Silica-Resin-Bentonite Nanocomposite and Its Application in Catalysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation
2.1.1. Preparation of Activated Bentonites
2.1.2. Preparation of the Activated Bentonite Nanocomposite
2.2. Catalyst Characterization
2.3. Activity Test
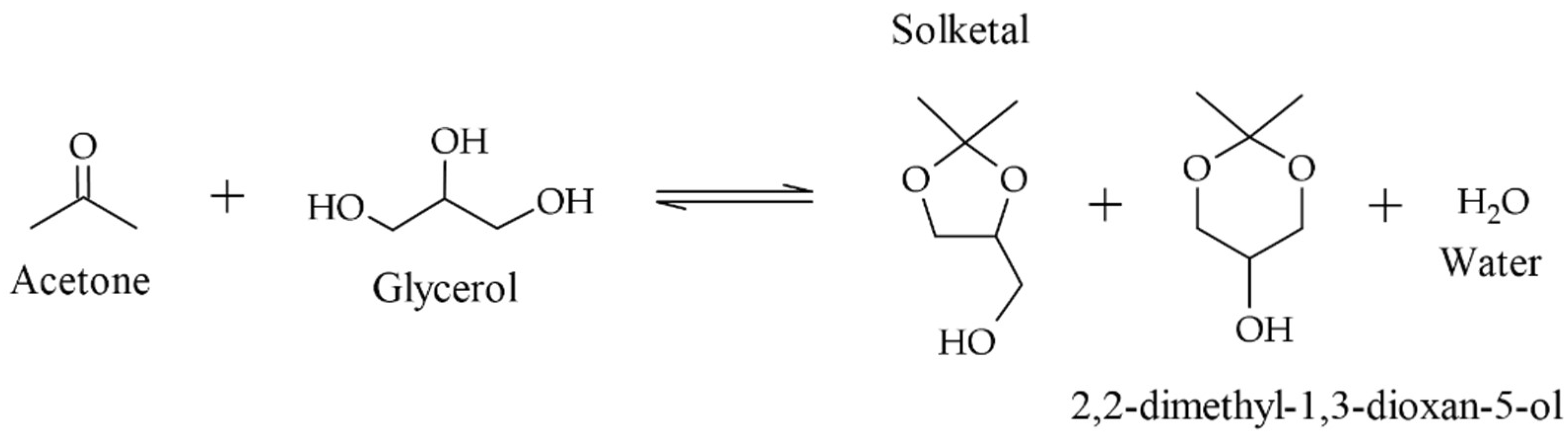
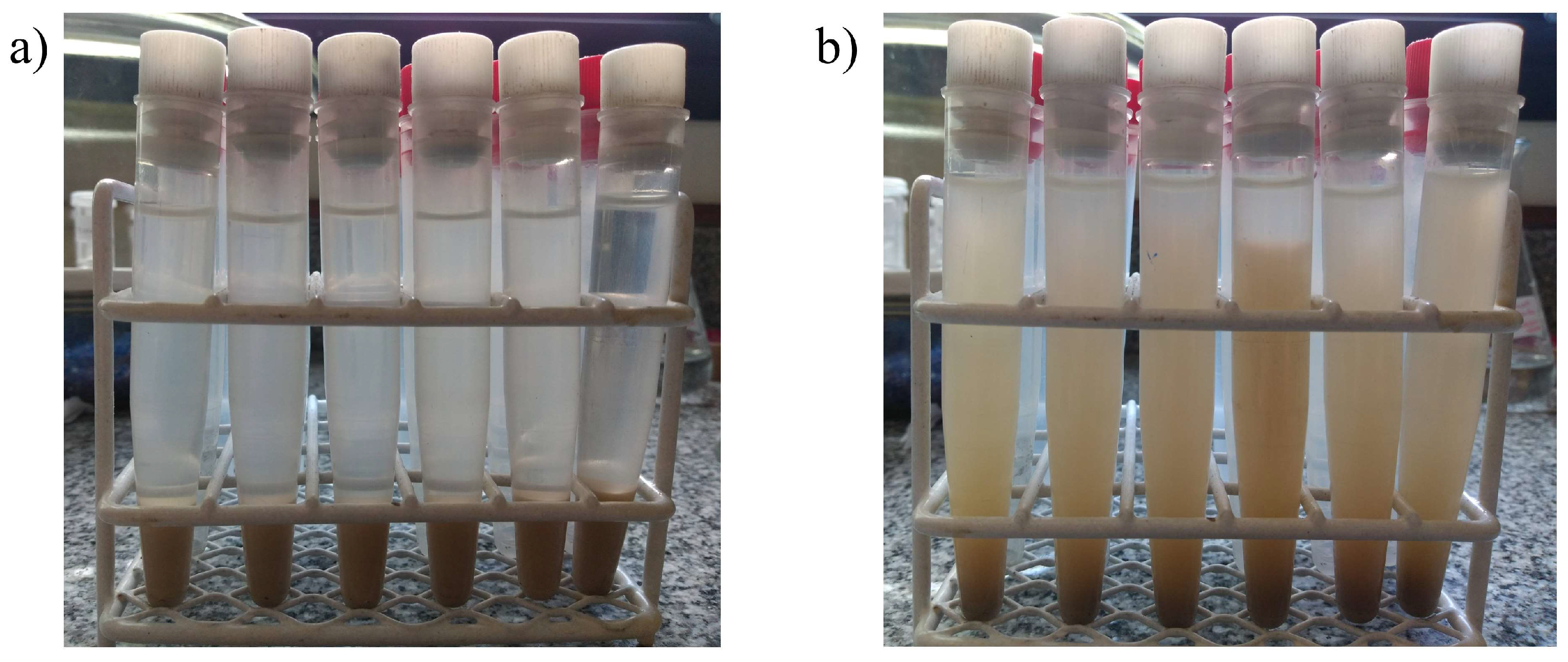
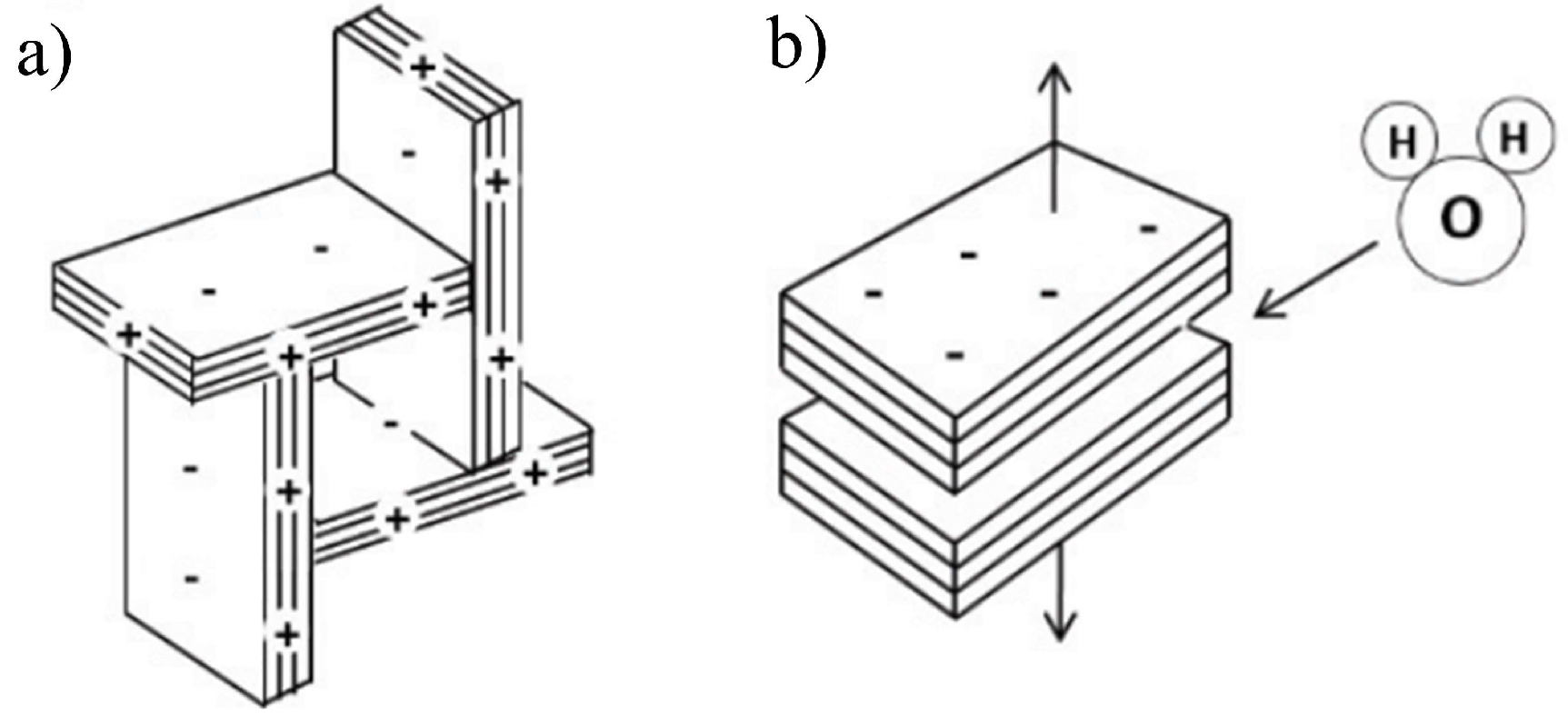
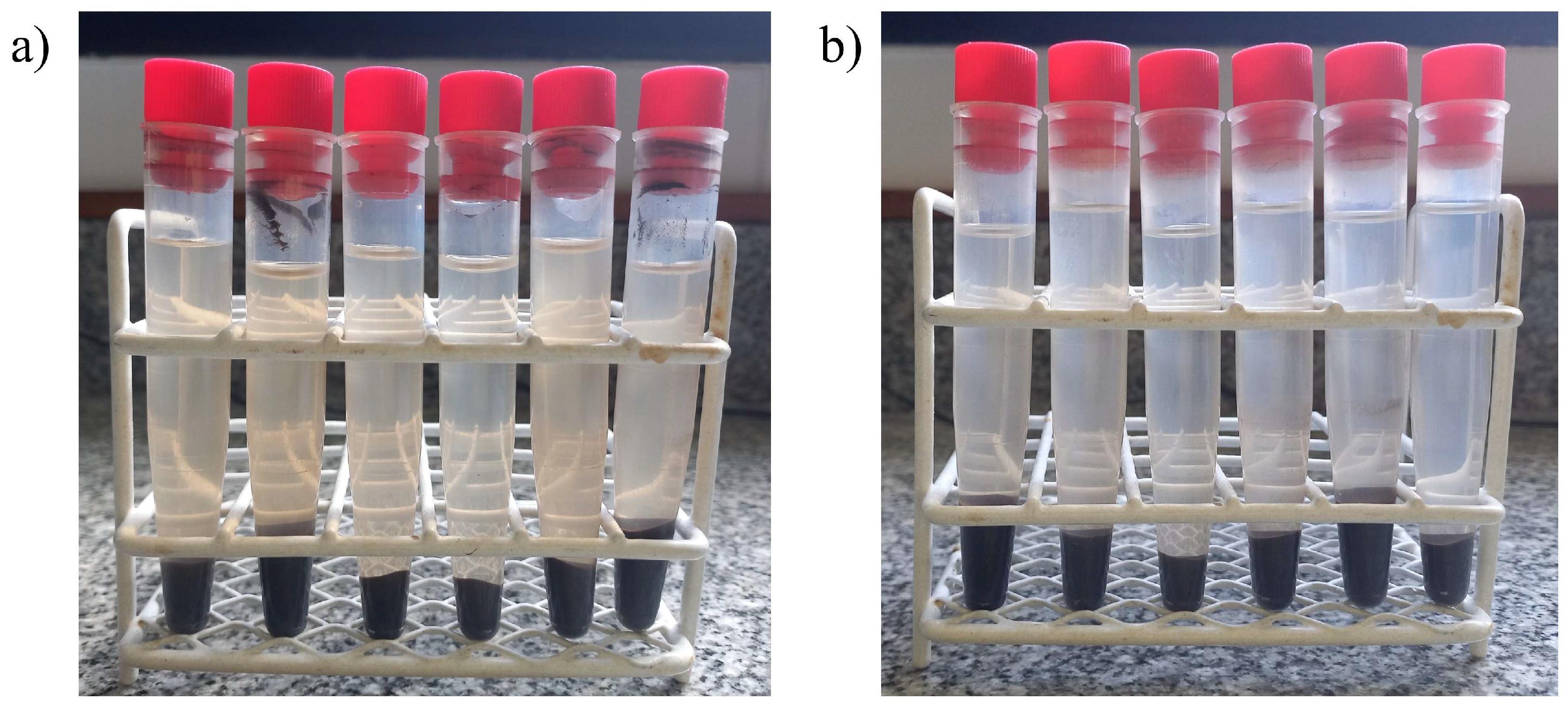
3. Results and Discussions
3.1. Precipitation Properties
3.2. Characterization Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Murray, H.H. Applied Clay Mineralogy; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Elfadly, A.M.; Zeid, I.F.; Yehia, F.Z.; Abouelela, M.M.; Rabie, A.M. Production of Aromatic Hydrocarbons from Catalytic Pyrolysis of Lignin over Acid-Activated Bentonite Clay. Fuel Process. Technol. 2017, 163, 1–7. [Google Scholar] [CrossRef]
- Rabie, A.M.; Mohammed, E.A.; Negm, N.A. Feasibility of Modified Bentonite as Acidic Heterogeneous Catalyst in Low Temperature Catalytic Cracking Process of Biofuel Production from Nonedible Vegetable Oils. J. Mol. Liq. 2018, 254, 260–266. [Google Scholar] [CrossRef]
- Nones, J.; Riella, H.G.; Trentin, A.G.; Nones, J. Effects of Bentonite on Different Cell Types: A Brief Review. Appl. Clay Sci. 2015, 105, 225–230. [Google Scholar] [CrossRef]
- Jlassi, K.; Chandran, S.; Poothanari, M.A.; Benna-Zayani, M.; Thomas, S.; Chehimi, M.M. Clay/Polyaniline Hybrid through Diazonium Chemistry: Conductive Nanofiller with Unusual Effects on Interfacial Properties of Epoxy Nanocomposites. Langmuir 2016, 32, 3514–3524. [Google Scholar] [CrossRef]
- Heydari, A.; Sheibani, H. Fabrication of Poly(β-Cyclodextrin-Co-Citric Acid)/Bentonite Clay Nanocomposite Hydrogel: Thermal and Absorption Properties. RSC Adv. 2015, 5, 82438–82449. [Google Scholar] [CrossRef]
- Ollier, R.; Rodriguez, E.; Alvarez, V. Unsaturated Polyester/Bentonite Nanocomposites: Influence of Clay Modification on Final Performance. Compos. Part A Appl. Sci. Manuf. 2013, 48, 137–143. [Google Scholar] [CrossRef]
- Rivas-Rojas, P.C.; Ollier, R.P.; Alvarez, V.A.; Huck-Iriart, C. Enhancing the Integration of Bentonite Clay with Polycaprolactone by Intercalation with a Cationic Surfactant: Effects on Clay Orientation and Composite Tensile Properties. J. Mater. Sci. 2021, 56, 5595–5608. [Google Scholar] [CrossRef]
- Seeni Meera, K.M.; Murali Sankar, R.; Murali, A.; Jaisankar, S.N.; Mandal, A.B. Sol-Gel Network Silica/Modified Montmorillonite Clay Hybrid Nanocomposites for Hydrophobic Surface Coatings. Colloids Surf. B Biointerfaces 2012, 90, 204–210. [Google Scholar] [CrossRef]
- Choudhury, P.R.; Mondal, P.; Majumdar, S. Synthesis of Bentonite Clay Based Hydroxyapatite Nanocomposites Cross-Linked by Glutaraldehyde and Optimization by Response Surface Methodology for Lead Removal from Aqueous Solution. RSC Adv. 2015, 5, 100838–100848. [Google Scholar] [CrossRef]
- Legarto, C.M.; Scian, A.; Lombardi, M.B. Preparation and Characterization of Bentonite Nanocomposites via Sol–Gel Process. SN Appl. Sci. 2019, 1, 1–7. [Google Scholar] [CrossRef]
- Tangestanifard, M.; Ghaziaskar, H.S. Arenesulfonic Acid-Functionalized Bentonite as Catalyst in Glycerol Esterification with Acetic Acid. Catalysts 2017, 7, 211. [Google Scholar] [CrossRef] [Green Version]
- Bergaya, F.; Theng, B.K.G.; Lagaly, G. Handbook of Clay Science; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Seher, H.; Geckeis, H.; Fanghänel, T.; Schäfer, T. Bentonite Nanoparticle Stability and the Effect of Fulvic Acids: Experiments and Modelling. Colloids Interfaces 2020, 4, 16. [Google Scholar] [CrossRef] [Green Version]
- Shamsuddin, R.M.; Verbeek, C.J.R.; Lay, M.C. Settling of Bentonite Particles in Gelatin Solutions for Stickwater Treatment. Procedia Eng. 2016, 148, 194–200. [Google Scholar] [CrossRef] [Green Version]
- Rutherford, D.W.; Chiou, C.T.; Eberl, D.D. Effects of Exchanged Cation on the Microporosity of Montmorillonite. Clays Clay Miner. 1997, 45, 534–543. [Google Scholar] [CrossRef]
- Torres, D.; Villarroel-Rocha, J.; Barrera, D.; Gutarra, A.; Sapag, K.; Citar, C. Effects of the Calcination Temperature and the Load of Sodium Carboxymethyl Cellulose in the Synthesis of Novel Bentonite Ceramic Foams. Rev. Av. Investig. Ing. 2017, 14, 133–144. [Google Scholar]
- Legarto, M.C.; Benito, D.; Scian, A.; Lombardi, M.B. Obtention and Characterization of a Hybrid Nanocomposite Monolith by Sol–Gel Process. Boletín Soc. Española Cerámica Vidr. 2021, 1–11. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. In Multimolecular. J. Am.Chem.Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Alexander, J.A.; Ahmad Zaini, M.A.; Surajudeen, A.; Aliyu, E.N.U.; Omeiza, A.U. Surface Modification of Low-Cost Bentonite Adsorbents—A Review. Part. Sci. Technol. 2019, 37, 534–545. [Google Scholar] [CrossRef]
- Timofeeva, M.N.; Panchenko, V.N.; Krupskaya, V.V.; Gil, A.; Vicente, M.A. Effect of Nitric Acid Modification of Montmorillonite Clay on Synthesis of Solketal from Glycerol and Acetone. Catal. Commun. 2017, 90, 65–69. [Google Scholar] [CrossRef]
- Alabarse, F.G.; Conceição, R.V.; Balzaretti, N.M.; Schenato, F.; Xavier, A.M. In-Situ FTIR Analyses of Bentonite under High-Pressure. Appl. Clay Sci. 2011, 51, 202–208. [Google Scholar] [CrossRef] [Green Version]
- Lambert, J.F.; Poncelet, G. Acidity in Pillared Clays: Origin and Catalytic Manifestations. Top. Catal. 1997, 4, 43–56. [Google Scholar] [CrossRef]
- Babaki, H.; Salem, A.; Jafarizad, A. Kinetic Model for the Isothermal Activation of Bentonite by Sulfuric Acid. Mater. Chem. Phys. 2008, 108, 263–268. [Google Scholar] [CrossRef]
- Komadel, P. Chemically Modified Smectites. Clay Miner. 2003, 38, 127–138. [Google Scholar] [CrossRef] [Green Version]
- Nanda, M.R.; Zhang, Y.; Yuan, Z.; Qin, W.; Ghaziaskar, H.S.; Xu, C. Catalytic Conversion of Glycerol for Sustainable Production of Solketal as a Fuel Additive: A Review. Renew. Sustain. Energy Rev. 2016, 56, 1022–1031. [Google Scholar] [CrossRef]
- Perez, F.M.; Nichio, N.; Pompeo, F. Thermodynamic Assessment of Chemical Equilibrium for the Synthesis of Solketal in the Liquid Phase. Chem. Eng. Technol. 2021, 44, 1356–1363. [Google Scholar] [CrossRef]
- Perez, F.M.; Legarto, C.; Lombardi, M.B.; Santori, G.F.; Pompeo, F.; Nichio, N.N. Activated Bentonite Nanocomposite for the Synthesis of Solketal from Glycerol in the Liquid Phase. Catalysts 2022, 12, 673. [Google Scholar] [CrossRef]
- Moreira, M.N.; Faria, R.P.V.; Ribeiro, A.M.; Rodrigues, A.E. Solketal Production from Glycerol Ketalization with Acetone: Catalyst Selection and Thermodynamic and Kinetic Reaction Study. Ind. Eng. Chem. Res. 2019, 58, 17746–17759. [Google Scholar] [CrossRef]
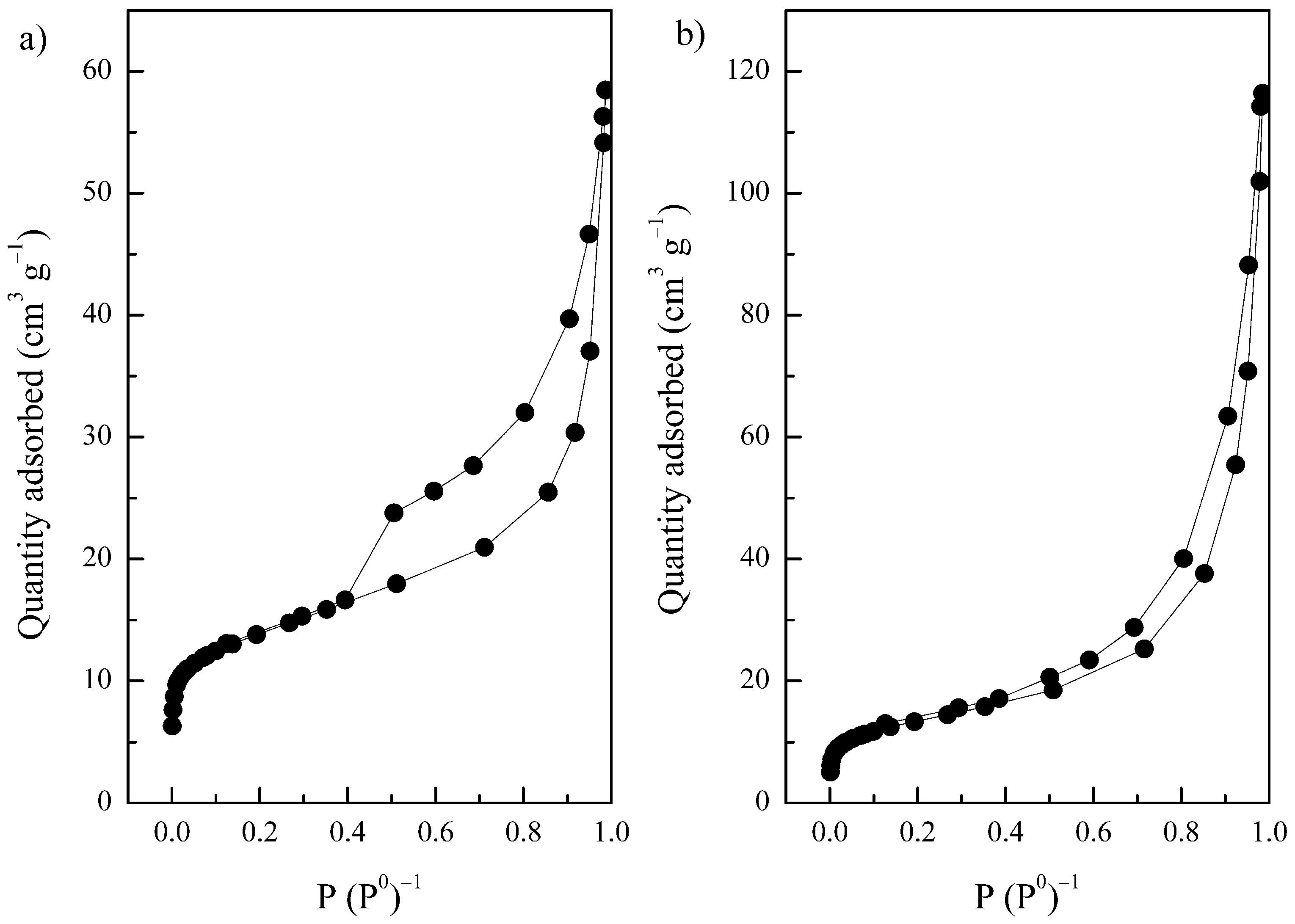

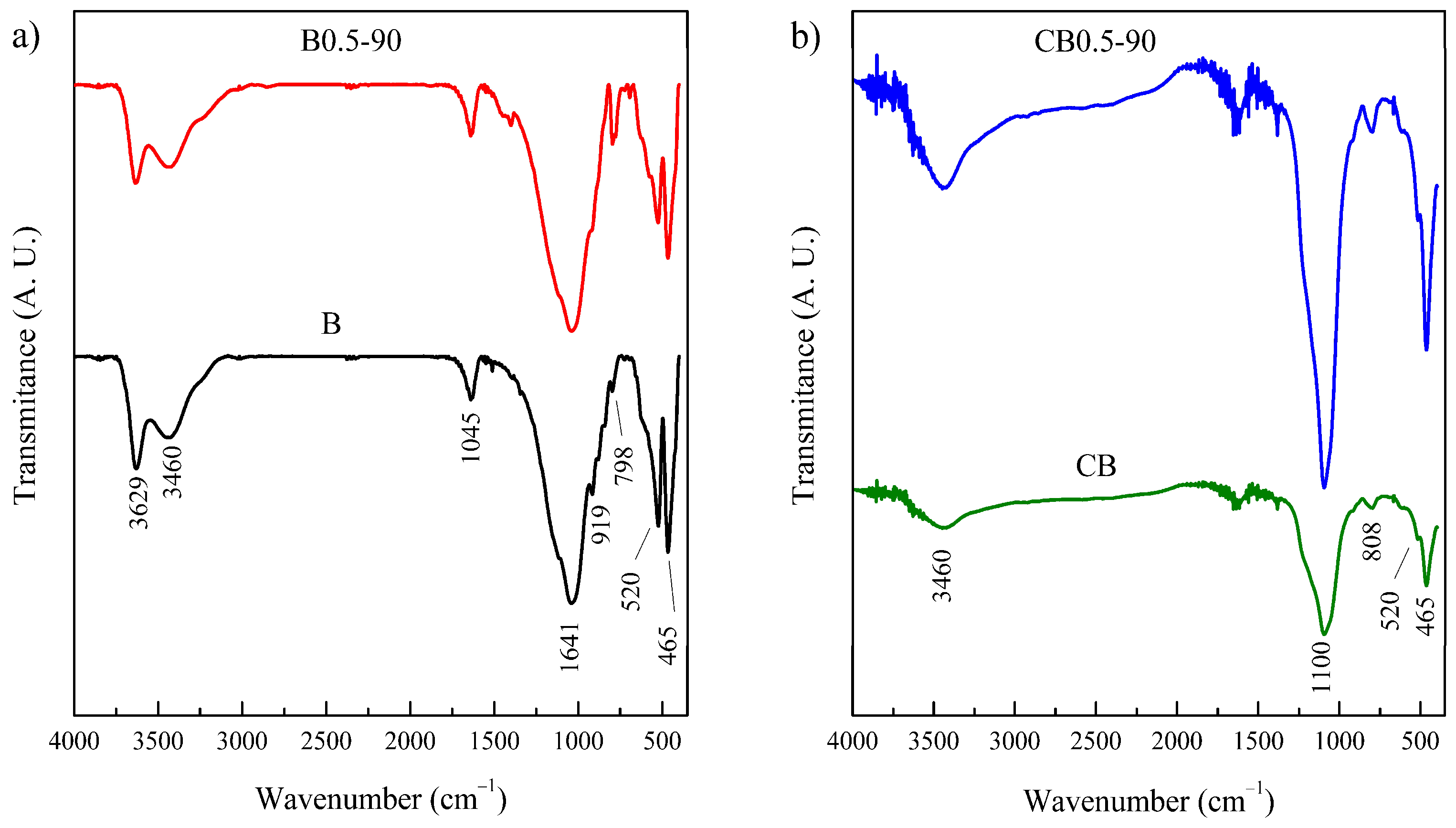
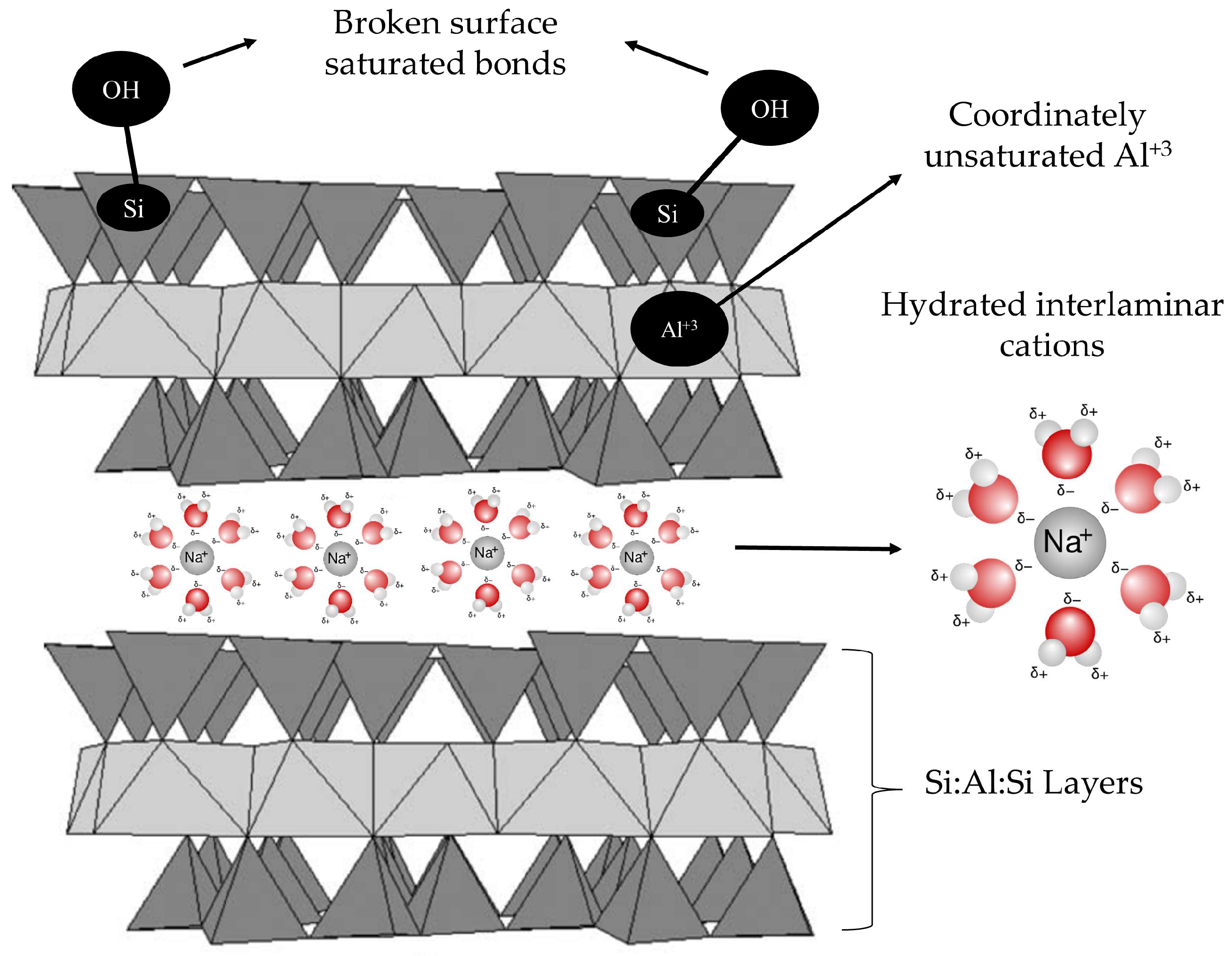

| Solid | SBET 1 | Vpore 2 | Micropores | Mesopores | ||
|---|---|---|---|---|---|---|
| Smicro 3 | Vmicro 3 | Smeso 4 | Vmeso 4 | |||
| B | 38.60 | 0.0823 | 10.42 | 0.00454 | 28.18 | 0.0778 |
| B0.15-30 | 46.98 | 0.0713 | 15.92 | 0.00686 | 31.06 | 0.0644 |
| B0.5-30 | 38.60 | 0.0598 | 12.57 | 0.00544 | 26.03 | 0.0544 |
| B0.5-90 | 40.10 | 0.0607 | 11.37 | 0.00425 | 28.73 | 0.0565 |
| CB | 45.07 | 0.1576 | 4.26 | 0.00314 | 40.81 | 0.1545 |
| CB0.5-90 | 42.28 | 0.1468 | 4.78 | 0.00356 | 37.50 | 0.1442 |
| Element in Oxide Form | Solid | |||||
|---|---|---|---|---|---|---|
| B | B0.15-30 | B0.5-30 | B0.5-90 | CB | CB0.5-90 | |
| SiO2 | 68.41 | 73.57 | 76.29 | 76.07 | 83.4 | 84.1 |
| Al2O3 | 23.26 | 21.29 | 18.99 | 20.11 | 12.2 | 13.0 |
| Na2O | 4.50 | 2.39 | 2.72 | 1.52 | 2.6 | 0.9 |
| MgO | 3.84 | 2.75 | 1.99 | 2.30 | 1.8 | 2.0 |
| Phase | 2θ (°) | PDF Card |
|---|---|---|
| Montmorillonite | 7.1; 19.7; 34.8; 54.2; 61.7 | 00-029-1498 |
| Gypsum | 11.4 | 00-021-0816 |
| Quartz | 20.8; 26.6 | 00-046-1045 |
| Feldspar | 28.4 | 00-020-0548 |
| Solid | Initial Potential (mV) |
|---|---|
| B | −62.7 |
| B0.15-30 | 384.5 |
| B0.5-30 | 374.1 |
| B0.5-90 | 496.3 |
| CB | −25.2 |
| CB0.5-90 | 450.1 |
| Result | Solid | |||||
|---|---|---|---|---|---|---|
| B | B0.15-30 | B0.5-30 | B0.5-90 | CB | CB0.5-90 | |
| Acid sites concentrations (mmol per gram of bentonite) | 1.44 | 2.38 | 2.93 | 4.1 | 1.40 | 3.9 |
| Glycerol conversion (1st use) * | 0 | 45.3 | 53.6 | 72 | 0 | 70.5 |
| Glycerol conversion (2nd use) * | 0 | 29.4 | 32.1 | 40.2 | 0 | 66.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perez, F.M.; Santori, G.F.; Pompeo, F.; Nichio, N.N. Silica-Resin-Bentonite Nanocomposite and Its Application in Catalysis. Minerals 2022, 12, 1486. https://doi.org/10.3390/min12121486
Perez FM, Santori GF, Pompeo F, Nichio NN. Silica-Resin-Bentonite Nanocomposite and Its Application in Catalysis. Minerals. 2022; 12(12):1486. https://doi.org/10.3390/min12121486
Chicago/Turabian StylePerez, Federico M., Gerardo F. Santori, Francisco Pompeo, and Nora N. Nichio. 2022. "Silica-Resin-Bentonite Nanocomposite and Its Application in Catalysis" Minerals 12, no. 12: 1486. https://doi.org/10.3390/min12121486
APA StylePerez, F. M., Santori, G. F., Pompeo, F., & Nichio, N. N. (2022). Silica-Resin-Bentonite Nanocomposite and Its Application in Catalysis. Minerals, 12(12), 1486. https://doi.org/10.3390/min12121486






