Selective Neodymium Enrichment of Sulfides as a “Fingerprint” of Late Processes of Ore-Formation: Insight into Sm-Nd Isotopes for Sulfides from Magmatic Cu-Ni-PGE Complexes and Hydrothermal Pb-Zn, Au-Mo, and Gold Deposits
Abstract
:1. Introduction
2. Geological Settings
2.1. Magmatic Cu-Ni-PGE and Fe-Ti-V Complexes of the Fennoscandian Shield
2.1.1. Pilgujärvi Cu-Ni Deposit
2.1.2. Kaula-Kotselvaara, Pechenga
2.1.3. Ahmavaara Deposit, Portimo Complex (Finland)
2.1.4. Monchegorsk Ore Field
2.1.5. Fedorovo-Pansky 2.5 Ga Layered Complex
2.2. Tokuzbay Gold Deposit (South Altai, Northwest China)
2.3. Qingchengzi Pb-Zn (Northeastern China)
2.4. Dahu Au-Mo Deposit
3. Samples and Methods
3.1. Sm-Nd Analytical Methods
3.2. ICP-MS
3.3. Coefficients Sulfide/Whole Rock
4. Results and Discussion
4.1. Forms of REE Occurrence in Sulfides
- -
- The isomorphic replacement of main cations in a lattice [9];
- -
- Silicate micro-inclusions within the sulfide with a certain REE composition [10];
- -
- -
- -
- -
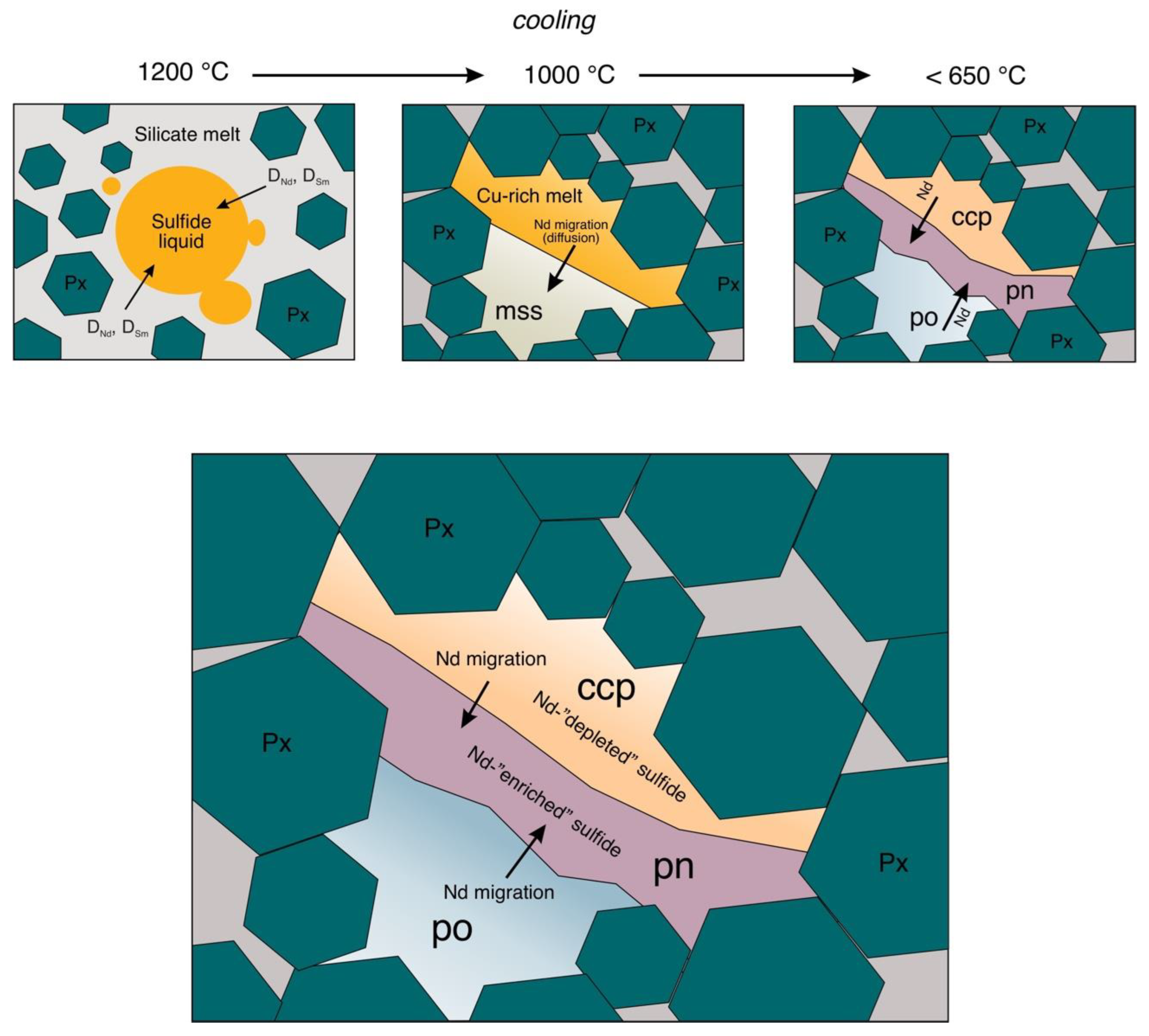
- Fluid inclusions with inherited REE composition from an ore-bearing melt [8,13,14,15,16,17,18,19,86]. Many hydrothermal ore deposits are known to be formed by the interaction of ore fluids with the host rocks. Thus, the isotopic composition of ores depends on the isotopic composition of the host rocks and ore-forming fluids [7,87,88]. Notably, despite the publications where the REE occurrence in the form of fluid inclusions in hydrothermally generated sulfides is postulated, there are no pictures of these inclusions. This is probably caused by the difficulties of the optical detection of such inclusions due to the non-transparency of a sulfide mineral and the incapability of opening the sulfide without breaking a fluid inclusion capsule. Having taken this reason into consideration, we suggested that heterophase inclusions in the form of sub-micron bubbles of fluids or melt may be a possible source of REE in sulfides [1]. However, the results of the computer micro-tomography of disseminated ore sulfides from the Pilgujärvi Cu-Ni deposit (Pechenga, Kola Peninsula) and ore gabbronorites from the platinum-bearing Fedorovo-Pansky complex (Kola Peninsula) did not support this hypothesis, as the studied sulfide minerals showed their homogeneity to the scale of one micron [89]. The absence of silicate micro-inclusions of a size bigger than one micron in the studied sulfides allows us to suggest the isomorphic form of REE occurrence in sulfides. On the other hand, there is a hypothesis that the composition of REE silicate micro-inclusions is a part of a general balance of REE fluid from which the sulfide had crystallized. So, the bulk composition of REE in a mineral may be treated as a composition of an ore-forming fluid [10,84,85]. Otherwise, the neodymium isotopic anomalies in sulfides may also be the result of segregation in lattice defects and similar defects may serve as channels for a swift diffusion of elements [90].
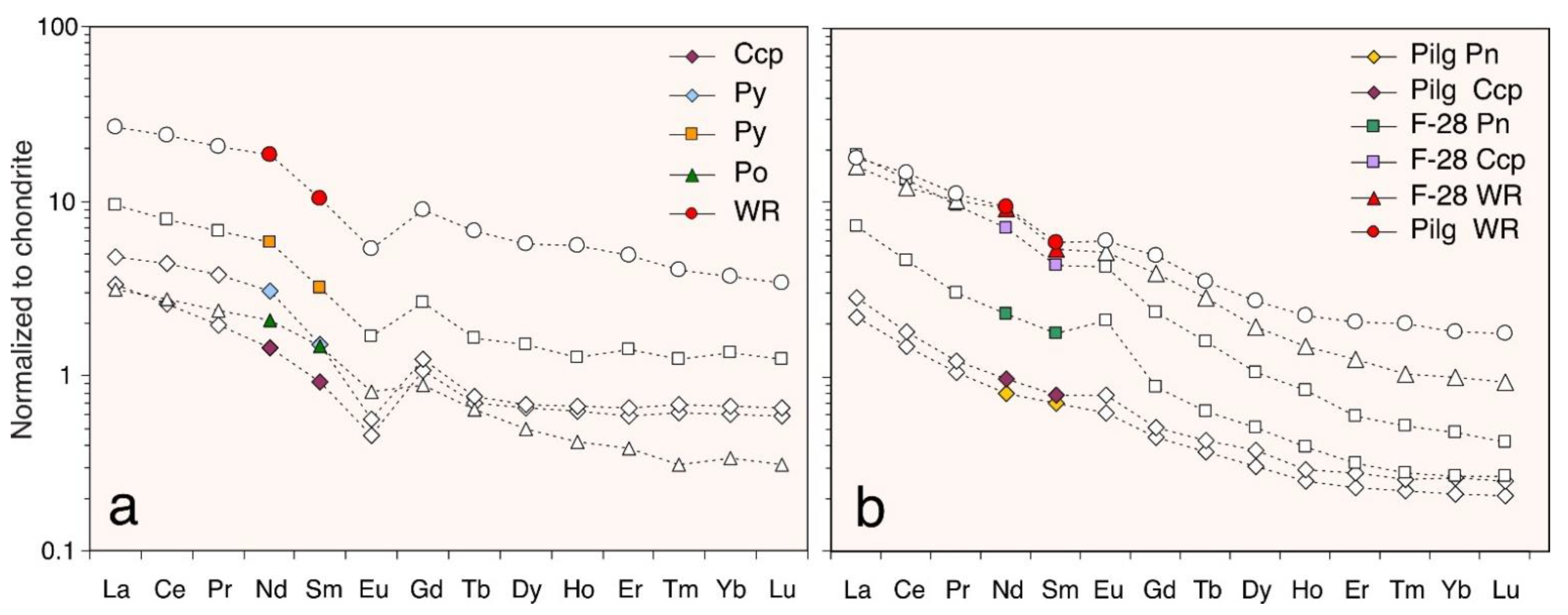
4.2. REE Distribution in Sulfides
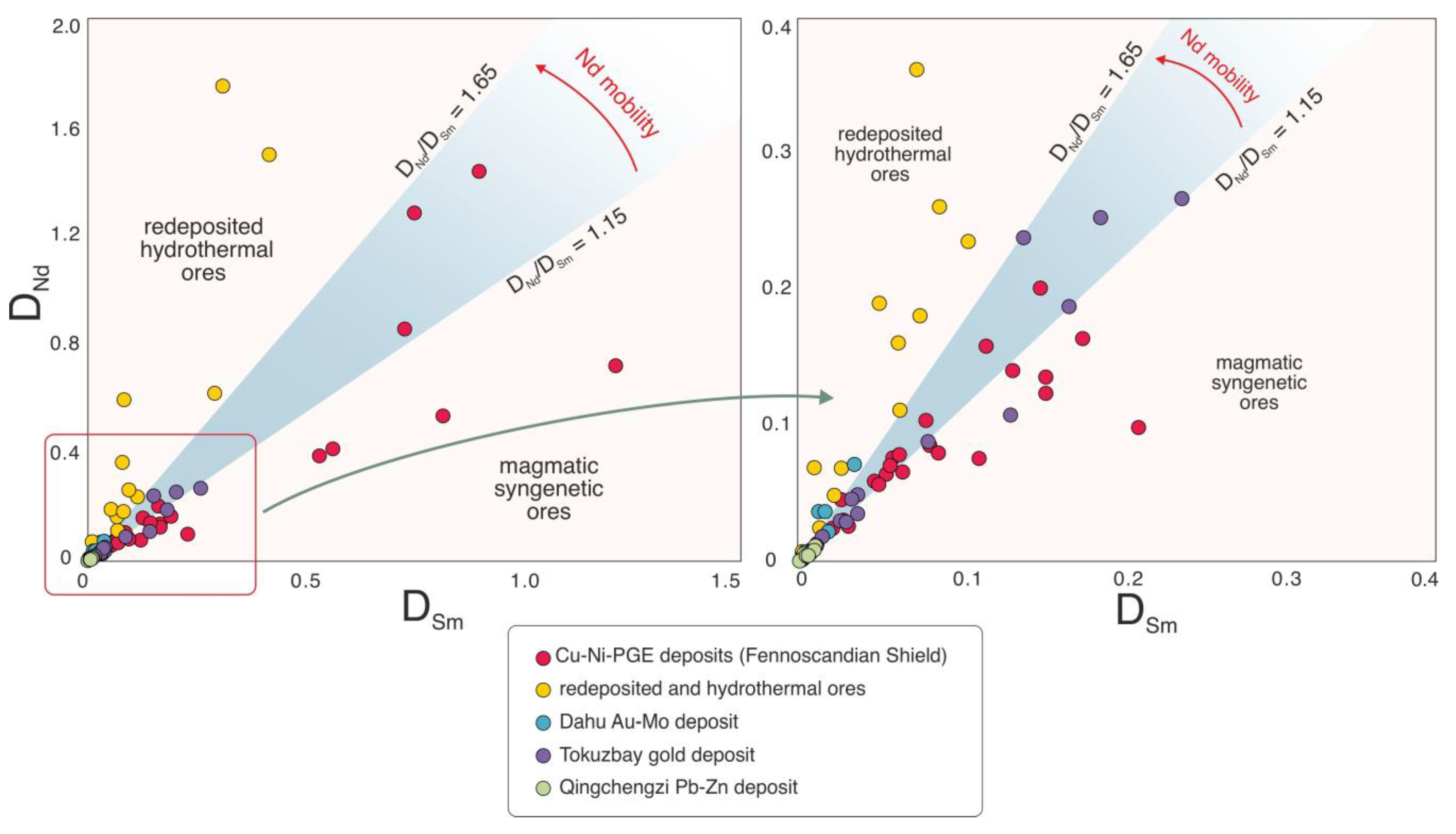
4.3. Selective Enrichment of Nd in Sulfides
4.4. Nd and Sm in Magmatic Sulfides
4.5. Nd and Sm in Hydrothermal Sulfides
5. Conclusions
- (1)
- The DNd/DSm ratio is shown to increase for the sulfide minerals of late processes, which correspond to the redeposition of ores or hydrothermal or metamorphic impact. This process causes relative Nd enrichment in relation to Sm and the consequent increase in the DNd/DSm ratio for the sulfide minerals of late processes.
- (2)
- Sulfides from magmatic Cu-Ni-PGE complexes feature a more characteristic selective Nd accumulation in a sequence of pyrite–chalcopyrite–pyrrhotine–pentlandite, which corresponds to the most probable sequence of ore formation in magmatic complexes.
- (3)
- The hydrothermal sulfides feature a more characteristic REE accumulation in fluid and silicate inclusions and in crystal lattice defects. The total effect of the Nd enrichment of such sulfides will be more observable than that of the sulfides from the magmatic complexes.
- (4)
- The mineral/rock partition coefficients for Nd and Sm (the DNd/DSm ratio) in sulfides may serve as a prospective tool for the reconstruction of the sulfide mineral formation and geochemical substantiation of possible sources of ore-forming fluids for the deposits of various genetic types.
Funding
Acknowledgments
Conflicts of Interest
References
- Serov, P.A.; Bayanova, T.B. The Sulfide/Silicate Coefficients of Nd and Sm: Geochemical “Fingerprints” for the Syn- and Epigenetic Cu-Ni-(PGE) Ores in the NE Fennoscandian Shield. Minerals 2021, 11, 1069. [Google Scholar] [CrossRef]
- Ekimova, N.A.; Serov, P.A.; Bayanova, T.B.; Elizarova, I.R.; Mitrofanov, F.P. New Data on Distribution of REEs in Sulfide Minerals and Sm-Nd Dating of Ore Genesis of Layered Basic Intrusions. Dokl. Earth Sci. 2011, 436, 28–31. [Google Scholar] [CrossRef]
- Steshenko, E.N.; Nikolaev, A.I.; Bayanova, T.B.; Drogobuzhskaya, S.V.; Chashchin, V.V.; Serov, P.A.; Lyalina, L.M.; Novikov, A.I. The Paleoproterozoic Kandalaksha Anorthosite Massif: New U–Pb (ID–TIMS) Data and Geochemical Features of Zircon. Dokl. Earth Sci. 2017, 477, 1454–1457. [Google Scholar] [CrossRef]
- Bayanova, T.; Korchagin, A.; Mitrofanov, A.; Serov, P.; Ekimova, N.; Nitkina, E.; Kamensky, I.; Elizarov, D.; Huber, M. Long-Lived Mantle Plume and Polyphase Evolution of Palaeoproterozoic PGE Intrusions in the Fennoscandian Shield. Minerals 2019, 9, 59. [Google Scholar] [CrossRef] [Green Version]
- Mitrofanov, F.P.; Bayanova, T.B.; Ludden, J.N.; Korchagin, A.U.; Chashchin, V.v.; Nerovich, L.I.; Serov, P.A.; Mitrofanov, A.F.; Zhirov, D.v. Origin and Exploration of the Kola PGE-bearing Province. In Ore Deposits: Origin, Exploration, and Exploitation; Decrée, S., Robb, L., Eds.; Wiley: New York, NY, USA, 2019; pp. 1–36. [Google Scholar]
- Aibai, A.; Deng, X.; Pirajno, F.; Han, S.; Liu, W.; Li, X.; Chen, X.; Wu, Y.; Liu, J.; Chen, Y. Origin of Ore-Forming Fluids of Tokuzbay Gold Deposit in the South Altai, Northwest China: Constraints from Sr–Nd–Pb Isotopes. Ore Geol. Rev. 2021, 134, 104165. [Google Scholar] [CrossRef]
- Ni, Z.Y.; Chen, Y.J.; Li, N.; Zhang, H. Pb-Sr-Nd Isotope Constraints on the Fluid Source of the Dahu Au-Mo Deposit in Qinling Orogen, Central China, and Implication for Triassic Tectonic Setting. Ore Geol. Rev. 2012, 46, 60–67. [Google Scholar] [CrossRef]
- Yang, J.H.; Zhou, X.H. Rb-Sr, Sm-Nd, and Pb Isotopes Systematics of Pyrite: Implications for the Age and Genesis of Lode Gold Deposits. Geology 2002, 29, 711–714. [Google Scholar] [CrossRef]
- Morgan, J.W.; Wandless, G.A. Rare Earth Element Distribution in Some Hydrothermal Minerals: Evidence for Crystallographic Control. Geochim Cosmochim Acta 1980, 44, 973–980. [Google Scholar] [CrossRef]
- Kong, P.; Deloule, E.; Palme, H. REE-Bearing Sulfide in Bishunpur (LL3.1), a Highly Unequilibrated Ordinary Chondrite. Earth Planet Sci. Lett. 2000, 177, 1–7. [Google Scholar] [CrossRef]
- Chen, G.; Shao, W.; Sun, D. Genetic Mineralogy of Gold Deposits in Jiaodong Region with Emphasis on Gold Prospecting; Chongqing Publishing House: Chongqing, China, 1989. [Google Scholar]
- Rimskaya-Korsakova, M.N.; Dubinin, A.V. Rare Earth Elements in Sulfides of Submarine Hydrothermal Vents of the Atlantic Ocean. Dokl. Earth Sci. 2003, 389, 432–436. [Google Scholar]
- Cao, Z.; Cao, H.; Tao, C.; Li, J.; Yu, Z.; Shu, L. Rare Earth Element Geochemistry of Hydrothermal Deposits from Southwest Indian Ridge. Acta Oceanol. Sin. 2012, 31, 62–69. [Google Scholar] [CrossRef]
- Jiao, Q.; Wang, L.; Deng, T.; Xu, D.; Chen, G.; Yu, D.; Ye, T.; Gao, Y. Origin of the Ore-Forming Fluids and Metals of the Hetai Goldfield in Guangdong Province of South China: Constraints from C-H-O-S-Pb-He-Ar Isotopes. Ore Geol. Rev. 2017, 88, 674–689. [Google Scholar] [CrossRef]
- Ruan, B.; Liao, M.; Sun, B.; Chen, C. Origin and Nature of Parental Magma and Sulfide Segregation of the Baixintan Magmatic Ni–Cu Sulfide Deposit, Southern Central Asian Orogenic Belt (Caob), Nw China: Insights from Mineral Chemistry of Chromite and Silicate Minerals. Minerals 2020, 10, 1050. [Google Scholar] [CrossRef]
- Wang, S.; Li, H.; Zhai, S.; Yu, Z.; Cai, Z. Geochemical Features of Sulfides from the Deyin-1 Hydrothermal Field at the Southern Mid-Atlantic Ridge near 15°S. J. Ocean Univ. China 2017, 16, 1043–1054. [Google Scholar] [CrossRef]
- Zeng, Z.; Ma, Y.; Yin, X.; Selby, D.; Kong, F.; Chen, S. Factors Affecting the Rare Earth Element Compositions in Massive Sulfides from Deep-Sea Hydrothermal Systems. Geochem. Geophys. Geosystems. 2015, 18, 1541–1576. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Yang, J.H.; Zeng, Q.D.; Xie, L.W.; Zhu, Y.S.; Li, R.; Li, B. Pyrite Rb-Sr, Sm-Nd and Fe Isotopic Constraints on the Age and Genesis of the Qingchengzi Pb-Zn Deposits, Northeastern China. Ore Geol. Rev. 2020, 117, 103324. [Google Scholar] [CrossRef]
- Zhao, K.-D.; Jiang, S.-Y. Rare Earth Element and Yttrium Analyses of Sulfides from the Dachang Sn-Polymetallic Ore Field, Guangxi Province, China: Implication for Ore Genesis. Geochem. J. 2007, 41, 121–134. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.; Yang, R.; Chen, W.; Liu, R.; Tao, P. Trace Element and REE Geochemistry of Sanshenjiang Gold Deposit, Southeastern Guizhou Province, China. Chin. J. Geochem. 2014, 33, 109–118. [Google Scholar] [CrossRef]
- Karykowski, B.T.; Maier, W.D.; Groshev, N.Y.; Barnes, S.-J.; Pripachkin, P.v.; McDonald, I.; Savard, D. Critical Controls on the Formation of Contact-Style PGE-Ni-Cu Mineralization: Evidence from the Paleoproterozoic Monchegorsk Complex, Kola Region, Russia. Econ. Geol. 2018, 113, 911–935. [Google Scholar] [CrossRef]
- Nerovich, L.I.; Bayanova, T.B.; Serov, P.A.; Elizarov, D.v. Magmatic Sources of Dikes and Veins in the Moncha Tundra Massif, Baltic Shield: Isotopic-Geochronologic and Geochemical Evidence. Geochem. Int. 2014, 52, 548–566. [Google Scholar] [CrossRef]
- Sharkov, E.v.; Chistyakov, A.v. Geological and Petrological Aspects of Ni-Cu-PGE Mineralization in the Early Paleoproterozoic Monchegorsk Layered Mafic-Ultramafic Complex, Kola Peninsula. Geol. Ore Depos. 2014, 56, 147–168. [Google Scholar] [CrossRef]
- Yang, S.-H.; Hanski, E.; Li, C.; Maier, W.D.; Huhma, H.; Mokrushin, A.v.; Latypov, R.; Lahaye, Y.; O’Brien, H.; Qu, W.-J. Mantle Source of the 2.44–2.50-Ga Mantle Plume-Related Magmatism in the Fennoscandian Shield: Evidence from Os, Nd, and Sr Isotope Compositions of the Monchepluton and Kemi Intrusions. Min. Depos. 2016, 51, 1055–1073. [Google Scholar] [CrossRef]
- Hanski, E.; Huhma, H.; Smolkin, V.F.; Vaasjoki, M. The Age of the Ferropicritic Volcanics and Comagmatic Ni-Bearing Intrusions at Pechenga, Kola Peninsula, U.S.S.R. Bull. Geol. Soc. Finl. 1990, 62, 123–133. [Google Scholar] [CrossRef]
- Walker, R.J.; Morgan, J.W.; Hanski, E.J.; Smolkin, V.F. Re-Os Systematics of Early Proterozoic Ferropicrites, Pechenga Complex, Northwestern Russia: Evidence for Ancient 187Os-Enriched Plumes. Geochim. Cosmochim. Acta 1997, 61, 3145–3160. [Google Scholar] [CrossRef]
- Sharkov, E.v; Smolkin, V.F. The Early Proterozoic Pechenga-Varzuga Belt: A Case of Precambrian Back-Arc Spreading. Precambrian Res. 1997, 82, 133–151. [Google Scholar] [CrossRef]
- Smolkin, V.F.; Lokhov, K.I.; Skublov, S.G.; Sergeeva, L.Y.; Lokhov, D.K.; Sergeev, S.A. Paleoproterozoic Keulik–Kenirim Ore-Bearing Gabbro–Peridotite Complex, Kola Region: A New Occurrence of Ferropicritic Magmatism. Geol. Ore Depos. 2018, 60, 142–171. [Google Scholar] [CrossRef]
- Neradovsky, Y.; Alekseeva, S.; Chernousenko, E. Mineralogy and Process Properties of Kolvitsky Titanomagnetite Ore. IOP Conf. Ser. Earth Environ. Sci. 2019, 262, 012050. [Google Scholar] [CrossRef]
- Voitekhovskiy, Y.L.; Neradovskiy, Y.N.; Grishin, N.N.; Rakitina, E.Y.; Kasikov, A.G. Kolvitsa Field (Geology, Material Composition of Ores). Vestn. MSTU 2014, 17, 271–278. [Google Scholar]
- Schissel, D.; Tsvetkov, A.A.; Mitrofanov, F.P.; Korchagin, A.U. Basal Platinum-Group Element Mineralization in the Federov Pansky Layered Mafic Intrusion, Kola Peninsula, Russia. Econ. Geol. 2002, 97, 1657–1677. [Google Scholar] [CrossRef]
- Groshev, N.; Karykowski, B. The Main Anorthosite Layer of the West-Pana Intrusion, Kola Region: Geology and U-Pb Age Dating. Minerals 2019, 9, 71. [Google Scholar] [CrossRef] [Green Version]
- Serov, P.A. Paleoproterozoic Pt-Pd Fedorovo-Pansky and Cu-Ni-Cr Monchegorsk Ore Complexes: Age, Metamorphism, and Crustal Contamination According to Sm-Nd Data. Minerals 2021, 11, 1410. [Google Scholar] [CrossRef]
- Amelin, Y.V.; Heaman, L.M.; Semenov, V.S. U-Pb Geochronology of Layered Mafic Intrusions in the Eastern Baltic Shield: Implications for the Timing and Duration of Paleoproterozoic Continental Rifting. Precambrian Res. 1995, 75, 31–46. [Google Scholar] [CrossRef]
- Huhma, H.; Cliff, R.A.; Perttunen, V.; Sakko, M. Sm-Nd and Pb Isotopic Study of Mafic Rocks Associated with Early Proterozoic Continental Rifting: The Peräpohja Schist Belt in Northern Finland. Contrib. Mineral. Petrol. 1990, 104, 369–379. [Google Scholar] [CrossRef]
- Halkoaho, T.A.A.; Alapieti, T.T.; Lahtinen, J.J. The Sompujärvi PGE Reef in the Penikat Layered Intrusion, Northern Finland. Miner. Pet. 1990, 42, 39–55. [Google Scholar] [CrossRef]
- Iljina, M.; Maier, W.D.; Karinen, T. PGE-(Cu-Ni) Deposits of the Tornio-Näränkävaara Belt of Intrusions (Portimo, Penikat, and Koillismaa). In Mineral Deposits of Finland; Elsevier: Amsterdam, Netherlands, 2015; pp. 133–164. ISBN 9780124104761. [Google Scholar]
- Hanski, E.; Walker, R.J.; Huhma, H.; Suominen, I. The Os and Nd Isotopic Systematics of c. 2.44 Ga Akanvaara and Koitelainen Mafic Layered Intrusions in Northern Finland. Precambrian Res. 2001, 109, 73–102. [Google Scholar] [CrossRef]
- Balashov, Y.A.; Bayanova, T.B.; Mitrofanov, F.P. Isotope Data on the Age and Genesis of Layered Basic-Ultrabasic Intrusions in the Kola Peninsula and Northern Karelia, Northeastern Baltic Shield. Precambrian Res. 1993, 64, 197–205. [Google Scholar] [CrossRef]
- Bayanova, T.B. Baddeleyite: A Promising Geochronometer for Alkaline and Basic Magmatism. Petrology 2006, 14, 187–200. [Google Scholar] [CrossRef]
- Bekker, A.; Grokhovskaya, T.L.; Hiebert, R.; Sharkov, E.v.; Bui, T.H.; Stadnek, K.R.; Chashchin, V.v.; Wing, B.A. Multiple Sulfur Isotope and Mineralogical Constraints on the Genesis of Ni-Cu-PGE Magmatic Sulfide Mineralization of the Monchegorsk Igneous Complex, Kola Peninsula, Russia. Min. Depos. 2016, 51, 1035–1053. [Google Scholar] [CrossRef]
- Chashchin, V.v.; Bayanova, T.B.; Mitrofanov, F.P.; Serov, P.A. Low-Sulfide PGE Ores in Paleoproterozoic Monchegorsk Pluton and Massifs of Its Southern Framing, Kola Peninsula, Russia: Geological Characteristic and Isotopic Geochronological Evidence of Polychronous Ore–Magmatic Systems. Geol. Ore Depos. 2016, 58, 37–57. [Google Scholar] [CrossRef]
- Hanski, E.; Huhma, H.; Vaasjoki, M. Geochronology of Northern Finland: A Summary and Discussion. Spec. Pap. Geol. Surv. Finl. 2001, 33, 255–279. [Google Scholar]
- Hanski, E.J. Evolution of the Palaeoproterozoic (2.50–1.95 Ga) Non-Orogenic Magmatism in the Eastern Part of the Fennoscandian Shield. In Reading the Archive of Earth’s Oxygenation; Melezhik, V.A., Prave, A.R., Fallick, A.E., Kump, L.R., Strauss, H., Lepland, A., Hanski, E.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 179–245. ISBN 978-3-642-29682-6. [Google Scholar]
- Smith, W.D.; Maier, W.D. The Geotectonic Setting, Age and Mineral Deposit Inventory of Global Layered Intrusions. Earth Sci. Rev. 2021, 220, 103736. [Google Scholar] [CrossRef]
- Lauri, L.S.; Mikkola, P.; Karinen, T. Early Paleoproterozoic Felsic and Mafic Magmatism in the Karelian Province of the Fennoscandian Shield. Lithos 2012, 151, 74–82. [Google Scholar] [CrossRef]
- Moilanen, M.; Hanski, E.; Konnunaho, J.; Yang, S.H.; Törmänen, T.; Li, C.; Zhou, L.M. Re-Os Isotope Geochemistry of Komatiite-Hosted Ni-Cu-PGE Deposits in Finland. Ore Geol. Rev. 2019, 105, 102–122. [Google Scholar] [CrossRef]
- Moilanen, M.; Hanski, E.; Konnunaho, J.; Törmänen, T.; Yang, S.-H.; Lahaye, Y.; O’Brien, H.; Illikainen, J. Composition of Iron Oxides in Archean and Paleoproterozoic Mafic-Ultramafic Hosted Ni-Cu-PGE Deposits in Northern Fennoscandia: Application to Mineral Exploration. Min. Depos. 2020, 55, 1515–1534. [Google Scholar] [CrossRef] [Green Version]
- Peltonen, P.; Brügmann, G. Origin of Layered Continental Mantle (Karelian Craton, Finland): Geochemical and Re–Os Isotope Constraints. Lithos 2006, 89, 405–423. [Google Scholar] [CrossRef]
- Puchtel, I.; Brügmann, G.; Hofmann, A.; Kulikov, V.; Kulikova, V. Os Isotope Systematics of Komatiitic Basalts from the Vetreny Belt, Baltic Shield: Evidence for a Chondritic Source of the 2.45 Ga Plume. Contrib. Miner. Petrol. 2001, 140, 588–599. [Google Scholar] [CrossRef]
- Vogel, D.C.; Vuollo, J.I.; Alapieti, T.T.; James, R.S. Tectonic, Stratigraphic, and Geochemical Comparisons between ca. 2500–2440Ma Mafic Igneous Events in the Canadian and Fennoscandian Shields. Precambrian Res. 1998, 92, 89–116. [Google Scholar] [CrossRef]
- Lahtinen, R.; Garde, A.A.; Victor, M. Paleoproterozoic Evolution of Fennoscandia and Greenland. Episodes 2008, 31, 20–28. [Google Scholar] [CrossRef] [Green Version]
- Melezhik, V.A.; Prave, A.R.; Fallick, A.E.; Hanski, E.J.; Lepland, A.; Kump, L.R.; Strauss, H. Reading the Archive of Earth’s Oxygenation; Frontiers in Earth Sciences; Melezhik, V., Prave, A.R., Hanski, E.J., Fallick, A.E., Lepland, A., Kump, L.R., Strauss, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 7, ISBN 978-3-642-29658-1. [Google Scholar]
- Abzalov, M.Z.; Both, R.A. The Pechenga Ni-Cu Deposits, Russia: Data on PGE and Au Distribution and Sulphur Isotope Compositions. Miner. Pet. 1997, 61, 119–143. [Google Scholar] [CrossRef]
- Balabonin, N.L.; Distler, V.V.; Dokuchaeva, V.S.; Neradovsky, Y.N.; Orsoev, D.A.; Osokin, A.S.; Filimonova, A.A.; Yakovlev, Y.N.; Yakovleva, A.K. Mineralogy of Sulfide Copper-Nickel Deposits of the Kola Peninsula; Gorbunov, G.I., Ed.; Nauka: Leningrad, Russia, 1981. [Google Scholar]
- Hickey, R.L.; Frey, F.A. Geochemical Characteristics of Boninite Series Volcanics: Implications for Their Source. Geochim. Cosmochim. Acta 1982, 46, 2099–2115. [Google Scholar] [CrossRef]
- Smolkin, V.F.; Mitrofanov, F.P. Layered Intrusions of the Monchegorsk Ore District: Petrology, Mineralization, and Deep Structure; Mitrofanov, F.P., Smolkin, V.F., Eds.; Kola Science Center RAS: Apatity, Russia, 2004. [Google Scholar]
- Bayanova, T.B.; Nerovich, L.I.; Mitrofanov, F.P.; Zhavkov, V.A.; Serov, P.A. The Monchetundra Basic Massif of the Kola Region: New Geological and Isotope Geochronological Data. Dokl. Earth Sci. 2010, 431, 288. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, G.; Chai, F.M. LA-ICP-MS U-Pb Ages and Geological Implications of the Habahe Pluton at the Southern Margin of the Altay, Xinjiang. Xinjiang Geology 30. Sci. Sin. Phys Mech Astron 2010, 30, 146–151. [Google Scholar]
- Zhou, N.-W.; Guo, X.-C.; He, G.-L. LA-ICP-MS Zircon U-Pb Ages of Two Types of Ore-Bearing Dykes in the Tuokuzibayi Gold Ore District in Habahe Area of Xinjiang and Their Geological Significance. Geol. Bull. China 2012, 31, 707–715. [Google Scholar]
- Cai, K.; Sun, M.; Yuan, C.; Zhao, G.; Xiao, W.; Long, X.; Wu, F. Geochronological and Geochemical Study of Mafic Dykes from the Northwest Chinese Altai: Implications for Petrogenesis and Tectonic Evolution. Gondwana Res. 2010, 18, 638–652. [Google Scholar] [CrossRef]
- Yan, S.; Chen, W.; Wang, Y.; Zhang, Z.; Chen, B. 40Ar/39Ar dating and its significance of the Ertix gold metallogenic belt in the Altay Oregon, Xinjiang. Acta Geol. Sin. 2004, 78, 500–506. [Google Scholar]
- Zhang, H.-F.; Ying, J.-F.; Tang, Y.-J.; Li, X.-H.; Feng, C.; Santosh, M. Phanerozoic Reactivation of the Archean North China Craton through Episodic Magmatism: Evidence from Zircon U–Pb Geochronology and Hf Isotopes from the Liaodong Peninsula. Gondwana Res. 2011, 19, 446–459. [Google Scholar] [CrossRef]
- Zhai, M.; Santosh, M. Metallogeny of the North China Craton: Link with Secular Changes in the Evolving Earth. Gondwana Res. 2013, 24, 275–297. [Google Scholar] [CrossRef]
- Fang, R.H. On the Ordered Liaohe Group Sequences. Liaoning Geol. 1993, 2, 97–119. [Google Scholar]
- Duan, X.; Zeng, Q.; Yang, J.; Liu, J.; Wang, Y.; Zhou, L. Geochronology, Geochemistry and Hf Isotope of Late Triassic Magmatic Rocks of Qingchengzi District in Liaodong Peninsula, Northeast China. J. Asian Earth Sci. 2014, 91, 107–124. [Google Scholar] [CrossRef]
- Fang, R.; He, S.; Fu, D. Nonferrous Metallic Ore Deposit in the East Liaoningsouth Jilin Early Proterozoic Rift. In Geology of Coloured Metallic Mineral Deposits in North Margin of North China Terrain and Neighbor Area; Rui, Z.Y., Shi, L.D., Fang, R.H., Eds.; Geological Publishing House: Beijing, China, 1994; pp. 54–64. [Google Scholar]
- Zhao, H.-X.; Jiang, S.-Y.; Frimmel, H.E.; Dai, B.-Z.; Ma, L. Geochemistry, Geochronology and Sr–Nd–Hf Isotopes of Two Mesozoic Granitoids in the Xiaoqinling Gold District: Implication for Large-Scale Lithospheric Thinning in the North China Craton. Chem. Geol. 2012, 294, 173–189. [Google Scholar] [CrossRef]
- Li, N.; Chen, Y.-J.; Fletcher, I.R.; Zeng, Q.-T. Triassic Mineralization with Cretaceous Overprint in the Dahu Au–Mo Deposit, Xiaoqinling Gold Province: Constraints from SHRIMP Monazite U–Th–Pb Geochronology. Gondwana Res. 2011, 20, 543–552. [Google Scholar] [CrossRef]
- Kalinin, A.A.; Kaulina, T.v.; Serov, P.A. Comparison of Isotope Data Obtained with Sm-Nd and Re-Os Methods for Minerals and Rocks from the Ozernoe Ore Occurrence, Salla-Kuolajarvi Belt. Vestn. MGTU 2021, 24, 5–13. [Google Scholar] [CrossRef]
- Raczek, I.; Jochum, K.P.; Hofmann, A.W. Neodymium and Strontium Isotope Data for USGS Reference Materials BCR-1, BCR-2, BHVO-1, BHVO-2, AGV-1, AGV-2, GSP-1, GSP-2 and Eight MPI-DING Reference Glasses. Geostand. Geoanal. Res. 2003, 27, 173–179. [Google Scholar] [CrossRef]
- Tanaka, T.; Togashi, S.; Kamioka, H.; Amakawa, H.; Kagami, H.; Hamamoto, T.; Yuhara, M.; Orihashi, Y.; Yoneda, S.; Shimizu, H.; et al. JNdi-1: A Neodymium Isotopic Reference in Consistency with LaJolla Neodymium. Chem. Geol. 2000, 168, 279–281. [Google Scholar] [CrossRef]
- Ludwig, K.R. Isoplot 3.75. A Geochronological Toolkit for Microsoft Excel. Berkeley Geochronol. Cent. Spec. Publ. 2012, 4, 71. [Google Scholar]
- Bouvier, A.; Vervoort, J.D.; Patchett, P.J. The Lu–Hf and Sm–Nd Isotopic Composition of CHUR: Constraints from Unequilibrated Chondrites and Implications for the Bulk Composition of Terrestrial Planets. Earth Planet Sci. Lett. 2008, 273, 48–57. [Google Scholar] [CrossRef]
- Goldstein, S.J.; Jacobsen, S.B. Nd and Sr Isotopic Systematics of River Water Suspended Material: Implications for Crustal Evolution. Earth Planet Sci. Lett. 1988, 87, 249–265. [Google Scholar] [CrossRef]
- Elizarova, I.R.; Bayanova, T.B. Mass-Spectrometric REE Analysis in Sulphide Minerals. J. Biol. Earth Sci. 2012, 2, 45–49. [Google Scholar] [CrossRef]
- Steshenko, E.N.; Bayanova, T.B.; Serov, P.A. The Paleoproterozoic Kandalaksha-Kolvitsa Gabbro-Anorthosite Complex (Fennoscandian Shield): New U–Pb, Sm–Nd, and Nd–Sr (ID-TIMS) Isotope Data on the Age of Formation, Metamorphism, and Geochemical Features of Zircon (LA-ICP-MS). Minerals 2020, 10, 254. [Google Scholar] [CrossRef] [Green Version]
- Kunakkuzin, E.; Borisenko, E.; Nerovich, L.; Serov, P.; Bayanova, T.; Elizarov, D. The Origin and Evolution of Ore-Bearing Rocks in the Loypishnun Deposit (Monchetundra Massif, NE Fennoscandian Shield): Isotope Nd-Sr and REE Geochemical Data. Minerals 2020, 10, 286. [Google Scholar] [CrossRef] [Green Version]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Jordens, A.; Cheng, Y.P.; Waters, K.E. A Review of the Beneficiation of Rare Earth Element Bearing Minerals. Min. Eng. 2013, 41, 97–114. [Google Scholar] [CrossRef]
- She, H.; Fan, H.; Yang, K.; Liu, X.; Li, X.; Dai, Z.-H. Carbonatitic Footprints in the Bayan Obo REEs Deposit as Seen from Pyrite Geochemistry. Precambrian Res. 2022, 379, 106801. [Google Scholar] [CrossRef]
- Borst, A.M.; Smith, M.P.; Finch, A.A.; Estrade, G.; Villanova-de-Benavent, C.; Nason, P.; Marquis, E.; Horsburgh, N.J.; Goodenough, K.M.; Xu, C.; et al. Adsorption of Rare Earth Elements in Regolith-Hosted Clay Deposits. Nat. Commun. 2020, 11, 4386. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, J.M.; Wheeler, J.; Harley, S.L.; Mariani, E.; Goodenough, K.M.; Crowley, Q.; Tatham, D. Lattice Distortion in a Zircon Population and Its Effects on Trace Element Mobility and U–Th–Pb Isotope Systematics: Examples from the Lewisian Gneiss Complex, Northwest Scotland. Contrib. Miner. Petrol. 2013, 166, 21–41. [Google Scholar] [CrossRef] [Green Version]
- Mao, G.; Hua, R.; Gao, J.; Li, W.; Zhao, K.; Long, G.; Lu, H. Existing Forms of REE in Gold-Bearing Pyrite of the Jinshan Gold Deposit, Jiangxi Province, China. J. Rare Earths 2009, 27, 1079–1087. [Google Scholar] [CrossRef]
- Silyanov, S.A.; Sazonov, A.M.; Tishin, P.A.; Lobastov, B.M.; Nekrasova, N.A.; Zvyagina, E.A.; Ryabukha, M.A. Trace Elements in Sulfides and Gold of the Olimpiada Deposit (Yenisei Ridge): Ore Substance Sources and Fluid Parameters. Russ. Geol. Geophys. 2021, 62, 306–323. [Google Scholar] [CrossRef]
- Bai, Z.J.; Zhong, H.; Hu, R.Z.; Zhu, W.G. Early Sulfide Saturation in Arc Volcanic Rocks of Southeast China: Implications for the Formation of Co-Magmatic Porphyry–Epithermal Cu–Au Deposits. Geochim. Cosmochim. Acta 2020, 280, 66–84. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Pirajno, F.; Qi, J.-P. The Shanggong Gold Deposit, Eastern Qinling Orogen, China: Isotope Geochemistry and Implications for Ore Genesis. J. Asian Earth Sci. 2008, 33, 252–266. [Google Scholar] [CrossRef]
- Pirajno, F. Hydrothermal Processes and Mineral Systems; Springer: Dordrecht, The Netherlands, 2009; ISBN 978-1-4020-8612-0. [Google Scholar]
- Serov, P.A.; Kadyrov, R.I.; Kalashnikov, A.O. Microtomography of Sulfide Minerals: Study of Internal Micro-Inclusions and the Consequences for Sm-Nd Dating of Ore-Genesis. In Ultramafic-Mafic Complexes: Geology, Structure, Ore Potential; Mokrushin, A.V., Ed.; Publishing House of the FRC KSC RAS: Apatity, Russia, 2022; pp. 91–93. [Google Scholar]
- Verberne, R.; Reddy, S.M.; Saxey, D.W.; Fougerouse, D.; Rickard, W.D.A.; Quadir, Z.; Evans, N.J.; Clark, C. Dislocations in Minerals: Fast-Diffusion Pathways or Trace-Element Traps? Earth Planet Sci. Lett. 2022, 584, 117517. [Google Scholar] [CrossRef]
- Serov, P.A.; Ekimova, N.A.; Bayanova, T.B.; Mitrofanov, F.P. Sulphide Minerals as New Sm-Nd Geochronometers for Ore Genesis Dating of Mafic-Ultramafic Layered Intrusions of Baltic Shield. LITHOSPHERE 2014, 4, 11–21. [Google Scholar]
- Mills, R.A.; Elderfield, H. Rare Earth Element Geo chemistry of Hydrothermal Deposits from the Active TAG Mound, 26°N Mid-Atlantic Ridge. Geochim. Cosmochim. Acta 1995, 59, 3511–3524. [Google Scholar] [CrossRef]
- Marques, A.F.A.; Barriga, F.; Chavagnac, V.; Fouquet, Y. Mineralogy, Geochemistry, and Nd Isotope Composition of the Rainbow Hydrothermal Field, Mid-Atlantic Ridge. Min. Depos. 2006, 41, 52–67. [Google Scholar] [CrossRef] [Green Version]
- Wohlers, A.; Wood, B.J. Uranium, Thorium and REE Partitioning into Sulfide Liquids: Implications for Reduced S-Rich Bodies. Geochim. Cosmochim. Acta 2017, 205, 226–244. [Google Scholar] [CrossRef]
- Kotelnikov, A.E.; Kolmakova, D.A.; Kotelnikova, E.M. Determination of the Copper-Nickel Ores Formation Sequence of the Kun-Manye Deposit (Amur Region). RUDN J. Eng. Res. 2020, 21, 48–57. [Google Scholar] [CrossRef]
- Essarraj, S.; Zoheir, B.; Steele-macinnis, M.; Frische, M.; Khalifa, A.; Ouadjou, A. Polymetallic Sulfide—Quartz Vein System in the Koudiat Aïcha Massive Sulfide Deposit, Jebilet Massif, Morocco: Microanalytical and Fluid Inclusion Approaches. Minerals 2022, 12, 1396. [Google Scholar] [CrossRef]
- Liu, Y.C.; Song, Y.C.; Fard, M.; Zhou, L.M.; Hou, Z.Q.; Kendrick, M.A. Pyrite Re-Os Age Constraints on the Irankuh Zn-Pb Deposit, Iran, and Regional Implications. Ore Geol. Rev. 2019, 104, 148–159. [Google Scholar] [CrossRef]
- Cave, B.; Lilly, R.; Hong, W. The Effect of Co-Crystallising Sulphides and Precipitation Mechanisms on Sphalerite Geochemistry: A Case Study from the Hilton Zn-Pb (Ag) Deposit, Australia. Minerals 2020, 10, 797. [Google Scholar] [CrossRef]
- Holwell, D.A.; Fiorentini, M.L.; Knott, T.R.; McDonald, I.; Blanks, D.E.; Campbell McCuaig, T.; Gorczyk, W. Mobilisation of Deep Crustal Sulfide Melts as a First Order Control on Upper Lithospheric Metallogeny. Nat. Commun. 2022, 13, 573. [Google Scholar] [CrossRef]
- Shelton, K.L.; Cavender, B.D.; Perry, L.E.; Schiffbauer, J.D.; Appold, M.S.; Burstein, I.; Fike, D.A. Stable Isotope and Fluid Inclusion Studies of Early Zn-Cu-(Ni-Co)-Rich Ores, Lower Ore Zone of Brushy Creek Mine, Viburnum Trend MVT District, Missouri, U.S.A.: Products of Multiple Sulfur Sources and Metal-Specific Fluids. Ore Geol. Rev. 2020, 118, 103358. [Google Scholar] [CrossRef]
- Stoltnow, M.; Lüders, V.; de Graaf, S.; Niedermann, S. A Geochemical Study of the Sweet Home Mine, Colorado Mineral Belt, USA: Formation of Deep Hydrothermal Vein–Type Molybdenum Greisen and Base Metal Mineralization. Min. Depos. 2022, 57, 801–825. [Google Scholar] [CrossRef]




| Sample | Rock and Geological Setting | Concentrations in Sulfide, ppm | Concentrations in Whole Rock, ppm | DNd/DSm | |||
|---|---|---|---|---|---|---|---|
| Sm | Nd | Sm | Nd | ||||
| Monchegorsk area (Kola Peninsula, Russia) | |||||||
| 1 | B58/111 Mix | Plagioclasite | 0.030 | 0.120 | 0.970 | 4.62 | 0.83 |
| 2 | B58/111 Pn | Plagioclasite | 0.109 | 0.350 | 0.970 | 4.62 | 0.67 |
| 3 | B70/111 Mix | Olivine orthopyroxenite | 0.034 | 0.188 | 0.041 | 0.131 | 1.73 |
| 4 | MT-3 Mix | Orthopyroxenite | 0.020 | 0.090 | 0.245 | 1.055 | 1.05 |
| 5 | P-1/109 Mix | Orthopyroxenite | 0.032 | 0.123 | 0.678 | 2.09 | 1.23 |
| 6 | P-1/109 Po | Orthopyroxenite | 0.018 | 0.095 | 0.678 | 2.09 | 1.73 |
| Fedorovo-Pansky complex (Kola Peninsula, Russia) | |||||||
| 7 | FPM-1 Ccp | Gabbronorite | 0.049 | 0.248 | 0.563 | 3.12 | 0.91 |
| 8 | FPM-1 Po | Gabbronorite | 0.028 | 0.176 | 0.563 | 3.12 | 1.14 |
| 9 | FPM-1 Po-2 | Gabbronorite | 0.073 | 0.294 | 1.132 | 6.01 | 0.75 |
| 10 | FPM-1 Ccp + Pn | Gabbronorite | 0.022 | 0.122 | 1.044 | 4.99 | 1.14 |
| 11 | FPM-1 Mix | Gabbronorite | 0.424 | 1.663 | 0.563 | 3.120 | 0.71 |
| 12 | MP-1 Po | Gabbronorite | 0.029 | 0.151 | 1.044 | 4.99 | 1.11 |
| 13 | BGF-616 Py + Pn | Gabbro | 0.153 | 0.912 | 1.313 | 5.77 | 1.36 |
| 14 | BGF-616 Py | Gabbro | 0.082 | 0.452 | 1.313 | 5.77 | 1.26 |
| 15 | BGF-616 Py | Gabbro | 0.157 | 0.934 | 2.49 | 8.41 | 1.76 |
| 16 | BGF-616 Ccp | Gabbro | 0.104 | 0.597 | 1.313 | 5.77 | 1.30 |
| Pechenga (Kola Peninsula, Russia) | |||||||
| 17 | Pilg-4/3 Pn | Massive ore (Pilgujärvi) | 0.040 | 0.210 | 0.26 | 1.700 | 0.80 |
| 18 | Pilg -4/3 Po | Massive ore (Pilgujärv) | 0.180 | 2.180 | 0.260 | 1.700 | 1.85 |
| 19 | Pilg-4/3 Mix | Massive ore (Pilgujärvi) | 0.070 | 1.050 | 0.260 | 1.700 | 2.29 |
| 20 | Pilg-4/3 Ccp | Massive ore (Pilgujärvi) | 0.040 | 0.230 | 0.260 | 1.700 | 0.88 |
| 21 | KT-10 Mix | Antigorite with sulfides (Kotselvaara) | 0.291 | 1.221 | 0.260 | 1.700 | 0.64 |
| 22 | KT-6 Mix | Sulfides from talc vein (Kotselvaara) | 0.055 | 0.167 | 0.260 | 1.700 | 0.46 |
| 23 | KT-8 Mix | Sulfides from a carbonate vein (Kotselvaara) | 0.046 | 0.278 | 0.260 | 1.700 | 0.92 |
| 24 | KT-9 Mix | Quartz-sulfide vein (Kotselvaara) | 0.135 | 0.701 | 0.260 | 1.700 | 0.79 |
| Finnish Group Intrusions, Finland | |||||||
| 25 | F-6 Py | Gabbronorite (Penikat) | 0.417 | 1.706 | 0.850 | 4.41 | 0.79 |
| 26 | F-4 Mix | Gabbronorite (Penikat) | 0.114 | 0.709 | 2.00 | 10.07 | 1.23 |
| 27 | F-4 Py | Gabbronorite (Penikat) | 0.117 | 0.767 | 2.00 | 10.07 | 1.31 |
| 28 | F-4 Ccp | Gabbronorite (Penikat) | 0.109 | 0.647 | 2.10 | 10.07 | 1.19 |
| 29 | F-4 Po | Gabbronorite (Penikat) | 0.301 | 2.020 | 2.00 | 10.07 | 1.32 |
| 30 | F-8 Ccp | Gabbronorite (Penikat) | 0.005 | 0.019 | 0.710 | 2.87 | 0.86 |
| 31 | F-8 Pn | Gabbronorite (Penikat) | 0.005 | 0.017 | 0.710 | 2.87 | 0.71 |
| 32 | F-8 Pn | Gabbronorite (Penikat) | 0.008 | 0.044 | 1.044 | 4.99 | 1.00 |
| 33 | F-8 Mix | Gabbronorite (Penikat) | 0.008 | 0.038 | 0.710 | 2.87 | 1.18 |
| 34 | F-28 Ccp | Massive ores (Ahmavaara) | 0.761 | 5.140 | 1.132 | 6.01 | 1.27 |
| 35 | F-28 Pn | Massive ores (Ahmavaara) | 0.151 | 0.842 | 1.132 | 6.01 | 1.05 |
| 36 | F-28 Po | Massive ores (Ahmavaara) | 0.073 | 0.394 | 1.132 | 6.01 | 1.02 |
| Qingchengzi Pb-Zn deposits, northeastern China (data from [18]) | |||||||
| 37 | Py | Pb-Zn ores (Qingchengzi) | 0.05 | 0.65 | 16.71 | 117.3 | 1.85 |
| 38 | Py | Pb-Zn ores (Qingchengzi) | 0.02 | 0.11 | 16.71 | 117.3 | 0.76 |
| 39 | Py | Pb-Zn ores (Qingchengzi) | 0.04 | 0.17 | 16.71 | 117.3 | 0.62 |
| 40 | Py | Pb-Zn ores (Qingchengzi) | 0.02 | 0.10 | 16.71 | 117.3 | 0.86 |
| 41 | Py | Pb-Zn ores (Qingchengzi) | 0.00 | 0.02 | 16.71 | 117.3 | 0.71 |
| 42 | Py | Pb-Zn ores (Qingchengzi) | 0.17 | 1.34 | 16.71 | 117.3 | 1.11 |
| 43 | Py | Pb-Zn ores (Qingchengzi) | 0.16 | 0.99 | 16.71 | 117.3 | 0.88 |
| 44 | Py | Pb-Zn ores (Qingchengzi) | 0.08 | 0.48 | 16.71 | 117.3 | 0.85 |
| 45 | Py | Pb-Zn ores (Qingchengzi) | 0.07 | 0.50 | 16.71 | 117.3 | 0.99 |
| 46 | Py | Pb-Zn ores (Qingchengzi) | 0.10 | 0.50 | 16.71 | 117.3 | 0.75 |
| Tokuzbay gold deposit (south Altai, northwest China) (data from [6]) | |||||||
| 47 | 26-1-3 Py | Disseminated ores (Stage-1) | 0.36 | 1.41 | 2.73 | 13.1 | 0.82 |
| 48 | S3 Py | Quartz–pyrite vein (Stage-2) | 0.1 | 0.46 | 2.73 | 13.1 | 0.96 |
| 49 | S1 Py | Quartz–pyrite vein (Stage-2) | 0.07 | 0.39 | 2.73 | 13.1 | 1.16 |
| 50 | TK-Py Py | Quartz–pyrite vein (Stage-2) | 0.08 | 0.38 | 2.73 | 13.1 | 0.99 |
| 51 | 5-3-83-4 Py | Quartz–pyrite vein (Stage-2) | 0.1 | 0.64 | 2.73 | 13.1 | 1.33 |
| 52 | 33-6-Py | Quartz–polymetallic sulfides vein (Stage-3) | 0.09 | 0.60 | 2.73 | 13.1 | 1.39 |
| 53 | 33-6-Ccp | Quartz–polymetallic sulfides vein (Stage-3) | 0.04 | 0.24 | 2.73 | 13.1 | 1.25 |
| 54 | I-py | Quartz–polymetallic sulfides vein (Stage-3) | 0.03 | 0.15 | 2.73 | 13.1 | 1.04 |
| 55 | 33-3 py | Quartz–polymetallic sulfides vein (Stage-3) | 0.22 | 1.15 | 2.73 | 13.1 | 1.09 |
| 56 | 33-3-ccp | Quartz–polymetallic sulfides vein (Stage-3) | 0.46 | 2.45 | 2.73 | 13.1 | 1.11 |
| 57 | 26-1-10 Py1 | Disseminated ores (Stage-1) | 0.59 | 2.57 | 3.14 | 10.18 | 1.34 |
| 58 | 26-1-a Py1 | Disseminated ores (Stage-1) | 0.75 | 2.71 | 3.14 | 10.18 | 1.11 |
| 59 | 26-1-1 Py1 | Disseminated ores (Stage-1) | 0.44 | 2.42 | 3.14 | 10.18 | 1.70 |
| Redeposited, metamorphic, and hydrothermally altered ores | |||||||
| 60 | KT-2 Mix | Disseminated ore (Kotselvaara) | 0.100 | 2.546 | 0.260 | 1.700 | 3.89 |
| 61 | KT-4 Mix | Massive ores (Kotselvaara) | 0.013 | 0.322 | 0.260 | 1.700 | 3.79 |
| 62 | F-27 Pn | Redeposited ores (Ahmavaara) | 0.192 | 4.990 | 2.49 | 8.41 | 7.70 |
| 63 | F-27 Ccp | Redeposited ores (Ahmavaara) | 0.183 | 3.040 | 2.49 | 8.41 | 4.95 |
| 64 | F-27 Po | Redeposited ores (Ahmavaara) | 0.263 | 1.975 | 2.49 | 8.41 | 2.22 |
| 65 | Ccp | Albitites Salla-Kuolajarvi (Karelia) [70] | 0.762 | 10.52 | 2.66 | 6.01 | 6.11 |
| 66 | TK-P1 Py3 | Quartz–polymetallic sulfides vein (Stage-3) Tokuzbay gold deposit [6] | 0.03 | 0.70 | 3.14 | 10.18 | 7.20 |
| 67 | 6-x-2 Py3 | Quartz–polymetallic sulfides vein (Stage-3) Tokuzbay gold deposit [6] | 0.04 | 0.25 | 3.14 | 10.18 | 1.93 |
| 68 | B66/111 Py | Ore-bearing norites Nyud-II | 0.029 | 0.168 | 1.322 | 3.46 | 2.21 |
| 69 | B66/111 Ccp | Ore-bearing norites Nyud-II | 0.082 | 0.556 | 1.322 | 3.46 | 2.59 |
| Dahu Au-Mo deposit (data from [7]) | |||||||
| 70 | 7-002-2 Py | Dahu Au-Mo deposit | 0.06 | 0.62 | 4.90 | 16.98 | 2.98 |
| 71 | 7-005-3 Py | Dahu Au-Mo deposit | 0.01 | 0.06 | 4.90 | 16.98 | 1.73 |
| 72 | DH-3 Py | Dahu Au-Mo deposit | 0.43 | 4.42 | 4.90 | 16.98 | 2.97 |
| 73 | DH07-1 Py | Dahu Au-Mo deposit | 0.13 | 1.16 | 4.90 | 16.98 | 2.58 |
| 74 | DH07 Py | Dahu Au-Mo deposit | 0.09 | 0.37 | 4.90 | 16.98 | 1.19 |
| 75 | 7-005-1 Py | Dahu Au-Mo deposit | 0.01 | 0.12 | 4.90 | 16.98 | 3.47 |
| 76 | 7-002-1 Py | Dahu Au-Mo deposit | 0.02 | 0.12 | 4.90 | 16.98 | 1.73 |
| 77 | 35-010-1 Py | Dahu Au-Mo deposit | 0.08 | 0.62 | 4.90 | 16.98 | 2.24 |
| 78 | DH-4 Gal | Dahu Au-Mo deposit | 0.03 | 0.11 | 4.90 | 16.98 | 1.06 |
| 79 | DH08-20 Gal | Dahu Au-Mo deposit | 0.37 | 3.06 | 4.90 | 16.98 | 2.39 |
| 80 | 35-010-2 Gal | Dahu Au-Mo deposit | 0.17 | 1.21 | 4.90 | 16.98 | 2.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serov, P.A. Selective Neodymium Enrichment of Sulfides as a “Fingerprint” of Late Processes of Ore-Formation: Insight into Sm-Nd Isotopes for Sulfides from Magmatic Cu-Ni-PGE Complexes and Hydrothermal Pb-Zn, Au-Mo, and Gold Deposits. Minerals 2022, 12, 1634. https://doi.org/10.3390/min12121634
Serov PA. Selective Neodymium Enrichment of Sulfides as a “Fingerprint” of Late Processes of Ore-Formation: Insight into Sm-Nd Isotopes for Sulfides from Magmatic Cu-Ni-PGE Complexes and Hydrothermal Pb-Zn, Au-Mo, and Gold Deposits. Minerals. 2022; 12(12):1634. https://doi.org/10.3390/min12121634
Chicago/Turabian StyleSerov, Pavel A. 2022. "Selective Neodymium Enrichment of Sulfides as a “Fingerprint” of Late Processes of Ore-Formation: Insight into Sm-Nd Isotopes for Sulfides from Magmatic Cu-Ni-PGE Complexes and Hydrothermal Pb-Zn, Au-Mo, and Gold Deposits" Minerals 12, no. 12: 1634. https://doi.org/10.3390/min12121634
APA StyleSerov, P. A. (2022). Selective Neodymium Enrichment of Sulfides as a “Fingerprint” of Late Processes of Ore-Formation: Insight into Sm-Nd Isotopes for Sulfides from Magmatic Cu-Ni-PGE Complexes and Hydrothermal Pb-Zn, Au-Mo, and Gold Deposits. Minerals, 12(12), 1634. https://doi.org/10.3390/min12121634






