The Recovery and Concentration of Spodumene Using Dense Media Separation
Abstract
1. Introduction
The Beneficiation of Hard-Rock Deposits Using Dense Media Separation
2. Materials and Methods
2.1. The Hidden Lake Pegmatites
2.2. Mineralogy and Head Assays
2.2.1. Sample Preparation
2.2.2. Elemental Analysis
2.2.3. QEMSCAN Operational Modes and Quality Control
2.2.4. X-ray Diffraction
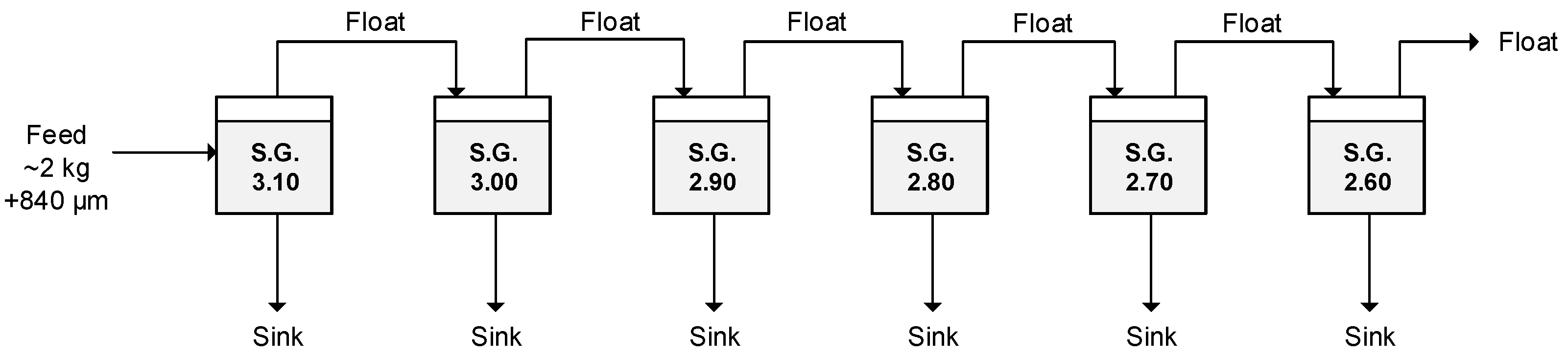
2.3. Heavy Liquid Separation (HLS)
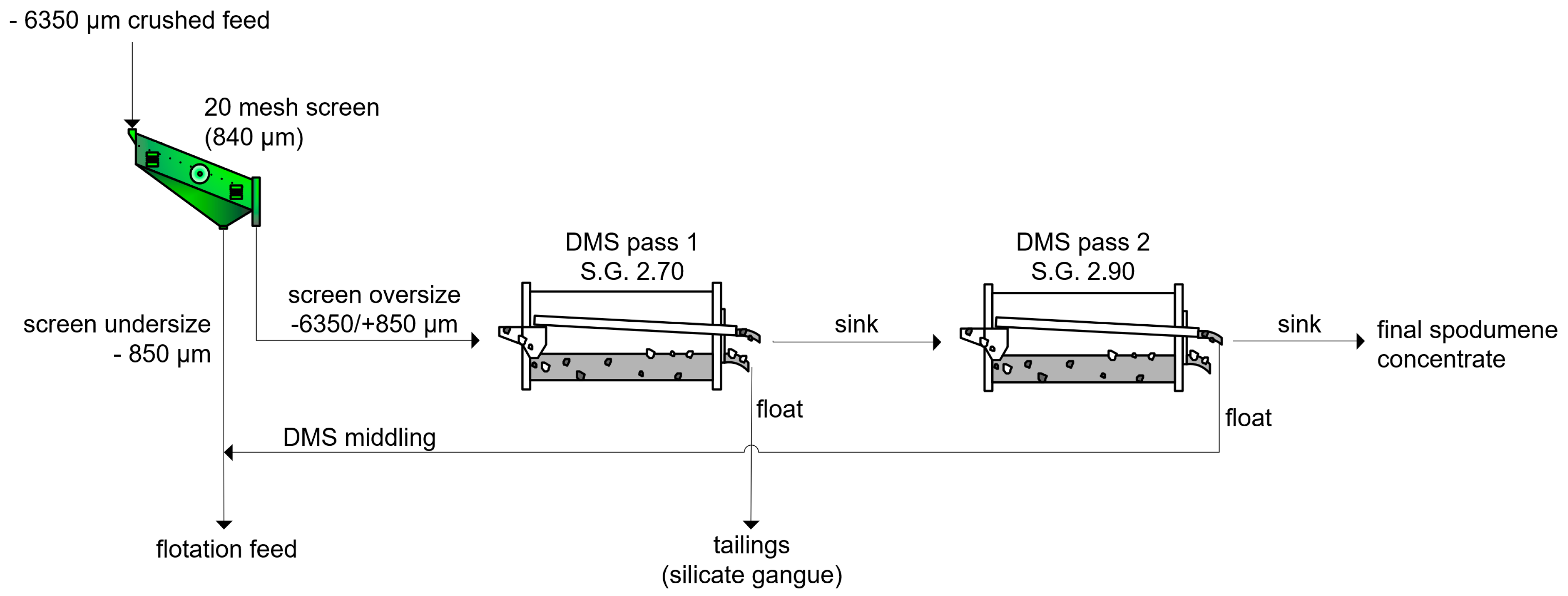
2.4. Dense Media Separation
3. Results and Discussion
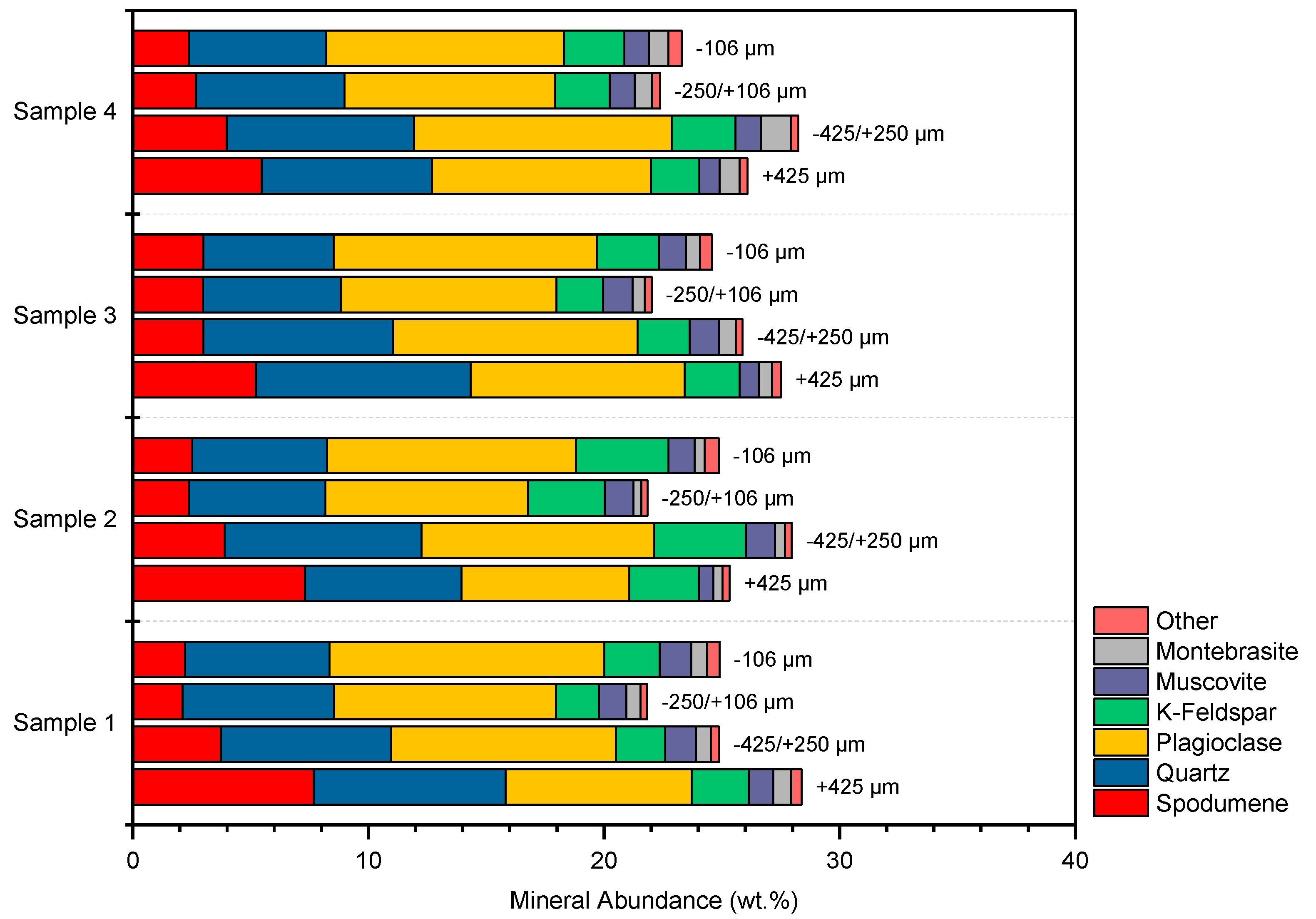
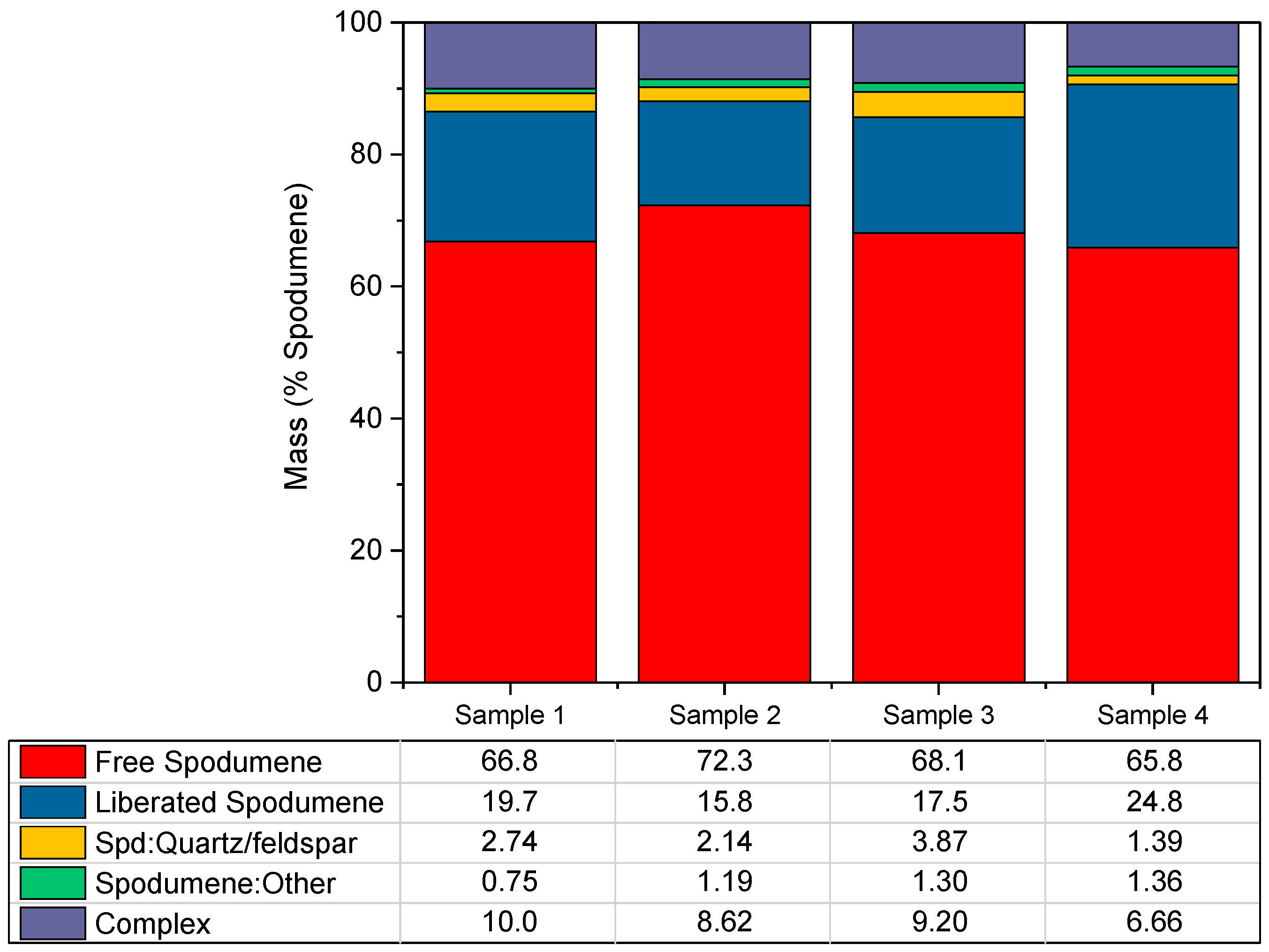
3.1. Mineralogy
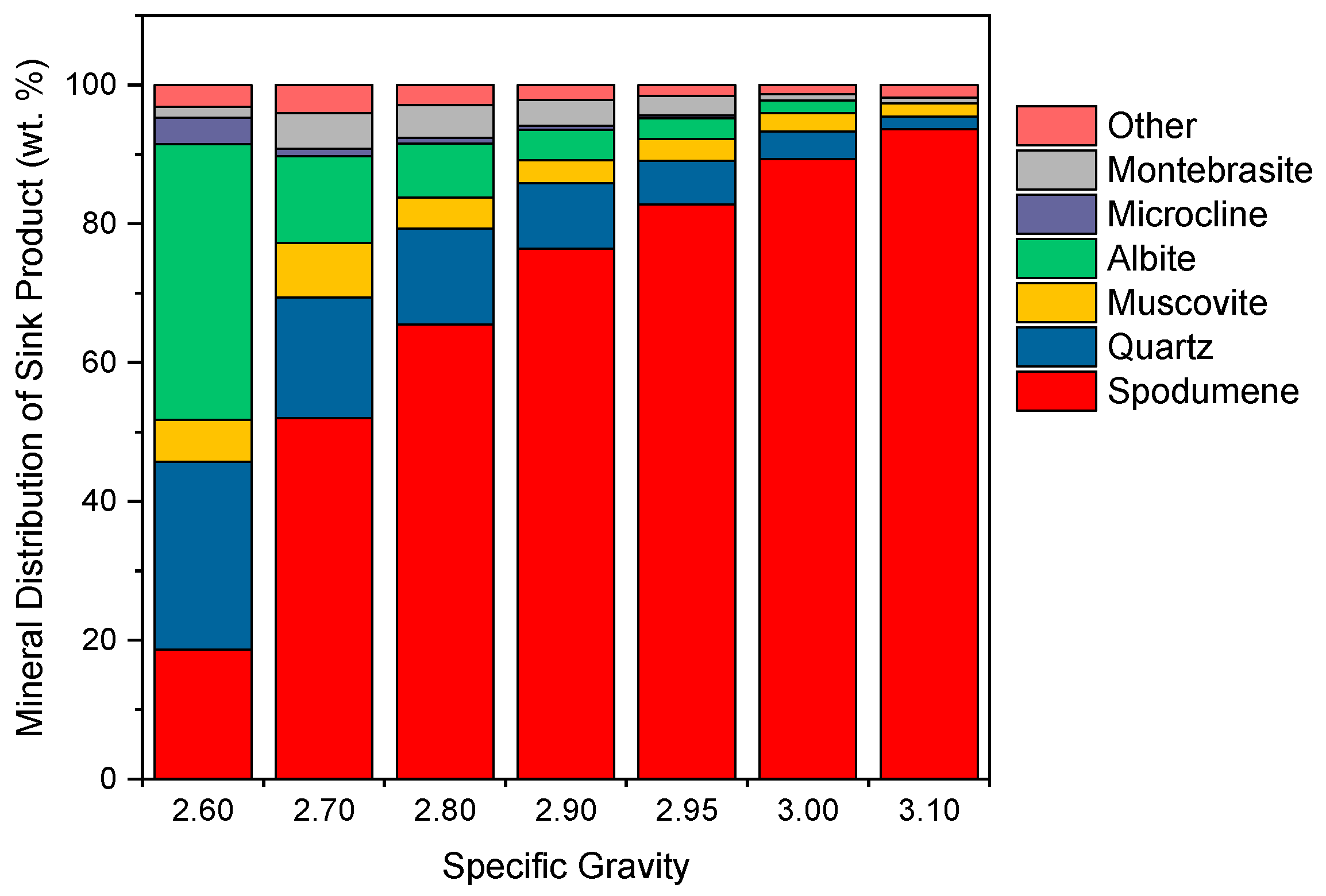
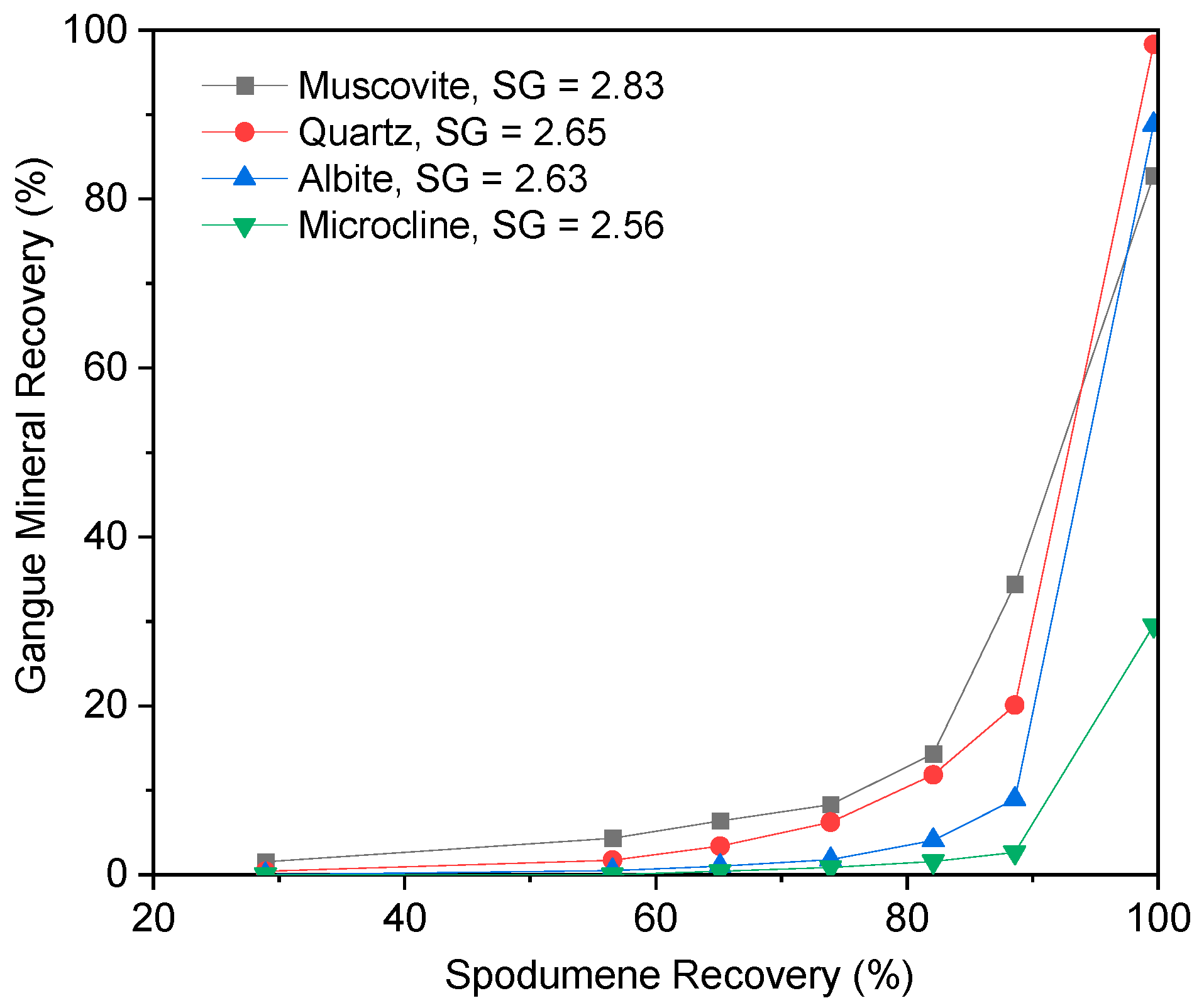
3.2. Heavy Liquid Separation
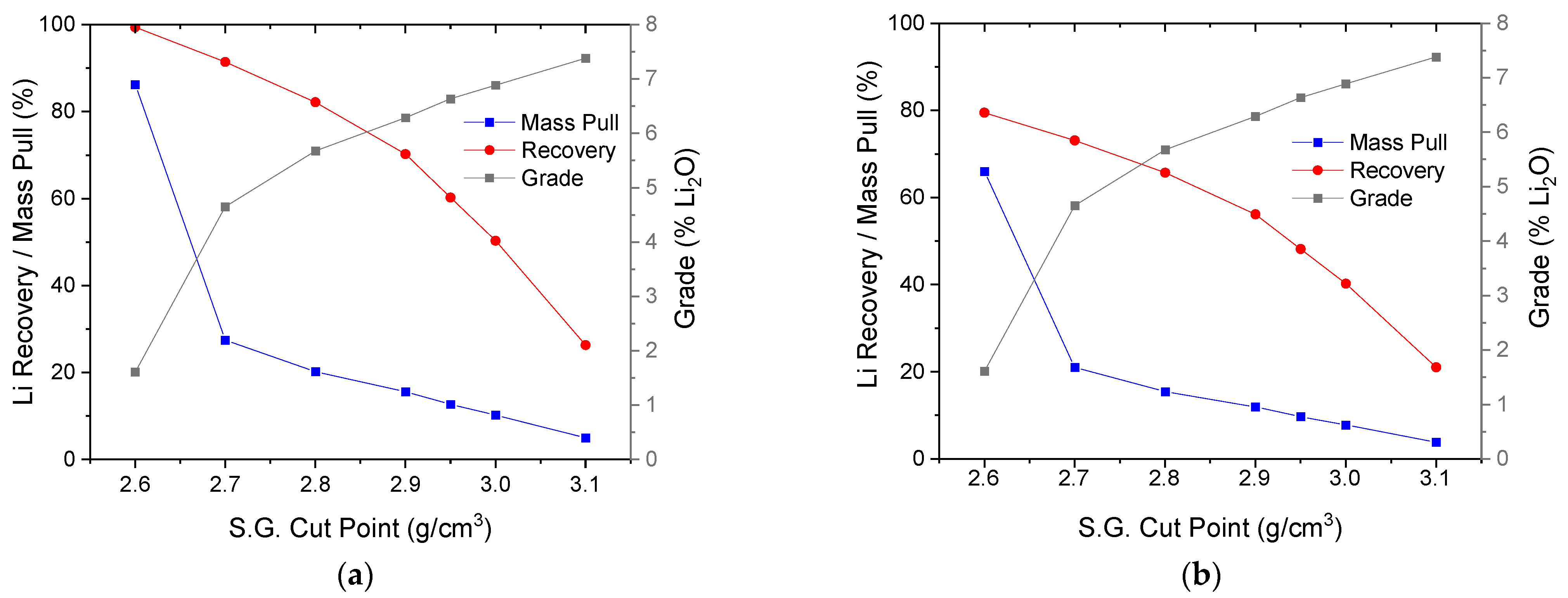
3.3. Dense Media Separation Testing
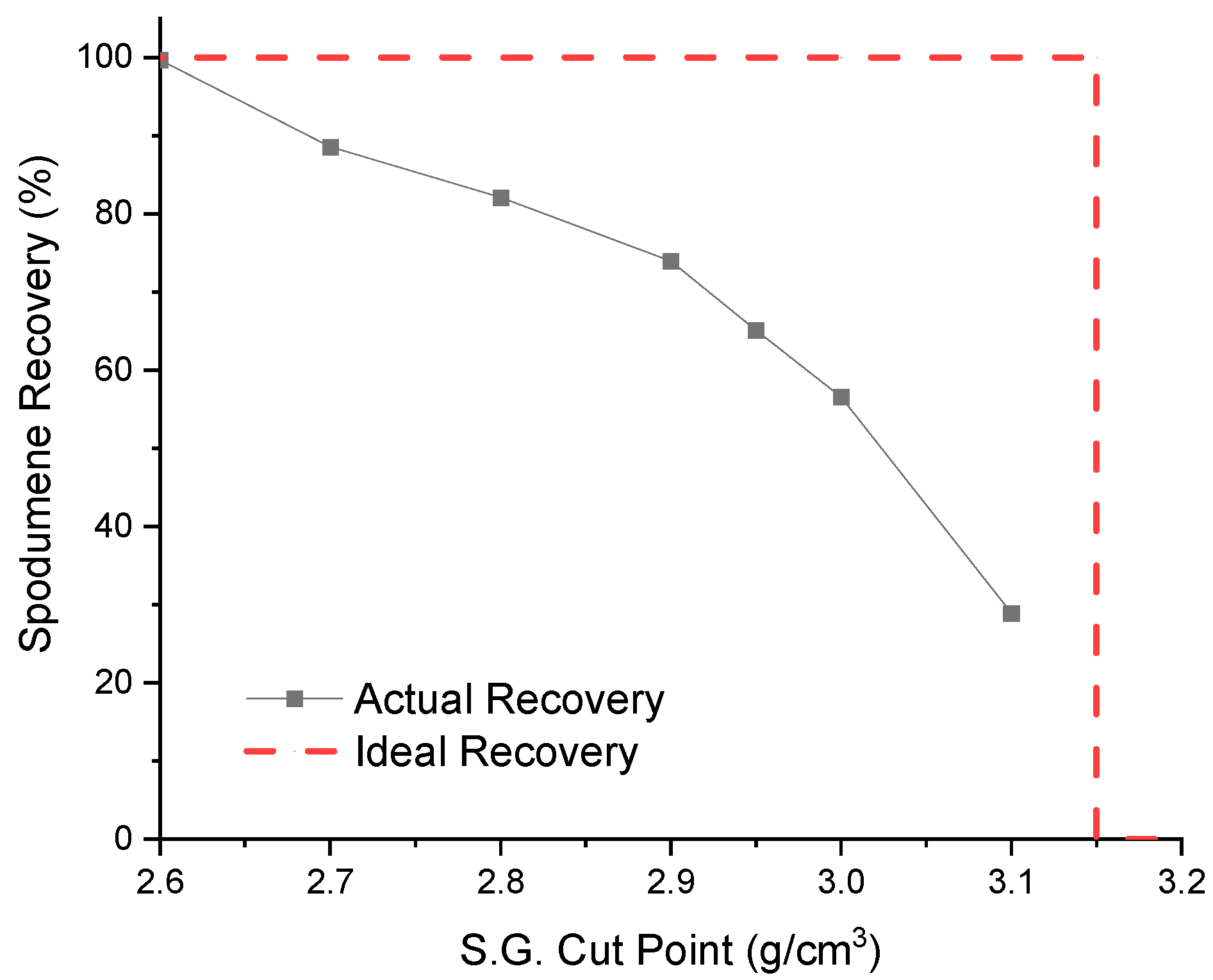
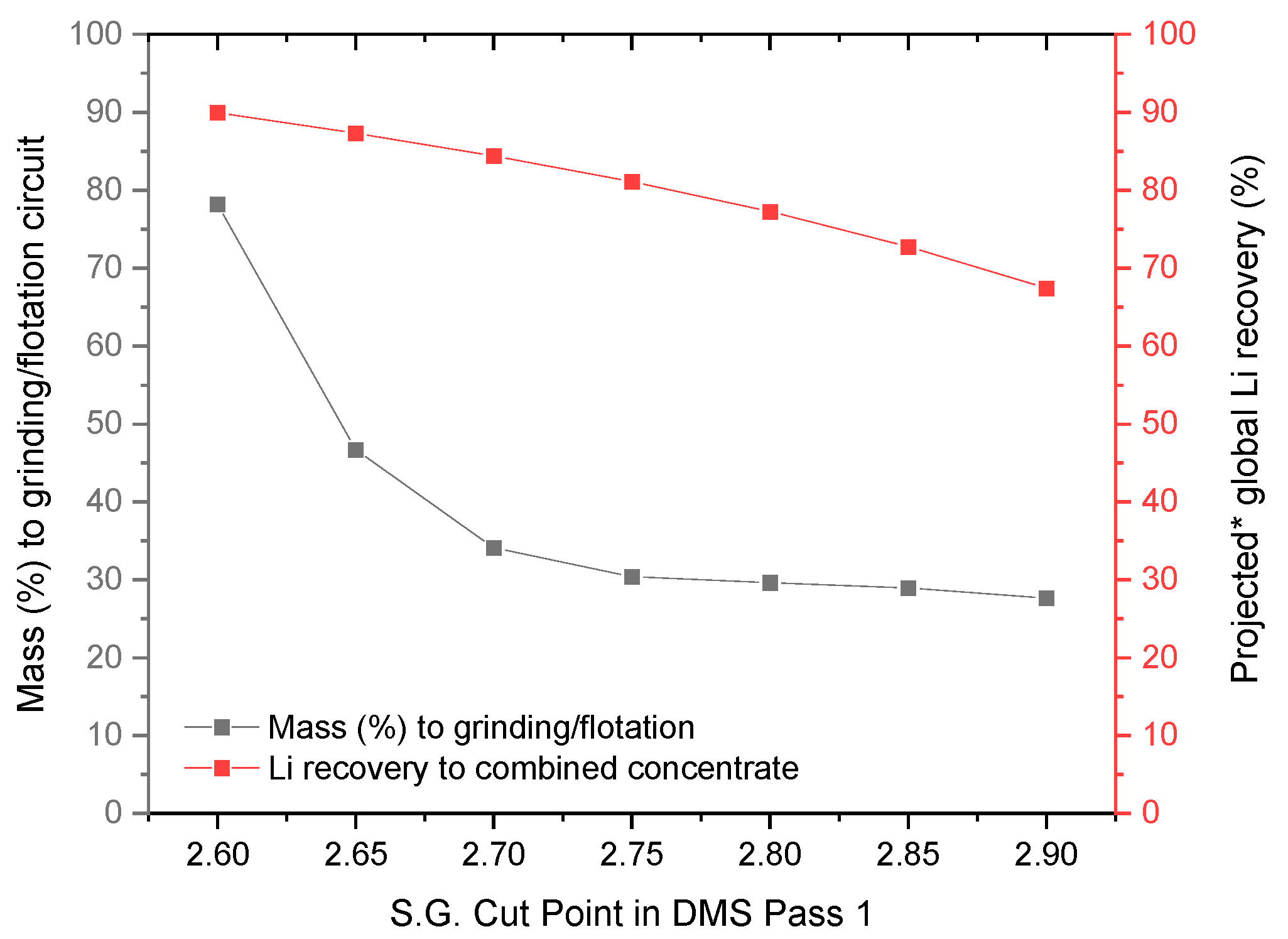
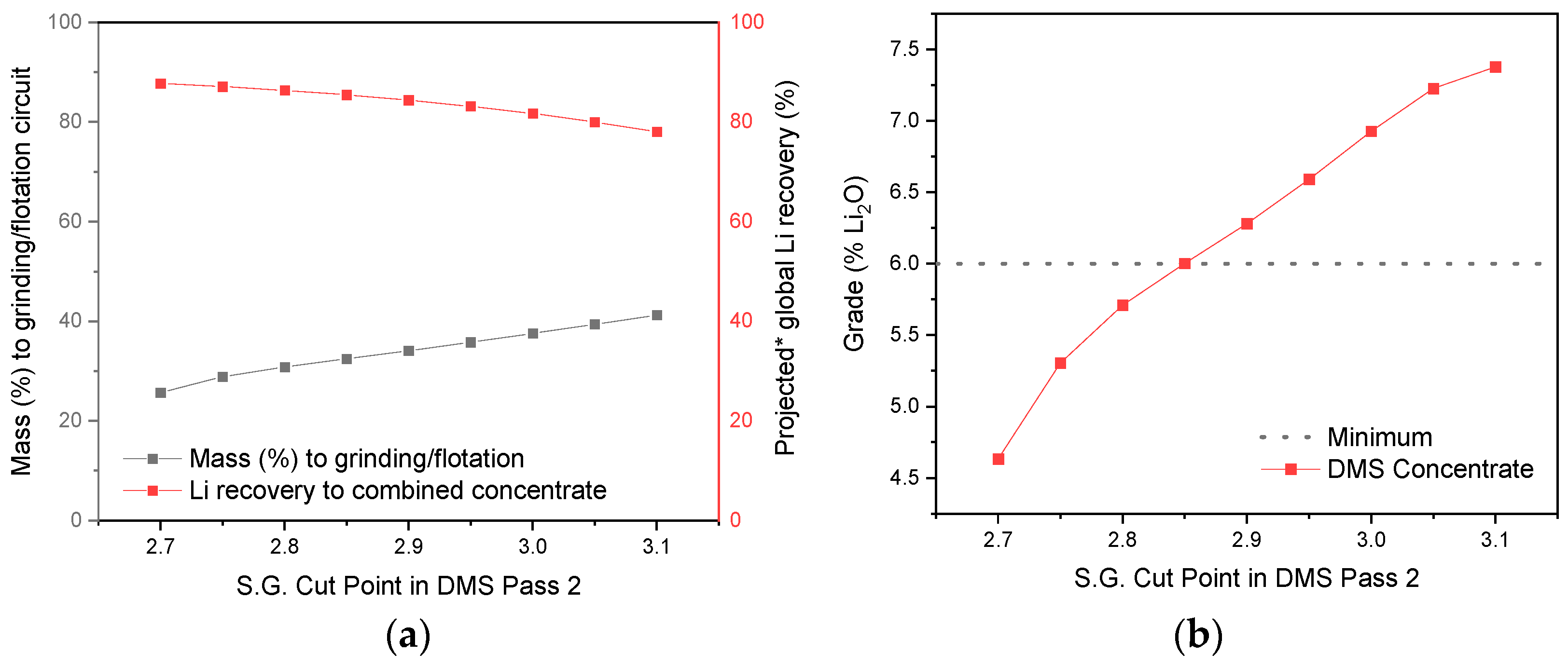
3.4. Effect of the S.G. Cut Point in DMS Operation
4. Summary and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jaskula, B. Lithium Statistics and Information; United States Geological Survey: Reston, VA, USA, 2021; p. 2. Available online: https://pubs.usgs.gov/periodicals/mcs2021/mcs2021-lithium.pdf (accessed on 16 February 2021).
- Jaskula, B. Lithium Statistics and Information; United States Geological Survey: Reston, VA, USA, 2020; p. 2. Available online: https://pubs.usgs.gov/periodicals/mcs2020/mcs2020-lithium.pdf (accessed on 16 February 2021).
- Jaskula, B. Lithium Statistics and Information; United States Geological Survey: Reston, VA, USA, 2019; p. 2. Available online: https://prd-wret.s3-us-west-2.amazonaws.com/assets/palladium/production/atoms/files/mcs-2019-lithi.pdf (accessed on 16 February 2021).
- Jaskula, B. Lithium Statistics and Information; United States Geological Survey: Reston, VA, USA, 2018; p. 2. Available online: https://s3-us-west-2.amazonaws.com/prd-wret/assets/palladium/production/mineral-pubs/lithium/mcs-2018-lithi.pdf (accessed on 16 February 2021).
- Jaskula, B. Lithium Statistics and Information; United States Geological Survey: Reston, VA, USA, 2017; p. 2. Available online: https://s3-us-west-2.amazonaws.com/prd-wret/assets/palladium/production/mineral-pubs/lithium/mcs-2017-lithi.pdf (accessed on 16 February 2021).
- Jaskula, B. Lithium Statistics and Information; United States Geological Survey: Reston, VA, USA, 2016; p. 2. Available online: https://s3-us-west-2.amazonaws.com/prd-wret/assets/palladium/production/mineral-pubs/lithium/mcs-2016-lithi.pdf (accessed on 16 February 2021).
- Jaskula, B. Lithium Statistics and Information; United States Geological Survey: Reston, VA, USA, 2015; p. 2. Available online: https://s3-us-west-2.amazonaws.com/prd-wret/assets/palladium/production/mineral-pubs/lithium/mcs-2015-lithi.pdf (accessed on 16 February 2021).
- Jaskula, B. Lithium Statistics and Information; United States Geological Survey: Reston, VA, USA, 2014; p. 2. Available online: https://s3-us-west-2.amazonaws.com/prd-wret/assets/palladium/production/mineral-pubs/lithium/mcs-2014-lithi.pdf (accessed on 16 February 2021).
- Jaskula, B. Lithium Statistics and Information; United States Geological Survey: Reston, VA, USA, 2013; p. 2. Available online: https://s3-us-west-2.amazonaws.com/prd-wret/assets/palladium/production/mineral-pubs/lithium/mcs-2013-lithi.pdf (accessed on 16 February 2021).
- Jaskula, B. Lithium Statistics and Information; United States Geological Survey: Reston, VA, USA, 2012; p. 2. Available online: https://s3-us-west-2.amazonaws.com/prd-wret/assets/palladium/production/mineral-pubs/lithium/mcs-2012-lithi.pdf (accessed on 16 February 2021).
- Jaskula, B. Lithium Statistics and Information; United States Geological Survey: Reston, VA, USA, 2011; p. 2. Available online: https://s3-us-west-2.amazonaws.com/prd-wret/assets/palladium/production/mineral-pubs/lithium/mcs-2011-lithi.pdf (accessed on 16 February 2021).
- Jaskula, B. Lithium Statistics and Information; United States Geological Survey: Reston, VA, USA, 2010; p. 2. Available online: https://s3-us-west-2.amazonaws.com/prd-wret/assets/palladium/production/mineral-pubs/lithium/mcs-2010-lithi.pdf (accessed on 16 February 2021).
- Tadesse, B.; Makuei, F.; Albijanic, B.; Dyer, L. The beneficiation of lithium minerals from hard rock ores: A review. Miner. Eng. 2019, 131, 170–184. [Google Scholar] [CrossRef]
- Bowell, R.J.; Lagos, L.; de los Hoyos, C.R.; Declercq, J. Classification and Characteristics of Natural Lithium Resources. Elements 2020, 16, 259–264. [Google Scholar] [CrossRef]
- Flexer, V.; Baspineiro, C.F.; Galli, C.I. Lithium recovery from brines: A vital raw material for green energies with a potential environmental impact in its mining and processing. Sci. Total Environ. 2018, 639, 1188–1204. [Google Scholar] [CrossRef] [PubMed]
- Tscherning, R.; Chapman, B. Navigating the emerging lithium rush: Lithium extraction from brines for clean-tech battery storage technologies. J. Energy Nat. Resour. Law 2021, 39, 13–42. [Google Scholar] [CrossRef]
- Liu, W.; Agusdinata, D.B. Interdependencies of lithium mining and communities sustainability in Salar de Atacama, Chile. J. Clean. Prod. 2020, 260, 120838. [Google Scholar] [CrossRef]
- Mohr, S.H.; Mudd, G.M.; Giurco, D. Lithium Resources and Production: Critical Assessment and Global Projections. Minerals 2012, 2, 65–84. [Google Scholar] [CrossRef]
- Ambrose, H.; Kendall, A. Understanding the future of lithium: Part 1, resource model. J. Ind. Ecol. 2019, 24, 80–89. [Google Scholar] [CrossRef]
- NRCan, Canada and U.S. Finalize Joint Action Plan on Critical Minerals Collaboration; Natural Resources Canada: Ottawa, ON, Canada, 2020. [Google Scholar]
- Gibson, C.E.; Aghamirian, M.; Grammatikopoulos, T. A review: The beneficiation of lithium minerals from hard rock deposits. Min. Eng. 2017, 69, 18–27. [Google Scholar]
- Çetinkaya, Z.; Bayat, O. Upgrading low-rank coals (Çan, Çanakkale/Turkey) by float-sink separation in dense media. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 42, 113–120. [Google Scholar] [CrossRef]
- Filippov, L.; Farrokhpay, S.; Lyo, L.; Filippova, I. Spodumene Flotation Mechanism. Minerals 2019, 9, 372. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, S.; Wang, W.; Zhang, J.; Yan, W.; Deng, J.; Feng, Q.; Huang, Y. The effects of Ca(II) and Mg(II) ions on the flotation of spodumene using NaOL. Miner. Eng. 2015, 79, 40–46. [Google Scholar] [CrossRef]
- Moon, K.S.; Fuerstenau, D.W. Surface crystal chemistry in selective flotation of spodumene (LiAl[SiO3]2) from other aluminosilicates. Int. J. Miner. Process. 2003, 72, 11–24. [Google Scholar] [CrossRef]
- Shu, K.; Xu, L.; Wu, H.; Tang, Z.; Luo, L.; Yang, J.; Xu, Y.; Feng, B. Selective flotation separation of spodumene from feldspar using sodium alginate as an organic depressant. Sep. Purif. Technol. 2020, 248, 117122. [Google Scholar] [CrossRef]
- Xie, R.; Zhu, Y.; Liu, J.; Li, Y.; Wang, X.; Shumin, Z. Research Status of Spodumene Flotation: A Review. Miner. Process. Extr. Metall. Rev. 2020, 42, 321–334. [Google Scholar] [CrossRef]
- Zhu, G.; Zhao, Y.; Zheng, X.; Wang, Y.; Zheng, H.; Lu, D. Surface features and flotation behaviors of spodumene as influenced by acid and alkali treatments. Appl. Surf. Sci. 2020, 507, 145058. [Google Scholar] [CrossRef]
- Legault-Seguin, E.; Mohns, C.; Rylatt, M. Dense Media Separation—An effective and robust pre-concentration technology. In Proceedings of the 48th Annual Canadian Minerals Processors Operators Conference, Ottawa, ON, Canada, 19–21 January 2016; pp. 381–400. [Google Scholar]
- Redeker, I.H. Flotation of feldspar, spodumene, quartz and mica from pegmatites in North Carolina, USA. In Proceedings of the 13th Canadian Mineral Processors Annual Meeting, Ottawa, ON, Canada, 20 January 1981. [Google Scholar]
- Far Resources. Hidden Lake Lithium Project, Northwest Territories. Available online: https://farresources.com/hidden-lake-lithium/ (accessed on 16 February 2021).
- Wills, B.A.; Finch, J.A. Wills’ Mineral Processing Technology: An Introduction to the Practical Aspects of Ore Treatment and Mineral Recovery, 8th ed.; Butterworth-Heinemann: Oxford, UK, 2016; ISSN 02678179. [Google Scholar]
- Amarante, M.M.; de Sousa, A.B.; Leite, M.M. Processing a spodumene ore to obtain lithium concentrates for addition to glass and ceramic bodies. Miner. Eng. 1999, 12, 433–436. [Google Scholar] [CrossRef]
- Rockwood Lithium. Spodumene Concentrate SC6.5. 2015. Available online: https://www.albemarle-lithium.com/pdf/402203.pdf/ (accessed on 18 March 2020).


| Element (wt.%) | Li | Li2O | Al | Ca | Fe | K | Mg | Na | P | Rb | Si |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample 1 | 0.62 | 1.33 | 8.84 | 0.19 | 0.25 | 1.71 | 0.04 | 3.35 | 0.59 | 0.08 | 34.0 |
| Sample 2 | 0.63 | 1.36 | 8.83 | 0.15 | 0.27 | 2.29 | 0.02 | 3.10 | 0.39 | 0.09 | 34.1 |
| Sample 3 | 0.60 | 1.29 | 8.83 | 0.20 | 0.25 | 1.67 | 0.03 | 3.41 | 0.61 | 0.08 | 34.0 |
| Sample 4 | 0.63 | 1.36 | 8.89 | 0.21 | 0.23 | 1.84 | 0.04 | 3.33 | 0.80 | 0.10 | 33.7 |
| Sample | Sample 1 | Sample 2 | Sample 3 | Sample 4 |
|---|---|---|---|---|
| Spodumene | 15.8 | 16.1 | 14.2 | 14.5 |
| Quartz | 27.9 | 26.5 | 28.5 | 27.3 |
| Plagioclase | 38.5 | 36.2 | 39.8 | 39.3 |
| K-Feldspar | 8.66 | 14.0 | 9.16 | 9.66 |
| Muscovite | 4.86 | 4.20 | 4.48 | 4.03 |
| Biotite | 0.02 | 0.03 | 0.01 | 0.01 |
| Clays | 1.09 | 0.91 | 0.93 | 1.07 |
| Apatite | 0.28 | 0.26 | 0.34 | 0.30 |
| Montebrasite | 2.68 | 1.54 | 2.36 | 3.69 |
| Other | 0.20 | 0.27 | 0.22 | 0.17 |
| Total | 100 | 100 | 100 | 100 |
| Element/Oxide, % | Li | Li2O | SiO2 | Al2O3 | Fe2O3 | MgO | CaO | Na2O | K2O | P2O5 | MnO | LOI | Sum |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Head Composite | 0.64 | 1.38 | 72.5 | 16.7 | 0.23 | 0.04 | 0.26 | 4.18 | 2.41 | 1.40 | 0.02 | 0.82 | 98.6 |
| Mineral Name | Mineral Formula | Abundance (wt. %) | Specific Gravity (S.G.) | Average S.G. | CC * in Water S.G. = 1.0 | CC * in Heavy Liquid S.G. = 2.5 |
|---|---|---|---|---|---|---|
| Spodumene | LiAlSi2O6 | 16.9 | 3.1–3.2 | 3.15 | 1.00 | 1.00 |
| Montebrasite | LiAl(PO4)(OH) | 1.40 | 2.98–3.04 | 3.01 | 1.07 | 1.27 |
| Albite (plagioclase) | Na(AlSi3O8) | 38.6 | 2.6–2.65 | 2.63 | 1.32 | 5.20 |
| Quartz | SiO2 | 23.8 | 2.65–2.66 | 2.65 | 1.30 | 4.26 |
| Microcline (k-feldspar) | K(AlSi3O8) | 11.0 | 2.54–2.57 | 2.56 | 1.38 | 11.8 |
| Muscovite | KAl2(AlSi3O10)(OH)2 | 6.28 | 2.77–2.88 | 2.83 | 1.18 | 2.00 |
| Other | - | 2.81 | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gibson, C.E.; Aghamirian, M.; Grammatikopoulos, T.; Smith, D.L.; Bottomer, L. The Recovery and Concentration of Spodumene Using Dense Media Separation. Minerals 2021, 11, 649. https://doi.org/10.3390/min11060649
Gibson CE, Aghamirian M, Grammatikopoulos T, Smith DL, Bottomer L. The Recovery and Concentration of Spodumene Using Dense Media Separation. Minerals. 2021; 11(6):649. https://doi.org/10.3390/min11060649
Chicago/Turabian StyleGibson, Charlotte E., Massoud Aghamirian, Tassos Grammatikopoulos, Darren L. Smith, and Lindsay Bottomer. 2021. "The Recovery and Concentration of Spodumene Using Dense Media Separation" Minerals 11, no. 6: 649. https://doi.org/10.3390/min11060649
APA StyleGibson, C. E., Aghamirian, M., Grammatikopoulos, T., Smith, D. L., & Bottomer, L. (2021). The Recovery and Concentration of Spodumene Using Dense Media Separation. Minerals, 11(6), 649. https://doi.org/10.3390/min11060649






