The Use of Acid Mine Drainage (AMD) in the Flotation of a Platinum-Group-Minerals-Bearing Merensky Ore
Abstract
1. Introduction
2. Materials and Methods
2.1. Ore Chemistry and Mineralogy
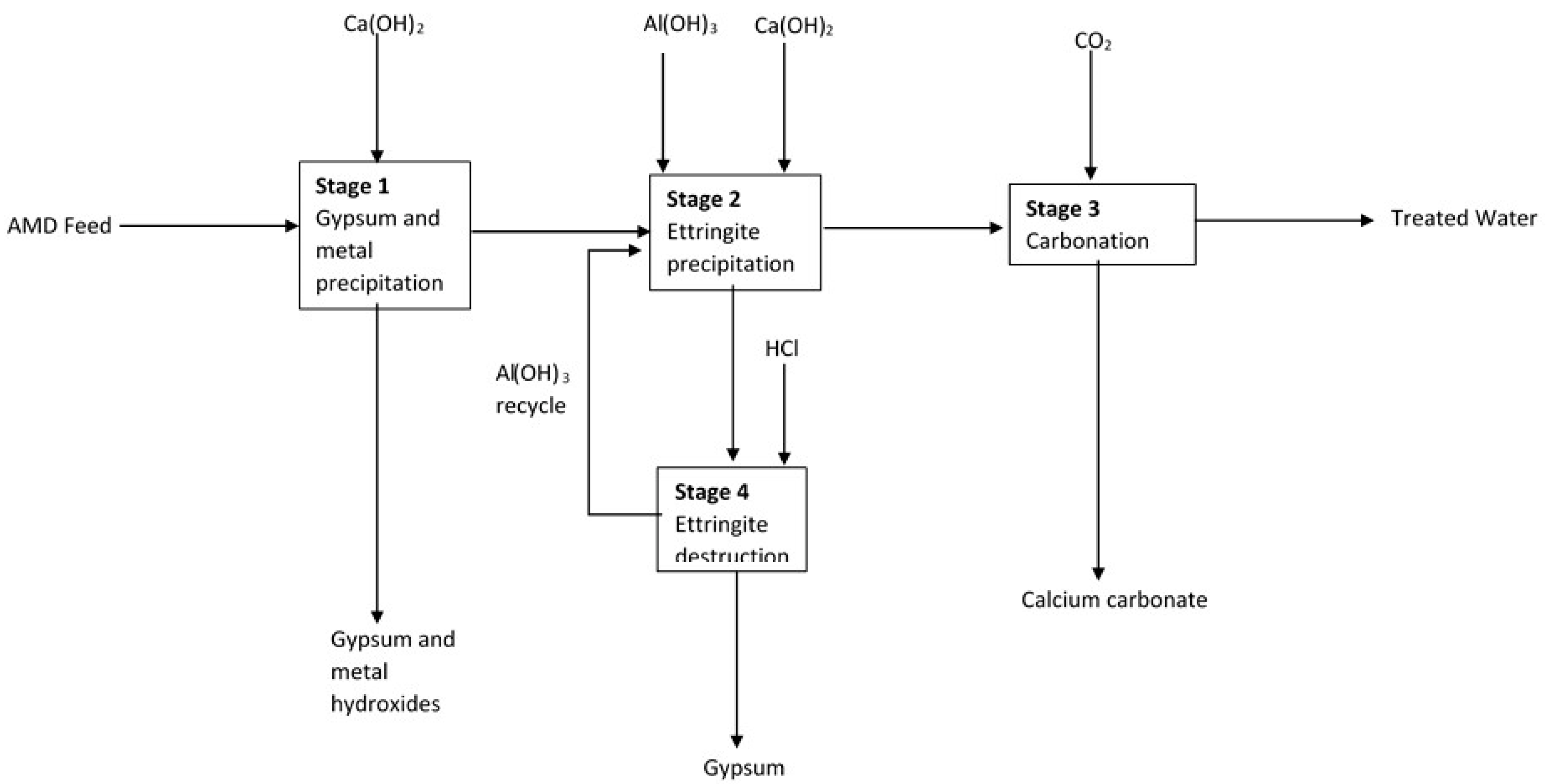
2.2. Water Chemistry
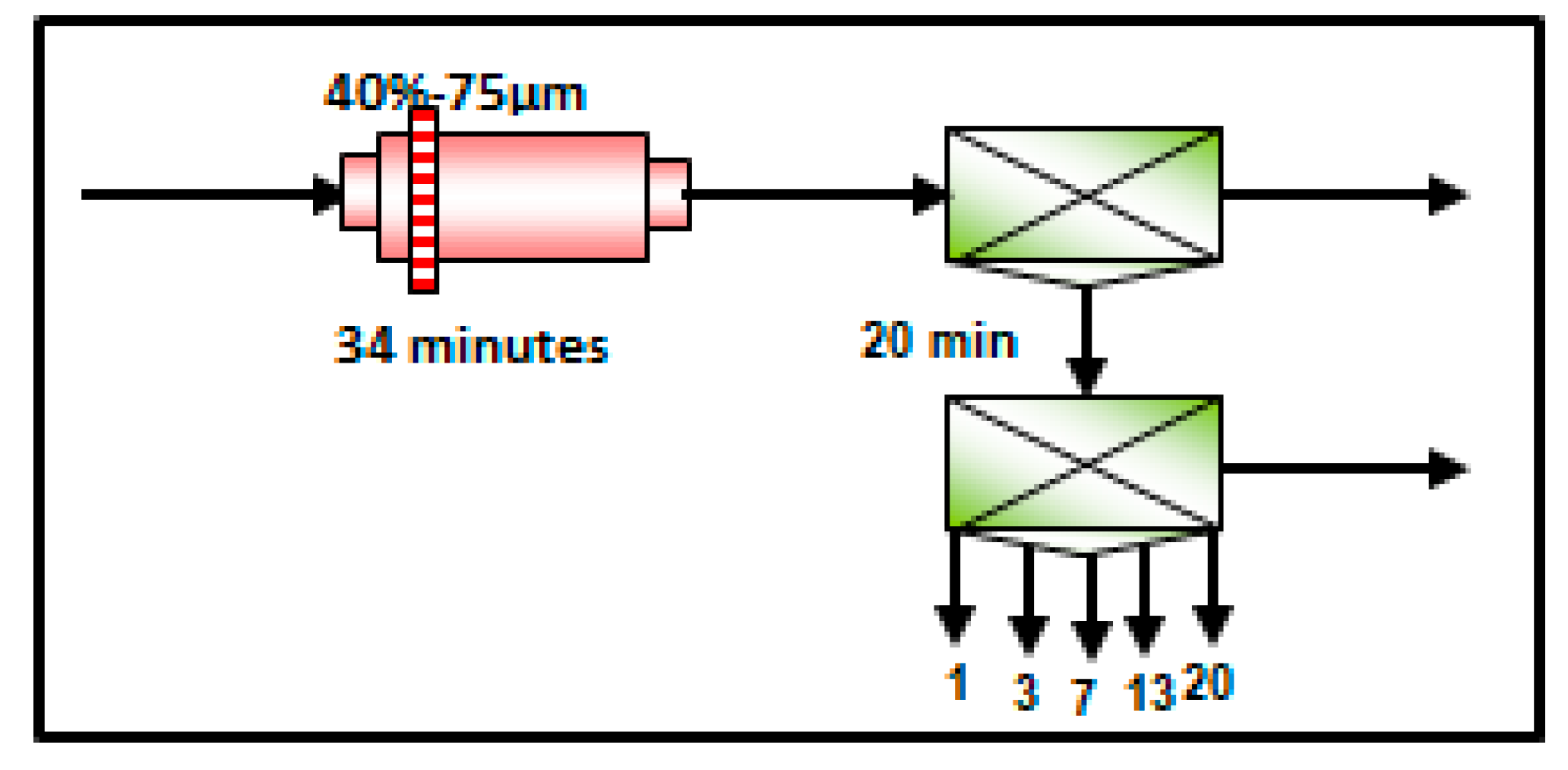
2.3. Flotation Tests and Procedure
3. Results and Discussion
3.1. Ore Chemistry and Mineralogical Results
3.2. Water Characterisation Results
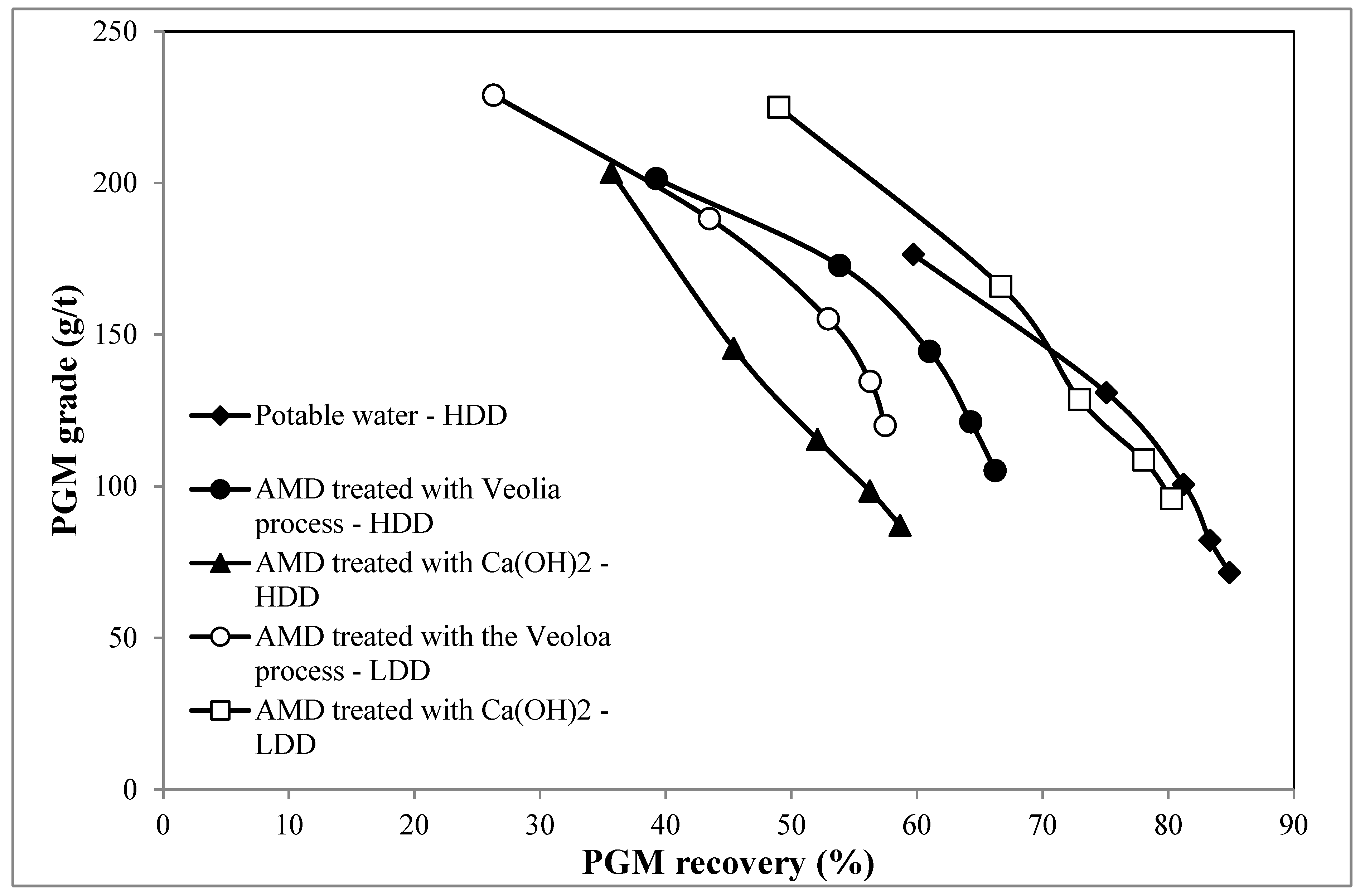
3.3. Effect of AMD Treated with Ca(OH)2 on the Flotation Response of the Merensky Ore
3.3.1. Effect of Ca2+ and SO42− Ions on the Flotation Performance of PGMs
3.3.2. Effect of Ca2+ and SO42− ions on the Flotation Performance of Ni
3.3.3. Effect of Ca2+ and SO42− Ions on the Flotation Performance of Cu
3.4. Effect of AMD-Treated Veolia Process on the Flotation Performance of the Merensky Ore
3.5. Effect of Depressant Dosage on the Flotation Performance of the Merensky Ore
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haggard, E.L.; Sheridan, C.M.; Harding, K.G. Quantification of water usage at a South African platinum processing plant. Water SA 2015, 41, 279–286. [Google Scholar] [CrossRef][Green Version]
- Park, J.H.; Han, Y.-S.; Ji, S.-W. Investigation of Mineral-Processing Wastewater Recycling Processes: A Pilot Study. Sustainability 2018, 10, 3069. [Google Scholar] [CrossRef]
- Levay, G.; Smart, R.S.C.; Skinner, W.M. The impact of water quality on flotation performance. J. S. Afr. Inst. Min. Metall. 2001, 101, 69–75. [Google Scholar]
- Jennett, J.C.; Wixson, B.G. Geochemistry, mining and the environment. Miner. Environ. 1983, 5, 39–53. [Google Scholar] [CrossRef]
- Fisher, W.; Rudy, S. Municipal wastewater utilization for froth flotation of copper and molybdenum sulfides. Soc. Min. Eng. AIME 1976, 264, 698–1702. [Google Scholar]
- Levay, G.; Schumann, R. A systematic approach to water quality management in the minerals processing industry. In Proceedings of the Water in Mining Conference, Brisbane, Australia, 14–16 November 2006; pp. 277–287. [Google Scholar]
- Maree, J.P.; Mujuru, M.; Bologo, V.; Daniels, N.; Mpholoane, D. Neutralisation treatment of AMD at affordable cost. Water SA 2013, 39, 2. [Google Scholar] [CrossRef]
- Bulatovic, S.M. Handbook of Flotation Reagents: Chemistry, Theory and Practice: Volume 1: Flotation of Sulfide Ores; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Wills, B.A.; Napier-Munn, T.J. Wills’ Mineral Processing Technology: An Introduction to the Practical Aspects of Ore Treatment; Elsevier Oxford: Oxford, UK, 2006. [Google Scholar]
- Ikumapayi, F.; Makitalo, M.; Johansson, B.; Rao, K.H. Recycling of process water in sulphide flotation: Effect of calcium and sulphate ions on flotation of galena. Miner. Eng. 2012, 39, 77–88. [Google Scholar] [CrossRef]
- Li, Y.; Lartey, C.; Song, S.; Li, Y.; Gerson, A.R. The fundamental roles of monovalent and divalent cations with sulfates on molybdenite flotation in the absence of flotation reagents. RSC Adv. 2018, 8, 23364–23371. [Google Scholar] [CrossRef]
- Corin, K.; Reddy, A.; Miyen, L.; Wiese, J.; Harris, P. The effect of ionic strength of plant water on valuable mineral and gangue recovery in a platinum bearing ore from the Merensky reef. Miner. Eng. 2010, 24, 131–137. [Google Scholar] [CrossRef]
- Manono, M.; Corin, K.; Wiese, J. An investigation into the effect of various ions and their ionic strength on the flotation performance of a platinum bearing ore from the Merensky reef. Miner. Eng. 2012, 36-38, 231–236. [Google Scholar] [CrossRef]
- Muzenda, E. An investigation into the effect of water quality on flotation performance. World Acad. Sci. Eng. Technol. 2010, 69, 237–241. [Google Scholar]
- Can, I.B.; Bıçak, Ö; Özçelik, S.; Can, M.; Ekmekçi, Z. Sulphate Removal from Flotation Process Water Using Ion-Exchange Resin Column System. Minerals 2020, 10, 655. [Google Scholar] [CrossRef]
- Filho, J.A.; Azevedo, A.; Etchepare, R.; Rubio, J. Removal of sulfate ions by dissolved air flotation (DAF) following precipitation and flocculation. Int. J. Miner. Process. 2016, 149, 1–8. [Google Scholar] [CrossRef]
- Ikumapayi, F.; Makitalo, M.; Johansson, B.; Rao, K.H. Recycling process water in sulfide flotation, Part A: Effect of calcium and sulfate on sphalerite recovery. Mining Met. Explor. 2012, 29, 183–191. [Google Scholar] [CrossRef]
- Dzingai, M.; Manono, M.; Corin, K. Simulating the Effect of Water Recirculation on Flotation through Ion-Spiking: Effect of Ca2+ and Mg2+. Minerals 2020, 10, 1033. [Google Scholar] [CrossRef]
- Dzingai, M.; Manono, M.S.; Corin, K.C. Probing the effect of water recycling on flotation through anion spiking using a low-grade Cu-Ni-PGM ore: The effect of NO3−, SO42− and S2O32−. Minerals 2021, 11, 340. [Google Scholar] [CrossRef]
- October, L.L.; Corin, K.C.; Manono, M.S.; Schreithofer, N.; Wiese, J.G. A fundamental study considering specific ion effects on the attachment of sulfide minerals to air bubbles. Miner. Eng. 2020, 151, 106313. [Google Scholar] [CrossRef]
- Mhonde, N.; Schreithofer, N.; Corin, K.; Mäkelä, M. Assessing the Combined Effect of Water Temperature and Complex Water Matrices on Xanthate Adsorption Using Multiple Linear Regression. Minerals 2020, 10, 733. [Google Scholar] [CrossRef]
- Kirjavainen, V.; Schreithofer, N.; Heiskanen, K. Effect of calcium and thiosulfate ions on flotation selectivity of nickel–copper ores. Miner. Eng. 2002, 15, 1–5. [Google Scholar] [CrossRef]
- Manono, M.S.; Corin, K.C. Considering Specific Ion Effects on Froth Stability in Sulfidic Cu-Ni-PGM Ore Flotation. Minerals 2022, 12, 321. [Google Scholar] [CrossRef]
- Manono, M.S.; Matibidi, K.; Corin, K.C.; Thubakgale, C.K.; Otunniyi, I.O.; Wiese, J.G. Specific Ion Effects on the Behavior of Mixtures of Sodium Iso-Butyl Xanthate and Sodium Diethyl Dithiophosphate during the Flotation of a Cu-Ni-PGM Ore: Effects of CaCl2 and NaCl. Environ. Sci. Proc. 2021, 6, 22. [Google Scholar]
- Manono, M.S.; Corin, K.C.; Wiese, J.G. The Behavior of Gangue During the Flotation of a Sulfidic PGM-Bearing Ore in Response to Various Monovalent and Divalent Ions in Process Water. Front. Chem. 2020, 8, 79. [Google Scholar] [CrossRef] [PubMed]
- Manono, M.; Corin, K.; Wiese, J. Perspectives from literature on the influence of inorganic electrolytes present in plant water on flotation performance. Physicochem. Probl. Miner. Process. 2018, 54, 1191–1214. [Google Scholar] [CrossRef]
- Manono, M.; Corin, K.; Wiese, J. The influence of electrolytes present in process water on the flotation behaviour of a Cu-Ni containing ore. Miner. Eng. 2016, 96–97, 99–107. [Google Scholar] [CrossRef]
- October, L.L.; Manono, M.S.; Corin, K.C.; Schreithofer, N.; Wiese, J.G. The Influence of Specific Ions and Oxyhydroxo Species in Plant Water on the Bubble–Particle Attachment of Pyrrhotite. ACS Omega 2021, 6, 28496–28506. [Google Scholar] [CrossRef]
- Ikumapayi, F.; Rao, K.H. Recycling Process Water in Complex Sulfide Ore Flotation: Effect of Calcium and Sulfate on Sulfide Minerals Recovery. Miner. Process. Extr. Met. Rev. 2014, 36, 45–64. [Google Scholar] [CrossRef]
- Boujounoui, K.; Abidi, A.; Baçaoui, A.; El Amari, K.; Yaacoubi, A. The influence of water quality on the flotation performance of complex sulphide ores: Case study at Hajar Mine, Morocco. J. S. Afr. Inst. Min. Met. 2015, 115, 1243–1251. [Google Scholar] [CrossRef]
- Wang, D. Flotation Reagent: Applied Surface Chemistry on Minerals Flotation and Energy Resources Beneficiation; Springer: Singapore, 2016. [Google Scholar]
- Rao, S.R. Surface Chemistry of Froth Flotation; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar] [CrossRef]
| Reagent | Supplier | Purity (%) |
|---|---|---|
| SIBX | Senmin | 100 |
| KU92 | Senmin | 100 |
| Sasfroth 200 | Sasol chemicals | ≥85 |
| CuSO4 | Sigma-Aldrich | ≥99 |
| Ca(OH)2 | Sigma-Aldrich | ≥95 |
| Location | Reagents and Dosages | Cond Iime (min) | Float Time (min) |
|---|---|---|---|
| Rougher | 20 g/t CuSO4 | 3 | |
| 100 g/t SIBX | 2 | ||
| 40 g/t KU92 | 2 | ||
| 50 g/t Sasfroth 200 | 1 | ||
| RC | 20 | ||
| Cleaner | 10 g/t KU92 | 2 | |
| 10 g/t Sasfroth 200 | 1 | ||
| CC1 | 1 | ||
| CC2 | 2 | ||
| CC3 | 4 | ||
| CC4 | 6 | ||
| CC5 | 7 |
| Water Type | Circuit | CuSO4 Dosage (g/t) | SIBX Dosage (g/t) | KU 92 Dosage (g/t) | Sasfroth 200 Dosage (g/t) |
| Potable water | Rougher | 20 | 100 | 40 | 50 |
| Cleaner | - | - | 10 | 10 | |
| AMD treated with Ca(OH)2—HDD. | Rougher | 20 | 100 | 40 | 50 |
| Cleaner | - | - | 10 | 10 | |
| AMD treated with Ca(OH)2—LDD. | Rougher | 20 | 100 | 20 | 50 |
| Cleaner | - | - | 5 | 10 | |
| AMD treated with the Veolia process—HDD | Rougher | 20 | 100 | 40 | 50 |
| Cleaner | - | - | 10 | 10 | |
| AMD treated with the Veolia process—LDD. | Rougher | 20 | 100 | 20 | 50 |
| Cleaner | - | - | 5 | 10 |
| PGMs | Cu | Ni | Fe | Mg | Si | Total S |
|---|---|---|---|---|---|---|
| (g/t) | (%) | (%) | (%) | (%) | (%) | (%) |
| 4.2 | 0.11 | 0.21 | 7.7 | 11.1 | 23.7 | 0.40 |
| Parameter | Unit | Potable Water | AMD Treated with Ca(OH)2 | AMD Treated with the Veolia Process |
|---|---|---|---|---|
| pH | 7.6 | 8.3 | 7.8 | |
| Na | ppm | 11.25 | 102.5 | 49.7 |
| K | ppm | 7.11 | 17 | 10.8 |
| Ca | ppm | 6.31 | 667.5 | 1202 |
| Mg | ppm | 2.65 | 148.5 | 0.7 |
| Cl | ppm | 5.82 | 66.1 | 481.6 |
| F | ppm | 0.02 | 0.02 | 0.02 |
| SO4 | ppm | <10 | 2730 | 109.0 |
| Conductivity | µS/cm | 163 | 3600 | 9350 |
| Water Type | Circuit | Mass | Grade | Recovery (%) | ||||
|---|---|---|---|---|---|---|---|---|
| % | PGMs (g/t) | Cu (%) | Ni (%) | PGMs (%) | Cu (%) | Ni (%) | ||
| Potable water (HDD) | Rougher | 8.4 ± 0.3 | 45.2 | 0.7 | 1.5 | 88.4 | 57.5 | 58.3 |
| Cleaner | 5.1 ± 0.4 | 71.6 | 1.2 | 2.3 | 84.9 | 56.9 | 54.9 | |
| AMD treated with Ca(OH)2 (HDD) | Rougher | 5.5 ± 0.2 | 56.4 | 1.1 | 1.7 | 67.8 | 57.0 | 47.7 |
| Cleaner | 3.1 ± 0.5 | 87.1 | 1.9 | 2.4 | 58.6 | 52.8 | 37.9 | |
| AMD treated with Ca(OH)2 (LDD) | Rougher | 6.5 ± 0.4 | 67.5 | 1.1 | 1.8 | 91.0 | 60.2 | 57.5 |
| Cleaner | 4.0 ± 0.5 | 96.0 | 1.7 | 2.7 | 80.3 | 57.6 | 54.6 | |
| AMD treated with the Veolia process (HDD) | Rougher | 4.8 ± 0.3 | 59.4 | 1.5 | 2.1 | 70.8 | 65.2 | 58.3 |
| Cleaner | 2.5 ± 0.3 | 105.2 | 2.7 | 3.8 | 66.2 | 62.2 | 55.4 | |
| AMD treated with the Veolia process (LDD) | Rougher | 5.4 ± 0.4 | 82.5 | 1.3 | 1.7 | 59.2 | 59.4 | 47.0 |
| Cleaner | 3.6 ± 0.5 | 120.1 | 1.9 | 2.5 | 57.5 | 58.7 | 45.2 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taguta, J.; Peku, Z.; Sehlotho, N.; Corin, K. The Use of Acid Mine Drainage (AMD) in the Flotation of a Platinum-Group-Minerals-Bearing Merensky Ore. Minerals 2022, 12, 1259. https://doi.org/10.3390/min12101259
Taguta J, Peku Z, Sehlotho N, Corin K. The Use of Acid Mine Drainage (AMD) in the Flotation of a Platinum-Group-Minerals-Bearing Merensky Ore. Minerals. 2022; 12(10):1259. https://doi.org/10.3390/min12101259
Chicago/Turabian StyleTaguta, Jestos, Zandile Peku, Nthapo Sehlotho, and Kirsten Corin. 2022. "The Use of Acid Mine Drainage (AMD) in the Flotation of a Platinum-Group-Minerals-Bearing Merensky Ore" Minerals 12, no. 10: 1259. https://doi.org/10.3390/min12101259
APA StyleTaguta, J., Peku, Z., Sehlotho, N., & Corin, K. (2022). The Use of Acid Mine Drainage (AMD) in the Flotation of a Platinum-Group-Minerals-Bearing Merensky Ore. Minerals, 12(10), 1259. https://doi.org/10.3390/min12101259







