RETRACTED: UV and Visible Light Induced Photodegradation of Reactive Red 198 Dye and Textile Factory Wastewater on Fe2O3/Bentonite/TiO2 Nanocomposite
Abstract
1. Introduction
2. Material and Methods
2.1. Chemicals and Reagents
2.2. Synthesis Steps and Characterization Analysis
2.3. Photocatalytic Experiments
3. Results and Discussion
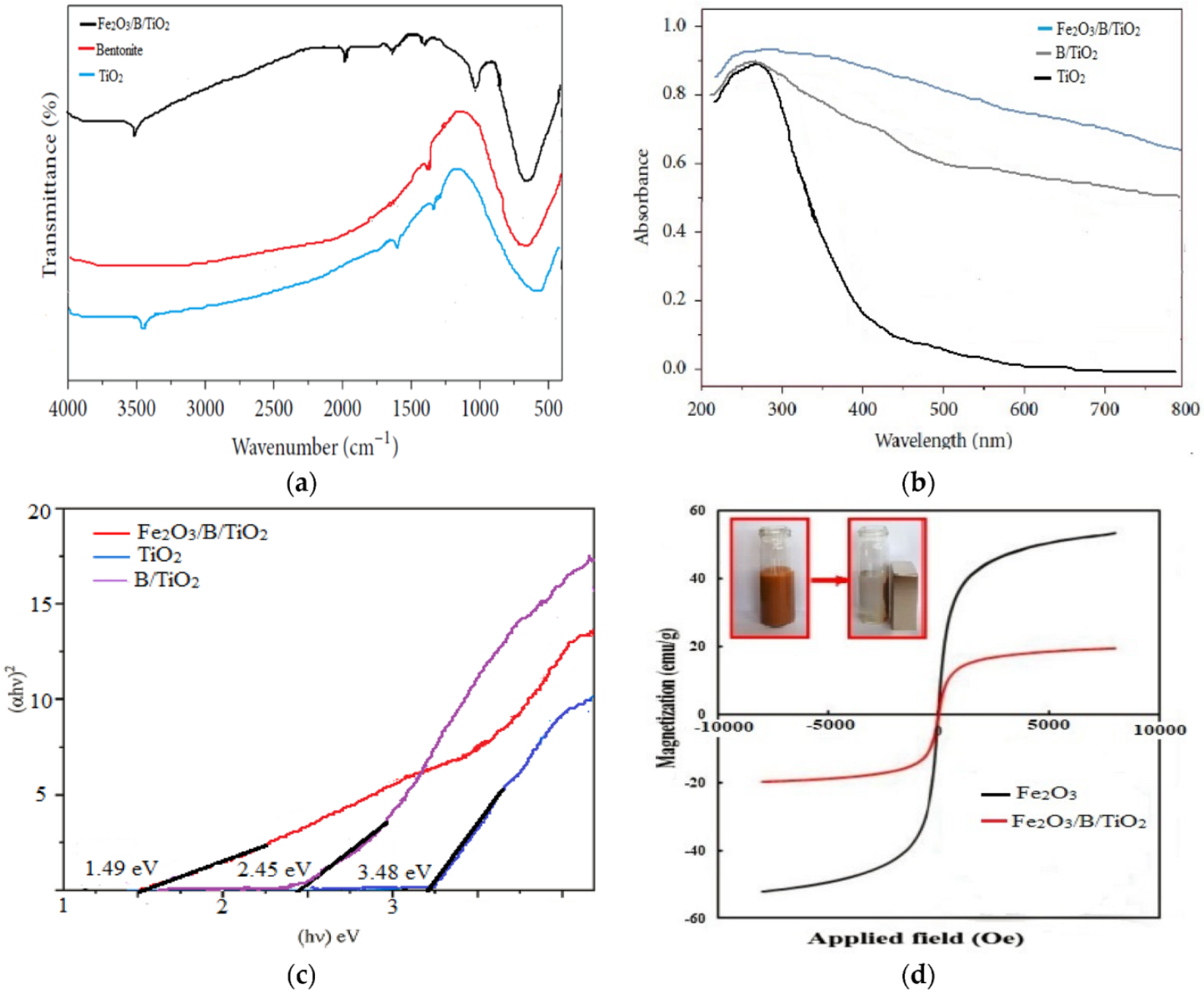
3.1. Characteristics of Fe2O3/B/TiO2 Catalyst
3.2. Comparison of RR198 Degradation by Two Methods Using UV Lamp and Visible Light
3.3. Effect of Different Parameters on RR198 Dye Degradation by Visible Light
3.4. Stability and Reusability of the Photocatalyst
3.5. Reaction Kinetics
3.6. Determining Energy Consumption
3.7. Effluent Toxicity Test
3.8. Experiments with Textile Factory Wastewater
3.9. Scavenger Test
3.10. Elucidation of the Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balarak, D.; Al-Musawi, T.J.; Mohammed, I.A.; Abasizadeh, H. The eradication of reactive black 5 dye liquid wastes using Azolla filiculoides aquatic fern as a good and an economical biosorption agent. SN Appl. Sci. 2020, 2, 1015. [Google Scholar] [CrossRef]
- Ruan, S.; Huang, W.; Zhao, M.; Song, H.; Gao, Z. A Z-scheme mechanism of the novel ZnO/CuO nn heterojunction for photocatalytic degradation of Acid Orange 7. Mater. Sci. Semicond. Process. 2020, 107, 104835. [Google Scholar] [CrossRef]
- Liu, X.; Yuan, B.; Zou, J.; Wu, L.; Dai, L.; Ma, H.; Li, K.; Ma, J. Cu (II)-enhanced degradation of acid orange 7 by Fe (II)-activated persulfate with hydroxylamine over a wide pH range. Chemosphere 2020, 238, 124533. [Google Scholar] [CrossRef] [PubMed]
- Sillanpää, M.; Mahvi, A.H.; Balarak, D.; Khatibi, A.D. Adsorption of Acid orange 7 dyes from aqueous solution using Polypyrrole/nanosilica composite: Experimental and modelling. Int. J. Environ. Anal. Chem. 2021, 101, 1–11. [Google Scholar]
- Jose, M.; Aswathi, P.; Sriram, K.; Parakh, P.; Prakash, H. Ion-exchange bonded H2Ti3O7 nanosheets-based magnetic nanocomposite for dye removal via adsorption and its regeneration via synergistic activation of persulfate. RSC Adv. 2016, 6, 80133–80144. [Google Scholar] [CrossRef]
- Dhanasekar, M.; Ratha, S.; Rout, C.S.; Bhat, S.V. Efficient sono-photocatalytic degradation of methylene blue using nickel molybdate nanosheets under diffused sunlight. J. Environ. Chem. Eng. 2017, 5, 2997–3004. [Google Scholar] [CrossRef]
- May-Lozano, M.; Mendoza-Escamilla, V.; Rojas-García, E.; López-Medina, R.; Romero, G.R.; Martinez-Delgadillo, S.A. Sonophotocatalytic degradation of Orange II dye using low cost photocatalyst. J. Clean. Prod. 2017, 148, 836–844. [Google Scholar] [CrossRef]
- Balarak, D.; Mostafapour, F.K.; Joghataei, A. Adsorption of Acid Blue 225 dye by Multi Walled Carbon Nanotubes: Determination of equilibrium and kinetics parameters. Pharm. Chem. 2016, 8, 138–145. [Google Scholar]
- Ghanbari, F.; Zirrahi, F.; Lin, K.; Kakavandi, B.; Hassani, A. Enhanced electro-peroxone using ultrasound irradiation for the degradation of organic compounds: A comparative study. J. Environ. Chem. Eng. 2020, 5, 104167. [Google Scholar] [CrossRef]
- Wang, C.Q.; Jiang, X.Y.; Huang, R.; Cao, Y.J.; Xu, J.; Han, Y.F. Copper/carbon composites from waste printed circuit boards as catalysts for Fenton-like degradation of Acid Orange 7 enhanced by ultrasound. AIChE J. 2019, 65, 1234–1244. [Google Scholar] [CrossRef]
- Ramos, D.; González, M.V.; Muñóz, R.; Cruz, J.S.D. Obtaining and Characterization of TiO2-GO Composites for Photocatalytic Applications. Int. J. Photoenergy 2020, 2020, 3489218. [Google Scholar] [CrossRef]
- Martins, P.M.; Ferreiraa, C.G.; Silva, A.R.; Magalhãesa, B. TiO2/graphene and TiO2/graphene oxide nanocomposites for photocatalytic applications: A computer modeling and experimental study. Compos. Part B 2018, 145, 39–46. [Google Scholar] [CrossRef]
- Basturk, E.; Işık, M.; Karatas, M. Removal of aniline (Methylene Blue) and azo (Reactive Red 198) dyes by photocatalysis via nano TiO2. Desalin Water Treat. 2019, 143, 306–313. [Google Scholar]
- Hasanzadeh, M.; Jorfi, S.; Ahmadi, M.; Jaafarzadeh, N. Hybrid Sono-photocatalytic degradation of Acid Brown 14 Using Persulphate and ZnO Nanoparticles: Feasibility and kinetic Study. Int. J. Environ. Anal. Chem. 2020, 100, 1–14. [Google Scholar] [CrossRef]
- Akpan, U.G.; Hameed, B.H. Parameters affecting the photocatalytic degradation of dyes using TiO2-based photocatalysts: A review. J. Hazard. Mater. 2009, 170, 520–529. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, G.; Akhtar, M.; Umar, A. Sonophotocatalytic degradation of methyl orange using ZnO nano-aggregates. J. Alloys Compd. 2015, 629, 167–172. [Google Scholar]
- Reza, K.M.; Kurny, A.; Gulshan, F. Parameters affecting the photocatalytic degradation of dyes using TiO2: A review. Appl Water Sci. 2017, 7, 1569–1578. [Google Scholar] [CrossRef]
- Elmolla, E.S.; Chaudhuri, M. Degradation of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution by the UV/ZnO photocatalytic process. J. Hazard. Mater. 2010, 173, 445–449. [Google Scholar] [CrossRef]
- Saleh, T.A.; Gondal, M.; Drmosh, Q.; Yamani, Z.; Al-Yamani, A. Enhancement in photocatalytic activity for acetaldehyde removal by embedding ZnO nano particles on multiwall carbon nanotubes. Chem. Eng. J. 2011, 166, 407–412. [Google Scholar] [CrossRef]
- Khan, M.A.N.; Siddique, M.; Wahid, F.; Khan, R. Removal of reactive blue 19 dye by sono, photo and sonophotocatalytic oxidation using visible light. Ultrason. Sonochem. 2015, 26, 370–377. [Google Scholar] [CrossRef]
- Khataee, A.; Karimi, A.; Zarei, M.; Joo, S.W. Eu-doped ZnO nanoparticles: Sonochemical synthesis, characterization, and sonocatalytic application. Ultrason. Sonochem. 2020, 67, 102822. [Google Scholar] [PubMed]
- Kakavandi, B.; Bahari, N.; Kalantary, R.R. Enhanced sono-photocatalysis of tetracycline antibiotic using TiO2 decorated on magnetic activated carbon (MAC@T) coupled with US and UV: A new hybrid system. Ultrason. Sonochem. 2019, 55, 75–85. [Google Scholar] [PubMed]
- Mirzaei, A.; Yerushalmi, L.; Chen, Z.; Haghighat, F.; Guo, J. Enhanced photocatalytic degradation of sulfamethoxazole by zinc oxide photocatalyst in the presence of fluoride ions: Optimization of parameters and toxicological evaluation. Water Res. 2018, 132, 241–251. [Google Scholar] [PubMed]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.-M. Photocatalytic degradation pathway of methylene blue in water. Appl. Catal. B 2001, 31, 145–157. [Google Scholar] [CrossRef]
- Eshaq, G.; Wang, S.; Sun, H.; Sillanpaa, M. Superior performance of FeVO4@CeO2 uniform core-shell nanostructures in heterogeneous Fenton-sonophotocatalytic degradation of 4-nitrophenol. J. Hazard. Mater. 2020, 382, 121059. [Google Scholar] [CrossRef]
- Azarpira, H.; Sadani, M.; Abtahi, M.; Vaezi, N.; Rezaei, S.Z. Photo-catalytic degradation of triclosan with UV/iodide/ZnO process: Performance, kinetic, degradation pathway, energy consumption and toxicology. J. Photochem. Photobiol. A 2019, 371, 423–432. [Google Scholar] [CrossRef]
- Isari, A.A.; Payan, A.; Fattahi, M.; Jorfi, S.; Kakavandi, B. Photocatalytic degradation of rhodamine B and real textile wastewater using Fe-doped TiO2 anchored on reduced graphene oxide (Fe-TiO2/rGO): Characterization and feasibility, mechanism and pathway studies. Appl. Surf. Sci. 2018, 62, 549–564. [Google Scholar] [CrossRef]
- Khan, S.A.; Arshad, Z.; Shahid, S.; Arshad, I.; Rizwan, K. Synthesis of TiO2/Graphene oxide nanocomposites for their enhanced photocatalytic activity against methylene blue dye and ciprofloxacin. Compos. Part B 2019, 175, 107120. [Google Scholar] [CrossRef]
- Ahmad, S.; Yasin, A. Photocatalytic degradation of deltamethrin by using Cu/TiO2/bentonite composite. Arab. J. Chem. 2020, 13, 8481–8488. [Google Scholar] [CrossRef]
- Szczepanik, B. Photocatalytic degradation of organic contaminants over clay TiO2 nano composites: A review. Appl. Clay Sci. 2017, 141, 227–239. [Google Scholar] [CrossRef]
- Silvestri, S.; Foletto, E.L. Preparation and characterization of Fe2O3/TiO2/clay plates and their use as photocatalysts. Ceram. Int. 2017, 43, 4057–14062. [Google Scholar] [CrossRef]
- Devi, L.G.; Kavitha, R. A review on non metal ion doped titania for the photocatalytic degradation of organic pollutants under UV/solar light: Role of photogenerated charge carrier dynamics in enhancing the activity. Appl. Catal. B Environ. 2013, 140–141, 559–587. [Google Scholar] [CrossRef]
- Ghorai, T.K.; Chakraborty, M.; Pramanik, P. Photocatalytic performance of nanophotocatalyst from TiO2 and Fe2O3 by mechanochemical synthesis. J. Alloys Compd. 2011, 509, 8158–8164. [Google Scholar]
- Uzunova-Bujnova, M.; Kralchevska, R.; Milanova, M.; Todorovska, R.; Hristov, D. Todorovsky, Crystal structure, morphology and photocatalytic activity of modified TiO2 and of spray-deposited TiO2 films. Catal. Today 2010, 151, 14–20. [Google Scholar] [CrossRef]
- Ziylan-Yavas, A.; Mizukoshi, Y.; Maeda, Y.; Ince, N.H. Supporting of pristine TiO2 with noble metals to enhance the oxidation and mineralization of paracetamol by sonolysis and sonophotolysis. Appl. Catal. A 2015, 172, 7–17. [Google Scholar]
- Zhu, H.; Jiang, R.; Fu, Y.; Guan, Y.; Xiao, L.; Yao, J. Effective photocatalytic decolorization of methyl orange utilizing TiO2/ZnO/chitosan nanocomposite films under simulated solar irradiation. Desalination 2012, 286, 41–48. [Google Scholar] [CrossRef]
- Kamranifar, M.; Al-Musawi, T.J.; Amarzadeh, M.; Qutob, M.; Arghavan, F.S. Quick adsorption followed by lengthy photodegradation using FeNi3@SiO2@ZnO: A promising method for complete removal of penicillin G from wastewater. J. Water Process Eng. 2021, 40, 101940. [Google Scholar]
- Rajiv, P.; Mengelizadeh, N.; McKay, G.; Balarak, D. Photocatalytic degradation of ciprofloxacin with Fe2O3 nanoparticles loaded on graphitic carbon nitride: Mineralisation, degradation mechanism and toxicity assessment. Int. J. Environ. Anal. Chem. 2021, 101, 1–15. [Google Scholar] [CrossRef]
- Arghavan, F.S.; Al-Musawi, T.J.; Allahyari, E.; Nasseh, N.; Hossein Panahi, A. Complete degradation of tamoxifen using FeNi3@SiO2@ZnO as a photocatalyst with UV light irradiation: A study on the degradation process and sensitivity analysis using ANN tool. Mater. Sci. Semicond. Process. 2021, 128, 105725. [Google Scholar] [CrossRef]
- Lin, S.H.; Chiou, C.H.; Chang, C.K.; Juang, R.S. Photocatalytic degradation of phenol on different phases of TiO2 particles in aqueous suspensions under UV irradiation. J. Environ. Manag. 2011, 92, 3098–3104. [Google Scholar] [CrossRef]
- Krishna Kumar, B.; Selvam, K.; Velmurugan, R.; Swaminathan, M. Influence of operational parameters on photomineralization of Acid Black 1 with ZnO. Desalin Water Treat. 2010, 24, 132–139. [Google Scholar] [CrossRef]
- Sarafraz, M.; Amini, M.M.; Adiban, M.; Eslami, A. Facile synthesis of mesoporous black N–TiO2 photocatalyst for efficient charge separation and the visible-driven photocatalytic mechanism of ibuprofen degradation. Mater. Sci. Semicond. Process. 2020, 120, 105258. [Google Scholar]
- Farzadkia, M.; Bazrafshan, E.; Esrafili, A.; Yang, J.-K.; Shirzad-Siboni, M. Photocatalytic degradation of Metronidazole with illuminated TiO2 nanoparticles. J. Environ. Health Sci. Eng. 2015, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Al-Musawi, T.J.; Mengelizadeh, N.; Taghavi, M.; Shehu, Z. Capability of copper-nickel ferrite nanoparticles loaded onto multi-walled carbon nanotubes to degrade acid blue 113 dye in the sonophotocatalytic treatment process. Environ. Sci. Pollut. Res. 2022, 29, 51703–51716. [Google Scholar] [CrossRef]
- Liu, X.; Li, W.; Hu, R.; Wei, Y. Synergistic degradation of acid orange 7 dye by using non-thermal plasma and g-C3N4/TiO2: Performance, degradation pathways and catalytic mechanism. Chemosphere 2020, 249, 126093. [Google Scholar] [CrossRef]
- Masoud, M.; Nourbakhsh, A.; Hassanzadeh-Tabrizi, S.A. Influence of modified CNT-Ag nanocomposite addition on photocatalytic degradation of methyl orange by mesoporous TiO2. Inorg. Nano-Met. Chem. 2017, 47, 1168–1174. [Google Scholar] [CrossRef]
- Bhavani, R.; Sivasamy, A. Sonocatalytic degradation of malachite green oxalate by a semiconductor metal oxide nanocatalyst. Ecotoxicol. Environ. Saf. 2016, 134, 403–411. [Google Scholar]
- Isari, A.A.; Mehregan, M.S.; Hayati, F.; Kalantary, R.R. Sono-photocatalytic degradation of tetracycline and pharmaceutical wastewater using WO3/CNT heterojunction nanocomposite under US and visible light irradiations: A novel hybrid system. J. Hazard. Mater. 2020, 390, 122050. [Google Scholar] [CrossRef]
- Khataee, A.; Rad, T.S.; Nikzat, S.A.; Hassani, M.H. Fabrication of NiFe layered double hydroxide/reduced graphene oxide (NiFe-LDH/rGO) nanocomposite with enhanced sonophotocatalytic activity for the degradation of moxifloxacin. Chem. Eng. J. 2019, 375, 122102. [Google Scholar] [CrossRef]
- Al-Musawi, T.J.; Mengelizadeh, N.; Sathishkumar, K.; Mohebi, S. Preparation of CuFe2O4/montmorillonite nanocomposite and explaining its performance in the sonophotocatalytic degradation process for ciprofloxacin. Colloid Interface Sci. Commun. 2021, 45, 100532. [Google Scholar]
- Balarak, D.; Rajiv, P.; Chandrika, K. Photocatalytic degradation of amoxicillin from aqueous solutions by titanium dioxide nanoparticles loaded on graphene oxide. Environ. Sci. Pollut. Res. 2021, 28, 49743–49754. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.; Mengelizadeh, N.; Saloot, M.K.; Shahbaksh, S. Facile synthesis of Fe3O4/ZnO/GO photocatalysts for decolorization of acid blue 113 under solar, visible and UV lights. Mater. Sci. Semicond. Process. 2022, 144, 106593. [Google Scholar] [CrossRef]
- Al-Musawi, T.J.; Rajiv, P.; Mengelizadeh, N.; Sadat Arghavan, F. Photocatalytic efficiency of CuNiFe2O4 nanoparticles loaded on multi-walled carbon nanotubes as a novel photocatalyst for ampicillin degradation. J. Mol. Liq. 2021, 337, 116470. [Google Scholar] [CrossRef]
- Dianati, R.A.; Mengelizadeh, N.; Zazouli, M.A. Photocatalytic degradation of bisphenol A by GO-TiO2 nanocomposite under ultraviolet light: Synthesis, effect of parameters and mineralisation. Int. J. Environ. Anal. Chem. 2022. [Google Scholar] [CrossRef]








| Material | Al2O3 | SiO2 | Fe2O3 | TiO2 | Na2O | MgO | CaO | MnO | K2O |
|---|---|---|---|---|---|---|---|---|---|
| Bentonite | 24.4 | 59.9 | 2.65 | ---- | 1.73 | 4.55 | 2.64 | 0.86 | 2.75 |
| Fe2O3/B/TiO2 | 20.7 | 34.7 | 20.4 | 11.6 | 1.65 | 4.39 | 2.6 | 0.85 | 2.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammadhosseini, S.; Al-Musawi, T.J.; Romero Parra, R.M.; Qutob, M.; Gatea, M.A.; Ganji, F.; Balarak, D. RETRACTED: UV and Visible Light Induced Photodegradation of Reactive Red 198 Dye and Textile Factory Wastewater on Fe2O3/Bentonite/TiO2 Nanocomposite. Minerals 2022, 12, 1417. https://doi.org/10.3390/min12111417
Mohammadhosseini S, Al-Musawi TJ, Romero Parra RM, Qutob M, Gatea MA, Ganji F, Balarak D. RETRACTED: UV and Visible Light Induced Photodegradation of Reactive Red 198 Dye and Textile Factory Wastewater on Fe2O3/Bentonite/TiO2 Nanocomposite. Minerals. 2022; 12(11):1417. https://doi.org/10.3390/min12111417
Chicago/Turabian StyleMohammadhosseini, Shakiba, Tariq J. Al-Musawi, Rosario Mireya Romero Parra, Mutaz Qutob, M. Abdulfadhil Gatea, Fatemeh Ganji, and Davoud Balarak. 2022. "RETRACTED: UV and Visible Light Induced Photodegradation of Reactive Red 198 Dye and Textile Factory Wastewater on Fe2O3/Bentonite/TiO2 Nanocomposite" Minerals 12, no. 11: 1417. https://doi.org/10.3390/min12111417
APA StyleMohammadhosseini, S., Al-Musawi, T. J., Romero Parra, R. M., Qutob, M., Gatea, M. A., Ganji, F., & Balarak, D. (2022). RETRACTED: UV and Visible Light Induced Photodegradation of Reactive Red 198 Dye and Textile Factory Wastewater on Fe2O3/Bentonite/TiO2 Nanocomposite. Minerals, 12(11), 1417. https://doi.org/10.3390/min12111417





