A Combined Re-Os and Pt-Os Isotope and HSE Abundance Study of Ru-Os-Ir Alloys from the Kunar and Unga Placer Deposits, the Taimyr Peninsula, Polar Siberia
Abstract
1. Introduction
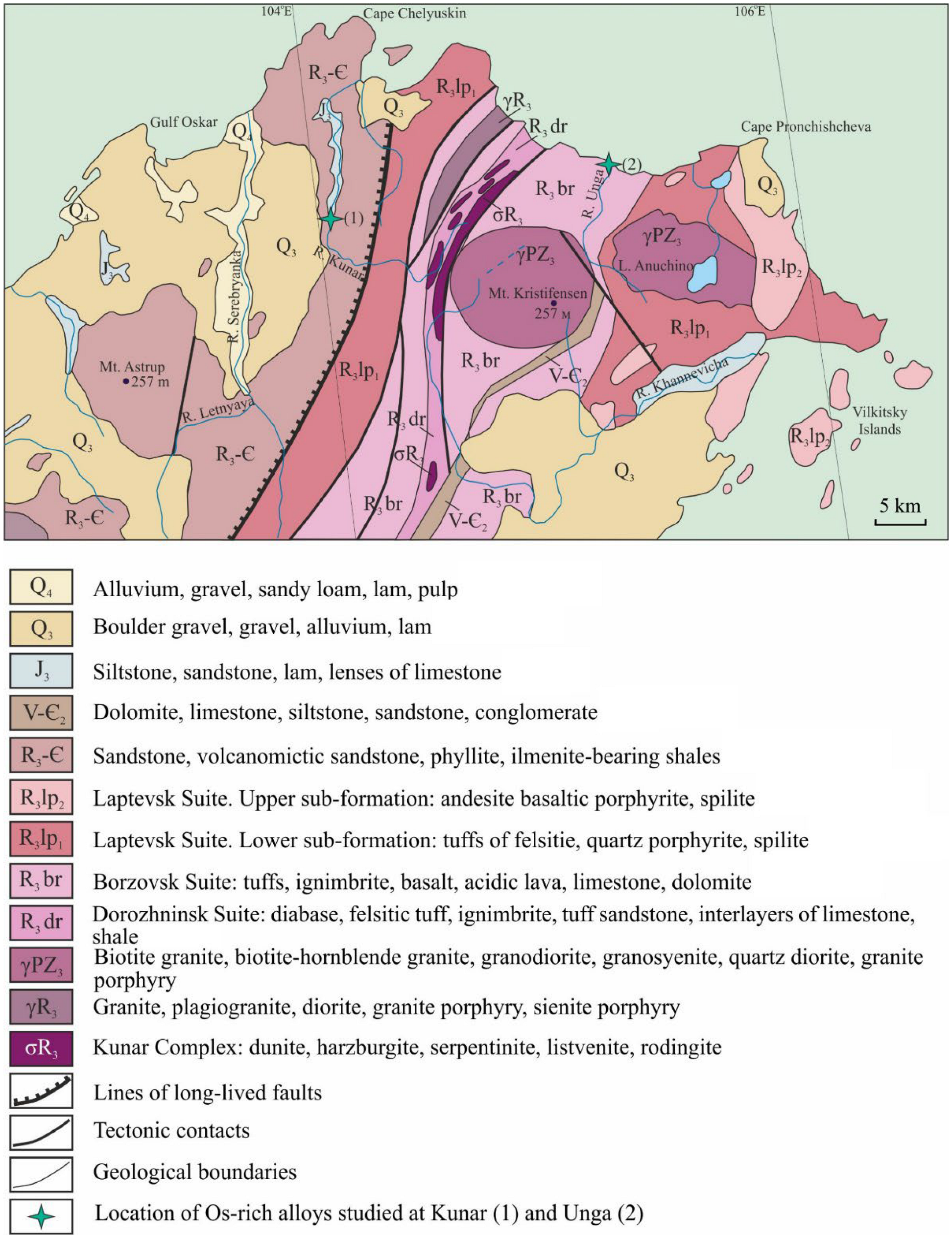
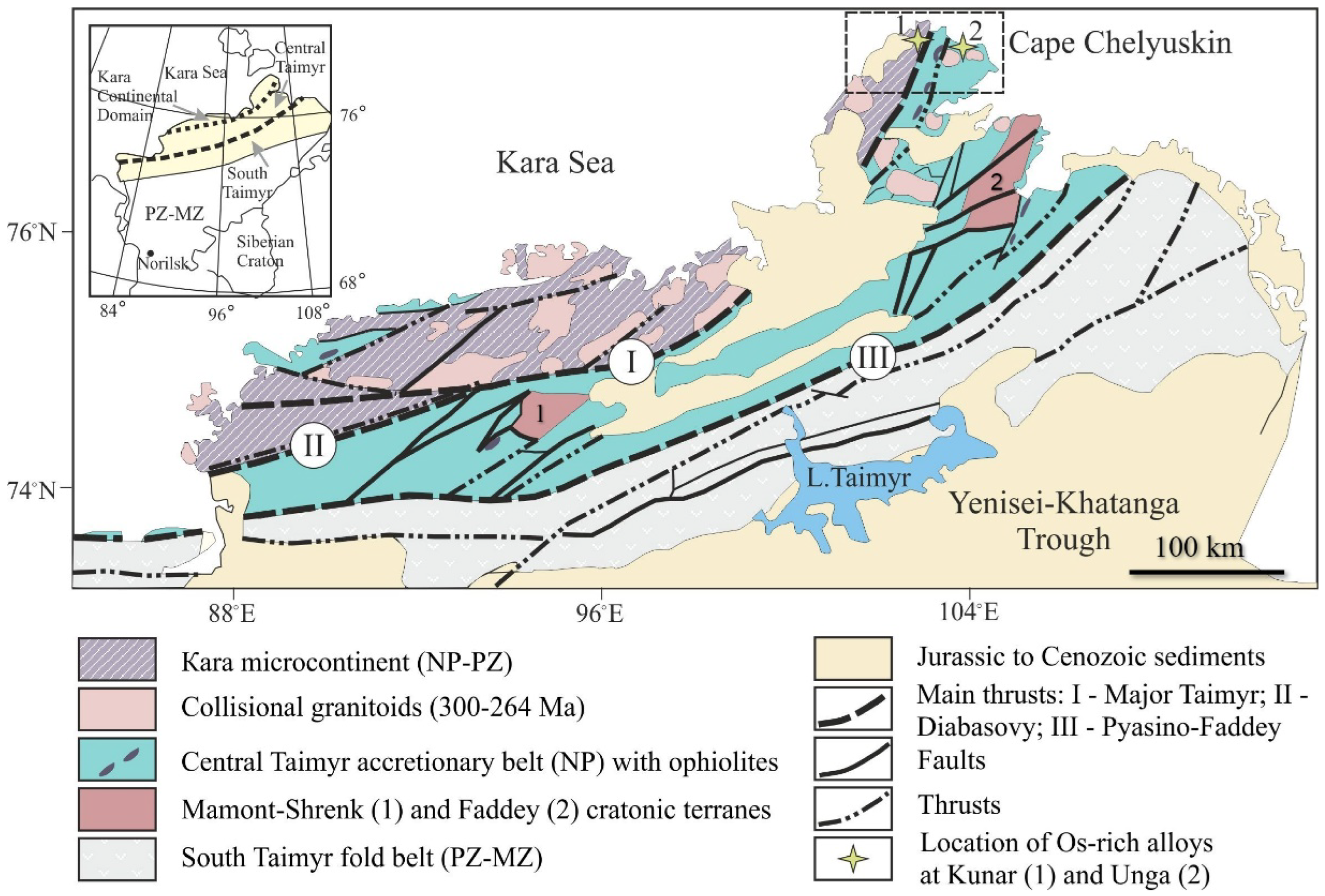
2. Geological Background and Sample Location
3. Analytical Techniques
4. Results
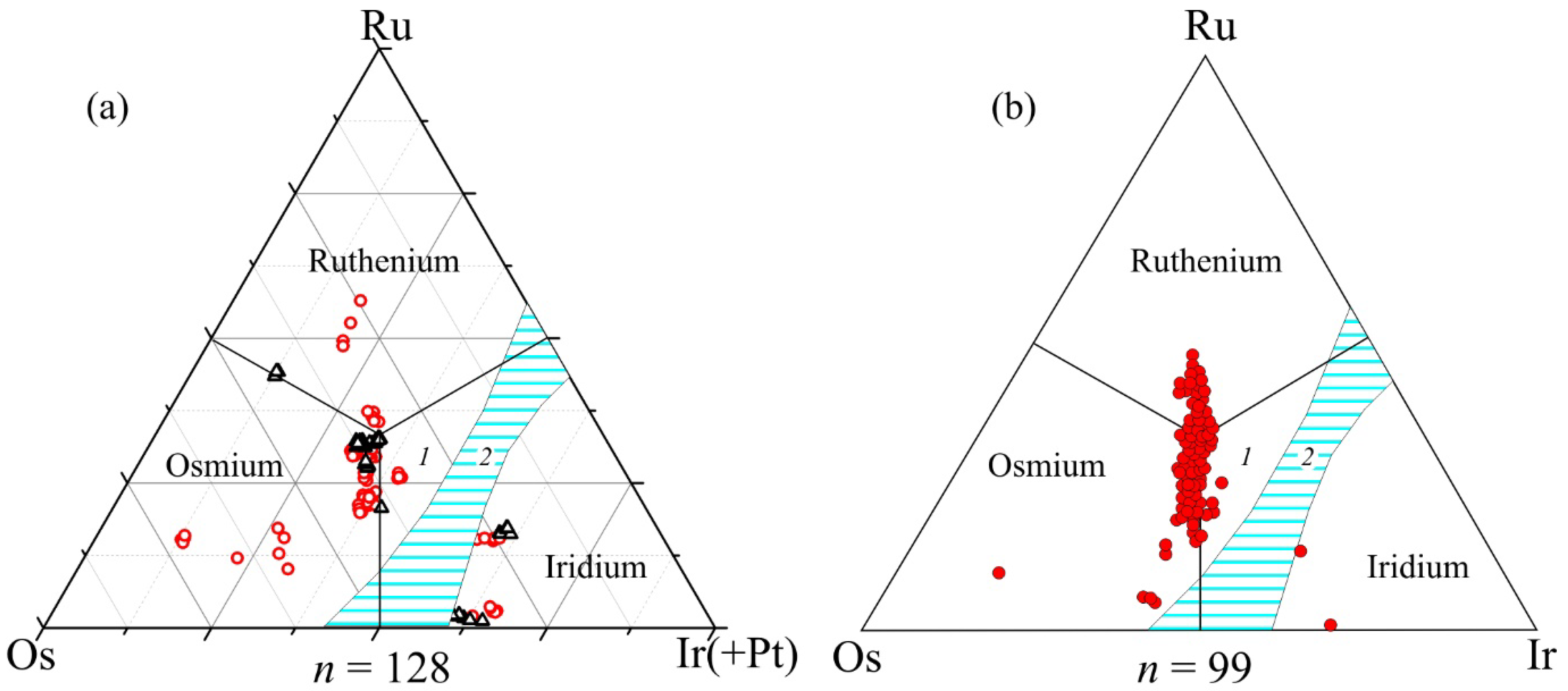
4.1. Composition of Ru-Os-Ir and Pt-Fe Alloys at Kunar and Unga
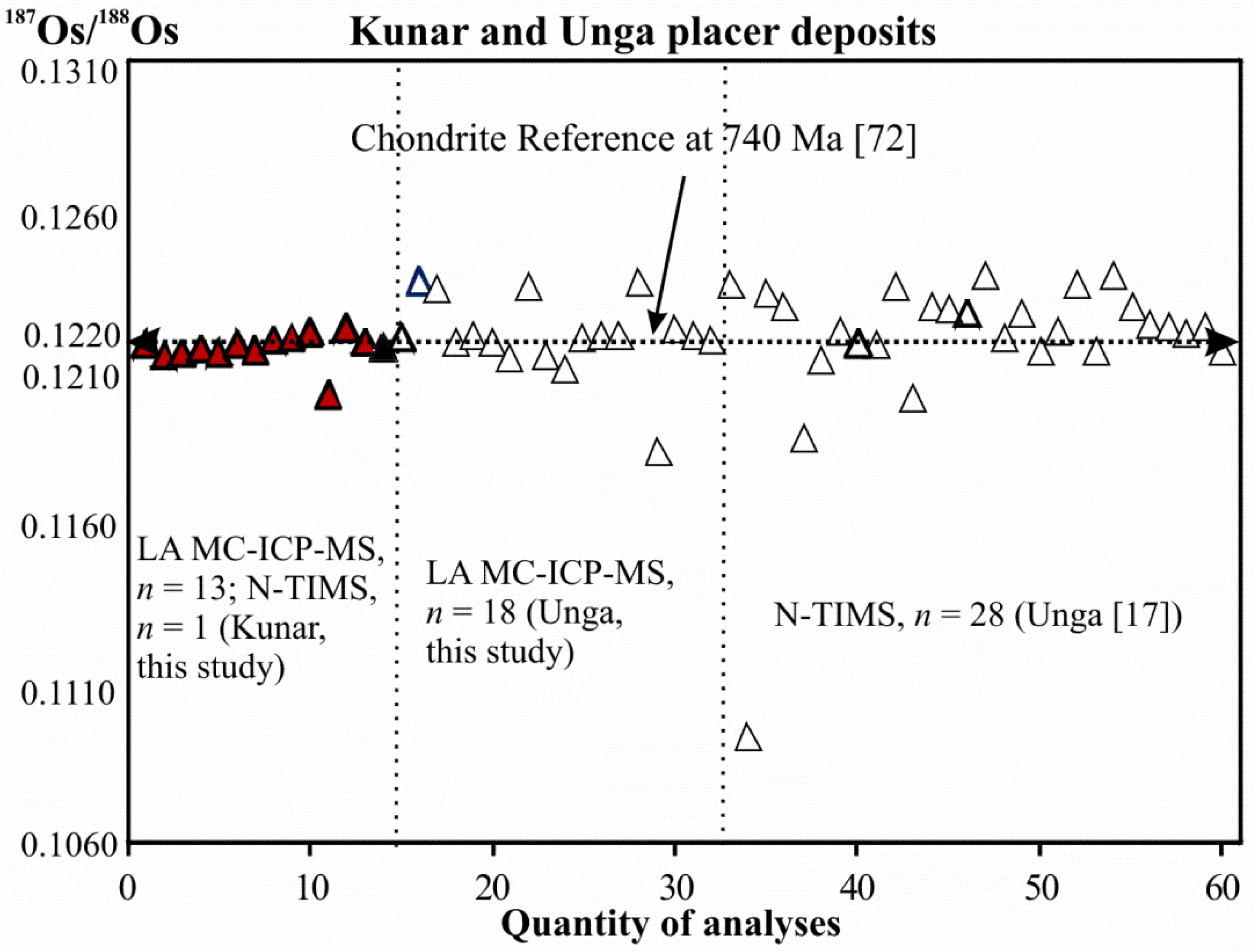
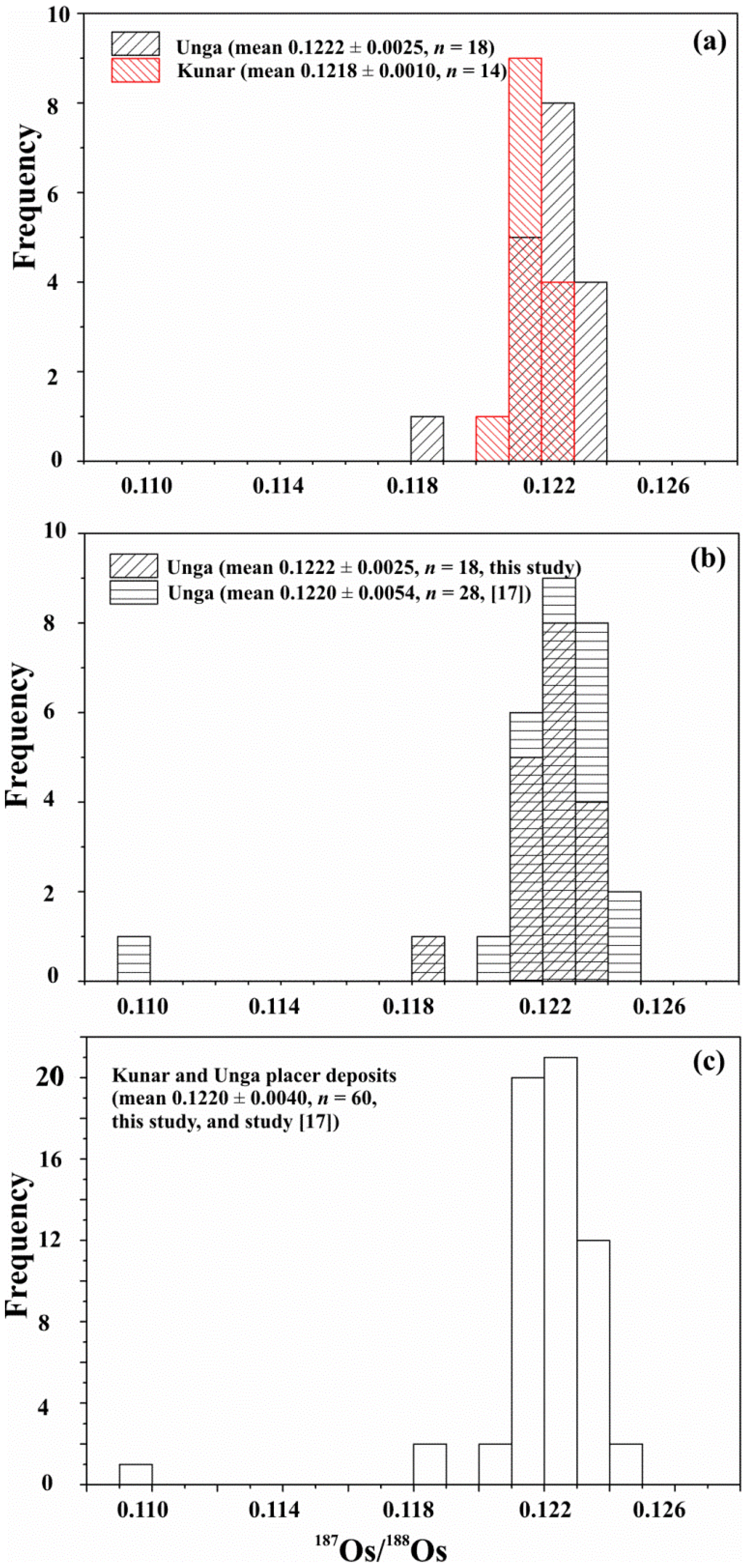
4.2. Osmium Isotope Data
5. Discussion
5.1. Provenance of the Ru-Os-Ir Alloys
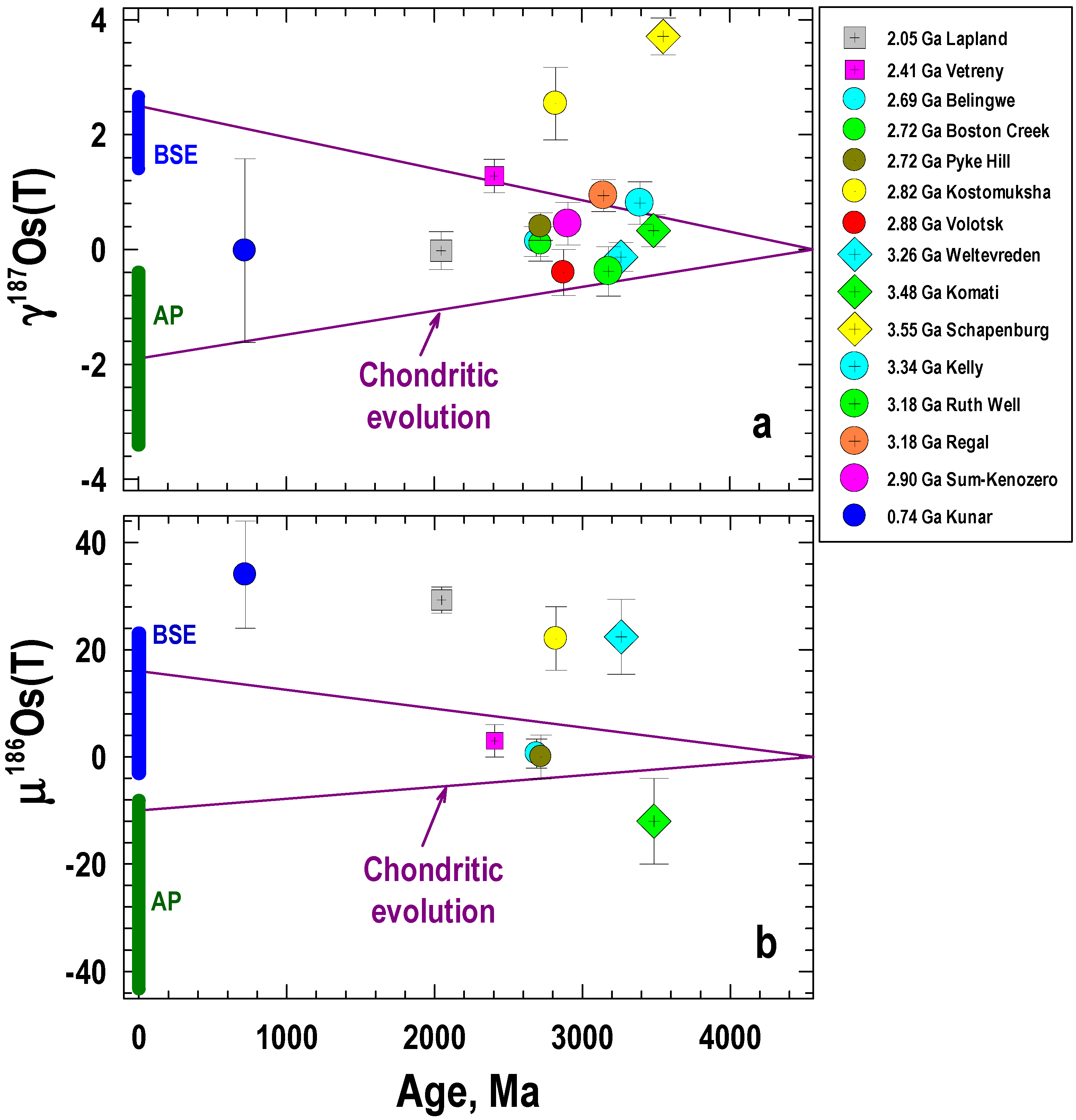
5.2. Os Isotopic Composition of the Mantle as Evidenced by the Ru-Os-Ir Alloys
6. Conclusions
- A multi-technique approach, including the use of electron microprobe analysis, negative thermal ionization mass-spectrometry (N-TIMS) and laser ablation multiple-collector inductively coupled plasma mass-spectrometry (LA MC-ICP-MS), provided a set of HSE abundance and Re-Os and Pt-Os isotope constraints on the origin of detrital Ru-Os-Ir alloys from placer deposits of the Kunar and Unga Rivers in the northern part of the Taimyr Peninsula in the Polar Siberia.
- The Ru-Os-Ir alloys from both localities show similar compositional signatures dominated by osmium and iridium over ruthenium and rutheniridosmine. The common occurrence of euhedral inclusions of ferroan platinum in Os-Ir-(Ru) alloys is indicative of their high-temperature origin. The primary nature of PGM studied is further supported by the presence of a ruthenium trend in the mineral compositions of Ru-Os-Ir alloys and the occurrence of euhedral inclusions of high-Mg olivine (Fo92–93) that fall within the compositional range of mantle (primitive) olivine (Fo 88–93).
- The LA MC-ICP-MS data from this study show similar average initial 187Os/188Os values for both PGM assemblages at Kunar and Unga (0.1218 ± 0.0010, γOs(740 Ma) = −0.18 ± 0.84, and 0.1222 ± 0.0025, γOs(740 Ma) = +0.10 ± 2.1, respectively). These values are identical, within uncertainty, to the initial 187Os/188Os value for the Ru-Os-Ir alloy obtained by N-TIMS (0.1218463 ± 0.0000015, γOs(740 Ma) = −0.1500 ± 0.0012). The average initial 187Os/188Os value of the Ru-Os-Ir alloys at Kunar and Unga are indicative of derivation from a source that evolved with a long-term chondritic Re/Os ratio; this source is within the range of those for the majority of komatiite and abyssal peridotite sources and chondritic meteorites.
- In contrast to the 187Os/188Os data, the initial 186Os/188Os value of 0.1198409 ± 0.0000012 obtained by N-TIMS for the same Ru-Os-Ir alloy sample T-2 at Kunar is 34 ± 10 ppm higher than this value in the chondritic reference of Brandon et al. [15] at that time, but is similar to the µ186Os value of +29 ± 2 in the source of the 2.05 Ga Lapland komatiite system [65]. This implies evolution of the Kunar mantle source with time-integrated suprachondritic Pt/Os ratio. The reason for such long-term Pt/Os enrichment in the Kunar mantle source is not yet clear and would require further investigation.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morgan, J.W.; Wandless, G.A.; Petrie, R.K.; Irving, A.J. Composition of the Earth’s upper mantle-I. Siderophile trace elements in ultramafic nodules. Tectonophysics 1981, 75, 47–67. [Google Scholar] [CrossRef]
- Barnes, S.-J.; Naldrett, A.J.; Gorton, M.P. The origin of the fractionation of platinum-group elements in terrestrial magmas. Chem. Geol. 1985, 53, 302–323. [Google Scholar] [CrossRef]
- Hauri, E.H. Osmium isotopes and mantle convection. Philos. Trans. R. Soc. Lond. A 2002, 360, 2371–2382. [Google Scholar] [CrossRef]
- Rehkämper, M.; Halliday, N.A.; Fitton, J.G.; Lee, D.-C.; Wieneke, M.; Arndt, N.T. Ir, Ru, Pt, and Pd in basalts and komatiites: New constraints for the geochemical behavior of the platinum-group elements in the mantle. Geochim. Et Cosmochim. Acta 1999, 63, 3915–3934. [Google Scholar] [CrossRef]
- Allegre, C.J.; Luck, J.-M. Osmium isotopes as petrogenetic and geological tracers. Earth Planet. Sci. Lett. 1980, 48, 148–154. [Google Scholar] [CrossRef]
- Walker, R.J.; Morgan, J.W.; Beary, E.; Smoliar, M.I.; Czamanske, G.K.; Horan, M.F. Applications of the 190Pt-186Os isotope system to geochemistry and cosmochemistry. Geochim. Et Cosmochim. Acta 1997, 61, 4799–4808. [Google Scholar] [CrossRef]
- Hattori, K.; Hart, S.R. Osmium-isotope ratios of platinum-group minerals associated with ultramafic intrusions: Os-isotopic evolution of the oceanic mantle. Earth Planet. Sci. Lett. 1991, 107, 499–514. [Google Scholar] [CrossRef]
- Hattori, K.; Cabri, L.J. Origin of platinum-group mineral nuggets inferred from an osmium-isotope study. Can. Mineral. 1992, 30, 289–301. [Google Scholar]
- Bird, J.M.; Meibom, A.; Frei, R.; Nagler, T.F. Osmium and lead isotopes of rare OsIrRu minerals: Derivation from the core-mantle boundary region? Earth Planet. Sci. Lett. 1999, 170, 83–92. [Google Scholar] [CrossRef]
- Malitch, K.N.; Thalhammer, O.A. Pt-Fe nuggets derived from clinopyroxenite-dunite massifs, Russia: A structural, compositional and osmium-isotope study. Can. Mineral. 2002, 40, 395–418. [Google Scholar] [CrossRef]
- Meibom, A.; Sleep, N.H.; Chamberlain, C.P.; Coleman, R.G.; Frei, R.; Hren, M.T.; Wooden, J.L. Re-Os isotopic evidence for long-lived heterogeneity and equilibration processes in the Earth’s upper mantle. Nature 2002, 419, 705–708. [Google Scholar] [CrossRef]
- Meibom, A.; Frei, R.; Sleep, N.H. Osmium isotopic compositions of Os-rich platinum group element alloys from the Klamath and Siskiyou Mountains. J. Geophys. Res. 2004, 109, B02203. [Google Scholar] [CrossRef]
- Kostoyanov, A.I.; Ivanov, D.Y.; Manoilov, V.V. Polycyclic formation of platinum-group minerals from placers of the Ural and Timan. Geochem. Int. 2003, 41, 534–544. [Google Scholar]
- Walker, R.J.; Brandon, A.D.; Bird, J.M.; Piccoli, P.M.; McDonough, W.F.; Ash, R.D. 187Os–186Os systematics of Os-Ir-Ry alloy grains from southwestern Oregon. Earth Planet. Sci. Lett. 2005, 230, 211–236. [Google Scholar] [CrossRef]
- Brandon, A.D.; Walker, R.J.; Puchtel, I.S. Platinum-osmium isotope evolution of the Earth’s mantle: Constraints from chondrites and Os-rich alloys. Geochim. Et Cosmochim. Acta 2006, 70, 2093–2103. [Google Scholar] [CrossRef]
- Pearson, D.G.; Parman, S.W.; Nowell, G.M. A link between large mantle melting events and continent growth seen in osmium isotopes. Nature 2007, 449, 202–205. [Google Scholar] [CrossRef]
- Malitch, K.N.; Badanina, I.Y.; Kostoyanov, A.I. Initial Os-isotopic composition of Os-Ir-Ru alloys from ultramafic massifs of the Polar Siberia. Dokl. Earth Sci. 2011, 440, 1343–1348. [Google Scholar] [CrossRef]
- Pašava, J.; Malec, J.; Griffin, W.L.; González-Jiménez, J.M. Re-Os isotopic constraints on the source of platinum-group minerals (PGMs) from the Vestřev pyrope-rich garnet placer deposit, Bohemian Massif. Ore Geol. Rev. 2015, 68, 117–126. [Google Scholar] [CrossRef]
- Badanina, I.Y.; Malitch, K.N.; Merkle, R.K.W.; Antonov, A.V.; Kapitonov, I.N.; Khiller, V.V. Chemical and isotopic composition of Os-rich alloys and sulfide from the Evander Goldfield of the Witwatersrand Basin (South Africa). Lithosphere 2016, 16, 129–144. (In Russian) [Google Scholar]
- Dijkstra, A.H.; Dale, C.W.; Oberthür, T.; Nowell, G.M.; Pearson, D.G. Osmium isotope compositions of detrital Os-rich alloys from the Rhine River provide evidence for a global late Mesoproterozoic mantle depletion event. Earth Planet. Sci. Lett. 2016, 452, 115–122. [Google Scholar] [CrossRef]
- Malitch, K.N.; Badanina, I.Y.; Belousova, E.A.; Murzin, V.V.; Velivetskaya, T.A. Origin of Ru-Os sulfides from the Verkh-Neivinsk ophiolite massif (Middle Urals, Russia): Compositional and S-Os isotope evidence. Minerals 2021, 11, 329. [Google Scholar] [CrossRef]
- Augustithis, S.S. Mineralogical and geochemical studies of the platiniferous dunite–birbirite–pyroxenite complex of Yubdo (Birbir), W. Ethiopia. Chem. Erde 1965, 24, 159–196. [Google Scholar]
- Cousins, C.A.; Kinloch, E.D. Some observations on textures and inclusions in alluvial platinoids. Econ. Geol. 1976, 71, 1377–1398. [Google Scholar] [CrossRef]
- Bowles, J.F.W. The development of platinum-group minerals in laterites. Econ. Geol. 1986, 81, 1278–1285. [Google Scholar] [CrossRef]
- Bowles, J.F.W. The development of platinum-group minerals (PGM) in laterites: Mineral morphology. Chron. Rech. Min. 1995, 520, 55–63. [Google Scholar]
- Bowles, J.F.W.; Suarez, S. Formation of alluvial platinum-group minerals: Present knowledge and the way ahead. Mineral. Mag. 2021, 85, 12–21. [Google Scholar] [CrossRef]
- Kuz’min, V.G.; Gavrish, A.V.; Smirnov, A.N. Conditions of distribution and formation of gold placers in the Severnaya Zemlya–Chelyuskin shelf zone. In Resources of Taimyr; Simonov, O.N., Malitch, N.S., Eds.; VSEGEI Press: Noril’sk, Russia; St.Petersburg, Russia, 1999; pp. 150–171. (In Russian) [Google Scholar]
- Gavrish, A.V. Mineral composition of placers of the Severnaya Zemlya-Chelyuskin gold-bearing shelf zone. In Resources of Taimyr; Simonov, O.N., Malitch, N.S., Eds.; VSEGEI Press: Noril’sk, Russia; St.Petersburg, Russia, 2000; pp. 160–184. (In Russian) [Google Scholar]
- Proskurnin, V.F. Mineragenic Analysis of the Taimyr–Severnaya Zemlya Region and Assessment of Its Gold-Forming Potential. Habilitation Thesis, All-Russia Geological Research Institute (VSEGEI), Saint Petersburg, Russia, 2013; p. 326. [Google Scholar]
- Proskurnin, V.F.; Vernikovsky, V.A.; Metelkin, D.V.; Petrushkov, B.S.; Vernikovskaya, A.E.; Gavrish, A.V.; Bagaeva, A.A.; Matushkin, N.Y.; Vinogradova, N.P.; Larionov, A.N. Rhyolite-granite association in the Central Taimyr zone: Evidence of accretionary-collisional events in the Neoproterozoic. Russ. Geol. Geophys. 2014, 55, 18–32. [Google Scholar] [CrossRef]
- Simonov, O.N.; Afanasenkov, A.P.; Samoilov, A.G.; Sidorov, I.I. Mineral resource base of the Taimyr National District. In Resources of Taimyr; Simonov, O.N., Malitch, N.S., Eds.; VSEGEI Press: Noril’sk, Russia; St.Petersburg, Russia, 1995; pp. 5–35. (In Russian) [Google Scholar]
- Malitch, N.S.; Tuganova, E.V.; Proskurnin, V.F. Metallogenic map of Gorny Taimyr at 1:500000 scale, 1. In Resources of Taimyr; Simonov, O.N., Malitch, N.S., Eds.; VSEGEI Press: Noril’sk, Russia; St.Petersburg, Russia, 2000; pp. 98–108. (In Russian) [Google Scholar]
- Malitch, K.N. Osmium isotope constraints on contrasting sources and prolonged melting in the Proterozoic upper mantle: Evidence from ophiolitic Ru-Os sulfides and Ru-Os-Ir alloys. Chem. Geol. 2004, 208, 157–173. [Google Scholar] [CrossRef]
- Zalyaleev, R.S.; Bezzubtsev, V.V. Chelyuskin ultramafic Belt (Eastern Taimyr). Geol. Geophys. 1975, 16, 132–133. (In Russian) [Google Scholar]
- Vernikovsky, V.A. Geodynamic Evolution of the Taimyr Folded Region; Publishing House of SB RAS, SRC UIGGM: Novosibirsk, Russia, 1996; p. 203. (In Russian) [Google Scholar]
- Malitch, K.N.; Belousova, E.A.; Griffin, W.L.; Badanina, I.Y.; Latypov, R.M.; Sluzhenikin, S.F. New insights on the origin of ultramafic-mafic intrusions and associated Ni-Cu-PGE sulfide deposits of the Noril’sk and Taimyr provinces, Russia: Evidence from radiogenic- and stable-isotope data. In Processes and Ore Deposits of Ultramafic-Mafic Magmas through Space and Time, 1st ed.; Mondal, S., Griffin, W.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 197–238. [Google Scholar]
- Malitch, K.N. Platinum-Group Elements in Clinopyroxenite-Dunite Massifs of the Eastern Siberia (Geochemistry, Mineralogy, and Genesis); St. Petersburg Cartographic Factory VSEGEI Press: Saint Petersburg, Russia, 1999; p. 296. (In Russian) [Google Scholar]
- Walker, R.J.; Prichard, H.M.; Ishiwatari, A.; Pimentel, M. The osmium isotopic composition of convecting upper mantle deduced from ophiolite chromites. Geochim. Et Cosmochim. Acta 2002, 66, 329–345. [Google Scholar] [CrossRef]
- Malitch, K.N.; Junk, S.A.; Thalhammer, O.A.R.; Melcher, F.; Knauf, V.V.; Pernicka, E.; Stumpfl, E.F. Laurite and ruarsite from podiform chromitites at Kraubath and Hochgrossen, Austria: New insights from osmium isotopes. Can. Mineral. 2003, 41, 331–352. [Google Scholar] [CrossRef]
- Tessalina, S.G.; Bourdon, B.; Gannoun, A.; Campas, F.; Birck, J.-L.; Allegre, C.J. Complex proterozoic to paleozoic history of the upper mantle recorded in the Urals lherzolite massifs by Re–Os and Sm–Nd systematics. Chem. Geol. 2007, 240, 61–84. [Google Scholar] [CrossRef]
- Nowell, G.M.; Pearson, D.G.; Parman, S.W.; Luguet, A.; Hanski, E. Precise and accurate 186Os/188Os and 187Os/188Os measurements by Multi-collector Plasma Ionisation Mass Spectrometry, part II: Laser ablation and its application to single-grain Pt–Os and Re–Os geochronology. Chem. Geol. 2008, 248, 394–426. [Google Scholar] [CrossRef]
- Marchesi, C.; González-Jiménez, J.M.; Gervilla, F.; Griffin, W.L.; O’Reilly, S.Y.; Proenza, J.A.; Pearson, N.J. In situ Re–Os isotopic analysis of platinum-group minerals from the Mayarí-Cristal ophiolitic massif (Mayarí-Baracoa Ophiolitic Belt, eastern Cuba): Implications for the origin of Os-isotope heterogeneities in podiform chromitites. Contrib. Mineral. Petrol. 2011, 161, 977–990. [Google Scholar] [CrossRef]
- Badanina, I.Y.; Malitch, K.N.; Belousova, E.A.; Murzin, V.V.; Lord, R.A. Osmium isotope systematics of Ru–Os–Ir alloys and Ru–Os sulfides of the dunite-harzburgite massifs: A synthesis of new data. Proc. Inst. Geol. Geochem. UB RAS 2014, 161, 167–172. (In Russian) [Google Scholar]
- González-Jiménez, J.M.; Griffin, W.L.; Gervilla, F.; Proenza, J.A.; O’Reilly, S.Y.; Pearson, N.J. Chromitites in ophiolites: How, where, when, why? Part I. A review and new ideas on the origin and significance of platinum-group minerals. Lithos 2014, 189, 127–139. [Google Scholar] [CrossRef]
- Tessalina, S.G.; Malitch, K.N.; Auge, T.; Puchkov, V.N.; Belousova, E.; McInnes, B.I. Origin of the Nizhny Tagil clinopyroxenite-dunite massif (Uralian Platinum Belt, Russia): Insights from PGE and Os isotope systematics. J. Petrol. 2015, 56, 2297–2318. [Google Scholar] [CrossRef]
- Badanina, I.Y.; Malitch, K.N.; Lord, R.A.; Belousova, E.A.; Meisel, T.C. Closed-system behaviour of the Re-Os isotope system recorded in primary and secondary PGM assemblages: Evidence from a mantle chromitite at Harold’s Grave (Shetland ophiolite Complex, Scotland). Ore Geol. Rev. 2016, 75, 174–185. [Google Scholar] [CrossRef]
- Prichard, H.M.; Barnes, S.J.; Dale, C.W.; Godel, B.; Fisher, P.C.; Nowell, G.M. Paragenesis of multiple platinum-group mineral populations in Shetland ophiolite chromitite: 3D X-ray tomography and in situ Os isotopes. Geochim. Et Cosmochim. Acta 2017, 216, 314–334. [Google Scholar] [CrossRef]
- Malitch, K.N.; Belousova, E.A.; Griffin, W.L.; Badanina, I.Y.; Knauf, V.V.; O’Reilly, S.Y.; Pearson, N.J. Laurite and zircon from the Finero chromitites (Italy): New insights into evolution of the subcontinental mantle. Ore Geol. Rev. 2017, 90, 210–225. [Google Scholar] [CrossRef]
- Luguet, A.; Nowell, G.M.; Pushkarev, E.; Ballhaus, C.; Wirth, R.; Schreiber, A.; Gottman, I. 190Pt-186Os geochronometer reveals open system behaviour of 190Pt-4He isotope system. Geochem. Perspect. Lett. 2019, 11, 44–48. [Google Scholar] [CrossRef]
- González-Jimenéz, J.M.; Mondal, S.K.; Ghosh, B.; Griffin, W.L.; O’Reilly, S.Y. Re-Os isotope systematics of sulfides in chromitites and host lherzolites of the Andaman ophiolite, India. Minerals 2020, 10, 686. [Google Scholar] [CrossRef]
- Malitch, K.N.; Puchtel, I.S.; Belousova, E.A.; Badanina, I.Y. Contrasting platinum-group mineral assemblages of the Kondyor massif (Russia): Implications for the sources of HSE in zoned-type ultramafic massifs. Lithos 2020, 367–377, 105800. [Google Scholar] [CrossRef]
- Bezzubtsev, V.V.; Zalyaleev, R.S.; Sakovich, A.B. Geological Map of the Mountainous Taimyr. Scale 1:500000, Explanatory Note; Leningrad Cartographic Factory VSEGEI Press: Krasnoyarsk, Russia, 1986; pp. 1–177. (In Russian) [Google Scholar]
- Zabiyaka, A.I.; Zabiyaka, I.D.; Vernikovsky, V.A.; Zlobin, M.N.; Serdyuk, S.S. Geology and Tectonic Evolution of North-Eastern Taimyr; Nauka: Novosibirsk, Russia, 1986; p. 144. (In Russian) [Google Scholar]
- Proskurnin, V.F. Magmatic formations of the Taimyr–Severnaya Zemlya folded system, their ore content and geodynamic features of formation. In Ore Potential of Magmatic Formations of Siberia; SNIIGIMS Press: Novosibirsk, Russia, 1991; pp. 33–39. (In Russian) [Google Scholar]
- Vernikovsky, V.A.; Metelkin, D.V.; Vernikovskaya, A.E.; Salnikova, E.B.; Kovach, V.P.; Kotov, A.B. The oldest island-arc complex of Taimyr: On the formation of the Central Taimyr accretion belt and paleogeodynamic reconstructions in the Arctic. Dokl. Akad. Nauk 2011, 436, 647–653. (In Russian) [Google Scholar] [CrossRef]
- Proskurnin, V.F.; Remizov, D.N.; Proskurnina, M.A. Late-Riphean ophiolite belts of Taimyr. In Geological Processes in Subduction, Collision and Sliding of Lithospheric Plates: Materials of the IV All-Russian Scientific Conference with International Participation; Dal’nauka: Vladivostok, Russia, 2018; pp. 73–75. [Google Scholar]
- Vernikovsky, V.A.; Vernikovskaya, A.E. Central Taimyr accretionary belt (Arctic Asia): Meso-Neoproterozoic tectonic evolution and Rodinia breakup. Precambrian Res. 2001, 110, 127–141. [Google Scholar] [CrossRef]
- Vernikovsky, V.A.; Vernikovskaya, A.E.; Lyapunov, S.M.; Neimark, L.A.; Proskurnin, V.F.; Chernykh, A.I.; Safonova, I.Y. Petrology, geochemistry, and tectonic settings of plagiogranites of the Chelyuskin Ophiolite Belt. Intern. Geol. Rev. 1994, 36, 961–974. [Google Scholar] [CrossRef]
- Sherman, M.L.; Bezzubtsev, V.V.; Malitch, N.S.; Markov, F.G.; Pogrebitsky, Y.E. (Eds.) Geological Map of the Mountainous Taimyr. Scale 1:500000. 6 sheets; Leningrad Cartographic Factory VSEGEI Press: Leningrad, Russia, 1988. (In Russian) [Google Scholar]
- Badanina, I.Y.; Malitch, K.N.; Murzin, V.V.; Khiller, V.V.; Glavatskikh, S.P. Mineralogical and geochemical particularities of PGE mineralization of the Verkh-Neivinsk dunite-harzburgite massif (Middle Urals, Russia). Proc. Inst. Geol. Geochem. UB RAS 2013, 160, 188–192. (In Russian) [Google Scholar]
- González-Jimenéz, J.M.; Locmelis, M.; Belousova, E.; Griffin, W.; Gervilla, F.; Kerestedjian, T.N.; O’Reilly, S.Y.; Pearson, N.J.; Sergeeva, I. Genesis and tectonic implications of podiform chromitites in the metamorphosed ultramafic massif of Dobromirtsi (Bulgaria). Gondwana Res. 2015, 27, 555–574. [Google Scholar] [CrossRef]
- Lorand, J.-P.; Alard, O. Platinum-group element abundances in the upper mantle: New constraints from in situ and whole-rock analyses of Massif Central xenoliths (France). Geochim. Et Cosmochim. Acta 2001, 65, 2789–2806. [Google Scholar] [CrossRef]
- Pearson, N.J.; Alard, O.; Griffin, W.L.; Jackson, S.E.; O’Reilly, S.Y. In situ measurement of Re-Os isotopes in mantle sulfides by laser ablation multicollector-inductively coupled plasma mass spectrometry: Analytical methods and preliminary results. Geochim. Cosmochim. Acta 2002, 66, 1037–1050. [Google Scholar] [CrossRef]
- Merkle, R.K.; Malitch, K.N.; Gräser, P.P.; Badanina, I.Y. Native osmium from the Guli Massif, Northern Siberia (Russia). Mineral. Petrol. 2012, 104, 115–127. [Google Scholar] [CrossRef]
- Creaser, R.A.; Papanastassiou, D.A.; Wasserburg, G.J. Negative thermal ion mass spectrometry of osmium, rhenium, and iridium. Geochim. Cosmochim. Acta 1991, 55, 397–401. [Google Scholar] [CrossRef]
- Puchtel, I.S.; Touboul, M.; Blichert-Toft, J.; Walker, R.J.; Brandon, A.D.; Nicklas, R.W.; Kulikov, V.S.; Samsonov, A.V. Lithophile and siderophile element systematics of the mantle at the Archean-Proterozoic boundary: Evidence from 2.4 Ga komatiites. Geochim. Et Cosmochim. Acta 2016, 180, 227–255. [Google Scholar] [CrossRef]
- Puchtel, I.S.; Mundl-Petermeier, A.; Horan, M.; Hanski, E.J.; Blichert-Toft, J.; Walker, R.J. Ultra-depleted 2.05 Ga komatiites of Finnish Lapland: Products of grainy late accretion or core-mantle interaction? Chem. Geol. 2020, 554, 119801. [Google Scholar] [CrossRef]
- Cohen, A.S.; Waters, F.G. Separation of osmium from geological materials by solvent extraction for analysis by thermal ionisation mass spectrometry. Anal. Chim. Acta 1996, 332, 269–275. [Google Scholar] [CrossRef]
- Birck, J.-L.; Roy-Barman, M.; Capmas, F. Re–Os isotopic measurements at femtomole level in natural samples. Geostand. Newsl. 1997, 20, 19–27. [Google Scholar] [CrossRef]
- Puchtel, I.S.; Brandon, A.D.; Humayun, M. Precise Pt-Re-Os isotope systematics of the mantle from 2.7 Ga komatiites. Earth Planet. Sci. Lett. 2004, 224, 157–174. [Google Scholar] [CrossRef]
- Puchtel, I.S.; Walker, R.J.; Brandon, A.D.; Nisbet, E.G. Pt-Re-Os and Sm-Nd isotope and HSE and REE systematics of the 2.7 Ga Belingwe and Abitibi komatiites. Geochim. Cosmochim. Acta 2009, 73, 6367–6389. [Google Scholar] [CrossRef]
- Shirey, S.B.; Walker, R.J. The Re-Os isotope system in cosmochemistry and high-temperature geochemistry. Annu. Rev. Earth Planet. Sci. 1998, 26, 423–500. [Google Scholar] [CrossRef]
- Smoliar, M.I.; Walker, R.J.; Morgan, J.W. Re-Os ages of group IIA, IIIA, IVA, and IVB meteorites. Science 1996, 271, 1099–1102. [Google Scholar] [CrossRef]
- Begemann, F.; Ludwig, K.R.; Lugmair, G.W.; Min, K.; Nyquist, L.E.; Patchett Renne, P.R.; Shih, C.-Y.; Villa, I.M.; Walker, R.J. Call for an improved set of decay constants for geochronological use. Geochim. Cosmochim. Acta 2001, 65, 111–121. [Google Scholar] [CrossRef]
- Harris, D.C.; Cabri, L.J. Nomenclature of platinum-group-element alloys: Review and revision. Can. Mineral. 1991, 29, 231–237. [Google Scholar]
- Cabri, L.J.; Feather, C.E. Platinum-iron alloys: A nomenclature based on a study of natural and synthetic alloys. Can. Mineral. 1975, 13, 117–126. [Google Scholar]
- Cabri, L.-J.; Harris, D.C.; Weiser, T.W. Mineralogy and distribution of platinum-group mineral (PGM) placer deposits of the world. Explor. Min. Geol. 1996, 5, 73–167. [Google Scholar]
- Cabri, L.-J.; Oberthür, T.; Keays, R.R. Origin and depositional history of platinum-group minerals in placers–A critical review of facts and fiction. Ore Geol. Rev. 2022, 144, 104733. [Google Scholar] [CrossRef]
- Boyd, F.R.; Mertzman, S.A. Composition and structure of the Kaapvaal lithosphere, southern Africa. In Magmatic Processes: Physicochemical Principles; Mysen, B.O., Ed.; Geochemical Society Special Publication: Washington, DC, USA, 1987; Volume 1, pp. 13–24. [Google Scholar]
- Gaul, O.F.; Griffin, W.L.; O’Reilly, S.Y.; Pearson, D.G. Mapping olivine compositions in the lithospheric mantle. Earth Planet. Sci. Lett. 2000, 182, 223–235. [Google Scholar] [CrossRef]
- Pearson, D.G.; Canil, D.; Shirey, S.B. Mantle samples included in volcanic rocks: Xenoliths and diamonds. In Treatise on Geochemistry; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2004; pp. 171–275. [Google Scholar]
- Hansen, M.; Anderko, K. Constitution of Binary Alloys, 2nd ed.; McGraw Hill: New York, NY, USA, 1958; p. 1175. [Google Scholar]
- Voronova, L.I.; Polyakova, V.P.; Savitskiy, E.M. Alloys of the system Pt–Os. Russ. Metall. 1984, 5, 201–203. [Google Scholar]
- Massalski, T.B.; Murray, J.L.; Bennet, L.H.; Baker, H. Binary Alloy Phase Diagrams; American Society for Metals: Russel Township, OH, USA, 1993; p. 2224. [Google Scholar]
- Bird, J.M.; Bassett, W.A. Evidence of a deep mantle history in terrestrial osmium-iridium-ruthenium alloys. J. Geophys. Res. 1980, 85, 5461–5470. [Google Scholar] [CrossRef]
- Rudashevsky, N.S.; Kostoyanov, A.I.; Rudashevsky, V.N. Mineralogical and isotope evidences of origin of the Alpine-type massifs (the Ust’-Bel’sky massif, Koryak Highland, as an example). Zap. Vseross. Mineral. Obs. 1999, 128, 11–28. (In Russian) [Google Scholar]
- Malitch, K.N.; Auge, T.; Badanina, I.Y.; Goncharov, M.M.; Junk, S.A.; Pernicka, E. Os-rich nuggets from Au-PGE placers of the Maimecha-Kotui Province, Russia: A multi-disciplinary study. Mineral. Petrol. 2002, 76, 121–148. [Google Scholar] [CrossRef]
- Ahmed, A.H.; Hanghøj, K.; Kelemen, P.B.; Hart, S.R.; Arai, S. Osmium isotope systematics of the Proterozoic and Phanerozoic ophiolitic chromitites: In-situ ion probe analysis of primary Os-rich PGM. Earth Planet. Sci. Lett. 2006, 245, 777–791. [Google Scholar] [CrossRef]
- Shi, R.; Alard, O.; Zhi, X.; O’Reilly, S.Y.; Pearson, N.J.; Griffin, W.L.; Zhang, M.; Chen, X. Multiple events in the Neo-Tethyan oceanic upper mantle: Evidence from Ru–Os–Ir alloys in the Luobusa and Dongqiao ophiolitic podiform chromitites, Tibet. Earth Planet. Sci. Lett. 2007, 261, 33–48. [Google Scholar] [CrossRef]
- Malitch, K.N.; Anikina, E.V.; Badanina, I.Y.; Belousova, E.A.; Pushkarev, E.V.; Khiller, V.V. Chemical composition and osmium isotope systematics of primary and secondary platinum-group mineral assemblages from high-Mg chromitite of the Nurali lherzolite massif, South Urals, Russia. Geol. Ore Depos. 2016, 58, 1–19. [Google Scholar] [CrossRef]
- Walker, R.J.; Horan, M.F.; Morgan, J.W.; Becker, H.; Grossman, J.N.; Rubin, A.E. Comparative 187Re–187Os systematics of chondrites: Implications regarding early solar system processes. Geochim. Cosmochim. Acta 2002, 66, 4187–4201. [Google Scholar] [CrossRef]
- Fischer-Gödde, M.; Becker, H.; Wombacher, F. Rhodium, gold and other highly siderophile element abundances in chondritic meteorites. Geochim. Cosmochim. Acta 2010, 74, 356–379. [Google Scholar] [CrossRef]
- Dobretsov, N.L.; Berzin, N.A.; Buslov, M.M. Opening and tectonic evolution of the Paleo-Asian ocean. Int. Geol. Rev. 1995, 37, 335–360. [Google Scholar] [CrossRef]
- Horan, M.F.; Walker, R.J.; Morgan, J.W.; Grossman, J.N.; Rubin, A.E. Highly siderophile elements in chondrites. Chem. Geol. 2003, 196, 5–20. [Google Scholar] [CrossRef]
- Brandon, A.D.; Humayun, M.; Puchtel, I.S.; Zolensky, M. Re–Os isotopic systematics and platinum group element composition of the Tagish Lake carbonaceous chondrite. Geochim. Cosmochim. Acta 2005, 69, 1619–1631. [Google Scholar] [CrossRef]
- Acken, D.; Brandon, A.D.; Humayun, M. High-precision osmium isotopes in enstatite and Rumuruti chondrites. Geochim. Cosmochim. Acta 2011, 75, 4020–4036. [Google Scholar] [CrossRef]
- Puchtel, I.S.; Brandon, A.D.; Humayun, M.; Walker, R.J. Evidence for the early differentiation of the core from Pt–Re–Os isotope systematics of 2.8-Ga komatiites. Earth Planet. Sci. Lett. 2005, 237, 118–134. [Google Scholar] [CrossRef]
- Puchtel, I.S.; Walker, R.J.; Touboul, M.; Nisbet, E.G.; Byerly, G.R. Insights into Early Earth from the Pt–Re–Os isotope and Highly Siderophile Element abundance systematics of Barberton komatiites. Geochim. Cosmochim. Acta 2014, 125, 394–413. [Google Scholar] [CrossRef]
- Puchtel, I.S.; Humayun, M.; Walker, R.J. Os–Pb–Nd isotope and highly siderophile and lithophile trace element systematics of komatiitic rocks from the Volotsk suite, SE Baltic Shield. Precambrian Res. 2007, 158, 119–137. [Google Scholar] [CrossRef]
- Puchtel, I.S.; Walker, R.J.; Anhaeusser, C.R.; Gruau, G. Re–Os isotope systematics and HSE abundances of the 3.5 Ga Schapenburg komatiites, South Africa: Hydrous melting or prolonged survival of primordial heterogeneities in the mantle? Chem. Geol. 2009, 262, 355–369. [Google Scholar] [CrossRef]
- Puchtel, I.S.; Blichert-Toft, J.; Touboul, M.; Horan, M.F.; Walker, R.J. The coupled 182W-142Nd record of early terrestrial mantle differentiation. Geochem. Geophys. Geosyst. 2016, 17, 2168–2193. [Google Scholar] [CrossRef]
- Puchtel, I.S.; Blichert-Toft, J.; Touboul, M.; Walker, R.J. 182W and HSE constraints from 2.7 Ga komatiites on the heterogeneous nature of the Archean mantle. Geochim. Cosmochim. Acta 2018, 228, 1–26. [Google Scholar] [CrossRef]
- Puchtel, I.S. Re-Os isotope and HSE abundance systematics of the 2.9 Ga komatiites and basalts from the Sumozero-Kenozero greenstone belt, SE Fennoscandian Shield: Implications for the mixing rates of the mantle. Petrology 2022, 30, 548–566. [Google Scholar] [CrossRef]
- Puchtel, I.S.; Nicklas, R.W.; Slagle, J.; Horan, M.; Walker, R.J.; Nisbet, E.G.; Locmelis, M. Early global mantle chemical and isotope heterogeneity revealed by the komatiite-basalt record: The Western Australia connection. Geochim. Cosmochim. Acta 2022, 320, 238–278. [Google Scholar] [CrossRef]
- Meisel, T.; Walker, R.J.; Irving, A.J.; Lorand, J.-P. Osmium isotopic compositions of mantle xenoliths: A global perspective. Geochim. Cosmochim. Acta 2001, 65, 1311–1323. [Google Scholar] [CrossRef]
- Day, J.M.D.; Walker, R.J.; Warren, J.M. 186Os–187Os and highly siderophile element abundance systematics of the mantle revealed by abyssal peridotites and Os-rich alloys. Geochim. Cosmochim. Acta 2017, 200, 232–254. [Google Scholar] [CrossRef]
- Snow, J.E.; Reisberg, L. Os isotopic systematics of the MORB mantle–results from altered abyssal peridotites. Earth Planet. Sci. Lett. 1995, 133, 411–421. [Google Scholar]
- Brandon, A.D.; Snow, J.E.; Walker, R.J.; Morgan, J.W.; Mock, T.D. 190Pt–186Os and 187Re–187Os systematics of abyssal peridotites. Earth Planet. Sci. Lett. 2000, 177, 319–335. [Google Scholar] [CrossRef]
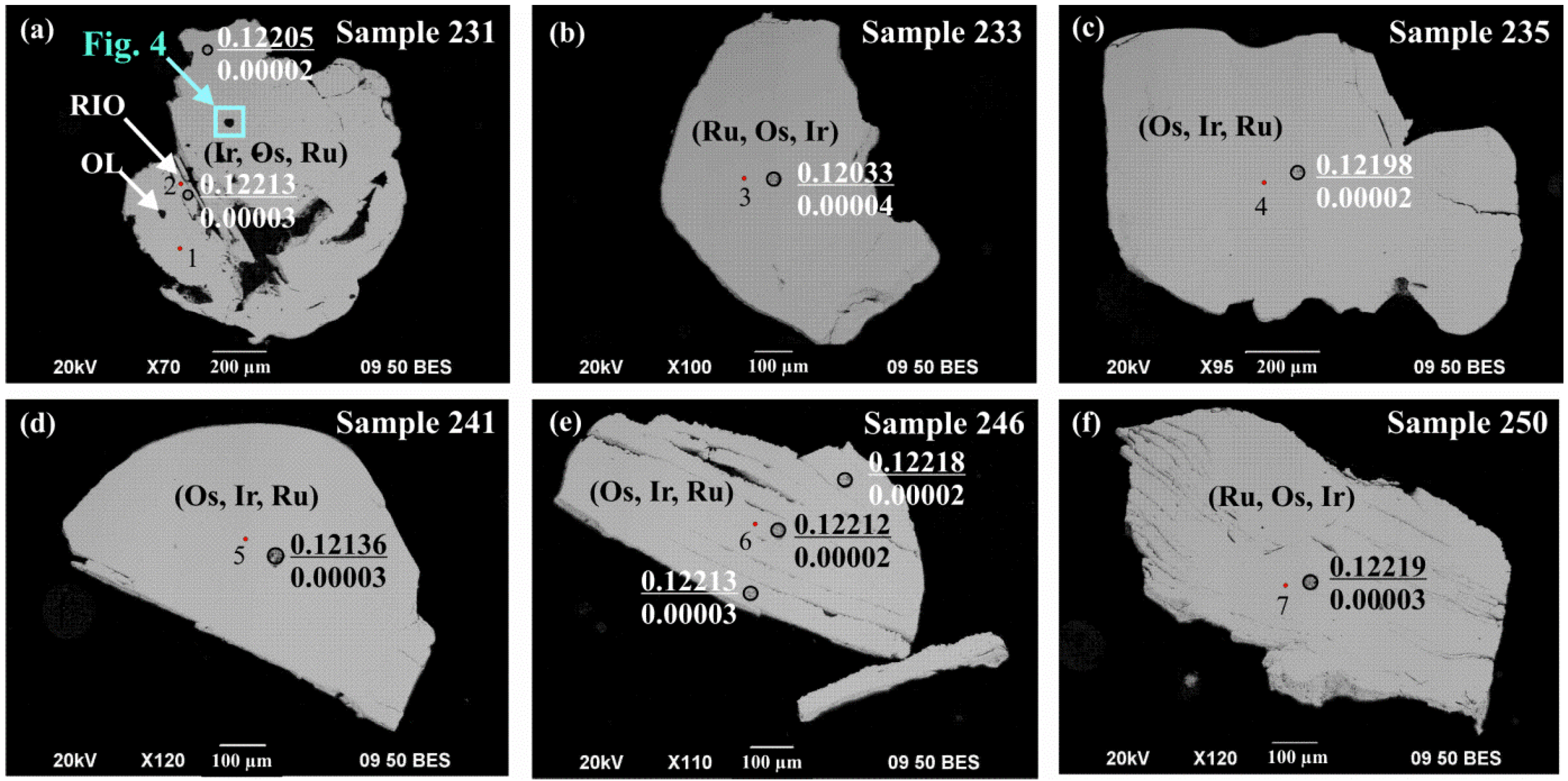

| Analysis# | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Sample# | 231 | 231 | 233 | 235 | 241 | 246 | 250 | 231 | 231 | 231 |
| PGM * | Ir | RIO | Ru | Os | Os | Os | Ru | Ir | (Pt, Fe) | (Pt, Fe) |
| Figure | 3a | 3a | 3b | 3c | 3d | 3e | 3f | 4a | 4a | 4a |
| Wt.% | ||||||||||
| Fe | 0.84 | 0.48 | “-” | 0.20 | 0.32 | 0.61 | 0.26 | 0.78 | 9.71 | 9.61 |
| Ni | 0.17 | “-” | “-” | “-” | “-” | “-” | “-” | 0.19 | “-” | “-” |
| Cu | “-” | “-” | “-” | “-” | “-” | “-” | “-” | “-” | 0.81 | 0.71 |
| Ru | 9.22 | 12.02 | 29.49 | 17.49 | 13.48 | 18.28 | 22.62 | 9.74 | “-” | “-” |
| Rh | “-” | “-” | “-” | “-” | “-” | “-” | “-” | “-” | 0.70 | 0.97 |
| Pd | “-” | “-” | “-” | “-” | “-” | “-” | “-” | “-” | “-” | “-” |
| Os | 25.25 | 42.87 | 53.85 | 42.96 | 44.29 | 41.66 | 38.49 | 23.81 | 0.34 | 0.25 |
| Ir | 56.29 | 43.08 | 12.49 | 35.19 | 39.64 | 36.64 | 35.13 | 57.10 | 1.81 | 2.06 |
| Pt | 7.51 | 0.97 | 3.82 | 3.68 | 1.71 | 2.37 | 2.03 | 7.77 | 86.33 | 86.28 |
| Total | 99.28 | 99.42 | 99.65 | 99.52 | 99.44 | 99.56 | 98.53 | 99.39 | 99.70 | 99.88 |
| AT.% | ||||||||||
| Fe | 2.62 | 1.48 | – | 0.59 | 0.98 | 1.78 | 0.74 | 2.43 | 26.87 | 26.60 |
| Ni | 0.51 | – | – | – | – | – | – | 0.56 | – | – |
| Cu | – | – | – | – | – | – | – | 1.97 | 1.73 | |
| Ru | 15.91 | 20.43 | 44.25 | 28.63 | 22.72 | 29.48 | 35.87 | 16.74 | – | – |
| Rh | – | – | – | – | – | – | 1.05 | 1.46 | ||
| Pd | – | – | – | – | – | – | – | – | – | – |
| Os | 23.16 | 38.73 | 42.93 | 37.37 | 39.67 | 35.70 | 32.43 | 21.75 | 0.28 | 0.20 |
| Ir | 51.08 | 38.51 | 9.85 | 30.29 | 35.14 | 31.06 | 29.29 | 51.60 | 1.45 | 1.65 |
| Pt | 6.72 | 0.85 | 2.97 | 3.12 | 1.49 | 1.98 | 1.67 | 6.92 | 68.38 | 68.36 |
| Analysis | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Sample# | 231-1 | 231-2 | 231-3 | 231-4 | 231-5 |
| Wt.% | |||||
| SiO2 | 41.26 | 41.23 | 40.96 | 40.86 | 41.01 |
| MgO | 51.74 | 51.60 | 50.03 | 50.36 | 50.08 |
| FeO | 6.50 | 6.53 | 8.08 | 7.80 | 8.13 |
| NiO | 0.44 | 0.57 | 0.65 | 0.63 | 0.67 |
| Total | 99.94 | 99.93 | 99.72 | 99.65 | 99.89 |
| Fo # | 93 | 93 | 92 | 92 | 92 |
| Apfu Cations on the basis of 4 atoms | |||||
| Si | 0.998 | 0.998 | 1.000 | 0.998 | 1.000 |
| Mg | 1.865 | 1.861 | 1.822 | 1.833 | 1.821 |
| Fe2+ | 0.131 | 0.132 | 0.165 | 0.159 | 0.166 |
| Ni | 0.009 | 0.011 | 0.013 | 0.012 | 0.013 |
| Sum | 3.003 | 3.002 | 3.000 | 3.002 | 3.000 |
| Sample#, Mineral *, Figure | Atomic Proportions | 187Re/188Os | 187Os/188Os | 187Os/188Os(T) | γ187Os(T) |
|---|---|---|---|---|---|
| Kunar | |||||
| 3-2, Os | (Os0.36Ru0.32Ir0.29Pt0.02Fe0.01) | 0.00016 ± 2 | 0.12194 ± 2 | 0.12194 | −0.07 |
| 229-1, Os | (Os0.34Ru0.32Ir0.31Pt0.02Fe0.01) | 0.00023 ± 2 | 0.12155 ± 3 | 0.12154 | −0.40 |
| 229-2, Os | (Os0.34Ru0.32Ir0.31Pt0.02Fe0.01) | 0.00034 ± 2 | 0.12164 ± 3 | 0.12163 | −0.33 |
| 229-3, Os | (Os0.34Ru0.32Ir0.32Pt0.01Fe0.01) | 0.00019 ± 2 | 0.12178 ± 3 | 0.12177 | −0.21 |
| 229-4, Os | (Os0.35Ru0.31Ir0.31Pt0.02Fe0.01) | 0.00022 ± 2 | 0.12165 ± 3 | 0.12164 | −0.32 |
| 230-1, Ir | (Ir0.59Os0.36Ru0.02Pt0.02Fe0.01) | 0.00026 ± 2 | 0.12194 ± 3 | 0.12193 | −0.08 |
| 230-2, Ir | (Ir0.59Os0.36Ru0.02Pt0.02Fe0.01) | 0.00043 ± 2 | 0.12174 ± 3 | 0.12174 | −0.24 |
| 231-1, Ir, Figure 3a | (Ir0.51Os0.23Ru0.16Pt0.07Fe0.03) | 0.000013 ± 23 | 0.12205 ± 3 | 0.12205 | +0.02 |
| 231-2, RIO, Figure 3a | (Ir0.39Os0.39Ru0.20Pt0.01Fe0.01) | 0.000044 ± 17 | 0.12213 ± 3 | 0.12213 | +0.08 |
| 232, Ir | (Ir0.62Os0.33Ru0.02Fe0.02Pt0.01) | 0.00032 ± 2 | 0.12231 ± 4 | 0.12231 | +0.23 |
| 233, Ru, Figure 3b | (Ru0.44Os0.43Ir0.10Pt0.03) | 0.000063 ± 23 | 0.12033 ± 4 | 0.12033 | −1.39 |
| 234, Os | (Os0.37Ru0.31Ir0.30Pt0.01Fe0.01) | 0.00016 ± 2 | 0.12244 ± 3 | 0.12244 | +0.34 |
| 235, Os, Figure 3c | (Os0.37Ir0.30Ru0.29Pt0.03Fe0.01) | 0.00069 ± 2 | 0.12199 ± 3 | 0.12198 | −0.04 |
| Mean (n = 13) | 0.1218 | −0.18 | |||
| 2SD (n = 13) | 0.0010 | 0.85 | |||
| Unga | |||||
| 236, Os | (Os0.39Ir0.34Ru0.26Pt0.01) | 0.00076 ± 3 | 0.12214 ± 2 | 0.12213 | +0.08 |
| 237-1, Ru | (Ru0.49Os0.31Ir0.19Pt0.01) | 0.00057 ± 4 | 0.12367 ± 4 | 0.12366 | +1.3 |
| 237-2, Ru | (Ru0.53Os0.28Ir0.18Pt0.01) | 0.00100 ± 4 | 0.12395 ± 3 | 0.12394 | +1.6 |
| 239-1, Os | (Os0.41Ir0.37Ru0.21Pt0.01) | 0.00029 ± 2 | 0.12198 ± 4 | 0.12197 | −0.05 |
| 239-2, Os | (Os0.41Ir0.37Ru0.21Pt0.01) | 0.00029 ± 2 | 0.12214 ± 2 | 0.12213 | +0.08 |
| 240, Ir | (Ir0.57Os0.25Ru0.15Pt0.02Fe0.01) | 0.000080 ± 15 | 0.12193 ± 3 | 0.12193 | −0.08 |
| 241, Os, Figure 3d | (Os0.40Ir0.35Ru0.23Pt0.01Fe0.01) | 0.00561 ± 10 | 0.12143 ± 3 | 0.12136 | −0.55 |
| 242, Ir | (Ir0.61Os0.31Ru0.03Pt0.03Rh0.02) | 0.00054 ± 2 | 0.12370 ± 4 | 0.12369 | +1.4 |
| 243, Os | (Os0.36Ir0.31Ru0.30Pt0.02Fe0.01) | 0.00032 ± 1 | 0.12154 ± 3 | 0.12153 | −0.41 |
| 244, Os | (Os0.40Ir0.35Ru0.22Pt0.02Fe0.01) | 0.00317 ± 2 | 0.12113 ± 3 | 0.12109 | −0.77 |
| 246-1, Os, Figure 3e | (Os0.36Ir0.31Ru0.29Pt0.02Fe0.02) | 0.00019 ± 2 | 0.12212 ± 3 | 0.12212 | +0.07 |
| 246-2, Os, Figure 3e | (Os0.36Ir0.31Ru0.29Pt0.02Fe0.02) | 0.00020 ± 2 | 0.12218 ± 3 | 0.12218 | +0.12 |
| 246-3, Os, Figure 3e | (Os0.36Ir0.31Ru0.29Pt0.02Fe0.02) | 0.00017 ± 2 | 0.12213 ± 2 | 0.12213 | +0.08 |
| 247, Os | (Os0.58Ir0.28Ru0.13Fe0.01) | 0.00021 ± 2 | 0.12389 ± 3 | 0.12389 | +1.5 |
| 248, Os | (Os0.71Ru0.16Ir0.13) | 0.000005 ± 15 | 0.11848 ± 3 | 0.11848 | −2.91 |
| 249, Os | (Os0.44Ir0.35Ru0.20Pt0.01) | 0.00023 ± 1 | 0.12237 ± 2 | 0.12237 | +0.28 |
| 250, Ru, Figure 3f | (Ru0.36Os0.32Ir0.29Pt0.02Fe0.01) | 0.00029 ± 1 | 0.12220 ± 3 | 0.12219 | +0.13 |
| 251, Os | (Os0.39Ru0.30Ir0.27Pt0.03Fe0.01) | 0.00056 ± 1 | 0.12200 ± 2 | 0.12200 | −0.03 |
| Mean (n = 18) | 0.1222 | +0.10 | |||
| 2SD (n = 18) | 0.0025 | 2.1 | |||
| Sample# | 187Re/188Os | 190Pt/188Os | 186Os/188Os | 187Os/188Os | μ186Os(T) | γ187Os(T) | TRD, Ga |
|---|---|---|---|---|---|---|---|
| T-2 | 0.00024 ± 10 | 0.000152 ± 26 | 0.1198411 ± 12 | 0.1218493 ± 15 | +34 ± 10 | −0.1500 ± 12 | 0.760 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malitch, K.N.; Puchtel, I.S.; Belousova, E.A.; Badanina, I.Y. A Combined Re-Os and Pt-Os Isotope and HSE Abundance Study of Ru-Os-Ir Alloys from the Kunar and Unga Placer Deposits, the Taimyr Peninsula, Polar Siberia. Minerals 2022, 12, 1463. https://doi.org/10.3390/min12111463
Malitch KN, Puchtel IS, Belousova EA, Badanina IY. A Combined Re-Os and Pt-Os Isotope and HSE Abundance Study of Ru-Os-Ir Alloys from the Kunar and Unga Placer Deposits, the Taimyr Peninsula, Polar Siberia. Minerals. 2022; 12(11):1463. https://doi.org/10.3390/min12111463
Chicago/Turabian StyleMalitch, Kreshimir N., Igor S. Puchtel, Elena A. Belousova, and Inna Yu. Badanina. 2022. "A Combined Re-Os and Pt-Os Isotope and HSE Abundance Study of Ru-Os-Ir Alloys from the Kunar and Unga Placer Deposits, the Taimyr Peninsula, Polar Siberia" Minerals 12, no. 11: 1463. https://doi.org/10.3390/min12111463
APA StyleMalitch, K. N., Puchtel, I. S., Belousova, E. A., & Badanina, I. Y. (2022). A Combined Re-Os and Pt-Os Isotope and HSE Abundance Study of Ru-Os-Ir Alloys from the Kunar and Unga Placer Deposits, the Taimyr Peninsula, Polar Siberia. Minerals, 12(11), 1463. https://doi.org/10.3390/min12111463






