Abstract
A rare crucible with an unusually large volume and a pot-shaped bottom was excavated at the Tiversk hillfort (late 13–14th century—1411 AD) in the North-Western Ladoga region (Russia). ICP-MS data showed that the crucible might be attributed to local technical ceramics. Because of its specific volume and shape, which are not typical for crucibles used in non-ferrous metallurgy in medieval Karelia, earlier it had been attributed to the technical ceramics used for the cementation of iron. The present research has revealed tin bronze metal alloy along with copper sulfide minerals recorded on the crucible walls, suggesting it might have been used in non-ferrous metal working. Thermal treatment of the crucible at temperatures above 1050 °C is evidenced by the heterogeneous composition of quartz, the thermal breakdown of biotite, recorded in the temper of the ceramic fabric, and Raman spectra characteristics of hematite.
1. Introduction
Methods of natural sciences have been applied to study archeological ceramics at least since the middle of the 20th century [1] (pp. 138–181). The development of analytical techniques and the growth of scientific interest in multidisciplinary investigations greatly contributed to the understanding of pottery technology of the past. Mineralogical analyses have become a standard routine for archeometric studies in deciphering the chemical and mineral composition of fabric in order to promote deep insight in provenance analysis as well as in different stages of the pottery production cycle, such as fabric preparation and thermal treatment [2,3,4,5,6].
The technical ceramics used in ancient metal working have received increasing attention in archeological research over the past several decades [7,8,9,10,11,12,13,14,15]. The investigation of metallurgical ceramics has led to numerous studies devoted to ancient metallurgy, usually concerning the analyses of final products, manufacturing techniques, or technical equipment and provenance studies. Metallurgical ceramics studies based on a mineralogical approach generally focus on the examination of crucible manufacturing technology and a reconstruction of the metallurgical procedure [8,10,16].
Mineralogical methods have already been applied to study Karelian medieval pottery from the hillforts located on the north-western shore of Lake Ladoga [17,18,19,20,21]. During the Middle Ages (10–15th century) the territory of the North-Western Ladoga area and the Karelian Isthmus were occupied by the Karelian people, whose material culture has a long history of archeological research and has previously been described [22,23,24,25,26,27,28]. The development of the Karelian people refers to the turn of I–II millennia AD, when archeological evidence showing Karelian material culture elements started to mark the territory, which further in the 13th century would turn into the “Land of Korela” of written sources [24] (pp. 35–36), [27] (pp. 75–79), [29] (pp. 323,327), [30] (p. 122), [31] (p. 54). The Land of Korela (Ethnonym korela, marking the Karelian people, first emerged in the text of a birch-bark manuscript of the late 11th century [32] (pp. 50–51), [33] (p. 41)) was incorporated into Novgorodian Rus and situated on its north-western border, thereby it was the first to meet foreign attack, which usually came from Swedish neighbors, and to defend the internal lands with the capital of Rus–Novgorod. The middle of the 12th century saw the start of a period of constant Russian–Swedish military conflicts, which is why the 12th century was the time when the Karelian people, sometimes with the help of the Novgorodians (as in the case of the Korela fortress (Korela or “Korel’skiy gorodok” was the political, administrative, trade, and manufacturing center of the Land of Korela [27] (pp. 220–234), [31])), began the building of fortresses on the Ladoga shore to strengthen the state border. Therefore, Karelian hillforts along with the chain of fortresses such as Ladoga, Oreshek, and Koporje made up “the stone shield” of Rus [30] (p. 3).
The 12–14th century is considered the age when Karelian culture flourished which is reflected in rich archeological materials [24] (p.37), [27] (p. 80), [31]. Fortresses hedged with stone walls and earth ramparts were usually placed at the high rocky shores of Lake Ladoga (Figure 1a), providing the possibility to see over the vast water areas to warn of the appearance of enemies. These hillforts remain the only well-studied type of settlement for the period of the 10–15th century; the other sites are presented by burial grounds, stray findings, and scarce objects of cult. Excavations of the hillforts (Figure 1a:1,3–7) have supplied collections of artifacts that provide evidence for various crafts and economic activities. The ceramic collection counts over 4400 fragments of pottery, including the sherds of crucibles (49 items) [25] (pp. 47–48,119). The most abundant ceramic material (2895 pcs) was obtained during the archeological survey of the Tiversk hillfort (Figure 1b) [25] (pp. 34–35).
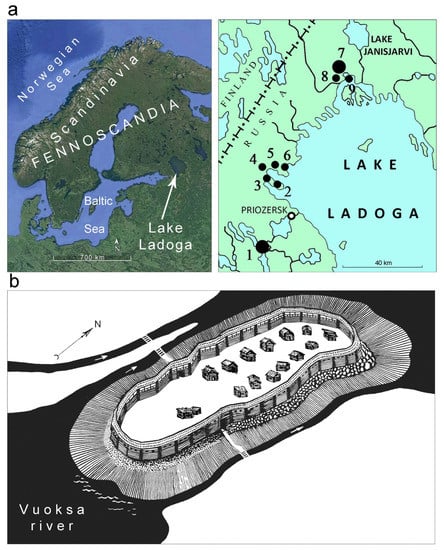
Figure 1.
(a) Maps showing Lake Ladoga and the medieval Karelian hillforts: 1—Tiversk; 2—Rantalinnamyaki; 3—Khyameenlakhti-Linnavuori; 4—Lopotti-Linnamyaki; 5—Soskua-Linnamyaki; 6—Tervu-Linnasaari; 7—Paaso; 8—Rautalakhti-Linnavuori; 9—Tokkarlakhti-Linnasaari. (b) Graphic reconstruction of the Tiversk hillfort by S.I. Kochkurkina and V.A. Bazegskiy [37] (pp. 18–19). The larger markers (a) stand for sites where more than 1000 m2 was excavated; the smaller ones stand for excavations less than 1000 m2. The bicolor mark of modern Priozersk points at the place of the Korela fortress location.
The Tiversk hillfort occupied the former island (now it is a part of the land due to the conducted dewatering of the territories) on the Vuoksa river. The first archeological research of the fortress was undertaken in the late 19th century by O.H. Appelgren [22] (pp. 98–106) and P.T. Schwindt [23] (pp. 85–90,93), and, as a result, a topographical plan of the site and a description of the fortifications were prepared; spot excavations in various parts of the fortress revealed stone foundations of dwellings. In 1891, P.T. Schwindt continued the research together with A. Hackman, studying the fortifications—walls and ramparts—of Tiversk, and in 1924 carrying on the survey with J. Rinne [34] (p. 70). Later the Tiversk hillfort was visited by archeologists mainly for exploratory purposes [26] (pp. 297–300), and the excavations were reorganized only in the 1970s. In 1971, A.N. Kirpichnikov examined the site with trial trenches (80 m2 in sum) [35]. The same year, the first large-scale investigations were started by S.I. Kochkurkina: during 1971–1974 the Tiversk fortress was excavated with 11 sites, together compiling 1620 m2 of its total square of 1 ha [25] (pp. 33–52), [36] (pp. 30–62). The survey shed light on the fortification, dwelling, and industrial structures of the hillfort, as well as the occupation and daily activities of its population. The hillfort was fortified with earth ramparts in its southern part and stone walls in its northern part; the living space was located mainly in the southern part—here were excavated 14 checkered foundations of houses, which created an additional line of defense [25] (pp. 40–47). The assemblage of artifacts points at agriculture, fishery, and cattle-breeding employment of Tiversk dwellers as well as their involvement in various crafts such as ferrous and non-ferrous metal working, pottery and textile production, and processing of wood, bone, and stone [24] (pp. 76–135). The collection dates to the late 13th and 14th century, defining the active period of the functioning of the fortress [25] (p. 51). Tiversk was burned and ruined during the Swedish attack in 1411 [29] (p. 402).
The rare crucible was at once distinguished among the crucibles from other Karelian hillforts due to its significantly large volume, thick walls, and unusual shape (Figure 2a). The crucible was found in the southern part of the Tiversk hillfort at excavation site I (5 m × 8 m), located close to the southern earth rampart. Almost the whole area of the site was covered with boulders and small stones, which formed a ruinated rectangular construction. Presumably, the perimeter of an undefined building foundation was discovered: for this suggestion stand the gathered pieces of clay cement, traditionally used in Karelian house building, found nearby. Sherds of the crucible were shattered within the described construction close to its south-eastern corner. The square of excavation site I was extended further with site IV (212 m2), mainly to the north and to the south, providing also a small increase in the excavated area in the eastern and western directions. No more constructions were revealed in the area of sites I and IV; a pit (2.6 m × 1.8 m and 0.4 m deep) with traces of ash and small pieces of charcoal was recorded 4 m to the south of the undefined foundation [25] (pp. 35–37).
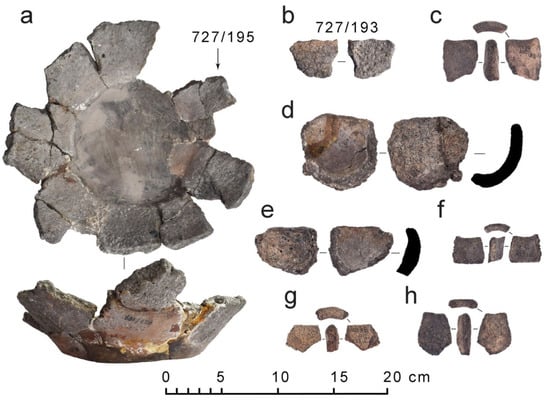
Figure 2.
Crucibles from the Tiversk hillfort: (a) the rare crucible of large volume (plan and profile view); (b) separated sherd of the same crucible; (c–h) fragments of common crucibles for non-ferrous metal working.
In contrast to thorough archeometric research of medieval Karelian kitchenware [17,18,19,20,21], the technical ceramics remain overlooked by archeologists, being only mentioned [27] (pp. 105,198–199,231,247,274–275) or preliminarily counted for pieces that could be attributed to crucibles [25] (pp. 47–48,119). Brief analysis has shown that most crucibles from Karelian hillforts are characterized by a small volume (circa max. 100 mL), round-bottom shape, occasionally the presence of glassy slags on the surface (Figure 2c–h), and their wall thickness parameter more frequently lies in the range of 0.8–1 cm. Some of the crucibles carry the imprints on the outer surfaces left by the tools used for manipulating them in the fire. These specific features suppose that they were destined for non-ferrous metal working. The evidence of non-ferrous metal working in medieval Karelia comes from the occurrence of industrial complexes, discovered at the settlements, and non-ferrous metal-working equipment (crucibles, molds, tools), which were even recorded in the graves [23] (pp. 32–34), [24] (pp. 80–81), [27] (pp. 104,301).
The crucible was attributed to the technical ceramics, as it is characterized by the signs of intensive thermal treatment including vitrification, bloating of the ceramic fabric, and numerous cracks (Figure 2a,b). However, based on the reconstruction of available remains of the crucible, its volume was estimated to be not less than 500 mL, which is notably larger than the typical volume of most crucibles (see Figure 2). Moreover, the rare crucible has a pot-shaped bottom with a flat base, which is rather typical of pot crucibles used for ferrous than for non-ferrous metal working. A previous study [24] (p. 127) based on visual analyses of the shape and the surface treatment of the vessel hypothesized that it was used for iron cementation in the steel production cycle. The procedure of steel production implied the usage of a pot-shaped vessel, filled with carbonizing agent (charcoal), sand, which must have been placed in the bottom as a pad, and pieces of iron or iron tools that should be cemented. The filled vessel was exposed to heating at the temperature of no less than 900 °C [38] (p. 52) so that the carbon from the charcoal diffused into the metal. The prolongation of the whole process could be from a few hours to days. Such findings of whole pots or their bottom sections filled with a melted mass of sand and metal slag were recorded in medieval Novgorod [39] (p. 14), as well as such technology of iron cementation itself [40] (p. 152).
During the excavations of the Tiversk hillfort, where the large crucible was found, a few industrial complexes were recognized [25] (p. 47), [27] (p. 200), [36] (p. 56). In the northern part of the settlement, rectangular cameras (roughly 2 m × 4 m in area) constructed of stone and built in the defensive wall were discovered, whereas a separated complex located between the foundations of dwellings—rectangular stone-built construction (9 × 4 m), presumably a forge—was opened in the central part of Tiversk. Various findings associated with metal working were recorded in these industrial complexes [25] (p. 41–42), [35], including numerous ceramic sherds and metal slags, copper ingots, iron tools, pieces of melted sand, and fragments of clay coating, which might be attributed to remains of fireplaces or furnaces built of stones and clay. Slags containing iron remains as well as slags with copper remains were recognized at the Tiversk hillfort, suggesting that both iron and non-ferrous metal production took place in the settlement.
The aim of the study is to decipher the functional usage of the rare crucible with an unusual large volume and a pot shape excavated at the Tiversk hillfort. To specify the use of the crucible for ferrous or non-ferrous metal working, the composition of metal alloy remains was studied. Moreover, we present the data on the chemical and mineral composition of the crucible fabric in order to make the provenance analysis and reconstruct the thermal treatment conditions. The obtained results provide deeper insight into the organization and technologies of ancient crafts.
2. Materials and Methods
The objects of the present case study were broken bottom fragments of a crucible with the overall traces of intensive thermal treatment (Figure 2a). The bottom of the crucible was reconstructed with gypsum so that the preserved part included 15 near-bottom fragments and 4 separated sherds belonging to the same vessel (Figure 2b). The reconstruction shows that the upper diameter of the crucible equals 23 cm, the bottom diameter is 14 cm, and the height of the preserved walls is up to 7 cm. The rim part is absent. The wall thickness varies from 1 cm to 1.3 cm.
The studied samples were taken directly from the wall fragment of the vessel (see Figure 2a, 727/195) and the separated sherd (see Figure 2b, 727/193). Three polished sections obtained by cross-section cut of the crucible fragments were studied by scanning electron microscopy with energy dispersive spectroscopy (SEM-EDS) and Raman spectroscopy methods. To examine the outer and inner surfaces of the crucible, four unpolished sections were cut along the crucible walls. For inductively coupled plasma mass spectrometry and X-ray powder diffraction analyses, the powder samples were prepared from the outer, inner, and central parts of the crucible, which were taken by cutting the crucible fragments along the walls.
Scanning electron microscopy with energy dispersive spectroscopy (SEM-EDS) was applied to determine the chemical and mineral composition of the fabric components and to characterize the specimen texture. The SEM-EDS study was carried out on the polished and unpolished sections with scanning electron microscope VEGA II LSH (Teskan, Brno, Czech Republic) and energy dispersive microanalyzer INCA Energy 350 (Oxford Instruments, Oxford, UK). The analyses were performed under the following analytical conditions: W cathode, 20 kV accelerating voltage, 20 nA beam current, 2 μm beam diameter, and a counting time of 90 s. The following standards were used: albite, MgO, Al2O3, SiO2, FeS2, orthoclase, wollastonite, Ti, Cr, Mn, Fe, Co, Ni, Cu, Zn, and Sn. SEM-EDS quantitative data and determination of the analysis accuracy were acquired and processed using the Microanalysis Suite Issue 12, INCA Suite version 4.07 (v.4.07, Oxford Instruments Analytical Limited, High Wycombe, UK); standard deviation (S) for Cu—0.5–0.8%, Sn—0.4–0.6%, Fe—0.2–0.4%, Zn—0.4–0.7%, and S—0.2–1.6%. The average composition of the clay matrix was determined by area analysis of the most homogeneous zones with the size of 100 μm × 100 μm to 300 μm ×300 μm, avoiding the temper-rich and the strongly altered areas. The mineral composition of the artificial additives was identified by the point SEM analysis.
Raman spectroscopy analysis was carried out using a dispersive Nicolet Almega XR Raman spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) with a green laser (532 nm, Nd-YAG (Thermo Fisher Scientific, Waltham, MA, USA). The spectra were collected at 2 cm−1 spectral resolution. A confocal microscope with a 50× objective was used to focus an excitation laser beam on the sample and to collect a Raman signal from a 2 μm diameter area. Raman spectra were acquired in the 85–4000 cm−1 spectral region, with the exposition time between 30 s and 100 s for each scan, depending on the signal intensity and laser power of 2–10 mW to prevent any sample degradation. A total of 40 Raman spectra were acquired from polished cross-sections of the crucible fragments. The Raman spectra were background corrected and fit with Lorentzian curves using OMNIC software (v.8.2, Thermo Fisher Scientific, Waltham, MA, USA) in order to obtain the quantitative characteristics of the Raman spectra, including the position, intensity, and full width at half maximum of the bands.
Inductively coupled plasma mass spectrometry (ICP-MS) was used to determine the content of trace and rare-earth elements in order to provide the provenance studies. The ICP-MS analysis was carried out using the Thermo Scientific X-SERIES-2 quadrupole mass-spectrometer (Thermo Fisher Scientific, Bremen, Germany) following the standard procedure [41] at the Centre for Collective Usage, Karelian Research Centre, RAS (Petrozavodsk, Russia). Five powder samples obtained from the outer and inner edges and central parts of the crucible fragments were decomposed using acid decomposition in an open system. The analysis was controlled by measurement of USGS standard BHVO-2 (Table S1).
The X-ray powder diffraction (XRD) method was applied to study the bulk mineral composition of various parts of the crucible fragments using the Thermo Scientific ARL X’TRA X-ray diffractometer (Thermo Fisher Scientific, Ecublens, Switzerland) employing Co Kα radiation (λ = 0.1790210 nm) at 35 kV and 35 mA. Samples were scanned from 2° to 90° 2θ, with a step size of 0.02° 2θ and a scan rate of 0.6° 2θ/min. The quantitative mineral composition of 5 samples was determined by the modeling of experimental diffraction curves using Siroquant software (v.3.0, Sietronics, Mitchell, Canberra, Australia).
3. Results
3.1. Ceramic Fabric
X-ray diffraction analysis indicated that the crucible was predominantly composed (in a modal percentage) of 32–39% quartz, 32–39% K-feldspar (orthoclase and microcline), and 14–26% albite (Table S2). The mica-like phase (muscovite) was recorded for the central part of the crucible and is considered to be the result of the transformation of clays due to the thermal treatment [42]. Additionally, dolomite was identified in the crucible edge areas, which is thought to be the result of contamination from the soil environment.
Microscopic investigation of the crucible cross-sections revealed ceramic fabric composed of a clay matrix and coarse rock fragments (Figure 3a). The clay matrix is more vitrified and vesicular at the edges of the crucible, especially at the inner surface, but more massive on the inside (Figure 3b–d). The boundaries between the edges and the central zone of the crucible are mostly gradual.
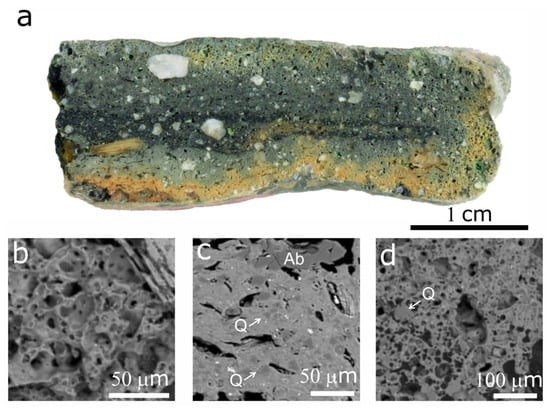
Figure 3.
Microscopic image of the crucible cross-cut section (a) and BSE images of the clay matrix from the inner edge (b), central zone (c), and outer edge (d) of the crucible. Ab—albite; Q—quartz.
EDS area analyses of the fabric showed the crucible was made of non-calcareous (concentration of CaO 3%) clays, predominantly of illite–montmorillonite with Al2O3 content varying from 15 to 26 wt.%, K2O content varying from 2 to 8 wt.%, and Na2O content varying from 1 to 3 wt.% (Table S3). No distinct differences in major oxide composition were recorded for the near-surface and central zones of the crucible walls, with the only exception of Fe2O3 total content, which increased toward the edges (Figure 4). Additionally, the outer edge is enriched in P2O5 (1.5–3 wt.%) and SO2 (3.2–5.6 wt.%). ICP-MS analysis showed that the Cu and Sn content increased from the outer to the inner edges from 34 ppm to 137 ppm and from 6 ppm to 36 ppm, respectively (Table S4). It is noteworthy that the Ba content in the inner edge is approximately twice higher (1155 ppm) than in the central zone (759 ppm) and the outer edge (678 ppm).
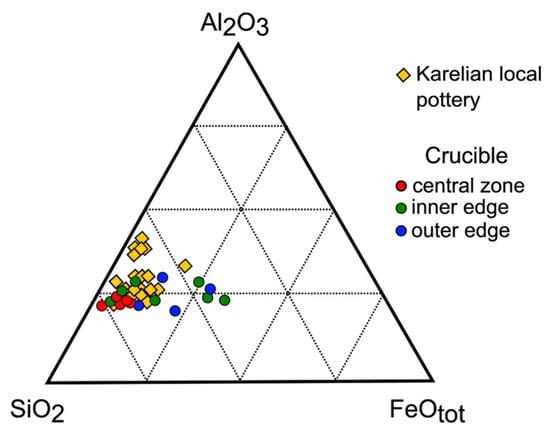
Figure 4.
SiO2–Al2O3–FeO ternary diagram demonstrating the clay matrix composition of the crucible and Karelian local pottery.
SEM-EDS analysis showed that the clay matrix contains silt grains (10–30 µm) presented dominantly by quartz with minor amounts of albite and K-feldspar and heavy minerals (zircon, titanite, apatite, monazite, ilmenite, and titanium oxide). The temper is presented by coarse grains ranging in size from 0.1 mm to 5 mm composed of quartz, albite, K-feldspar, and mica with the accessory minerals of epidote, apatite, monazite, and zircon. The mineral composition of the rock fragments is assumed to correspond to the felsic and intermediate igneous rocks.
3.2. Mineral Chemistry and Structural Characteristics
In order to specify the high-temperature treatment and reconstruct the heating conditions, the minerals, which can provide information about temperature treatment and atmosphere, were studied in detail.
Quartz was presented in the crucible fabric as variously sized fragments, ranging from 10 to 20 µm in the ceramic matrix and up to 1 mm in the temper. Quartz clasts in the clay matrix mostly showed a gradual boundary with the surrounding environment, indicating that they were partially molten (Figure 3c).
Coarse quartz clasts occurred as the temper appears to be internally cracked and occasionally shows dissolution interfaces with the surrounding ceramic paste. Some of the clasts recorded on the outer edges of the crucible are characterized by heterogeneous compositions. The composition of heterogeneity produced patchy and concentric zoning patterns. Figure 5 shows that the concentric zoning is defined by a pure quartz core in the central part of the clast, which is surrounded by a mantle presented by quartz incorporating about 5% of a mixture of CaO (2%), FeO (2%), and Al2O3 (1%) (Table S5). In the outer rim of the clast, the concentrations of the admixtures increased, and the SiO2 content decreased to 60 wt.%. Raman spectroscopy analysis showed the occurrence of quartz in all parts of the zoning clast, though the intensity of the quartz band 465 cm−1 decreased and its full width at half maximum increased from the core to the rims.
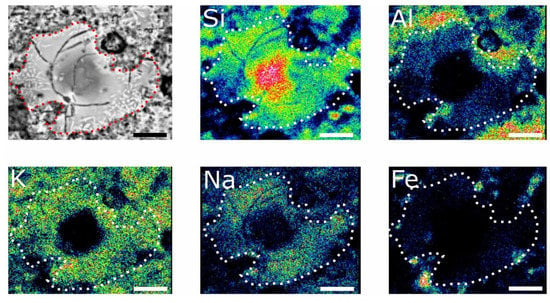
Figure 5.
BSE image of altered quartz clast from the outer edge of the crucible (top left) and element distribution maps. Scale bar corresponds to 40 µm. Brightness of each map is normalized to its maximum.
Mica-like grains compose the temper of the crucible and occur as single grains or intergrowths with quartz or feldspars. Depending on the grain size and location in the crucible body, the mica grains show various degrees of thermal transformation during heating. Large grains with the size of 500–900 µm from the central zone of the crucible are characterized by exfoliation and enlargement of the cleavage planes (Figure 6a), whereas the smaller mica grains (<200 µm) from the crucible edges show the signs of partial melting (Figure 6b), namely the melted boundaries and bubbles developed due to the OH- release from the mica structure. Additionally, microcline lamellas were recorded between the cleavage planes by Raman spectroscopy analysis (Figure 6c).
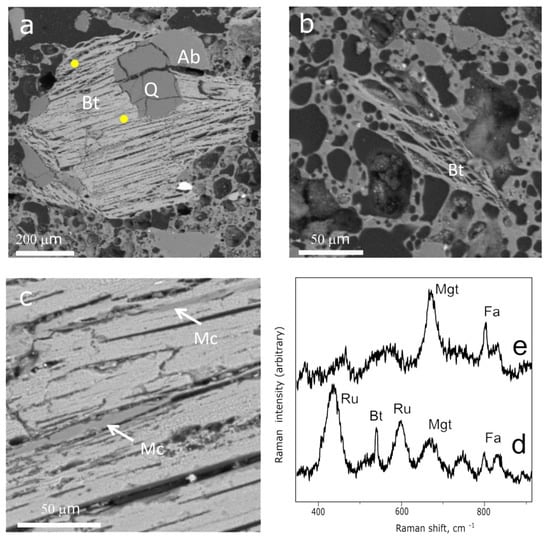
Figure 6.
BSE image of biotite from the central zone (a) and the outer edge; (b) of the crucible; SEM image of microcline lamella between the cleavage planes of biotite shown in (a); (c); Raman spectra obtained from the central zone; (d) and margin; (e) of biotite shown in (a). The yellow dots mark the point Raman analysis. Abbreviations: Ab—albite; Bt—biotite; Fa—fayalite; Mc—microcline; Mgt—magnetite; Q—quartz; Ru—rutile.
The composition of most of the mica grains recorded in the crucible is characterized by the low K2O (1–4 wt.%) and SiO2 (26–32 wt.%) content, which decreased from the center to the margins of the grains (Table S5). The low K2O and SiO2 content indicate that the composition of mica is notably changed due to the thermal treatment. Only a few SEM-EDS analyses obtained from the center of the large mica grains with high K2O content of about 8 wt.% might be attributed to biotite. The relics of biotite (annite–phlogopite) were also identified by Raman spectroscopy analysis according to the characteristic band at 556 cm−1 [43]. The band at 556 cm−1 attributed to biotite was recorded in the central part of the large mica grain together with rutile identified by the characteristic bands located at 440 and 609 cm−1, magnetite identified by the band at 676 cm−1, and fayalite identified by characteristic bands located at 815 and 838 cm−1 (Figure 6d). The Raman spectra obtained from the margins of the biotite grains as well as from the whole, relatively small biotite grains (Figure 6b) from the crucible edges are presented only by magnetite and fayalite (Figure 6e). Moreover, fayalite inclusions were also identified at the margins of the mica grains. Thus, the mineral assemblage pseudomorphing the biotite is presented by rutile, magnetite, fayalite, and microcline.
Raman spectroscopy was used to identify the iron oxide minerals, which provide information about the firing conditions. Among the iron oxides, hematite and magnetite were recorded in the inner and outer edges of the crucible. Hematite appeared as reddish irregular grains with the size circa 5–25 µm dispersed in the ceramic paste (Figure 7a) or a thin film on the mineral surface. The Raman spectra of hematite from the crucible fabric are presented by the characteristic bands at 407, 514, 607, and 665 cm−1 (Figure 7e). The intensity ratio (680/620) is used as a measure of the relative temperature of the hematite formation [44,45,46]. The intensity ratio (670/610) of the hematite from the crucible varies from 2.5 to 3. Additionally, magnetite, which occurred in the ceramic paste as rounded grains with a size of up to 250 µm, was identified by characteristic Raman bands at 547 and 676 cm−1 (Figure 7b,f).
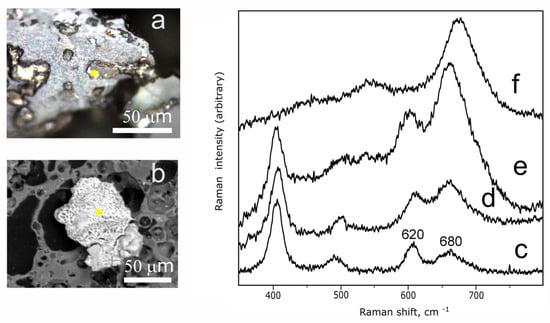
Figure 7.
BSE image of hematite (a) and magnetite (b); Raman spectra of hematite from local Karelian pottery fired at temperatures below 900 °C; (c) and above 1000 °C; (d) [21] and hematite shown in (a); (e) and magnetite shown in (b); (f) from the crucible fabric. The yellow dots mark the point Raman analysis.
3.3. Metal Alloys and Sulfides
Table 1 summarizes the metal alloys, metal sulfides, and oxides recorded in the crucible. The SEM-EDS study revealed 89 metal prills in the crucible fabric of the inner and outer surfaces, and only a few prills occurred further away from the edges. The size of most of the metal prills ranged from 2 to 20 µm, though single large prills with a size of up to 75 µm were also recorded (Figure 8a–d).

Table 1.
Composition of metal phases recorded in the crucible by SEM-EDS and Raman spectroscopy analyses.
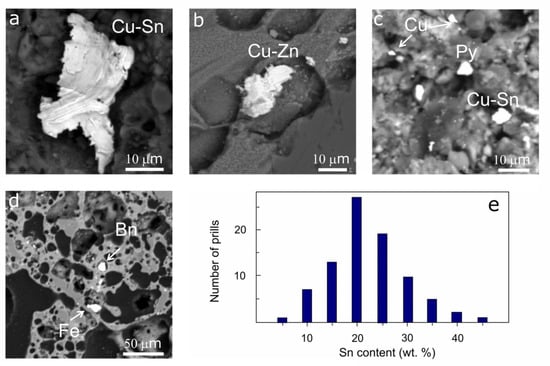
Figure 8.
BSE images of metal alloy prills (a,b) and metal sulfide minerals (c,d) recorded in the crucible. Histogram (e) shows the prill frequency over bronze alloy compositions. Abbreviations: Cu-Sn—binary tin bronze; Cu-Zn—brass; Cu—copper; Fe—iron; Bn—bornite; Py—pyrite.
The composition of most of the metal prills corresponds to the Cu-Sn alloys, with the Sn content varying from 8 to 44 wt.% and the most abundant composition being about 20 wt.% (Figure 8e) (Table S6). The Fe content in the bronze prills varies from 0 to 5 wt.%, but in single prills it increases up to 11 wt.%. Additionally, two prills are presented by Cu-Zn alloys with the Cu/Zn = 1.5, probably corresponding to brass (Figure 8b). It is noteworthy that one of the prills is characterized by a high Ni content (20 wt.%). Moreover, a few pure iron (5) and copper (2) prills were recognized in the ceramic paste of the crucible (Figure 8c,d).
Along with metal prills, sulfide minerals were recorded in the ceramic fabric of the inner and outer walls of the crucible, including bornite Cu5FeS4, covellite CuS, and sphalerite ZnS. Additionally, intermediate sulfide phases with the composition of FeS1.2–1.6 and Cu2FeS were recognized in the inner edge of the crucible. As the composition of these intermediate phases show a lack of sulfur they might be assumed to be the products of the oxidation of sulfide minerals. Single grains of cassiterite and chromite were recognized on the outer surface of the crucible. It is noteworthy that a significant amount of barite and ilmenite were recorded in the ceramic fabric of the inner and outer surfaces of the crucible.
4. Discussion
The data obtained by the complex analysis of the crucible fabric mineralogy allow the discussion of the crucible’s technological peculiarities in further detail, speculating about the functional determination and thermal treatment parameters of the crucible, its provenance origin, and the characteristics of the metal residues recorded on the surfaces.
4.1. Crucible Shape and Functional Use
The studied crucible drastically differs from other crucibles excavated at the Tiversk hillfort, as well as from all crucibles found in Karelian hillforts. The large volume of the preserved part exceeds the standard volume of a common crucible for non-ferrous metal working in at least five times (compare the volumes of 500 mL of the rare one to circa 100 mL of a larger representative of the crucible collection—see Figure 2a,d). As is mentioned above, the studied crucible has a pot-shaped near-bottom section, which is similar to the lower third of a usual cooking pot with an S-shape (This means that the contour of the vessel’s body from its bottom to the rim repeats the shape of the Latin letter S. It is the most widespread shape of cooking wheel-thrown ceramics in the north-western area of medieval Rus in the 10–15th century.) profile. Based on the similarity of the shape together with the traces of intensive fire treatment on the walls, S.I. Kochkurkina suspected that the crucible must have been used for steel production [24] (p. 127), as similar pot-shaped vessels with slag had already been recorded [38] (p. 52), [39] (p. 14). The present study revealed tin bronze prills on the crucible walls, suggesting that the crucible might have been utilized in non-ferrous metal working.
Nonetheless, it is important to stress that medieval crucibles for bronze production usually had a closed shape to avoid the oxidizing atmosphere [14], which may alter the technological procedure of metal melting, leading to the loss of metal and formation of slags. However, there are a few examples of crucibles with a shallow, open shape used for non-ferrous metal melting [8,47]. Although we could not confirm that the crucible had a shallow form due to the absence of the body and rim parts, some indicators such as the simultaneous presence of hematite and magnetite may evidence that the redox conditions were not stable.
Concerning the large volume of the crucible, it is worth mentioning that in the late Middle Ages the production of non-ferrous metals in ancient Rus had been rapidly developing, which led to the increase of raw material imports and resulted in the emergence of volume crucibles. Crucibles of large volumes, which allowed the melting of a few kilograms of bronze, were recognized in Novgorod—the trade and industrial center of medieval Rus [14]. These crucibles were dated to the late 14th–early 15th century, which corresponds to the period when the Tiversk hillfort was functioning. It was the time when non-ferrous metal working in Novgorod achieved industrial scales [14], which is also indicated by the associated findings: ingots, wire, and scrap of non-ferrous metals. Therefore, the use of the studied crucible with a relatively large volume for non-ferrous metal does not contradict the archeological context of the technical ceramics used in late medieval Rus metal working.
However, we cannot exclude the possibility that the crucible could have been used for both the cementation of iron and the alloying of copper, probably in different times. If the crucible had been used for iron cementation, the remains of charcoal and iron on the crucible walls might be suspected to have been preserved. No charcoal remains were recorded in the crucible (This could be explained by the further utilization of the crucible—bronze melting—which obviously resulted in the total burning-out of organic residues.), and only a few iron prills trapped in the crucible body were detected. The iron prills are unlikely to be the result of iron reduction from copper alloys because no evidence of strong reducing conditions were detected in the crucible (see Section 4.3). Therefore, the iron prills might be assumed to be the remains of iron cementation. An additional argument supporting this hypothesis comes from the occurrence of Mn (about 1%) in some iron prills. In Karelia, the bog iron ores are widely spread and have been used for iron production from ancient times [26] (pp. 75–76), [27] (p. 301). The bog ores are enriched in Mn, with the Mn content varying from 1 to 3 wt.% [48], so the high concentration of Mn may originate from the raw material—domestic iron ores—and the iron prills might be assumed to be the remains of iron cementation.
4.2. Provenance Analysis
The chemical composition of the crucible fabric provides information about its provenance. The data on the chemical composition of the clay matrix defined by area SEM-EDS analysis were compared to the clay matrix composition of 28 samples of medieval Karelian ceramic kitchenware studied earlier [19]. The major oxide composition of the crucible fabric is similar to that of local pottery (Figure 4), which is predominantly made of non-calcareous illite–montmorillonite clays. The data suggest that the technical ceramics and the household pottery were manufactured using rather similar clay, though part of the kitchenware items were made of more refractory kaolin clay. It is noteworthy that the iron total content in the crucible outer and inner surfaces is higher than the iron total content in its central zones. The effect might be explained by contamination from the burial environment or thermal treatment. Additionally, the P2O5 and SO2 enrichment of the fabric from the crucible outer edge might be assumed to be due to the wooden fuel or charcoal, as these oxides are typical of wood ash [49].
Trace and REE content in the crucible fragments determined by ICP-MS analysis were compared with the data on 62 ceramic samples of Karelian kitchenware studied previously [20,50]. Ti-Y and Ti-ΣREE bivariate diagrams have been constructed to discriminate the local and imported pottery from Karelian hillforts [20]. Data on crucible composition were also analyzed comparatively using wheel-thrown ceramics from Novgorod and the Oreshek hillfort as reference material. The choice of reference material is due to the fact that Novgorod was the capital of Novgorodian Rus and is considered to be the possible center of pottery manufacture. The Oreshek hillfort was the center of Ingria Land, which was a close neighbor to Karelian territory and demonstrated a strong connection with Karelian material culture that is also reflected in the typological similarity of ceramic sets [51] (pp. 87–90). The diagrams (Figure 9) reveal that most of the Karelian ceramics refer to local raw materials. Only five individual pots characterized by distinct Y and ΣREE contents have been attributed to imported pottery, which were obviously brought to Karelian settlements. In Ti-Y and Ti-ΣREE bivariate diagrams the bulk compositions of crucible fragments fall into the field of local Karelian pottery, testifying the unity of their origin (Figure 9). Thus, the data suppose the local production of the crucible. Moreover, the crucible was made of illite–montmorillonite clays with a temper composed of felsic or intermediate igneous rock fragments that is typical for the local Karelian pottery of the studied period, as the bedrock exposures and deposits of these rocks are widely spread in the territory of the North-Western Ladoga region.
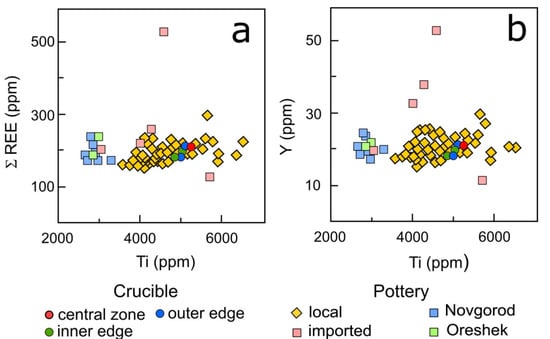
Figure 9.
Ti-Σ REE (a) and Ti-Y (b) bivariate diagrams illustrating the fabric composition of the crucible compared with various ceramic groups: local pottery of Karelian hillforts, imported pots, and ceramics from Novgorod and Oreshek.
The obtained data reveal that the crucible is made of non-refractory clays. Moreover, the crucible and local pottery from the Tiversk hillfort have a similar amount of temper with similar composition. Therefore, only the large walls’ thickness of about 1.3 cm might have compensated for the lack of refractoriness.
4.3. Thermal Treatment Parameters
Deciphering the thermal treatment conditions of non-calcareous ceramics is a challenge as significant modifications of the starting minerals during heating are not usually observed [52]. It was expected that the crucible was subjected to thermal treatment at the temperatures of at least 900 °C and above, as it was hypothesized to be used in metal working. Evidence in support of the high-temperature treatment of the crucible is as follows:
- (1)
- The high contents of K2O (9%), Al2O3 (7%), and Na2O (6%) recorded in the rim surrounding the quartz core (Figure 5) are not typical for the natural quartz, in which the content of a foreign element incorporated in quartz lattice usually does not exceed 1% [53]. Therefore, the rims might correspond to the glass with partially preserved crystalline quartz, indicating the alteration of quartz during heat treatment. Similar effects have been reported for ceramics experiencing high-temperature firing above 950 °C [10,12,54,55].
- (2)
- The evolution of biotite characteristics, mainly optical properties, has been used to estimate the firing temperature [56,57]. SEM and Raman spectroscopy analysis revealed that the biotite grains composing the crucible temper were partially or completely pseudomorphed by magnetite and fayalite rarely together with rutile. Additionally, microcline lamellas were recorded between the cleavage planes (Figure 6c). The thermal decomposition of biotite starts at temperatures above 850 °C [58] and finishes at temperatures above 1050 °C [56]. Experimental studies have shown various reactions [59,60] for the biotite thermal breakdown depending on the composition of biotite and the surrounding environment. The products of biotite decomposition might include magnetite, K-feldspar, and fayalite recorded in the mineral assemblage pseudomorphing mica in the crucible temper. Additionally, biotite thermal decomposition might be accompanied by the precipitation of tiny rutile needles [61]. Therefore, we assume that the mineral assemblage pseudomorphing mica in the crucible temper together with the absence of mica relics in most of the mica grains is likely to be the result of the thermal decomposition of biotite occurring at temperatures above 1000 °C.
- (3)
- The evolution of Raman spectra of hematite during heating provides information about the relative temperature of hematite formation and is widely used in archeometry to estimate the firing temperature [44,45,46]. The model experiments [46] showed that the heating of hematite was accompanied with the intensity increase of a band centered at 680 cm−1 in hematite Raman spectra. The band intensity ratio (680/620), which increases with a firing temperature rise, was used to estimate the firing temperature of wheel-thrown pottery [21] from Karelian hillforts. It has been shown [21] that the intensity ratio (680/620) for most of the local pottery samples varied from 0.8 to 1.5 (Figure 7c). A maximum ratio (680/620) of 1.8 was recorded for hematite from the pot made of refractory kaolin clay, which was subjected to the high-temperature firing (Figure 7d). The low temperature limit above 1000 °C was supported by the presence of mullite in this sample, which formed during the decomposition of muscovite at temperatures above 1050 °C [62]. The ratio (680/620) of the hematite from the crucible is characterized by a significantly higher ratio (680/620) ranging from 2.6 to 3.0 (Figure 7e), so it might be assumed that the crucible experienced a thermal treatment at temperature at least above 1050 °C.
The study of the various iron oxides provides information about the firing atmosphere [44,45,46]. The mineral markers of both oxidizing, e.g., hematite and oxidized sulfide phases, and reducing or partially reducing conditions, e.g., magnetite and sulfide minerals, were detected in the crucible, so the atmosphere parameters are assumed to be variable.
In summary, the study of minerals indicating the thermal treatment provides strong evidence that the crucible experienced heating at temperatures above 1050 °C at variable redox conditions.
4.4. Bronze Production Techniques
The chemical composition of metal alloys and the occurrence of metal sulfide and oxide mineral phases provide information on metallurgical processes. The obtained data show that the crucible was used to produce binary Cu-Sn bronze, with variable Sn content from 8 to 44 wt.% and the most abundant composition of about 20 wt.%. Only two brass, two copper, and five iron prills were recorded compared with the 80 tin bronze prills.
Previous studies of non-ferrous metal working in medieval Karelia were mainly focused on the analysis of chemical composition and manufacture technology of final products [24] (pp. 80–125), [25] (pp. 127–136), [63,64,65], but there is no information about the composition of slags and metal remains in technical ceramics. Therefore, we compared the composition of metal alloys recorded in the crucible with that of the jewelry findings (75 pcs) [64] from Karelian hillforts. According to the chemical composition of alloys, the jewelry items were divided into various groups. Most artifacts were made of bronze (54%), with the dominating composition of ternary Cu-Pb-Sn alloys. Moderate amounts of jewelry pieces were made of brass (17%) and multicomponent alloys (18%), containing Zn, Sn, and Pb. Equal amounts (4% for each group) of jewelry findings had the composition of binary Cu-Pb bronze and Ag alloys, whereas the pure copper artifacts presented only 3 % of the assemblage.
Only two findings from Karelian hillforts (Paaso and Soskua-Linnamyaki), a Karelian-type oval brooch (fibula) and a pendant also used for chains [64], were made of binary Cu-Sn bronzes, with the Sn content of 12% and 22%, respectively. The bronze composition recorded in these jewelry findings is similar to the bronze alloys recorded in the crucible.
The main techniques of bronze production [16] (p. 591) include the “alloying of two fresh metals (copper and tin); cementation (copper metal with tin ore); co-smelting (copper and tin ore); and recycling (possibly involving addition of fresh metal (or tin ore))”. It has been reported that in Novgorodian Rus the bronze was mainly produced from imported non-ferrous metal by alloying and recycling (In this case the term recycling stands for adding non-ferrous scrap into copper alloy for remelting.) [11,66]. The obtained data on the composition of metal alloys from the crucible do not contradict the idea of alloying, but the presence of copper sulfide minerals and accessory minerals typical for the skarn copper ores, e.g., cassiterite and barite, gives rise to the question of whether the raw ores could also be used as additives during bronze alloying. Therefore, we may carefully assume that the crucible was used in bronze production, which might involve not only alloying but also different techniques of smelting metals and ores. This conclusion is supported by the occurrence of copper sulfide deposits and bedrock exposures located near the Tiversk hillfort [67]. In contrast, our data suppose that the studied crucible was unlikely to have been used for recycling (by this term, we imply adding bronze scrap—broken or used objects into alloy for remelting), because we did not record traces of Pb and Zn in the alloys of prills, which were supposed to appear as most of the analyzed Karelian jewelry pieces were made of alloys containing Pb and Zn. Bronze prills detected in the studied crucible have the composition of binary Cu-Sn bronze with variable Sn content. Obviously, further studies of typical crucible collections as well as slags are needed to decipher the specific features of non-ferrous metal working in medieval Karelia.
5. Conclusions
Mineralogical analysis of the rare crucible excavated at the Tiversk hillfort in the North-Western Ladoga region (Russia) suggests that it was used for bronze production, though it is characterized by a large volume and a pot-shaped bottom, which are not typical for crucibles used for non-ferrous metal working. The evidence comes from the occurrence of tin bronze prills recorded in the crucible. The composition of bronze and sulfide minerals detected in the crucible supposes that the melting process for which this crucible has been used might involve not only alloying but also the possible smelting of metals and ores. Nevertheless, in light of the present data, it is difficult to state clearly whether the crucible could have been used for both bronze production and iron cementation, as very few signs (rare iron prills) of potential iron cementation were found. However, we cannot exclude the possibility of multifunctional usage of the crucible as the bottom part of the vessel, which might preserve specific slags, is absent.
ICP-MS data showed that the crucible can be attributed to local technical ceramics. The obtained data reveal that the crucible is made of non-refractory clays, which were normally used to produce ceramic kitchenware in medieval Karelia. Moreover, the composition and amount of temper are similar for both the crucible and household ceramics. Therefore, only the large walls’ thickness of about 1.3 cm might have compensated for this lack of raw material refractoriness.
The reconstruction of the thermal treatment conditions based on the study of minerals indicated that the heating ratio provides strong evidence that the crucible was exposed to temperatures above 1050 °C at variable redox conditions. The established temperatures are in accordance with the limits of bronze melting. The present study also contributes to the estimation of the firing temperature for the ceramics made of non-calcareous clays.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/min11060648/s1: Table S1: ICP-MS BHVO2 standard results; Table S2: X-ray analysis data on mineral composition (mod. %) of the crucible ceramic paste; Table S3: Major element compositions (wt.%) of the crucible clay matrix obtained by microprobe area analysis; Table S4: Trace element compositions (ppm) of the crucible fabric obtained by ICP-MS analysis; Table S5: Microprobe analysis data for representative mica and quartz from the crucible; Table S6: Microprobe analysis data for metal alloys of the prills (I), sulfide minerals (II), and metal oxide minerals (III) recorded in the crucible.
Author Contributions
Conceptualization, I.M.S. and S.A.S.; investigation, I.M.S. and S.Y.C.; data curation, S.Y.C. and I.M.S.; writing—original draft, I.M.S. and S.Y.C.; writing—review and editing, S.A.S. and I.M.S.; visualization, S.Y.C. and I.M.S.; supervision, S.A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by state assignment to the Institute of Geology, Karelian Research Centre RAS program AAAA-A18-118020290084-7 “Mineralogical and geological heritage of the South-Eastern part of the Fennoscandinavian shield” and the Institute of Linguistics, Literature and History, Karelian Research Centre of RAS program AAAA-A18-118012490341-4 “Complex studies of archeological heritage in Karelia and neighbor territories (Mesolithic–Middle Ages)”.
Data Availability Statement
Archeological ceramics of Karelia: geochemical studies. Available online: http://illhportal.krc.karelia.ru/section.php?plang=r&id=3729.
Acknowledgments
We are grateful to A.S. Paramonov, S.V. Burdyukh, and S.N. Ivashevskaya (IG KRC RAS, Petrozavodsk, Russia) for their assistance in the analytical investigations. O.B. Lavrov and O.S. Sibilev are thanked for their fruitful discussion of the study. The authors thank the anonymous reviewers for their very careful revisions and checking of the manuscript. Their comments and suggestions greatly helped to improve the text.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shepard, A.O. Ceramics for the Archaeologist; Carnegie Institution of Washington publication: Washington, DC, USA, 1956; 414p. [Google Scholar]
- Maniatis, Y.; Tite, M.S. Technological examination of Neolithic-Bronze Age pottery from central and southeast Europe and from the Near East. J. Archaeol. Sci. 1981, 8, 59–76. [Google Scholar] [CrossRef]
- Freestone, I.C. Applications and potential of electron probe micro-analysis in technological and provenance investigations of ancient ceramics. Archaeometry 1982, 24, 99–116. [Google Scholar] [CrossRef]
- Tite, M.S. Pottery production, distribution, and consumption—The contribution of the physical sciences. J. Archaeol. Method Theory 1999, 6, 181–233. [Google Scholar] [CrossRef]
- Ionescu, C.; Hoeck, V.; Simon, V. Effect of the temperature and the heating time on the composition of an illite-smectite clay: An XRPD study. Studia UBB Chemia 2011, 2, 69–78. [Google Scholar]
- Eramo, G. Ceramic technology: How to recognize clay processing. Archaeol. Anthropol. Sci. 2020, 12, 164. [Google Scholar] [CrossRef]
- Rehren, T. Crucibles as reaction vessels in ancient metallurgy. In Mining and Metal Production through the Ages; Craddock, P.T., Lang, J., Eds.; British Museum Press: London, UK, 2003; pp. 207–215. [Google Scholar]
- Bayley, J.; Rehren, T. Towards a functional and typological classification of crucibles. In Metals and Mines Studies in Archaeometallurgy; La Niece, S., Hook, D., Craddock, P., Eds.; Archetype Books: London, UK, 2007; pp. 46–55. [Google Scholar]
- Hein, A.; Kilikoglou, V.; Kassianidou, V. Chemical and mineralogical examination of metallurgical ceramics from a late bronze age copper smelting site in Cyprus. J. Archaeol. Sci. 2007, 34, 141–154. [Google Scholar] [CrossRef]
- Martinón-Torres, M.; Freestone, I.; Hunt, A.; Rehren, T. Mass-produced mullite crucibles in medieval Europe: Manufacture and material properties. J. Am. Ceram. Soc. 2008, 91, 2071–2074. [Google Scholar] [CrossRef]
- Eniosova, N.V.; Mitoyan, R.A.; Saracheva, T.G. Chemical composition of jewelry metal in the Middle Ages and the routes of its import to the territory of the Ancient Rus. In Non-Ferrous and Precious Metals and Their Alloys in the Territory of Eastern Europe in the Middle Ages; Konovalov, A.A., Eniosova, N.V., Mitoyan, R.A., Saracheva, T.G., Eds.; Vostochnaya literatura: Moscow, Russia, 2008; pp. 107–162. (In Russian) [Google Scholar]
- Martinón-Torres, M.; Rehren, T. Post-Medieval crucible production and distribution: A study of materials and materialities. Archaeometry 2009, 51, 49–74. [Google Scholar] [CrossRef]
- Thornton, C.P.; Rehren, T. A truly refractory crucible from fourth millennium Tepe Hissar, Northeast Iran. J. Archaeol. Sci. 2009, 36, 2700–2712. [Google Scholar] [CrossRef]
- Eniosova, N.V.; Rehren, T. Melting vessels of Novgorod jewelers. In Novgorodian Archaeological Readings—3, Proceedings of the Archaeology of Medieval City, the 75th Anniversary of Archaeological Study of Novgorod, Novgorod the Great, Russia, 25–28 September 2007; Rybina, E.A., Ed.; Novgorod State Museum: Novgorod the Great, Russia, 2011; pp. 243–254. (In Russian) [Google Scholar]
- Zavyalov, V.I.; Lopatina, O.A.; Sudakov, V.V. Technological analysis of medieval crucibles from Pereyaslavl Ryazanskiy. Russ. Archaeol. 2018, 3, 43–51. (In Russian) [Google Scholar]
- Rademakers, F.W.; Rehren, T. Seeing the forest for the trees: Assessing technological variability in ancient metallurgical crucible assemblages. J. Archaeol. Sci. Rep. 2016, 7, 588–596. [Google Scholar] [CrossRef]
- Potasheva, I.M.; Svetov, S.A. Geochemical research in archaeology: ICP-MS analysis of wheel-thrown pottery samples found in ancient Karelian hillforts. Writ. Karelian Res. Cent. Russ. Acad. Sci. 2013, 3, 136–142. (In Russian) [Google Scholar]
- Potasheva, I.M.; Svetov, S.A. ICP-MS analysis of ancient ceramics as identification method of clay source and pottery production area. Proc. Petrozavodsk State Univ. 2014, 4, 71–77. (In Russian) [Google Scholar]
- Summanen, I.M.; Chazhengina, S.Y.; Svetov, S.A. Mineralogy and technological analysis of ceramics (on materials from medieval sites of the North-Western Ladoga area). Proc. Russ. Mineral. Soc. 2017, 146, 108–123. (In Russian) [Google Scholar]
- Summanen, I.M.; Chazhengina, S.Y.; Svetov, S.A. Geochemical (ICP-MS, LA-ICP-MS) and mineralogical studies. In Ceramics of Medieval Karelia (on the Materials from Archaeological Sites of X–XV cc.); Karelian Research Centre of RAS: Petrozavodsk, Russia, 2019; pp. 215–265. (In Russian) [Google Scholar]
- Chazhengina, S.Y.; Summanen, I.M.; Svetov, S.A. Raman spectroscopy for firing condition characterization: Case study of Karelian medieval pottery. J. Raman Spectrosc. 2020, 51, 1894–1902. [Google Scholar] [CrossRef]
- Appelgren, H.O. Suomen muinaislinnat: Tutkimus vertailevan muinaistieteen alalla. In Suomen Muinaismuistoyhdistyksen Aikakauskirja; Seuran Kirjapainossa: Helsinki, Finland, 1891; Volume 12, 237p. (In Finnish) [Google Scholar]
- Schwindt, T. Tietoja Karjalan rautakaudesta ja sitä seuraavilta ajoilta Käkisalmen kihlakunnan alalta saatujen löytöjen mukaan. In Suomen Muinaismuistoyhdistyksen Aikakauskirja; Suomen Muinaismuistoyhdistys: Helsinki, Finland, 1893; Volume 13, 206p. (In Finnish) [Google Scholar]
- Kochkurkina, S.I. Ancient Korela; Nauka: Leningrad, Russia, 1982; 216p. (In Russian) [Google Scholar]
- Kochkurkina, S.I. Ancient Karelian Hillforts of the Medieval Epoch; Vzlet: Petrozavodsk–Saint-Petersburg, Russia, 2010; 264p. (In Russian) [Google Scholar]
- Uino, P. Ancient Karelia. Archaeological studies. In Suomen Muinaismuisto-Yhdistyksen Aikakauskirja; Suomalaisen Kirjallisuuden Seuran Kirjapainossa: Helsinki, Finland, 1997; Volume 104, 426p, (In English/Finnish). [Google Scholar]
- Saksa, A.I. Ancient Karelia in the Late First and the Early Second Millennia AD. Origins, History and Culture of the Chronicles of the Karelian Land; Nestor-istorija: St. Petersburg, Russia, 2010; 398p. (In Russian) [Google Scholar]
- Belskiy, S.V. Kyulyalakhti-Kalmistomyaki Burial Ground in North-Western Ladoga Area (Archaeological Survey in 2006-2009); Nauka: Saint-Petersburg, Russia, 2012; 240p. (In Russian) [Google Scholar]
- Tikhomirov, M.N.; Nasonov, A.N. (Eds.) The first Novgorodian Chronicle of Older and Younger Recensions; Akademia Nauk SSSR: Moscow, Leningrad, Russia, 1950; 561p. (In Russian) [Google Scholar]
- Kirpichnikov, A.N. Stone Fortresses of Novgorodian Land; Nauka: Leningrad, Russia, 1984; 276p. (In Russian) [Google Scholar]
- Kirpichnikov, A.N. Historical and archaeological survey of ancient Korela (“Korel’skiy gorod” of 14th century). In Finno-Ugrians and Slavs; Nauka: Leningrad, Russia, 1979; pp. 52–73. (In Russian) [Google Scholar]
- Yanin, V.L.; Zaliznyak, A.A. Novgorodian Birch Bark Manuscripts (from Excavations of 1977–1983); Nauka: Moscow, Russia, 1986; 312p. (In Russian) [Google Scholar]
- Yanin, V.L. Essays on the History of Medieval Novgorod; Jazyki slavjanskih kul’tur: Moscow, Russia, 2008; 400p. (In Russian) [Google Scholar]
- Rinne, J. Suomen Keskiaikaiset Mäkilinnat; K.F. Puromiehen kirjapaino oy: Helsinki, Finland, 1914; Volume 1. (In Finnish) [Google Scholar]
- Kirpichnikov, A.N.; Petrenko, V.P. Tiverskiy gorodok. Brief Commun. Inst. Archaeol. 1974, 139, 106–113. (In Russian) [Google Scholar]
- Kochkurkina, S.I. Archaeological Sites of Korela of V–XV cc.; Nauka: Leningrad, Russia, 1981; 160p. (In Russian) [Google Scholar]
- Kochkurkina, S.I. Ancient Karelians; Karelia: Petrozavodsk, Russia, 1987; 72p. (In Russian) [Google Scholar]
- Kolchin, B.A. Iron metallurgy and metal in the Ancient Rus (pre-Mongolian period). In Materials and Studies on Archaeology of the USSR; Akademia Nauk SSSR: Moscow, Russia, 1953; Volume 32, 261p. (In Russian) [Google Scholar]
- Kolchin, B.A. Metal working craft of Novgorod the Great (production, technology). In Materials and Studies on Archaeology of the USSR; Akademia Nauk SSSR: Moscow, Russia, 1959; Volume 65, pp. 7–121. (In Russian) [Google Scholar]
- Smith, C.S. The Discovery of Carbon in Steel. Technol. Cult. 1964, 5, 149–175. [Google Scholar] [CrossRef]
- Svetov, S.A.; Stepanova, A.V.; Chazhengina, S.Y.; Svetova, E.N.; Rybnikova, Z.P.; Mikhailova, A.I.; Paramonov, A.S.; Utitsyna, V.L.; Ekhova, M.V.; Kolodey, B.S. Precision geochemical (ICP-MS, LA-ICP-MS) analysis of rock and mineral composition: The method and accuracy estimation in the case study of early precambrian mafic complexes. Writ. Karelian Res. Cent. Russ. Acad. Sci. 2015, 7, 54–73. (In Russian) [Google Scholar]
- Wang, G.; Wang, H.; Zhang, N. In situ high temperature X-ray diffraction study of illite. Appl. Clay Sci. 2017, 146, 254–263. [Google Scholar] [CrossRef]
- Wang, A.; Freeman, J.J.; Jolliff, B.L. Understanding the Raman spectral features of phyllosilicates. J. Raman Spectrosc. 2015, 46, 829–845. [Google Scholar] [CrossRef]
- Zoppi, A.; Lofrumento, C.; Castellucci, E.M.; Migliorini, M.G. The Raman spectrum of hematite: Possible indicator for a compositional or firing distinction among Terra Sigillata wares. Ann. Chim. 2005, 95, 239–246. [Google Scholar] [CrossRef]
- Leon, Y.; Lofrumento, C.; Zoppi, A.; Carles, R.; Castellucci, E.M.; Sciau, P. Micro-Raman investigation of terra sigillata slips: A comparative study of central Italian and southern Gaul productions. J. Raman Spectrosc. 2010, 41, 1550–1555. [Google Scholar] [CrossRef]
- Cianchetta, I.; Maish, J.; Saunders, D.; Walton, M.; Mehta, A.; Foran, B.; Trentelman, K. Investigating the firing protocol of Athenian pottery production: A Raman study of replicate and ancient sherds. J. Raman Spectrosc. 2015, 46, 996–1002. [Google Scholar] [CrossRef]
- Mongiatti, A.; Montero-Ruiz, I. Rediscovering famous assemblages: A rare Bronze Age crucible from El Argar, Spain. Archaeometry 2020, 62, 329–345. [Google Scholar] [CrossRef]
- Glebova-Kulbah, G.O.; Kratz, K.O. Karelian ASSR: Mineral deposits. In Geology of USSR; Antropov, P.Y., Ed.; State Scientific Publishing House on Geology and Environment Protection: Moscow, Russia, 1962; Volume 37, pp. 72–103. (In Russian) [Google Scholar]
- Tite, M.S.; Shortland, A.; Maniatis, Y.; Kavoussanaki, D.; Harris, S.A. The composition of the soda-rich and alkali plant ashes used in the production of glass. J. Archaeol. Sci. 2006, 33, 1284–1292. [Google Scholar] [CrossRef]
- Archaeological Ceramics of Karelia: Geochemical Studies. Available online: http://illhportal.krc.karelia.ru/section.php?plang=r&id=3729 (accessed on 6 May 2021).
- Summanen, I.M. Ceramics of Medieval Karelia (on the Materials from Archaeological Sites of X–XV cc.); Karelian Research Centre of RAS: Petrozavodsk, Russia, 2019; 265p. (In Russian) [Google Scholar]
- Gliozzo, E. Ceramic technology. How to reconstruct the firing process. Archaeol. Anthropol. Sci. 2020, 12, 260. [Google Scholar] [CrossRef]
- Scotford, D.M. A test of aluminium in quartz as a geothermometer. Amer. Mineral. 1975, 60, 139–142. [Google Scholar]
- Ohya, Y.; Takahashi, Y.; Murata, M.; Nakagawa, Z.; Hamano, K. Acoustic Emission from a Porcelain Body During Cooling. J. Am. Ceram. Soc. 1999, 82, 445–448. [Google Scholar] [CrossRef]
- Ionescu, C.; Hoeck, V. Firing-induced transformations in Copper Age ceramics from NE Romania. Eur. J. Mineral. 2011, 23, 937–958. [Google Scholar] [CrossRef]
- Riccardi, M.P.; Messiga, B.; Duminuco, P. An approach to the dynamics of clay firing. Appl. Clay Sci. 1999, 15, 393–409. [Google Scholar] [CrossRef]
- Allepuz, E.T. Colour transformation and textural change in biotite: Some remarks for the interpretation of firing technology in greyware pottery thin-sections. Minerals 2021, 11, 428. [Google Scholar] [CrossRef]
- Vedder, W.; Wilkins, R.W.T. Dehydroxylation and rehydroxylation, oxidation and reduction of micas. Am. Mineral. 1969, 54, 482–509. [Google Scholar]
- Eugster, H.P.; Wones, D.R. Stability relations of the ferruginous biotite, annite. J. Petrol. 1962, 3, 81–125. [Google Scholar] [CrossRef]
- Rutherford, M.J. An experimental determination of iron biotite-alkali feldspar equilibria. J. Petrol. 1969, 10, 381–408. [Google Scholar] [CrossRef]
- Henry, D.J.; Guidotti, C.V. Titanium in biotite from metapelitic rocks: Temperature effects, crystal-chemical controls, and petrologic applications. Am. Mineral. 2002, 87, 375–382. [Google Scholar] [CrossRef]
- Olivares, M.; Zuluaga, M.C.; Ortega, L.A.; Murelaga, X.; Alonso-Olazabal, A.; Urteaga, M.; Amundaray, L.; Alonso-Martin, I.; Etxebarria, N. Characterisation of fine wall and eggshell Roman pottery by Raman spectroscopy. J. Raman Spectrosc. 2010, 41, 1543–1549. [Google Scholar] [CrossRef]
- Vasyljeva, E.S. Characteristics of copper alloys from Paaso and Tiversk hillforts. In Ancient Korela; Kochkurkina, S.I., Ed.; Nauka: Leningrad, Russia, 1982; pp. 185–188. (In Russian) [Google Scholar]
- Eniosova, N.V. New data on chemical composition of non-ferrous and precious metal from sites of annalistic korela. In Ancient Karelian Hillforts of the Medieval Epoch; Kochkurkina, S.I., Ed.; Vzlet: Petrozavodsk–Saint-Petersburg, Russia, 2010; pp. 248–262. (In Russian) [Google Scholar]
- Eniosova, N.V. Manufacture technique and chemical composition of jewelry metal of sites in South-Eastern Ladoga region and the Onega Lake basin. In Archaeology of Medieval Karelia; Kochkurkina, S.I., Ed.; Karelian Research Centre of RAS: Petrozavodsk, Russia, 2017; pp. 117–138. (In Russian) [Google Scholar]
- Konovalov, A.A. Non-ferrous metal (copper and its alloys) in the products of Novgorod in the 10th-15th centuries. In Non-Ferrous and Precious Metals and Their Alloys in the Territory of Eastern Europe in the Middle Ages; Konovalov, A.A., Eniosova, N.V., Mitoyan, R.A., Saracheva, T.G., Eds.; Vostochnaya literatura: Moscow, Russia, 2008; pp. 107–162. (In Russian) [Google Scholar]
- Mikhailov, V.P.; Golovanov, Y.B.; Fedyuk, Z.N. Non-ferrous metal. Copper. In Mineral Resources of Republic of Karelia, 1st ed.; Mikhailov, V.P., Aminov, V.N., Eds.; Karelia: Petrozavodsk, Russia, 2005; Volume 3, pp. 92–104. (In Russian) [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).