Systematic Evaluation for the Impact of the Geological Conditions on the Adsorption Affinities of Calcite as an Adsorbent of Zn2+ Ions from Aqueous Solutions: Experimental and Theoretical Studies
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Materials and Characterization
2.2. Batch Adsorption Studies
2.3. Theoretical Traditional and Advanced Equilibrium Studies
3. Results and Discussion
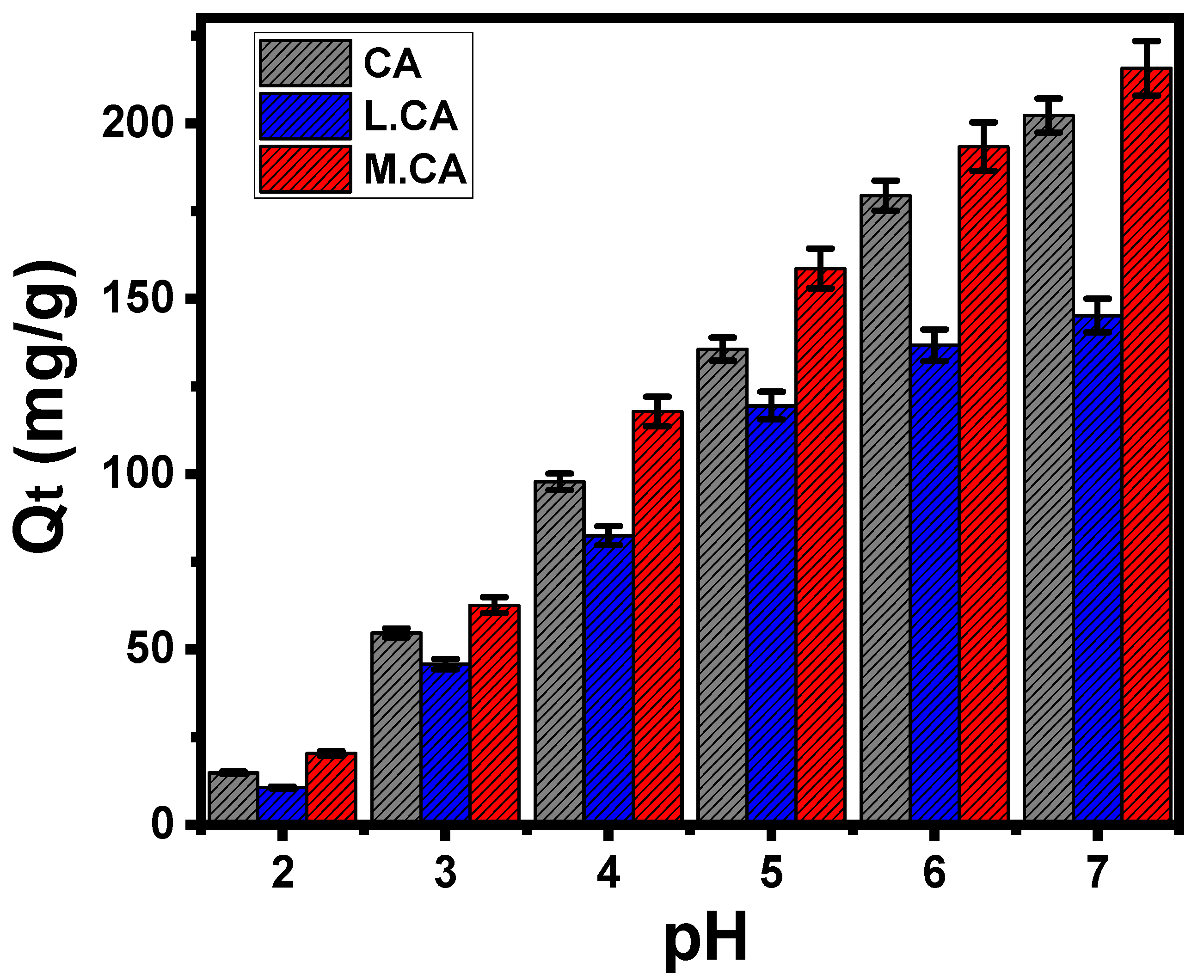
3.1. Effect of pH
3.2. Kinetic Studies
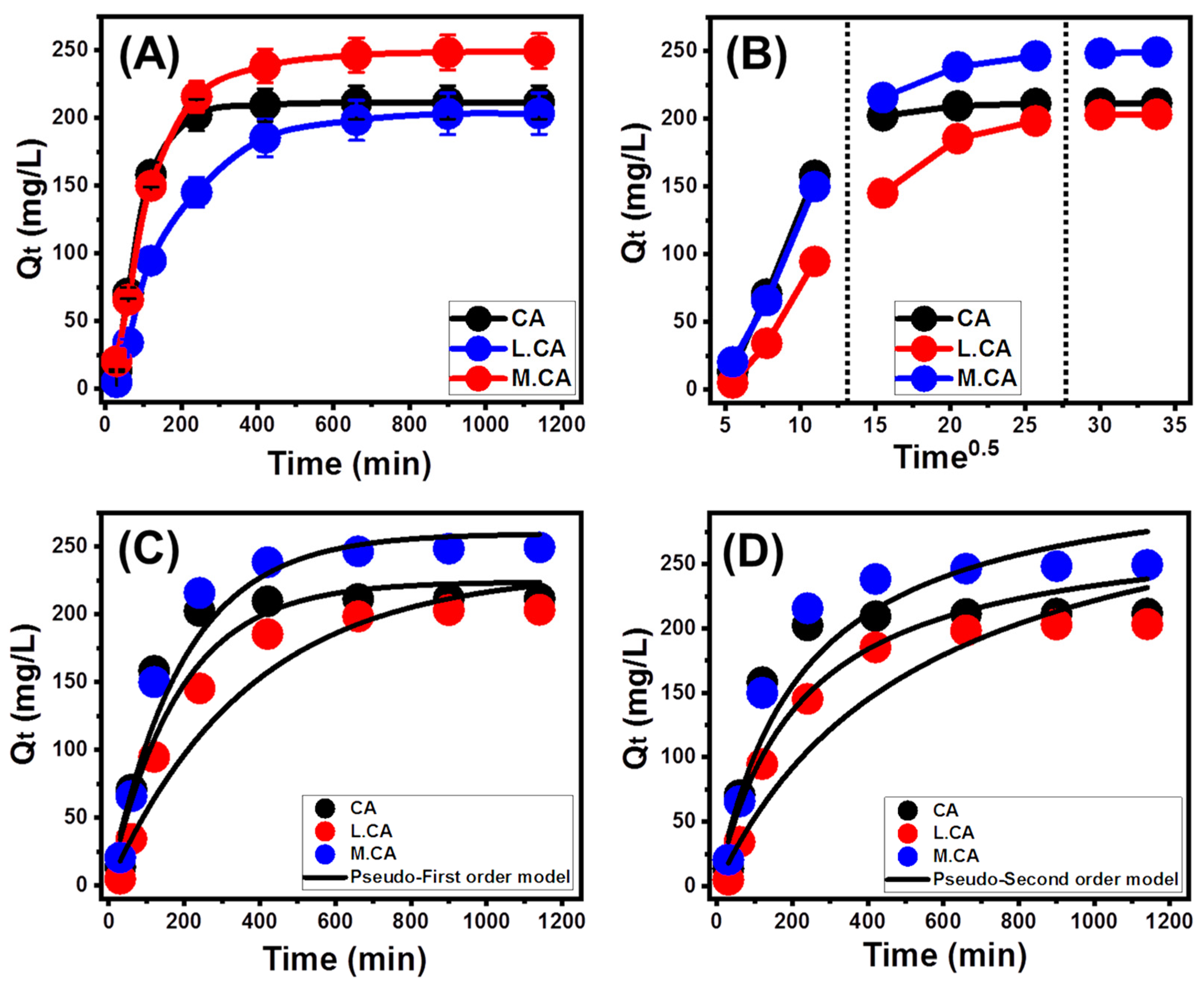
3.2.1. Effect of Contact Time
3.2.2. Intra-Particle Diffusion Behavior
3.2.3. Kinetic Modeling
3.3. Equilibrium Studies
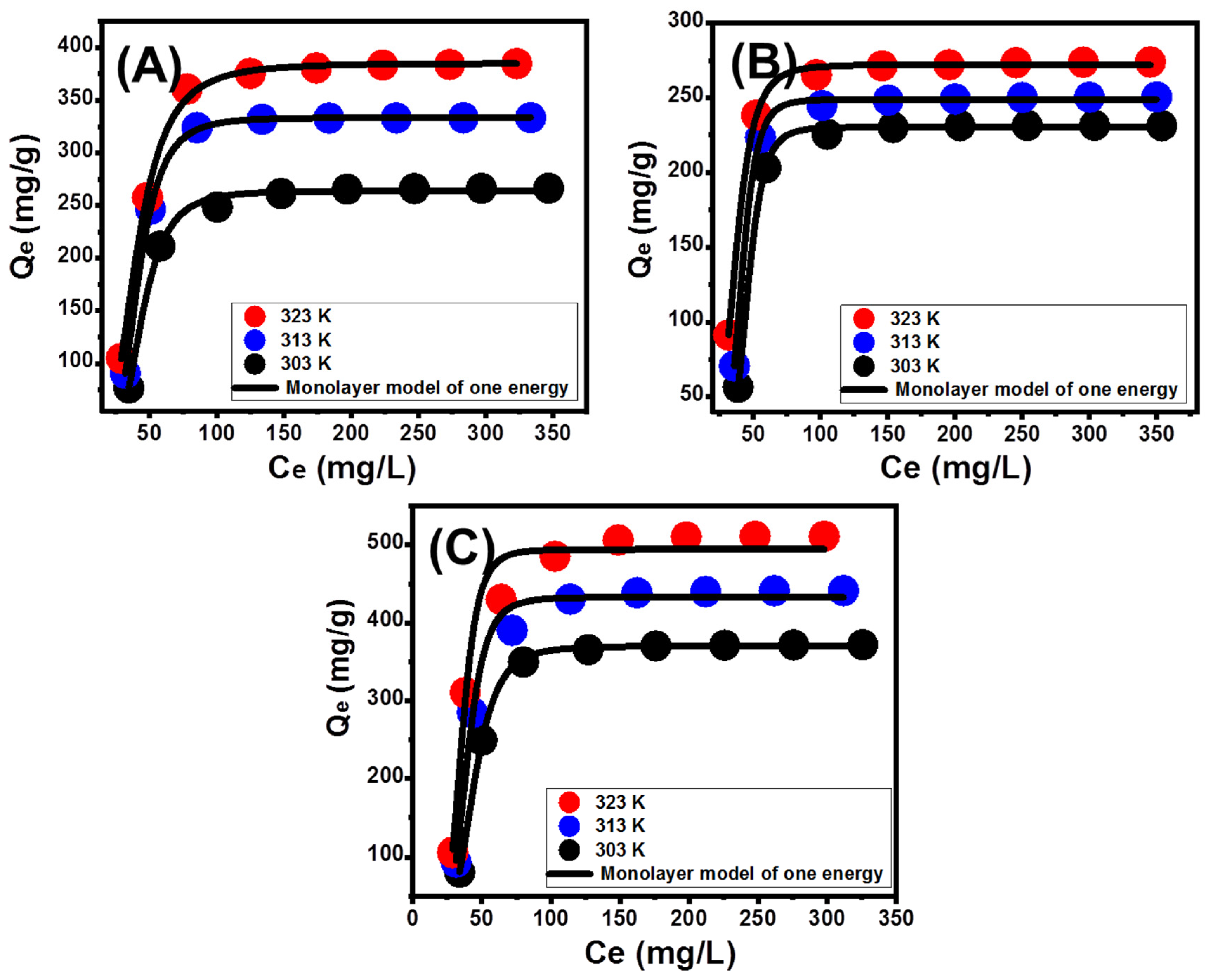
3.3.1. Effect of Zn (II) Concentrations
3.3.2. Giles’s Classification
3.3.3. Classic Isotherm Models
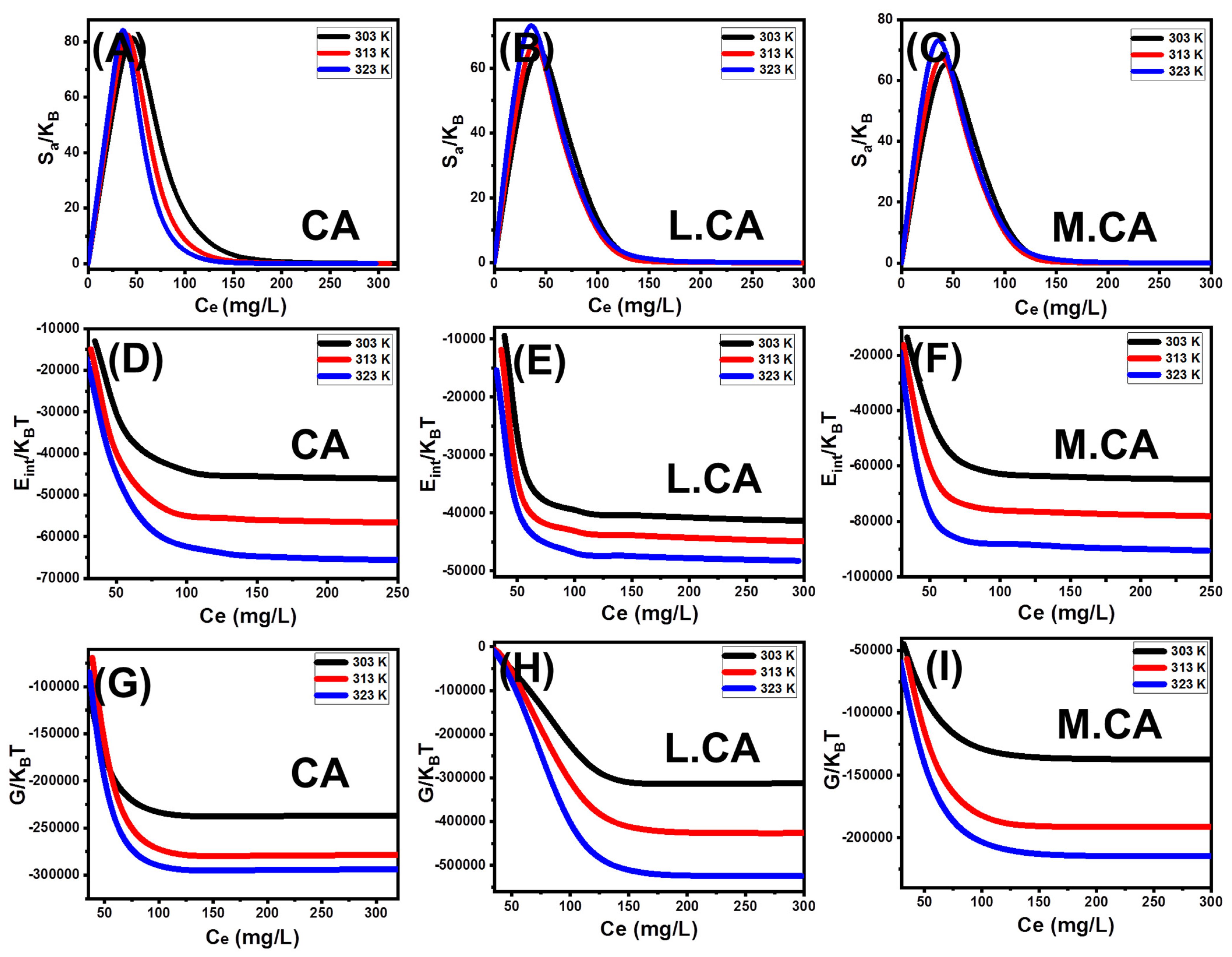
3.3.4. Advanced Isotherm Models
Steric Properties
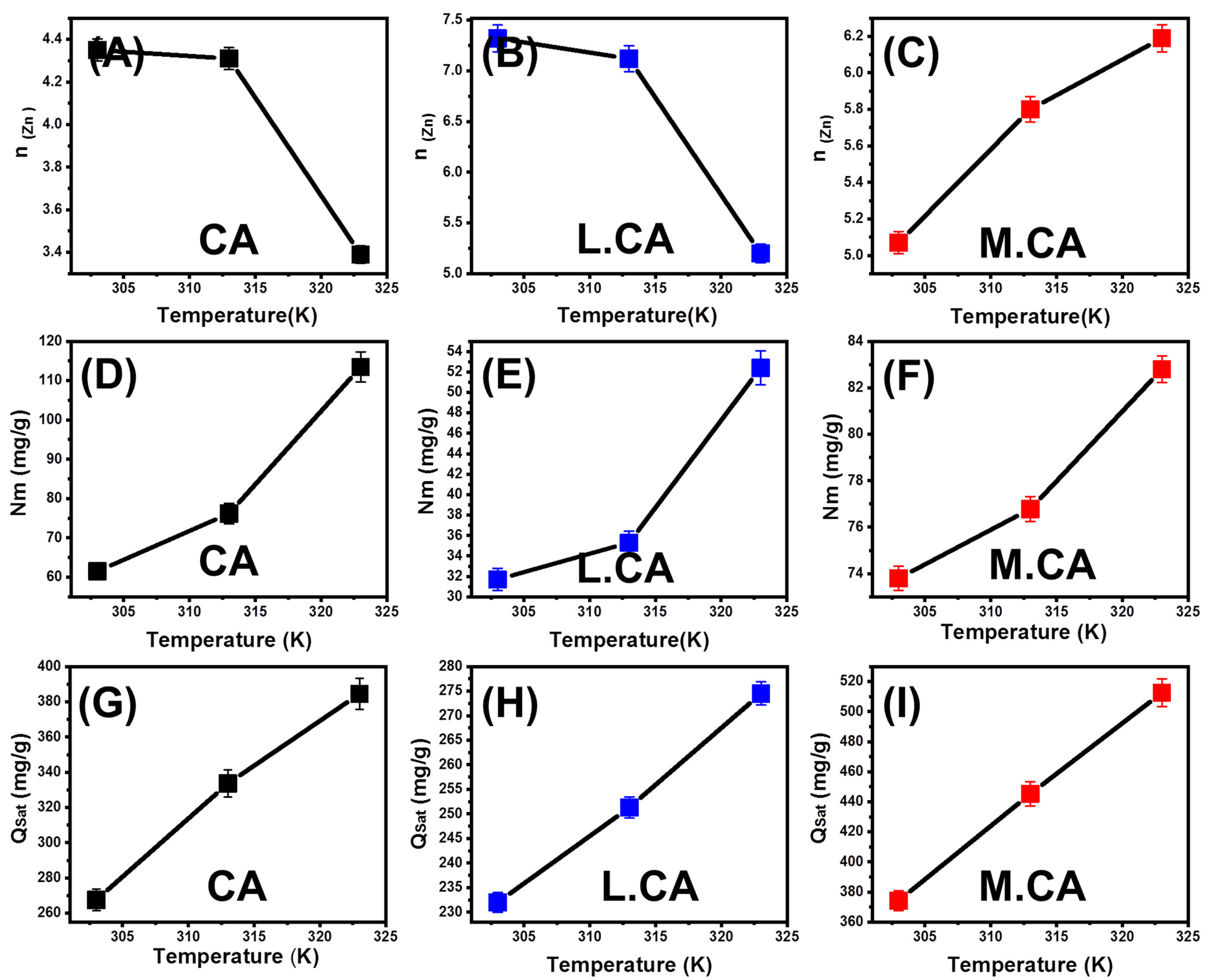
- Number of Adsorbed Zn (II) (n (Zn)) per Site
- Occupied Active Site Density (Nm (Zn))
- Adsorption Capacity at the Saturation State the Calcite Phases (Qsat (Zn))
Energetic Properties
- Retention Energy
- Thermodynamic Functions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salam, M.A.; Mokhtar, M.; Albukhari, S.M.; Baamer, D.F.; Palmisano, L.; Jaremko, M.; Abukhadra, M.R. Synthesis and Characterization of Green ZnO@ polynaniline/Bentonite Tripartite Structure (G. Zn@ PN/BE) as Adsorbent for As (V) Ions: Integration, Steric, and Energetic Properties. Polymers 2022, 14, 2329. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, J.; El-Sherbeeny, A.M.; AlHammadi, A.A.; Park, W.-H.; Abukhadra, M.R. Insight into the adsorption and oxidation activity of a ZnO/piezoelectric quartz core-shell for enhanced decontamination of ibuprofen: Steric, energetic, and oxidation studies. Chem. Eng. J. 2022, 431, 134312. [Google Scholar] [CrossRef]
- Sayed, I.R.; Farhan, A.M.; AlHammadi, A.A.; El-Sayed, M.I.; El-Gaied, I.M.A.; El-Sherbeeny, A.M.; Al Zoubi, W.; Ko, Y.G.; Abukhadra, M.R. Synthesis of novel nanoporous zinc phosphate/hydroxyapatite nano-rods (ZPh/HPANRs) core/shell for enhanced adsorption of Ni2+ and Co2+ ions: Characterization and application. J. Mol. Liq. 2022, 360, 119527. [Google Scholar] [CrossRef]
- Javaheri, F.; Kheshti, Z.; Ghasemi, S.; Altaee, A. Enhancement of Cd2+ removal from aqueous solution by multifunctional mesoporous silica: Equilibrium isotherms and kinetics study. Sep. Purif. Technol. 2019, 224, 199–208. [Google Scholar] [CrossRef]
- Jiang, Y.; Abukhadra, M.R.; Refay, N.M.; Sharaf, M.F.; El-Meligy, M.A.; Awwad, E.M. Synthesis of chitosan/MCM-48 and β-cyclodextrin/MCM-48 composites as bio-adsorbents for environmental removal of Cd2+ ions; kinetic and equilibrium studies. React. Funct. Polym. 2020, 154, 104675. [Google Scholar] [CrossRef]
- Igiri, B.E.; Okoduwa, S.I.; Idoko, G.O.; Akabuogu, E.P.; Adeyi, A.O.; Ejiogu, I.K. Toxicity and Bioremediation of Heavy Metals Contaminated Ecosystem from Tannery Wastewater: A Review. J. Toxicol. 2018, 2018, 2568038. [Google Scholar] [CrossRef] [Green Version]
- Samieifard, R.; Landi, A.; Pourreza, N. Adsorption of Cd, Co and Zn from multi-ionic solutions onto Iranian sepiolite isotherms. Cent. Asian J. Environ. Sci. Technol. Innov. 2021, 2, 102–118. [Google Scholar]
- Pan, J.; Gao, B.; Guo, K.; Gao, Y.; Xu, X.; Yue, Q. Insights into selective adsorption mechanism of copper and zinc ions onto biogas residue-based adsorbent: Theoretical calculation and electronegativity difference. Sci. Total Environ. 2022, 805, 150413. [Google Scholar] [CrossRef]
- Wu, K.; Meng, Y.; Gong, Y.; Wu, L.; Liu, W.; Ding, X. Drinking water elements constituent profiles and health risk assessment in Wuxi, China. Environ. Monit. Assess. 2022, 194, 1–13. [Google Scholar] [CrossRef]
- Zhu, Z.; Wu, Y.; Hu, C.; Zhang, L.; Ding, H.; Zhu, Y.; Fan, Y.; Deng, H.; Zhou, X.; Tang, S. Elimination of zinc ions from aqueous solution by a hydroxylapatite-biochar composite material with the hierarchical porous microstructures of sugarcane waste. J. Clean. Prod. 2022, 362, 132483. [Google Scholar] [CrossRef]
- O’Connor, K.F.; Al-Abed, S.R.; Hordern, S.; Pinto, P.X. Assessing the efficiency and mechanism of zinc adsorption onto biochars from poultry litter and softwood feedstocks. Bioresour. Technol. Rep. 2022, 18, 101039. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Mazumder, M.J.; Al-Attas, O.; Husain, T. Heavy metals in drinking water: Occurrences, implications, and future needs in developing countries. Sci. Total Environ. 2016, 569–570, 476–488. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, S.H. Application of Ethiopian bentonite for water treatment containing zinc. Emerg. Contam. 2022, 8, 113–122. [Google Scholar] [CrossRef]
- Cherono, F.; Mburu, N.; Kakoi, B. Adsorption of lead, copper and zinc in a multi-metal aqueous solution by waste rubber tires for the design of single batch adsorber. Heliyon 2021, 7, e08254. [Google Scholar] [CrossRef] [PubMed]
- Ofudje, E.A.; Adedapo, A.E.; Oladeji, O.B.; Sodiya, E.F.; Ibadin, F.H.; Zhang, D. Nano-rod hydroxyapatite for the uptake of nickel ions: Effect of sintering behaviour on adsorption parameters. J. Environ. Chem. Eng. 2021, 9, 105931. [Google Scholar] [CrossRef]
- El-Sherbeeny, A.M.; Ibrahim, S.M.; AlHammadi, A.A.; Soliman, A.T.A.; Shim, J.-J.; Abukhadra, M.R. Effective retention of radioactive Cs+ and Ba2+ ions using β-cyclodextrin functionalized diatomite (β-CD/D) as environmental adsorbent; characterization, application, and safety. Surf. Interfaces 2021, 26, 101434. [Google Scholar] [CrossRef]
- Kadeche, A.; Ramdani, A.; Adjdir, M.; Guendouzi, A.; Taleb, S.; Kaid, M.; Deratani, A. Preparation, characterization and application of Fe-pillared bentonite to the removal of Coomassie blue dye from aqueous solutions. Res. Chem. Intermed. 2020, 46, 4985–5008. [Google Scholar] [CrossRef]
- Chen, Y.; Nie, Z.; Gao, J.; Wang, J.; Cai, M. A novel adsorbent of bentonite modified chitosan-microcrystalline cellulose aerogel prepared by bidirectional regeneration strategy for Pb(II) removal. J. Environ. Chem. Eng. 2021, 9, 105755. [Google Scholar] [CrossRef]
- Albukhari, S.M.; Salam, M.A.; Abukhadra, M.R. Effective retention of inorganic Selenium ions (Se (VI) and Se (IV)) using novel sodalite structures from muscovite; characterization and mechanism. J. Taiwan Inst. Chem. Eng. 2021, 120, 116–126. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, J.; Qiao, Y.; Luan, Z. Effect of Mg (II), Mn (II), and Fe (II) doping on the mechanical properties and electronic structure of calcite. Mater. Today Commun. 2022, 2022, 103725. [Google Scholar] [CrossRef]
- Raju, C.; Anitha, J.; Kalyani, R.M.; Satyanandam, K.; Jagadeesh, P. Sorption of cobalt using marine macro seaweed graciliariacorticatared algae powder. Mater. Today Proc. 2021, 44, 1816–1827. [Google Scholar] [CrossRef]
- Bin Jumah, M.N.; Eid, M.H.; Al-Huqail, A.A.; Mohammad, M.A.; Bin-Murdhi, N.S.; Abu-Taweel, G.M.; Altoom, N.; Allam, A.A.; AbuKhadra, M.R. Enhanced remediation of As (V) and Hg (II) ions from aqueous environments using β-cyclodextrin/MCM-48 composite: Batch and column studies. J. Water Process. Eng. 2021, 42, 102118. [Google Scholar] [CrossRef]
- Vieira, Y.; Netto, M.S.; Lima, É.C.; Anastopoulos, I.; Oliveira, M.L.; Dotto, G.L. An overview of geological originated materials as a trend for adsorption in wastewater treatment. Geosci. Front. 2022, 101150, 101150. [Google Scholar] [CrossRef]
- Luo, C.; Yang, X.; Li, J. Mechanical Properties of Single-Crystal Calcite and Their Temperature and Strain-Rate Effects. Materials 2022, 15, 4613. [Google Scholar] [CrossRef]
- Sabry, M.; Alazab, H.A.; Gad, A.; El-Faramawy, N. Thermoluminescence properties of natural Egyptian calcite. J. Lumin. 2021, 238, 118273. [Google Scholar] [CrossRef]
- Carchini, G.; Hussein, I.; Al-Marri, M.J.; Shawabkeh, R.; Mahmoud, M.; Aparicio, S. A theoretical study of gas adsorption on calcite for CO2 enhanced natural gas recovery. Appl. Surf. Sci. 2020, 504, 144575. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Anbalagan, G. Spectroscopic characterization of natural calcite minerals. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2001, 68, 656–664. [Google Scholar] [CrossRef]
- Tangarfa, M.; Hassani, N.S.A.; Alaoui, A. Behavior and Mechanism of Tannic Acid Adsorption on the Calcite Surface: Isothermal, Kinetic, and Thermodynamic Studies. ACS Omega 2019, 4, 19647–19654. [Google Scholar] [CrossRef] [Green Version]
- Ban, M.; Luxbacher, T.; Lützenkirchen, J.; Viani, A.; Bianchi, S.; Hradil, K.; Rohatsch, A.; Castelvetro, V. Evolution of calcite surfaces upon thermal decomposition, characterized by electrokinetics, in-situ XRD, and SEM. Colloids Surf. A Physicochem. Eng. Asp. 2021, 624, 126761. [Google Scholar] [CrossRef]
- Ulian, G.; Moro, D.; Valdrè, G. Elastic properties of heterodesmic composite structures: The case of calcite CaCO3 (space group R3¯c). Compos. Part C Open Access 2021, 6, 100184. [Google Scholar] [CrossRef]
- Vivas, E.L.; Cho, K. Efficient adsorptive removal of Cobalt(II) ions from water by dicalcium phosphate dihydrate. J. Environ. Manag. 2021, 283, 111990. [Google Scholar] [CrossRef] [PubMed]
- Abukhadra, M.R.; AlHammadi, A.A.; Khim, J.S.; Ajarem, J.S.; Allam, A.A. Enhanced decontamination of Levofloxacin residuals from water using recycled glass based a green zinc oxide/mesoporous silica nanocomposite; adsorption and advanced oxidation studies. J. Clean. Prod. 2022, 356, 131836. [Google Scholar] [CrossRef]
- Yuan, M.; Gu, Z.; Xia, S.; Zhao, J.; Wang, X. In-situ remediation of zinc contaminated soil using phosphorus recovery product: Hydroxyapatite/calcium silicate hydrate (HAP/C–S–H). Chemosphere 2021, 286, 131664. [Google Scholar] [CrossRef] [PubMed]
- El Qada, E. Kinetic Behavior of the Adsorption of Malachite Green Using Jordanian Diatomite as Adsorbent. Jordanian J. Eng. Chem. Ind. (JJECI) Res. Pap. 2020, 3, 1–10. [Google Scholar] [CrossRef]
- Salam, M.A.; Abukhadra, M.R.; Mostafa, M. Effective decontamination of As (V), Hg (II), and U (VI) toxic ions from water using novel muscovite/zeolite aluminosilicate composite: Adsorption behavior and mechanism. Environ. Sci. Pollut. Res. 2020, 27, 13247–13260. [Google Scholar] [CrossRef]
- Lin, X.; Xie, Y.; Lu, H.; Xin, Y.; Altaf, R.; Zhu, S.; Liu, D. Facile preparation of dual La-Zr modified magnetite adsorbents for efficient and selective phosphorus recovery. Chem. Eng. J. 2021, 413, 127530. [Google Scholar] [CrossRef]
- Huang, Y.; Li, S.; Chen, J.; Zhang, X.; Chen, Y. Adsorption of Pb (II) on mesoporous activated carbons fabricated from water hyacinth using H3PO4 activation: Adsorption capacity, kinetic and isotherm studies. Appl. Surf. Sci. 2014, 293, 160–168. [Google Scholar] [CrossRef]
- Sherlala, A.; Raman, A.; Bello, M.; Buthiyappan, A. Adsorption of arsenic using chitosan magnetic graphene oxide nanocomposite. J. Environ. Manag. 2019, 246, 547–556. [Google Scholar] [CrossRef]
- Huang, Y.; Zeng, X.; Guo, L.; Lan, J.; Zhang, L.; Cao, D. Heavy metal ion removal of wastewater by zeolite-imidazolate frameworks. Sep. Purif. Technol. 2018, 194, 462–469. [Google Scholar] [CrossRef]
- Jasper, E.E.; Ajibola, V.O.; Onwuka, J.C. Nonlinear regression analysis of the sorption of crystal violet and methylene blue from aqueous solutions onto an agro-waste derived activated carbon. Appl. Water Sci. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Ashraf, M.-T.; AlHammadi, A.A.; El-Sherbeeny, A.M.; Alhammadi, S.; Al Zoubi, W.; Ko, Y.G.; Abukhadra, M.R. Synthesis of cellulose fibers/Zeolite-A nanocomposite as an environmental adsorbent for organic and inorganic selenium ions; Characterization and advanced equilibrium studies. J. Mol. Liq. 2022, 360, 119573. [Google Scholar] [CrossRef]
- Giles, C.H.; MacEwan, T.H.; Nakhwa, S.N.; Smith, D. Studies in adsorption. Part XI. A system of classification of solution adsorption isotherms, and its use in diagnosis of adsorption mechanisms and in measurement of specific surface areas of solids. J. Chem. Soc. 1960, 111, 3973–3993. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; Dardir, F.M.; Shaban, M.; Ahmed, E.A.; Soliman, M.F. Superior removal of Co2+, Cu2+ and Zn2+ contaminants from water utilizing spongy Ni/Fe carbonate–fluorapatite; preparation, application and mechanism. Ecotoxicol. Environ. Saf. 2018, 157, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Shaban, M.; Abukhadra, M.R.; Shahien, M.G.; Khan, A.A.P. Upgraded modified forms of bituminous coal for the removal of safranin-T dye from aqueous solution. Environ. Sci. Pollut. Res. 2017, 24, 18135–18151. [Google Scholar] [CrossRef]
- Dawodu, F.; Akpomie, G.; Abuh, M. Equilibrium Isotherm Studies on the Batch Sorption of Copper (II) ions from Aqueous Solution unto Nsu Clay. Int. J. Sci. Eng. Res. 2012, 3, 1–7. [Google Scholar]
- Mobarak, M.; Ali, R.A.; Seliem, M.K. Chitosan/activated coal composite as an effective adsorbent for Mn(VII): Modeling and interpretation of physicochemical parameters. Int. J. Biol. Macromol. 2021, 186, 750–758. [Google Scholar] [CrossRef]
- Hua, P.; Sellaoui, L.; Franco, D.; Netto, M.S.; Dotto, G.L.; Bajahzar, A.; Belmabrouk, H.; Bonilla-Petriciolet, A.; Li, Z. Adsorption of acid green and procion red on a magnetic geopolymer based adsorbent: Experiments, characterization and theoretical treatment. Chem. Eng. J. 2020, 383, 123113. [Google Scholar] [CrossRef]
- Dhaouadi, F.; Sellaoui, L.; Reynel-Ávila, H.E.; Landín-Sandoval, V.; Mendoza-Castillo, D.I.; Jaime-Leal, J.E.; Lima, E.C.; Bonilla-Petriciolet, A.; Lamine, A.B. Adsorption mechanism of Zn2+, Ni2+, Cd2+, and Cu2+ ions by carbon-based adsorbents: Interpretation of the adsorption isotherms via physical modelling. Environ. Sci. Pollut. Res. 2021, 28, 30943–30954. [Google Scholar] [CrossRef]
- Sellaoui, L.; Ali, J.; Badawi, M.; Bonilla-Petriciolet, A.; Chen, Z. Understanding the adsorption mechanism of Ag+ and Hg2+ on functionalized layered double hydroxide via statistical physics modeling. Appl. Clay Sci. 2020, 198, 105828. [Google Scholar] [CrossRef]
- Lacombe, O. Calcite Deformation Twins: From Crystal Plasticity to Applications in Geosciences. Geosciences 2022, 12, 280. [Google Scholar] [CrossRef]
- Dhaouadi, F.; Sellaoui, L.; Badawi, M.; Reynel-Ávila, H.E.; Mendoza-Castillo, D.I.; Jaime-Leal, J.E.; Bonilla-Petriciolet, A.; Ben Lamine, A. Statistical physics interpretation of the adsorption mechanism of Pb2+, Cd2+ and Ni2+ on chicken feathers. J. Mol. Liq. 2020, 319, 114168. [Google Scholar] [CrossRef]
- Sellaoui, L.; Guedidi, H.; SarraWjihi, S.; Reinert, L.; Knani, S.; Duclaux, L.; Ben Lamine, A. Experimental and theoretical studies of adsorption of ibuprofen on raw and two chemically modified activated carbons: New physicochemical interpretations. RSC Adv. 2016, 6, 12363–12373. [Google Scholar] [CrossRef]
- Yue, H.; Shang, Z.; Xu, P.; Feng, D.; Li, X. Preparation of EDTA modified chitooligosaccharide/sodium alginate/Ca2+ physical double network hydrogel by using of high-salinity oilfield produced water for adsorption of Zn2+, Ni2+ and Mn2+. Sep. Purif. Technol. 2022, 280, 119767. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; Bakry, B.M.; Adlii, A.; Yakout, S.M.; El-Zaidy, M.E. Facile conversion of kaolinite into clay nanotubes (KNTs) of enhanced adsorption properties for toxic heavy metals (Zn2+, Cd2+, Pb2+, and Cr6+) from water. J. Hazard. Mater. 2019, 374, 296–308. [Google Scholar] [CrossRef]
- Biswas, S.; Bal, M.; Behera, S.K.; Sen, T.K.; Meikap, B.C. Process Optimization Study of Zn2+ Adsorption on Biochar-Alginate Composite Adsorbent by Response Surface Methodology (RSM). Water 2019, 11, 325. [Google Scholar] [CrossRef] [Green Version]
- Bao, S.; Tang, L.; Li, K.; Ning, P.; Peng, J.; Guo, H.; Zhu, T.; Liu, Y. Highly selective removal of Zn(II) ion from hot-dip galvanizing pickling waste with amino-functionalized Fe3O4@SiO2 magnetic nano-adsorbent. J. Colloid Interface Sci. 2016, 462, 235–242. [Google Scholar] [CrossRef]
- Tian, W.; Rong, Y.; Li, D.; Tian, J.; Lin, N.; Wang, Z. Self-templated formation and characterization of polyhedral CoS hollow nanocage (HNC) for heavy metal ions (Ag+, Cd2+, Cu2+, Pb2+ and Zn2+) removal in aqueous solutions. J. Phys. Chem. Solids 2022, 162, 110516. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, X.; Wang, Y.; Song, X.; Fang, D.; Ge, S. The sorption of single- and multi-heavy metals in aqueous solution using enhanced nano-hydroxyapatite assisted with ultrasonic. J. Environ. Chem. Eng. 2021, 9, 105240. [Google Scholar] [CrossRef]
- Ni, P.; Fox, J.T. Synthesis and appraisal of a hydroxyapatite/pectin hybrid material for zinc removal from water. RSC Adv. 2019, 9, 21095–21105. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Gong, Y.; Zhao, D.; Dang, Z.; Lin, Z. Enhanced removal of zinc and cadmium from water using carboxymethyl cellulose-bridged chlorapatite nanoparticles. Chemosphere 2021, 263, 128038. [Google Scholar] [CrossRef]


| Kinetic Models | |||
|---|---|---|---|
| Model | Parameters | Values | |
| CA | Pseudo-first-order | K1 (1/min) | 0.0027 |
| Qe(Cal) (mg/g) | 232.06 | ||
| R2 | 0.93 | ||
| X2 | 5.6 | ||
| Pseudo-second-order | k2 (mg/g min) | 5.35 × 10−6 | |
| Qe(Cal) (mg/g) | 342.5 | ||
| R2 | 0.91 | ||
| X2 | 7.2 | ||
| L.CA | Pseudo-first-order | K1 (1/min) | 0.0054 |
| Qe(Cal) (mg/g) | 223.8 | ||
| R2 | 0.92 | ||
| X2 | 5.4 | ||
| Pseudo-second-order | k2 (mg/g min) | 1.61 × 10−5 | |
| Qe(Cal) (mg/g) | 284.2 | ||
| R2 | 0.89 | ||
| X2 | 8.3 | ||
| M.CA | Pseudo-first-order | K1 (1/min) | 0.0051 |
| Qe(Cal) (mg/g) | 259.6 | ||
| R2 | 0.96 | ||
| X2 | 4.2 | ||
| Pseudo-second-order | k2 (mg/g min) | 1.35 × 10−5 | |
| Qe(Cal) (mg/g) | 329.2 | ||
| R2 | 0.94 | ||
| X2 | 6.7 | ||
| 303 K | 313 K | 323 K | |||
|---|---|---|---|---|---|
| CA | Langmuir model | Qmax (mg/g) | 352.15 | 442.3 | 506.24 |
| b(L/mg) | 0.0138 | 0.0146 | 0.0153 | ||
| R2 | 0.84 | 0.822 | 0.86 | ||
| X2 | 5.7 | 6.5 | 4.8 | ||
| Freundlich model | 1/n | 0.39 | 0.39 | 0.39 | |
| kF (mg/g) | 30.46 | 38.39 | 44.45 | ||
| R2 | 0.73 | 0.71 | 0.75 | ||
| X2 | 6.8 | 7.4 | 6.2 | ||
| D-R model | β (mol2/KJ2) | 0.0055 | 0.0049 | 0.0042 | |
| Qm (mg/g) | 278.2 | 351.7 | 399.23 | ||
| R2 | 0.97. | 0.97 | 0.97 | ||
| X2 | 1.03 | 1.62 | 1.62 | ||
| E (KJ/mol) | 9.46 | 10.1 | 10.7 | ||
| L.CA | Langmuir model | Qmax (mg/g) | 323.27 | 329.07 | 339.7 |
| b(L/mg) | 0.01 | 0.014 | 0.019 | ||
| R2 | 0.81 | 0.80 | 0.83 | ||
| X2 | 6.77 | 6.21 | 5.7 | ||
| Freundlich model | 1/n | 0.44 | 0.24 | 0.32 | |
| kF (mg/g) | 20.23 | 65.9 | 45.6 | ||
| R2 | 0.67 | 0.51 | 0.68 | ||
| X2 | 8.78 | 9.2 | 8.5 | ||
| D-R model | β (mol2/KJ2) | 0.0073 | 0.0062 | 0.0055 | |
| Qm (mg/g) | 247.69 | 265.09 | 285.46 | ||
| R2 | 0.93 | 0.92 | 0.95 | ||
| X2 | 3.9 | 3.55 | 1.75 | ||
| E (KJ/mol) | 8.27 | 8.98 | 9.5 | ||
| M.CA | Langmuir model | Qmax (mg/g) | 535.3 | 630.35 | 727.5 |
| b(L/mg) | 0.01 | 0.011 | 0.012 | ||
| R2 | 0.81 | 0.81 | 0.82 | ||
| X2 | 7.8 | 7.5 | 6.7 | ||
| Freundlich model | 1/n | 0.47 | 0.47 | 0.47 | |
| kF (mg/g) | 27.98 | 34.4 | 40.4 | ||
| R2 | 0.71 | 0.72 | 0.73 | ||
| X2 | 9.2 | 9.5 | 8.8 | ||
| D-R model | β (mol2/KJ2) | 0.0063 | 0.0053 | 0.0045 | |
| Qm (mg/g) | 396.5 | 467.4 | 537 | ||
| R2 | 0.96 | 0.96 | 0.96 | ||
| X2 | 3.01 | 4.01 | 4.58 | ||
| E (KJ/mol) | 8.16 | 9.7 | 10.5 | ||
| Advanced Isotherm Model | ||||
|---|---|---|---|---|
| Steric and Energetic Parameters | ||||
| 303 K | 313 K | 323 K | ||
| CA | R2 | 0.998 | 0.999 | 0.999 |
| X2 | 0.09 | 0.002 | 0.06 | |
| n | 4.35 | 4.31 | 3.39 | |
| Nm (mg/g) | 61.5 | 76.17 | 113.46 | |
| Qsat (mg/g) | 267.5 | 333.62 | 384.62 | |
| C1/2 (mg/L) | 42.43 | 39.95 | 38.83 | |
| ΔE (kJ/mol) | 10.49 | 10.99 | 11.42 | |
| L.CA | R2 | 0.999 | 0.999 | 0.999 |
| X2 | 0.019 | 0.016 | 0.016 | |
| n | 7.32 | 7.12 | 5.247 | |
| Nm (mg/g) | 31.7 | 35.3 | 52.4 | |
| Qsat (mg/g) | 232.0 | 251.3 | 274.5 | |
| C1/2 (mg/L) | 45.15 | 40.86 | 36.11 | |
| ΔE (kJ/mol) | 10.33 | 10.93 | 11.61 | |
| M.CA | R2 | 0.999 | 0.995 | 0.998 |
| X2 | 0.021 | 0.62 | 1.76 | |
| n | 5.07 | 5.8 | 6.19 | |
| Nm (mg/g) | 73.8 | 76.78 | 82.8 | |
| Qsat (mg/g) | 374.2 | 445.3 | 512.5 | |
| C1/2 (mg/L) | 43.56 | 38.94 | 35.4 | |
| ΔE (kJ/mol) | 10.4 | 11.1 | 11.67 | |
| Adsorbents | Qmax | References |
|---|---|---|
| ECSDNH | 160.19 | [53] |
| Kaolinite nanotubes (KNTs) | 204.8 | [54] |
| Biochar–Alginate composite | 120 | [55] |
| Amino–Fe3O4@SiO2 | 169.5 | [56] |
| CoS HNCs | 151.51 | [57] |
| nHAP | 285 | [58] |
| HAP/pectin | 333.3 | [59] |
| CMC-CAP | 141.1 | [60] |
| CA | 384.6 | This study |
| L.CA | 274.5 | This study |
| M.CA | 512.6 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasser, N.; El-Sayed, M.I.; Othman, S.I.; Allam, A.A.; Al-Labadi, I.G.; Abukhadra, M.R.; Bellucci, S. Systematic Evaluation for the Impact of the Geological Conditions on the Adsorption Affinities of Calcite as an Adsorbent of Zn2+ Ions from Aqueous Solutions: Experimental and Theoretical Studies. Minerals 2022, 12, 1635. https://doi.org/10.3390/min12121635
Nasser N, El-Sayed MI, Othman SI, Allam AA, Al-Labadi IG, Abukhadra MR, Bellucci S. Systematic Evaluation for the Impact of the Geological Conditions on the Adsorption Affinities of Calcite as an Adsorbent of Zn2+ Ions from Aqueous Solutions: Experimental and Theoretical Studies. Minerals. 2022; 12(12):1635. https://doi.org/10.3390/min12121635
Chicago/Turabian StyleNasser, Nourhan, Mohamed I. El-Sayed, Sarah I. Othman, Ahmed A. Allam, Ibrahim G. Al-Labadi, Mostafa R. Abukhadra, and Stefano Bellucci. 2022. "Systematic Evaluation for the Impact of the Geological Conditions on the Adsorption Affinities of Calcite as an Adsorbent of Zn2+ Ions from Aqueous Solutions: Experimental and Theoretical Studies" Minerals 12, no. 12: 1635. https://doi.org/10.3390/min12121635






