Infrared Spectroscopic Analysis of the Inorganic Components from Teeth Exposed to Psychotherapeutic Drugs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. ATR-FTIR Spectroscopy
2.3. X-ray Diffraction Analysis
2.4. Scanning Electron Microscopy and Energy-Dispersive X-ray Spectroscopy
2.5. Statistical Analysis
3. Results
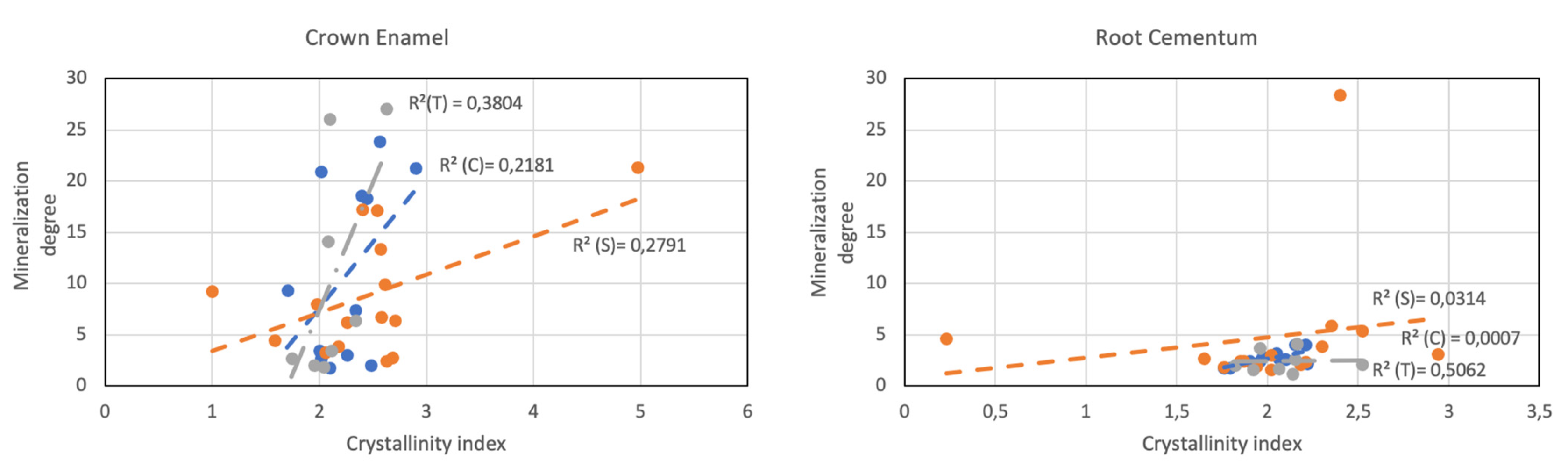
3.1. ATR-FTIR Analysis
3.2. ATR-FTIR Analysis of Selected Samples
3.3. Crystallographic Conservation of Enamel and Cementum
3.4. Surface Morphology and Ca/P Ratios
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sweet, D. Forensic dental identification. Forensic Sci Int. 2010, 201, 3–4. [Google Scholar] [CrossRef]
- Pretty, I.A.; Sweet, D. A look at forensic dentistry-Part I. The role of teeth in the determination of human identity. Br. Dent. J. 2001, 190, 359–366. [Google Scholar] [CrossRef] [Green Version]
- Krishan, K.; Kanchan, T.; Garg, A.K. Dental Evidence in Forensic Identification–An Overview, Methodology and Present Status. Open Dent. J. 2015, 9, 250–256. [Google Scholar] [CrossRef] [Green Version]
- Tinoco, R.L.; Martins, E.C.; Daruge, E., Jr.; Daruge , E.; Prado , F.B. ; Caria , P.H. Dental anomalies and their value in human identification: A case report. J. Forensic Odontostomatol. 2010, 28, 39–43. [Google Scholar] [PubMed]
- Gupta, P.; Kaur, H.; Shankari, G.S.; Jawanda, M.; Sahi, N. Human age estimation from tooth cementum and dentin. J. Clin. Diagn. Res. 2014, ZC8, 7–10. [Google Scholar] [CrossRef]
- Combes, C.; Cazalbou, S.; Rey, C.C. Apatite Biominerals. Minerals 2016, 6, 34. [Google Scholar] [CrossRef] [Green Version]
- Lacruz, R.S.; Habelitz, S.; Wright, J.T.; Paine, M.L. Dental enamel formation and implications for oral health and disease. Physiol. Rev. 2017, 97, 939–993. [Google Scholar] [CrossRef]
- Simmer, J.P.; Hu, J.C. Dental enamel formation and its impact on clinical dentistry. J. Dent. Educ. 2001, 65, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Abou Neel, E.A.; Aljabo, A.; Strange, A.; Ibrahim, S.; Coathup, M.; Young, A.M.; Bozec, L.; Mudera, V. Demineralization-remineralization dynamics in teeth and bone. Int. J. Nanomed. 2016, 11, 4743–4763. [Google Scholar] [CrossRef]
- Hayashi, O.; Chiba, T.; Shimoda, S.; Momoi, Y. Demineralization and Remineralization Phenomena of Human Enamel in Acid Erosion Model. J. Hard Tissue Biol. 2016, 25, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Al Shehab, A.H.; Al Hazoom, A.A,; Alowa, M.H.; Al Ali, H.A.; Abdulmohsen, A.A.; Farooq, I. Effect of bristle stiffness of manual toothbrushes on normal and demineralized human enamel-An in vitro profilometric study. Int. J. Dent. Hyg. 2018, 16, e128–e132. [Google Scholar] [CrossRef]
- Shamim, T.; Ipe Varghese, V.; Shameena, P.M.; Sudha, S. Age estimation: A dental approach. JPAFMAT 2006, 6, 14–16. [Google Scholar]
- Verma, M.; Verma, N.; Sharma, R.; Sharma, A. Dental age estimation methods in adult dentitions: An overview. J. Forensic Dent. Sci. 2019, 11, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Nanci, A. Ten Cate’s Oral Histology: Development, Structure, and Function, 9th ed.; Mosby: Maryland Heights, MO, USA, 2008. [Google Scholar]
- Bodart, F.; Deconninck, G.; Martin, M.T. Large scale study of tooth enamel. IEEE Trans. Nucl. Sci. 1981, 28, 1401–1403. [Google Scholar] [CrossRef]
- Gelse, K.; Pöschl, E.; Aigner, T. Collagens--structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallet-Regí, M.; González-Calbet, J.M. Calcium phosphates as substitution of bone tissues. Prog. Solid State Chem. 2004, 32, 1–31. [Google Scholar] [CrossRef]
- Eanes, E.D. Enamel apatite: Chemistry, structure and properties. J. Dent. Res. 1979, 58, 829–836. [Google Scholar] [CrossRef]
- Elliot, J.C.; Holcomb, D.W.; Young, R.A. Infrared determination of the degree of substitution of hydroxyl by carbonate ions in human dental enamel. Calcif. Tissue Int. 1985, 37, 372–375. [Google Scholar] [CrossRef]
- Pasteris, J.; Wopenka, B.; Valsami-Jones, E. Bone and Tooth Mineralization: Why Apatite? Elements 2008, 4, 97–104. [Google Scholar] [CrossRef]
- Mathew, M.; Takagi, S. Structures of Biological Minerals in Dental Research. J. Res. Natl. Inst. Stand Technol. 2001, 106, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Shellis, R.P.; Barbour, M.E.; Jones, S.B.; Addy, M. Effects of pH and acid concentration on erosive dissolution of enamel, dentine, and compressed hydroxyapatite. Eur. J. Oral Sci. 2010, 118, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Cummins, D. The development and validation of a new technology, based upon 1.5% arginine, an insoluble calcium compound and fluoride, for everyday use in the prevention and treatment of dental caries. J. Dent. 2013, 41 (Suppl. S2), S1–S11. [Google Scholar] [CrossRef] [Green Version]
- Farooq, I.; Bugshan, A. The role of salivary contents and modern technologies in the remineralization of dental enamel: A narrative review. F1000Research 2020, 9, 171. [Google Scholar] [CrossRef]
- Vainionpaa, R.; Tuulaniemi, K.; Pesonen, P.; Laitala, M.-L.; Anttonen, V. Erosive tooth wear and use of psychoactive substances among Finnish prisoners. BMC Oral Health 2019, 19, 97. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, L.; Diebolder, R.; Hibst, R.; Zezell, D.M. Infrared absorption bands of enamel and dentin tissues from human and bovine teeth. Appl. Spectrosc. Rev. 2003, 38, 1–14. [Google Scholar] [CrossRef]
- Ramakrishnaiah, R.; Rehman, G.U.; Basavarajappa, S.; Al Khuraif, A.A.; Durgesh, B.H.; Khan, A.S.; Rehman, I. Applications of Raman spectroscopy in dentistry: Analysis of tooth structure. Appl. Spectrosc. Rev. 2015, 50, 332–350. [Google Scholar] [CrossRef]
- Movasaghi, Z.; Rehman, S.; Rehman, I. Fourier Transform Infrared (FTIR) Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2008, 43, 134–179. [Google Scholar] [CrossRef]
- France, C.; Sugiyama, N.; Aguayo, E. Establishing a preservation index for bone, dentin, and enamel bioapatite mineral using ATR-FTIR. J. Archaeol. Sci. Rep. 2020, 33, 102551. [Google Scholar] [CrossRef]
- Tramini, P.; Bonnet, B.; Sabatier, R.; Maury, L. A method of age estimation using Raman microspectrometry imaging of the human dentin. Forensic Sci. Int. 2001, 118, 1–9. [Google Scholar] [CrossRef]
- Krafft, C.; Sergo, V. Biomedical applications of Raman and infrared spectroscopy to diagnose tissues. Spectroscopy 2006, 20, 195–218. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, X.; Liu, Y.W.; Wang, Y. A Fourier Transform Infrared spectroscopy analysis of carious dentin from transparent zone to normal zone. Caries Res. 2014, 48, 320–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karteva, E.; Manchorova, N. Root Dentin Analysis from using Fourier-Transform Infrared Spectroscopy with Attenuated Total Reflectance (FTIR-ATR). IJRS 2019, 8. [Google Scholar] [CrossRef]
- Grunenwald, A.; Keyser, C.; Sautereau, A.M.; Crubézy, E.; Ludes, B.; Drouet, C. Revisiting carbonate quantification in apatite (bio)minerals: A validated FTIR methodology. J. Archaeol. Sci. 2014, 49, 134–141. [Google Scholar] [CrossRef] [Green Version]
- Shepel, D.; Goreacioc, T.; Lupascu, T.; Filippov, M.; Rusu, M. Method of Infrared Spectra Registration of Activated Carbons in Potassium Bromide Pellets. Chem. J. Mold. 2015, 10, 113–115. [Google Scholar] [CrossRef]
- Miller, L.M.; Vairavamurthy, V.; Chance, M.R.; Mendelsohn, R.; Paschalis, E.P.; Betts, F.; Boskey, A.L. In situ analysis of mineral content and crystallinity in bone using infrared micro-spectroscopy of the PO43- vibration. Biochim. Biophys. Acta 2001, 1527, 11–19. [Google Scholar] [CrossRef]
- Boskey, A.L.; Mendelsohn, R. Infrared spectroscopic characterization of mineralized tissues. Vib. Spectrosc. 2005, 38, 107–114. [Google Scholar] [CrossRef] [Green Version]
- Behroozibakhsh, M.; Hajizamani, H.; Shekofteh, K.; Otadi, M.; Ghavami-Lahiji, M.; Nazari, N.S.F. Comparative assessment of the crystalline structures of powder and bulk human dental enamel by X-ray diffraction analysis. J. Oral Biosci. 2019, 61, 173–178. [Google Scholar] [CrossRef]
- Paschalis, E.P.; DiCarlo, E.; Betts, F.; Sherman, P.; Mendelsohn, R.; Boskey, A.L. FTIR microspectroscopic analysis of human osteonal bone. Calcif. Tissue Int. 1996, 59, 480–487. [Google Scholar] [CrossRef]
- Rey, C.; Shimizu, M.; Collins, B.; Glimcher, M.J. Resolution-enhanced Fourier transform infrared spectroscopy study of the environment of phosphate ions in the early deposits of a solid phase of calcium-phosphate in bone and enamel, and their evolution with age. I: Investigations in the upsilon4 PO4 domain. Calcif. Tissue Int. 1990, 46, 384–394. [Google Scholar] [CrossRef]
- Young, R.A.; Mackie, P.E. Crystallography of human tooth enamel: Initial structure refinement. Mater. Res. Bull. 1980, 15, 17–29. [Google Scholar] [CrossRef]
- Alghunaim, N.S. In situ synthesis and investigation poly (methyl methacrylate)/polycarbonate nanocomposites incorporated with copper oxide nanoparticles. Results Phys. 2020, 19, 103368. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Sun, X.; Kishen, A.; Deng, X.; Yang, X.; Wang, H.; Cong, C.; Wang, Y.; Wu, M. Biomimetic remineralization of demineralized enamel with nano-complexes of phosphorylated chitosan and amorphous calcium phosphate. J. Mater. Sci. Mater. Med. 2014, 25, 2619–2628. [Google Scholar] [CrossRef] [PubMed]
- Tzaphlidou, M.; Zaichick, V. Calcium, phosphorus, calcium-phosphorus ratio in rib bone of healthy humans. Biol. Trace Elem. Res. 2003, 93, 63–74. [Google Scholar] [CrossRef]
- Bailey, M.J.; Coe, S.; Grant, D.M.; Grime, G.W.; Jeynes, C. Accurate determination of the Ca:P ratio in rough hydroxyapatite samples by SEM-EDS, PIXE and RBS—a comparative study. X-ray Spectrom. 2009, 38, 343–347. [Google Scholar] [CrossRef] [Green Version]
- Ten Cate, A.R. Oral Histology, Development. Structure, and Function, 4th ed.; Mosby: St. Louis, MO, USA, 1994. [Google Scholar]
- Allan, J.H. Investigations into the mineralization pattern of human dental enamel. J. Dent. Res. 1959, 38, 1096–1128. [Google Scholar] [CrossRef]
- Kubala, E.; Strzelecka, P.; Grzegocka, M.; Lietz-Kijak, D.; Gronwald, H.; Skomro, P.; Kijak, E. A Review of Selected Studies That Determine the Physical and Chemical Properties of Saliva in the Field of Dental Treatment. Biomed Res. Int. 2018, 2018, 6572381. [Google Scholar] [CrossRef]
- Penel, G.; Leroy, G.; Rey, C.; Sombret, B.; Huvenne, J.P.; Bres, E. Infrared and Raman microspectrometry study of fluor-fluor-hydroxy and hydroxy-apatite powders. Mater. Sci. Mater. Med. 1997, 8, 271–276. [Google Scholar] [CrossRef]
- Rey, C.; Shimizu, M.; Collins, B.; Glimcher, M.J. Resolution-enhanced Fourier transform infrared spectroscopy study of the environment of phosphate ion in the early deposits of a solid phase of calcium phosphate in bone and enamel and their evolution with age: 2. Investigations in the nu3PO4 domain. Calcif. Tissue Int. 1991, 49, 383–388. [Google Scholar] [CrossRef]
- Leventouri, T.; Antonakos, A.; Kyriacou, A.; Venturelli, R.; Liarokapis, E.; Perdikatsis, V. Crystal structure studies of human dental apatite as a function of age. Int. J. Biomater. 2009, 2009, 698547. [Google Scholar] [CrossRef] [Green Version]
- Malhotra, R.; Kapoor, A.; Grover, V.; Kaushal, S. Nicotine and periodontal tissues. J. Indian Soc. Periodontol. 2010, 14, 72–79. [Google Scholar] [CrossRef]
- Kong, J.; Yu, S. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim. Biophys. Sin. (Shanghai) 2007, 39, 549–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rey, C.; Collins, B.; Goehl, T.; Dickson, I.R.; Glimcher, M.J. The carbonate environment in bone-mineral a resolution-enhanced Fourier-Transform Infrared-Spectroscopy study. Calcif. Tissue Int. 1989, 45, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Lussi, A.; Carvalho, T.S. Analyses of the Erosive Effect of Dietary Substances and Medications on Deciduous Teeth. PLoS ONE 2015, 10, e0143957. [Google Scholar] [CrossRef] [PubMed]
- Beniash, E.; Stifler, C.A.; Sun, C.Y.; Jung, G.S.; Qin, Z.; Buehler, M.J.; Gilbert, P. The hidden structure of human enamel. Nat. Commun. 2019, 10, 4383. [Google Scholar] [CrossRef] [Green Version]
- Reyes-Gasga, J.; Martínez-Piñeiro, E.L.; Rodríguez-Álvarez, G.; Tiznado-Orozco, G.E.; García-García, R.; Brès, E.F. XRD and FTIR crystallinity indices in sound human tooth enamel and synthetic hydroxyapatite. Mater Sci Eng C Mater Biol Appl. 2013, 33, 4568–4574. [Google Scholar] [CrossRef]





| Group | Age (Years) | Sex | Crown Enamel | Root Cementum | ||||
|---|---|---|---|---|---|---|---|---|
| Crystallinity Index | Mineralization Degree | Carbonation Degree | Crystallinity Index | Mineralization Degree | Carbonation Degree | |||
| C | 18 | F | 2.02 | 20.97 | 0.16 | 2.21 | 4.08 | 0.17 |
| 20 | M | 2.39 | 18.62 | 0.02 | 2.06 | 2.72 | 0.26 | |
| 32 | F | 2.48 | 2.04 | 0.24 | 1.90 | 2.47 | 0.33 | |
| 33 | F | 2.26 | 3.03 | 0.27 | 2.22 | 2.26 | 0.04 | |
| 33 | F | 2.34 | 7.45 | 0.21 | 1.97 | 2.89 | 0.26 | |
| 39 | M | 2.10 | 1.77 | 0.28 | 1.79 | 1.79 | 0.05 | |
| 47 | M | 2.90 | 21.34 | 0.13 | 2.15 | 4.07 | 0.23 | |
| 48 | F | 2.56 | 23.87 | 0.13 | 2.05 | 3.23 | 0.25 | |
| 53 | F | 1.70 | 9.37 | 0.22 | 2.10 | 2.66 | 0.29 | |
| 56 | M | 2.02 | 2.72 | 0.27 | 1.76 | 1.81 | 0.32 | |
| 65 | M | 2.00 | 3.48 | 0.35 | 1.92 | 2.11 | 0.05 | |
| 68 | M | 2.44 | 18.36 | 0.15 | 1.96 | 2.47 | 0.17 | |
| S | 21 | F | 2.63 | 2.47 | 0.24 | 2.52 | 5.44 | 0.02 |
| 23 | M | 2.71 | 6.44 | 0.23 | 2.35 | 5.88 | 0.04 | |
| 24 | F | 1.00 | 9.24 | 0.16 | 1.76 | 1.86 | 0.32 | |
| 26 | M | 2.68 | 2.79 | 0.26 | 1.85 | 2.44 | 0.37 | |
| 29 | F | 1.98 | 7.98 | 0.14 | 2.18 | 2.11 | 0.34 | |
| 32 | M | 2.57 | 13.44 | 0.18 | 1.65 | 2.69 | 0.02 | |
| 40 | F | 2.58 | 6.80 | 0.01 | 2.02 | 1.65 | 0.08 | |
| 43 | F | 1.58 | 4.48 | 0.27 | 2.21 | 2.35 | 0.03 | |
| 55 | F | 2.06 | 3.28 | 0.20 | 2.94 | 3.14 | 0.23 | |
| 55 | F | 2.26 | 6.25 | 0.01 | 1.87 | 2.46 | 0.24 | |
| 56 | F | 2.40 | 17.29 | 0.16 | 2.30 | 3.92 | 0.18 | |
| 59 | F | 4.97 | 21.37 | 0.11 | 2.02 | 3.05 | 0.20 | |
| 59 | M | 2.18 | 3.86 | 0.19 | 1.94 | 1.94 | 0.04 | |
| 66 | F | 2.54 | 17.22 | 0.15 | 0.23 | 4.62 | 0.17 | |
| 78 | M | 2.61 | 9.95 | 0.16 | 2.40 | 28.44 | 3.94 | |
| M | 61 | F | 2.34 | 6.42 | 0.18 | 1.92 | 1.64 | 0.15 |
| S-A | 62 | M | 2.11 | 3.44 | 0.54 | 1.96 | 3.71 | 0.08 |
| S-CT | 32 | M | 2.63 | 27.06 | 0.13 | 2.16 | 4.17 | 0.25 |
| S-AA | 60 | M | 1.95 | 2.05 | 0.31 | 1.82 | 2.07 | 0.08 |
| S-M | 25 | M | 2.08 | 14.16 | 0.01 | 2.14 | 1.25 | 0.07 |
| 40 | M | 1.74 | 2.75 | 0.03 | 2.06 | 1.75 | 0.13 | |
| 47 | F | 2.10 | 26.08 | 0.11 | 2.15 | 2.66 | 0.20 | |
| 43 | M | 2.04 | 1.92 | 0.29 | 2.52 | 2.15 | 0.40 | |
| Sample | #C(33)c | #S-M(40)c | #C(56)r | #S-AA(60)r | #S(29)r | #S(59)r | #S-M(25)r | #S-A(62)r |
|---|---|---|---|---|---|---|---|---|
| Ca | 37.24 | 50.7 | 32.96 | 42.68 | 37.18 | 25.61 | 25.5 | 27.48 |
| P | 15.59 | 15.8 | 13.97 | 13.9 | 15.54 | 15.35 | 12.93 | 16.2 |
| Ca/P | 2.39 | 3.21 | 2.36 | 3.07 | 2.39 | 1.67 | 1.97 | 1.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diez, C.; Rojo, M.Á.; Martín-Gil, J.; Martín-Ramos, P.; Garrosa, M.; Córdoba-Diaz, D. Infrared Spectroscopic Analysis of the Inorganic Components from Teeth Exposed to Psychotherapeutic Drugs. Minerals 2022, 12, 28. https://doi.org/10.3390/min12010028
Diez C, Rojo MÁ, Martín-Gil J, Martín-Ramos P, Garrosa M, Córdoba-Diaz D. Infrared Spectroscopic Analysis of the Inorganic Components from Teeth Exposed to Psychotherapeutic Drugs. Minerals. 2022; 12(1):28. https://doi.org/10.3390/min12010028
Chicago/Turabian StyleDiez, Camila, Maria Ángeles Rojo, Jesús Martín-Gil, Pablo Martín-Ramos, Manuel Garrosa, and Damián Córdoba-Diaz. 2022. "Infrared Spectroscopic Analysis of the Inorganic Components from Teeth Exposed to Psychotherapeutic Drugs" Minerals 12, no. 1: 28. https://doi.org/10.3390/min12010028
APA StyleDiez, C., Rojo, M. Á., Martín-Gil, J., Martín-Ramos, P., Garrosa, M., & Córdoba-Diaz, D. (2022). Infrared Spectroscopic Analysis of the Inorganic Components from Teeth Exposed to Psychotherapeutic Drugs. Minerals, 12(1), 28. https://doi.org/10.3390/min12010028







