Insight into the Crystal Structures and Physical Properties of the Uranium Borides UB1.78±0.02, UB3.61±0.041 and UB11.19±0.13
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Chemical Analyses and X-ray Diffraction
3.2. Magnetic Susceptibility
3.3. Nuclear Magnetic Resonance
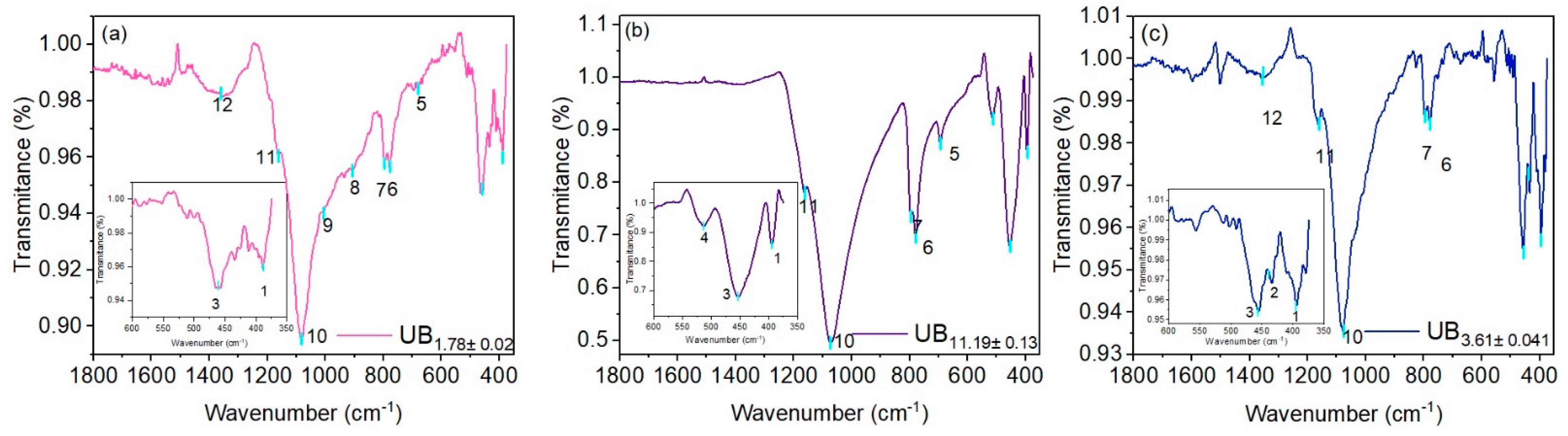
3.4. Infrared Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lupinetti, A.J.; Fife, J.L.; Garcia, E.; Dorhout, P.K.; Abney, K.D. Low-temperature synthesis of uranium tetraboride by solid-state metathesis reactions. Inorg. Chem. 2002, 41, 2316–2318. [Google Scholar] [CrossRef]
- Gossé, S.; Alpettaz, T.; Géneau, C.; Allegri, P. High temperature thermochemistry of (U-Pu)O2 MOX fuel with B4C absorber—Application to severe accidents in SFR. In Proceedings of the International Conference on Fast Reactors and Related Fuel Cycles: Safe Technologies and Sustainable Scenarios, Paris, France, 4–7 March 2013. [Google Scholar]
- Kardoulaki, E.; White, J.T.; Byler, D.D.; Frazer, D.M.; Shivprasad, A.P.; Saleh, T.A.; Gong, B.; Yao, T.; Lian, J.; McClellan, K.J. Thermophysical and mechanical property assessment of UB2 and UB4 sintered via spark plasma sintering. J. Alloys Compd. 2020, 818, 153216. [Google Scholar] [CrossRef]
- Howlett, B.W. A Note on the Uranium-Boron Alloy System. J. Inst. Met. 1959, 88, 91–92. [Google Scholar]
- Chevalier, P.Y.; Fischer, E. Thermodynamic modelling of the C-U and B-U binary systems. J. Nucl. Mater. 2001, 288, 100–129. [Google Scholar] [CrossRef]
- Brewer, L.; Sawyer, D.L.; Templeton, D.H.; Dauben, C.H. A study of the refractory borides. J. Am. Ceram. Soc. 1951, 34, 173–179. [Google Scholar] [CrossRef]
- Yamamoto, E.; Honma, T.; Haga, Y.; Inada, Y.; Aoki, D.; Suzuki, N.; Settai, R.; Sugawara, H.; Sato, H.; Yoshichika, Ō. Electrical and thermal properties of UB2. J. Phys. Soc. Jpn. 1999, 68, 972. [Google Scholar] [CrossRef]
- Yamamoto, E.; Honma, T.; Haga, Y.; Inada, Y.; Aoki, D.; Tokiwa, Y.; Suzuki, N.; Miyake, K.; Yoshichika, Ō. Magnetoresistance and de Haas Van Alphen effect in UB2. J. Phys. Soc. Jpn. 1999, 68, 3347–3351. [Google Scholar] [CrossRef]
- Galatanu, A.; Yamamoto, E.; Hagab, Y.; Onuki, Y. Magnetic behaviour of UB4 at high temperatures. Phys. B Condens. Matter. 2006, 378, 999–1000. [Google Scholar] [CrossRef]
- Troć, R.; Wawryk, R.; Pikul, A.; Shitsevalova, N. Physical properties of cage-like compound UB12. Philos. Mag. 2015, 95, 2343–2363. [Google Scholar] [CrossRef]
- Samsel-Czekała, M.; Talik, E.; Tróc, R.; Shitsevalova, N. Electronic structure of cage-like compound UB12—Theory and XPS experiment. J. Alloys Compd. 2014, 615, 446–450. [Google Scholar] [CrossRef]
- Chipaux, R. Contribution a l’étude de la cristallochimie et des propriétés electroniques des borures dactinides. Ph.D. Thesis, Université d’Aix-Marseille II, Marseille, France, 1988. [Google Scholar]
- Massiot, D.; Farnan, I.; Gautier, N.; Trumeau, D.; Trokiner, A.; Coutures, J.P. 71Ga and 69Ga nuclear magnetic resonance study of β-Ga2O3: Resolution of four—and six-fold coordinated Ga sites in static conditions. Solid State Nucl. Magn. Reson. 1995, 4, 241–248. [Google Scholar] [CrossRef]
- Martel, L.; Griveau, J.-C.; Eloirdi, R.; Selfslag, C.; Colineau, E.; Caciuffo, R. Ferromagnetic ordering in NpAl2: Magnetic susceptibility and 27Al nuclear magnetic resonance. J. Magn. Magn. Mater. 2015, 387, 72–76. [Google Scholar] [CrossRef]
- Massiot, D.; Fayon, F.; Capron, M.; King, I.; Le Calvé, S.; Alonso, B.; Durand, J.O.; Bujoli, B.; Gan, Z.; Hoatson, G. Modelling one—and two-dimensional solid-state NMR spectra. Magn. Reson. Chem. 2002, 40, 70–76. [Google Scholar] [CrossRef]
- Angeli, F.; Charpentier, T.; De Ligny, D.; Cailleteau, C. Boron speciation in soda-lime borosilicate glasses containing zirconium. J. Am. Ceram. Soc. 2010, 93, 2693–2704. [Google Scholar] [CrossRef]
- Flotow, H.E.; Osborne, D.W.; O’Hare, P.A.G.; Settle, J.L.; Mrazek, F.C.; Hubbard, W.N. Uranium Diboride: Preparation, Enthalpy of Formation at 298.15°K, Heat Capacity from 1° to 350°K, and Some Derived Thermodynamic Properties. J. Chem. Phys. 1969, 51, 583–592. [Google Scholar] [CrossRef]
- Akopov, G.; Mak, W.H.; Koumoulis, D.; Yin, H.; Owens-Baird, B.; Yeung, M.T.; Muni, M.H.; Lee, S.; Roh, I.; Sobell, Z.C.; et al. Synthesis and characterization of single-phase metal dodecaboride solid solutions: Zr1−xYxB12 and Zr1−xUxB12. J. Am. Chem. Soc. 2019, 141, 9047–9062. [Google Scholar] [CrossRef]
- Menovsky, A.; Franse, J.J.M.; Klaasse, J.C.P. The crystal growth of uranium tetraboride UB4 from the melt. J. Cryst. Growth 1984, 70, 519–522. [Google Scholar] [CrossRef]
- Dancausse, J.-P.; Gering, E.; Heathman, S.; Benedict, U.; Gerward, L.; Staun Olsen, S.; Hulliger, F. Compression study of uranium borides UB2, UB4 and UB12 by synchrotron X-ray diffraction. J. Alloys Compd. 1992, 189, 205–208. [Google Scholar] [CrossRef]
- Blum, P.; Bertaux, F. Contribution à l’etude des borures à teneur elevée en bore. Acta Cryst. 1954, 7, 81–86. [Google Scholar] [CrossRef]
- Matkovich, V.I.; Economy, J.; Giese jr., R.F. Barrett, R. The structure of metallic dodecaborides. Acta Cryst. 1965, 19, 1056–1058. [Google Scholar] [CrossRef]
- Jossou, E.; Oladimeji, D.; Malakkal, L.; Middleburgh, S.; Szpunar, B.; Szpunar, J. First-principles study of defects and fission product behavior in uranium diboride. J. Nucl. Mater. 2017, 494, 147–156. [Google Scholar] [CrossRef]
- Chipaux, R.; Cecilia, G.; Beauvy, M.; Tróc, R. Capacité thermique a haute temperature de UBe13, ThBe13 et UB4. J. Less Common Met. 1986, 121, 347–351. [Google Scholar] [CrossRef]
- Turner, J.; Martini, F.; Buckley, J.; Phillips, G.; Middleburgh, S.C.; Abram, T.J. Synthesis of candidate advanced technology fuel: Uranium diboride (UB2) via carbo/borothermic reduction of UO2. J. Nucl. Mater. 2020, 540, 152388. [Google Scholar] [CrossRef]
- Guo, H.; Wang, J.; Chen, D.; Tian, W.; Cao, S.; Chen, D.; Tan, C.; Deng, Q.; Qin, Z. Boro/carbothermal reduction synthesis of uranium tetraboride and its oxidation behavior in dry air. J. Am. Ceram. Soc. 2019, 102, 1049–1056. [Google Scholar] [CrossRef]
- Evitts, L.J.; Middleburgh, S.C.; Kardoulaki, E.; Ipatova, I.; Rushton, M.J.D.; Lee, W.E. Influence of boron isotope ratio on the thermal conductivity of uranium diboride (UB2) and zirconium diboride (ZrB2). J. Nucl. Mater. 2020, 528, 151892. [Google Scholar] [CrossRef]
- Okada, Y.; Tokumaru, Y. Precise determination of lattice parameter and thermal expansion coefficient of silicon between 300 and 1500 K. J. Appl. Phys. 1984, 56, 314–320. [Google Scholar] [CrossRef]
- Czopnik, A.; Shitsevalova, N.; Pluzhnikov, V.; Krivchikov, A.; Paderno, Y.; Onuki, Y. Low-temperature thermal properties of yttrium and lutetium dodecaborides. J. Phys. Condens. Matter. 2005, 17, 5971–5985. [Google Scholar] [CrossRef]
- Chachkhiani, Z.B.; Chachkhiani, L.G.; Chechernikov, V.I.; Slovyanskikh, V.K. Magnetic properties of thorium and uranium borides and U1−xThxB4 solid solutions. Sov. Phys. J. 1982, 25, 621–623. [Google Scholar] [CrossRef]
- Kasaya, M.; Iga, F.; Katoh, K.; Takegahara, K.; Kasuya, T. Magnetic and transport properties of uranium borides, UB12, Ulr3B2 and UOs3B2. J. Magn. Magn. Mater. 1990, 90, 521–522. [Google Scholar] [CrossRef]
- Troć, R.; Trzebiatowski, W.; Piprek, K. Magnetic properties of uranium borides and of uranium beryllide UBe13 Bull. Acad. Polon. Sci. Ser. Sci. Chim. 1971, 19, 427. [Google Scholar]
- Wallash, A.; Crow, J.; Fisk, Z. Susceptibility, magnetization, and specific heat in the paramagnetic and ferromagnetic regions of (Y1−xUx)B4. J. Appl. Phys. 1985, 57, 3143–3145. [Google Scholar] [CrossRef][Green Version]
- Mackenzie, K.J.D.; Smith, M.E. Multinuclear Solid-State NMR of Inorganic Materials; Cahn, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Angel Wong, Y.-T.; Bryce, D.L. Recent Advances in 11B Solid-State Nuclear Magnetic Resonance Spectroscopy of Crystalline Solids. Annu. Rep. NMR Spectrosc. 2018, 93, 213–279. [Google Scholar]
- Fukushima, E.; Strubeing, V.O.; Hill, H.H. Induced Paramagnetism in Uranium Alloys: A Boron NMR Study of Alloys Formed between UB4 and YB4. J. Phys. Soc. Jpn. 1975, 39, 921–926. [Google Scholar] [CrossRef]
- Creyghton, J.H.N.; Locher, P.R.; Buschow, K.H.J. Nuclear magnetic resonance of 11B at the three boron sites in rare-earth tetraborides. Phys. Rev. B 1973, 7, 4829–4843. [Google Scholar] [CrossRef]
- Crystal Structures, 2nd ed.; Interscience: New York, NY, USA, 1964; Volume 2.
- Fisk, Z.; Cooper, A.S.; Schmidt, P.H.; Castellano, R.N. Preparation and lattice parameters of the rare earth tetraborides. Mater. Res. Bull. 1972, 7, 285–288. [Google Scholar] [CrossRef]
- Roger, J.; Babizhetskyy, V.; Jardin, R.; Halet, J.-F.; Guérin, R. Solid state phase equilibria in the ternary Nd–Si–B system at 1270 K. J. Alloys Compd. 2006, 415, 73–84. [Google Scholar] [CrossRef]
- Kato, K.; Kawada, I.; Oshima, C.; Kawai, S. Lanthanum tetraboride. Acta Cryst. B 1974, B30, 2933–2934. [Google Scholar] [CrossRef]
- Zalkin, A.; Templeton, D.H. The crystal structures of CeB4 ThB4 and UB4. Acta Cryst. 1953, 6, 269–272. [Google Scholar] [CrossRef]
- Giese, R.F., Jr.; Matkovich, V.I.; Economy, J. The crystal structure of YB4. Z. Krist. 1965, 122, 423–432. [Google Scholar] [CrossRef]
- Jäger, B.; Paluch, S.; Wolf, W.; Herzig, P.; Zogał, O.J.; Shitsevalova, N.; Paderno, Y. Characterization of the electronic properties of YB4 and YB6 using 11B NMR and first-principles calculations. J. Alloys Compd. 2004, 383, 232–238. [Google Scholar] [CrossRef]
- Baek, S.H.; Suh, B.J.; Pavarini, E.; Borsa, F.; Barnes, R.G.; Bud’ko, S.L.; Canfield, P.C. NMR spectroscopy of the normal and superconducting states of MgB2 and comparison to AlB2. Phys. Rev. B 2002, 66, 104510. [Google Scholar] [CrossRef]
- Żogał, O.J.; Florian, P.; Massiot, D.; Paluch, S.; Shitsevalova, N.; Borshchevsky, D.F. 11B and 89Y MAS NMR and magnetic characterization of YB4. Solid State Commun. 2009, 149, 693–696. [Google Scholar] [CrossRef]
- Schmitt, R.; Blaschkowski, B.; Eichele, K.; Meyer, H.-J. Calcium TetraborideDoes It Exist? Synthesis and Properties of a Carbon-Doped Calcium Tetraboride That Is Isotypic with the Known Rare Earth Tetraborides. Inorg. Chem. 2006, 45, 3067–3073. [Google Scholar]
- Nagamatsu, J.; Nakagawa, N.; Muranaka, T.; Zenitani, Y.; Akimitsu, J. Superconductivity at 39 K in magnesium diboride. Nature 2001, 410, 63–64. [Google Scholar] [CrossRef]
- Buschow, K.H.J.; Creyghton, J.H.N. Magnetic Properties of Rare Earth Tetraborides. J. Chem. Phys. 1972, 57, 3910–3914. [Google Scholar] [CrossRef]
- Fojud, Z.; Herzig, P.; Żogał, O.J.; Pietraszko, A.; Dukhnenko, A.; Jurga, S.; Shitsevalova, N. Electric-field-gradient tensor and boron site-resolved 11B NMR in single-crystalline YB12. Phys. Rev. B 2007, 75, 184102. [Google Scholar] [CrossRef]
- Carvajal Nuñez, U.; Martel, L.; Prieur, D.; Lopez Honorato, E.; Eloirdi, R.; Farnan, I.; Vitova, T.; Somers, J. Coupling XRD, EXAFS, and 13C NMR to study the effect of the carbon stoichiometry on the local structure of UC1±x. Inorg. Chem. 2013, 19, 11669–11676. [Google Scholar] [CrossRef]
- Kroumova, E.; Aroyo, M.I.; Perez Mato, J.M.; Kirov, A.; Capillas, C.; Ivantchev, S.; Wondratschek, H. Bilbao Crystallographic Server: Useful databases and tools for phase transitions studies. In Phase Transitions 76; Nos. 1-2; 2003; pp. 155–170. [Google Scholar] [CrossRef]
- Lopez-Bezanilla, A. f-Orbital based Dirac states in a two-dimensional uranium compound. J. Phys. Mater. 2020, 3, 024002. [Google Scholar] [CrossRef]
- Werheit, H.; Filipov, V.; Shirai, K.; Dekura, H.; Shitsevalova, N.; Schwarz, U.; Armbrüster, M. Raman scattering and isotopic phonon effects in dodecaborides. J. Phys. Condens. Matter 2011, 23, 065403. [Google Scholar] [CrossRef]
- Bohnen, K.-P.; Heid, R.; Renker, B. Phonon Dispersion and Electron-Phonon Coupling in MgB2 and AlB2. Phys. Rev. Lett. 2001, 86, 5771–5774. [Google Scholar] [CrossRef]
- Kong, Y.; Dolgov, O.V.; Jepsen, O.; Andersen, O.K. Electron-phonon interaction in the normal and superconducting states of MgB2. Phys. Rev. B 2001, 64, 020501. [Google Scholar] [CrossRef]
- Kortus, J.; Mazin, I.I.; Belashchenko, K.D.; Antropov, V.P.; Boyer, L.L. Superconductivity of metallic boron in MgB2. Phys. Rev. Lett. 2001, 86, 4656–4659. [Google Scholar] [CrossRef] [PubMed]
- Alarco, J.A.; Chou, A.; Talbot, P.C.; Mackinnon, I.D.R. Phonon Modes of MgB2: Super-lattice Structures and Spectral Response. Phys. Chem. Chem. Phys. 2014, 16, 24443–24456. [Google Scholar] [CrossRef] [PubMed]
- Sundar, C.S.; Bharathi, A.; Premila, M.; Kalavathi, S.; Reddy, G.L.N.; Sastry, V.S.; Hariharan, Y.; Radhakrishnan, T.S. Infrared absorption in superconducting MgB2. arXiv 2001, arXiv:0104354. [Google Scholar]
- Surucu, G.; Ozısık, H.; Deligoz, E.; Shein, I.R.; Matovnikov, A.V.; Mitroshenkov, N.V.; Morozov, A.V.; Novikov, V.V. Electronic and thermodynamic properties of lanthanum tetraboride on low-temperature experimental and ab-initio calculation data. J. Alloys Compd. 2020, 862, 158020. [Google Scholar] [CrossRef]
- Werheit, H.; Filipov, V.; Shitsevalova, N.; Armbrüster, M.; Schwarz, U.; Ievdokimova, A.; Muratov, V.; Gurin, V.N.; Korsukova, M.M. Raman scattering in rare earths tetraborides. Solid State Sci. 2014, 31, 24–32. [Google Scholar] [CrossRef]
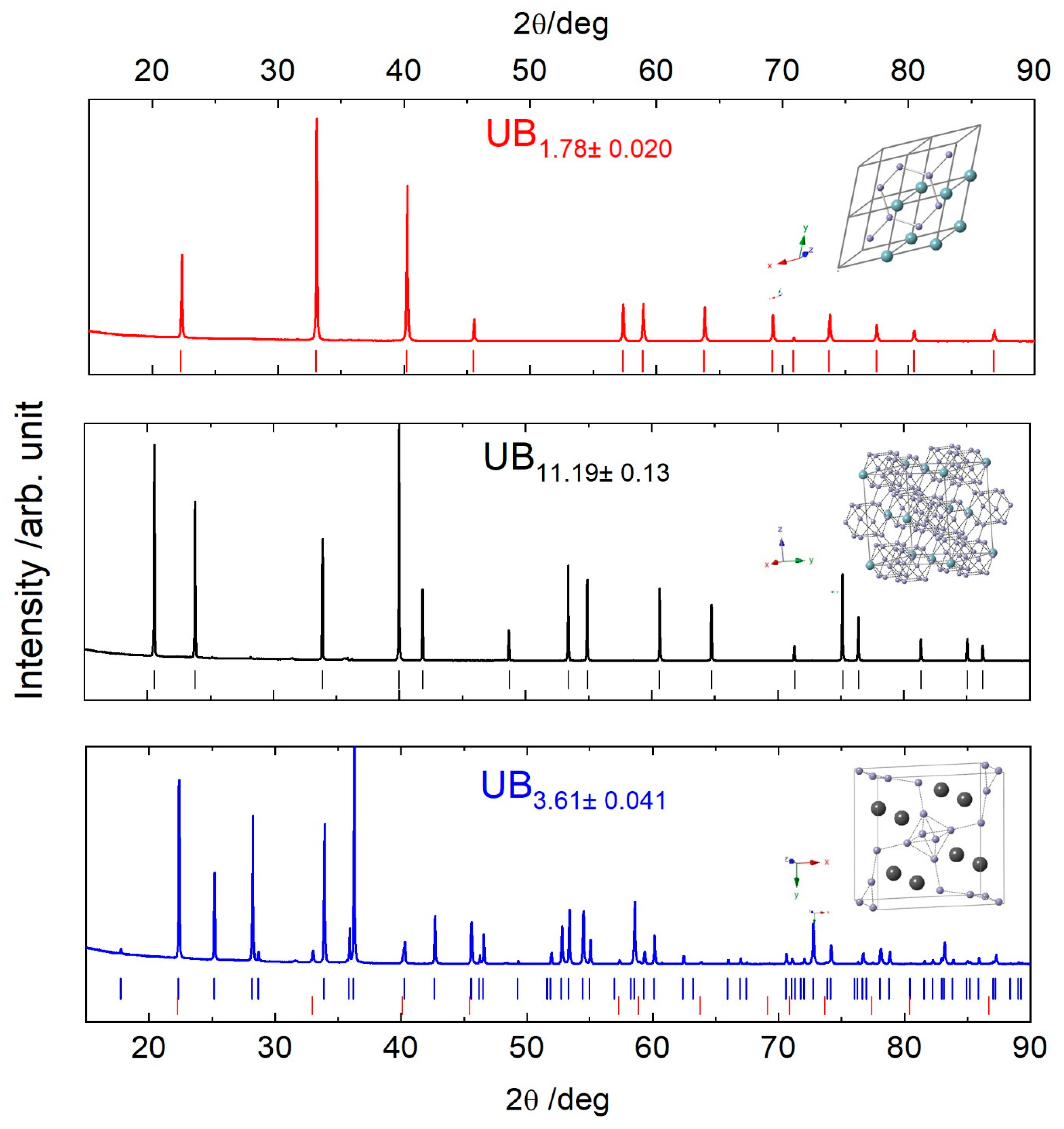
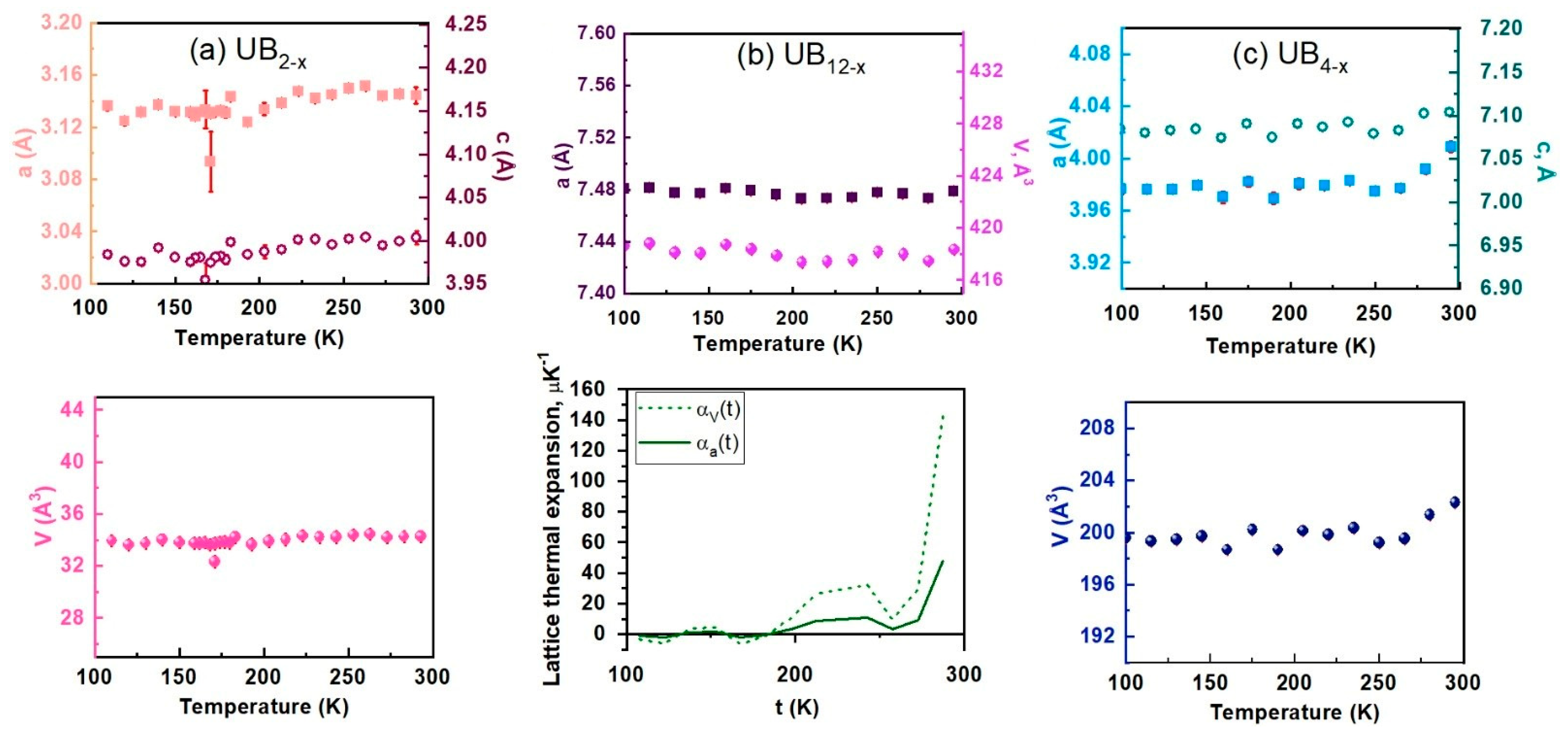
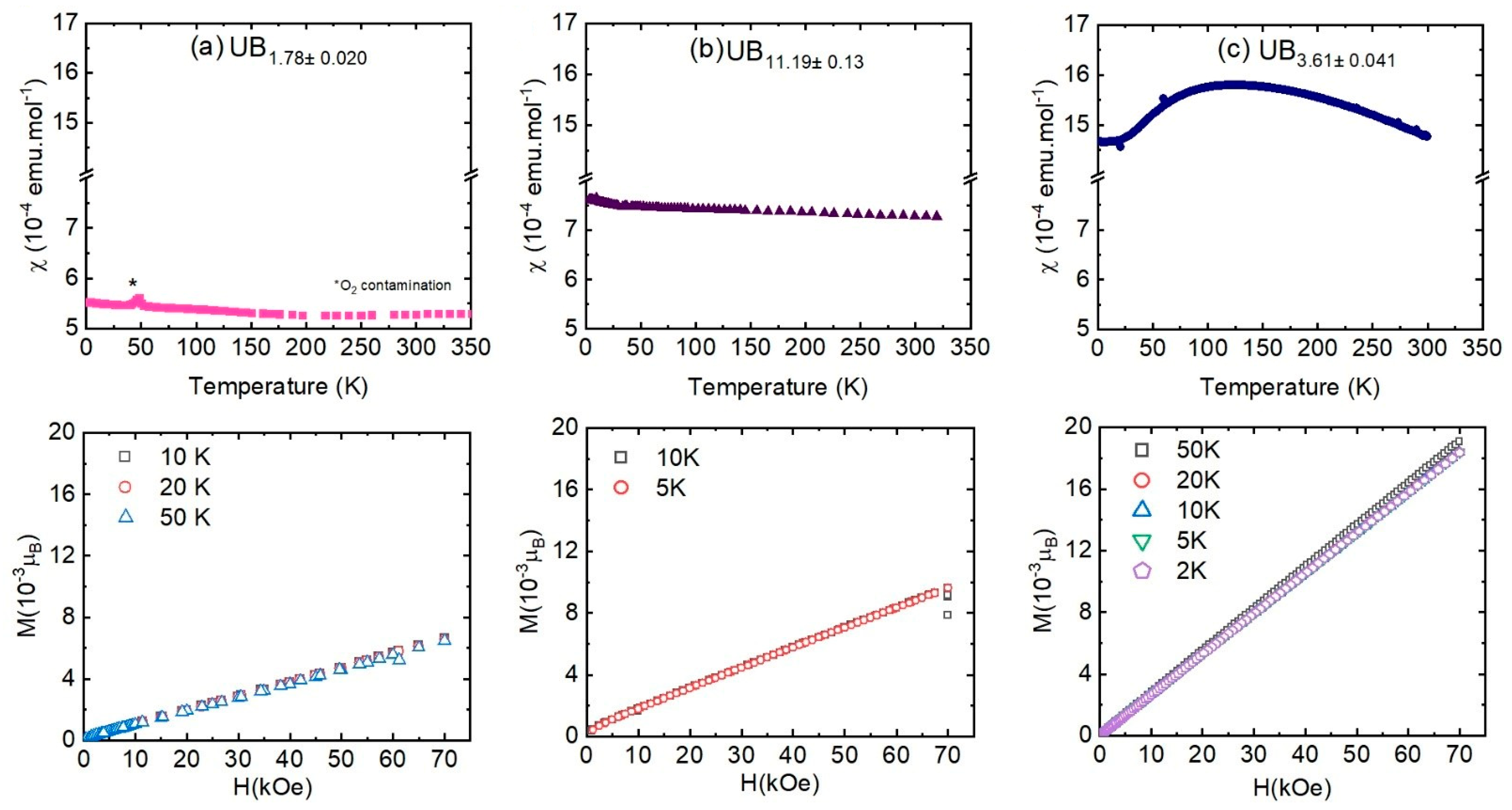
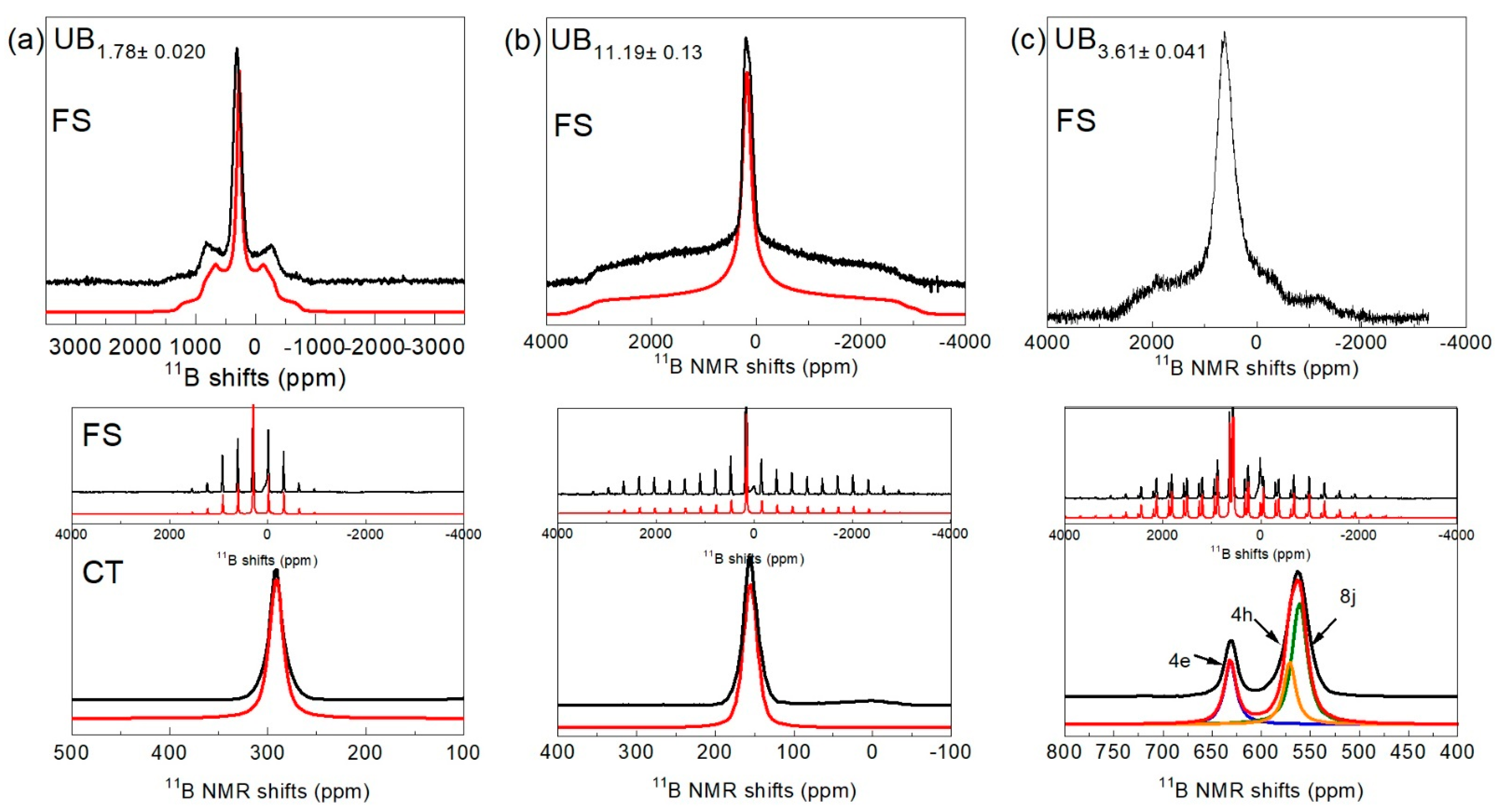
| Samples | UB2−x | UB4−x | UB12−x |
|---|---|---|---|
| U/B (at/at) | 1.780 ± 0.020 | 3.613 ± 0.041 | 11.19 ± 0.13 |
| Oxygen content (mg/g) | <200 * |
| Name | UB1.78 | UB11.19 | UB3.61 |
|---|---|---|---|
| Structure | hexagonal | cubic | tetragonal |
| Space group n° | P6/mmm | Fm-3m | P4/mbm |
| a = b/Å | 3.1225 (1) | 7.4715 (1) | 7.0777 (4) |
| c/Å | 3.9858 (2) | - | 3.9783 (2) |
| α/deg | 90 | 90 | 90 |
| β/deg | - | - | - |
| γ/deg | 120 | - | - |
| Rwp | 8.38 | 10.24 | 9.01 |
| Gof | 2.81 | 3.16 | 3.05 |
| Composition/% | 100 | 100 | 95.1 (+UB2) |
| Shortest U-U spacing Å | 3.12 | 5.28 | 3.65 |
| UB2 | UBX | UB4 | UBX | UB12 | UBX | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| a (Å) | c (Å) | Ref. | X | a (Å) | c (Å) | Ref. | X | a (Å) | Ref. | X | |
| 1 | 3.1225 | 3.9858 | P.S. | 1.78 (2) | 7.0573 | 3.9008 | [20] | 7.468 | [21] 1 | ||
| 2 | 3.1302 (3) | 3.9878 (3) | [20] | 7.0777 | 3.9783 | P.S. | 3.613 (41) | 7.4715 | P.S. | 11.19 (13) | |
| 3 | 3.132 | 3.986 | [7] 2 | 7.080 | 3.978 | [21] | 7.473 | [22] | |||
| 4 | 3.139 | 3.994 | [23] | 7.079 (1) | 3.983 (1) | [24] | 7.474 | [10] 3, [11] | |||
| 5 | 3.133 | 3.986 | [25] | 2.02 | 7.0795 | 3.9794 | [26] 4 | 7.475 | [18,20] | 16 | |
| 6 | 3.084 | 4.020 | [27] | 7.0764 | 3.9811 | [19] | 3.98 | ||||
| 7 | 3.136 (6) | 3.988 (8) | 7.08 | 3.98 | [9] | ||||||
| 8 | 3.1309 (5) | 3.9837 (5) | [17] | 1.79 (6) | |||||||
| Compounds | UB2−x | UB4−x | UB12−x | |||||
|---|---|---|---|---|---|---|---|---|
| Parameter | a | c | V | a | c | V | a | V |
| slope | (1.0648 ± 1.8127) 10−5 | (1.3111 ± 0.2538) 10−4 | 0.0043 ± 0.0016 | (7.3755 ± 2.9109) 10−5 | (7.1264 ± 3.0399) 10−5 | (8.83 ± 3.64) 10−3 | (−2.3 ± 1) 10−5 | −4.82 ± 1.53) 10−3 |
| intercept | 3.1172 ± 0.0040 | 3.9641 ± 0.0057 | 33.0589 ± 0.2981 | 7.072 ± 0.006 | 3.9662 ± 0.0061 | 198.087 ± 0.7423 | 7.4814 ± 0.0021 | 419.0161 ± 0.2836 |
| Adj-R2 | 0.5727 | 0.5067 | 0.2054 | 0.2942 | 0.2570 | 0.2725 | 0.2206 | 0.4076 |
| Compound Name | 11BK (ppm) | FWHM (ppm) | CQ (kHz) | hQ | |
|---|---|---|---|---|---|
| UB1.78 | B1 | 303.6 a | -- | 298.4 a | 0.2 a |
| 291.6 b | 16.8 | ||||
| UB3.61 | B1 (4e) | 631.5 | 14.2 | 390 b | 1 b |
| B2 (4h) | 571 | 14 | 560 b | 0.9 b | |
| B3 (8j) | 561.2 | 18 | 560 b | 0.8 b | |
| UB11.19 | B1 | 164.3 a | -- | 774.7 a | 0.8 a |
| 155.4 b | 21.2 | ||||
| AlB2 [18] | −10 ± 5 | 1.08 | 0 | ||
| ZrB2 [18] | −29 | -- | -- | ||
| MgB2 [45] | 40 ± 10 | 1.67 | 0 | ||
| YB4 [44,46] | 4e | 34.7 | 1.14 | ||
| 4h | 12.6 | 1.46 | |||
| 8j | 5.4 | 1.04 | |||
| LaB4 [37,47] | 4e | 42 | 0.69 | 0 | |
| 4h | 47 | 1.1 | 0 | ||
| 8j | 18 | 0.8 | 0.5 | ||
| NdB4 [37,46] | 4e | 3300 (±10–15%)) | 0.84 | 0 | |
| 4h | 2300 (±10–15%) | 0.89 | 0.5 | ||
| 8j | 2600 (±10–15%) | 1.244 | 0 | ||
| ZrB12 [18] | 10 | 1.083 | 0.98 | ||
| YB12 [18] | 25 | 1.08 | 0.93 |
| Present Study | Literature | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UB1.78 | UB3.61 | UB11.19 | UB4 [53] | ZrB12 [54] | UB12 [54] | UB2 [23] | MgB2 | |||||
| 1 | 388 | 395 | 394 | dd | 315 | T1u | 0 | E1u | 393 | E1u [55] | 322/327 # | |
| 2 | -- | 438 | 350 | T2u | 198.3 | A2u | 481 | A2u | 394/405 # | |||
| 3 | 463 | 456 | 453 | dd | 358 | T1u | 206.6 | E2g | 598 | |||
| 4 | 500 | -- | 513 | 399 | T1g | 478.1 | B1g | 794 | E1u [56] | 335 # | ||
| 5 | 680 | -- | 693 | dd | 608 | Eu | 498.2 | A2u | 401 # | |||
| 6 | 778 | 778 | 778 | dd | 722 | A2g | 518.8 | |||||
| 7 | 796 | 796 | 795 | dd | 796 | Eg | 639.9 | 621 | E1u [57] | 320 # | ||
| 8 | 907 | -- | -- | T2u | 661.6 | A2u | 390 # | |||||
| 9 | 1002 | -- | -- | T1g | 693.6 | |||||||
| 10 | 1083 | 1077 | 1073 | T1u | 729.1 | |||||||
| 11 | 1162 | 1162 | 1162 | T2g | 801.7 | 748 | ||||||
| 12 | 1360 | 1354 | -- | T1u | 854.2 | |||||||
| A2u | 982.4 | |||||||||||
| Eg | 1010.1 | 971 | ||||||||||
| A1g | 1078.3 | 1039 | ||||||||||
| T2g | 1084.4 | 1088 | ||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martel, L.; Charpentier, T.; Amador Cedran, P.; Selfslag, C.; Naji, M.; Griveau, J.-C.; Colineau, E.; Eloirdi, R. Insight into the Crystal Structures and Physical Properties of the Uranium Borides UB1.78±0.02, UB3.61±0.041 and UB11.19±0.13. Minerals 2022, 12, 29. https://doi.org/10.3390/min12010029
Martel L, Charpentier T, Amador Cedran P, Selfslag C, Naji M, Griveau J-C, Colineau E, Eloirdi R. Insight into the Crystal Structures and Physical Properties of the Uranium Borides UB1.78±0.02, UB3.61±0.041 and UB11.19±0.13. Minerals. 2022; 12(1):29. https://doi.org/10.3390/min12010029
Chicago/Turabian StyleMartel, Laura, Thibault Charpentier, Pedro Amador Cedran, Chris Selfslag, Mohamed Naji, Jean-Christophe Griveau, Eric Colineau, and Rachel Eloirdi. 2022. "Insight into the Crystal Structures and Physical Properties of the Uranium Borides UB1.78±0.02, UB3.61±0.041 and UB11.19±0.13" Minerals 12, no. 1: 29. https://doi.org/10.3390/min12010029
APA StyleMartel, L., Charpentier, T., Amador Cedran, P., Selfslag, C., Naji, M., Griveau, J.-C., Colineau, E., & Eloirdi, R. (2022). Insight into the Crystal Structures and Physical Properties of the Uranium Borides UB1.78±0.02, UB3.61±0.041 and UB11.19±0.13. Minerals, 12(1), 29. https://doi.org/10.3390/min12010029






