) * | 40 | 1.43 ± 0.02 | 799 ± 16 | 16.2 ± 0.8 | 16.2 ± 0.8 | 6.7 ± 0.2 | 0 |
| Air (control) ** | 45 | 1.71 ± 0.1 | 656 ± 22 | 2.8 ± 0.7 | 3.9 ± 0.8 | 2.3 ± 0.2 | 90 |
| Molasses ** | 1.61 ± 0.04 | 735 ± 9 | 7.9 ± 0.7 | 8.0 ± 0.7 | 2.6 ± 0.05 | 0 | |
| CO2 ** | 1.40 ± 0.05 | 788 ± 20 | 20.3 ± 1.1 | 20.4 ± 1.1 | 4.5 ± 0.3 | 0 | |
| Experiment | Mass Yield, % | Content, % | Ss oxidation, % | |||
|---|---|---|---|---|---|---|
| Carbon Source | T, °C | Fe | As | Ss | ||
| Aeration (control) * | 40 | 66.5 | 9.6 | 3.1 | 6.3 | 78.9 |
| Aeration (control) ** | 45 | 77 | 17.4 | 5.2 | 14.2 | 39.1 |
| Molasses ** | 73 | 14.6 | 4.6 | 8.5 | 66.0 | |
| CO2 ** | 60 | 10.6 | 3.1 | 6.4 | 79.0 | |
| Microorganisms | Electron Donor | Temperature, °C (Optimum/Upper Limit) | Carbon Nutrition |
|---|---|---|---|
| Bacteria of the genus Leptospirillum | Fe2+ | 28–50/45–60 | Autotroph |
| Acidithiobacillus caldus | S0 | 45/52 | Autotroph |
| Bacteria of the genus Sulfobacillus | Fe2+, S0 | 38–55/55–60 | Mixotroph |
| Archea of the genus Ferroplasma | Fe2+ | 35–42/45–51 | Mixotrophs, heterotrophs |
| Archea of the genus Acidiplasma | Fe2+, S0 | 45–54/60–65 | Mixotrophs, heterotrophs |
| Archea of the genus Cuniculiplasma | Organic compounds | 37–40/45–48 | Heterotrophs |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bulaev, A.; Boduen, A. Carbon Sources as a Factor Determining the Activity of Microbial Oxidation of Sulfide Concentrate at Elevated Temperature. Minerals 2022, 12, 110. https://doi.org/10.3390/min12020110
Bulaev A, Boduen A. Carbon Sources as a Factor Determining the Activity of Microbial Oxidation of Sulfide Concentrate at Elevated Temperature. Minerals. 2022; 12(2):110. https://doi.org/10.3390/min12020110
Chicago/Turabian StyleBulaev, Aleksandr, and Anna Boduen. 2022. "Carbon Sources as a Factor Determining the Activity of Microbial Oxidation of Sulfide Concentrate at Elevated Temperature" Minerals 12, no. 2: 110. https://doi.org/10.3390/min12020110
APA StyleBulaev, A., & Boduen, A. (2022). Carbon Sources as a Factor Determining the Activity of Microbial Oxidation of Sulfide Concentrate at Elevated Temperature. Minerals, 12(2), 110. https://doi.org/10.3390/min12020110






