Elaboration of a Phytoremediation Strategy for Successful and Sustainable Rehabilitation of Disturbed and Degraded Land
Abstract
:1. Introduction
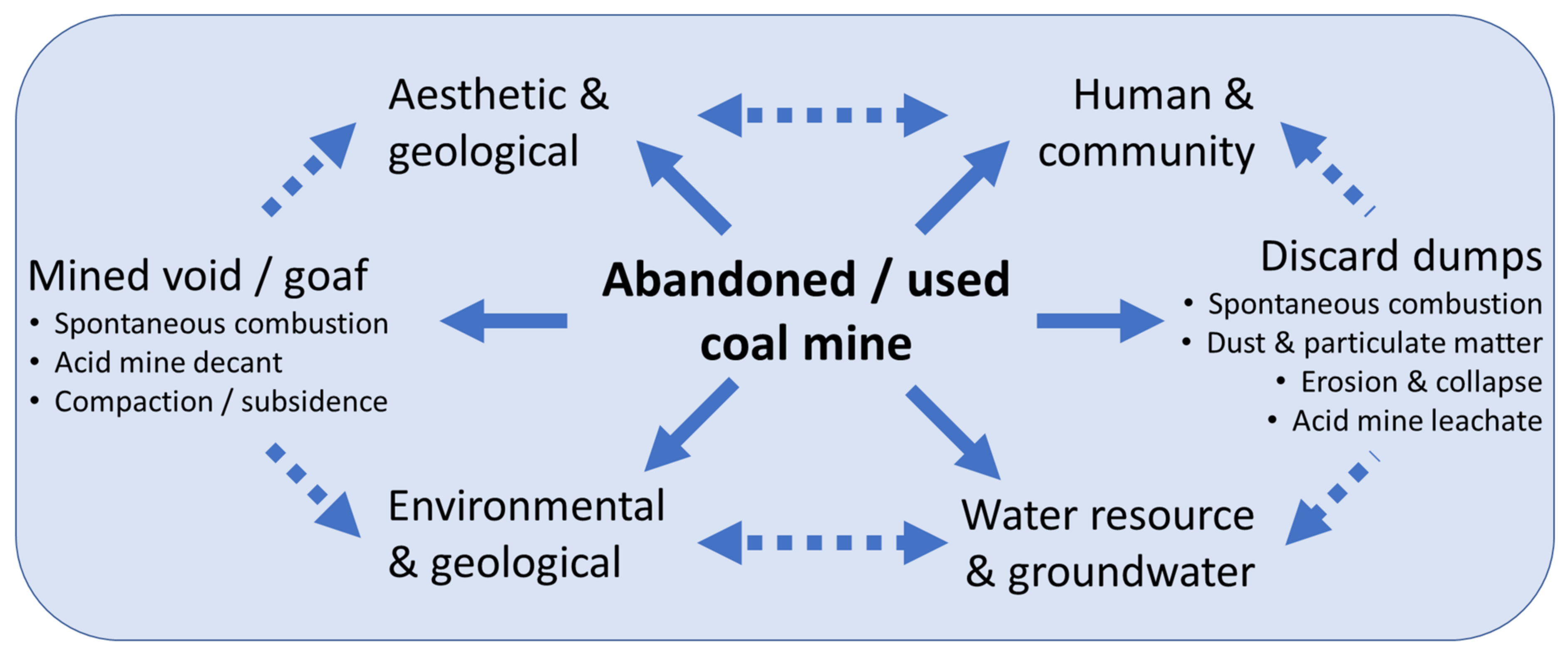
2. Impact of Mining-Induced Land Disturbance and Appropriate Stewardship
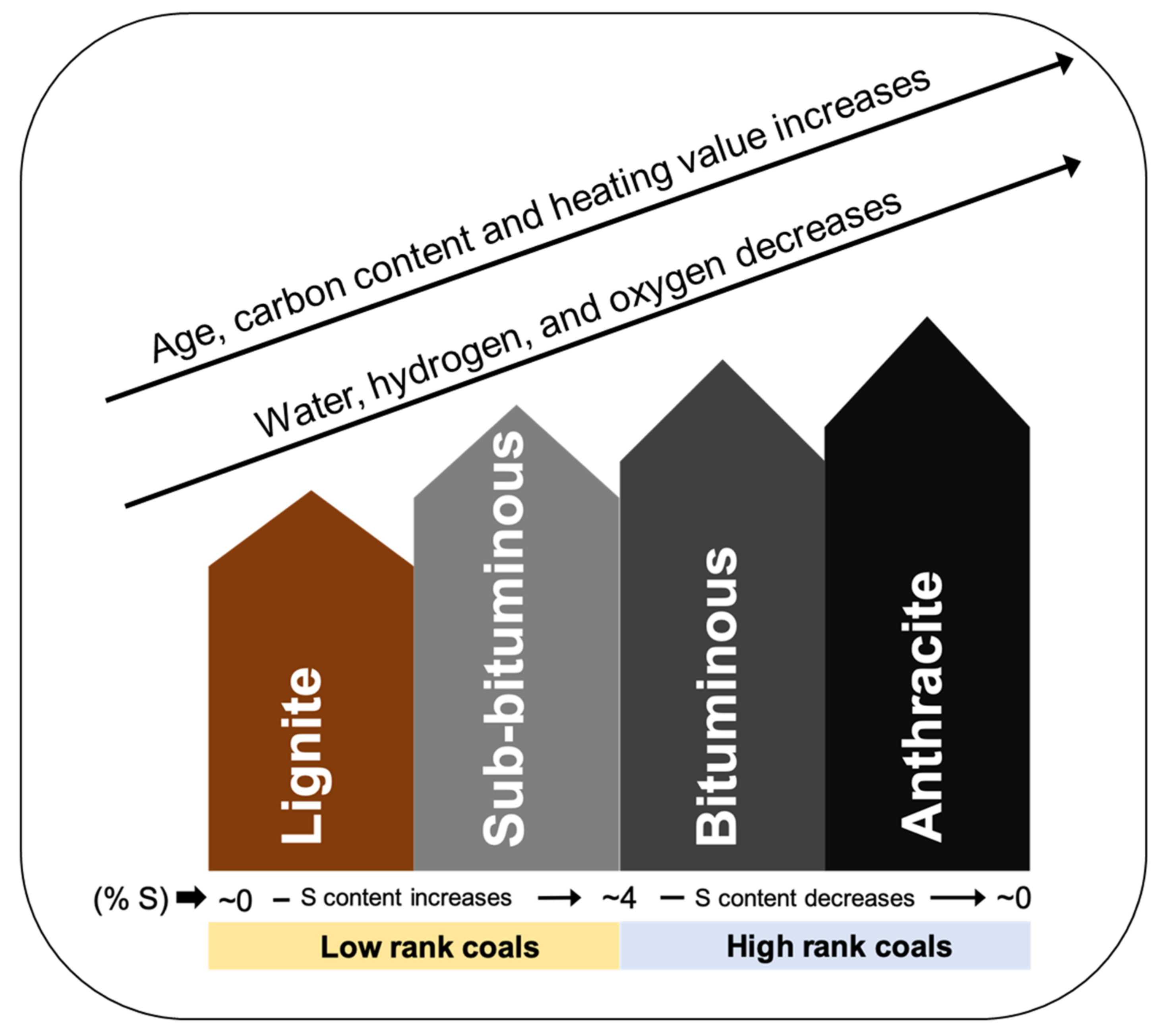
3. Biological Processing of Coal and Coal By-Product
3.1. Coal Bio-Liquefaction
3.2. Bio-Conversion of Coal to Methane
3.3. Humic Substance Production
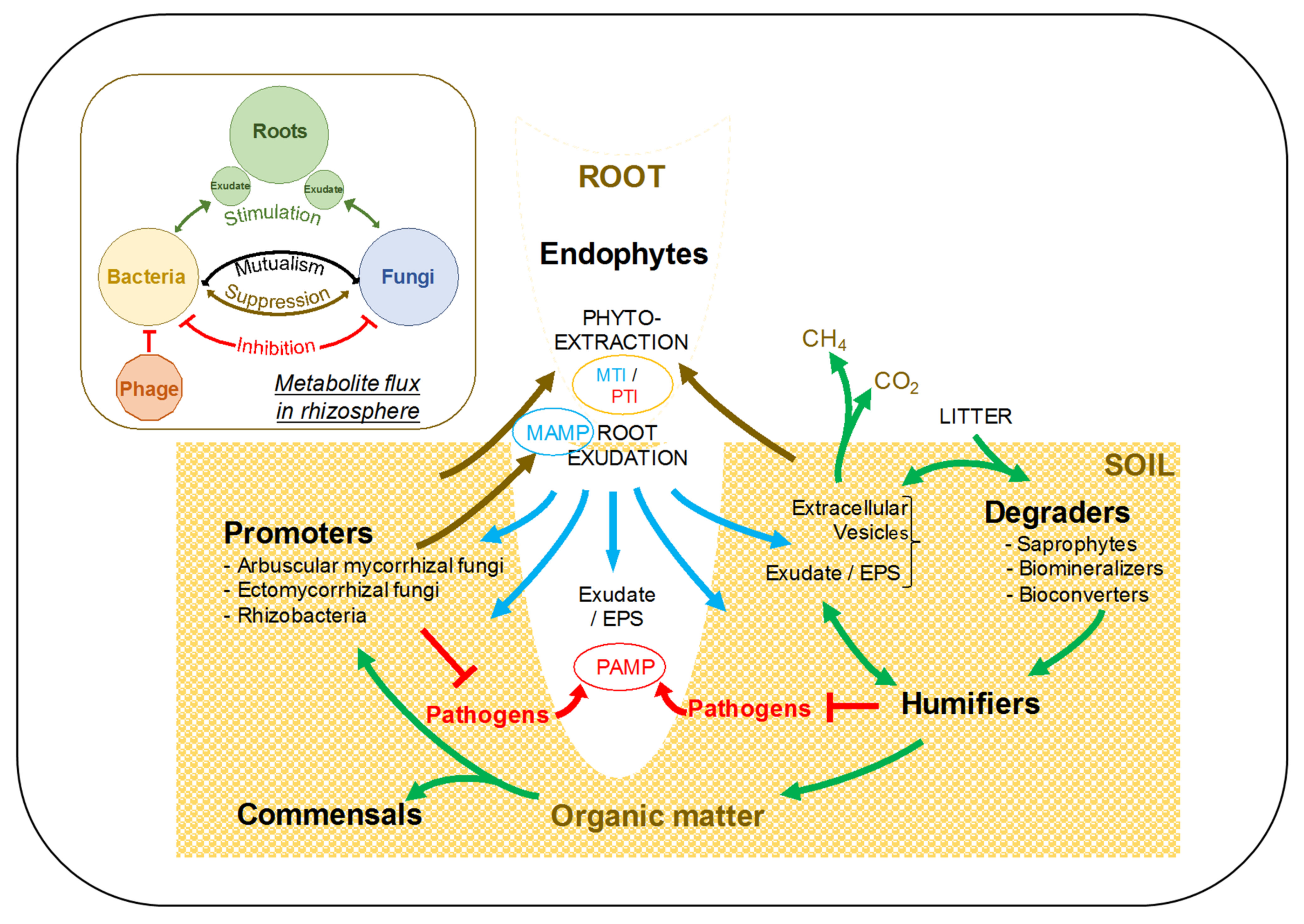
4. Phytoremediation: A Strategy for Successful and Sustainable Reclamation
4.1. On the Origin, Supramolecular Aggregation, and Mode of Action of Humic Substances
4.2. Humification: A Dynamic Equilibrium That Sustains Soil Organic Matter
4.3. Mycorrhizal Fungi and Plant Growth Promoting Rhizobacteria: Essential Biocatalysts for Successful Phytoremediation
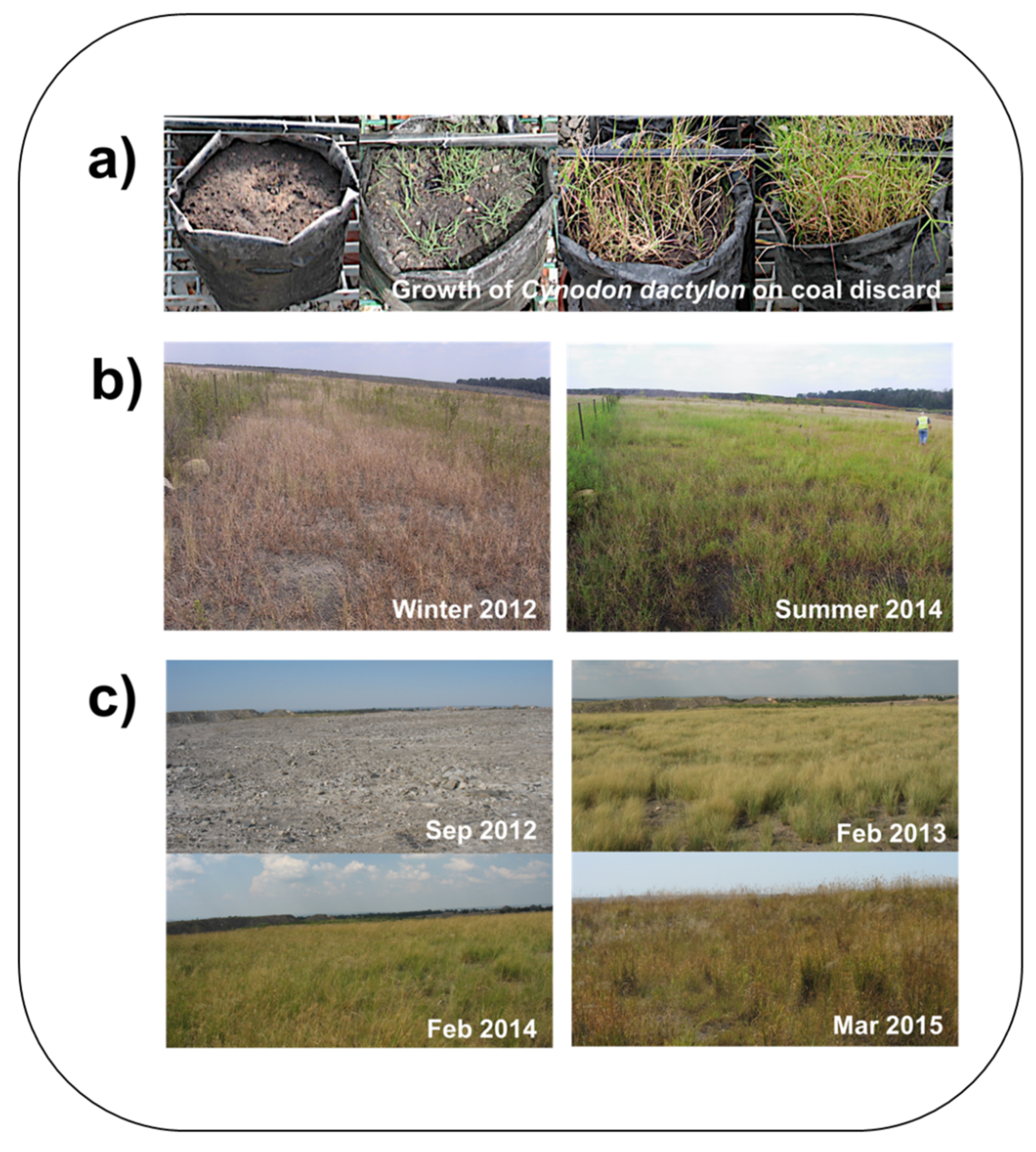
4.4. Towards Sustainable Rehabilitation: The Fungcoal Process
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pone, J.D.N.; Hein, K.A.A.; Stracher, G.B.; Annegarn, H.J.; Robert, B. The spontaneous combustion of coal and its by-products in the Witbank and Sasolburg coalfields of South Africa. Int. J. Coal Geol. 2007, 72, 124–140. [Google Scholar] [CrossRef]
- Eberhard, A. The future of South African coal: Market, investment, and policy challenges. Program Energy Sustain. Dev. 2011, 20, 1–30. [Google Scholar] [CrossRef]
- Simpson, G.B.; Badenhorst, J.; Jewitt, G.P.W.; Berchner, M.; Davies, E. Competition for land: The water-energy-food nexus and coal mining in Mpumalanga Province, South Africa. Front. Environ. Sci. 2019, 7, 86. [Google Scholar] [CrossRef]
- Bureau for Food and Agricultural Policy. The BFAP Baseline—Agricultural Outlook 2012–2021; BFAP: Pretoria, South Africa, 2012; p. 112. Available online: http://www.bfap.co.za/wp-content/uploads/2018/08/BFAP_Baseline_2012.pdf (accessed on 16 March 2021).
- Bureau for Food and Agricultural Policy. Evaluating the Impact of Coal Mining on Agriculture in the Delmas, Ogies and Leandra Districts: A Focus on Maize Production. Report Compiled for the Maize Trust; BFAP: Pretoria, South Africa, 2012; p. 47. Available online: https://www.bfap.co.za/the-impact-of-coal-mining-on-agriculture/ (accessed on 17 March 2021).
- Du Preez, C.C.; van Huyssteen, C.W.; Mnkeni, P.N.S. Land Use and soil organic matter in South Africa 1: A review on spatial variability and the influence of rangeland stock production. S. Afr. J. Sci. 2011, 107, 354. [Google Scholar] [CrossRef]
- Du Preez, C.C.; van Huyssteen, C.W.; Mnkeni, P.N.S. Land use and soil organic matter in South Africa 2: A review on the influence of arable crop production. S. Afr. J. Sci. 2011, 107, 358. [Google Scholar] [CrossRef] [Green Version]
- Sanderman, J.; Hengl, T.; Fiske, G.J. Soil carbon debt of 12,000 years of human land use. Proc. Natl. Acad. Sci. USA 2017, 114, 9575–9580. [Google Scholar] [CrossRef] [Green Version]
- Calinger, K.; Calhoon, E.; Chang, H.-C.; Whitacre, J.; Wenzel, J.; Comita, L.; Queenborough, S. Historic mining and agriculture as indicators of occurrence and abundance of widespread invasive plant species. PLoS ONE 2015, 10, e0128161. [Google Scholar] [CrossRef] [PubMed]
- Corbett, E.; Anderson, R.; Rodgers, C. Prairie revegetation of a strip mine in Illinois: Fifteen years after establishment. Restor. Ecol. 1996, 4, 346–354. [Google Scholar] [CrossRef]
- Adibee, N.; Osanloo, M.; Rahmanpour, M. Adverse effects of coal mine waste dumps on the environment and their management. Environ. Earth Sci. 2013, 70, 1581–1592. [Google Scholar] [CrossRef]
- Dhar, S.B.; Dutta, M. Changing land use pattern in the Raniganj Coal Belt and its sustainable management: A case study of Mangalpur Opencast Colliery. Curr. World Environ. 2020, 15, 76–88. [Google Scholar] [CrossRef]
- Langkamp, P.J. Potential conflict between the coal and arable land resources in Australia: A case for corporate responsiveness. Environ. Manag. 1985, 9, 49–60. [Google Scholar] [CrossRef]
- Oskarsson, P.; Lahiri-Dutt, K.; Wennström, P. From incremental dispossession to a cumulative land grab: Understanding territorial transformation in India’s North Karanpura Coalfield. Dev. Change 2019, 50, 1485–1508. [Google Scholar] [CrossRef] [Green Version]
- Brevik, E.C.; Sauer, T.J. The past, present, and future of soils and human health studies. Soil 2015, 1, 35–46. [Google Scholar] [CrossRef] [Green Version]
- Phillips, H.A. Pollution of the atmosphere. Nature 1882, 27, 127. [Google Scholar] [CrossRef]
- Sharma, A.; Sumbali, G. Ecobiology of coal mines and spoils. J. Appl. Nat. Sci. 2019, 11, 624–631. [Google Scholar] [CrossRef]
- Simpson, G.B.; Jewitt, G.P.W. The development of the water-energy-food nexus as a framework for achieving resource security: A Review. Front. Environ. Sci. 2019, 7, 8. [Google Scholar] [CrossRef] [Green Version]
- Katzur, J.; Haubold-Rosar, M. Amelioration and reforestation of sulfurous mine soils in Lusatia (Eastern Germany). Water Air Soil Pollut. 1996, 91, 17–32. [Google Scholar] [CrossRef]
- Li, M.S. Ecological restoration of mineland with particular reference to the metalliferous mine wasteland in China: A review of research and practice. Sci. Total Environ. 2006, 357, 38–53. [Google Scholar] [CrossRef]
- Truter, W.J.; Rethman, N.F.G.; Potgieter, C.E.; Kruger, R.A. Re-vegetation of cover soils and coal discard material ameliorated with Class F fly ash. In Proceedings of the Collected Abstracts, 2009 World of Coal Ash (WOCA) Conference, Lexington, KY, USA, 9–12 May 2011; Available online: http://energy.caer.uky.edu/AshSymposium/Agenda09.asp (accessed on 30 June 2020).
- Cowan, A.K.; Lodewijks, H.M.; Sekhohola, L.M.; Edeki, O.G. In situ bioremediation of South African coal discard dumps. In Proceedings of the Mine Closure 2016 11th International Conference on Mine Closure, Perth, Australia, 15–17 March 2016; Fourie, A.B., Tibbett, M., Eds.; Australian Centre for Geomechanics: Perth, Australia, 2016; pp. 501–509. [Google Scholar] [CrossRef] [Green Version]
- Lima, A.T.; Mitchella, K.; O’Connella, D.W.; Verhoeven, J.; Van Cappellen, P. The legacy of surface mining: Remediation, restoration, reclamation and rehabilitation. Environ. Sci. Policy 2016, 66, 227–233. [Google Scholar] [CrossRef]
- Kundu, N.K.; Ghose, M.K. Shelf life of stock-piled topsoil of an opencast coal mine. Environ. Conser. 1997, 24, 24–30. [Google Scholar] [CrossRef]
- Glaser, B.; Lehmann, J.; Zech, W. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal—A review. Biol. Fert. Soils 2002, 35, 219–230. [Google Scholar] [CrossRef]
- Stahl, P.D.; Williams, S.E.; Christensen, M. Efficacy of native vesicular-arbuscular mycorrhizal fungi after severe soil disturbance. New Phytol. 1988, 110, 347–354. [Google Scholar] [CrossRef]
- Claassens, S.; Jansen Van Rensburg, P.J.; Van Rensburg, L. Soil microbial community structure of coal mine discard under rehabilitation. Water Air Soil Pollut. 2006, 174, 355–366. [Google Scholar] [CrossRef]
- Claassens, S.; Riedel, K.J.; Van Rensburg, L.; Bezuidenhout, J.J.; Jansen van Rensburg, P.J. Microbial community function and structure on coal mine discard under rehabilitation. S. Afr. J. Plant Soil 2006, 23, 105–112. [Google Scholar] [CrossRef]
- Šourková, M.; Frouz, J.; Fettweis, U.; Bens, O.; Hüttl, R.; Šantrůčková, H. Soil development and properties of microbial biomass succession in reclaimed post mining sites near Sokolov (Czech Republic) and near Cottbus (Germany). Geoderma 2005, 129, 73–80. [Google Scholar] [CrossRef]
- Igbinigie, E.E.; Aktins, S.; Van Breugel, Y.; Van Dyke, S.; Davies-Coleman, M.T.; Rose, P.D. Fungal biodegradation of hard coal by a newly reported isolate, Neosartorya fischeri. Biotechnol. J. 2008, 3, 1407–1416. [Google Scholar] [CrossRef]
- Igbinigie, E.E.; Mutambanengwe, C.Z.; Rose, P.D. Phyto-bioconversion of hard coal in the Cyanodon dactylon/coal rhizosphere. Biotechnol. J. 2010, 5, 292–303. [Google Scholar] [CrossRef] [Green Version]
- Mukasa-Mugerwa, T.T.; Dames, J.F.; Rose, P.D. The role of a plant/fungal consortium in the degradation of bituminous hard coal. Biodegradation 2011, 22, 129–141. [Google Scholar] [CrossRef]
- Sekhohola, L.M.; Isaacs, M.L.; Cowan, A.K. Fungal colonization and enzyme-mediated metabolism of waste coal by Neosartorya fischeri strain ECCN 84. Biosci. Biotechnol. Biochem. 2014, 78, 1797–1802. [Google Scholar] [CrossRef]
- Sekhohola, L.M.; Cowan, A.K. Biological conversion of low-grade coal discard to a humic substance-enriched soil-like material. Int. J. Coal Sci. Technol. 2017, 4, 183–190. [Google Scholar] [CrossRef]
- Rose, P.D.; Igbinigie, E.E.; Mukasa-Mugerwa, T.; Dames, J. Beneficiation of Coal. S. Afr. Patent No. RSA2010/023542010, 26 October 2011. [Google Scholar]
- Lottermoser, B.G. Recycling, reuse and rehabilitation of mine wastes. Elements 2011, 7, 405–410. [Google Scholar] [CrossRef]
- Zásterová, P.; Marschalkoa, M.; Niemieca, D.; Durćáka, J.; Bulkob, R.; Vlček, J. Analysis of possibilities of reclamation waste dumps after coal mining. Procedia Earth Planet. Sci. 2015, 15, 656–662. [Google Scholar] [CrossRef] [Green Version]
- Nadudvari, A.A.; Kozielska, B.; Abramowicz, A.; Fabianska, M.; Ciesielczuk, J.; Cabała, J.; Krzykawski, T. Heavy metal- and organic-matter pollution due to self-heating coal-waste dumps in the Upper Silesian Coal Basin (Poland). J. Hazard. Mater. 2021, 412, 125244. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, K.; Mal, U. Evaluation of contamination of manganese in groundwater from overburden dumps of Lower Gondwana coal mines. Environ. Earth Sci. 2021, 80, 1–15. [Google Scholar] [CrossRef]
- Cogho, V.E. Optimum Coal Mine: Striving towards a ‘zero effluent’ mine. J. S. Afr. Inst. Min. Metall. 2012, 112, 119–126. [Google Scholar]
- Li, W.; Wang, D.; Li, H. Environmental engineering issues induced by abandoned coal mine hidden disasters. IOP Conf. Ser. Earth Environ. Sci. 2019, 237, 022039. [Google Scholar] [CrossRef]
- Agboola, O.; Babatunde, D.E.; Fayomi, O.S.I.; Sadiku, E.R.; Popoola, P.; Moropeng, L.; Yahaya, A.; Mamudu, O.-A. A review on the impact of mining operation: Monitoring, assessment and management. Results Eng. 2020, 8, 100181. [Google Scholar] [CrossRef]
- Cui, X.; Peng, S.; Lines, L.R.; Zhu, G.; Hu, Z.; Cui, F. Understanding the capability of an ecosystem nature-restoration in coal mined area. Sci. Rep. 2019, 9, 19690. [Google Scholar] [CrossRef]
- Jawarkar, A.A.; Jambhulkar, H.P. Phytoremediation of coal mine spoil dump through integrated biotechnology approach. Bioresour. Technol. 2008, 99, 4732–4741. [Google Scholar] [CrossRef]
- Pierwoła, J.; Ciesielczuk, J.; Misz-Kennan, M.; Fabiańska, M.J.; Bielińska, A.; Kruszewski, Ł. Structure and thermal history of the Wełnowiec Dump, Poland: A municipal dump rehabilitated with coal waste. Int. J. Coal Geol. 2018, 197, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Ran, Z.; Pan, Y.; Liu, W. Co-disposal of coal gangue and red mud for prevention of acid mine drainage generation from self-heating gangue dumps. Minerals 2020, 10, 1081. [Google Scholar] [CrossRef]
- Valero, N.; Melgarejo, L.M.; Ramírez, R. Effect of low-rank coal inoculated with coal solubilizing bacteria on edaphic materials used in post-coal-mining land reclamation: A greenhouse trial. Chem. Biol. Technol. Agric. 2016, 3, 20. [Google Scholar] [CrossRef] [Green Version]
- Coppin, N.J. An ecologist in mining—A retrospective of 40 years in mine closure and reclamation. In Proceedings of the Eighth International Seminar on Mine Closure; Tibbett, M., Fourie, A.B., Digby, C., Eds.; Australian Centre for Geomechanics: Crawley, Australia, 2013; pp. 295–310. [Google Scholar]
- Limpitlaw, D.; Briel, A. Post-mining land use opportunities in developing countries—A review. J. S. Afr. Inst. Min. Metall. 2015, 114, 1–5. [Google Scholar]
- Unger, C.J.; Everingham, J.A.; Bond, C.J. Transition or transformation: Shifting priorities and stakeholders in Australian mined land rehabilitation and closure. Australas. J. Environ. Manag. 2020, 27, 84–113. [Google Scholar] [CrossRef]
- Sekhohola, L.M.; Igbinigie, E.E.; Cowan, A.K. Biological degradation and solubilization of coal. Biodegradation 2013, 24, 305–318. [Google Scholar] [CrossRef]
- Ghani, M.J.; Rajoka, M.; Akhtar, K. Investigations in fungal solubilization of coal: Mechanisms and significance. Biotechnol. Bioprocess Eng. 2015, 20, 634–642. [Google Scholar] [CrossRef]
- Reich-Walber, M.; Meyrahn, H.; Lenz, U. Rheinbraun’s concept for power generation based on biotechnologically converted lignite. Fuel Process. Technol. 1997, 52, 267–277. [Google Scholar] [CrossRef]
- Hölker, U.; Mönkemann, H.; Höfer, M. A system to analyze the complex physiological states of coal solubilizing fungi. Fuel Process. Technol. 1997, 52, 65–71. [Google Scholar] [CrossRef]
- Mönkemann, H.; Hölker, U.; Höfer, M. Components of the ligninolytic system of Fusarium oxysporum and Trichoderma atroviride. Fuel Process. Technol. 1997, 52, 73–77. [Google Scholar] [CrossRef]
- Ralph, J.P.; Catcheside, D.E.A. Transformations of low rank coal by Phanerochaete chrysosporium and other wood-rot fungi. Fuel Process. Technol. 1997, 52, 79–93. [Google Scholar] [CrossRef]
- Fakoussa, R.; Frost, P. In vivo-decolorization of coal-derived humic acids by laccase-excreting fungus Trametes versicolor. Appl. Microbiol. Biotechnol. 1999, 52, 60–65. [Google Scholar] [CrossRef]
- Kwiatos, N.; Jędrzejczak-Krzepkowska, M.; Strzelecki, B.; Bielecki, S. Improvement of efficiency of brown coal biosolubilization by novel recombinant Fusarium oxysporum laccase. AMB Express 2018, 8, 133. [Google Scholar] [CrossRef] [PubMed]
- Kwiatos, N.; Jędrzejczak-Krzepkowska, M.; Krzemińska, A.; Delavari, A.; Paneth, P.; Bielecki, S. Evolved Fusarium oxysporum laccase expressed in Saccharomyces cerevisiae. Sci. Rep. 2020, 10, 3244. [Google Scholar] [CrossRef] [PubMed]
- Crawford, D.L.; Gupta, R.K. Characterization of extracellular bacterial enzymes which depolymerize a soluble lignite coal polymer. Fuel 1991, 70, 577–580. [Google Scholar] [CrossRef]
- Campbell, J.A.; Stewart, D.L.; McCulloch, M.; Lucke, R.B.; Bean, R.M. Biodegradation of coal-related model compounds. Am. Chem. Soc. Div. Fuel Chem. Prep. 1988, 33, 514. [Google Scholar]
- Hölker, U.; Schmiers, H.; Große, S.; Winkelhöfer, M.; Polsakiewicz, M.; Ludwig, S.; Dohse, J.; Höfer, M. Solubilization of low-rank coal by Trichoderma atroviride: Evidence for the involvement of hydrolytic and oxidative enzymes by using 14C-labelled lignite. J. Ind. Microbiol. Biotechnol. 2002, 28, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.L.; Yang, J.S.; Chen, W.X. Production of alkaline materials, surfactants and enzymes by Penicillium decumbens strain P6 in association with lignite degradation/solubilization. Fuel 2006, 85, 1378–1382. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, J.; Li, B.; Wang, E.; Yuan, H. An esterase from Penicillium decumbens P6 involved in lignite depolymerization. Fuel 2018, 214, 416–422. [Google Scholar] [CrossRef]
- Sudheer, P.D.V.N.; David, Y.; Chae, C.G.; Kim, Y.J.; Baylon, M.G.; Baritugo, K.-A.; Kim, T.W.; Kim, M.-S.; Na, J.G.; Park, S.J. Advances in the biological treatment of coal for synthetic natural gas and chemicals. Korean J. Chem. Eng. 2016, 33, 2788–2801. [Google Scholar] [CrossRef]
- Olawale, J.T.; Edeki, O.G.; Cowan, A.K. Bacterial degradation of coal discard and geologically weathered coal. Int. J. Coal Sci. Technol. 2020, 7, 405–416. [Google Scholar] [CrossRef] [Green Version]
- Ghani, M.J.; Akhtar, K.; Khaliq, S.; Akhtar, N.; Ghauri, M.A. Characterization of humic acids produced from fungal liquefaction of low-grade Thar coal. Process Biochem. 2021, 107, 1–12. [Google Scholar] [CrossRef]
- Gupta, R. Advanced coal characterization: A review. Energy Fuels 2007, 21, 451–460. [Google Scholar] [CrossRef]
- Ulrich, G.; Bower, S. Active methanogenesis and acetate utilization in Powder River Basin coals. United States. Int. J. Coal Geol. 2008, 76, 25–33. [Google Scholar] [CrossRef]
- Lozano, C.J.S.; Mendoza, M.V.; de Arango, M.C.; Monroy, E.F.C. Microbiological characterization and specific methanogenic activity of anaerobe sludges used in urban solid waste treatment. Waste Manag. 2009, 29, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Jani, K.; Thite, V.; Dhar, S.K.; Shouche, Y. Geochemistry shapes bacterial communities and their metabolic potentials in tertiary coalbed. Geomicrobiol. J. 2019, 36, 179–187. [Google Scholar] [CrossRef]
- Zhubanova, A.A.; Xiaohui, Q.; Ualieva, P.S.; Abdieva, G.Z.; Tastambek, K.T.; Kayrmanova, G.K.; Akimbekov, N.S. Metagenomic analysis reveals correlation between microbiome structure and Leonardite characteristics from Kazakhstan coal deposits. Eurasian Chem.-Technol. J. 2019, 21, 135–141. [Google Scholar] [CrossRef]
- Green, M.S.; Flanegan, K.C.; Gilcrease, P.C. Characterization of a methanogenic consortium enriched from a coalbed methane well in the Powder River Basin, U.S.A. Int. J. Coal Geol. 2008, 76, 34–45. [Google Scholar] [CrossRef]
- Harris, S.H.; Smith, R.L.; Barker, C.E. Microbial and chemical factors influencing methane production in laboratory incubations of low-rank subsurface coals. Int. J. Coal Geol. 2008, 76, 46–51. [Google Scholar] [CrossRef]
- Johnson, E.R.; Klasson, K.T.; Basu, R.; Volkwein, J.C.; Clausen, E.C.; Gaddy, J.L. Microbial conversion of high-rank coals to methane. Appl. Biochem. Biotechnol. 1994, 45–46, 329–338. [Google Scholar] [CrossRef]
- Volkwein, J.C.; Schoeneman, A.L.; Clausen, E.G.; Gaddy, J.L.; Johnson, E.R.; Basu, R.; Ju, N.; Klasson, K.T. Biological production of methane from bituminous coal. Fuel Process Technol. 1994, 40, 339–345. [Google Scholar] [CrossRef]
- Budwill, K. Microbial methanogenesis and its role in enhancing coalbed methane recovery. In CSEG Recorder; Alberta Research Council: Edmonton, AB, Canada, 2003. [Google Scholar]
- Jones, E.J.P.; Voytek, M.A.; Warwick, P.D.; Corum, M.D.; Cohn, A.; Bunnell, J.E.; Clark, A.C.; Orem, W.H. Bioassay for estimating the biogenic methane-generating potential of coal samples. Int. J. Coal Geol. 2008, 76, 138–150. [Google Scholar] [CrossRef]
- Panow, A.; FitzGerald, J.M.P.; Mainwaring, D.E. Mechanisms of biologically-mediated methane evolution from black coal. Fuel Process. Technol. 1997, 52, 115–125. [Google Scholar] [CrossRef]
- Pérez, M.; Romero, L.I.; Nebot, E.; Sales, D. Colonisation of a porous sintered-glass support in anaerobic thermophilic bioreactors. Bioresour. Technol. 1997, 59, 177–183. [Google Scholar] [CrossRef]
- Gupta, A.; Birendra, K. Biogasification of coal using different sources of microorganisms. Fuel 2000, 79, 103–105. [Google Scholar] [CrossRef]
- Chang, B.V.; Chang, S.W.; Yuan, S.Y. Anaerobic degradation of polycyclic aromatic hydrocarbons in sludge. Adv. Environ. Res. 2003, 7, 623–628. [Google Scholar] [CrossRef]
- Hazrin-Chong, N.H.; Marjo, C.E.; Das, T.; Rich, A.M.; Manefield, M. Surface analysis reveals biogenic oxidation of sub-bituminous coal by Pseudomonas fluorescens. Appl. Microbiol. Biotechnol. 2014, 98, 6443–6452. [Google Scholar] [CrossRef] [PubMed]
- Levania, M.; Cheema, S.; Sarma, P.M.; Ganapathi, R. Methanogenic potential of a thermophilic consortium enriched from coal mine. Int. Biodeterior. Biodegrad. 2014, 93, 177–185. [Google Scholar] [CrossRef]
- Valero, N.; Gómez, L.; Pantoja, M.; Ramirez, R. Production of humic substances through coal solubilizing bacteria. Braz. J. Microbiol. 2014, 45, 911–918. [Google Scholar] [CrossRef] [Green Version]
- Romanowska, I.; Strzelecki, B.; Bielecki, S. Biosolubilization of Polish brown coal by Gordonia alkanivorans S7 and Bacillus mycoides NS1020. Fuel Process. Technol. 2015, 131, 430–436. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Cui, X.; Zhang, Y.; Yu, Z. Bioconversion of coal to methane by microbial communities from soil and from an opencast mine in the Xilingol grassland of northeast China. Biotechnol. Biofuels 2019, 12, 236. [Google Scholar] [CrossRef] [Green Version]
- Opara, A.; Adams, D.; Free, M.L.; McLennan, J.; Hamilton, J. Microbial production of methane and carbon dioxide from lignite, bituminous coal, and coal waste materials. Int. J. Coal Geol. 2012, 96–97, 1–8. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, Y.; Pandey, R.; Harpalani, S. Characterizing microbial communities dedicated for conversion of coal to methane in-situ and ex-situ. Int. J. Coal Geol. 2015, 146, 145–154. [Google Scholar] [CrossRef] [Green Version]
- He, H.; Han, Y.; Jin, D.; Leng, Y.; Sun, Q.; Shen, L.; Tao, X. Microbial consortium in a non-production biogas coal mine of eastern China and its methane generation from lignite. Energy Source Part A 2016, 38, 1377–1384. [Google Scholar] [CrossRef]
- Mayumi, D.; Mochimaru, H.; Tamaki, H.; Yamamoto, K.; Yoshioka, H.; Suzuki, Y.; Kamagata, Y.; Sakata, S. Methane production from coal by a single methanogen. Science 2016, 354, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zhao, S.; Xia, D.; Wang, L.; Lv, J.; Yu, H.; Jiao, X. Efficient utilization of coal slime using anaerobic fermentation technology. Bioresour. Technol. 2021, 332, 125072. [Google Scholar] [CrossRef]
- O’Keefe, J.M.K.; Bechtel, A.; Christanis, K.; Dai, S.; DiMichele, W.A.; Eblef, C.F.; Esterle, J.S.; Mastalerz, M.; Raymond, A.L.; Valentim, B.V.; et al. On the fundamental difference between coal rank and coal type. Int. J. Coal Geol. 2013, 118, 58–87. [Google Scholar] [CrossRef]
- Giannouli, A.; Stavros, K.; Siavalas, G.; Chatziapostolou, A.; Christanis, K.; Papazisimou, S.; Papanicolaou, C.; Foscolos, A. Evaluation of Greek low-rank coals as potential raw material for the production of soil amendments and organic fertilizers. Int. J. Coal Geol. 2009, 477, 383–393. [Google Scholar] [CrossRef]
- Van de Venter, H.A.; Furter, M.; Dekker, J.; Cronje, I.J. Stimulation of seedling root growth by coal-derived sodium humate. Plant Soil 1991, 138, 17–21. [Google Scholar] [CrossRef]
- Janoš, P. Separation methods in the chemistry of humic substances. J. Chromatog. A 2003, 983, 1–18. [Google Scholar] [CrossRef]
- Zhou, L.; Yuan, L.; Zhao, B.; Li, Y.; Lin, Z. Structural characteristics of humic acids derived from Chinese weathered coal under different oxidizing conditions. PLoS ONE 2019, 14, e0217469. [Google Scholar] [CrossRef] [Green Version]
- Cheng, G.; Niu, Z.; Zhang, C.; Zhang, X.; Li, X. Extraction of humic acid from lignites by KOH-hydrothermal method. Appl. Sci. 2019, 9, 1356. [Google Scholar] [CrossRef] [Green Version]
- Yildirim, M.; Özbayoğlu, G. Production of ammonium nitrohumate from Elbistan lignite and its use as a coal binder. Fuel 1997, 76, 385–389. [Google Scholar] [CrossRef]
- Kurniati, E.; Muljani, S.; Virgani, D.G.; Neno, B.P. Humic acid isolations from lignite by ion exchange method. J. Phys. Conf. Ser. 2018, 953, 012234. [Google Scholar] [CrossRef]
- Doskočil, L.; Grasset, L.; Válková, D.; Pekar, M. Hydrogen peroxide oxidation of humic acids and lignite. Fuel 2014, 134, 406–413. [Google Scholar] [CrossRef]
- Chen, H.; Kim, H.U.; Weng, H.; Browse, J. Malonyl-CoA synthetase, encoded by ACYL ACTIVATING ENZYME13, is essential for growth and development of Arabidopsis. Plant Cell 2011, 23, 2247–2262. [Google Scholar] [CrossRef] [Green Version]
- Hofrichter, M.; Fakoussa, R.M. Microbial degradation and modification of coal. In Lignin, Humic Substances and Coal; Steinbüchel, A., Ed.; Wiley-VCH: Weinheim, Germany, 2004; pp. 399–425. [Google Scholar]
- David, Y.; Baylon, M.G.; Pamidimarri, S.D.V.N.; Baritugo, K.-A.; Chae, C.G.; Kim, Y.J.; Kim, T.W.; Kim, M.-S.; Na, J.G.; Park, S.J. Screening of microorganisms able to degrade low-rank coal in aerobic conditions: Potential coal biosolubilization mediators from coal to biochemicals. Biotechnol. Bioprocess Eng. 2017, 22, 178–185. [Google Scholar] [CrossRef]
- Akimbekov, N.; Digel, I.; Abdieva, G.; Ualieva, P.; Tastambek, K. Lignite biosolubilization and bioconversion by Bacillus sp.: The collation of analytical data. Biofuels 2021, 12, 247–258. [Google Scholar] [CrossRef]
- Akimbekov, N.; Digel, I.; Qiaoa, X.; Tastambeka, K.; Zhubanova, A. Lignite biosolubilization by Bacillus sp. RKB 2 and characterization of its products. Geomicrobiol. J. 2020, 37, 255–261. [Google Scholar] [CrossRef]
- Titilawo, Y.; Masudi, W.L.; Olawale, J.T.; Sekhohola-Dlamini, L.M.; Cowan, A.K. Coal-degrading bacteria display characteristics typical of plant growth promoting rhizobacteria. Processes 2020, 8, 1111. [Google Scholar] [CrossRef]
- KluIáková, M.; Pavlíková, M. Lignitic humic acids as environmentally-friendly adsorbent for heavy metals. J. Chem. 2017, 2017, 7169019. [Google Scholar] [CrossRef]
- De Souza, F.; Braganc, S.R. Extraction and characterization of humic acid from coal for the application as dispersant of ceramic powders. J. Mater. Res. Technol. 2018, 7, 254–260. [Google Scholar] [CrossRef]
- Akimbekov, N.; Qiao, X.; Digel, I.; Abdieva, G.; Ualieva, P.; Zhubanova, A. The Effect of Leonardite-derived amendments on soil microbiome structure and potato yield. Agriculture 2020, 10, 147. [Google Scholar] [CrossRef]
- Gong, G.; Xu, L.; Zhang, Y.; Liu, W.; Wang, M.; Zhao, Y.; Yuan, X.; Li, Y. Extraction of fulvic acid from lignite and characterization of its functional groups. ACS Omega 2020, 5, 27953–27961. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.J.; Oh, M.S.; Rehman, J.U.; Yoon, H.Y.; Kim, J.-H.; Shin, J.; Shin, S.G.; Bae, H.; Jeon, J.-R. Effects of microbes from coal-related commercial humic substances on hydroponic crop cultivation: A microbiological view for agronomical use of humic substances. J. Agric. Food Chem. 2021, 69, 805–814. [Google Scholar] [CrossRef]
- Yang, F.; Du, Q.; Sui, L.; Cheng, K. One-step fabrication of artificial humic acid-functionalized colloid-like magnetic biochar for rapid heavy metal removal. Bioresour. Technol. 2021, 328, 124825. [Google Scholar] [CrossRef]
- Wang, C.-F.; Fan, X.; Zhang, F.; Wang, S.-Z.; Zhao, Y.-P.; Zhao, X.-Y.; Zhao, W.; Zhu, T.-G.; Lu, J.-L.; Wei, X.-Y. Characterization of humic acids extracted from a lignite and interpretation for the mass spectra. RSC Adv. 2017, 7, 20677. [Google Scholar] [CrossRef] [Green Version]
- Perminova, I.V. From green chemistry and nature-like technologies towards ecoadaptive chemistry and technology. Pure Appl. Chem. 2019, 91, 851–864. [Google Scholar] [CrossRef]
- Bankowski, P.; Zou, L.; Hodges, R. A case study on stabilization and reuse of geopolymer-encapsulated brown coal fly ash. Int. J. Sus. Dev. Plann. 2006, 1, 76–90. [Google Scholar] [CrossRef]
- Mucsi, G.; Molnár, Z.; Kumar, S. Geopolymerisation of mechanically activated lignite and brown coal fly ash. Acta Phys. Pol. A 2014, 126, 994–998. [Google Scholar] [CrossRef]
- Yi, C.; Ma, H.; Chen, H.; Wang, J.; Shi, J.; Li, Z.; Yu, M. Preparation and characterization of coal gangue geopolymers. Constr. Build. Mater. 2018, 187, 318–326. [Google Scholar] [CrossRef]
- Lipczynska-Kochany, E. Humic substances, their microbial interactions and effects on biological transformations of organic pollutants in water and soil: A review. Chemosphere 2018, 202, 420–437. [Google Scholar] [CrossRef] [PubMed]
- Sachkova, A.S.; Kovel, E.S.; Churilov, G.N.; Stom, D.I.; Kudryasheva, N.S. Biological activity of carbonic nano-structures-comparison via enzymatic bioassay. J. Soils Sediments 2019, 19, 2689–2696. [Google Scholar] [CrossRef] [Green Version]
- Sondreal, E.A.; Wiltsee, G.A. Low-rank coal: Its present and future role in the United States. Annu. Rev. Energy 1984, 9, 473–479. [Google Scholar] [CrossRef]
- American Society for Testing and Materials. Annual Book of ASTM Standards, Section 5, Petroleum Products, Lubricants, and Fossil Fuels, v. 05.05, Gaseous Fuels; Coal and Coke; American Society for Testing and Materials: Philadelphia, PA, USA, 1999; pp. 155–584. [Google Scholar]
- Mochida, I.; Okuma, O.; Yoon, S.H. Chemicals from direct coal liquefaction. Chem. Rev. 2014, 114, 1637–1672. [Google Scholar] [CrossRef]
- Haider, K.; Martin, J.P. Synthesis and transformation of phenolic compounds by Epicoccum nigrum in relation to humic acid formation. Soil Sci. Soc. Am. J. 1967, 31, 766–772. [Google Scholar] [CrossRef]
- Crawford, D.L.; Gupta, R.K. Influence of cultural parameters on the depolymerization of a soluble lignite coal polymer by Pseudomonas cepacia DLC-071. Resour. Conserv. Recycl. 1991, 5, 245–254. [Google Scholar] [CrossRef]
- Macheroux, P.; Schmid, J.; Amrhein, N.; Schaller, A. A unique reaction in a common pathway: Mechanism and function of chorismate synthase in the shikimate pathway. Planta 1999, 207, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Gerke, J. Concepts and misconceptions of humic substances as the stable part of soil organic matter: A review. Agronomy 2018, 8, 76. [Google Scholar] [CrossRef] [Green Version]
- Hänninen, K. Historical and current progress in understanding the origin and structure of humic substances. Chem. Ecol. 2010, 26, 1–11. [Google Scholar] [CrossRef]
- Misztal, P.K.; Hewitt, C.N.; Wildt, J.; Blande, J.D.; Eller, A.S.D.; Fares, S.; Gentner, D.R.; Gilman, J.B.; Graus, M.; Greenberg, J.; et al. Atmospheric benzenoid emissions from plants rival those from fossil fuels. Sci. Rep. 2015, 5, 12064. [Google Scholar] [CrossRef] [Green Version]
- Dakora, F.D.; Phillips, D.A. Root exudates as mediators of mineral acquisition in low-nutrient environments. Plant Soil 2002, 245, 35–47. [Google Scholar] [CrossRef]
- Jin, C.W.; You, G.Y.; He, Y.F.; Tang, C.; Wu, P.; Zheng, S.J. Iron deficiency-induced secretion of phenolics facilitates the reutilization of root apoplastic iron in red clover. Plant Physiol. 2007, 144, 278–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zwetsloot, M.J.; Kessler, A.; Bauerle, T.L. Phenolic root exudate and tissue compounds vary widely among temperate forest tree species and have contrasting effects on soil microbial respiration. New Phytol. 2018, 218, 530–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zwetsloot, M.J.; Ucros, J.M.; Wickings, K.; Wilhelm, R.C.; Sparks, J.; Buckley, D.H.; Bauerle, T.L. Prevalent root-derived phenolics drive shifts in microbial community composition and prime decomposition in forest soil. Soil Biol. Biochem. 2020, 145, 107797. [Google Scholar] [CrossRef]
- Wu, D.; Wei, Z.M.; Qu, F.T.; Mohamed, T.A.; Zhu, L.J.; Zhao, Y.; Jia, L.M.; Zhao, R.; Liu, L.J.; Li, P. Effect of Fenton pretreatment combined with bacteria inoculation on humic substances formation during lignocellulosic biomass composting derived from rice straw. Bioresour. Technol. 2020, 303, 122849. [Google Scholar] [CrossRef]
- Wu, D.; Xia, T.; Zhang, Y.; Wei, Z.; Qu, F.; Zheng, G.; Song, C.; Zhao, Y.; Kang, K.; Yang, H. Identifying driving factors of humic acid formation during rice straw composting based on Fenton pretreatment with bacterial inoculation. Bioresour. Technol. 2021, 337, 125403. [Google Scholar] [CrossRef]
- Ye, Z.; Ding, H.; Yin, Z.; Ping, W.; Ge, J. Evaluation of humic acid conversion during composting under amoxicillin stress: Emphasizes the driving role of core microbial communities. Bioresour. Technol. 2021, 337, 125483. [Google Scholar] [CrossRef]
- Pollegioni, L.; Tonin, F.; Rosini, E. Lignin-degrading enzymes. FEBS J. 2015, 282, 1190–1213. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Wang, S.; Niu, Q.; Yan, H.; Li, G.; Zhu, Q.; Li, Q. Illite/smectite clay regulating laccase encoded genes to boost lignin decomposition and humus formation in composting habitats revealed by metagenomics analysis. Bioresour. Technol. 2021, 338, 125546. [Google Scholar] [CrossRef]
- Li, F.; Zhao, H.; Shao, R.; Zhang, X.; Yu, H. Enhanced Fenton reaction for xenobiotic compounds and lignin degradation fueled by quinone redox cycling by lytic polysaccharide monooxygenases. J. Agric. Food Chem. 2021, 69, 7104–7114. [Google Scholar] [CrossRef]
- Grinhut, T.; Hadar, Y.; Chen, Y. Degradation and transformation of humic substances by saprotrophic fungi: Processes and mechanisms. Fungal Biol. Rev. 2007, 21, 179–189. [Google Scholar] [CrossRef]
- Qi, H.; Zhai, W.; Du, Y.; Zhao, Y.; Wei, Z.; Wu, J.; Xie, X.; Yang, H.; Wu, D.; Guo, T. Core bacterial community driven conversion of fulvic acid components during composting with adding manganese dioxide. Bioresour. Technol. 2021, 337, 125495. [Google Scholar] [CrossRef] [PubMed]
- Kallenbach, C.M.; Frey, S.D.; Grandy, A.S. Direct evidence for microbial-derived soil organic matter formation and its ecophysiological controls. Nat. Commun. 2016, 7, 13630. [Google Scholar] [CrossRef] [PubMed]
- Baveye, P.C.; Wander, M. The (bio)chemistry of soil humus and humic substances: Why is the “new view” still considered novel after more than 80 years? Front. Environ. Sci. 2019, 7, 27. [Google Scholar] [CrossRef] [Green Version]
- Waksman, S.A. Humus. Origin, Chemical Composition, and Importance in Nature; The Williams & Wilkins Company: Baltimore, MD, USA, 1936; pp. 1–508. [Google Scholar]
- Salt, D.E.; Smith, R.D.; Raskin, I. Phytoremediation. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 643–668. [Google Scholar] [CrossRef]
- Pilon-Smits, E. Phytoremediation. Annu. Rev. Plant Biol. 2005, 56, 15–39. [Google Scholar] [CrossRef]
- Masciandaro, G.; Macci, C.; Ceccanti, B.; Doni, S. Organic matter–microorganism–plant in soil bioremediation: A synergic approach. Rev. Environ. Sci. Biotechnol. 2013, 12, 399419. [Google Scholar] [CrossRef]
- Kurade, M.B.; Ha, Y.-H.; Xiong, J.-Q.; Govindwar, S.P.; Jang, M.; Jeon, B.-H. Phytoremediation as a green biotechnology tool for emerging environmental pollution: A step forward towards sustainable rehabilitation of the environment. Chem. Eng. J. 2021, 415, 129040. [Google Scholar] [CrossRef]
- Wei, Z.; Van Le, Q.; Peng, W.; Yang, Y.; Yang, H.; Gu, H.; Lam, S.S.; Sonne, C. A review on phytoremediation of contaminants in air, water and soil. J. Hazard. Mater. 2021, 403, 123658. [Google Scholar] [CrossRef] [PubMed]
- Upcraft, T.; Guo, M. Phytoremediation value chains and modeling. In Sustainable Remediation of Contaminated Soil and Groundwater: Materials, Processes, and Assessment, 1st ed.; Hou, D., Ed.; Elsevier: Oxford, UK, 2020; pp. 325–366. [Google Scholar] [CrossRef]
- Naylor, D.; Sadler, N.; Bhattacharjee, A.; Graham, E.B.; Anderton, C.R.; McClure, R.; Lipton, M.; Hofmockel, K.S.; Jansson, J.K. Soil microbiomes under climate change and implications for carbon cycling. Annu. Rev. Environ. Resour. 2020, 45, 29–59. [Google Scholar] [CrossRef]
- Jansson, J.K.; Hofmockel, K.S. The soil microbiome—From metagenomics to metaphenomics. Curr. Opin. Microbiol. 2018, 43, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, W.W. Rhizosphere processes and management in plant-assist bioremediation (phytoremediation) of soils. Plant Soil 2009, 321, 385–408. [Google Scholar] [CrossRef]
- Brundrett, M.C. Coevolution of roots and mycorrhizas of land plants. New Phytol. 2002, 154, 275–304. [Google Scholar] [CrossRef] [PubMed]
- Vives-Peris, V.; de Ollas, C.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Root exudates: From plant to rhizosphere and beyond. Plant Cell Rep. 2020, 39, 3–17. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, J.-M. Plant immunity triggered by microbial molecular signatures. Mol. Plant 2010, 3, 783–793. [Google Scholar] [CrossRef] [Green Version]
- Pérez-de-Luque, A.; Tille, S.; Johnson, I.; Pascual-Pardo, D.; Ton, J.; Cameron, D.D. The interactive effects of arbuscular mycorrhiza and plant growth-promoting rhizobacteria synergistically enhance host plant defences against pathogens. Sci. Rep. 2017, 7, 16409. [Google Scholar] [CrossRef]
- Piccolo, A. The supramolecular structure of humic substances. Soil Sci. 2001, 166, 810–832. [Google Scholar] [CrossRef] [Green Version]
- Simpson, A.J.; Kingery, W.L.; Hayes, M.H.; Spraul, M.; Humpfer, E.; Dvortsak, P.; Kerssebaum, R.; Hofmann, M. Molecular structures and associations of humic substances in the terrestrial environment. Naturwissenschaften 2002, 89, 84–88. [Google Scholar] [CrossRef]
- Sutton, R.; Sposito, G. Molecular structure in soil humic substances: The new view. Environ. Sci. Technol. 2005, 39, 9009–9015. [Google Scholar] [CrossRef]
- Piccolo, A.; Nardi, S.; Concheri, G. Macromolecular changes of humic substances induced by interaction with organic acids. Eur. J. Soil Sci. 1996, 47, 319–328. [Google Scholar] [CrossRef]
- Piccolo, A.; Nardi, S.; Concheri, G. Micelle-like conformation of humic substances as revealed by size exclusion chromatography. Chemosphere 1996, 33, 595–602. [Google Scholar] [CrossRef]
- Nardi, S.; Reniero, F.; Concheri, G. Soil organic matter mobilization by root exudates of three maize hybrids. Chemosphere 1997, 35, 2237–2244. [Google Scholar] [CrossRef]
- Lehtonen, K.; Hänninen, K.; Ketola, M. Structurally bound lipids in peat humic acids. Org. Geochem. 2001, 32, 33–43. [Google Scholar] [CrossRef]
- Trubetskoj, O.A.; Richard, C.; Guyot, G.; Voyard, G.; Trubetskaya, O.E. Analysis of electrophoretic soil humic acids fractions by reversed-phase high performance liquid chromatography with on-line absorbance and fluorescence detection. J. Chromatog. A 2012, 1243, 62–68. [Google Scholar] [CrossRef]
- Trubetskoi, O.A.; Trubetskaya, O.E. Reversed-phase high-performance liquid chromatography of the stable electrophoretic fractions of soil humic acids. Eurasia Soil Sci. 2015, 48, 148–156. [Google Scholar] [CrossRef]
- Wershaw, R.L. A new model for humic materials and their interactions with hydrophobic organic chemicals in soil-water and sediment-water systems. J. Contam. Hydrol. 1986, 1, 29–45. [Google Scholar] [CrossRef]
- Semenov, V.M.; Tulina, A.S.; Semenova, N.A.; Ivannikova, L.A. Humification and nonhumification pathways of the organic matter stabilization in soil: A review. Eurasian Soil Sci. 2013, 46, 355–368. [Google Scholar] [CrossRef]
- Badri, D.V.; Vivanco, J.M. Regulation and function of root exudates. Plant Cell. Environ. 2009, 32, 666–681. [Google Scholar] [CrossRef]
- Jones, D.L.; Nguyen, C.; Finlay, R.D. Carbon flow in the rhizosphere: Carbon trading at the soil-root interface. Plant Soil 2009, 321, 5–33. [Google Scholar] [CrossRef]
- Canarini, A.; Kaiser, C.; Merchant, A.; Richter, A.; Wanek, W. Root exudation of primary metabolites: Mechanisms and their roles in plant responses to environmental stimuli. Front. Plant Sci. 2019, 10, 157. [Google Scholar] [CrossRef] [Green Version]
- Ivanov, S.; Austin, J.; Berg, R.H.; Harrison, M.J. Extensive membrane systems at the host-arbuscular mycorrhizal fungus interface. Nat. Plants 2019, 5, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Roth, R.; Hillmer, S.; Funaya, C.; Chiapello, M.; Schumacher, K.; Lo Presti, L.; Regine Kahmann, R.; Paszkowski, U. Arbuscular cell invasion coincides with extracellular vesicles and membrane tubules. Nat. Plants 2019, 5, 204–211. [Google Scholar] [CrossRef] [PubMed]
- De Palma, M.; Ambrosone, A.; Leone, A.; Del Gaudio, P.; Ruocco, M.; Turiák, L.; Bokka, R.; Fiume, I.; Tucci, M.; Pocsfalvi, G. Plant roots release small extracellular vesicles with antifungal activity. Plants 2020, 9, 1777. [Google Scholar] [CrossRef]
- Deatherage, B.L.; Cookson, B.T. Membrane vesicle release in bacteria, eukaryotes, and archaea: A conserved yet underappreciated aspect of microbial life. Infect. Immun. 2012, 80, 1948–1957. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Gao, J.; He, Y.; Jiang, L. Plant extracellular vesicles. Protoplasma 2020, 257, 3–12. [Google Scholar] [CrossRef]
- Panepinto, J.; Komperda, K.; Frases, S.; Park, Y.-D.; Djordjevic, J.T.; Casadevall, A.; Williamson, P.R. Sec6-dependent sorting of fungal extracellular exosomes and laccase of Cryptococcus neoformans. Mol. Microbiol. 2009, 71, 1165–1176. [Google Scholar] [CrossRef]
- Da Silva, R.P.; Puccia, R.; Rodrigues, M.L.; Oliveira, D.L.; Joffe, L.S.; César, G.V.; Nimrichter, L.; Goldenberg, S.; Alves, L.R. Extracellular vesicle-mediated export of fungal RNA. Sci. Rep. 2015, 5, 7763. [Google Scholar] [CrossRef] [Green Version]
- Samuel, M.; Bleackley, M.; Anderson, M.; Mathivanan, S. Extracellular vesicles including exosomes in cross kingdom regulation: A viewpoint from plant-fungal interactions. Front. Plant Sci. 2015, 6, 766. [Google Scholar] [CrossRef] [Green Version]
- Herkert, P.F.; Amatuzzi, R.F.; Alves, L.R.; Rodrigues, M.L. Extracellular vesicles as vehicles for the delivery of biologically active fungal molecules. Curr. Protein Pept. Sci. 2019, 20, 1027–1036. [Google Scholar] [CrossRef]
- Rizzo, J.; Rodrigues, M.L.; Janbon, G. Extracellular vesicles in fungi: Past, Present, and future perspectives. Front. Cell Infect. Microbiol. 2020, 10, 346. [Google Scholar] [CrossRef]
- Sakiyama, T.; Ueno, H.; Homma, H.; Numata, O.; Kuwabara, T. Purification and characterization of a hemolysin-like protein, Sll1951, a nontoxic member of the rtx protein family from the cyanobacterium Synechocystis sp. strain PCC 6803. J. Bacteriol. 2006, 188, 3535–3542. [Google Scholar] [CrossRef] [Green Version]
- Joffe, L.S.; Nimrichter, L.; Rodrigues, M.L.; Del Poeta, M. Potential roles of fungal extracellular vesicles during infection. mSphere 2016, 1, e00099-16. [Google Scholar] [CrossRef] [Green Version]
- Albuquerque, P.C.; Nakayasu, E.S.; Rodrigues, M.L.; Frases, S.; Casadevall, A.; Zancope-Oliveira, R.M.; Almeida, I.C.; Nosanchuk, J.D. Vesicular transport in Histoplasma capsulatum: An effective mechanism for trans-cell wall transfer of proteins and lipids in ascomycetes. Cell Microbiol. 2008, 10, 1695–1710. [Google Scholar] [CrossRef] [Green Version]
- Deng, Z.; Rong, Y.; Teng, Y.; Mu, J.; Zhuang, X.; Tseng, M.; Samykutty, A.; Zhang, L.; Yan, J.; Miller, D.; et al. Broccoli-derived nanoparticle inhibits mouse colitis by activating dendritic cell AMP-activated protein kinase. Mol. Ther. 2017, 25, 1641–1654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, G.; Kang, G.; Wang, S.; Huang, Y.; Cai, Q. Extracellular vesicles: Emerging players in plant defense against pathogens. Front. Plant Sci. 2021, 12, 757925. [Google Scholar] [CrossRef] [PubMed]
- Regente, M.; Pinedo, M.; San Clemente, H.; Balliau, T.; Jamet, E.; de la Canal, L. Plant extracellular vesicles are incorporated by a fungal pathogen and inhibit its growth. J. Exp. Bot. 2017, 68, 5485–5495. [Google Scholar] [CrossRef] [PubMed]
- Hansen, L.L.; Nielsen, M.A. Plant exosomes: Using an unconventional exit to prevent pathogen entry? J. Exp. Bot. 2018, 69, 59–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meldolesi, J. Exosomes and ectosomes in intercellular communication. Curr. Biol. 2018, 28, R435–R444. [Google Scholar] [CrossRef] [Green Version]
- Teng, Y.; Ren, Y.; Sayed, M.; Hu, X.; Lei, C.; Kumar, A.; Hutchins, E.; Mu, J.; Deng, Z.; Luo, C.; et al. Plant-derived exosomal microRNAs shape the gut microbiota. Cell Host Microbe 2018, 24, 637–652.e8. [Google Scholar] [CrossRef] [Green Version]
- Combarnous, Y.; Nguyen, T.M.D. Cell communications among microorganisms, plants, and animals: Origin, evolution, and interplays. Int. J. Mol. Sci. 2020, 21, 8052. [Google Scholar] [CrossRef]
- Vincent, D.; Rafiqi, M.; Job, D. The multiple facets of plant–fungal interactions revealed through plant and fungal secretomics. Front. Plant Sci. 2020, 10, 1626. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.; Catchpole, R.; Forterre, P. Extracellular membrane vesicles in the three domains of life and beyond. FEMS Microbiol. Rev. 2019, 43, 273–303. [Google Scholar] [CrossRef] [PubMed]
- Canellas, L.P.; Olivares, F.L. Physiological responses to humic substances as plant growth promoter. Chem. Biol. Technol. Agric. 2014, 1, 3. [Google Scholar] [CrossRef] [Green Version]
- Susic, M. Replenishing humic acids in agricultural soils. Agronomy 2016, 6, 45. [Google Scholar] [CrossRef] [Green Version]
- Wershaw, R.L. Evaluation of conceptual models of natural organic matter (humus) from a consideration of the chemical and biochemical processes of humification. US Geol. Surv. Sci. Investig. Rep. 2004, 5121, 44. Available online: http://pubs.usgs.gov/sir/2004/5121/pdf/sir2004-5121.pdf (accessed on 29 April 2021).
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 61–68. [Google Scholar] [CrossRef]
- Tipping, E. WHAMC—A chemical equilibrium model and computer code for waters, sediments, and soils incorporating a discrete site/electrostatic model of ion-binding by humic substances. Comput. Geosci. 1994, 20, 973–1023. [Google Scholar] [CrossRef]
- Nardi, S.; Concheri, G.; Pizzeghello, D.; Sturaro, A.; Rella, R.; Parvoli, G. Soil organic matter mobilization by root exudates. Chemosphere 2000, 41, 653–658. [Google Scholar] [CrossRef]
- Piccolo, A. Special issue on: Humic molecules in soils. J. Geochem. Explor. 2013, 129, vii. [Google Scholar] [CrossRef]
- Puglisi, E.; Fragoulis, G.; Ricciuti, P.; Cappa, F.; Spaccini, R.; Piccolo, A.; Trevisan, M.; Crecchio, C. Effects of a humic acid and its size-fractions on the bacterial community of soil rhizosphere under maize (Zea mays L.). Chemosphere 2009, 77, 829–837. [Google Scholar] [CrossRef]
- Trevisan, S.; Francioso, O.; Quaggiotti, S.; Nardi, S. Humic substances biological activity at the plant-soil interface: From environmental aspects to molecular factors. Plant Signal. Behav. 2010, 5, 635–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamman, B.; Koning, G.; van de Venter, H.A. Cell-wall extension as a mode of action of coal-derived humates. S. Afr. J. Bot. 1999, 65, 197–202. [Google Scholar] [CrossRef] [Green Version]
- Elmongy, M.S.; Zhou, H.; Cao, Y.; Liu, B.; Xia, Y. The effect of humic acid on endogenous hormone levels and antioxidant enzyme activity during in vitro rooting of evergreen azalea. Sci. Hortic. 2018, 227, 234–243. [Google Scholar] [CrossRef]
- Galambos, N.; Compant, S.; Moretto, M.; Sicher, C.; Puopolo, G.; Wäckers, F.; Sessitsch, A.; Pertot, I.; Perazzolli, M. Humic acid enhances the growth of tomato promoted by endophytic bacterial strains through the activation of hormone-, growth-, and transcription-related processes. Front. Plant Sci. 2020, 11, 582267. [Google Scholar] [CrossRef] [PubMed]
- Shah, Z.H.; Rehman, H.M.; Akhtar, T.; Alsamadany, H.; Hamooh, B.T.; Mujtaba, T.; Daur, I.; Al Zahrani, Y.; Alzahrani, H.A.S.; Ali, S.; et al. Humic substances: Determining potential molecular regulatory processes in plants. Front. Plant Sci. 2018, 9, 263. [Google Scholar] [CrossRef] [Green Version]
- Adani, F.; Spagnol, M.; Genevini, P. Biochemical origin and refractory properties of humic acid extracted from the maize plant. Biogeochemistry 2006, 78, 85–96. [Google Scholar] [CrossRef]
- Widhalm, J.R.; Jaini, R.; Morgan, J.A.; Dudareva, N. Rethinking how volatiles are released from plant cells. Trends Plant Sci. 2015, 20, 545–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senesi, N.; Plaza, C.; Brunettia, G.; Polo, A. A comparative survey of recent results on humic-like fractions in organic amendments and effects on native soil humic substances. Soil Biol. Biochem. 2007, 39, 1244–1262. [Google Scholar] [CrossRef]
- Olaetxea, M.; De Hita, D.; Garcia, A.; Fuentes, M.; Baigorri, R.; Mora, V.; Garica, M.; Urrutia, O.; Erro, J.; Zamarreño, A.M.; et al. Hypothetical framework integrating the main mechanisms involved in the promoting action of rhizospheric humic substances on plant root- and shoot-growth. Appl. Soil Ecol. 2018, 123, 521–537. [Google Scholar] [CrossRef]
- Olk, D.C.; Bloom, P.R.; Perdue, E.M.; McKnight, D.M.; Chen, Y.; Farenhorst, A.; Harir, M. Environmental and agricultural relevance of humic fractions extracted by alkali from soils and natural waters. J. Environ. Qual. 2019, 48, 217–232. [Google Scholar] [CrossRef]
- Perminova, I.V.; García-Mina, J.-M.; Knicker, H.; Miano, T. Humic substances and nature-like technologies. Learning from nature: Understanding humic substances structures and interactions for the development of environmentally friendly, nature-like technologies. J. Soils Sediments 2019, 19, 2663–2664. [Google Scholar] [CrossRef] [Green Version]
- Bezuglova, O.S.; Gorovtsov, A.V.; Polienko, E.A.; Zinchenko, V.E.; Grinko, A.V.; Lykhman, V.A.; Dubinina, M.N.; Demidov, A. Effect of humic preparation on winter wheat productivity and rhizosphere microbial community under herbicide-induced stress. J. Soils Sediments 2019, 19, 2665–2675. [Google Scholar] [CrossRef]
- Santos, L.F.; Olivares, F.L. Plant microbiome structure and benefits for sustainable agriculture. Curr. Plant Biol. 2021, 26, 100198. [Google Scholar] [CrossRef]
- Nardi, S.; Pizzeghello, D.; Muscolo, A.; Vianello, A. Physiological effects of humic substances on higher plants. Soil Biol. Biochem. 2002, 34, 1527–1536. [Google Scholar] [CrossRef]
- Mora, V.; Bacaicoa, E.; Zamarreño, A.M.; Aguirre, E.; Garnica, M.; Fuentes, M.; GarcíaMina, J.M. Action of humic acid on promotion of cucumber shoot growth involves nitrate-related changes associated with the root-to-shoot distribution of cytokinins, polyamines and mineral nutrients. J. Plant Physiol. 2010, 167, 633–642. [Google Scholar] [CrossRef]
- García, A.C.; Souza, L.G.A.; Pereira, M.G.; Castro, R.N.; García-Mina, J.M.; Zonta, E.; Lisboa, F.J.G.; Berbara, R.L.L. Structure-property-function relationship in humic substances to explain the biological activity in plants. Sci. Rep. 2016, 6, 20798. [Google Scholar] [CrossRef] [Green Version]
- Jindo, K.; Olivares, F.L.; Malcher, D.J.P.; Sánchez-Monedero, M.A.; Kempenaar, C.; Canellas, L.P. From lab to field: Role of humic substances under open-field and greenhouse conditions as biostimulant and biocontrol agent. Front. Plant Sci. 2020, 11, 426. [Google Scholar] [CrossRef] [PubMed]
- Elmongy, M.S.; Wang, X.; Zhou, H.; Xia, Y. Humic acid and auxins induced metabolic changes and differential gene expression during adventitious root development in azalea microshoots. HortScience 2020, 55, 926–935. [Google Scholar] [CrossRef]
- García, A.C.; Castro, T.A.V.T.; Santos, L.A.; Tavares, O.C.H.; Castro, R.N.; Berbara, R.L.L.; García-Mina, J.M. Structure-property-function relationship of humic substances in modulating the root growth of plants: A review. J. Environ. Qual. 2019, 48, 1622–1632. [Google Scholar] [CrossRef]
- Castro, T.A.V.T.; Berbara, R.L.L.; Tavares, O.C.H.; da Graça Mello, D.F.; Pereira, E.G.; da Costa Barros de Souza, C.; Espinosa, L.M.; García, A.C. Humic acids induce a eustress state via photosynthesis and nitrogen metabolism leading to a root growth improvement in rice plants. Plant Physiol. Biochem. 2021, 162, 171–184. [Google Scholar] [CrossRef]
- Park, S.; Kim, K.S.; Kim, J.-T.; Kang, D.; Sung, K. Effects of humic acid on phytodegradation of petroleum hydrocarbons in soil simultaneously contaminated with heavy metals. J. Environ. Sci. 2011, 23, 2034–2041. [Google Scholar] [CrossRef]
- Chen, M.; Xu, P.; Zeng, G.; Yang, C.; Huang, D.; Zhang, J. Bioremediation of soils contaminated with polycyclic aromatic hydrocarbons, petroleum, pesticides, chlorophenols and heavy metals by composting: Applications, microbes and future research needs. Biotechnol. Adv. 2015, 33, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Pukalchik, M.; Mercl, F.; Panova, M.; Břendová, K.; Terekhova, V.A.; Tlustoš, P. The improvement of multi-contaminated sandy loam soil chemical and biological properties by the biochar, wood ash, and humic substances amendments. Environ. Pollut. 2017, 229, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zheng, J.; Han, Z.; Chen, H. Bioremediation of crude oil-contaminated soil by hydrocarbon-degrading microorganisms immobilized on humic acid-modified biofuel ash. J. Chem. Technol. Biotechnol. 2019, 94, 1904–1912. [Google Scholar] [CrossRef]
- Da Silva, I.G.S.; de Almeida, F.C.G.; da Rocha e Silva, N.M.P.; Casazza, A.A.; Converti, A.; Sarubbo, L.A. Soil bioremediation: Overview of technologies and trends. Energies 2020, 13, 4664. [Google Scholar] [CrossRef]
- Tang, Y.; Li, X.; Dong, B.; Huang, J.; Wei, Y.; Dai, X.; Dai, L. Effect of aromatic repolymerization of humic acid-like fraction on digestate phytotoxicity reduction during high-solid anaerobic digestion for stabilization treatment of sewage sludge. Water Res. 2018, 143, 436–444. [Google Scholar] [CrossRef]
- Coles, C.A.; Yong, R.N. Humic acid properties and implications for treatment of wastewater containing metals. In Geo-Environmental Engineering: Integrated Management of Groundwater and Contaminated Land; Yong, R.N., Thomas, H.R., Eds.; British Geotechnical Association: Cardiff, UK, 2004; pp. 29–36. [Google Scholar] [CrossRef]
- Kochany, J.; Lipczynska-Kochany, E. Fenton reaction in the presence of humates. Treatment of highly contaminated wastewater at neutral pH. Environ. Technol. 2007, 28, 1007–1013. [Google Scholar] [CrossRef]
- Tang, K.; Casas, M.E.; Ooi, G.T.H.; Kaarsholm, K.M.S.; Bester, K.; Andersen, H.R. Influence of humic acid addition on the degradation of pharmaceuticals by biofilms in effluent wastewater. Int. J. Hyg. Environ. Health 2017, 220, 604–610. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Tang, B.; Xie, H. Treatment of waste gases by humic acid. Energy Fuels 2015, 29, 1269–1278. [Google Scholar] [CrossRef]
- Bearden, B.N.; Petersen, L. Influence of arbuscular mycorrhizal fungi on soil structure and aggregate stability of a vertisol. Plant Soil 2000, 218, 173–183. [Google Scholar] [CrossRef]
- Medina, A.; Roldán, R.; Azcón, R. The effectiveness of arbuscular-mycorrhizal fungi and Aspergillus niger or Phanerochaete chrysosporium treated organic amendments from olive residues upon plant growth in a semi-arid degraded soil. J. Environ. Manag. 2010, 91, 2547–2553. [Google Scholar] [CrossRef]
- McAllister, C.B.; Garcia-Romera, I.; Martin, J.; Godeas, A.; Ocampo, J.A. Interaction between Aspergillus niger van Tiegh. and Glomus mosseae (Nicol. & Gerd.) Gerd. & Trappe. New Phytol. 1995, 129, 309–316. [Google Scholar]
- Phillips, R.P.; Brzostek, E.; Midgley, M.G. The mycorrhizal-associated nutrient economy: A new framework for predicting carbon–nutrient couplings in temperate forests. New Phytol. 2013, 199, 41–51. [Google Scholar] [CrossRef]
- Hou, W.; Lian, B.; Dong, H.; Jiang, H.; Wu, X. Distinguishing ectomycorrhizal and saprophytic fungi using carbon and nitrogen isotopic compositions. Geosci. Front. 2012, 3, 351–356. [Google Scholar] [CrossRef] [Green Version]
- Almonacid, L.; Fuentes, A.; Ortiz, J.; Salas, C.; Garcia-Romera, I.; Ocampo, J.; Arriagada, C. Effect of mixing soil saprophytic fungi with organic residues on the response of Solanum lycopersicum to arbuscular mycorrhizal fungi. Soil Use Manag. 2015, 31, 155–164. [Google Scholar] [CrossRef]
- Lagos, C.; Larsen, J.; Fuentes, A.; Herrera, H.; García-Romera, I.; Campos-Vargas, R.; Arriagada, C. Inoculation of Triticum aestivum L. (Poaceae) with plant-growth-promoting fungi alleviates plant oxidative stress and enhances phenanthrene dissipation in soil. Agronomy 2021, 11, 411. [Google Scholar] [CrossRef]
- Hedden, P.; Sponsel, V. A century of gibberellin research. J. Plant Growth Regul. 2015, 34, 740–760. [Google Scholar] [CrossRef] [Green Version]
- Gusmiaty, M.R.A.; Payangan, R.Y. Production of IAA (indole acetic acid) of the rhizosphere fungus in the Suren community forest stand. IOP Conference Series: Earth Environ. Sci. 2019, 343, 012058. [Google Scholar] [CrossRef]
- Kumla, J.; Suwannarach, N.; Matsui, K.; Lumyong, S. Biosynthetic pathway of indole-3-acetic acid in ectomycorrhizal fungi collected from northern Thailand. PLoS ONE 2020, 15, e0227478. [Google Scholar] [CrossRef] [Green Version]
- Leontovyčová, H.; Trdá, L.; Dobrev, P.I.; Šašek, V.; Gay, L.; Balesdent, M.H.; Burketová, L. Auxin biosynthesis in the phytopathogenic fungus Leptosphaeria maculans is associated with enhanced transcription of indole-3-pyruvate decarboxylase LmIPDC2 and tryptophan aminotransferase LmTAM1. Res. Microbiol. 2020, 171, 174–184. [Google Scholar] [CrossRef]
- Santoyo, G.; Moreno-Hagelsieb, G.; Orozco-Mosqueda, M.C.; Glick, B.R. Plant growth-promoting bacterial endophytes. Microbiol. Res. 2016, 183, 92–99. [Google Scholar] [CrossRef]
- Saldajeno, M.G.B.; Hyakumachi, M. Arbuscular mycorrhizal interactions with rhizobacteria or saprotrophic fungi and its implications to biological control of plant diseases. In Mycorrhizal Fungi: Soil, Agriculture and Environmental Implications; Fulton, S.M., Ed.; Nova Science Publishers: New York, NY, USA, 2011; pp. 187–212. [Google Scholar]
- Lugtenberg, B.; Kamilova, F. Plant-growth-promoting rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef] [Green Version]
- Glick, B.R. Using soil bacteria to facilitate phytoremediation. Biotechnol. Adv. 2010, 28, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R. Plant growth-promoting bacteria: Mechanisms and applications. Scientifca 2012, 2012, 963401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of arbuscular mycorrhizal fungi in plant growth regulation: Implications in abiotic stress tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frey, S.D. Mycorrhizal fungi as mediators of soil organic matter dynamics. Annu. Rev. Ecol. Evol. Syst. 2019, 50, 237–259. [Google Scholar] [CrossRef]
- Saleem, M.; Hu, J.; Jousset, A. More than the sum of its parts: Microbiome biodiversity as a driver of plant growth and soil health. Annu. Rev. Ecol. Evol. Syst. 2019, 50, 145–168. [Google Scholar] [CrossRef]
- Shi, K.; Liu, Y.; Chen, P.; Li, Y. Contribution of lignin peroxidase, manganese peroxidase, and laccase in lignite degradation by mixed white-rot fungi. Waste Biomass Valoriz. 2021, 12, 3753–3763. [Google Scholar] [CrossRef]
- Feng, X.; Sun, J.; Xie, Y. Degradation of Shanxi lignite by Trichoderma citrinoviride. Fuel 2021, 291, 120204. [Google Scholar] [CrossRef]
- He, W.; Megharaj, M.; Subashchandrabose, S.R.; Wu, C.-Y.; Dai, C.-C. Endophyte-assisted phytoremediation: Mechanisms and current application strategies for soil mixed pollutants. Crit. Rev. Biotechnol. 2020, 40, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Lukešová, A. Soil Algae in brown coal and lignite post-mining areas in central Europe (Czech Republic and Germany). Restor. Ecol. 2001, 9, 341–350. [Google Scholar] [CrossRef]
- Chamizo, S.; Mugnai, G.; Rossi, F.; Certini, G.; De Philippis, R. Cyanobacteria inoculation improves soil stability and fertility on different textured soils: Gaining insights for applicability in soil restoration. Front. Environ. Sci. 2018, 6, 49. [Google Scholar] [CrossRef]
- Condron, L.; Stark, C.; O’Callaghan, M.; Clinton, P.; Huang, Z. The role of microbial communities in the formation and decomposition of soil organic matter. In Soil Microbiology and Sustainable Crop Production; Dixon, G.R., Tilston, E.L., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 81–118. [Google Scholar] [CrossRef]
- Misz-Kennan, M.; Fabiańska, M.J. Application of organic petrology and geochemistry to coal waste studies. Int. J. Coal Geol. 2011, 88, 1–23. [Google Scholar] [CrossRef]
- Gargallo-Garriga, A.; Preece, C.; Urban, O.; Sardans, J.; Oravec, M.; Peñuelas, J. Root exudate metabolomes change under drought and show limited capacity for recovery. Sci. Rep. 2018, 8, 12696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furey, G.N.; Tilman, D. Plant biodiversity and the regeneration of soil fertility. Proc. Natl. Acad. Sci. USA 2021, 118, e2111321118. [Google Scholar] [CrossRef] [PubMed]
- Niu, Q.; Meng, Q.; Yang, H.; Wang, Y.; Li, X.; Li, G.; Li, Q. Humification process and mechanisms investigated by Fenton-like reaction and laccase functional expression during composting. Bioresour. Technol. 2021, 341, 125906. [Google Scholar] [CrossRef] [PubMed]
- Köbö, K. Microbiological studies on the humification process (Part 1). Effect of microbes on the transformation. Soil Sci. Plant Nutr. 1955, 1, 53–54. [Google Scholar] [CrossRef]
- Köbö, K.; Takai, Y. Microbiological studies on the humification process (Part 2). On the aerobic decomposition of the fresh plant residue in the case of single inoculation. Soil Sci. Plant Nutr. 1955, 1, 55–56. [Google Scholar] [CrossRef] [Green Version]
- Terrer, C.; Phillips, R.P.; Hungate, B.A.; Rosende, J.; Pett-Ridge, J.; Craig, M.E.; van Groenigen, K.J.; Keenan, T.F.; Sulman, B.N.; Stocker, B.D.; et al. A trade-off between plant and soil carbon storage under elevated CO2. Nature 2021, 591, 599–603. [Google Scholar] [CrossRef]
- Bastos, A.; Fleischer, K. Fungi are key to CO2 response of soil. Nature 2021, 591, 532–534. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sekhohola-Dlamini, L.M.; Keshinro, O.M.; Masudi, W.L.; Cowan, A.K. Elaboration of a Phytoremediation Strategy for Successful and Sustainable Rehabilitation of Disturbed and Degraded Land. Minerals 2022, 12, 111. https://doi.org/10.3390/min12020111
Sekhohola-Dlamini LM, Keshinro OM, Masudi WL, Cowan AK. Elaboration of a Phytoremediation Strategy for Successful and Sustainable Rehabilitation of Disturbed and Degraded Land. Minerals. 2022; 12(2):111. https://doi.org/10.3390/min12020111
Chicago/Turabian StyleSekhohola-Dlamini, Lerato M., Olajide M. Keshinro, Wiya L. Masudi, and A. Keith Cowan. 2022. "Elaboration of a Phytoremediation Strategy for Successful and Sustainable Rehabilitation of Disturbed and Degraded Land" Minerals 12, no. 2: 111. https://doi.org/10.3390/min12020111
APA StyleSekhohola-Dlamini, L. M., Keshinro, O. M., Masudi, W. L., & Cowan, A. K. (2022). Elaboration of a Phytoremediation Strategy for Successful and Sustainable Rehabilitation of Disturbed and Degraded Land. Minerals, 12(2), 111. https://doi.org/10.3390/min12020111






