Confusion between Carbonate Apatite and Biological Apatite (Carbonated Hydroxyapatite) in Bone and Teeth
Abstract
:1. Introduction
2. Background
2.1. Definition of a Mineral
2.2. Apatite Group in the Classic Standard Mineralogy Textbook, Dana’s System of Mineralogy
2.3. Apatite Group and Solid-Solution Series
2.4. Crystal Structure of Apatite
3. Biological Apatite
3.1. Initial Research on Biological Apatite
3.2. Distribution of Biological Apatite
3.3. Indeterminate Composition of Biological Apatite and Differences among Animal Species, Tissues, and Body Sites
3.4. Problem of Carbonate Ions in the Crystal Structure of Biological Apatite
3.5. Crystal Structure Analysis of Enamel Apatite
3.6. Carbonate Ions in Biological Apatite Are B-Type
3.7. Bioapatite Is Hydroxyapatite Containing Carbonic Acid
4. Discussion
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nesse, W. Introduction to mineralogy. Geol. Mag. 2002, 139, 491–492. [Google Scholar]
- Nickel, E.H. The definition of a mineral. Can. Mineral. 1995, 33, 689–690. [Google Scholar]
- International Mineralogical Association: The Commission on New Minerals, Nomenclature and Classification (CNMNC). Available online: https://www.ima-mineralogy.org/CNMNC_Strategy.htm (accessed on 12 November 2021).
- JW., J. A system of mineralogy. Nature 1892, 46, 217–218. [Google Scholar]
- Dana, J.D.; Dana, E.S.; Gaines, R.V.; Dana, J.D. Dana’s New Mineralogy: The System of Mineralogy of James Dwight Dana and Edward Salisbury Dana, Entirely Rewritten and Greatly Enlarged, 8th ed.; Gaines, R.V., Skinner, H.C.W., Foord, E.E., Mason, B., Eds.; Wiley-Interscience: New York, NY, USA, 1997; pp. 697–970. [Google Scholar]
- Definition & Classification of Minerals. Available online: http://earthresources.sakura.ne.jp/er/Min_D%26C.html (accessed on 12 November 2021).
- Minerals Arranged by the Nickel-Strunz (Ver10) Classification System. Available online: http://www.webmineral.com/strunz.shtml (accessed on 12 November 2021).
- White, T.J.; Zhili, D. Structural derivation and crystal chemistry of apatites. Acta Crystallogr. B 2003, 59, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Pasero, M.; Kampf, A.R.; Ferraris, C.; Pekov, I.V.; Rakovan, J.; White, T.J. Nomenclature of the apatite supergroup minerals. Eur. J. Mineral. 2010, 22, 163–179. [Google Scholar] [CrossRef]
- Hughes, J.M.; Rakovan, J.F. Structurally robust, chemically diverse: Apatite and apatite supergroup minerals. Elements 2015, 11, 165–170. [Google Scholar] [CrossRef]
- Pan, Y.; Fleet, M.E. Compositions of the apatite-group minerals: Substitution mechanisms and controlling factors. Rev. Mineral. Geochem. 2002, 48, 13–49. [Google Scholar] [CrossRef]
- Mehmel, M. Über die Struktur des Apatits. Zeits Kristal 1930, 75, 323–331. [Google Scholar] [CrossRef]
- Náray-Szabó, S. Ein auf der Kristallstruktur basierendes Silicatsystem. Z. Physik Chem. Ab 1930, 9, 356–377. [Google Scholar] [CrossRef]
- Elliott, J.C. Structure and Chemistry of the Apatites and Other Calcium Orthophosphates, 1st ed.; Elsevier: Amsterdam, The Netherlands, 1994; pp. 111–117. [Google Scholar]
- Hughes, J.M.; Rakovan, J. The crystal structure of apatite, Ca5(PO4)3(F, OH, Cl). Rev. Mineral. Geochem. 2002, 48, 1–12. [Google Scholar] [CrossRef]
- Combes, C.; Cazalbou, S.; Rey, C. Apatite biominerals. Minerals 2016, 6, 34. [Google Scholar] [CrossRef] [Green Version]
- Kay, M.I.; Young, R.A.; Posner, A.S. Crystal structure of hydroxyapatite. Nature 1964, 204, 1050–1052. [Google Scholar] [CrossRef] [PubMed]
- Lowenstam, H.A.; Weiner, S. On Biomineralization, 1st ed.; Oxford University Press: New York, NY, USA, 1989; pp. 135–188. [Google Scholar]
- Hendrick, S.B.; Hill, W.L.; Jacob, K.D.; Jefferson, M.E. Structural characteristics of apatite-like substances and composition of phosphate rock and bone as determined from microscopical and X-ray diffraction examinations. Ind. Eng. Chem. 1931, 23, 1412–1418. [Google Scholar] [CrossRef]
- Shearer, G. The X-ray microscope. Br. J. Radiol. 1936, 9, 30–37. [Google Scholar] [CrossRef]
- Driessens, F.C.M. Mineral aspects of sentistry in monographs. In Oral Science, 1st ed.; Myers, H.M., Ed.; Karger: New York, NY, USA, 1982; Volume 10, pp. 53–57. [Google Scholar]
- LeGeros, R.Z. Calcium phosphates in oral biology and medicine. In Monographs in Oral Science, 1st ed.; Howard, M.M., Ed.; Karger: Basel, Switzerland, 1991; Volume 15, pp. 108–129. [Google Scholar]
- Sakae, T.; Suzuki, K.; Kozawa, Y. A Short review of studies on chemical and physical properties of enamel crystallites. In Tooth Enamel Microstructure, 1st ed.; Koenigswald, W.V., Sander, P.M., Eds.; Bookfield: Rotterdam, The Netherlands, 1997; Volume 1, pp. 31–39. [Google Scholar]
- Sakae, T.; Nakada, H.; LeGeros, J.P. Historical review of biological apatite crystallography. J. Hard. Tissue Biol. 2015, 24, 111–122. [Google Scholar] [CrossRef] [Green Version]
- Rowles, S.L. Chemistry of the mineral phase of dentin. In Structural and Chemical Organization of Teeth, 1st ed.; Miles, A.E.W., Ed.; Academic Press: New York, NY, USA, 1967; pp. 201–246. [Google Scholar]
- Broudevold, T.; Söremark, R. Chemistry of the mineral phase of enamel. In Structural and Chemical Organization of Teeth, 1st ed.; Miles, A.E.W., Ed.; Academic Press: New York, NY, USA, 1967; Volume 18, pp. 247–277. [Google Scholar]
- Foley, B.; Greiner, M.; McGlynn, G.; Schmahl, W.W. Anatomical variation of human bone bioapatite crystallography. Crystals 2020, 10, 859. [Google Scholar] [CrossRef]
- Eisenberger, S.; Lehrman, A.; Turner, W.D. The basic calcium phosphates and related systems. Some theoretical and practical aspects. Chem. Rev. 1940, 26, 257–296. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Effect of carbonate on the lattice parameters of apatite. Nature 1965, 206, 403–404. [Google Scholar] [CrossRef] [PubMed]
- LeGeros, R.Z.; LeGeros, J.P. Phosphate minerals in human tissues. In Phosphate Minerals, 1st ed.; Nriagu, J.O., Moore, P.B., Eds.; Springer: Berlin/Heidelberg, Germany, 1984; Volume 12, pp. 351–385. [Google Scholar]
- Weatherell, J.A.; Robinson, C.; Hallsworth, A.S. Variations in the chemical composition of human enamel. J. Dent. Res. 1974, 53, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Suwa, T.; Sakae, T.; Nakada, H.; LeGeros, R.Z.; Kobayash, K. Variation in composition of bone surrounding implants. Key Eng. Mater. 2006, 309−311, 19–22. [Google Scholar] [CrossRef]
- Nakada, H.; Numata, Y.; Sakae, T.; Kimura-Suda, H.; Tanimoto, Y.; Saeki, H.; Teranishi, M.; Kato, T.; LeGeros, R.Z. Changes in bone quality associated with the mineralization of new bone formed around implants-using XPS, polarized microscopy, and FTIR imaging. J. Hard. Tissue Biol. 2010, 19, 101–110. [Google Scholar] [CrossRef] [Green Version]
- Simmons, L.M.; Al-Jawad, M.; Kilcoyne, S.H.; Wood, D.J. Distribution of enamel crystallite orientation through an entire tooth crown studied using synchrotron X-ray diffraction. Eur. J. Oral Sci. 2011, 119, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Simmons, L.M.; Montgomery, J.; Beaumont, J.; Davis, G.R.; Al-Jawad, M. Mapping the spatial and temporal progression of human dental enamel biomineralization using synchrotron X-ray diffraction. Arch. Oral Biol. 2013, 58, 1726–1734. [Google Scholar] [CrossRef]
- Besnard, C.; Harper, R.A.; Moxham, T.E.J.; James, J.D.; Storm, M.; Salvati, E.; Landini, G.; Shelton, R.M.; Korsunsky, A.M. 3D analysis of enamel demineralization in human dental caries using a high-resolution, large field of view synchrotron X-ray micro-computed tomography. Mater. Today 2021, 27, 102418. [Google Scholar]
- Dowsett, M.; Wiesinger, R.; Adriaens, M. X-ray diffraction. In Spectroscopy, Diffraction and Tomography in Art and Heritage Science, 1st ed.; Adriaens, M., Dowsett, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 6, pp. 161–207. [Google Scholar]
- Eimar, H.; Ghadimi, E.; Marelli, B.; Vali, H.; Nazhat, S.N.; Amin, W.M.; Torres, J.; Ciobanu, O.; Albuquerque, R.F.J.; Tamimi, F. Regulation of enamel hardness by its crystallographic dimensions. Acta Biomater. 2012, 8, 3400–3410. [Google Scholar] [CrossRef]
- Cuy, J.L.; Mann, A.B.; Livi, K.J.; Teaford, M.F.; Weihs, T.P. Nanoindentation mapping of the mechanical properties of human molar tooth enamel. Arch. Oral Biol. 2002, 47, 281–291. [Google Scholar] [CrossRef]
- Talal, A.; Hamid, S.K.; Khan, M.; Khan, A.S. Structure of biological apatite: Bone and tooth. In Handbook of Ionic Substituted Hydroxyapatites, 1st ed.; Khan, A.S., Chaudhry, A.A., Eds.; Woodhead Publishing: Cambridge, UK, 2020; Volume 1, pp. 1–19. [Google Scholar]
- Vallet-Regi, M.; Navarrete, D.A. Chapter 1: Biological apatites in bone and teeth. In Nanoceramics in Clinical Use: From Materials to Applications, 2nd ed.; O’Brien, P., Ed.; Royal Society of Chemistry: Cambridge, UK, 2015; Volume 1, pp. 1–29. [Google Scholar]
- McConnell, D. Apatite-Its Crystal Chemistry, Mineralogy, Utilization, and Geologic and Biologic Occurrences, 1st ed.; Springer: Wien, Austria, 1973; pp. 56–80. [Google Scholar]
- Vasil’eva, Z.V. On the role of manganese in apatites. Mem. All·Union Mineral Soc. 1958, 87, 455–468. (In Russian) [Google Scholar]
- Elliott, J.C. Recent progress in the chemistry, crystal chemistry and structure of the apatites. Calcif. Tissue Res. 1969, 3, 293–370. [Google Scholar] [CrossRef]
- LeGeros, R.Z.; Trautz, O.R.; Klein, E.; LeGeros, J.P. Two types of carbonate substitution in the apatite structure. Experientia 1969, 25, 5–7. [Google Scholar] [CrossRef]
- Cacciotti, I. Cationic and anionic substitutions in hydroxyapatite. In Handbook of Bioceramics and Biocomposites, 1st ed.; Antoniac, I., Ed.; Springer: Cham, Switzerland, 2016; Volume 1, pp. 145–211. [Google Scholar]
- Kim, Y.; Izumi, F. Structure refinements with a new version of the Rietveld-refinement program RIETAN. J. Ceram. Soc. Jpn. 1994, 102, 401–404. [Google Scholar] [CrossRef] [Green Version]
- Young, R.A.; Mackie, P.E. Crystallography of human tooth enamel: Initial structure refinement. Mater. Res. Bull. 1980, 15, 17–29. [Google Scholar] [CrossRef]
- Weatherell, J.A.; Widmann, S.M.; Hamm, S.M. Sampling of enamel particles by means of strong acids for density measurements. Arch. Oral Biol. 1996, 11, 107–111. [Google Scholar] [CrossRef]
- Nozaki, Y.; Sakae, T. Estimation of the site occupancy ratio of carbonate ions in the apatite lattice and the water content ratio among the density fractions of human tooth enamel. Nihon Univ. J. Oral Sci. 2001, 27, 73–79. [Google Scholar]
- Ouladdiaf, B.; Rodriguez-Carvajal, J.; Goutaudier, C.; Ouladdiaf, S.; Grosgogeat, B.; Pradelle, N.; Colon, P. Crystal structure of human tooth enamel studied by neutron diffraction. Mater. Res. Express 2015, 2, 025401. [Google Scholar] [CrossRef]
- DeRocher, K.A.; Smeets, P.J.M.; Goodge, B.H.; Zachman, M.J.; Balachandran, P.V.; Stegbauer, L.; Cohen, M.J.; Gordon, L.M.; Rondinelli, J.M.; Kourkoutis, L.F.; et al. Chemical gradients in human enamel crystallites. Nature 2020, 583, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Frank-Kamenetskaya, O.V. Structure, chemistry and synthesis of carbonate apatites-the main components of dental and bone tissues. In Minerals as Advanced Materials I, 1st ed.; Sergey, V.K., Ed.; Springer: Berlin, Germany, 2008; Volume 1, pp. 241–252. [Google Scholar]
- Rey, C.; Collins, B.; Goehl, T.; Dickson, I.R.; Glimcher, M.J. The carbonate environment in bone mineral: A resolution-enhanced Fourier transform infrared spectroscopy study. Calcif. Tissue Int. 1989, 45, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Leventouri, T.; Antonakos, A.; Kyriacou, A.; Venturelli, R.; Liarokapis, E.; Perdikatsis, V. Crystal structure studies of human dental apatite as a function of age. Int. J. Biomater. 2009, 2009, 698547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spizzirri, P.G.; Cochrane, N.J.; Prawer, S.; Reynolds, E.C. A comparative study of carbonate determination in human teeth using Raman spectroscopy. Caries Res. 2012, 46, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Grunenwald, A.; Keyser, C.; Sautereau, A.M.; Crubézy, E.; Ludes, B.; Drouet, C. Revisiting carbonate quantification in apatite (bio) minerals: A validated FTIR methodology. J. Archaeol. Sci. 2014, 49, 134–141. [Google Scholar] [CrossRef] [Green Version]
- Madupalli, H.; Pavan, B.; Tecklenburg, M.M.J. Carbonate substitution in the mineral component of bone: Discriminating the structural changes, simultaneously imposed by carbonate in A and B sites of apatite. J. Solid. State Chem. 2017, 255, 27–35. [Google Scholar] [CrossRef]
- Siddiqi, S.A.; Azhar, U. 6-Carbonate substituted hydroxyapatite. In Woodhead Publishing Series in Biomaterials Handbook of Ionic Substituted Hydroxyapatites, 1st ed.; Khan, A.S., Chaudhry, A.A., Eds.; Woodhead Publishing: Cambridge, UK, 2020; Volume 1, pp. 149–173. [Google Scholar]
- Ishikawa, K. Carbonate apatite bone replacement: Learn from the bone. J. Ceram. Soc. Jpn. 2019, 127, 595–601. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, K.; Matsuya, S.; Lin, X.; Lei, Z.; Yuasa, T.; Miyamoto, Y. Fabrication of low crystalline B-type carbonate apatite block from low crystalline calcite block. J. Ceram. Soc. Jpn. 2010, 118, 341–344. [Google Scholar] [CrossRef] [Green Version]
- Euw, V.S.; Wang, Y.; Laurent, G.; Drouet, C.; Babonneauet, F.; Nassif, N.; Azaïs, T. Bone mineral: New insights into its chemical composition. Sci. Rep. 2019, 9, 8456. [Google Scholar]
- Legros, R.; Balmain, N.; Bonel, G. Age-related changes in mineral of rat and bovine cortical bone. Calcif. Tissue Int. 1987, 41, 137–144. [Google Scholar] [CrossRef] [PubMed]

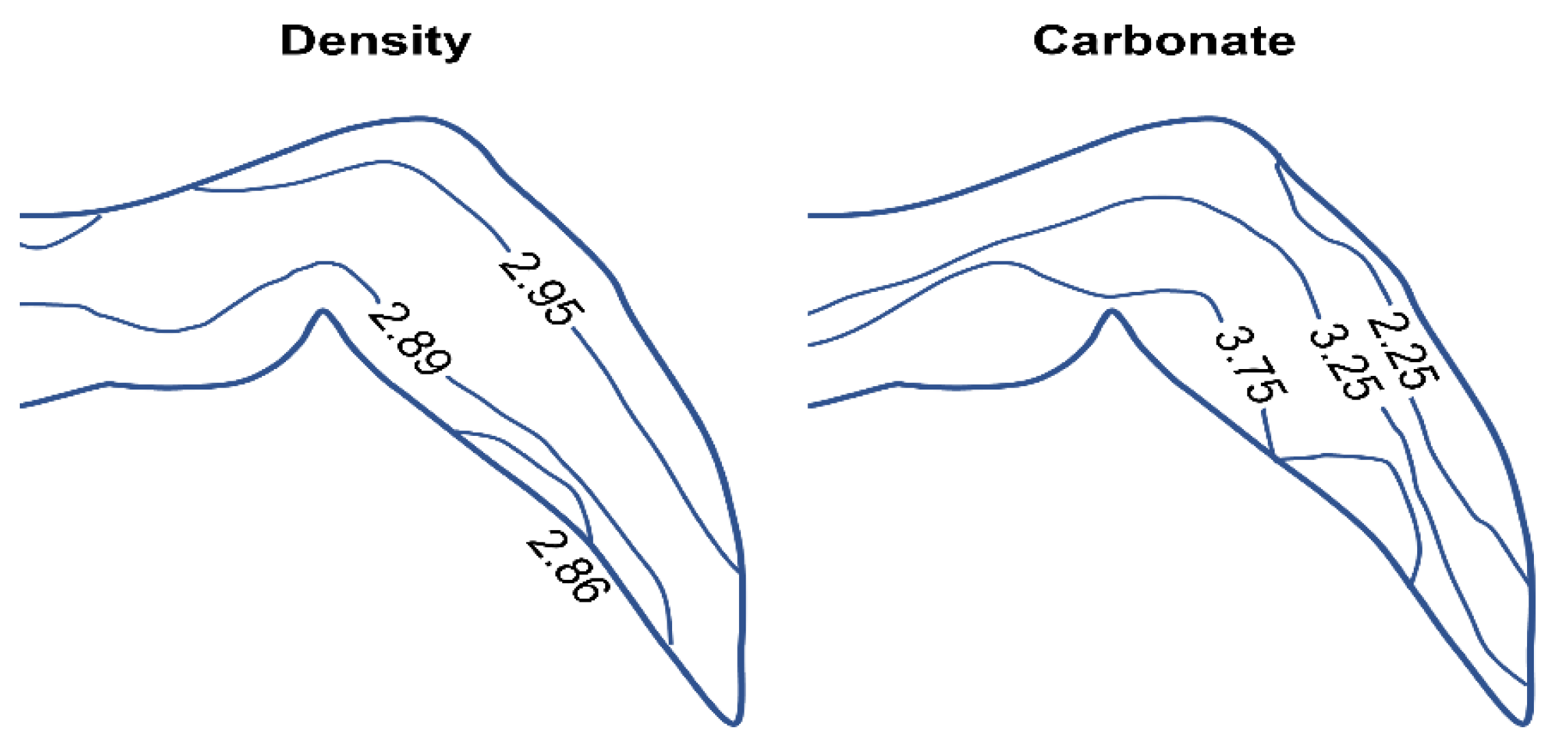
| Composition wt% | Enamel | Dentin | Bone |
|---|---|---|---|
| Calcium, Ca2+ | 36.5 | 27.5 | 24.5 |
| Phosphorus, as P | 17.7 | 13.0 | 11.5 |
| (Ca/P) by weight | 2.06 | 2.12 | 2.33 |
| (Ca/P) molar | 1.58 | 1.62 | 1.80 |
| Sodium, Na+ | 0.30 | 0.60 | 0.70 |
| Potassium, K+ | 0.08 | 0.05 | 0.02 |
| Magnesium, Mg2+ | 0.34 | 0.81 | 0.55 |
| Carbonate, CO32− | 3.5 | 5. | 6.0 |
| Fluoride, F− | 0.01 | 0.02 | 0.02 |
| Chloride, Cl− | 0.30 | 0.01 | 0.10 |
| Ignition products (950 °C) | Apatite + β-TCP | Apatite + β-TCP | Apatite + CaO |
| Constituents | Enamel % | Dentin % |
|---|---|---|
| Ca10 (PO4)6 (OH)1.59 (CO)0.15 (Cl)0.1(F)0.01 | 70 | - |
| Ca9Mg(HPO4) (PO4)6 | 10 | 47 |
| Ca8.5 Na1.5(PO4)4.5(CO3)2.5 | 20 | 20 |
| Ca9(PO4)4.5(CO3)1.5(OH)1.5 | - | 30 |
| Ca8(citrate) (PO4)4.5H2O | - | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kono, T.; Sakae, T.; Nakada, H.; Kaneda, T.; Okada, H. Confusion between Carbonate Apatite and Biological Apatite (Carbonated Hydroxyapatite) in Bone and Teeth. Minerals 2022, 12, 170. https://doi.org/10.3390/min12020170
Kono T, Sakae T, Nakada H, Kaneda T, Okada H. Confusion between Carbonate Apatite and Biological Apatite (Carbonated Hydroxyapatite) in Bone and Teeth. Minerals. 2022; 12(2):170. https://doi.org/10.3390/min12020170
Chicago/Turabian StyleKono, Tetsuro, Toshiro Sakae, Hiroshi Nakada, Takashi Kaneda, and Hiroyuki Okada. 2022. "Confusion between Carbonate Apatite and Biological Apatite (Carbonated Hydroxyapatite) in Bone and Teeth" Minerals 12, no. 2: 170. https://doi.org/10.3390/min12020170
APA StyleKono, T., Sakae, T., Nakada, H., Kaneda, T., & Okada, H. (2022). Confusion between Carbonate Apatite and Biological Apatite (Carbonated Hydroxyapatite) in Bone and Teeth. Minerals, 12(2), 170. https://doi.org/10.3390/min12020170






