Chemical and Mineralogical Characterization of Montevive Celestine Mineral
Abstract
1. Introduction
2. Materials and Methods
2.1. Celestine Ore
2.2. Mineral Samples
2.3. Microscopy
2.4. Thermogravimentric Analysis
2.5. X-ray Fluorescence and X-ray Diffraction
3. Results and Discussion
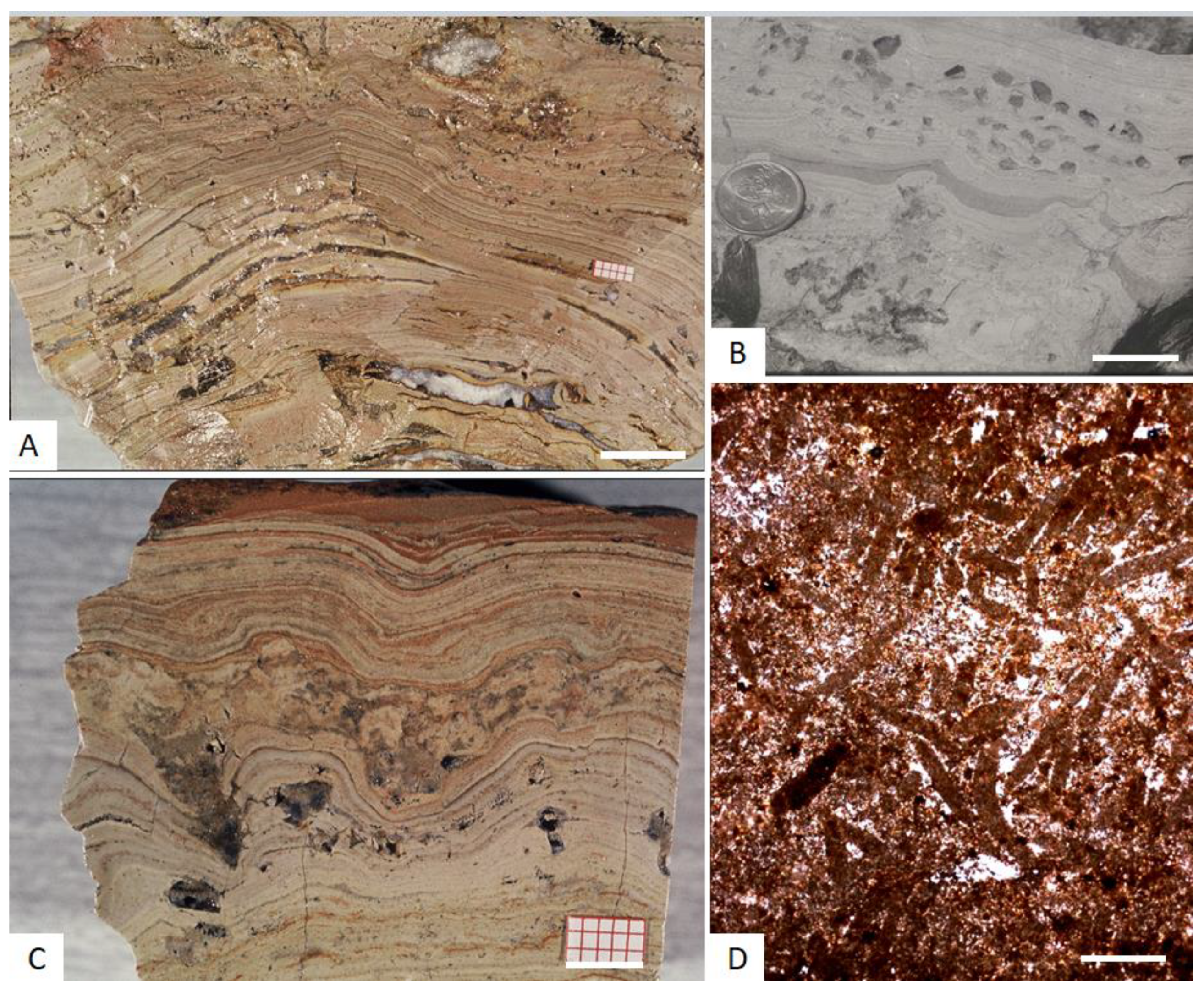
3.1. Optical Microscopy
3.2. Granulometry Analysis
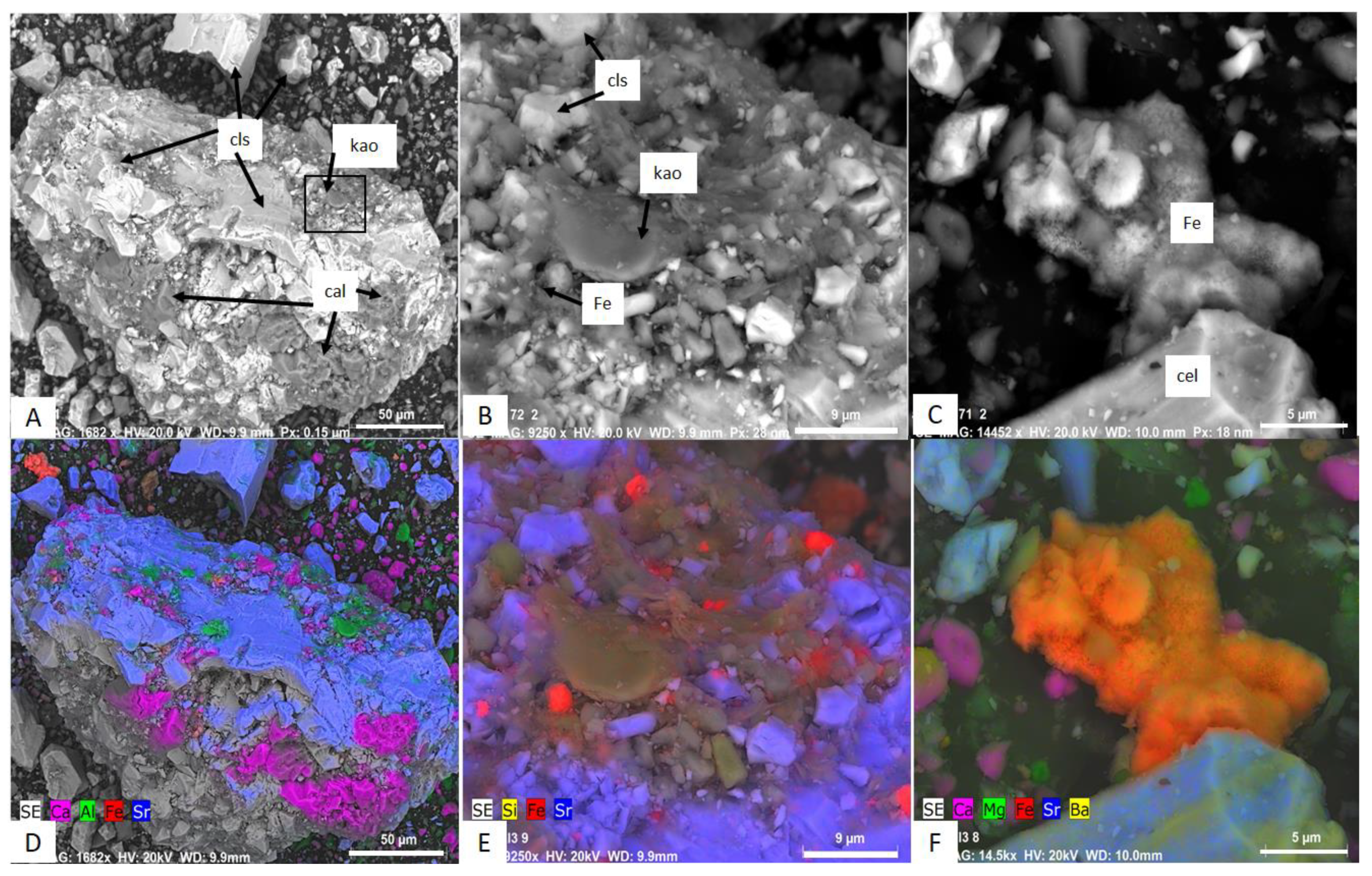
3.3. Scanning Electron Microscopy
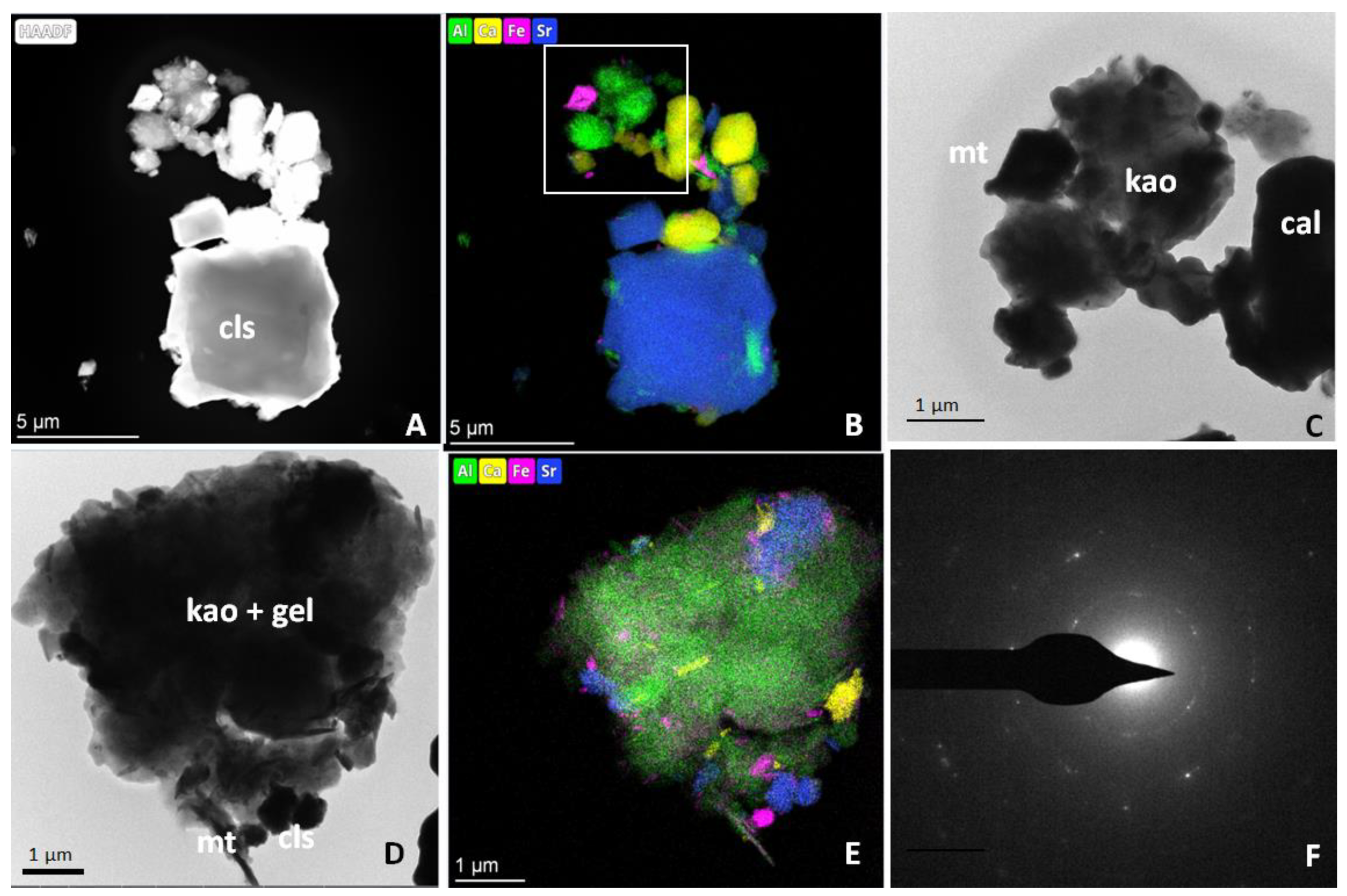
3.4. Transmission Electron Microscopy
3.5. X-ray Fluorescence
Correlation Analysis
3.6. X-ray Diffraction
3.6.1. Sulfates
3.6.2. Carbonates
3.6.3. Silicates
3.6.4. Other Minerals
3.6.5. Correlation Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nesse, W.D. Introduction to Mineralogy; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
- Butler, J.C. Selected Aspects of the Crystal Chemistry of BaSO4, SrSO4 and PbSO4 Selected Aspects of the Crystal Chemistry of BaSO4, SrSO4 and PbSO4. Ph.D. Thesis, Universidad de Ohio, Athens City, OH, USA, 1941. [Google Scholar]
- Chang, L.L.Y.; Howie, R.A.; Zussman, J. Rock-forming Minerals, 5B: Non Silicates, 2nd. ed.; Longman: London, UK, 1996. [Google Scholar]
- Colville, A.A.; Staudhammer, K. A refinement of the structure barite. Am. Mineral. 1967, 52, 1877–1880. [Google Scholar]
- Deer, W.A.; Howie, R.A.; Zussman, J. An Introduction to the Rock Fonning Minerals; Wiley Online Library, Ed.; EEUU: New York, NY, USA, 1967. [Google Scholar] [CrossRef]
- Forjanes, P.; Astilleros, J.M.; Fernández-Díaz, L. The formation of barite and celestine through the replacement of gypsum. Minerals 2020, 10, 189. [Google Scholar] [CrossRef]
- Hanor, J.S. Frequency distribution of compositions in the barite-celestine series. Am. Mineral. 1968, 53, 1215–1222. [Google Scholar]
- Cohén, Á. Minas y Mineros de Granada (Siglos XIX y XX). Granada, España: Diputación de Granada. 2002. Available online: https://dialnet.unirioja.es/servlet/libro?codigo=86054 (accessed on 4 September 2022).
- Hanor, J. A model for the origin of large carbonate and evaporite-hosted celestine (SrSO4) deposits. J. Sediment. Res. 2004, 74, 168–175. [Google Scholar] [CrossRef]
- Martin, J.M.; Ortega Huertas, M.; Torres Ruiz, J. Genesis and evolution of strontium deposits of the granada basin (southeastern spain)—Evidence of diagenetic replacement of a stromatolite belt. Sediment. Geol. 1984, 39, 281–298. [Google Scholar] [CrossRef]
- Taberner, C.; Marshall, J.D.; Hendry, J.P.; Pierre, C.; Thirlwall, M.F. Celestine formation, bacterial sulphate reduction and carbonate cementation of eocene reefs and basinal sediments (igualada, NE spain). Sedimentology 2002, 49, 171–190. [Google Scholar] [CrossRef]
- Riding, R.; Braga, J.C.; Martín, J.M.; Sánchez-Almazo, I.M. Mediterranean Messinian Salinity Crisis: Constraints from a coeval marginal basin, Sorbas, southeastern Spain. Mar. Geol. 1998, 146, 1–20. [Google Scholar] [CrossRef]
- Wallace, A.F. Organic interfaces enhance strontium content of marine barite. Proc. Natl. Acad. Sci. USA 2019, 116, 13161–13162. [Google Scholar] [CrossRef] [PubMed]
- Alía, J.M.; Edwards, H.G.M.; López-Andrés, S.; González-Martín, P.; García-Navarro, F.J.; Mansour, H.R. FT-raman study of three synthetic solid solutions formed by orthorhombic sulfates: Celestine-barytes, barytes-anglesite and celestine-anglesite. Spectrosc. Lett. 2000, 33, 323–336. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, E.; Yu, S. High-pressure raman study on the BaSO4-SrSO4 series. Solid State Commun. 2009, 149, 2050–2052. [Google Scholar] [CrossRef]
- García-Veigas, J.; Rosell, L.; Cendón, D.I.; Gibert, L.; Martín, J.M.; Torres-Ruiz, J.; Ortí, F. Large celestine orebodies formed by early-diagenetic replacement of gypsified stromatolites (upper miocene, montevive-escúzar deposit, granada basin, spain). Ore Geol. Rev. 2015, 64, 187–199. [Google Scholar] [CrossRef]
- James, R.W.; Wood, W.A. The crystal structure of barytes, celestine and anglesite. Proc. R. Soc. Lond. 1925, 109, 598–620. [Google Scholar]
- Perri, E.; Gindre-Chanu, L.; Caruso, A.; Cefalà, M.; Scopelliti, G.; Tucker, M. Microbial-mediated pre-salt carbonate deposition during the Messinian salinity crisis (Calcare di Base fm., Southern Italy). Mar. Pet. Geol. 2017, 88, 235–250. [Google Scholar] [CrossRef]
- European Union. Critical Raw Materials for Strategic Technologies and Sectors in the Eua Foresight Study; Publications Office of the European Union: Luxemburg, 2020; Available online: https://ec.europa.eu/docsroom/documents/42881 (accessed on 4 September 2022). [CrossRef]
- Free and Open Patent and Scholary Search. Available online: http://www.lens.org (accessed on 4 September 2022).
- Ober, J.A. Mineral commodity summaries 2016. In Mineral Commodity Summaries; U.S. Geological Survey: Reston, VA, USA, 2016; p. 205. [Google Scholar]
- Jiménez-Peñalvarez, J.D. Nieve, oro, sal, Estroncio. Historia de la Minería en Granada. 2008. Available online: https://www.ugr.es/~jorgejp/InvestDoc/mineria.pdf (accessed on 4 September 2022).
- Melgarejo, J.C.; Proenza, J.A.; Galí, S.; Llovet, X. Técnicas de caracterización mineral y su aplicación en exploración y explotación minera. Boletín De La Soc. Geológica Mex. 2010, 62, 1–23. [Google Scholar] [CrossRef]
- Petruk, W. Applied Mineralogy in the Mining Industry; Elsevier Science BV: Amsterdam, The Netherlands; New York, NY, USA, 2000. [Google Scholar]
- Vassilev, S.V.; Tascón, J.M.D. Methods for characterization of inorganic and mineral matter in coal: A critical overview. Energy Fuels 2003, 17, 271–281. [Google Scholar] [CrossRef]
- Cheary, R.W.; Coelho, A.A.; Cline, J.P. Fundamental parameters line profile fitting in laboratory diffractometers. J. Res. Natl. Inst. Stand. Technol. 2004, 109, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Girard, A.; Stekiel, M.; Spahr, D.; Morgenroth, W.; Wehinger, B.; Milman, V.; Nguyen-Thanh, T.; Mirone, A.; Minelli, A.; Paolasin, L.; et al. Structural, elastic and vibrational properties of celestine, SrSO4, from synchrotron x-ray diffraction, thermal diffuse scattering and raman scattering. J. Phys. Condens. Matter 2019, 31, 055703. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Navarro, A.B. XRD2DScan: New software for polycrystalline materials characterization using two-dimensional X-ray diffraction. J. Appl. Crystallogr. 2006, 39, 905–909. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, A.B.; Alvarez-Lloret, P.; Ortega-Huertas, M.; Rodriguez-Gallego, M. Automatic crystal size determination in the micrometer range from spotty X-ray diffraction rings of powder samples. J. Am. Ceram. Soc. 2006, 89, 2232–2238. [Google Scholar] [CrossRef]
- Salazar, S.; Bravo, J.; Castellanos Román, M. Identificación de la Fracción Mineral del Aerosol Atmosférico, en Una Zona Urbana de la Ciudad de México Por Medio de Difracción y Fluorescencia de Rayos-X. Atmósfera 2009, 2. [Google Scholar]
- Conesa Ferrer, J.A. Curso Básico de Análisis Térmico; Editorial Club Universiatrio: Alicante, Spain, 2000. [Google Scholar]
- Manzaneda Cábala, J.R. Aplicación de Microscopía en el Procesamiento de Minerales Por Flotación. Ph.D. Thesis, University of Lima, Lima, Peru, 2010. [Google Scholar]
- Igathinathane, C.; Ulusoy, U.; Pordesimo, L.O. Comparison of particle size distribution of celestine mineral by machine vision ΣVolume approach and mechanical sieving. Powder Technol. 2012, 215, 137–146. [Google Scholar] [CrossRef]
- Ballester, A.; Verdejo, L.F.; Sancho, J. Metalurgia Extractiva (Volumen 1); Síntesis: Madrid, Spain, 2014. [Google Scholar]
- Calero de Hoces, M. Aprovechamiento de Recursos Geológicos Andaluces. Concentración de Celestina. Ph.D. Thesis, Universidad de Granada, Granada, Spain, 1994. [Google Scholar]
- Garcés de los Fayos Toruner, F. Procedimiento Para Enriquecimiento en Estroncio de Mineral de Celestina. 1986. Available online: https://www.igme.es/PanoramaMinero/Historico/2003_04/estronci03.pdf (accessed on 4 September 2022).
- Santos, H.; Neumann, R.; Ávila, C. Mineral Quantification with Simultaneous Refinement of Ca-Mg Carbonates Non-Stoichiometry by X-ray Diffraction, Rietveld Method. Minerals 2017, 7, 164. [Google Scholar] [CrossRef]
- Garske, D.; Peacor, D.R. Refinement of the strontium of celestine. Z. Krist. –Cryst. Mater. 1965, 121, 204. [Google Scholar]
- Ball, T.K.; Booth, S.J.; Nickless, E.F.P.; Smith, R.T. Geochemical prospecting for baryte and celestine using a portable radioisotope fluorescence analyzer. J. Geochem. Explor. 1979, 11, 277–284. [Google Scholar] [CrossRef]
- Li, K.; Li, G.; Zhao, G.; Lu, Y.; Shao, K. Method for Simultaneously Detecting Fluorite, Barite and Celestine, Involves Preparing Artificial Standard Material Followed by Preparing Standard Sample, Scanning Standard Sample, Establishing Standard Curve and Detecting Content. Patent CN108051468A, 18 May 2018. Available online: https://patents.google.com/patent/CN108051468A/en (accessed on 4 September 2022).
- Liu, C.L.; Zhang, L.; Xu, L.J.; Su, Z.M.; Xie, T.P.; Wang, Y.A. Sr speciation in producing SrCO3 with celestine. Acta Geochim. 2014, 33, 244–247. [Google Scholar] [CrossRef]
- López-Quirós, A.; Barbier, M.; Martín, J.M.; Puga-Bernabéu, Á.; Guichet, X. Diagenetic evolution of Tortonian temperate carbonates close to evaporites in the Granada Basin (SE Spain). Sediment. Geol. 2016, 335, 180–196. [Google Scholar] [CrossRef]
- García-del-Cura, M.A.; Sanz-Montero, M.E.; La Iglesia, A.; Ordóñez, S. Carbonate facies and sedimentological model of travertine-tufa quaternary deposits in the Betic Cordillera (SE Spain). In Proceedings of the 27 th IAS Meeting of Sedimentology, Alghero, Italy, 20–23 September 2009; p. 518. [Google Scholar]
- Roncal-Herrero, T.; Astilleros, J.M.; Bots, P.; Rodríguez-Blanco, J.D.; Prieto, M.; Benning, L.G.; Fernández-Díaz, L. Reaction pathways and textural aspects of the replacement of anhydrite by calcite at 25 °C. Am. Mineral. 2017, 120, 1270–1278. [Google Scholar] [CrossRef]

| Size Fraction | Grain Size | Mean Diameter (mm) | E60 | E69 | E92 | |||
|---|---|---|---|---|---|---|---|---|
| Fraction | %Mass | Fraction | %Mass | Fraction | %Mass | |||
| >10 mm | >10 | 0.12 | 12.07 | 0.29 | 29.08 | 0.33 | 32.52 | |
| 6 | 10–5 mm | 7.50 | 0.32 | 43.77 | 0.21 | 49.93 | 0.25 | 57.18 |
| 5 | 5–3 mm | 3.51 | 0.28 | 83.43 | 0.20 | 80.52 | 0.17 | 80.88 |
| 4 | 2–1 mm | 1.50 | 0.12 | 83.43 | 0.10 | 80.52 | 0.07 | 80.88 |
| 3 | 1.0–0.5 mm | 0.75 | 0.05 | 88.40 | 0.07 | 87.04 | 0.04 | 85.11 |
| 2 | 500–315 µm | 0.41 | 0.02 | 91.57 | 0.03 | 91.58 | 0.02 | 88.35 |
| 1 | 315–200 µm | 0.20 | 0.04 | 94.64 | 0.04 | 94.73 | 0.04 | 91.40 |
| <100 µm | 0.05 | 0.05 | 98.81 | 0.05 | 98.83 | 0.07 | 96.93 | |
| Sample | SrSO4 | BaSO4 | Fe2O3 | SiO2 | Al2O3 | CaCO3 | MgCO3 |
|---|---|---|---|---|---|---|---|
| E60 | 63.0 | 1.1 | 3.1 | 7.7 | 3.2 | 20.6 | 2.5 |
| E60_1 | 60.3 | 1.1 | 4.1 | 8.6 | 3.6 | 20.6 | 2.6 |
| E60_2 | 61.9 | 1.2 | 3.7 | 7.6 | 3.1 | 20.6 | 2.3 |
| E60_3 | 64.3 | 1.1 | 3.6 | 6.5 | 2.7 | 20.1 | 2.1 |
| E60_4 | 64.7 | 1.7 | 3.1 | 6.6 | 2.7 | 20.5 | 2.1 |
| E60_5 | 64.4 | 1.0 | 2.8 | 6.4 | 2.6 | 19.5 | 2.1 |
| E60_6 | 71.5 | 1.7 | 2.4 | 6.1 | 2.5 | 14.4 | 1.9 |
| E69 | 70.5 | 1.2 | 1.9 | 5.5 | 0.0 | 16.0 | 2.0 |
| E69_1 | 64.0 | 1.2 | 2.4 | 6.4 | 2.6 | 20.6 | 3.1 |
| E69_2 | 62.6 | 1.3 | 2.3 | 6.9 | 2.8 | 20.8 | 3.3 |
| E69_3 | 63.3 | 1.1 | 2.2 | 6.9 | 2.8 | 20.5 | 3.3 |
| E69_4 | 64.3 | 1.1 | 1.8 | 5.5 | 2.3 | 21.9 | 3.0 |
| E69_5 | 68.5 | 1.2 | 1.5 | 3.9 | 1.6 | 19.5 | 2.3 |
| E69_6 | 67.1 | 0.9 | 0.8 | 2.5 | 1.0 | 24.0 | 1.9 |
| E70 | 52.2 | 1.2 | 0.4 | 0.9 | 0.3 | 46.6 | 0.9 |
| E80 | 74.9 | 0.3 | 0.7 | 5.4 | 0.0 | 17.3 | 0.9 |
| E90 | 95.8 | 1.9 | 0.1 | 0.4 | 0.3 | 0.0 | 0.4 |
| E92 | 90.7 | 1.4 | 0.1 | 2.2 | 0.4 | 2.8 | 0.7 |
| E92_1 | 89.8 | 1.5 | 0.8 | 2.5 | 0.5 | 3.6 | 0.9 |
| E92_2 | 88.2 | 1.5 | 0.7 | 2.6 | 0.4 | 4.3 | 0.8 |
| E92_3 | 88.9 | 1.5 | 0.6 | 2.4 | 0.4 | 3.4 | 0.8 |
| E92_4 | 88.7 | 1.5 | 0.5 | 2.1 | 0.4 | 3.6 | 0.7 |
| E92_5 | 90.4 | 1.5 | 0.5 | 2.3 | 0.4 | 3.3 | 0.6 |
| E92_6 | 88.4 | 1.5 | 0.4 | 2.9 | 0.4 | 5.6 | 0.5 |
| SrSO4 | BaSO4 | Fe2O3 | SiO2 | Al2O3 | CaCO3 | MgCO3 | |
|---|---|---|---|---|---|---|---|
| SrSO4 | 1.000 | 0.590 | −0.711 | −0.681 | −0.699 | −0.940 | −0.525 |
| BaSO4 | 0.590 | 1.000 | −0.294 | −0.451 | −0.198 | −0.595 | −0.226 |
| Fe2O3 | −0.711 | −0.294 | 1.000 | 0.921 | 0.909 | 0.454 | 0.631 |
| SiO2 | −0.681 | −0.451 | 0.921 | 1.000 | 0.862 | 0.413 | 0.679 |
| Al2O3 | −0.699 | −0.198 | 0.909 | 0.862 | 1.000 | 0.442 | 0.702 |
| CaCO3 | −0.940 | −0.595 | 0.454 | 0.413 | 0.442 | 1.000 | 0.287 |
| MgCO3 | −0.525 | −0.226 | 0.631 | 0.679 | 0.702 | 0.287 | 1.000 |
| Calcite | Celestine | Strontianite | Quartz | Dolomite | Kaolinite | Illite | Paragonite | |
|---|---|---|---|---|---|---|---|---|
| E60 | 24.4 | 59.1 | 1.8 | 5.1 | 0.5 | 5.8 | 0.0 | 2.4 |
| E60_1 | 27.1 | 55.7 | 1.9 | 5.4 | 0.2 | 6.0 | 0.1 | 2.7 |
| E60_2 | 27.3 | 55.9 | 1.5 | 4.5 | 0.6 | 6.1 | 0.0 | 2.7 |
| E60_3 | 23.3 | 60.2 | 1.6 | 3.3 | 1.4 | 5.2 | 0.0 | 2.3 |
| E60_4 | 23.5 | 63.0 | 1.4 | 4.0 | 0.7 | 4.3 | 0.0 | 2.4 |
| E60_5 | 24.8 | 59.1 | 2.5 | 4.2 | 1.0 | 4.7 | 0.0 | 2.5 |
| E60_6 | 18.3 | 67.9 | 1.8 | 4.2 | 0.7 | 4.4 | 0.0 | 1.8 |
| E69 | 24.2 | 58.7 | 1.8 | 5.1 | 0.6 | 6.0 | 0.0 | 2.4 |
| E69_1 | 25.9 | 54.9 | 3.1 | 4.7 | 4.2 | 5.0 | 0.0 | 2.6 |
| E69_2 | 24.0 | 53.9 | 2.8 | 4.4 | 5.6 | 4.6 | 0.0 | 2.4 |
| E69_3 | 23.9 | 55.8 | 2.9 | 4.2 | 5.0 | 4.8 | 0.0 | 2.4 |
| E69_4 | 25.9 | 59.0 | 2.2 | 4.1 | 4.4 | 3.9 | 0.0 | 2.6 |
| E69_5 | 21.3 | 66.1 | 1.9 | 3.2 | 4.2 | 2.6 | 0.0 | 2.1 |
| E69_6 | 23.2 | 67.1 | 0.3 | 4.2 | 3.2 | 1.4 | 0.0 | 2.3 |
| E70 | 57.3 | 39.3 | 0.7 | 1.2 | 0.4 | 0.2 | 0.1 | 5.7 |
| E80 | 28.4 | 67.3 | 0.7 | 1.0 | 1.3 | 0.2 | 0.0 | 2.8 |
| E90 | 1.8 | 92.3 | 0.8 | 0.5 | 3.4 | 0.3 | 0.0 | 0.2 |
| E92 | 3.9 | 91.9 | 1.1 | 0.5 | 0.0 | 2.1 | 0.0 | 0.4 |
| E92_1 | 3.6 | 93.2 | 0.7 | 1.2 | 0.0 | 1.2 | 0.0 | 0.4 |
| E92_2 | 2.7 | 94.6 | 0.2 | 1.9 | 0.0 | 0.2 | 0.0 | 0.3 |
| E92_3 | 3.2 | 93.0 | 0.1 | 3.1 | 0.0 | 0.4 | 0.0 | 0.3 |
| E92_4 | 3.8 | 92.9 | 0.1 | 1.9 | 0.0 | 0.5 | 0.6 | 0.4 |
| E92_5 | 3.2 | 93.0 | 0.1 | 2.6 | 0.0 | 0.4 | 0.6 | 0.3 |
| E92_6 | 4.6 | 92.4 | 0.1 | 2.0 | 0.4 | 0.5 | 0.5 |
| Calcite | Celestine | Strontianite | Quartz | Dolomite | Kaolinite | Illite | Paragonite | |
|---|---|---|---|---|---|---|---|---|
| Calcite | 1.000 | −0.940 | 0.474 | 0.384 | 0.213 | 0.416 | −0.374 | 0.805 |
| Celestine | −0.940 | 1.000 | −0.711 | −0.623 | −0.374 | −0.674 | 0.432 | −0.840 |
| Strontianite | 0.474 | −0.711 | 1.000 | 0.653 | 0.611 | 0.810 | −0.444 | 0.541 |
| Quartz | 0.384 | −0.623 | 0.653 | 1.000 | 0.259 | 0.841 | −0.211 | 0.429 |
| Dolomite | 0.213 | −0.374 | 0.611 | 0.259 | 1.000 | 0.210 | −0.257 | 0.077 |
| Kaolinite | 0.416 | −0.674 | 0.810 | 0.841 | 0.210 | 1.000 | −0.370 | 0.598 |
| Illite | −0.374 | 0.432 | −0.444 | −0.211 | −0.257 | −0.370 | 1.000 | −0.342 |
| Paragonite | 0.805 | −0.840 | 0.541 | 0.429 | 0.077 | 0.598 | −0.342 | 1.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ariza-Rodríguez, N.; Rodríguez-Navarro, A.B.; Calero de Hoces, M.; Martin, J.M.; Muñoz-Batista, M.J. Chemical and Mineralogical Characterization of Montevive Celestine Mineral. Minerals 2022, 12, 1261. https://doi.org/10.3390/min12101261
Ariza-Rodríguez N, Rodríguez-Navarro AB, Calero de Hoces M, Martin JM, Muñoz-Batista MJ. Chemical and Mineralogical Characterization of Montevive Celestine Mineral. Minerals. 2022; 12(10):1261. https://doi.org/10.3390/min12101261
Chicago/Turabian StyleAriza-Rodríguez, Noemi, Alejandro B. Rodríguez-Navarro, Mónica Calero de Hoces, Jose Manuel Martin, and Mario J. Muñoz-Batista. 2022. "Chemical and Mineralogical Characterization of Montevive Celestine Mineral" Minerals 12, no. 10: 1261. https://doi.org/10.3390/min12101261
APA StyleAriza-Rodríguez, N., Rodríguez-Navarro, A. B., Calero de Hoces, M., Martin, J. M., & Muñoz-Batista, M. J. (2022). Chemical and Mineralogical Characterization of Montevive Celestine Mineral. Minerals, 12(10), 1261. https://doi.org/10.3390/min12101261










