Source and Evolution of Subduction–Related Hot Springs Discharged in Tengchong Geothermal Field, Southwest China: Constrained by Stable H, O, and Mg Isotopes
Abstract
:1. Introduction
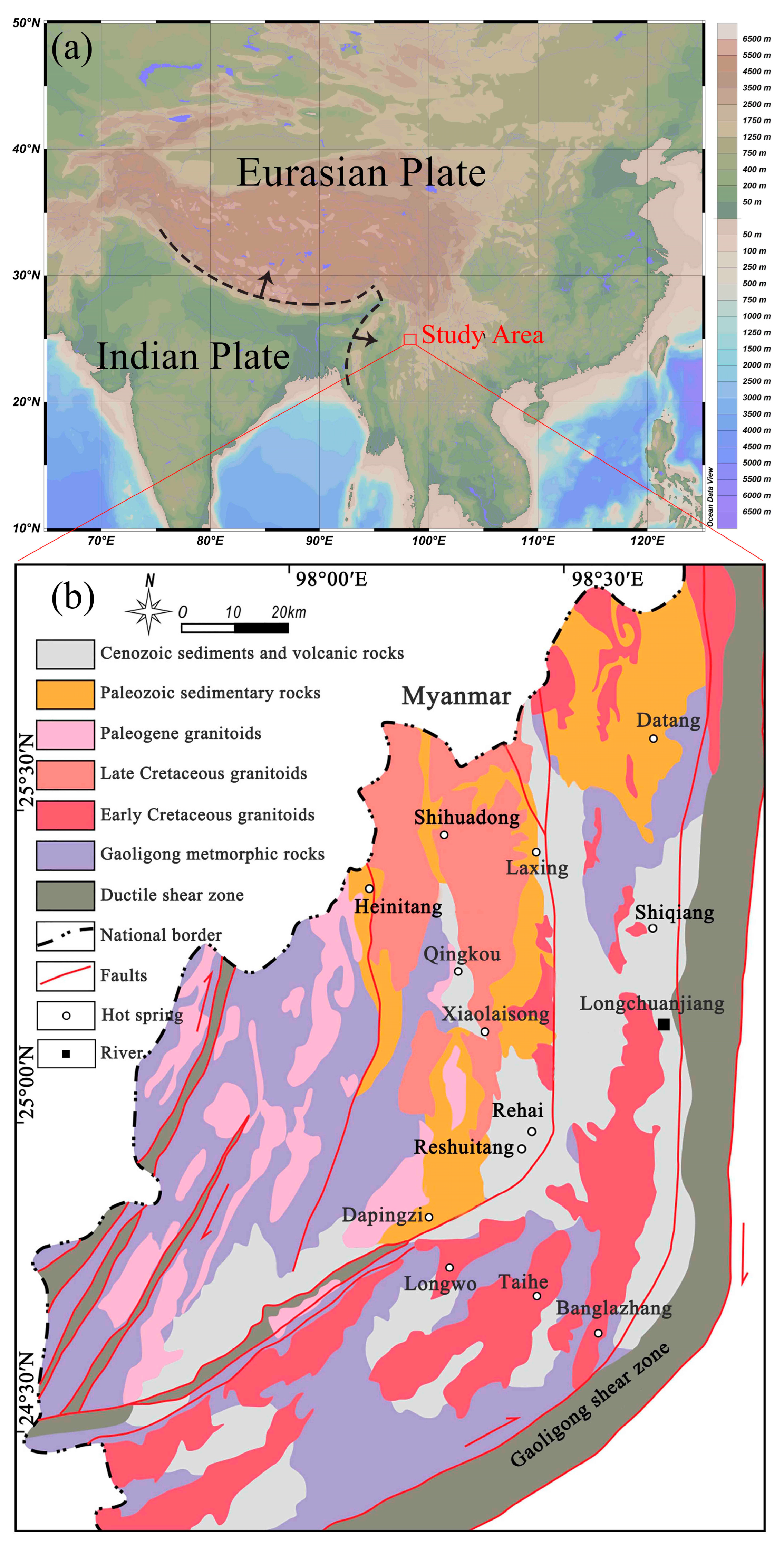
2. Geological Setting
3. Materials and Methods
3.1. Sampling
3.2. Analytical Methods
3.3. Calculations and Statistical Analyses
4. Results
4.1. Chemical Compositions of the Tengchong Hydrothermal Fluids
4.2. Stable H and O Isotopes
4.3. Mg Isotopes
5. Discussion
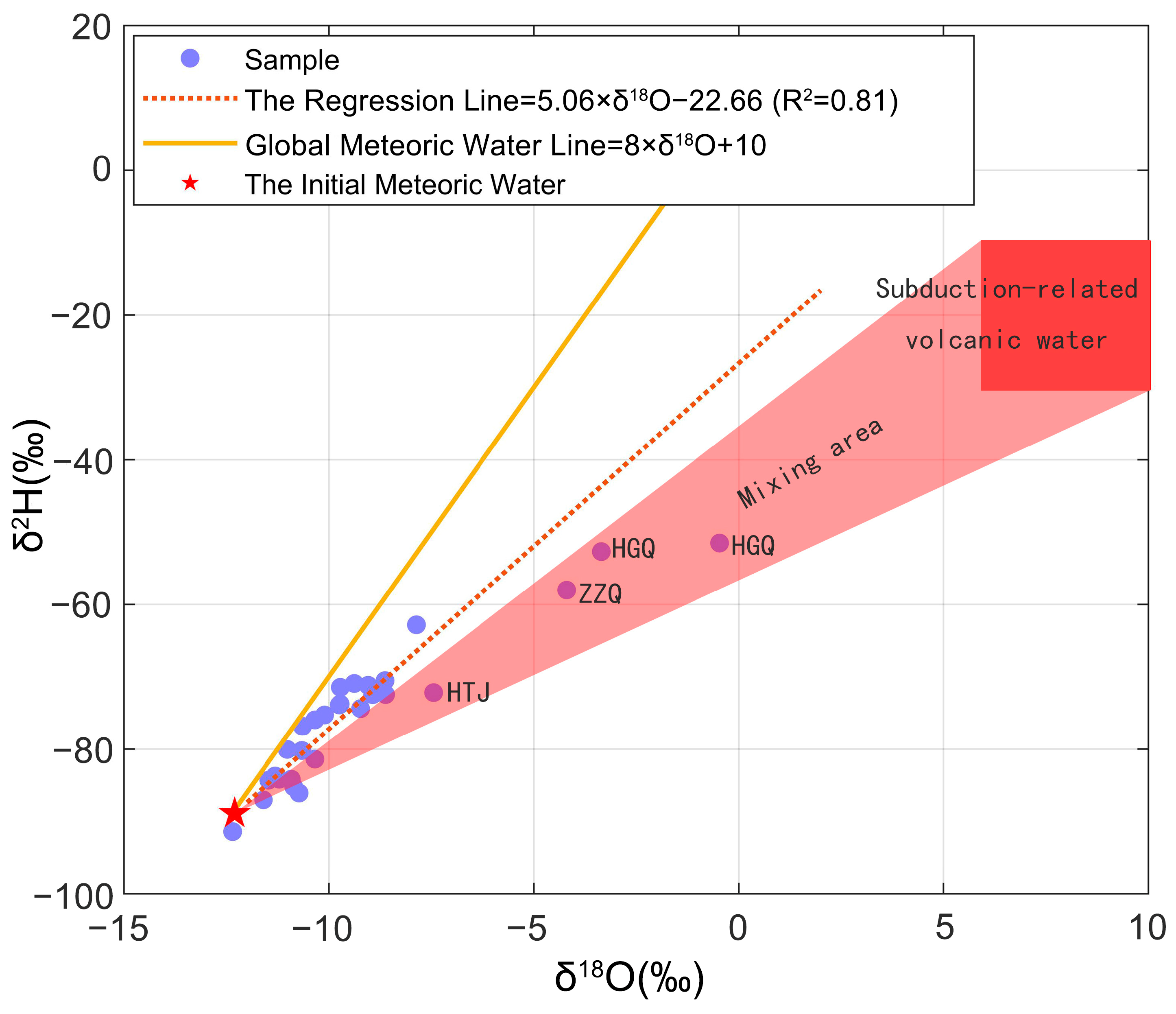
5.1. Source of Vent Fluid
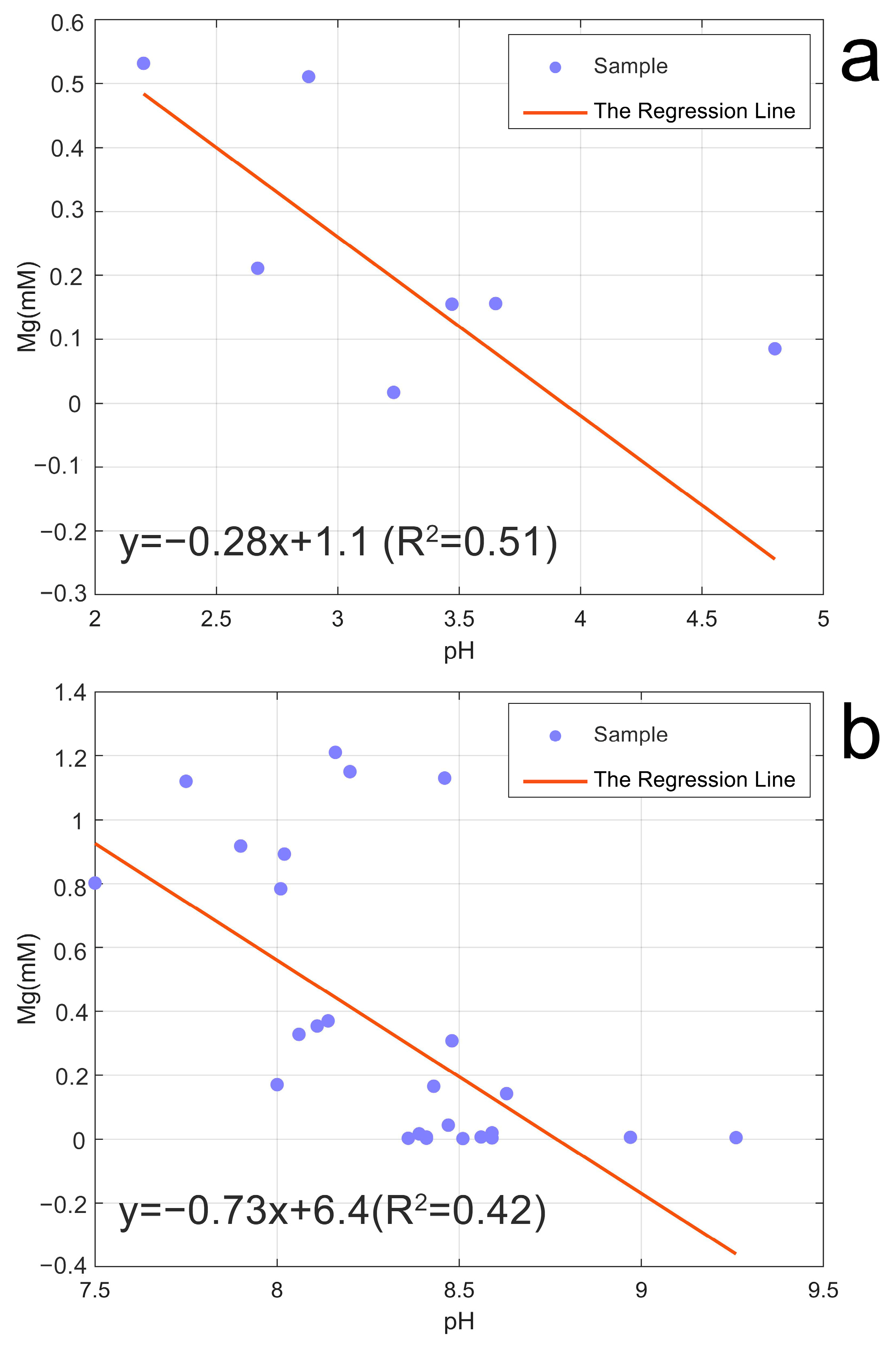
5.2. Magnesium Is Contributed by Percolated Meteoric Water
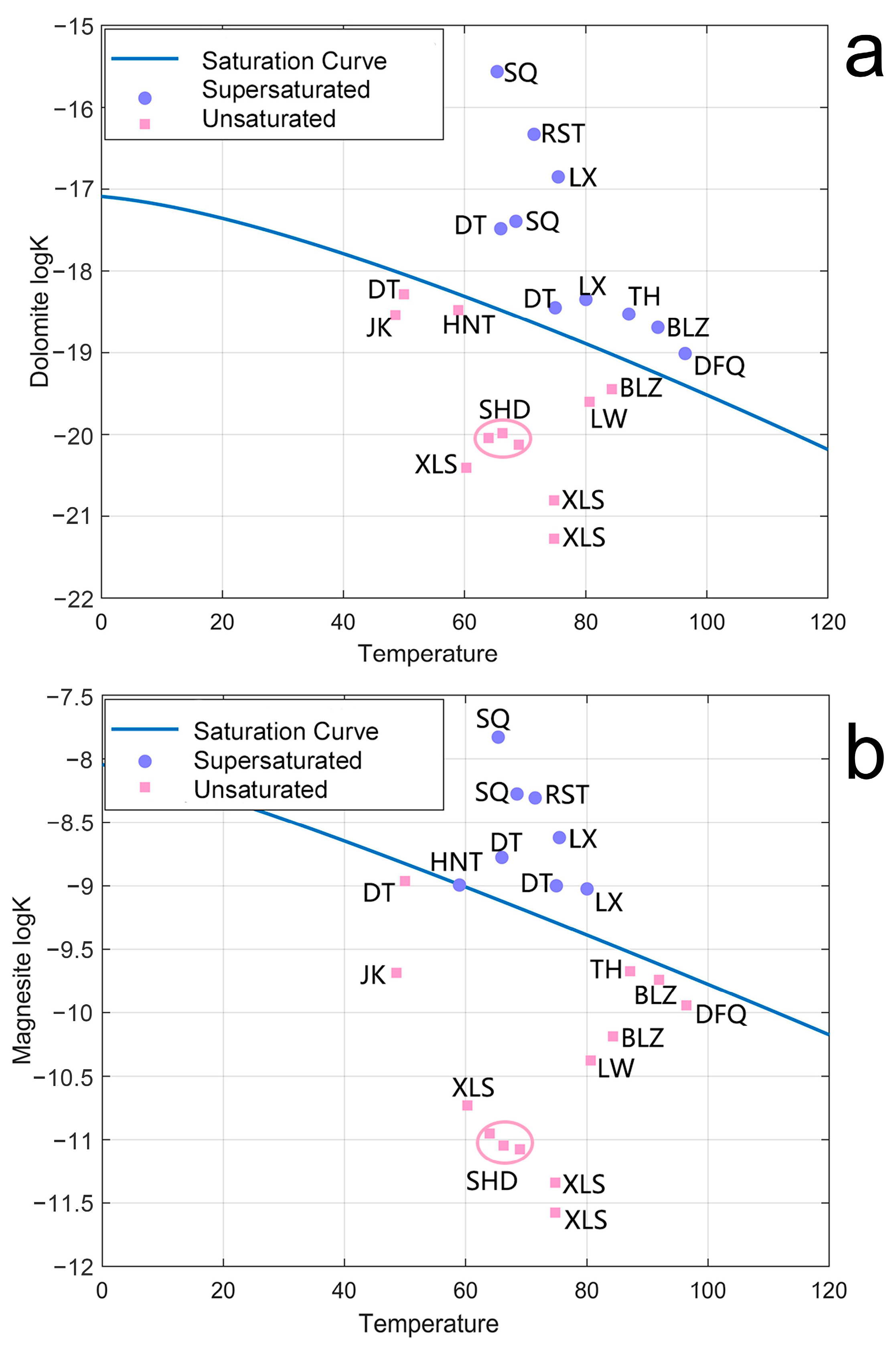
5.3. Precipitation Dissolution Equilibrium of Mg
5.4. Mg Isotope Fractionation Caused by Precipitation of Carbonate
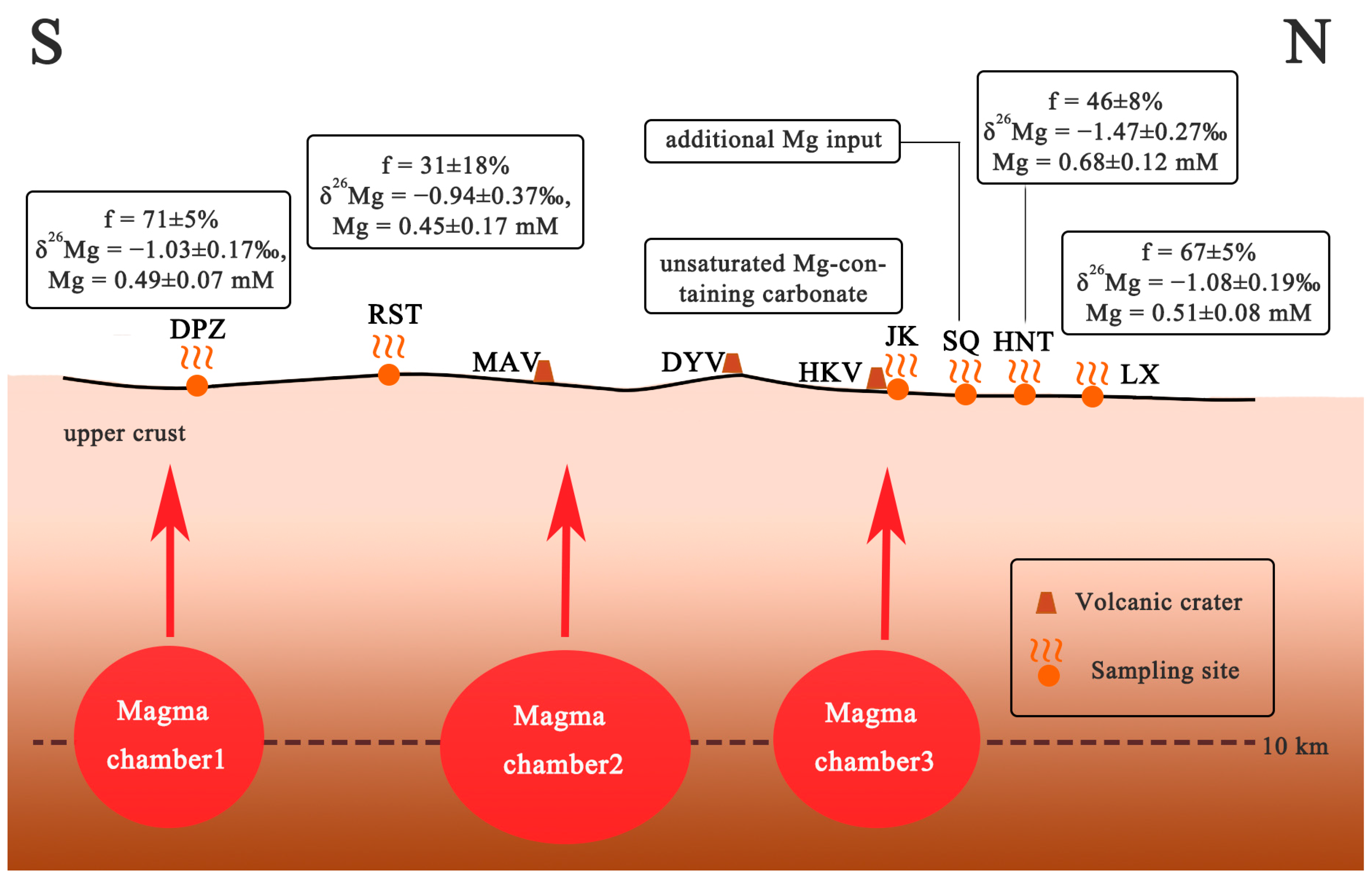
5.5. Implication for Water and Mg Cycles in the Tengchong Geothermal Systems
6. Conclusions
- (1)
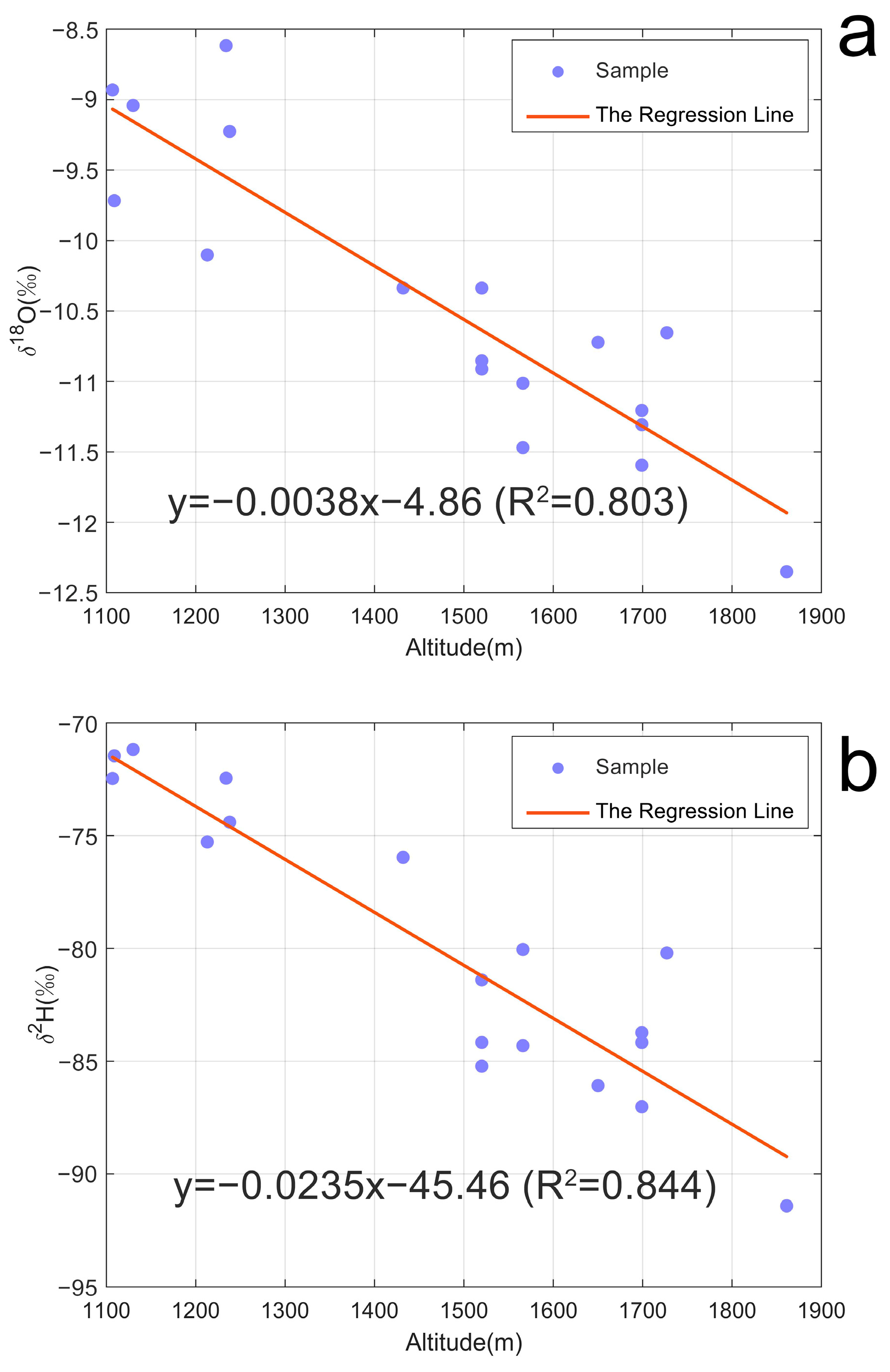
- The δ2H of the Tengchong hydrothermal fluid samples falls in a range from −91‰ to −51‰, with an average of −75.12‰. The δ18O values vary from −12.3‰ to −0.5‰, showing an average of −9.24‰. The hydrogen and oxygen isotopic compositions illustrate that meteoric water is the primary water source of the Tengchong hydrothermal fluids. However, the samples from the Rehai were affected by the mixing process of the subduction–related volcanic water. Additionally, the δ2H and δ18O values show a good correlation with the altitude, and the equation for the altitude effect of local hydrogen and oxygen isotopes in Tengchong is proposed: ; .
- (2)
- We measured the Mg isotopic composition of the hydrothermal fluids in Tengchong for the first time. The δ26Mg values (−0.97 ± 0.04‰~0.17 ± 0.01‰) varied considerably in different vents, and they showed lighter Mg isotopic compositions compared to host rock, indicating Mg isotopes fractionation during hydrothermal circulation. The relationship between the Mg concentration and the δ26Mg values suggests that the water–rock reaction is responsible, which dominantly controls the Mg content in the Tengchong hydrothermal system. By simulating the precipitation dissolution of the Mg-containing carbonates, we found that this process can significantly change the δ26Mg values. The δ26Mg values and Mg contents of hydrothermal fluids before the carbonate precipitation were about −1.13 ± 0.25‰ and 0.533 ± 0.11 mM. The removal rates of Mg ranged from 31 to 71%. In contrast, SQ exhibits extremely high δ26Mg values and Mg content, which suggests an input of additional Mg sources, which are possibly influenced by the upper crust.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- German, C.R.; Seyfried, W.E. 8.7-Hydrothermal Processes, Treatise on Geochemistry, 2nd ed.; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Oxford, UK, 2014; pp. 191–233. [Google Scholar]
- Mottl, M.J.; Wheat, C. Hydrothermal circulation through mid-ocean ridge flanks: Fluxes of heat and magnesium. Geochim. Cosmochim. Acta 1994, 58, 2225–2237. [Google Scholar] [CrossRef]
- Tian, J.; Pang, Z.; Guo, Q.; Wang, Y.; Li, J.; Huang, T.; Kong, Y. Geochemistry of geothermal fluids with implications on the sources of water and heat recharge to the Rekeng high-temperature geothermal system in the Eastern Himalayan Syntax. Geothermics 2018, 74, 92–105. [Google Scholar] [CrossRef]
- Liu, C.-M.; Song, S.-R.; Chen, Y.-L.; Tsao, S. Characteristics and Origins of Hot Springs in the Tatun Volcano Group in Northern Taiwan. Terr. Atmos. Ocean. Sci. 2011, 22, 475–489. [Google Scholar] [CrossRef] [Green Version]
- Haymon, R.M.; Fornari, D.J.; Edwards, M.H.; Carbotte, S.; Wright, D.; Macdonald, K.C. Hydrothermal vent distribution along the East Pacific Rise crest (9°09′–54′ N) and its relationship to magmatic and tectonic processes on fast-spreading mid-ocean ridges. Earth Planet. Sci. Lett. 1991, 104, 513–534. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.-G.; Yu, M.-Z.; Qiu, Z.; Loh, P.-S.; Chen, C.-T.A.; Garbe-Schönberg, D.; Schmidt, M.; Wang, X.; Ye, Y. High-Ca vent fluids discharged from the Lutao arc volcanic hydrothermal system are associated with albitization and recycling of marine carbonate. Chem. Geol. 2021, 585, 12058. [Google Scholar] [CrossRef]
- Tian, J.; Pang, Z.; Wang, Y.; Guo, Q. Fluid geochemistry of the Cuopu high temperature geothermal system in the eastern Himalayan syntaxis with implication on its genesis. Appl. Geochem. 2019, 110, 104422. [Google Scholar] [CrossRef]
- Guo, Q.; Liu, M.; Li, J.; Zhang, X.; Wang, Y. Acid hot springs discharged from the Rehai hydrothermal system of the Tengchong volcanic area (China): Formed via magmatic fluid absorption or geothermal steam heating? Bull. Volcanol. 2014, 76, 1–12. [Google Scholar] [CrossRef]
- Eom, J.; Yoshimura, T.; Araoka, D.; Gamo, T.; Kawahata, H. Magnesium isotopic composition of submarine vent fluids from arc and back-arc hydrothermal systems in the western Pacific. Chem. Geol. 2020, 551, 119767. [Google Scholar] [CrossRef]
- Von Damm, K.; Bray, A.; Buttermore, L.; Oosting, S. The geochemical controls on vent fluids from the Lucky Strike vent field, Mid-Atlantic Ridge. Earth Planet. Sci. Lett. 1998, 160, 521–536. [Google Scholar] [CrossRef]
- Guo, Q. Hydrogeochemistry of high-temperature geothermal systems in China: A review. Appl. Geochem. 2012, 27, 1887–1898. [Google Scholar] [CrossRef]
- Teng, F.-Z.; Li, W.-Y.; Ke, S.; Marty, B.; Dauphas, N.; Huang, S.; Wu, F.-Y.; Pourmand, A. Magnesium isotopic composition of the Earth and chondrites. Geochim. Cosmochim. Acta 2010, 74, 4150–4166. [Google Scholar] [CrossRef]
- Li, W.-Y.; Teng, F.-Z.; Wing, B.A.; Xiao, Y. Limited magnesium isotope fractionation during metamorphic dehydration in metapelites from the Onawa contact aureole, Maine. Geochem. Geophys. Geosyst. 2014, 15, 408–415. [Google Scholar] [CrossRef]
- Wang, S.J.; Teng, F.Z.; Li, S.G.; Hong, J.A. Magnesium isotopic systematics of mafic rocks during continental subduction. Geochim. Cosmochim. Acta 2014, 143, 34–48. [Google Scholar] [CrossRef]
- Dauphas, N.; Teng, F.-Z.; Arndt, N.T. Magnesium and iron isotopes in 2.7 Ga Alexo komatiites: Mantle signatures, no evidence for Soret diffusion, and identification of diffusive transport in zoned olivine. Geochim. Cosmochim. Acta 2010, 74, 3274–3291. [Google Scholar] [CrossRef]
- Tipper, E.T.; Galy, A.; Bickle, M.J. Riverine evidence for a fractionated reservoir of Ca and Mg on the continents: Implications for the oceanic Ca cycle. Earth Planet. Sci. Lett. 2006, 247, 267–279. [Google Scholar] [CrossRef]
- Voigt, M.; Pearce, C.R.; Fries, D.M.; Baldermann, A.; Oelkers, E.H. Magnesium isotope fractionation during hydrothermal seawater-basalt interaction. Geochim. Cosmochim. Acta 2020, 272, 21–35. [Google Scholar] [CrossRef]
- Li, W.-Y.; Teng, F.-Z.; Ke, S.; Rudnick, R.L.; Gao, S.; Wu, F.-Y.; Chappell, B. Heterogeneous magnesium isotopic composition of the upper continental crust. Geochim. Cosmochim. Acta 2010, 74, 6867–6884. [Google Scholar] [CrossRef]
- Higgins, J.; Schrag, D. Constraining magnesium cycling in marine sediments using magnesium isotopes. Geochim. Cosmochim. Acta 2010, 74, 5039–5053. [Google Scholar] [CrossRef]
- Huang, K.-J.; Teng, F.-Z.; Plank, T.; Staudigel, H.; Hu, Y.; Bao, Z.-Y. Magnesium isotopic composition of altered oceanic crust and the global Mg cycle. Geochim. Cosmochim. Acta 2018, 238, 357–373. [Google Scholar] [CrossRef]
- Berner, R.A.; Lasaga, A.C.; Garrels, R.M. The carbonate-silicate geochemical cycle and its effect on atmospheric carbon dioxide over the past 100 million years. Am. J. Sci. 1983, 283, 641–683. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, X.; Liu, Y.; Xu, H.; Wu, Y.; Zhuo, L. Major, trace and rare earth elements geochemistry of geothermal waters from the Rehai high-temperature geothermal field in Tengchong of China. Appl. Geochem. 2020, 119, 104639. [Google Scholar] [CrossRef]
- Li, J.; Guo, Q.; Wang, Y. Evaluation of temperature of parent geothermal fluid and its cooling processes during ascent to surface: A case study in Rehai geothermal field, Tengchong. Earth Sci.-J. China Univ. Geosci. 2015, 40, 1576–1584. [Google Scholar]
- Guo, Q.; Liu, M.; Li, J.; Zhou, C. Geochemical Genesis of Arsenic in the Geothermal Waters from the Rehai Hydrothermal System, Southwestern China. Procedia Earth Planet. Sci. 2017, 17, 49–52. [Google Scholar] [CrossRef]
- Guo, Q.H.; Wang, Y.X. Geochemistry of hot springs in the Tengchong hydrothermal areas, Southwestern China. J. Volcanol. Geotherm. Res. 2012, 215, 61–73. [Google Scholar] [CrossRef]
- Tian, H.-C.; Yang, W.; Li, S.-G.; Ke, S.; Duan, X.-Z. Low δ26Mg volcanic rocks of Tengchong in Southwestern China: A deep carbon cycle induced by supercritical liquids. Geochim. Cosmochim. Acta 2018, 240, 191–219. [Google Scholar] [CrossRef]
- Shi, L.-H.; Yuan, J.-Y.; Lin, L.-H.; Liu, X.-S.; Ai, M.-Q.; Jin, F.-F.; Wang, P.-L.; Chen, X.-G. Gas geochemistry of hot springs at the Tengchong field, Southwest China: Controlled by the spatial distribution of magmatic chamber. J. Volcanol. Geotherm. Res. 2020, 402, 106998. [Google Scholar] [CrossRef]
- Tong, W.; Zhang, M. Geothermics in Tengchong; Science Press: Beijing, China, 1989; (In Chinese with English abstract). [Google Scholar]
- Zhang, M.; Guo, Z.; Sano, Y.; Zhang, L.; Sun, Y.; Cheng, Z.; Yang, T.F. Magma-derived CO2 emissions in the Tengchong volcanic field, SE Tibet: Implications for deep carbon cycle at intra-continent subduction zone. J. Southeast Asian Earth Sci. 2016, 127, 76–90. [Google Scholar] [CrossRef]
- Shangguan, Z.; Bai, C.; Sun, M. Mantle-derived magmatic gas releasing features at the Rehai area, Tengchong county, Yunnan Province, China. Sci. China Ser. D Earth Sci. 2000, 43, 132–140. [Google Scholar] [CrossRef]
- Lei, J.; Zhao, D.; Su, Y. Insight into the origin of the Tengchongintraplate volcano and seismotectonics in southwest China from local and teleseismic data. J. Geophys. Res. Solid Earth 2009, 114. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Wang, Z. Plate interactions, crustal deformation and magmatism along the eastern margins of the Tibetan Plateau. Tectonophysics 2018, 740–741, 10–26. [Google Scholar] [CrossRef]
- Lei, J.; Xie, F.; Fan, Q.; Santosh, M. Seismic imaging of the deep structure under the Chinese volcanoes: An overview. Phys. Earth Planet. Inter. 2013, 224, 104–123. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Lou, H.; Wu, J.-P.; Bai, Z.-M.; Huangfu, G.; Qin, J.-Z. Seismological study on the crustal structure of Tengchong volcanic-geothermal area. Acta Seism. Sin. 2002, 15, 247–259. [Google Scholar] [CrossRef]
- Zhou, M.-F.; Robinson, P.T.; Wang, C.Y.; Zhao, J.-H.; Yan, D.-P.; Gao, J.-F.; Malpas, J. Heterogeneous mantle source and magma differentiation of quaternary arc-like volcanic rocks from Tengchong, SE margin of the Tibetan Plateau. Contrib. Miner. Pet. 2011, 163, 841–860. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.Y.; Chan, W.W.; Mooney, W.D. Three-dimensional velocity structure of crust and upper mantle in southwestern China and its tectonic implications. J. Geophys. Res. Solid Earth 2003, 108. [Google Scholar] [CrossRef] [Green Version]
- Liao, Z.J.; Guo, G.Y. Geology of the Tengchong geothermal field and surrounding area, west Yunnan, China. Geothermics 1986, 15, 339–345. [Google Scholar]
- Li, N.; Zhao, Y.-W.; Zhang, L.-Y.; Wang, J.-L. The quaternary eruptive sequence of the Tengchong volcanic group, southwestern China. Lithos 2020, 354, 105173. [Google Scholar] [CrossRef]
- Xu, C.; Zhao, G.; He, Y.; Li, D. Geochemistry Of Cenozoic Volcanic Rocks From Tengchong, Western Yunnan. Mar. Geol. Quat. Geol. 2012, 32, 65–76. [Google Scholar] [CrossRef]
- Wang, E.; Burchfiel, B. Interpretation of Cenozoic tectonics in the right-lateral accommodation zone between the Ailao Shan shear zone and the eastern Himalayan syntaxis. Int. Geol. Rev. 1997, 39, 191–219. [Google Scholar] [CrossRef]
- Wang, G.; Wan, J.; Wang, E.; Zheng, D.; Li, F. Late Cenozoic to recent transtensional deformation across the Southern part of the Gaoligong shear zone between the Indian plate and SE margin of the Tibetan plateau and its tectonic origin. Tectonophysics 2008, 460, 1–20. [Google Scholar] [CrossRef]
- Bai, D.; Meju, M.A.; Liao, Z. Magnetotelluric images of deep crustal structure of the Rehai geothermal field near Tengchong, southern China. Geophys. J. Int. 2001, 147, 677–687. [Google Scholar] [CrossRef] [Green Version]
- Li, D.P.; Luo, Z.H.; Liu, J.Q.; Chen, Y.L.; Jin, Y. Magma Origin and Evolution of Tengchong Cenozoic Volcanic Rocks from West Yunnan, China: Evidence from Whole Rock Geochemistry and Nd-Sr-Pb Isotopes. Acta Geol. Sin.-Engl. Ed. 2012, 86, 867–878. [Google Scholar]
- Zou, H.; Shen, C.-C.; Fan, Q.; Lin, K. U-series disequilibrium in young Tengchong volcanics: Recycling of mature clay sediments or mudstones into the SE Tibetan mantle. Lithos 2014, 192–195, 132–141. [Google Scholar] [CrossRef]
- Chen, T.F. The petrology of the volcanic rocks in Tengchong, Yunnan. Sediment. Geol. Tethyan Geol. 2003, 23, 56–61. [Google Scholar]
- Zhou, Z.; Xiang, C.; Yang, H. Geochemistry of the Isotopes in the Volcanic Rocks in Tengchong. China. J. Seismol. Res. 2000, 23, 194–200. [Google Scholar]
- Zhang, Z.; Liu, S.; Zhao, F. Geochemistry of thermal waters in the Tengchong volcanic geothermal area, West Yunnan Province, China. Geothermics 1987, 16, 169–179. [Google Scholar]
- Xie, J.-C.; Zhu, D.-C.; Dong, G.; Zhao, Z.-D.; Wang, Q.; Mo, X. Linking the Tengchong Terrane in SW Yunnan with the Lhasa Terrane in southern Tibet through magmatic correlation. Gondwana Res. 2016, 39, 217–229. [Google Scholar] [CrossRef]
- Luo, L.; Wen, H.; Capezzuoli, E. Travertine deposition and diagenesis in Ca-deficiency perched hot spring systems: A case from Shihuadong, Tengchong, China. Sediment. Geol. 2021, 414, 105827. [Google Scholar] [CrossRef]
- An, Y.; Wu, F.; Xiang, Y.; Nan, X.; Yu, X.; Yang, J.; Yu, H.; Xie, L.; Huang, F. High-precision Mg isotope analyses of low-Mg rocks by MC-ICP-MS. Chem. Geol. 2014, 390, 9–21. [Google Scholar] [CrossRef]
- Dick, J.M. CHNOSZ: Thermodynamic calculations and diagrams for geochemistry. Front. Earth Sci. 2019, 7, 180. [Google Scholar] [CrossRef] [Green Version]
- Foster, G.L.; von Strandmann, P.P.; Rae, J. Boron and magnesium isotopic composition of seawater. Geochem. Geophys. Geosyst. 2010, 11. [Google Scholar] [CrossRef]
- Ling, M.-X.; Sedaghatpour, F.; Teng, F.-Z.; Hays, P.; Strauss, J.; Sun, W. Homogeneous magnesium isotopic composition of seawater: An excellent geostandard for Mg isotope analysis. Rapid Commun. Mass Spectrom. 2011, 25, 2828–2836. [Google Scholar] [CrossRef] [PubMed]
- Tipper, E.; Galy, A.; Gaillardet, J.; Bickle, M.; Elderfield, H.; Carder, E. The magnesium isotope budget of the modern ocean: Constraints from riverine magnesium isotope ratios. Earth Planet. Sci. Lett. 2006, 250, 241–253. [Google Scholar] [CrossRef]
- Tan, H.; Zhang, Y.; Zhang, W.; Kong, N.; Zhang, Q.; Huang, J. Understanding the circulation of geothermal waters in the Tibetan Plateau using oxygen and hydrogen stable isotopes. Appl. Geochem. 2014, 51, 23–32. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, Y.; Liu, W. O, H, and Sr isotope evidences of mixing processes in two geothermal fluid reservoirs at Yangbajing, Tibet, China. Environ. Earth Sci. 2009, 59, 1589–1597. [Google Scholar] [CrossRef]
- Sasaki, K.; Morita, J.; Iwaki, C.; Ueda, A. Geochemical evaluation of geothermal resources in Toyama Prefecture, Japan, based on the chemical and isotopic characteristics of hot spring waters. Geothermics 2021, 93, 102071. [Google Scholar] [CrossRef]
- Pan, S.; Kong, Y.; Wang, K.; Ren, Y.; Pang, Z.; Zhang, C.; Wen, D.; Zhang, L.; Feng, Q.; Zhu, G.; et al. Magmatic origin of geothermal fluids constrained by geochemical evidence: Implications for the heat source in the northeastern Tibetan Plateau. J. Hydrol. 2021, 603, 126985. [Google Scholar] [CrossRef]
- Giggenbach, W. Isotopic shifts in waters from geothermal and volcanic systems along convergent plate boundaries and their origin. Earth Planet. Sci. Lett. 1992, 113, 495–510. [Google Scholar] [CrossRef]
- Hedenquist, J.W.; Lowenstern, J.B. The role of magmas in the formation of hydrothermal ore deposits. Nature 1994, 370, 519–527. [Google Scholar] [CrossRef]
- Taylor, B. Bismarck Sea: Evolution of a back-arc basin. Geology 1979, 7, 171–174. [Google Scholar] [CrossRef]
- Ohmoto, H. Stable isotope geochemistry of ore deposits. Rev. Mineral. Geochem. 1986, 16, 491–559. [Google Scholar]
- Fan, Y.; Chen, Y.; Li, X.; Li, W.; Li, Q. Characteristics of water isotopes and ice-snowmelt quantification in the Tizinafu River, north Kunlun Mountains, Central Asia. Quat. Int. 2015, 380-381, 116–122. [Google Scholar] [CrossRef]
- Craig, H. Isotopic Variations in Meteoric Waters. Science 1961, 133, 1702–1703. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Kong, Y.; Li, J.; Tian, J. An Isotopic Geoindicator in the Hydrological Cycle. Procedia Earth Planet. Sci. 2017, 17, 534–537. [Google Scholar] [CrossRef]
- Gat, J.R. Oxygen And Hydrogen Isotopes In The Hydrologic Cycle. Annu. Rev. Earth Planet. Sci. 1996, 24, 225–262. [Google Scholar] [CrossRef] [Green Version]
- Billarent, C.A.; Levresse, G.; Ferrari, L.; Inguaggiato, C.; Inguaggiato, S.; Hernández, P.E.; Hernández, E.A.; Corbo, C.F.; Carrera, H.J.; Arias, P.A. Deciphering origins and pathways of low-enthalpy geothermal waters in the unconventional geothermal system of Juchipila graben (Central Mexico). Geothermics 2021, 94, 102076. [Google Scholar] [CrossRef]
- Craig, H.; Gordon, L.I.; Horibe, Y. Isotopic exchange effects in the evaporation of water: 1. Low-temperature experimental results. J. Geophys. Res. Earth Surf. 1963, 68, 5079–5087. [Google Scholar] [CrossRef]
- Horita, J.; Wesolowski, D.J. Liquid-vapor fractionation of oxygen and hydrogen isotopes of water from the freezing to the critical temperature. Geochim. Cosmochim. Acta 1994, 58, 3425–3437. [Google Scholar] [CrossRef]
- Hua, Y.; Zhang, S.; Li, M.; Wu, T.; Zou, C.; Liu, L. Magma system beneath Tengchong volcanic zone inferred from local earthquake seismic tomography. J. Volcanol. Geotherm. Res. 2019, 377, 1–16. [Google Scholar] [CrossRef]
- Gourcy, L.; Groening, M.; Aggarwal, P. Stable Oxygen and Hydrogen Isotopes in Precipitation, Isotopes in the Water Cycle; Springer: Dordrecht, The Netherland, 2005; pp. 39–51. [Google Scholar]
- Clark, I.D.; Fritz, P. Environmental Isotopes in Hydrogeology; CRC Press: Boca Raton, USA, 2013. [Google Scholar]
- Wimpenny, J.; Gíslason, S.R.; James, R.H.; Gannoun, A.; Von Strandmann, P.A.P.; Burton, K.W. The behaviour of Li and Mg isotopes during primary phase dissolution and secondary mineral formation in basalt. Geochim. Cosmochim. Acta 2010, 74, 5259–5279. [Google Scholar] [CrossRef]
- Von Damm, K. Seafloor hydrothermal activity: Black smoker chemistry and chimneys. Annu. Rev. Earth Planet. Sci. 1990, 18, 173–204. [Google Scholar] [CrossRef]
- Alt, J.C. Subseafloor processes in mid-ocean ridge hydrothennal systems. Seafloor Hydrothermal Syst. Phys. Chem. Biol. Geol. Interact. 1995, 91, 85–114. [Google Scholar]
- Seyfried, W.; Bischoff, J.L. Hydrothermal transport of heavy metals by seawater: The role of seawater/basalt ratio. Earth Planet. Sci. Lett. 1977, 34, 71–77. [Google Scholar] [CrossRef]
- Bach, W.; Paulick, H.; Garrido, C.J.; Ildefonse, B.; Meurer, W.P.; Humphris, S.E. Unraveling the sequence of serpentinization reactions: Petrography, mineral chemistry, and petrophysics of serpentinites from MAR 15 degrees N (ODP Leg 209, Site 1274). Geophys. Res. Lett. 2006, 33. [Google Scholar] [CrossRef] [Green Version]
- Wimpenny, J.; Yin, Q.-Z.; Tollstrup, D.; Xie, L.-W.; Sun, J. Using Mg isotope ratios to trace Cenozoic weathering changes: A case study from the Chinese Loess Plateau. Chem. Geol. 2014, 376, 31–43. [Google Scholar] [CrossRef]
- Ryu, J.-S.; Vigier, N.; Decarreau, A.; Lee, S.-W.; Lee, K.-S.; Song, H.; Petit, S. Experimental investigation of Mg isotope fractionation during mineral dissolution and clay formation. Chem. Geol. 2016, 445, 135–145. [Google Scholar] [CrossRef]
- Li, W.; Beard, B.L.; Li, C.; Xu, H.; Johnson, C.M. Experimental calibration of Mg isotope fractionation between dolomite and aqueous solution and its geological implications. Geochim. Cosmochim. Acta 2015, 157, 164–181. [Google Scholar] [CrossRef]
- Pearce, C.R.; Saldi, G.D.; Schott, J.; Oelkers, E.H. Isotopic fractionation during congruent dissolution, precipitation and at equilibrium: Evidence from Mg isotopes. Geochim. Cosmochim. Acta 2012, 92, 170–183. [Google Scholar] [CrossRef]
- Dong, A.; Zhu, X. Mg isotope geochemical cycle in supergene environment. Adv. Earth Sci. 2016, 31, 43–58. [Google Scholar]
- Young, E.; Galy, A. The Isotope Geochemistry and Cosmochemistry of Magnesium. Rev. Miner. Geochem. 2004, 55, 197–230. [Google Scholar] [CrossRef]
- Teng, F.Z. Magnesium isotope geochemistry. Rev. Mineral. Geochem. 2017, 82, 219–287. [Google Scholar] [CrossRef]
- Teng, F.Z.; Li, W.Y.; Rudnick, R.L.; Gardner, L.R. Contrasting lithium and magnesium isotope fractionation during continental weathering. Earth Planet. Sci. Lett. 2010, 300, 63–71. [Google Scholar] [CrossRef]
- Liu, S.A.; Teng, F.Z.; He, Y.S.; Ke, S.; Li, S.G. Investigation of magnesium isotope fractionation during granite differentiation: Implication for Mg isotopic composition of the continental crust. Earth Planet. Sci. Lett. 2010, 297, 646–654. [Google Scholar] [CrossRef]
- Teng, F.-Z.; Yang, W.; Rudnick, R.L.; Hu, Y. Heterogeneous magnesium isotopic composition of the lower continental crust: A xenolith perspective. Geochem. Geophys. Geosyst. 2013, 14, 3844–3856. [Google Scholar] [CrossRef]
- Yang, W.; Teng, F.-Z.; Li, W.-Y.; Liu, S.-A.; Ke, S.; Liu, Y.-S.; Zhang, H.-F.; Gao, S. Magnesium isotopic composition of the deep continental crust. Am. Miner. 2016, 101, 243–252. [Google Scholar] [CrossRef]


| Site | Sample | δ 2H | Stdev (‰) | δ 18O | Stdev (‰) |
|---|---|---|---|---|---|
| DT | 015−DT−3 | −83.74 | 0.07 | −11.31 | 0.01 |
| DT | 015−DT−4 | −84.17 | 0.16 | −11.21 | 0.02 |
| DT | 2020−DT−L1 | −87.02 | 0.88 | −11.59 | 0.09 |
| SQ | 015−SQ−3 | −81.40 | 0.12 | −10.34 | 0 |
| SQ | 2020−SQ−L1 | −84.17 | 0.25 | −10.91 | 0.03 |
| SQ | 2020−SQ−L3 | −85.22 | 0.33 | −10.85 | 0.11 |
| BLZ | 2020−BLZB3−L1 | −72.45 | 0.13 | −8.62 | 0.16 |
| BLZ | 2020−BLZ−L1 | −74.40 | 0.16 | −9.23 | 0.05 |
| DFQ | 2020−DFQ−L2 | −75.28 | 0.07 | −10.10 | 0.07 |
| TH | 2020−TH−L2 | −72.46 | 0.08 | −8.93 | 0.12 |
| LX | 2020−LX−L1 | −86.08 | 0.37 | −10.72 | 0.04 |
| HGQ | 015−HGQ−1 | −52.68 | 0.46 | −3.35 | 0.19 |
| HGQ | 015−HGQ−2 | −51.50 | 0.08 | −0.47 | 0.12 |
| HGQ | 2020−HGQ−L2 | −70.92 | 0.05 | −9.38 | 0.08 |
| HGQ | 2020−HGQ−L4 | −62.82 | 0.33 | −7.87 | 0.06 |
| ZZQ | 015−ZZQ−1 | −58.01 | 0.27 | −4.20 | 0.06 |
| HTJ | 015−HTJ−2 | −72.18 | 0.25 | −7.45 | 0.07 |
| RST | 2020−RST−L2 | −71.17 | 0.87 | −9.04 | 0.04 |
| DPZ | 015−DPZ−1 | −70.53 | 0.06 | −8.63 | 0.04 |
| LW | 2020−LW−L2 | −71.46 | 0.06 | −9.72 | 0.05 |
| SHD | 2020−SHD−L3 | −91.40 | 0.07 | −12.35 | 0.13 |
| QK | 2020−QK−L1 | −80.20 | 0.46 | −10.65 | 0.08 |
| XLS | 2020−XLS−L1 | −84.31 | 0.47 | −11.47 | 0.14 |
| XLS | 2020−XLS−L3 | −80.05 | 0.04 | −11.01 | 0.08 |
| HNT | 015−HNT−3 | −73.76 | 0.21 | −9.72 | 0.10 |
| HNT | 015−HNT−5 | −73.89 | 0 | −9.74 | 0.08 |
| river | 2020−RLJ−L1 | −75.96 | 0.13 | −10.34 | 0.07 |
| Site | Sample | δ25Mg DSM-3‰ | 2σ | δ26Mg DSM-3‰ | 2σ |
|---|---|---|---|---|---|
| DPZ | 015−DPZ−1 | −0.17 | 0.02 | −0.33 | 0.01 |
| HGQ | 015−HGQ−1 | −0.36 | 0.03 | −0.70 | 0.04 |
| HGQ | 015−HGQ−3 | −0.32 | 0.02 | −0.62 | 0.02 |
| HNT | 015−HNT−1 | −0.51 | 0.01 | −0.97 | 0.04 |
| LX | 015−LX−1 | −0.19 | 0.02 | −0.37 | 0.03 |
| SQ | 015−SQ−1 | 0.09 | 0.05 | 0.17 | 0.01 |
| QK | 2020−QK−L1 | −0.12 | 0.02 | −0.23 | 0.05 |
| river | 2020−RLJ−L1 | −0.67 | 0.05 | −1.30 | 0.08 |
| river | 2020−RLJ−L1 | −0.70 | 0.02 | −1.36 | 0.04 |
| RST | 2020−RST−L2 | −0.40 | 0.04 | −0.67 | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, J.; Cao, H.; Guo, Y.; Chen, X. Source and Evolution of Subduction–Related Hot Springs Discharged in Tengchong Geothermal Field, Southwest China: Constrained by Stable H, O, and Mg Isotopes. Minerals 2022, 12, 1490. https://doi.org/10.3390/min12121490
Yuan J, Cao H, Guo Y, Chen X. Source and Evolution of Subduction–Related Hot Springs Discharged in Tengchong Geothermal Field, Southwest China: Constrained by Stable H, O, and Mg Isotopes. Minerals. 2022; 12(12):1490. https://doi.org/10.3390/min12121490
Chicago/Turabian StyleYuan, Jingying, Haigang Cao, Yuping Guo, and Xuegang Chen. 2022. "Source and Evolution of Subduction–Related Hot Springs Discharged in Tengchong Geothermal Field, Southwest China: Constrained by Stable H, O, and Mg Isotopes" Minerals 12, no. 12: 1490. https://doi.org/10.3390/min12121490
APA StyleYuan, J., Cao, H., Guo, Y., & Chen, X. (2022). Source and Evolution of Subduction–Related Hot Springs Discharged in Tengchong Geothermal Field, Southwest China: Constrained by Stable H, O, and Mg Isotopes. Minerals, 12(12), 1490. https://doi.org/10.3390/min12121490








