Abstract
The mining industry is facing emerging challenges as a result of the increase in energy consumption and environmental demands. These facts have promoted the use of renewable energy sources, such as wind, geothermal and, mainly, solar energy. This paper discusses the role of solar energy (UV-VIS-NIR) in leaching processes, evaluating its potential application in metal extraction from sulfide minerals, based on photochemical mechanisms that promote the regeneration of ferric iron or the so called ferrous iron cycling. The present paper discusses the possibility that ultraviolet, visible light and near infrared irradiation (e.g., sunlight provided) can assist the leaching processes in two main ways: by the oxidation of sulfide minerals through in-situ generated Fenton-like reactions, and by the photochemical activation of semiconductor minerals that contain transition metals (Fe, Cu, and Cr, among others). Thus, this paper provides theoretical support to move towards the future application of photoleaching, which consist of a leaching process assisted by UV, VIS, and NIR irradiation. This technology can be considered a promising mineral processing route, using direct photochemical solar energy that can reduce the energy consumption (electricity, fuels) and the environmental impact, opening an opportunity for an alternative method of metal extraction from sulfide ores.
1. Introduction
Today, the mining industry is facing new challenges and transformations due to economic and environmental issues. The world’s leading copper mining companies are facing the progressive decrease in ore grades, reaching an uneconomical condition for the pyrometallurgical route, after years of exploitation of their natural deposits [1]. At the same time, a significant decline is observed in the oxidized mineral deposits, which are conventionally processed using the hydrometallurgical route. In fact, Chilean annual copper concentrate production is projected to increase from 4.3 million tonnes in 2019 to 6.3 million tonnes in 2030. This forecast also includes a decrease in copper production from 1.6 million tonnes in 2019 to 0.75 million tonnes in 2030, in the form of cathode, due to the reduction in copper oxide ores [2] (Figure 1).
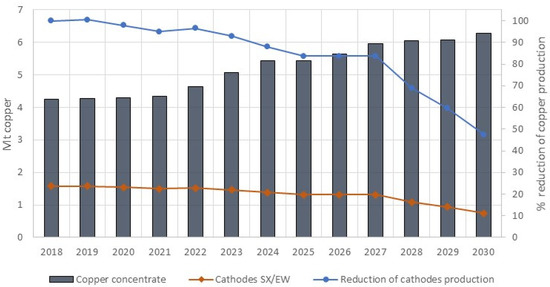
Figure 1.
Projected production of copper concentrates (excluding smelting and refining), Cathodes by solvent extraction/electrowinning (SX/EW) and reduction of cathodes production in Chile 2019–2030 by COCHILCO [2].
This context will force around 75% of concentrate production to be exported by 2030, but will also generate an available capacity of treatment plants of leaching solution, which are typically solvent extraction with electrowinning plants. Therefore, there is an opportunity to promote the development of alternative mineral processing technologies based on hydrometallurgical processes.
Other challenges faced by the mining industry are finding alternative energy sources aiming at cost saving, and reducing the environmental impact. Currently, the mining industry is investing in renewable energy sources such as wind, geothermal and, mainly, solar (photovoltaic and thermosolar) energy, which has the greatest potential for future development in this industry [3,4]. Coincidentally, the world’s major mining industries and deposits, located in countries such as Chile, Peru, the United States of America, Mexico and Canada, among others, are often placed in areas with high solar irradiation, provided by sunlight (photochemical source of ultraviolet (UV), visible (VIS) and near infrared irradiation (NIR)) [5,6]. The combination of factors such as mineral deposits and solar energy creates an unbeatable opportunity for developing novel solar assisted technologies for processing metal extraction, particularly to be applied in hydrometallurgical processes. The goal of this paper is to discuss the potential role of the solar energy (UV-VIS-NIR) as an assistant for leaching of sulfide minerals (e.g., CuFeS2 and others) presenting a theoretical support in relation to the effect of the solar energy (UV-VIS-NIR irradiation) on the oxidative decomposition mechanisms of mineral sulfides through in-situ generated photo-Fenton-like reactions.
2. Global Trends in the Mining Industry: Renewable Power Sources and Solar Energy
If we take Chile as a reference as the major copper-producing country in the world [7], it can be seen from the predictions provided in economic studies that electrical consumption by the copper mining industry will increase in the next decade (2020–2030), from 23.6 to 33.1 TWh [8]. This significant increase is mainly due to the decrease of ore grades and the increasing complexity of ore bodies required to be processed more intensively to keep the same production level of metal recovered (Cu, Mo, among others) [9,10]. This scenario will promote the use of renewable energy sources, such as solar, wind, and geothermal energy [11]. In this sense, the future projection based on solar and storage technologies should be highlighted, as in the cases of electrical energy storage (EES) for copper operations, photo-voltaic electricity (PV) in copper electrowinning and solar-thermal technologies, and most notably, concentrated solar power (CSP) for copper mining operations [12,13].
When considering, for example, the world’s largest copper producers, such as Chile (Escondida, El Teniente), Peru (Cerro Verde II, Quellaveco), China (Zijinshan gold-copper mine), United States (Morenci), and Australia (Olympic Dam), it can be seen that in some countries, coincidentally, industrial areas have high levels of average irradiance, which in some cases are far above 2000 kWh·m−2 year−1 [14,15].
It is important to note that solar energy in mining processing is for the most part used as a resource for solar and storage technologies (EES) and thermo-solar technologies (CSP). It is also feasible to explore its application as a direct photonic source for photochemical processes. Additionally, photons provided by sunlight (mainly UV and VIS radiation; additionally NIR light in photocatalysis) can be absorbed by a semiconductor material, such as natural doped compounds, sulfide minerals, and transition metal oxides, which are obtained from the ore bodies [16,17,18,19,20], generating excited states that give rise to a sequence of photochemical reactions (photooxidation/photoreduction) that can be exploited to improve or assist the oxidative dissolution of these sulfide minerals and accelerate ferric iron/ferrous cycling [21,22].
Considering the important role that solar energy can play in the mining industry, an opportunity arises for developing alternative hydrometallurgical processes for the treatment of sulfide minerals and copper concentrates through a complementary leaching procedure using UV-VIS-NIR radiation, which can eventually be obtained from solar energy [23]. This paper presents a comprehensive analysis that proposes the use of UV-VIS-NIR irradiation as an assistant to the oxidative decomposition mechanism of sulfide minerals (chalcopyrite, pyrite, molybdenite, chalcocite, galena, sphalerite, and others), using thermodynamic analysis and previous experimental results. The present study demonstrates the promising possibilities for the use of solar energy in the leaching of metals such as Cu, Fe, Cd and others, from a diverse range of sulfide mineral ores.
3. The Role of the Free Radicals Generated by Solar Irradiation, in the Composition of the Sulfide Orebodies and Rocks
It has been widely reported that the UV-VIS-NIR irradiation can accelerate the oxidation mechanisms through generation of transitory species such as free radicals, as well as charge carriers (electron and hole pairs) in photo-assisted processes [24]. This is also possible through radical species that possess very powerful oxidizing capacities [25]. A large diversity of radicals have been studied, including primarily reactive oxygen species (ROS), reactive nitrogen species (RNS), reactive sulfur species (RSS), and reactive chloride species (RCS), as shown in Table 1 [26].

Table 1.
Standard electrode potentials in aqueous medium (E° v/s SHE) of the most common free radicals and common oxidants. SHE: standard hydrogen electrode [26,27].
Compared to the chemical oxidizing agents most commonly used in leaching processes, for example, the radicals continue to have a strong oxidizing power. Some of their radical groups, such as ROS, RSS, and RNS, have the second strongest oxidizing potential after fluorine or hydrogen peroxide, namely HO˙, SO4˙−, RNS, among others (Table 1) [27].
In the hydrosphere or Earth shells and solid substrates, the free radicals drive various mechanisms for the oxidation of transition metal compounds; mostly Fe, Cu, Cr, among others [28]. In many cases, the physicochemical characteristics of the solid substrates (minerals, rocks, orebodies, and others) and their interaction with the environment (sunlight, ligands, oxygen, water, electrolytes, and others) can activate the environmental self-cleaning behavior, through a similar pathway to the advanced oxidation mechanisms [29,30]. As an example, we can mention the oxidation of pyrite (FeS2) that plays an important role in the cycling of iron (Fe(III)/Fe(II)) on the Earth’s crust and is responsible for acid mine drainage (AMD), usually for the minerals containing sulfide compounds [31,32,33].
Furthermore, in metal reservoirs in the Earth’s crust, free radicals are involved in a variety of chemical and photochemical processes that result in a continuous change of the composition of environmental compartments, in the metal ions’ stability, and in the transportation of metal ions in aquatic systems (Cr(III) and Cr(VI), Fe(II) and Fe(III), and others) [34,35]. The UV and VIS irradiation can excite the water soluble Fe(III) hydroxo-complexes in colloidal systems (heterogeneous systems), through the ligand-to-metal charge transfer (LMCT) mechanism [36], generating photochemical excited center Fe(II) compounds and spontaneous production of HO˙ radical, in the same way as advanced photocatalytic processes.
3.1. Oxidative Dissolution of Sulfide Minerals Promoted by Fenton-Like Mechanisms
As mentioned above, the natural oxidation of sulfide minerals plays a crucial role in the redox cycling of transition metals in geochemical systems (water and deposits). It has been established that the oxidative dissolution mechanism of sulfides is the primary cause of acid mine drainage. AMD is understood as the oxidative dissolution of metallic sulfides when exposed to oxygen and water due to geological and mining activities [37,38]. Gil-Lozano et al. (2017) proved that the dissolution of sulfide minerals, particularly pyrite dissolution by AMD, is mediated by the Fenton-like mechanism (Equation (5)) [31]. This pathway is promoted by the self-generated hydrogen peroxide and iron ions released from the mineral. In addition, it is proposed that the radical oxygen species (ROS) are always present as transient by-products and they can actively participate in the generation of dissolution products (Equations (1)–(5)) [39].
Fe2+(superficial) + O2(g) ↔ Fe3+(superficial) + O2˙−
Fe2+(superficial) + O2˙− + 2H+ ↔ Fe3+(superficial) + H2O2(aqueous)
Fe3+(superficial) + H2O ↔ Fe2+(superficial) + HO˙(absorbed) + H+
HO˙(absorbed) + HO˙(absorbed) ↔ H2O2(aqueous)
H2O2(aqueous) + Fe2+(aqueous) ↔ Fe3+(aqueous) + HO˙(absorbed) + OH−
Auspiciously, the oxidative decomposition mechanism of sulfide minerals AMD mediated by Fenton-like reactions would be used to enhance the leaching process. The combined effect of UV-VIS-NIR irradiation along with adequate control of parameters such as pH, hydrogen peroxide concentration, and iron dissolved species, could be used to accelerate the Fe(III)/Fe(II) cycling and promote ROS generation. We propose that one way to promote efficient control of Fe(III)/Fe(II) cycling that simultaneously avoids the formation of iron hydroxo-complexes can be the use of photochemical energy (UV-VIS-NIR irradiation) to degrade these metal complexes (e.g., [Fe(OH)]2+) [40]. At the same time, the UV radiation can promote simultaneous redox reactions, such as the photoreduction of aqueous species Fe(III) to Fe(II), catalyzing the iron cycling [41].
This is one of the highly remarkable aspects of the present revision, since it has been proved that the oxidative decomposition mechanism of sulfide minerals by AMD, such as pyrite (FeS2), galena (PbS), sphalerite (ZnS), chalcopyrite (CuFeS2), and arsenopyrite (FeAsS), among others, is driven by similar oxidation mechanisms, particularly by the Fenton-like reaction [42].
3.2. The Role of Semiconductive Properties of Sulfide Compounds in Photochemical Processes
Photocatalysts are solid materials that have semiconductive properties allowing them to be activated by specific radiation, according to their band gap values, e.g., giving rise to a sequence of photochemical reactions. The most used catalysts include a variety of transition metal oxide, such as TiO2 or ZnO [43]. However, chalcogenides as CdS, ZnS, MoS2, and WS2 have also been used due to their low band gap allowing absorption in the visible spectrum [44,45,46,47,48]. In addition, copper sulfides have been employed in photovoltaic and photoelectrochemical solar cells with high energy conversion efficiencies, including CdS, Cu2S, CuInS2, MoS2, and other sulfides [49,50,51]. It is important to consider that some of the metallurgical products, mainly concentrates, contain semiconductor materials, primarily sulfide minerals (Chalcopyrite, Chalcocite, Covellite, Pyrite, Bornite) along with transition metal oxidized compounds (Copper oxides, oxides, and hydroxides of iron), natural transition metal compounds and gangue [52,53,54]. These compounds can, eventually, be activated under specific UV-VIS-NIR irradiation acting as a photocatalyst in solar assisted leaching process [55].
In relation to solar photovoltaic technologies, chalcogenides such as CdS, Cu2S, or MoS2 have also been used due to their low band gap that allows absorption in the visible spectrum, as mentioned above [56]. However, in this latter case, photocorrosion can take place decreasing the photocatalytic efficiency and promoting metal leaching from the metallic sulfide (MS) (Equations (6)–(14)) [57,58,59]. Briefly, the metallic sulfide can photocorrode in aqueous media through two main pathways:
- Direct photooxidation by holes
MS + hv ↔ h+(MS) + e−(MS)
MS + 2h+(MS) + hv ↔ M2+ + S0
- b.
- Indirect photooxidation by oxygen, mediated by RSS
MS + h+(MS) + hv ↔ M2+ + S˙−
S0 + e−(MS) + hv ↔ S˙−
S˙− + O2 + hv ↔ SO2˙−
SO2˙− + h+(MS) + hv ↔ SO2
SO2 + H2O + hv ↔ HSO3− + H+
HSO3− + H2O + h+(MS) + hv ↔ SO42− + 3H+
MS + 2O2 + hv ↔ M2+ + SO42−
Considering the above, we propose that sulfide leaching can be enforced under specific irradiation and adequate control of physico-chemical parameters (pH, intensity of UV-VIS-NIR radiation, ligands, electrolytes, and others), by means of inducing photocorrosion of natural doped compounds (e.g., sulfide minerals) [60] and transition metal oxides, thus promoting the metal release (Fe, Cd, Cu, and others), which can play an assistant role in the sulfide dissolution mechanism through the Fenton-like reaction [61,62].
4. The Role of the radiation (UV-VIS-NIR) in the Sulfide Photoleaching Process
As mentioned in the literature, sulfide leaching processes generally involve additional costs associated with the highly corrosive and abrasive environments that are frequently found in these processes. The importance of designing an efficient hydrometallurgical process means to avoid excessive costs of energy consumption, resulting in fuel efficiency and in equipment lifetime (i.e., reactors) [63,64]. The addition of chemical and biological oxidizing agents requires subsequent chemical treatments to purify the leaching pregnant solutions and to separate the refractory products [65].
In contrast to earlier treatments, the use of solar energy (UV-VIS-NIR) as a direct activator of photochemical oxidative mechanisms could reduce the energy requirements because it will allow work under mild conditions in aqueous or acidic media. Likewise, sunlight can be used in a complementary way to leaching or bioleaching processes, accelerating the oxidative decomposition of sulfide minerals. The combined effect of visible light (UV-VIS-NIR) and photoactive compounds (i.e., natural doped minerals) in leaching or bioleaching processes has demonstrated their potential for increasing the leaching rates [66]. Consequently, it is to be expected that an increase in the leaching (or bioleaching) rates could reduce the processing time and the associated energy requirements.
Here we raise the possibility that the UV, VIS, and NIR irradiation can improve the leaching processes in two main ways, as follows.
4.1. The UV, VIS and NIR Irradiation Can Support the Oxidation of Sulfide Minerals through the Use of In-Situ Generated Fenton-Like Reactions
As shown in the previous sections, the dissolution mechanisms of refractory solid species such as pyrite in AMD is mediated by Fenton-like reactions, i.e., they make use of the formation of free radicals with high oxidative capacity (mainly ROS and RSS) mediated by the presence of a transition metal (mainly Fe or Cu) that catalyze the formation of these radicals. In the case of AMD, the formation of radical species, particularly ROS, is mediated by the interaction of H2O2 with ferric and ferrous ions, where Fe(III) and Fe(II) are provided by pyrite (or chalcopyrite) leaching under acidic oxidizing conditions, as shown in Figure 2 and Figure 3 [67,68,69,70].
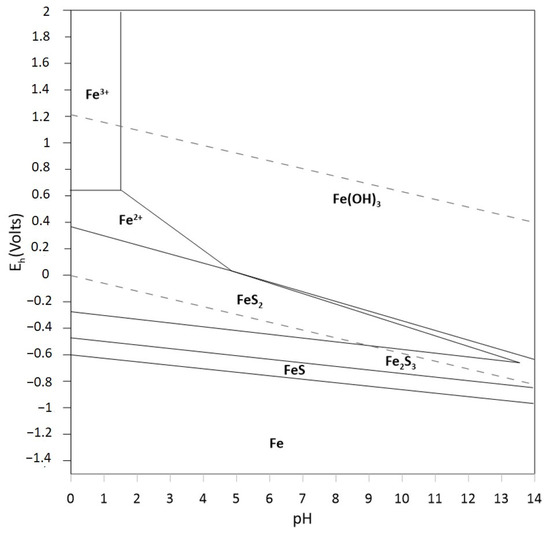
Figure 2.
Pourbaix diagram, system Fe-S-H2O at 25 °C, 1 mol L−1 Cu2+, Fe and S (HSC Chemistry 9.0, Metso Outotec, Helsinki, Finland).
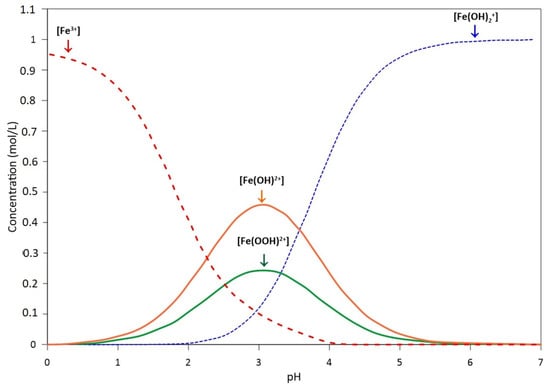
Figure 3.
Chemical speciation of iron in water. Red line: [Fe3+] v/s pH; blue line: [Fe(OH)2+] v/s pH; green line [Fe(OOH)2+] and orange line: [Fe(OH)2+]. Arrows shows the maximum concentration of each species (Ocatve-Gui 5.2.0, University of Wisconsin-Madison and University of Texas, United Stated of America).
The homolysis of hydrogen peroxide in an aqueous medium at acidic pH (below pH 3) and in the absence of irradiation or organic ligands, includes the generation of HO·, HO2· and O2·−, radicals [71]. This is catalyzed by the redox process of the iron, which is also present in the aqueous medium [72,73].
However, the availability of ferrous ions in the aqueous phase to catalyze Fenton-like reactions is limited by the formation of iron hydroxo-complexes (see Figure 2) and their oxidation to Fe(III). The combination of hydrogen peroxide, UV-VIS radiation and the Fe(III)/Fe(II) pair produces a greater amount of hydroxyl radicals than its pair without radiation [74]. Essentially, the photolysis of the ferric ion complexes takes place when the radiation is in the UV-VIS spectrum. For example, [Fe (OH)]2+, which predominates in the range between pH 2 and pH 4 (see Figure 3), is being irradiated with wavelengths between 290 nm and 400 nm and decomposes according to Equation (15) [75]:
[Fe(OH)]2+ + hv ↔ Fe2+ + HO˙
Also, incident radiation (UV) causes photochemical regeneration of ferrous ions by photoreduction of ions [76]. The newly generated ferrous ions react again with hydrogen peroxide, generating hydroxyl radical and ferric ion as shown in Equations (17) and (18):
Fe3+ + hv ↔ Fe2+ (global photoreduction)
Fe3+ + H2O + hv ↔ Fe2+ + HO˙ + H+
Fe3+ + H2O2 + hv ↔ Fe2+ + HO2˙ + H+
It has been reported that the photo-Fenton process is more efficient than the Fenton process, and, in addition, the use of sunlight (UV-VIS-NIR) is a good source of photochemical energy in the UV-VIS range to generate photo-Fenton reactions [77].
It is important to note that Fenton-like processes are not only mediated by the Fe(III)/Fe(II) couple as a catalyst, but they also occur with other transition metals such as copper (Equations (19) and (20)), since these metals are a suitable medium for leaching [77]. Metal sulfides of economic interest, such as chalcopyrite, release Cu(II) ions into the medium under certain acidic oxidizing conditions (see Figure 4) [78,79,80]. These transition metals can also catalyze the decomposition of hydrogen peroxide, generating hydroxyl radicals through the Fenton-like mechanism.
Cu+ + H2O2 ↔ Cu2+ + HO˙ + OH−
Cu2+ + H2O + hv ↔ Cu2+ + HO˙ + H+
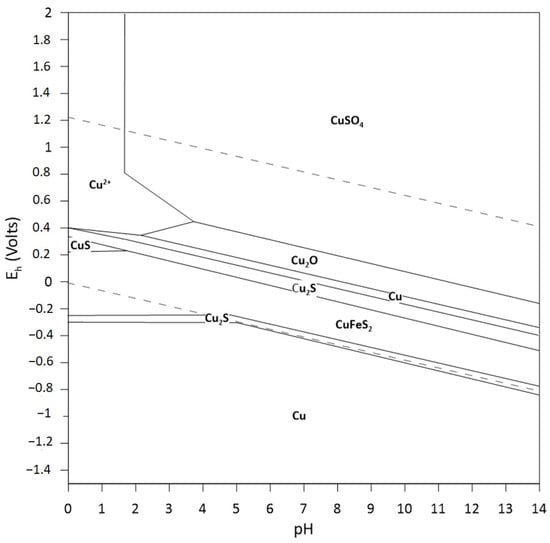
Figure 4.
Pourbaix diagram, system Fe-Cu-S-H2O at 25 °C, 1 mol L-1 Cu, Fe and S (HSC Chemistry 9.0, Metso Outotec, Helsinki, Finland).
4.2. The role of UV, VIS and NIR Irradiation in Photochemical Reactions in Aqueous Media for Semiconductors Minerals That Contain Transition Metals (Fe, Cu, Cr, and Others)
As mentioned earlier, the photochemical reactions induced by solar light can play a crucial role in the composition of the environment on the Earth’s crust, including the effect on rocks, complexes, and ore bodies, among others. These solid materials include semiconductors minerals such as transition metal chalcogenides, natural doped compounds, and transition metal oxides, which can act as a photocatalyst under specific UV-VIS, even NIR radiation (sunlight), that are taking part directly or indirectly in photoredox reactions [46].
It is interesting to note that in both photochemical processes, namely photocatalysis and photocorrosion, photodecomposition of semiconductors can occur under certain thermodynamic conditions in the electrolytic medium [81,82,83]. Photodecomposition is a consequence of simultaneous reactions of photooxidation and photoreduction in the surface of the semiconductor, mediated by charge carriers (e−(MS) and h+(MS)) generated on the surface by photochemical activation (Figure 5A,B), whose mechanism in a binary compound can be represented by the following equations:
MS + h+(MS) ↔ M+x + S + e−(MS) (photooxidation)
MS + e−(MS) ↔ M + S−z + h+(MS) (photoreduction)
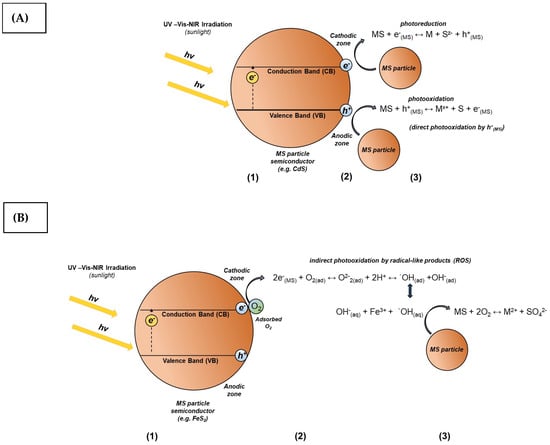
Figure 5.
Scheme of photodecomposition mechanism of semiconductor (binary semiconductor MS) in electrolytic medium by: charge carriers (A) and ROS or RSS (B). Stages: (1) photoactivation of SC particles, (2) charge carriers and radicals generation, (3) photodecomposition of SC particles by charge carriers and ROS or RSS.
The metal ions released (M+x) from transition metal chalcogenides (MS) can be promoted, and the sulfoxy species (represented by S−z) that produce intermediate by-products such as H2O2, ROS (by the interaction of adsorbed molecular oxygen O2(g) with the MS surface), and RSS are by means of photocatalytic mechanisms.
The leaching mechanism of sulfide mineral, mostly chalcopyrite, involves the generation of intermediates such as metal deficient polysulfide, elemental sulfur, polysulfides and jarosite type compounds. They contribute to the formation of the passivation layer, acting as a natural barrier for further dissolution of the mineral (CuFeS2) [84,85,86]. However, radical-like oxidation products, such as ROS and RSS, can even attack the sulfur layer zones and metal deficient polysulfide in the semiconductor surface, and oxidize them to sulfate species (Figure 5B). Some of these compounds, namely metal-deficient polysulfide, are semiconductor materials which can be photodecomposed under specific irradiation. This also contributes to an increase in the permeability of the passivating layer and consequently, improving the leaching kinetics of sulfide minerals (e.g., CuFeS2 and others) [87]. Nevertheless, a future specific kinetic study is required to describe the leaching of chalcopyrite under UV-VIS-NIR irradiation.
In addition, thermodynamic and kinetics criteria for such photodecomposition processes should be considered. Further specific thermodynamic study is necessary to determine the susceptibility of each semiconductor to photodecomposition, under specific irradiation. We propose that this photodecomposition mechanism of natural doped semiconductors could be used to enforce the oxidative dissolution (or decomposition) of these sulfide minerals.
The proposed solar assisted leaching mechanism is directly related to the photocatalytic contribution of semiconductive materials, namely minerals and natural doped compounds. Another interesting aspect herein is that, due to the effects of UV, VIS, and NIR irradiation, the photogenerated electrons or charge carriers in the surface of the semiconductor can act in a variety of photoredox reactions, especially in the photoreduction of ferric by ferrous ions, as demonstrated by Yang et al. (2017) [88] in the bioleaching process. They observed that the ferrous iron regeneration was promoted by visible light, and at the same time the ferrous iron consumption rate was slower than dark bioleaching. Consequently, bioleaching under visible light was increased.
The direct effect of the solar irradiation in Cu and Fe leaching was studied by Yepsen et al. (2018) [54] using minerals such as chalcopyrite, covellite and chalcocite, which are a natural metal-doped transition. They showed that metal leaching increases by effect of UV-VIS-NIR irradiation, compared to the absence of irradiation (dark process). These experiments were carried out under controlled conditions, in order to isolate the effect of radiation on the metal leaching. This approach would propose that UV, VIS, and NIR irradiation are contributing factors in leaching mechanisms.
This previous research in fact demonstrated the growing interest in the use of solar light (UV-VIS-NIR) applied to hydrometallurgical processes and their promising combined effect that has been proven to work in, for example, bioleaching assisted by UV-VIS irradiation and copper concentrate leaching. Therefore, the solar assisted leaching process could be an interesting alternative in the current scenario for Chilean copper production, for example, where concentrates produced by the copper sulfide processing plants can be treated in photoreactors located near the SX-EW plants with the available capacity to produce cathodes as a final product. Likewise, these processing plants are located in areas that have high levels of irradiance (UV-VIS-NIR). In this sense, the experience of the solar platform of Almeria could be used as a reference for the mining industry of Chile, focusing on a more sustainable way to produce a high-quality copper product, directly using solar energy in the extraction process [89,90].
Future studies should focus on optimal reactor designs that include a solar energy concentrator. In addition, these studies should determine the kinetics parameters for different copper sulfide minerals in order to assess the required equipment capacity of the technology. The following should also be considered: (i) photochemical characterization of semiconductor compounds; (ii) thermodynamic study of susceptibility of each semiconductor to photodecomposition; (iii) the quantification of reagent consumption, namely, adequate doses of H2O2 which are decisive for ROS generation by an induced photo-Fenton reaction; (iv) chemical speciation of Cu and Fe in the leaching solution; (v) the identification of solid content in the photoreactor including semiconductor minerals containing transition metals (optical, chemical and mineralogical composition); (vi) the study of kinetics parameters; and (vii) the establishment of solid-liquid separation stages parameters. Furthermore, all of these studies would be necessary for future expansion on pilot and industrial scales.
5. Conclusions
This article presents a discussion of the role of UV-VIS-NIR irradiation in solar assisted leaching process. According to an extensive analysis of the leaching mechanisms of sulfide minerals, we propose that the UV, VIS, and NIR irradiation (e.g., collected from sunlight) can be used to enhance sulfide mineral leaching (or bioleaching). Under UV-VIS-NIR irradiation, a natural semiconductor, such as natural doper sulfide compounds, can be photodecomposed under determined thermodynamic conditions by means of redox mechanisms. The charge carriers generated in the surface of the semiconductor, namely electrons (e−) and holes (h+), can initiate a sequence of reactions, and they can assist the oxidative dissolution of sulfide compounds by means of photooxidation and photoreduction reactions. The photogenerated electrons under irradiation can photoreduce the transition metal ions in the aqueous media, which are mostly ferrous iron. Simultaneously, the UV-VIS-NIR irradiation can accelerate the ferric iron/ferrous iron cycling, avoiding the formation of iron hydroxo-complexes (e.g., [Fe (OH)]2+), which decreases the availability of iron species, as previously reported [88]. The radicals generated as an intermediate product, namely ROS and RSS, can attack the semiconductor particles, oxidizing them until sulfate [87]. Moreover, the hydrogen peroxide in the presence of transition metal ions (Cu, Fe, others) can promote an additional source of ROS related to Fenton-like and photo-Fenton processes which involve AMD dissolution mechanisms. In order to optimize the leaching performance, kinetics and thermodynamics factors should be considered to optimize the effect of UV-VIS-NIR irradiation in each semiconductor (or sulfide mineral).
This study allows us to show a promising alternative for using photochemical solar energy (UV-VIS-NIR) in the extraction of sulfide minerals through a solar assisted leaching process (photoleaching) involving lower demand for energy (fuels or electricity) and less environmental impact compared to conventional pyrometallurgical processes. Hence, this study can be used to support further studies to move forward into the industrial application of this process.
Author Contributions
Conceptualization/methodology/investigation/writing—original draft preparation/founding acquisition, O.Y.; writing—review and editing/supervision E.A.; conceptualization/methodology/investigation/writing—original draft preparation R.Y.; writing—review and editing/supervision/founding acquisition, H.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research was founded by FONDECYT/3190383 and CONICYT-PIA/AFB180004.
Acknowledgments
The authors acknowledge the financial support of the Agencia Nacional de Investigación y Desarrollo ANID Chile, sponsored the FONDECYT/3190383 and CONICYT-PIA/AFB180004.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Burkin, A.R. Chemical Hydrometallurgy: Theory and Principles, 1st ed.; Imperial College Press: London, UK, 2001. [Google Scholar]
- Comisión Chilena del Cobre COCHILCO. Proyección del Consumo de Energía Eléctrica en la Minería del Cobre 2019–2030. Available online: https://www.cochilco.cl/Paginas/Estudios/Mercados%20de%20metales%20e%20insumos%20estrat%C3%A9gicos/Energ%C3%ADa.aspx (accessed on 1 December 2020).
- Ali, S.; Giurco, D.; Arndt, N.; Nickless, E.; Brown, G.; Demetriades, A.; Durrheim, R.; Enriquez, M.A.; Kinnaird, J.; Littleboy, A.; et al. Mineral supply for sustainable development requires resource governance. Nat. Cell Biol. 2017, 543, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Nasirov, A.; Agostini, C.A. Mining experts’ perspectives on the determinants of solar technologies adoption in the Chilean mining industry. Renew. Sustain. Energy Rev. 2018, 95, 194–202. [Google Scholar] [CrossRef]
- Choi, Y.; Song, J. Review of photovoltaic and wind power systems utilized in the mining industry. Renew. Sustain. Energy Rev. 2017, 75, 1386–1391. [Google Scholar] [CrossRef]
- Murray, C.; Platzer, W.; Petersen, J. Potential for solar thermal energy in the heap bioleaching of chalcopyrite in Chilean copper mining. Miner. Eng. 2017, 100, 75–82. [Google Scholar] [CrossRef]
- Lagos, G.; Peters, D.; Lima, M.; Jara, J.J. Potential copper production through 2035 in Chile. Miner. Econ. 2020, 33, 43–56. [Google Scholar] [CrossRef]
- Brantes, R. Forecast for electricity consumption in the copper mining industry, 2018–2029. J. Min. Eng. Res. 2019, 1, 35–44. [Google Scholar] [CrossRef] [Green Version]
- Norgate, T.; Jahanshahi, S. Low Grade Ores-Smelt, Leach or Concentrate? Miner. Eng. 2010, 23, 65–73. [Google Scholar] [CrossRef]
- Harmsen, J.; Roes, A.; Patel, M. The impact of copper scarcity on the efficiency of 2050 global renewable energy scenarios. Energy 2013, 50, 62–73. [Google Scholar] [CrossRef]
- Harmsen, J.; Jonker, G. Engineering for Sustainability, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Amusat, O.; Shearing, P.; Fraga, E.S. System Design of Renewable Energy Generation and Storage Alternatives for Large Scale Continuous Processes. Comput. Aided Chem. Eng. 2015, 37, 2279–2284. [Google Scholar] [CrossRef]
- Moreno-Leiva, S.; Haas, J.; Junne, T.; Valencia, F.; Godin, H.; Kracht, W.; Nowak, W.; Eltrop, L. Renewable energy in copper production: A review on systems design and methodological approaches. J. Clean. Prod. 2020, 246, 118978. [Google Scholar] [CrossRef]
- Haas, J.; Moreno-Leiva, S.; Junne, T.; Chen, P.-J.; Pamparana, G.; Nowak, W.; Kracht, W.; Ortiz, J.M. Copper mining: 100% solar electricity by 2030? Appl. Energy 2020, 262, 114506. [Google Scholar] [CrossRef]
- Pfenninger, S.; Staffell, I. Long-term patterns of European PV output using 30 years ofvalidated hourly reanalysis and satellite data. Energy 2016, 114, 1251–1265. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, G.; Yan, J.; Wen, Y.; Zhuang, Z.; Yu, Y. Two-Dimensional Transition Metal Oxides and Chalcogenides for Advanced Photocatalysis: Progress, Challenges, and Opportunities. Sol. RRL 2020, 5, 2000403. [Google Scholar] [CrossRef]
- Li, Y.; Lu, A.; Wang, C.; Wu, X. Characterization of natural sphalerite as a novel visible light-driven photocatalyst. Sol. Energy Mater. Sol. Cells 2008, 92, 953–959. [Google Scholar] [CrossRef]
- Xia, D.; Li, Y.; Huang, G.; Fong, C.C.; An, T.; Li, G.; Yip, H.Y.; Zhao, H.; Lu, A.; Wong, P.K. Visible-light-driven inactivation of Escherichia coli K-12 over thermal treated natural pyrrhotite. Appl. Catal. B Environ. 2015, 176-177, 749–756. [Google Scholar] [CrossRef]
- Garrido-Ramírez, E.; Theng, B.; Mora, M.L. Clays and oxide minerals as catalysts and nanocatalysts in Fenton-like reactions—A review. Appl. Clay Sci. 2010, 47, 182–192. [Google Scholar] [CrossRef]
- Andreozzi, R.; Caprio, V.; Insola, A.; Marotta, R. Advanced oxidation processes (AOP) for water purification and recovery. Catal. Today 1999, 53, 51–59. [Google Scholar] [CrossRef]
- Villegas-Guzman, P.; Giannakis, S.; Rtimi, S.; Grandjean, D.; Bensimon, M.; de Alencastro, L.F.; Torres-Palma, R.; Pulgarin, C. A green solar photo-Fenton process for the elimination of bacteria and micropollutants in municipal wastewater treatment using mineral iron and natural organic acids. Appl. Catal. B Environ. 2017, 219, 538–549. [Google Scholar] [CrossRef]
- Park, S.; Yoon, T.-I. The effects of iron species and mineral particles on advanced oxidation processes for the removal of humic acids. Desalination 2007, 208, 181–191. [Google Scholar] [CrossRef]
- Sang, Y.; Liu, H.; Umar, A. Photocatalysis from UV/Vis to Near-Infrared Light: Towards Full Solar-Light Spectrum Activity. ChemCatChem 2014, 7, 559–573. [Google Scholar] [CrossRef]
- Wayner, D.D.M.; McPhee, D.J.; Griller, D. Oxidation and reduction potentials of transient free radicals. J. Am. Chem. Soc. 1988, 110, 132–137. [Google Scholar] [CrossRef]
- Legrini, O.; Oliveros, E.; Braun, A.M. Photochemical processes for water treatment. Chem. Rev. 1993, 93, 671–698. [Google Scholar] [CrossRef]
- Armstrong, D.A.; Huie, R.E.; Lymar, S.; Koppenol, W.H.; Merényi, G.; Neta, P.; Stanbury, D.M.; Steenken, S.; Wardman, P. Standard electrode potentials involving radicals in aqueous solution: Inorganic radicals. Bioinorg. React. Mech. 2013, 9, 59–61. [Google Scholar] [CrossRef] [Green Version]
- Brillas, E.; Sirés, I.; Oturan, M.A. Electro-Fenton Process and Related Electrochemical Technologies Based on Fenton’s Reaction Chemistry. Chem. Rev. 2009, 109, 6570–6631. [Google Scholar] [CrossRef]
- Cieśla, P.; Kocot, P.; Mytych, P.; Stasicka, Z. Homogeneous photocatalysis by transition metal complexes in the environment. J. Mol. Catal. A Chem. 2004, 224, 17–33. [Google Scholar] [CrossRef]
- Nagendrappa, G. An appreciation of free radical chemistry Part 4. Free radicals in atmospheric chemistry. Resonance 2005, 10, 61–72. [Google Scholar] [CrossRef]
- Stochel, G.; Brindell, M.; Macyk, W.; Stasicka, Z. Bioinorganic Photochemistry, 1st ed.; Wiley: Chichester, UK, 2009. [Google Scholar]
- Gil Lozano, C.; Davila, A.F.; Losa-Adams, E.; Fairén, A.G.; Gago-Duport, L. Quantifying Fenton reaction pathways driven by self-generated H2O2 on pyrite surfaces. Sci. Rep. 2017, 7, srep43703. [Google Scholar] [CrossRef] [PubMed]
- Evangelou, V.P.; Zhang, Y.L. A review: Pyrite oxidation mechanisms and acid mine drainage prevention. Crit. Rev. Environ. Sci. Technol. 1995, 25, 141–199. [Google Scholar] [CrossRef]
- Brown, A.D.; Jurinak, J.J. Mechanism of Pyrite Oxidation in Aqueous Mixtures. J. Environ. Qual. 1989, 18, 545–550. [Google Scholar] [CrossRef]
- Stasicka, Z. Transition metal complexes as solar photocatalysts in the environment: A short review of recent development. Adv. Inorg. Chem. 2011, 63, 291–343. [Google Scholar]
- Cieśla, P.; Mytych, P.; Kocot, P.; Stasicka, Z. Role of Iron and Chromium Complexes in Environmental Self-cleaning Processes. Sep. Sci. Technol. 2007, 42, 1651–1666. [Google Scholar] [CrossRef]
- Szaciłowski, K.; Chmura, A.; Stasicka, Z. Interplay between iron complexes, nitric oxide and sulfur ligands: Structure, (photo)reactivity and biological importance. Coord. Chem. Rev. 2005, 249, 2408–2436. [Google Scholar] [CrossRef]
- Bronswijk, J.J.B.; Nugroho, K.; Aribawa, I.B.; Groenenberg, J.E.; Ritsema, C.J. Modeling of oxygen transport and pyrite oxidation in acid sulfate soils. J. Environ. Qual. 1993, 22, 544–554. [Google Scholar] [CrossRef]
- Moses, C.O.; Herman, J.S. Pyrite oxidation at circumneutral pH. Geochim. Cosmochim. Acta 1991, 55, 471–482. [Google Scholar] [CrossRef]
- Chandra, A.P.; Gerson, A.R. Pyrite (FeS2) oxidation: A sub-micron synchrotron investigation of the initial steps. Geochim. Cosmochim. Acta 2011, 75, 6239–6254. [Google Scholar] [CrossRef]
- Borda, M.J.; Elsetinow, A.R.; Strongin, D.R.; Schoonen, M.A. A mechanism for the production of hydroxyl radical at surface defect sites on pyrite. Geochim. Cosmochim. Acta 2003, 67, 935–939. [Google Scholar] [CrossRef]
- Bossmann, S.H.; Oliveros, E.; Göb, S.; Siegwart, S.; Dahlen, E.P.; Payawan, J.L.; Straub, M.; Wörner, A.M.; Braun, A.M. New Evidence against Hydroxyl Radicals as Reactive Intermediates in the Thermal and Photochemically Enhanced Fenton Reactions. J. Phys. Chem. A 1998, 102, 5542–5550. [Google Scholar] [CrossRef]
- Lueder, U.; Jørgensen, B.B.; Kappler, A.; Schmidt, C. Fe(III) Photoreduction producing Fe-aq(2+) in oxic freshwater sediment. Environ. Sci. Technol. 2020, 54, 862–869. [Google Scholar] [CrossRef]
- Rimstidt, J.; Vaughan, D.J. Pyrite oxidation: A state-of-the-art assessment of the reaction mechanism. Geochim. Cosmochim. Acta 2003, 67, 873–880. [Google Scholar] [CrossRef]
- Xu, Y.; Schoonen, M.A. The absolute energy positions of conduction and valence bands of selected semiconducting minerals. Am. Miner. 2000, 85, 543–556. [Google Scholar] [CrossRef]
- Ye, X.; Chen, Y.; Ling, C.; Zhang, J.; Meng, S.; Fu, X.; Wang, X.; Chen, S. Chalcogenide photocatalysts for selective oxidation of aromatic alcohols to aldehydes using O2 and visible light: A case study of CdIn2S4, CdS and In2S3. Chem. Eng. J. 2018, 348, 966–977. [Google Scholar] [CrossRef]
- Raubach, C.; De Santana, Y.; Ferrer, M.; Buzolin, P.; Sambrano, J.; Longo, E. Photocatalytic activity of semiconductor sulfide heterostructures. Dalton Trans. 2013, 42, 11111–11116. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.; Yu, J.; Lin, J.; Yu, J.; Li, P. Preparation and Photocatalytic Behavior of MoS2 and WS2 Nanocluster Sensitized TiO2. Langmuir 2004, 20, 5865–5869. [Google Scholar] [CrossRef]
- Zaanen, J.; Sawatzky, G.A.; Allen, J.W. Band gaps and electronic structure of transition-metal compounds. Phys. Rev. Lett. 1985, 55, 418–421. [Google Scholar] [CrossRef]
- Chaure, N.; Bordas, S.; Samantilleke, A.P.; Chaure, S.; Haigh, J.; Dharmadasa, I. Investigation of electronic quality of chemical bath deposited cadmium sulphide layers used in thin film photovoltaic solar cells. Thin Solid Films 2003, 437, 10–17. [Google Scholar] [CrossRef]
- Bacho, I.M.; Courte, M.; Fan, H.J.; Fichou, D. Conformal Cu2S-coated Cu2O nanostructures grown by ion exchange reaction and their photoelectrochemical properties. Nanotechnology 2015, 26, 185401. [Google Scholar] [CrossRef]
- Wilhelm, T.; Berenguier, B.; Aggour, M.; Kanis, M.; Lewerenz, H.-J. Efficient CuInS2 (CIS) solar cells by photoelectrochemical conditioning. Comptes Rendus Chim. 2006, 9, 294–300. [Google Scholar] [CrossRef]
- Terakura, K.; Williams, A.R.; Oguchi, T.; Kübler, J. Transition-Metal Monoxides: Band or Mott Insulators. Phys. Rev. Lett. 1984, 52, 1830–1833. [Google Scholar] [CrossRef]
- Yakubovich, O.; Khasanova, N.; Antipov, E. Mineral-inspired materials: Synthetic phosphate analogues for battery applications. Minerals 2020, 10, 524. [Google Scholar] [CrossRef]
- Yepsen, O.; Yáñez, J.; Mansilla, H.D. Photocorrosion of copper sulfides: Toward a solar mining industry. Sol. Energy 2018, 171, 106–111. [Google Scholar] [CrossRef]
- Zawadzki, P.; Baranowski, L.L.; Peng, H.; Toberer, E.S.; Ginley, D.S.; Tumas, W.; Zakutayev, A.; Lany, S. Evaluation of photovoltaic materials within the Cu-Sn-S family. Appl. Phys. Lett. 2013, 103, 253902. [Google Scholar] [CrossRef]
- Costa, M.B.; Medina, M.; Andrade, M.A.S., Jr.; Coelho, D.; Mascaro, L.H. Transition metal chalcogenides for photoelectrochemical water splitting. Mater. Res. Found. 2020, 71, 1–42. [Google Scholar]
- Awatani, T.; McQuillan, A.J. Adsorbed Thiosulfate Intermediate of Cadmium Sulfide Aqueous Photocorrosion Detected and Characterized by in Situ Infrared Spectroscopy. J. Phys. Chem. B 1998, 102, 4110–4113. [Google Scholar] [CrossRef]
- Meissner, D.; Memming, R.; Kastening, B. Photoelectrochemistry of cadmium sulfide. 1. Reanalysis of photocorrosion and flat-band potential. J. Phys. Chem. 1988, 92, 3476–3483. [Google Scholar] [CrossRef]
- Fermín, D.; Ponomarev, E.; Peter, L. A kinetic study of CdS photocorrosion by intensity modulated photocurrent and photoelectrochemical impedance spectroscopy. J. Electroanal. Chem. 1999, 473, 192–203. [Google Scholar] [CrossRef]
- Ma, X.; Li, H.; Liu, T.; Du, S.; Qiang, Q.; Wang, Y.; Yin, S.; Sato, T. Comparison of photocatalytic reaction-induced selective corrosion with photocorrosion: Impact on morphology and stability of Ag-ZnO. Appl. Catal. B Environ. 2017, 201, 348–358. [Google Scholar] [CrossRef] [Green Version]
- Devi, L.G.; Raju, K.A.; Kumar, S.G.; Rajashekhar, K.E. Photo-degradation of di azo dye Bismarck Brown by advanced photo-Fenton process: Influence of inorganic anions and evaluation of recycling efficiency of iron powder. J. Taiwan Inst. Chem. Eng. 2011, 42, 341–349. [Google Scholar] [CrossRef] [Green Version]
- Duan, L.; Jiang, N.; Lu, N.; Shang, K.; Li, J.; Wu, Y. Synergetic effect of TiO2 and Fe3+ as co-catalysts for enhanced phenol degradation in pulsed discharge system. Appl. Catal. B Environ. 2018, 221, 521–529. [Google Scholar] [CrossRef]
- Mahmoud, A.; Cézac, P.; Hoadley, A.; Contamine, F.; D’Hugues, P. A review of sulfide minerals microbially assisted leaching in stirred tank reactors. Int. Biodeterior. Biodegrad. 2017, 119, 118–146. [Google Scholar] [CrossRef]
- Dreisinger, D. Copper leaching from primary sulfides: Options for biological and chemical extraction of copper. Hydrometallurgy 2006, 83, 10–20. [Google Scholar] [CrossRef]
- Hyvarinen, I.; Hamalainen, M. HydroCopper((TM))—A new technology producing copper directly from concentrate. Hydrometallurgy 2005, 77, 61–65. [Google Scholar] [CrossRef]
- Yazici, E.; Yilmaz, E.; Ahlatci, F.; Celep, O.; Deveci, H. Recovery of silver from cyanide leach solutions by precipitation using Trimercapto-s-triazine (TMT). Hydrometallurgy 2017, 174, 175–183. [Google Scholar] [CrossRef]
- Zhou, S.; Gan, M.; Zhu, J.; Li, Q.; Jie, S.; Yang, B.; Liu, X. Catalytic effect of light illumination on bioleaching of chalcopyrite. Bioresour. Technol. 2015, 182, 345–352. [Google Scholar] [CrossRef]
- Schoonen, M.A.; Harrington, A.D.; Laffers, R.; Strongin, D.R. Role of hydrogen peroxide and hydroxyl radical in pyrite oxidation by molecular oxygen. Geochim. Cosmochim. Acta 2010, 74, 4971–4987. [Google Scholar] [CrossRef]
- Cohn, C.A.; Pak, A.; Strongin, D.; Schoonen, M.A. Quantifying hydrogen peroxide in iron-containing solutions using leuco crystal violet. Geochem. Trans. 2005, 6, 47. [Google Scholar] [CrossRef]
- Chandra, A.; Gerson, A. The mechanisms of pyrite oxidation and leaching: A fundamental perspective. Surf. Sci. Rep. 2010, 65, 293–315. [Google Scholar] [CrossRef]
- Ameta, S.; Ameta, R. Advanced oxidation processes for wastewater treatment. In Emerging Green Chemical Technology, 1st ed.; Academic Press: London, UK, 2018. [Google Scholar]
- Wiegand, H.L.; Orths, C.T.; Kerpen, K.; Lutze, H.V.; Schmidt, T.C. Investigation of the iron-peroxo complex in the Fenton reaction: Kinetic indication, decay kinetics and hydroxyl radical yields. Environ. Sci. Technol. 2017, 51, 14321–14329. [Google Scholar] [CrossRef] [PubMed]
- Voelker, B.M.; Morel, F.M.M.; Sulzberger, B. Iron Redox Cycling in Surface Waters: Effects of Humic Substances and Light. Environ. Sci. Technol. 1997, 31, 1004–1011. [Google Scholar] [CrossRef] [Green Version]
- Ruppert, G.; Bauer, R. UV-O3, UV-H2O2, UV-TiO2 and the photo-Fenton reaction comparison of advanced oxidation processes for wastewater treatment. Chemosphere 1994, 28, 1447–1454. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Ntampegliotis, K.; Riga, A.; Karayannis, V.; Bontozoglou, V.; Papapolymerou, G. Decolorization kinetics of Procion H-exl dyes from textile dyeing using Fenton-like reactions. J. Hazard. Mater. 2006, 136, 75–84. [Google Scholar] [CrossRef]
- Ghiselli, G.; Jardim, W.F.; Litter, M.I.; Mansilla, H.D. Destruction of EDTA using Fenton and photo-Fenton-like reactions under UV-A irradiation. J. Photochem. Photobiol. A Chem. 2004, 167, 59–67. [Google Scholar] [CrossRef]
- Nichela, D.A.; Berkovic, A.M.; Costante, M.R.; Juliarena, M.P.; Einschlag, F.S.G. Nitrobenzene degradation in Fenton-like systems using Cu(II) as catalyst. Comparison between Cu(II)- and Fe(III)-based systems. Chem. Eng. J. 2013, 228, 1148–1157. [Google Scholar] [CrossRef]
- Droguett, C.; Salazar, R.; Brillas, E.; Sirés, I.; Carlesi, C.; Marco, J.F.; Thiam, A. Treatment of antibiotic cephalexin by heterogeneous electrochemical Fenton-based processes using chalcopyrite as sustainable catalyst. Sci. Total. Environ. 2020, 740, 140154. [Google Scholar] [CrossRef]
- Ltaïef, A.H.; Pastrana-Martínez, L.M.; Ammar, S.; Gadri, A.; Faria, J.; Silva, A. Mined pyrite and chalcopyrite as catalysts for spontaneous acidic pH adjustment in Fenton and LED photo-Fenton-like processes. J. Chem. Technol. Biotechnol. 2017, 93, 1137–1146. [Google Scholar] [CrossRef]
- Meissner, D.; Benndorf, C.; Memming, R. Photocorrosion of cadmium sulfide: Analysis by photoelectron spectroscopy. Appl. Surf. Sci. 1987, 27, 423–436. [Google Scholar] [CrossRef]
- Gerischer, H. Photodecomposition of semiconductors thermodynamics, kinetics and application to solar cells. Faraday Discuss. Chem. Soc. 1980, 70, 137–151. [Google Scholar] [CrossRef]
- Davenport, W.G. Encyclopedia of Materials: Science and Technology, 2nd ed.; Pergamon: Oxford, UK, 2001. [Google Scholar]
- Harmer, S.; Thomas, J.E.; Fornasiero, D.; Gerson, A.R. The evolution of surface layers formed during chalcopyrite leaching. Geochim. Cosmochim. Acta 2006, 70, 4392–4402. [Google Scholar] [CrossRef]
- Parker, A.J.; Paul, R.L.; Power, G.P. Electrochemistry of the oxidative leaching of copper from chalcopyrite. J. Electroanal. Chem. Interfacial Electrochem. 1981, 118, 305–316. [Google Scholar] [CrossRef]
- Parker, A.; Klauber, C.; Kougianos, A.; Watling, H.; van Bronswijk, W. An X-ray photoelectron spectroscopy study of the mechanism of oxidative dissolution of chalcopyrite. Hydrometallurgy 2003, 71, 265–276. [Google Scholar] [CrossRef]
- Liang, D.X.; Li, J.; Li, L.; Pang, G.S. Fenton Degradation of Methylene Blue by CuFeS2 Ultrafine Powders. Key Eng. Mater. 2014, 609–610, 449–454. [Google Scholar] [CrossRef]
- Yang, B.; Lin, M.; Fang, J.; Zhang, R.; Luo, W.; Wang, X.; Liao, R.; Wu, B.; Wang, J.; Gan, M.; et al. Combined effects of jarosite and visible light on chalcopyrite dissolution mediated by Acidithiobacillus ferrooxidans. Sci. Total Environ. 2020, 698, 134175. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.P.; Roccamante, M.; Amorim, C.C.; Oller, I.; Pérez, J.A.S.; Malato, S. New trend on open solar photoreactors to treat micropollutants by photo-Fenton at circumneutral pH: Increasing optical pathway. Chem. Eng. J. 2020, 385, 123982. [Google Scholar] [CrossRef]
- Ruiz-Delgado, A.; Plaza-Bolaños, P.; Oller, I.; Malato, S.; Aguera, A. Advanced evaluation of landfill leachate treatments by low and high-resolution mass spectrometry focusing on micro contaminant removal. J. Hazard. Mater. 2019, 384, 121372. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).