Continuous Bioleaching of Arsenic-Containing Copper-Zinc Concentrate and Shift of Microbial Population under Various Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Flotation Concentrate
2.2. Experimental Setup and Biooxidation
2.3. Sampling and Analysis
2.4. Microbial Population Analysis
2.5. Data Processing
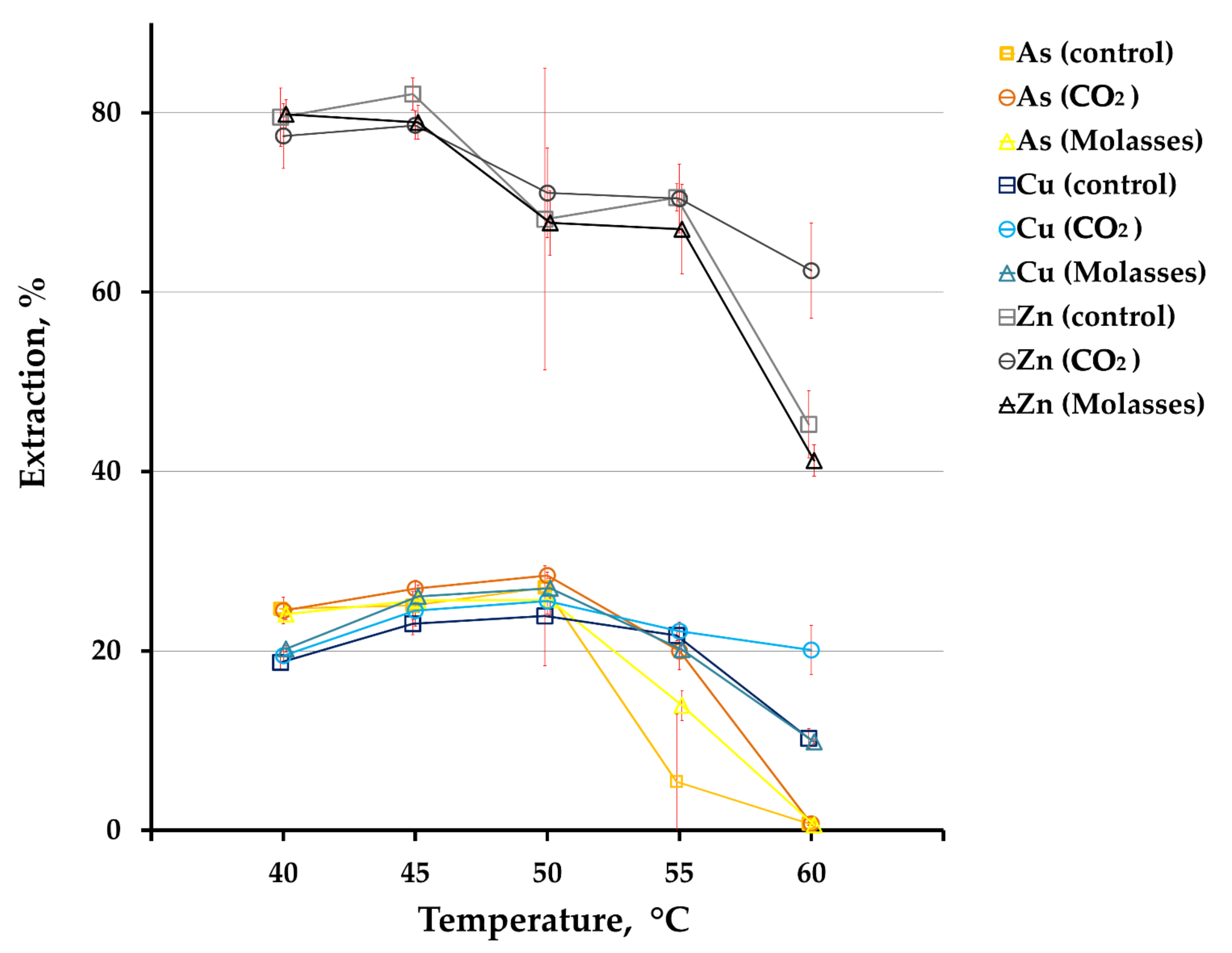
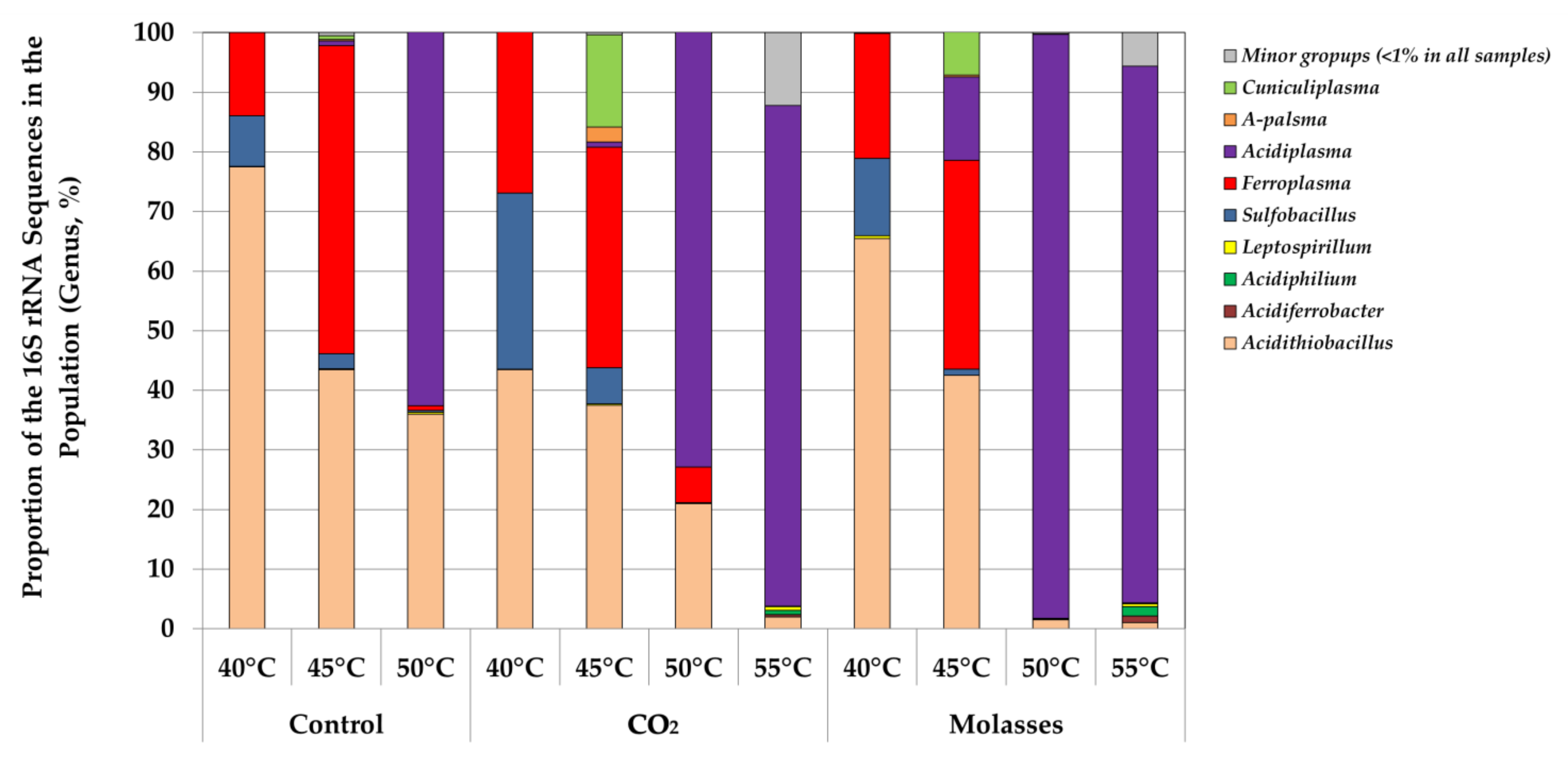
3. Results
3.1. Concentrate Bioleaching
3.2. Microbial Population Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Watling, H.R. The bioleaching of sulphide minerals with emphasis on copper sulphides—A review. Hydrometallurgy 2006, 84, 81–108. [Google Scholar] [CrossRef]
- Baba, A.A.; Ayinla, K.I.; Adekola, F.A.; Ghosh, M.K.; Ayanda, O.S.; Bale, R.B.; Sheik, A.R.; Pradhan, S.R. A Review on Novel Techniques for Chalcopyrite Ore Processing. Int. J. Min. Eng. Miner. Process. 2012, 1, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Pietrzyk, S.; Tora, B. Trends in global copper mining—A review. IOP Conf. Ser. Mater. Sci. Eng. 2018, 427, 012002. [Google Scholar] [CrossRef]
- Filippou, D.; St-Germain, P.; Grammatikopoulos, T. Recovery of metal values from copper-arsenic minerals and other related resources. Miner. Process. Extr. Metall. Rev. 2007, 28, 247–298. [Google Scholar] [CrossRef]
- Diaz, J.A.; Serrano, J.; Leiva, E. Bioleaching of Arsenic-Bearing Copper Ores. Minerals 2018, 8, 215. [Google Scholar] [CrossRef] [Green Version]
- Yagudin, R.A.; Yagudina, Y.R.; Zimin, A.V.; Nemchinova, L.A.; Yurlova, N.A. Improvement of quality of copper concentrate at “Uchalinsky Mining and Concentrating Plant”. Gorn. Zhurnal. 2010, 10, 52–56. [Google Scholar]
- Conner, K.; Anderson, C. Enargite treatments and pressure oxidation of concentrates. J. Metall. Eng. 2013, 10, 115–123. [Google Scholar]
- Salomon-De-Friedberg, H.; Robinson, T.; Lossin, A.; Omaynikova, V. Developing copper arsenic resources with CESL technology. In Proceedings of the COM 2014, 53rd Annual Conference of Metallurgists, Vancouver, BC, Canada, 28 September–1 October 2014. [Google Scholar]
- Tongamp, W.; Takasaki, Y.; Shibayama, A. Arsenic removal from copper ores and concentrates through alkaline leaching in NaHS media. Hydrometallurgy 2009, 98, 213–218. [Google Scholar] [CrossRef]
- Curreli, L.; Garbarino, C.; Ghiani, M.; Orru, G. Arsenic leaching from a gold bearing enargite flotation concentrate. Hydrometallurgy 2009, 96, 258–263. [Google Scholar] [CrossRef]
- Awe, S.A.; Sandstrom, A. Selective leaching of arsenic and antimony from a tetrahedrite rich complex sulphide concentrate using alkaline sulphide solution. Miner. Eng. 2010, 23, 1227–1236. [Google Scholar] [CrossRef]
- Li, T.; Zhang, Y.; Zhang, B.; Zhang, J.; Qin, W. Selective leaching of arsenic from enargite concentrate using alkaline leaching in the presence of pyrite. Hydrometallurgy 2018, 181, 143–147. [Google Scholar] [CrossRef]
- Safarzadeh, M.S.; Moats, M.S.; Miller, J.D. Recent Trends in the Processing of Enargite Concentrates. Miner. Processing Extr. Metall. Rev. Int. J. 2014, 35, 283–367. [Google Scholar] [CrossRef]
- Tongamp, W.; Takasaki, Y.; Shibayama, A. Precipitation of arsenic as Na3AsS4 from Cu3AsS4–NaHS–NaOH leach solutions. Hydrometallurgy 2010, 105, 42–46. [Google Scholar] [CrossRef]
- Marsden, J.O.; House, C.I. The Chemistry of Gold Extraction, 2nd ed.; Society for Mining, Metallurgy, and Exploration, Inc.: Littleton, CO, USA, 2006; 682p. [Google Scholar]
- Gentina, J.C.; Acevedo, F. Application of bioleaching to copper mining in Chile. Electron. J. Biotechnol. 2013, 16, 12. [Google Scholar] [CrossRef]
- Johnson, D.B. Biomining-biotechnologies for extracting and recovering metals from ores and waste materials. Curr. Opin. Biotechnol. 2014, 30, 24–31. [Google Scholar] [CrossRef]
- Roberto, F.F. Commercial heap biooxidation of refractory gold ores-Revisiting Newmont’s successful deployment at Carlin. Miner. Eng. 2017, 106, 2–6. [Google Scholar] [CrossRef]
- Gericke, M.; Neale, J.W.; van Staden, P.J. A Mintek perspective of the past 25 years in minerals bioleaching. J. S. Afr. Inst. Min. Metall. 2009, 109, 567–585. [Google Scholar]
- Morin, D.H.R.; d’Hugues, P. Bioleaching of a cobalt containing pyrite in stirred reactors: A case study from laboratory scale to industrial application. In Biomining; Rawlings, D.E., Johnson, B.D., Eds.; Springer: Berlin, Germany, 2007; pp. 35–55. [Google Scholar]
- Neale, J.; Seppälä, J.; Laukka, A.; van Aswegen, P.; Barnett, S.; Gericke, M. The MONDO Minerals Nickel Sulfide Bioleach Project: From Test Work to Early Plant Operation. Solid State Phenom. 2017, 262, 28–32. [Google Scholar] [CrossRef]
- Kondrat’eva, T.F.; Pivovarova, T.A.; Tsaplina, I.A.; Fomchenko, N.V.; Zhuravleva, A.E.; Muravov, M.I.; Melamud, V.S.; Bulaev, A.G. Diversity of the communities of acidophilic chemolithotrophic microorganisms in natural and technogenic ecosystems. Microbiology 2012, 81, 1–24. [Google Scholar] [CrossRef]
- Johnson, D.B. The Evolution, Current Status, and Future Prospects of Using Biotechnologies in the Mineral Extraction and Metal Recovery Sectors. Minerals 2018, 8, 343. [Google Scholar] [CrossRef] [Green Version]
- Brierley, J.A. Response of microbial systems to thermal stress in heap-biooxidation pretreatment of refractory gold ores. Hydrometallurgy 2003, 71, 13–19. [Google Scholar] [CrossRef]
- Plumb, J.J.; Hawkes, R.B.; Franzmann, P.D. The microbiology of moderately thermophilic and transiently thermophilic ore heaps. In Biomining; Rawlings, D.E., Johnson, B.D., Eds.; Springer: Berlin, Germany, 2007; pp. 217–235. [Google Scholar]
- Riekkola-Vanhanen, M. Talvivaara black schist bioheapleaching demonstration plant. Adv. Mater. Res. 2007, 20, 30–33. [Google Scholar] [CrossRef]
- Rawlings, D.E.; Coram, N.J.; Gardner, M.N.; Deane, S.M. Thiobacillus caldus and Leptospirillum ferrooxidans are widely distributed in continuous flow biooxidation tanks used to treat a variety of metal containing ores and concentrates. Process Metall. 1999, 9, 777–786. [Google Scholar]
- Okibe, N.; Gericke, M.; Hallberg, K.B.; Johnson, D.B. Enumeration and characterization of acidophilic microorganisms isolated from a pilot plant stirred tank bioleaching operation. Appl. Environ. Microbiol. 2003, 69, 1936–1943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dopson, M.; Lindstrom, E.B. Analysis of community composition during moderately thermophilic bioleaching of pyrite, arsenical pyrite, and chalcopyrite. Microb. Ecol. 2004, 48, 19–28. [Google Scholar] [CrossRef]
- Van Hille, R.P.; van Wyk, N.; Froneman, T.; Harrison, S.T.L. Dynamic evolution of the microbial community in BIOX leaching tanks. Adv. Mater. Res. 2013, 825, 331–334. [Google Scholar] [CrossRef]
- Bulaev, A.; Melamud, V.; Boduen, A. Bioleaching of non-ferrous metals from arsenic-bearing sulfide concentrate. Solid State Phenom. 2020, 299, 1064–1068. [Google Scholar] [CrossRef]
- Vakylabad, A.B. A comparison of bioleaching ability of mesophilic and moderately thermophilic culture on copper bioleaching from flotation concentrate and smelter dust. Int. J. Miner. Process. 2011, 101, 94–99. [Google Scholar] [CrossRef]
- Liu, H.; Xia, J.; Nie, Z.; Liu, L.; Wang, L.; Ma, C.; Zheng, L.; Zhao, Y.; Wen, W. Comparative study of S, Fe and Cu speciation transformation during chalcopyrite bioleaching by mixed mesophiles and mixed thermophiles. Miner. Eng. 2017, 106, 22–32. [Google Scholar] [CrossRef]
- Elkina, Y.A.; Bulaev, A.G.; Melnikova, E.A.; Melamud, V.S. Bioleaching of enargite and tennantite by moderately thermophilic acidophilic microorganisms. Microbiology (Mikrobiologiya) 2020, 89, 413–424. [Google Scholar] [CrossRef]
- Munoz, J.A.; Blazquez, M.L.; Gonzalez, F.; Balleste, A.; Acevedo, F.; Gentina, J.C.; Gonzalez, P. Electrochemical study of enargite bioleaching by mesophilic and thermophilic microorganisms. Hydrometallurgy 2006, 84, 175–186. [Google Scholar] [CrossRef]
- Bulaev, A.; Nechaeva, A.; Elkina, Y.; Melamud, V. Effect of carbon sources on pyrite-arsenopyrite concentrate bio-oxidation and growth of microbial population in stirred tank reactors. Microorganisms 2021, 9, 2350. [Google Scholar] [CrossRef] [PubMed]
- Bulaev, A.; Boduen, A. Carbon sources as a factor determining the activity of microbial oxidation of sulfide concentrate at elevated temperature. Minerals 2022, 12, 110. [Google Scholar] [CrossRef]
- Filippova, N.A. Fazovyi Analiz Rud i Produktov ikh Pererabotki (Phase Analysis of Ores and Products of Their Processing); Khimiya: Moscow, Russian, 1975; 280p. (In Russian) [Google Scholar]
- Reznikov, A.A.; Mulikovskaya, E.P.; Sokolov, I.Y. Metody Analiza Prirodnykh Vod (Methods for Analysis of Natural Waters); Nedra: Moscow, Russian, 1970; 140p. (In Russian) [Google Scholar]
- Surovskaya, I.A.; Titov, V.I.; Brodskaya, V.M.; Vasil’ev, P.I.; Lipshits, B.M.; Elentukh, B.M. Tekhnicheskii Analiz Tsvetnoi Metallurgii (Technical Analysis in Nonferrous Metallurgy); Metallurgizdat: Moscow, Russian, 1957; p. 567. (In Russian) [Google Scholar]
- Pimenov, N.V.; Merkel, A.Y.; Samylina, O.S.; Kanapatskii, T.A.; Tikhonova, E.N.; Vlasova, M.A.; Tarnovetskii, I.Y.; Malakhova, T.V. Structure of Microbial Mats in the Mramornaya Bay (Crimea) Coastal Areas. Microbiology (Mikrobiologiya) 2018, 87, 681–691. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pẽa, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335. [Google Scholar] [CrossRef] [Green Version]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckneret, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, Y.; Ballester, B.; Blazquez, M.L.; Gonzalez, F.; Munoz, J.A. New information on the chalcopyrite bioleaching mechanism at low and high temperature. Hydrometallurgy 2003, 71, 47–56. [Google Scholar] [CrossRef]
- Jyothi, N.; Sudha, K.N.; Natarajan, K.A. Electrochemical aspects of selective bioleaching of sphalerite and chalcopyrite from mixed sulphides. Int. J. Miner. Process. 1989, 27, 189–203. [Google Scholar] [CrossRef]
- Schippers, A.; Sand, W. Bacterial leaching of metal sulfides proceeds by two indirect mechanisms via thiosulfate or via polysulfides and sulfur. Appl. Environ. Microbiol. 1999, 65, 319–321. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, A.; Cezac, P.; Hoadley, A.F.A.; Contaminea, F.; D’Hugues, P. A review of sulfide minerals microbially assisted leaching in stirred tank reactors. Int. Biodeterior. Biodegrad. 2017, 119, 118–146. [Google Scholar] [CrossRef]
- Elkina, Y.A.; Melamud, V.S.; Bulaev, A.G. Bioleaching of a Copper-Zinc Concentrate with High Arsenic Content. Microbiology 2021, 90, 78–86. [Google Scholar] [CrossRef]
- Bulaev, A.; Elkina, Y.; Melamud, V. Copper and zinc bioleaching from arsenic-containing polymetallic concentrate. In Proceedings of the 19th International Multidisciplinary Scientific GeoConference (SGEM 2019), Vienna, Austria, 9–11 December 2019. [Google Scholar] [CrossRef]
- Elkina, Y.A.; Melamud, V.S.; Bulaev, A.G. Effect of organic nutrients on bioleaching of low-grade copper concentrate at different temperatures. IOP Conf. Ser. Earth Environ. Sci. 2021, 677, 042076. [Google Scholar] [CrossRef]
- Nancucheo, I.; Johnson, D.B. Production of glycolic acid by chemolithotrophic iron- and sulfur-oxidizing bacteria and its role in delineating and sustaining acidophilic sulfide mineral-oxidizing consortia. Appl. Environ. Microbiol. 2010, 76, 461–467. [Google Scholar] [CrossRef] [Green Version]
- Dopson, M.; Lindstrom, E.B. Potential role of Thiobacillus caldus in arsenopyrite bioleaching. Appl. Environ. Microbiol. 1999, 65, 36–40. [Google Scholar] [CrossRef] [Green Version]
- Bulaev, A.G. Pyrrhotite biooxidation by moderately thermophilic acidophilic microorganisms. Microbiology 2020, 89, 510–519. [Google Scholar] [CrossRef]
- Artykova, A.V.; Melamud, V.S.; Boduen, A.Y.; Bulaev, A.G. Two-stage leaching of copper-zinc concentrate containing tennantite. IOP Conf. Ser. Earth Environ. Sci. 2020, 548, 062042. [Google Scholar] [CrossRef]
| Content, % | |||||||
|---|---|---|---|---|---|---|---|
| As | Fe | Cu | Zn | Stotal | Ssulfate | S0 | Ssulfide |
| 1.7 | 24.4 | 6.2 | 7.3 | 35.1 | 2.6 | 0.6 | 31.9 |
| T, °C | Carbon Source | pH | Eh, mV | Concentration, g/L | |
|---|---|---|---|---|---|
| Fe3+ | Fe2+ | ||||
| 40 | CO2 | 1.08 ± 0.03 | 776 ± 12 | 16.32 ± 0.50 | 0.04 ± 0.06 |
| Molasses | 1.04 ± 0.02 | 778 ± 9 | 15.51 ± 0.55 | 0 | |
| Aeration (control) | 1.05 ± 0.01 | 773 ± 9 | 15.18 ± 0.54 | 0 | |
| 45 | CO2 | 1.04 ± 0.03 | 802 ± 29 | 18.70 ± 0.76 | 0.03 ± 0.06 |
| Molasses | 1.08 ± 0.04 | 804 ± 26 | 15.76 ± 0.29 | 0 | |
| Aeration (control) | 1.05 ± 0.05 | 792 ± 35 | 15.79 ± 0.27 | 0.06 ± 0.08 | |
| 50 | CO2 | 1.04 ± 0.04 | 805 ± 10 | 17.56 ± 0.67 | 0.06 ± 0.08 |
| Molasses | 1.02 ± 0.07 | 800 ± 4 | 14.64 ± 0.13 | 0.14 ± 0.00 | |
| Aeration (control) | 0.97 ± 0.08 | 802 ± 8 | 16.13 ± 0.51 | 0.03 ± 0.06 | |
| 55 | CO2 | 1.26 ± 0.03 | 832 ± 5 | 9.59 ± 0.54 | 0.35 ± 0.08 |
| Molasses | 1.37 ± 0.04 | 804 ± 4 | 4.97 ± 0.18 | 1.54 ± 0.11 | |
| Aeration (control) | 1.91 ± 0.22 | 760 ± 28 | 1.12 ± 0.57 | 3.22 ± 0.00 | |
| 60 | CO2 | 2.34 ± 0.07 | 580 ± 10 | 0.06 ± 0.02 | 1.10 ± 0.46 |
| Molasses | 2.52 ± 0.07 | 573 ± 9 | 0.09 ± 0.09 | 0.28 ± 0.04 | |
| Aeration (control) | 2.52 ± 0.04 | 572 ± 5 | 0.11 ± 0.06 | 0.66 ± 0.18 | |
| T, °C | Carbon Source | Concentration, g/L | ||
|---|---|---|---|---|
| As | Cu2+ | Zn2+ | ||
| 40 | CO2 | 0.42 ± 0.03 | 1.21 ± 0.05 | 5.65 ± 0.26 |
| Molasses | 0.41 ± 0.01 | 1.25 ± 0.02 | 5.83 ± 0.12 | |
| Aeration (control) | 0.42 ± 0.00 | 1.10 ± 0.04 | 5.80 ± 0.02 | |
| 45 | CO2 | 0.46 ± 0.02 | 1.52 ± 0.11 | 5.74 ± 0.12 |
| Molasses | 0.44 ± 0.02 | 1.62 ± 0.08 | 5.76 ± 0.14 | |
| Aeration (control) | 0.43 ± 0.03 | 1.43 ± 0.08 | 5.99 ± 0.13 | |
| 50 | CO2 | 0.48 ± 0.01 | 1.58 ± 0.09 | 5.19 ± 0.37 |
| Molasses | 0.44 ± 0.03 | 1.67 ± 0.07 | 4.94 ± 0.26 | |
| Aeration (control) | 0.46 ± 0.01 | 1.48 ± 0.35 | 4.97 ± 1.23 | |
| 55 | CO2 | 0.34 ± 0.04 | 1.38 ± 0.06 | 5.14 ± 0.28 |
| Molasses | 0.24 ± 0.03 | 1.25 ± 0.06 | 4.89 ± 0.36 | |
| Aeration (control) | 0.09 ± 0.13 | 1.35 ± 0.04 | 5.15 ± 0.11 | |
| 60 | CO2 | 0.01 ± 0 | 1.25 ± 0.17 | 4.55 ± 0.39 |
| Molasses | 0.01 ± 0 | 0.61 ± 0.02 | 3.00 ± 0.13 | |
| Aeration (control) | 0.01 ± 0 | 0.64 ± 0.07 | 3.30 ± 0.27 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elkina, Y.; Nechaeva, A.; Artykova, A.; Kolosoff, A.; Bugubaeva, A.; Melamud, V.; Mardanov, A.; Bulaev, A. Continuous Bioleaching of Arsenic-Containing Copper-Zinc Concentrate and Shift of Microbial Population under Various Conditions. Minerals 2022, 12, 592. https://doi.org/10.3390/min12050592
Elkina Y, Nechaeva A, Artykova A, Kolosoff A, Bugubaeva A, Melamud V, Mardanov A, Bulaev A. Continuous Bioleaching of Arsenic-Containing Copper-Zinc Concentrate and Shift of Microbial Population under Various Conditions. Minerals. 2022; 12(5):592. https://doi.org/10.3390/min12050592
Chicago/Turabian StyleElkina, Yuliya, Aleksandra Nechaeva, Alena Artykova, Aleksandr Kolosoff, Aliya Bugubaeva, Vitaliy Melamud, Andrey Mardanov, and Aleksandr Bulaev. 2022. "Continuous Bioleaching of Arsenic-Containing Copper-Zinc Concentrate and Shift of Microbial Population under Various Conditions" Minerals 12, no. 5: 592. https://doi.org/10.3390/min12050592
APA StyleElkina, Y., Nechaeva, A., Artykova, A., Kolosoff, A., Bugubaeva, A., Melamud, V., Mardanov, A., & Bulaev, A. (2022). Continuous Bioleaching of Arsenic-Containing Copper-Zinc Concentrate and Shift of Microbial Population under Various Conditions. Minerals, 12(5), 592. https://doi.org/10.3390/min12050592






