Proposition of a Thermogravimetric Method to Measure the Ferrous Iron Content in Metallurgical-Grade Chromite
Abstract
1. Introduction
2. Materials and Methods
2.1. Origin of the Raw Material
2.2. Analytical Methods
3. Results
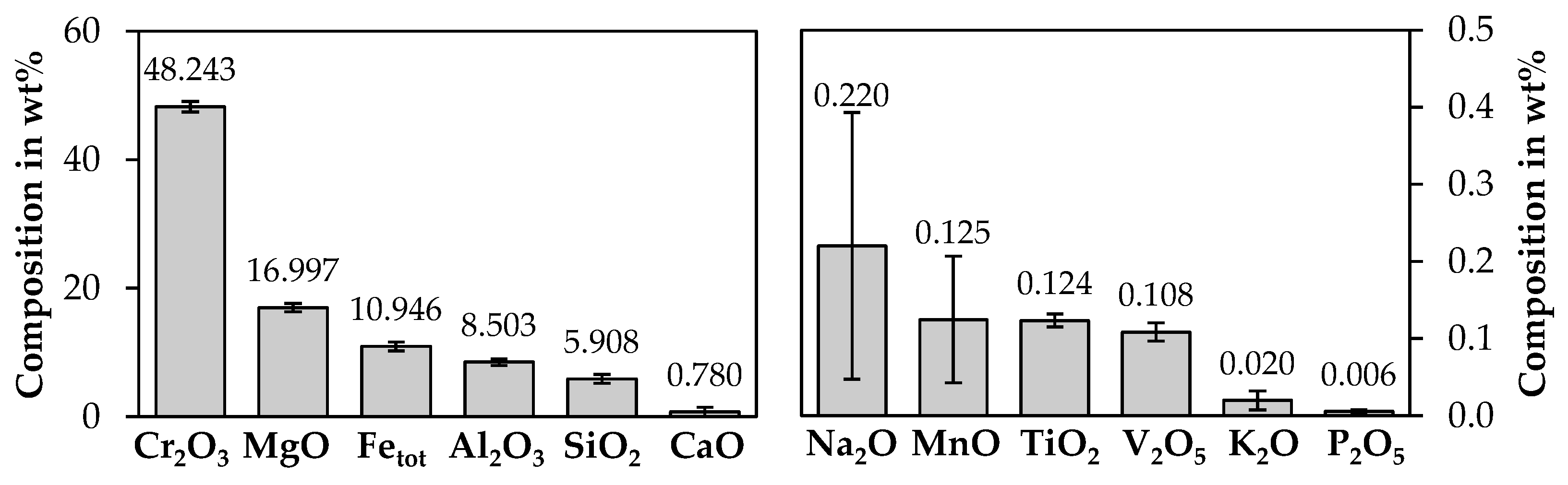
3.1. Bulk Chemical Analysis of Chromite Concentrate
3.2. Mineralogical Investigation of the Chromite Concentrate
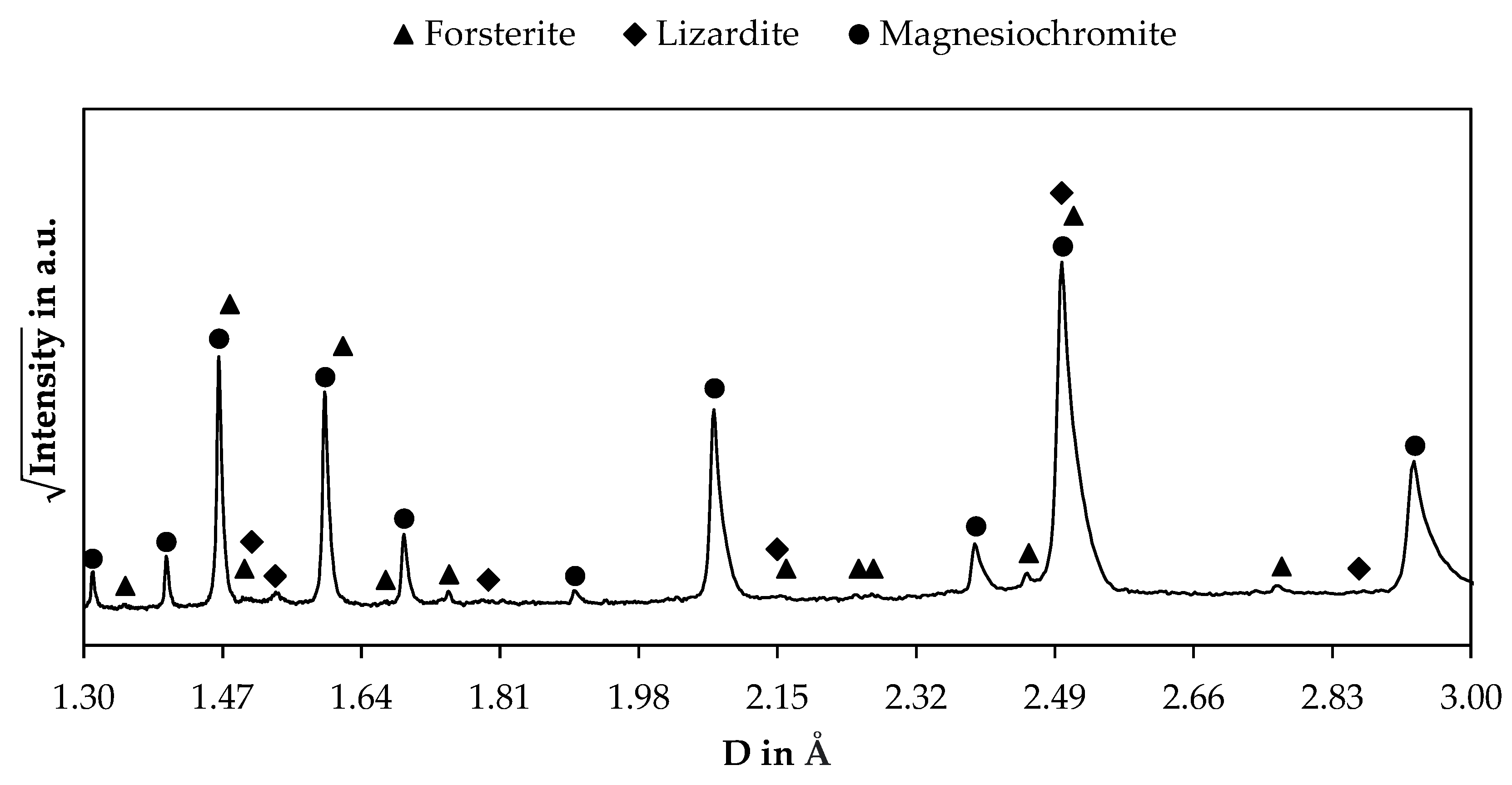
3.2.1. X-ray Diffraction
3.2.2. Raman Microscopy
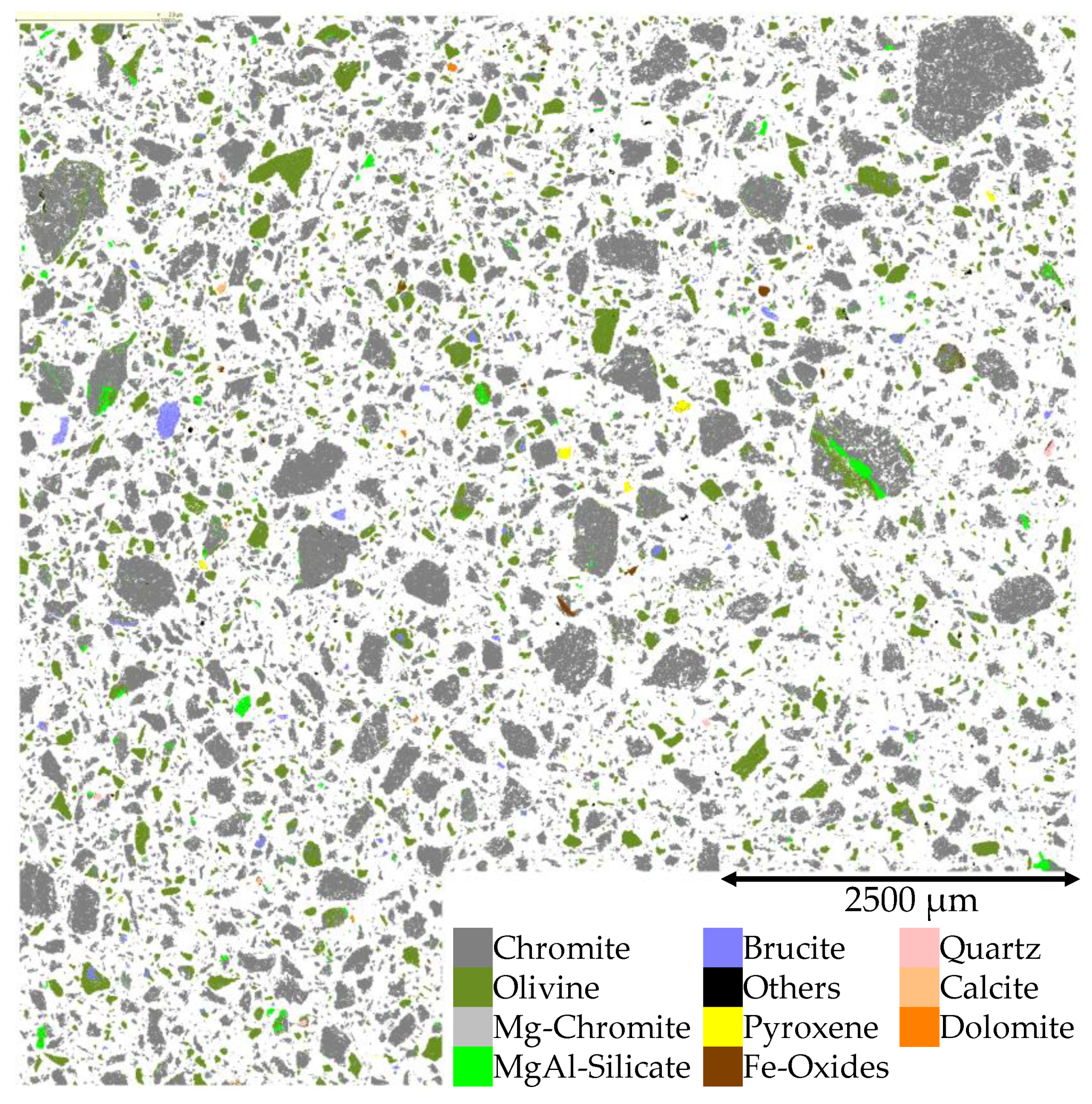
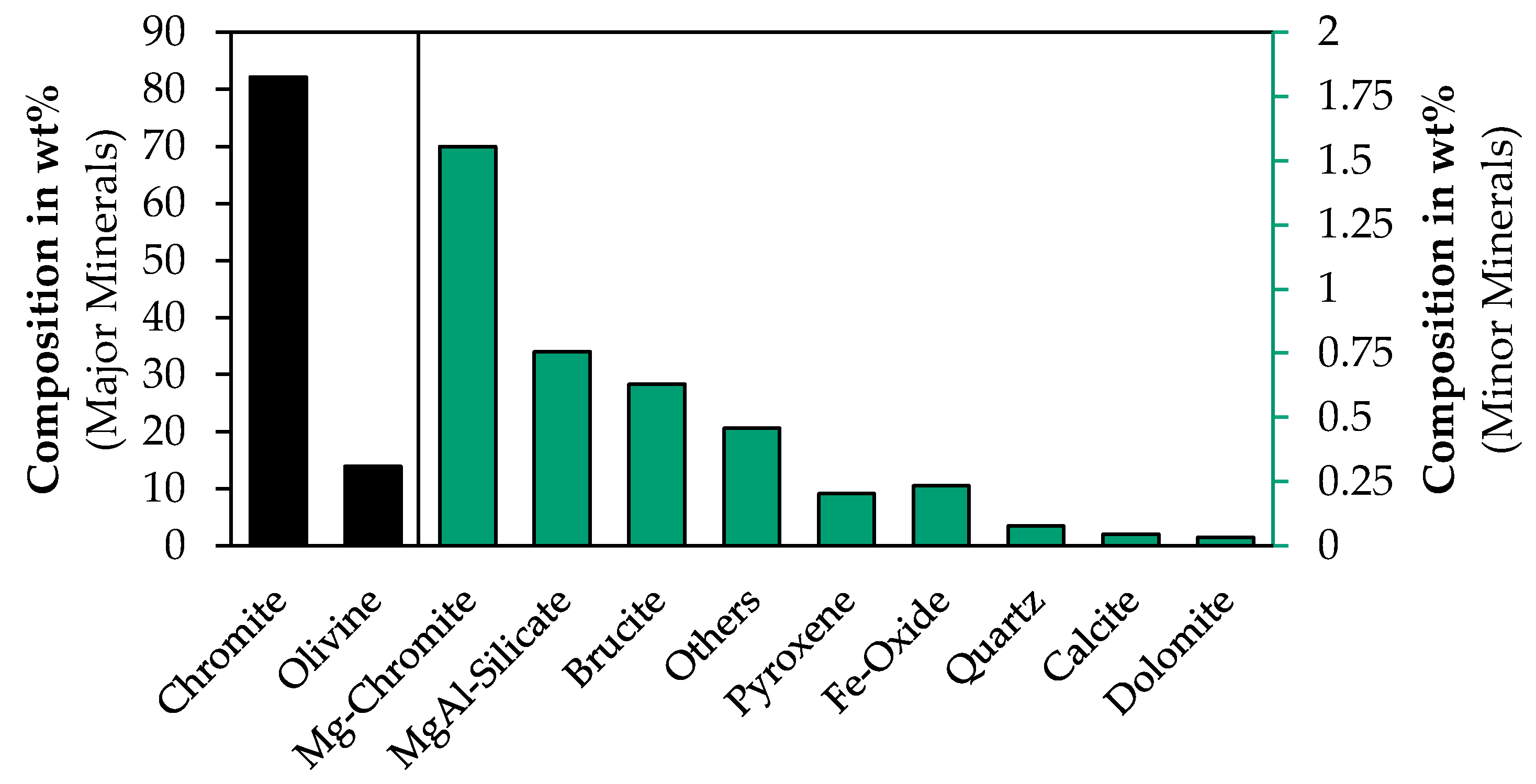
3.2.3. QEMSCAN®
3.3. Analysis of the Ferric/Ferrous Ratio
3.3.1. Mössbauer Spectroscopy
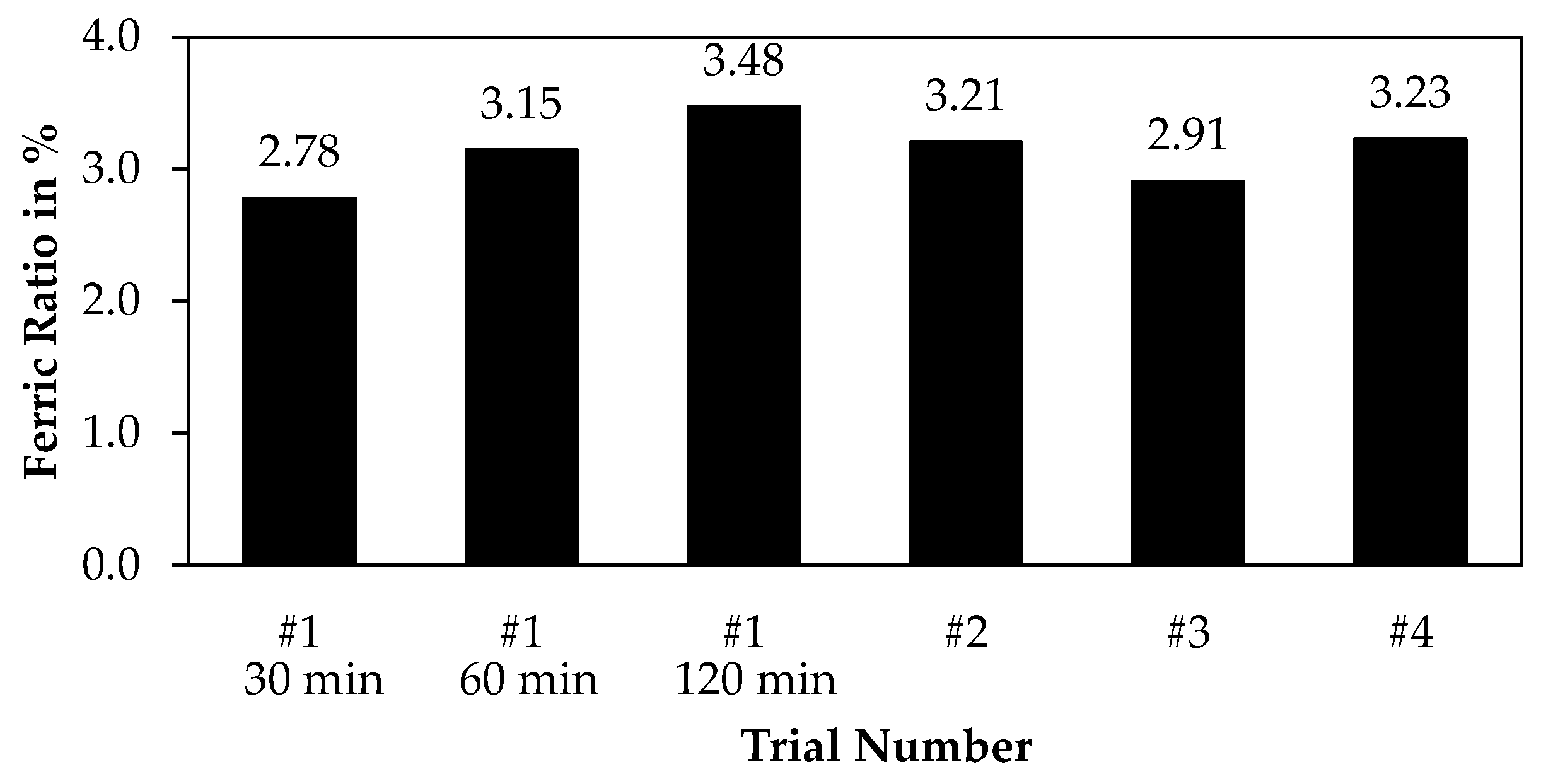
3.3.2. Wet Chemical Methods
3.4. Thermogravimetry of Chromite Concentrate to Measure the Content of Ferrous Iron
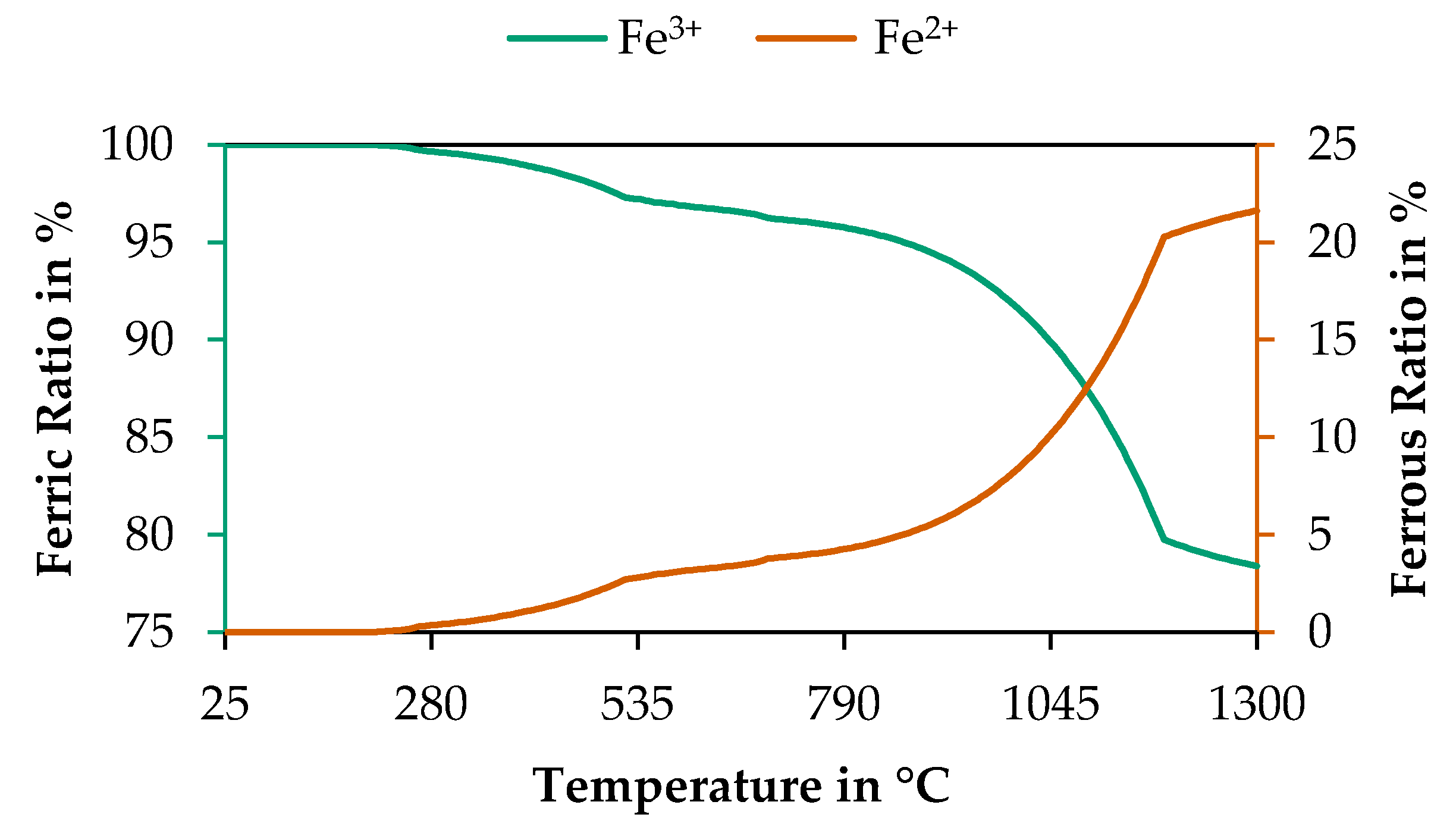
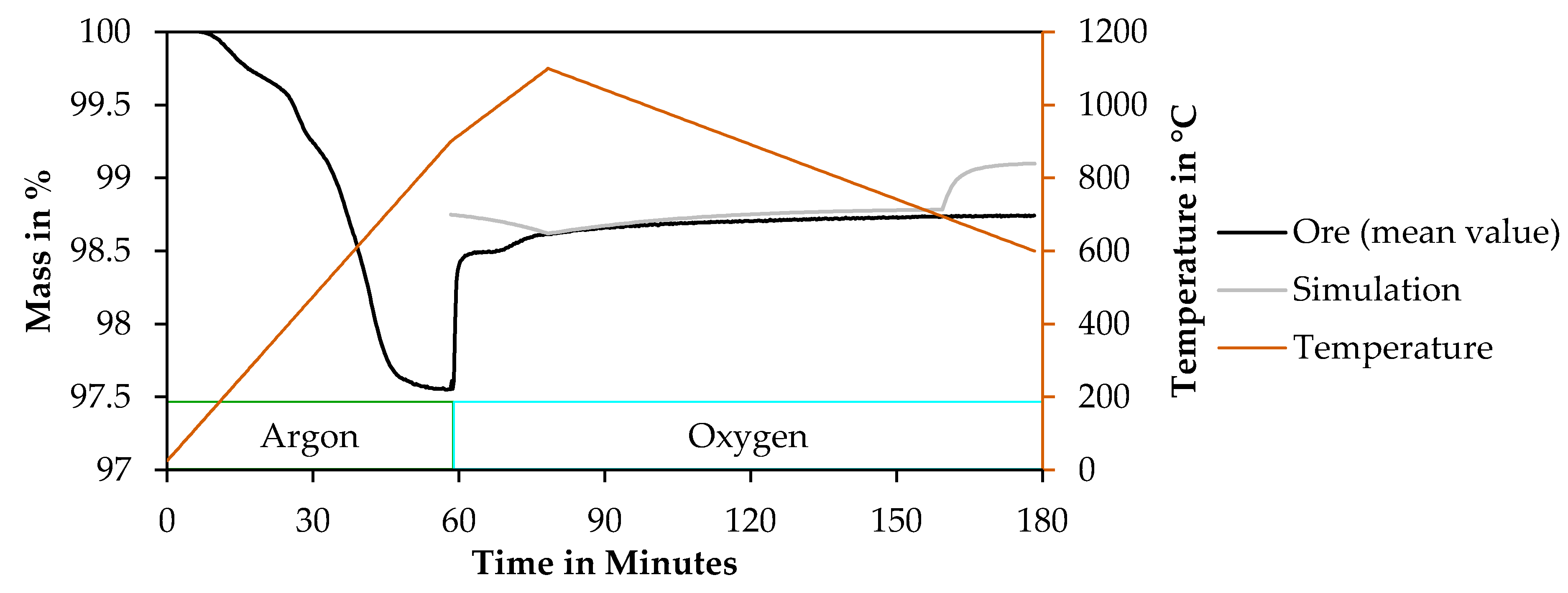
3.4.1. Determination of a Suitable Thermogravimetrical Program
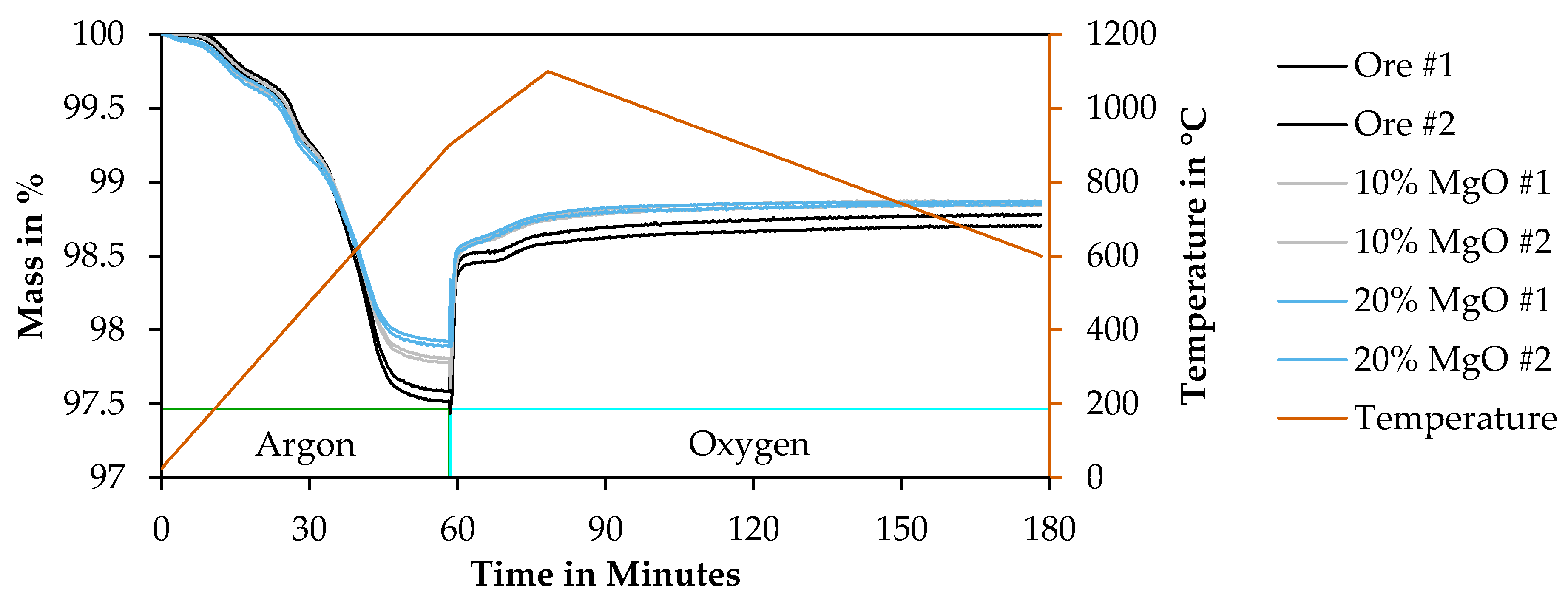
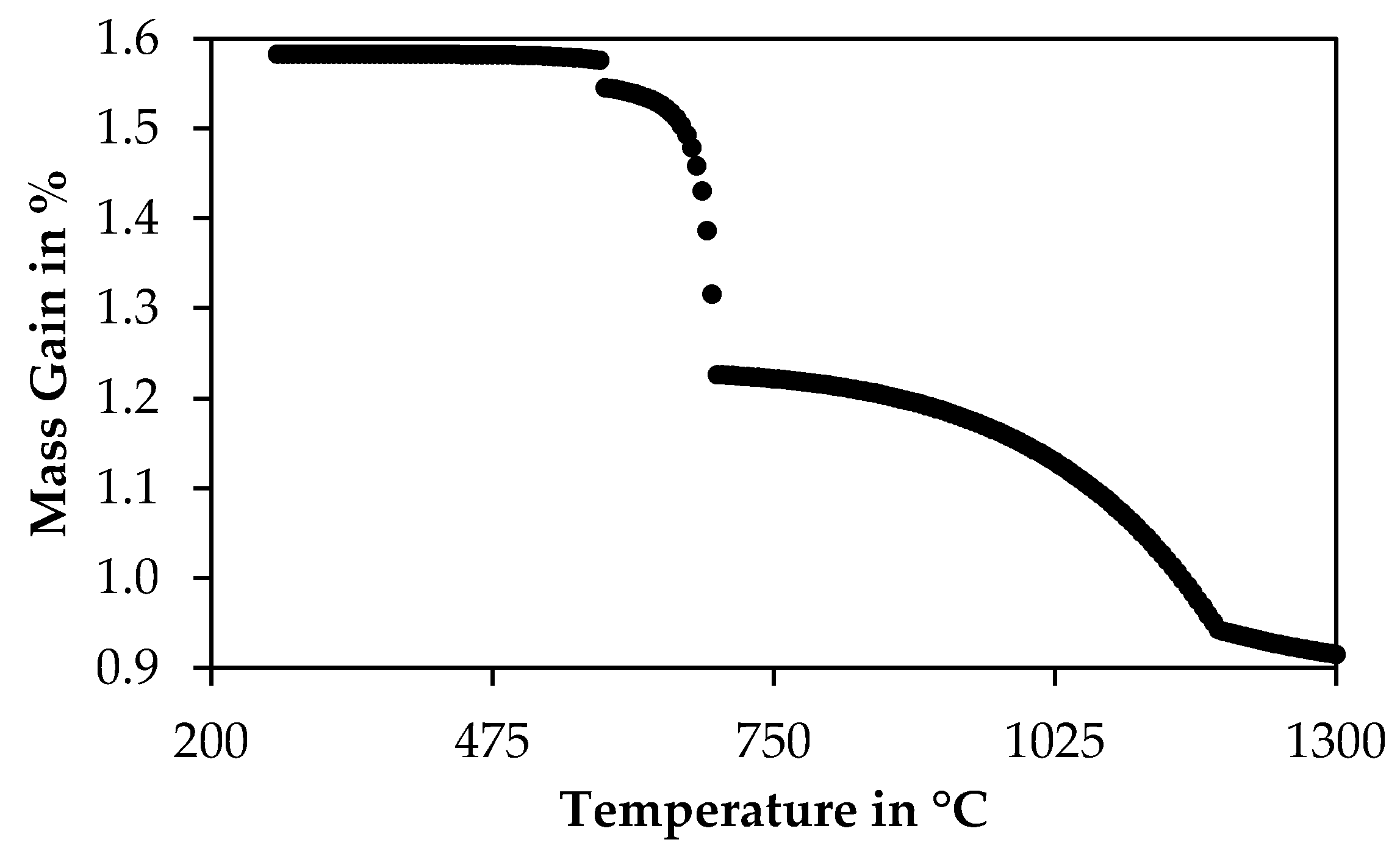
3.4.2. Results of Thermogravimetry Measurements
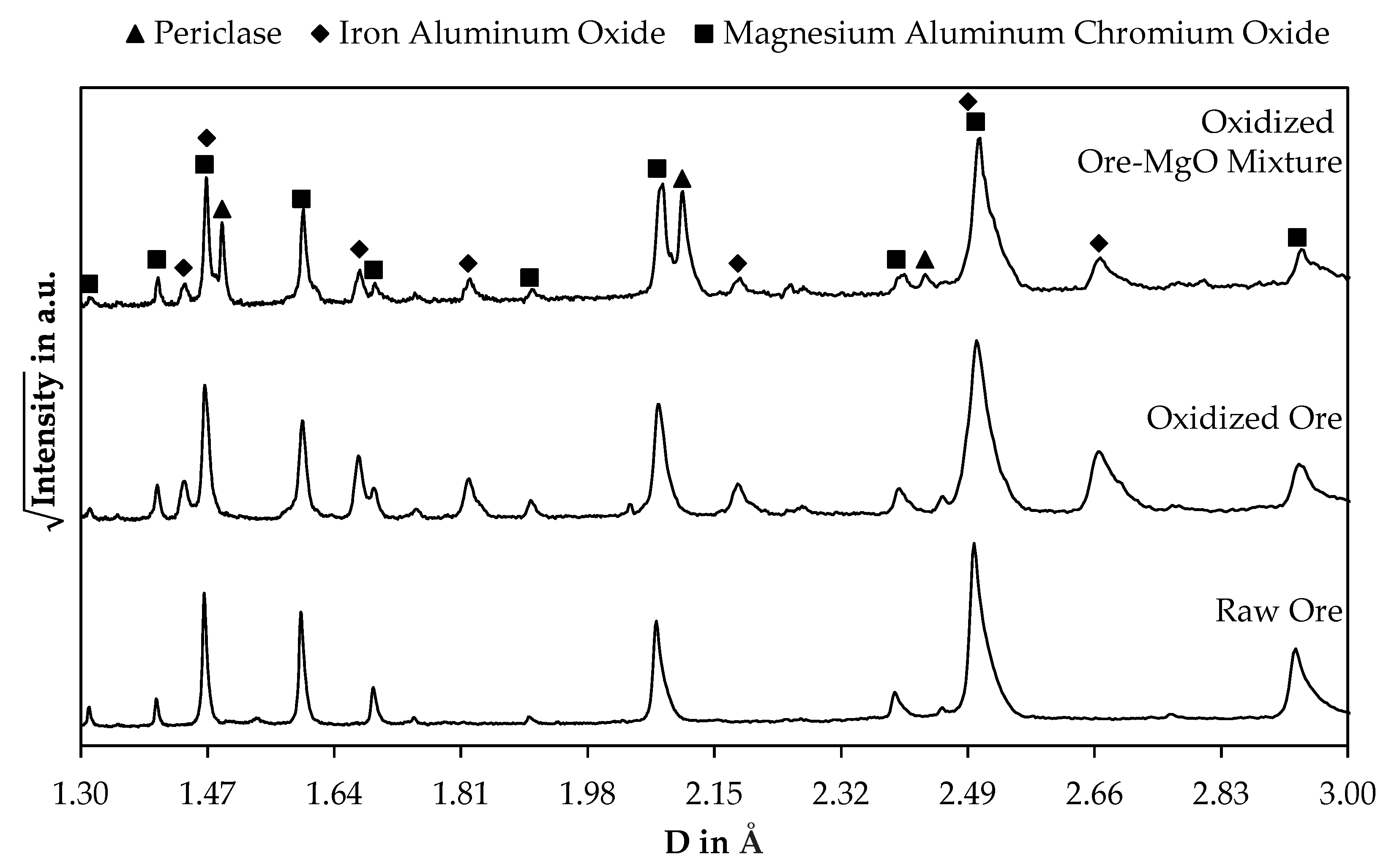
3.5. X-ray Diffraction of Oxidized Chromite Concentrate
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sweeten, N.J.; Verryn, S.M.C.; Oberholzer, J.; Zietsman, J.H. Chrome ore mineralogy and the furnace mass and energy balance. J. S. Afr. Inst. Min. Metall. 2018, 118, 637–643. [Google Scholar] [CrossRef]
- Tangstad, M.; Brynjulfsen, T.; Ringdalen, E. Reduction of Agglomerated Manganese Ores in a 150 kW Pilot Scale Furnace. In Celebrating the Megascale: Proceedings of the Extraction and Processing Division Symposium on Pyrometallurgy in Honor of David G.C. Robertson; Mackey, P.J., Grimsey, E.J., Jones, R.T., Brooks, G.A., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 149–156. ISBN 978-3-319-48591-1. [Google Scholar]
- Tangstad, M.; Ringdalen, E.; Manilla, E.; Davila, D. Pilot Scale Production of Manganese Ferroalloys Using Heat-Treated Mn-Nodules. JOM 2017, 69, 358–364. [Google Scholar] [CrossRef][Green Version]
- Olsen, S.E.; Tangstad, M.; Lindstad, T. Production of Manganese Ferroalloys; Tapir Akademisk Forlag: Trondheim, Norway, 2007; ISBN 9788251921916. [Google Scholar]
- Reid, H.R. Notes on the Operation of a Two-Stage Ferrochrome Silicide Process. J. S. Afr. Inst. Min. Metall. 1967, 68, 132–141. [Google Scholar]
- Zhang, A.; Monaghan, B.J.; Longbottom, R.J.; Nusheh, M.; Bumby, C.W. Reduction Kinetics of Oxidized New Zealand Ironsand Pellets in H2 at Temperatures up to 1443 K. Metall. Mater. Trans. B 2020, 51, 492–504. [Google Scholar] [CrossRef]
- Guo, S.; Li, W.; Peng, J.; Niu, H.; Huang, M.; Zhang, L.; Zhang, S.; Huang, M. Microwave-absorbing characteristics of mixtures of different carbonaceous reducing agents and oxidized ilmenite. Int. J. Miner. Process. 2009, 93, 289–293. [Google Scholar] [CrossRef]
- Pan, J.; Yang, C.; Zhu, D. Solid State Reduction of Preoxidized Chromite-iron Ore Pellets by Coal. ISIJ Int. 2015, 55, 727–735. [Google Scholar] [CrossRef]
- Zhao, B.; Hayes, P.C. Effects of oxidation on the microstructure and reduction of chromite pellets. In Proceedings of the Twelfth International Ferroalloys Congress, INFACON XII, Helsinki, Finland, 6–9 June 2010; pp. 263–274. [Google Scholar]
- Du Preez, S.P.; Beukes, J.P.; Paktunc, D.; van Zyl, P.G.; Jordaan, A. Recycling pre-oxidized chromite fines in the oxidative sintered pellet production process. J. S. Afr. Inst. Min. Metall. 2019, 119, 207–215. [Google Scholar] [CrossRef]
- Ye, M.F.; Wu, G.L. Preparation of Oxidized Pellets with Chrome Ore. In 9th International Symposium on High-Temperature Metallurgical Processing; Hwang, J.-Y., Jiang, T., Kennedy, M.W., Gregurek, D., Wang, S., Zhao, B., Yücel, O., Keskinkilic, E., Downey, J.P., Peng, Z., Eds.; Springer: Cham, Switzerland, 2018; pp. 775–784. ISBN 978-3-319-72137-8. [Google Scholar]
- Erwee, M.; Swanepoel, S.; Reynolds, Q. The Importance of Controlling the Chemistry of Pre-Oxidized Chromite Pellets for Submerged Arc Furnace FeCr Smelting: A Study on Furnace Si Control. In Proceedings of the Sixteenth International Ferroalloys Congress, INFACON XVI, Trondheim, Norway, 27–29 September 2021. [Google Scholar]
- Bidyananda, M.; Mitra, S. Room temperature 57Fe Mössbauer characteristics of chromites from the Nuggihalli schist belt, Dharwar craton, southern India. Curr. Sci. 2004, 86, 1293–1297. [Google Scholar]
- Lenaz, D.; Andreozzi, G.; Bidyananda, M.; Princivalle, F. Oxidation degree of chromite from Indian ophiolites: A crystal chemical and 57Fe Mössbauer study. Period. Mineral. 2014, 83, 241–255. [Google Scholar] [CrossRef]
- Schofield, P.F.; Smith, A.D.; Scholl, A.; Doran, A.; Covey-Crump, S.J.; Young, A.T.; Ohldag, H. Chemical and oxidation-state imaging of mineralogical intergrowths: The application of X-ray photo-emission electron microscopy (XPEEM). Coord. Chem. Rev. 2014, 277–278, 31–43. [Google Scholar] [CrossRef]
- Evans, D.M. Chromite compositions in nickel sulphide mineralized intrusions of the Kabanga-Musongati-Kapalagulu Alignment, East Africa: Petrologic and exploration significance. Ore Geol. Rev. 2017, 90, 307–321. [Google Scholar] [CrossRef]
- Danilenko, I.A.; Zamyatin, D.A.; Votyakov, S.L.; Chashchukhin, I.S. Local analysis of oxidation state of iron in oxide minerals using electron microprobe. In Physics, Technologies and Innovation (PTI-2019): Proceedings of the VI International Young Researchers’ Conference, Ekaterinburg, Russia, 20–23 May 2019; AIP Publishing: College Park, MD, USA, 2019; pp. 020017-1–020017-8. [Google Scholar]
- Uysal, I.; Akmaz, R.M.; Saka, S.; Kapsiotis, A. Coexistence of compositionally heterogeneous chromitites in the Antalya–Isparta ophiolitic suite, SW Turkey: A record of sequential magmatic processes in the sub-arc lithospheric mantle. Lithos 2016, 248–251, 160–174. [Google Scholar] [CrossRef]
- Fanlo, I.; Gervilla, F.; Mateo, E.; Irusta, S. X-ray photoelectron spectroscopy characterization of natural chromite from Mercedita Mine (Eastern Cuba): Quantification of the Fe3+/Fe2+ ratio. Eur. J. Mineral. 2008, 20, 125–129. [Google Scholar] [CrossRef]
- Monkawa, A.; Mikouchi, T.; Koizumi, E.; Sugiyama, K.; Miyamoto, M. Determination of the Fe oxidation state of the Chassigny kaersutite: A microXANES spectroscopic study. Meteorit. Planet. Sci. 2006, 41, 1321–1329. [Google Scholar] [CrossRef]
- Wilke, M.; Farges, F.; Petit, P.E.; Brown, G.E.; Martin, F. Oxidation state and coordination of Fe in minerals: An Fe K- XANES spectroscopic study. Am. Mineral. 2001, 86, 714–730. [Google Scholar] [CrossRef]
- Saikkonen, R.J.; Rautiainen, I.A. Determination of ferrous iron in rock and mineral samples by three volumetric methods. Bull. Geol. Soc. Finl. 1993, 65, 59–64. [Google Scholar] [CrossRef]
- Langa, M.M.; Jugo, P.J.; Leybourne, M.I.; Grobler, D.F.; Adetunji, J.; Skogby, H. Chromite chemistry of a massive chromitite seam in the northern limb of the Bushveld Igneous Complex, South Africa: Correlation with the UG-2 in the eastern and western limbs and evidence of variable assimilation of footwall rocks. Miner. Depos. 2021, 56, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Zorzi, J.E.; Perottoni, C.A.; Cruz, R.C.D. Determination of ferrous and ferric iron from total iron content and thermogravimetric analysis. J. Therm. Anal. Calorim. 2019, 136, 1879–1886. [Google Scholar] [CrossRef]
- Berryman, E.J.; Paktunc, D.; Kingston, D.; Beukes, J.P. Composition and Cr- and Fe-speciation of dust generated during ferrochrome production in a DC arc furnace. Clean. Eng. Technol. 2021, 6, 100386. [Google Scholar] [CrossRef]
- Du, Y.; Chrysochoou, M. Microstructural analyses of Cr(VI) speciation in chromite ore processing residue from the soda ash process. J. Hazard. Mater. 2020, 393, 122385. [Google Scholar] [CrossRef] [PubMed]
- Quintiliani, M.; Andreozzi, G.; Graziani, G. Fe2+ and Fe3+ quantification by different approaches and fO2 estimation for Albanian Cr-spinels. Am. Mineral. 2006, 91, 907–916. [Google Scholar] [CrossRef]
- Uysal, I.; Zaccarini, F.; Sadiklar, M.B.; Tarkian, M.; Thalhammer, O.A.R.; Garuti, G. The podiform chromitites in the Dagküplü and Kavak mines, Eskisehir ophiolite (NW-Turkey): Genetic implications of mineralogical and geochemical data. Geol. Acta 2009, 7, 351–362. [Google Scholar] [CrossRef]
- Uysal, I.; Zaccarini, F.; Sadiklar, M.B.; Garuti, G.; Meisel, T.; Tarkian, M.; Bernhardt, H.J. Cr-PGE Mineralizations in Turkey: Evaluation For their Future Potential. In Proceedings of the 27 Meeting of the Spanish Mineralogical Society, Jaen, Spain, 11–14 September 2007; p. 112. [Google Scholar]
- Thayer, T.P. Principal features and origin of podiform chromite deposits, and some observations on the Guelman-Soridag District, Turkey. Econ. Geol. 1964, 59, 1497–1524. [Google Scholar] [CrossRef]
- Deutsches Institut für Normung. Bodenbeschaffenheit—Bestimmung des Carbonatgehaltes—Volumetrisches Verfahren; DIN EN ISO 10693:2014; Deutsches Institut für Normung: Berlin, Germany, 2014. [Google Scholar]
- Lagarec, K.; Rancourt, D.G. Extended Voigt-based analytic lineshape method for determining N-dimensional correlated hyperfine parameter distributions in Mössbauer spectroscopy. Nucl. Instrum. Methods Phys. Res. B 1997, 129, 266–280. [Google Scholar] [CrossRef]
- Rancourt, D.G.; Ping, J.Y. Voigt-based methods for arbitrary-shape static hyperfine parameter distributions in Mössbauer spectroscopy. Nucl. Instrum. Methods Phys. Res. B 1991, 58, 85–97. [Google Scholar] [CrossRef]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.A.; Eriksson, G.; Gheribi, A.E.; Hack, K.; Jung, I.-H.; Kang, Y.-B.; Melançon, J.; et al. FactSage thermochemical software and databases, 2010–2016. Calphad 2016, 54, 35–53. [Google Scholar] [CrossRef]
- Gasik, M. (Ed.) Handbook of Ferroalloys; Elsevier/Butterworth-Heinemann: Amsterdam, The Netherlands, 2013; ISBN 9780080977539. [Google Scholar]
- Gu, F.; Wills, B.A. Chromite- mineralogy and processing. Miner. Eng. 1988, 1, 235–240. [Google Scholar] [CrossRef]
- Tripathy, S.K.; Singh, V.; Ramamurthy, Y. Improvement in Cr:Fe Ratio of Indian Chromite Ore for Ferro Chrome Production. Int. J. Min. Eng. Miner. Process. 2012, 1, 101–106. [Google Scholar] [CrossRef]
- International Mineralogical Association. The New IMA List of Minerals—A Work in Progress; International Mineralogical Association: Montpellier, France, 2020. [Google Scholar]
- Webmineral. Mineralogy Database. Available online: Webmineral.com (accessed on 14 July 2021).
- Carbonin, S.; Menegazzo, G.; Lenaz, D.; Princivalle, F. Crystal chemistry of two detrital Cr-spinels with unusually low values of oxygen positional parameter: Oxidation mechanism and possible origin. N. Jahrb. Miner. Mh. 1999, 8, 359–371. [Google Scholar]
- Carbonin, S.; Russo, U.; Giusta, A.D. Cation distribution in some natural spinels from X-ray diffraction and Mössbauer spectroscopy. Mineral. Mag. 1996, 60, 355–368. [Google Scholar] [CrossRef]
- Jastrzębska, I.; Szczerba, J.; Błachowski, A.; Stoch, P. Structure and microstructure evolution of hercynite spinel (Fe2+Al2O4) after annealing treatment. Eur. J. Mineral. 2017, 29, 62–71. [Google Scholar] [CrossRef]
- Jastrzębska, I.; Szczerba, J.; Stoch, P.; Błachowski, A.; Ruebenbauer, K.; Prorok, R.; Śnieżek, E. Crystal structure and Mössbauer study of FeAl2O4. Nukleonika 2015, 60, 47–49. [Google Scholar] [CrossRef]
- Larsson, L.; O’Neill, H.S.C.; Annersten, H. Crystal chemistry of synthetic hercynite (FeAl2O4) from XRD structural refinements and Mössbauer spectroscopy. Eur. J. Mineral. 1994, 6, 39–52. [Google Scholar] [CrossRef]
- Jastrzębska, I.; Bodnar, W.; Witte, K.; Burkel, E.; Stoch, P.; Szczerba, J. Structural properties of Mn-substituted hercynite. Nukleonika 2017, 62, 95–100. [Google Scholar] [CrossRef]
- Long, G.J.; Grandjean, F. Mössbauer Spectroscopy Applied to Inorganic Chemistry; Springer: Boston, MA, USA, 1989; ISBN 978-1-4899-2291-5. [Google Scholar]
- Porsch, K.; Kappler, A. FeII oxidation by molecular O2 during HCl extraction. Environ. Chem. 2011, 8, 190–197. [Google Scholar] [CrossRef]
- Baba, A.A.; Monsuru, J.A.; Olaoluwa, D.T.; Balogun, A.F.; Abdulganiyu, Y. Enrichment of a nigerian fayalite ore by a hydrochloric acid solution. J. Chem. Technol. Metall. 2017, 52, 572–578. [Google Scholar]
- Mahmoud, M.H.H.; Afifi, A.A.I.; Ibrahim, I.A. Reductive leaching of ilmenite ore in hydrochloric acid for preparation of synthetic rutile. Hydrometallurgy 2004, 73, 99–109. [Google Scholar] [CrossRef]
- Olanipekun, E. A kinetic study of the leaching of a Nigerian ilmenite ore by hydrochloric acid. Hydrometallurgy 1999, 53, 1–10. [Google Scholar] [CrossRef]
- International Organization for Standardization. Iron Ores—Determination of Acid-Soluble Iron(II) Content—Titrimetric Method; ISO 9035:1989(E); International Organization for Standardization: Geneva, Switzerland, 1989. [Google Scholar]


| Mineral | Formula [38] |
|---|---|
| Aragonite | CaCO3 |
| Brucite | Mg(OH)2 |
| Calcite | CaCO3 |
| Diopside | CaMgSi2O6 |
| Dozyite | Mg7Al2(Al2Si4)O15(OH)12 |
| Enstatite | Mg2Si2O6 |
| Fayalite | Fe2SiO4 |
| Forsterite | Mg2SiO4 |
| Lizardite | Mg3Si2O5(OH)4 |
| Magnesiochromite | MgCr2O4 |
| Magnesite | Mg(CO3) |
| Quartz | SiO2 |
| Site | Isomer Shift in mm/s | Quadrupole Splitting in mm/s | Gaussian Line Width in mm/S | Area in % |
|---|---|---|---|---|
| Fe2+ tetr. | 0.913 | 0.411 0.879 1.419 | 0.15 0.24 0.45 | 76 |
| Fe3+ oct. | 0.347 | 0.492 | 0.07 | 20 |
| Fe3+ tetr. | 0.211 | 1.044 | 0.12 | 4 |
| Trial | Mass of Ore in g | Ore Size in µm | Volume HCl in mL | Molar Concentration in Mol/L | Leaching Time in Minutes |
|---|---|---|---|---|---|
| 1 | 100 | <63 | 500 | 2 | 120 |
| 2 | 100 | <63 | 1000 | 2 | 60 |
| 3 | 100 | x50 = 192.20 | 500 | 2 | 60 |
| 4 | 100 | <63 | 500 | 4 | 60 |
| Segment | Temperature in °C | Heating Rate in K/min | Atmosphere |
|---|---|---|---|
| 1 | Room Temperature–900 | 15 | Argon |
| 2 | 900–1100 | 10 | Oxygen |
| 3 | 1100–600 | −5 | Oxygen |
| Sample | Ore #1 | Ore #2 | 10% MgO #1 | 10% MgO #2 | 20% MgO #1 | 20% MgO #2 |
|---|---|---|---|---|---|---|
| Ore/wt% | 100 | 100 | 90 | 90 | 80 | 80 |
| MgO/wt% | 0 | 0 | 10 | 10 | 20 | 20 |
| Mass after calcination/% | 97.5924 ±0.0058 | 97.5228 ±0.0052 | 97.8145 ±0.0049 | 97.7840 ±0.0050 | 97.8965 ±0.0057 | 97.9304 ±0.0047 |
| Mass after oxidation/% | 98.7762 ±0.0034 | 98.7019 ±0.0026 | 98.8689 ±0.0024 | 98.8438 ±0.0018 | 98.8485 ±0.0037 | 98.8690 ±0.0016 |
| Mass gain due to oxidation/% | 1.1839 | 1.1791 | 1.0544 | 1.0598 | 0.9520 | 0.9385 |
| Sample | Ore #1 | Ore #2 | 10% MgO #1 | 10% MgO #2 | 20% MgO #1 | 20% MgO #2 |
|---|---|---|---|---|---|---|
| FeO-content in wt% | 10.632 | 10.589 | 9.470 | 9.518 | 8.550 | 8.429 |
| Fe2+-content in wt% | 8.265 | 8.231 | 7.361 | 7.398 | 6.646 | 6.552 |
| Fetot-content in wt% | 10.946 | 10.946 | 9.851 | 9.851 | 8.757 | 8.757 |
| Fe2+-ratio in % | 75.503 | 75.196 | 74.717 | 75.099 | 75.896 | 74.820 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sommerfeld, M.; Friedrich, B. Proposition of a Thermogravimetric Method to Measure the Ferrous Iron Content in Metallurgical-Grade Chromite. Minerals 2022, 12, 109. https://doi.org/10.3390/min12020109
Sommerfeld M, Friedrich B. Proposition of a Thermogravimetric Method to Measure the Ferrous Iron Content in Metallurgical-Grade Chromite. Minerals. 2022; 12(2):109. https://doi.org/10.3390/min12020109
Chicago/Turabian StyleSommerfeld, Marcus, and Bernd Friedrich. 2022. "Proposition of a Thermogravimetric Method to Measure the Ferrous Iron Content in Metallurgical-Grade Chromite" Minerals 12, no. 2: 109. https://doi.org/10.3390/min12020109
APA StyleSommerfeld, M., & Friedrich, B. (2022). Proposition of a Thermogravimetric Method to Measure the Ferrous Iron Content in Metallurgical-Grade Chromite. Minerals, 12(2), 109. https://doi.org/10.3390/min12020109







