Using Calcined Marls as Non-Common Supplementary Cementitious Materials—A Critical Review
Abstract
1. Introduction
2. General Background of Supplementary Cementitious Materials (SCMs)
2.1. Introduction
2.2. Types of SCM
2.2.1. Fly Ash (FA)
2.2.2. Blast Furnace Slag
2.2.3. Calcined Clays
2.2.4. SCM Based on Calcined Clay and Limestone
2.2.5. Natural SCMs
3. Calcined Marls as SCMs
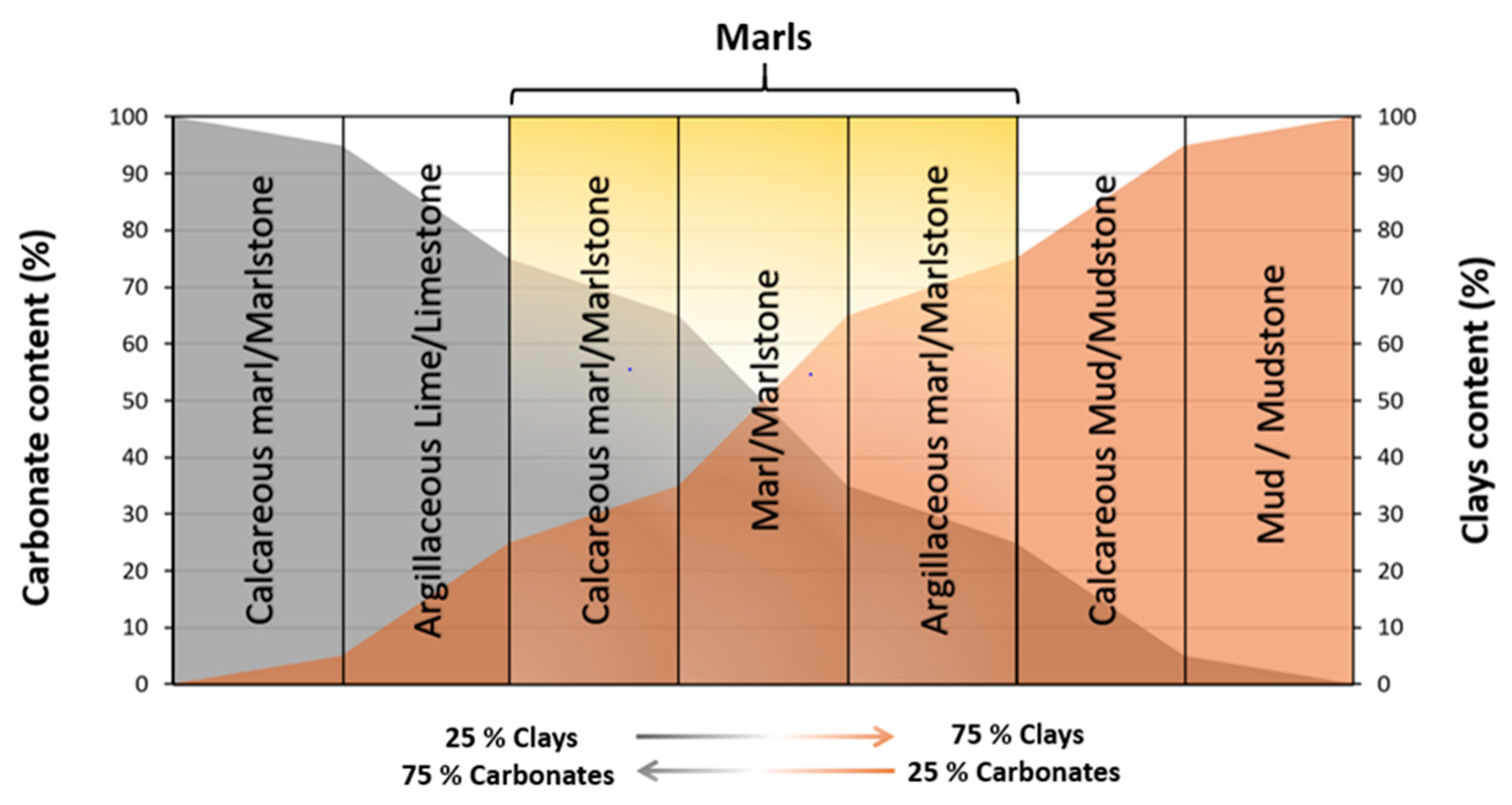
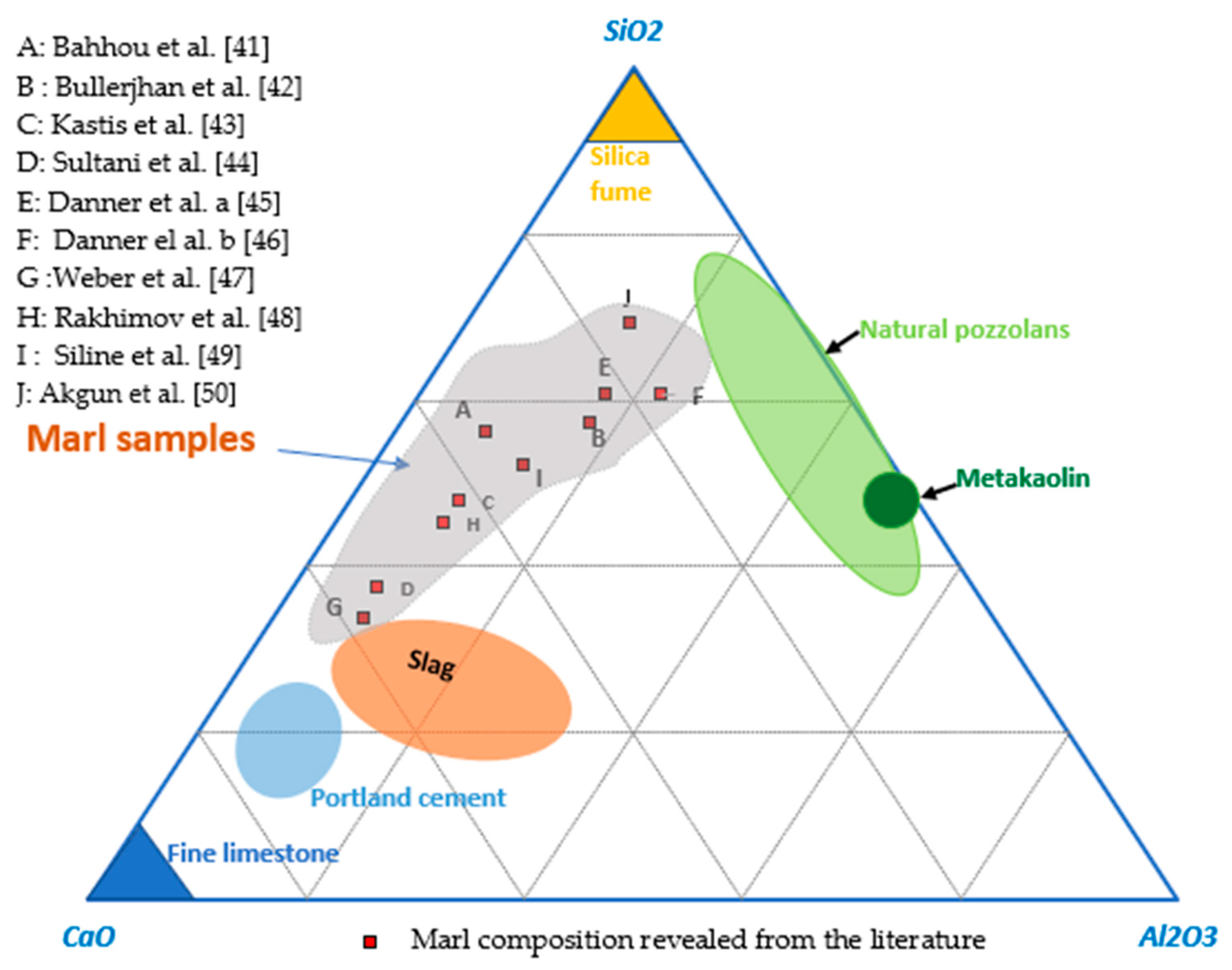
3.1. Characteristics of Marls
3.2. Activation Methods
3.2.1. Thermal Activation
3.2.2. Chemical Activation
3.2.3. Mechanical Activation
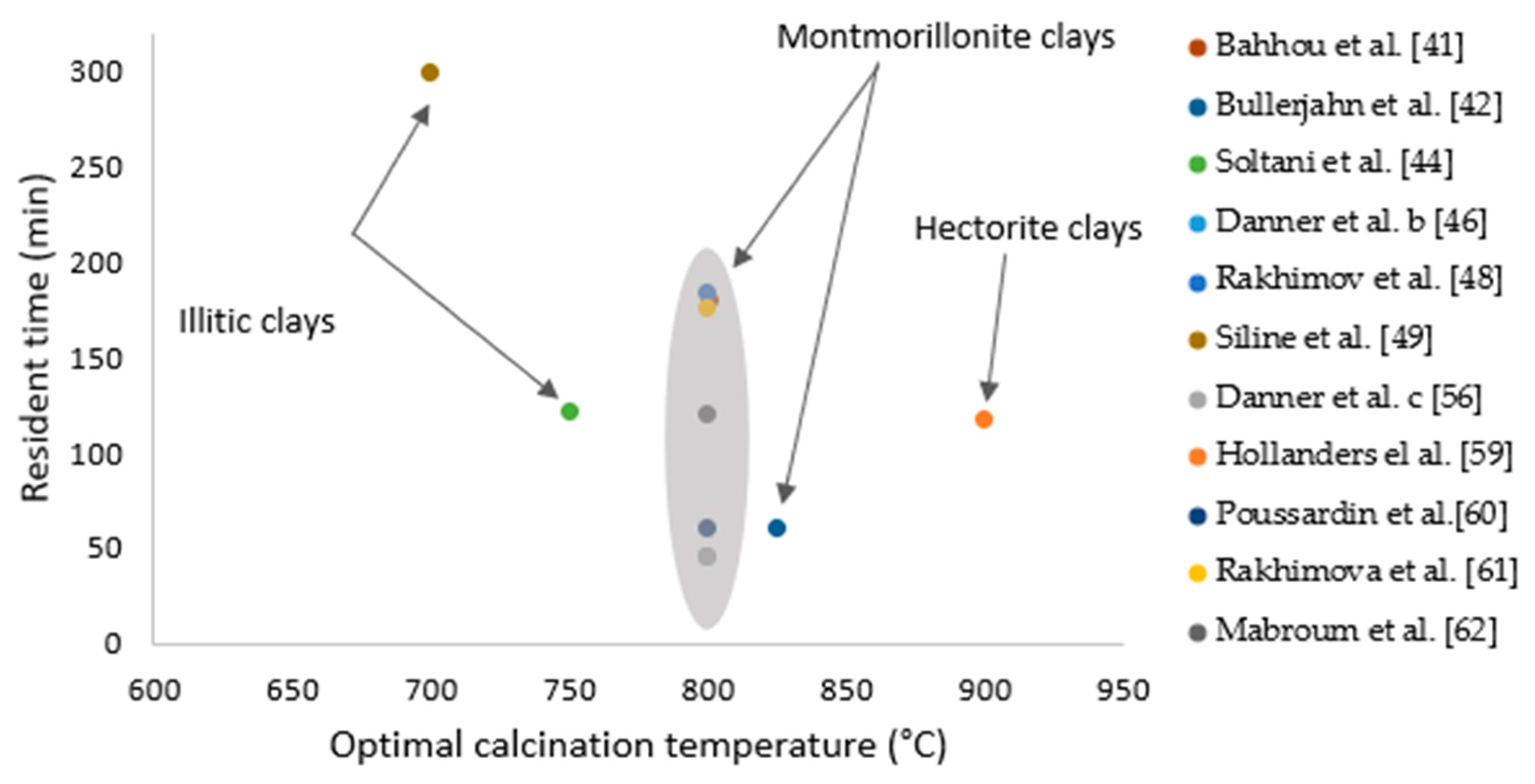
3.3. Factors Impacting the Quality of Calcined Marls
3.4. SCMs Characterization Techniques and Reactivity Tests
3.5. Calcined Marl Hydration and Pozzolanic Reactions
3.6. Kinetic of Calcined Marl-Based Cements
3.7. Impact of Calcined Marls on Mechanical Performance and Durability
3.8. Environmental Benefits of Using Calcined Marl as SCMs in Cement Production
4. Insights, Recommendations, and Conclusions
- The findings have used thermal treatment as an activation method: 800 °C was often the effective calcination temperature, which guarantees both maximum dehydroxylarion and moderation of decarbonation.
- The type of clay minerals existing in the marl matters. Their dehydroxylation, linked to a deterioration of the crystalline structure, is a preliminary requirement for highly effective reactions to generate reactive compounds.
- Many different parameters strongly affect the pozzolanic activity, including its fineness, w/c, chemical composition, calcination conditions and additives.
- Adding plasticizers is an overriding procedure to compromise the amount of water absorbed by the calcined marl, thus controlling the fluidity of the paste. However, a new approach of formulating new plasticizers needs to be found for this particular material in order to overcome intercalation issues.
- Complementary tests are needed to evaluate the reactivity of these calcined marl-based cements, as the existing ones may be affected by the high calcium carbonate content.
- Most of the calcined marls generate reactive silica and alumina phases after their calcination. Those phases are implicated in the production of calcium silicate hydrates through hydration of Portland cement along with the formation of calcium hydrocarboaluminates hydrates.
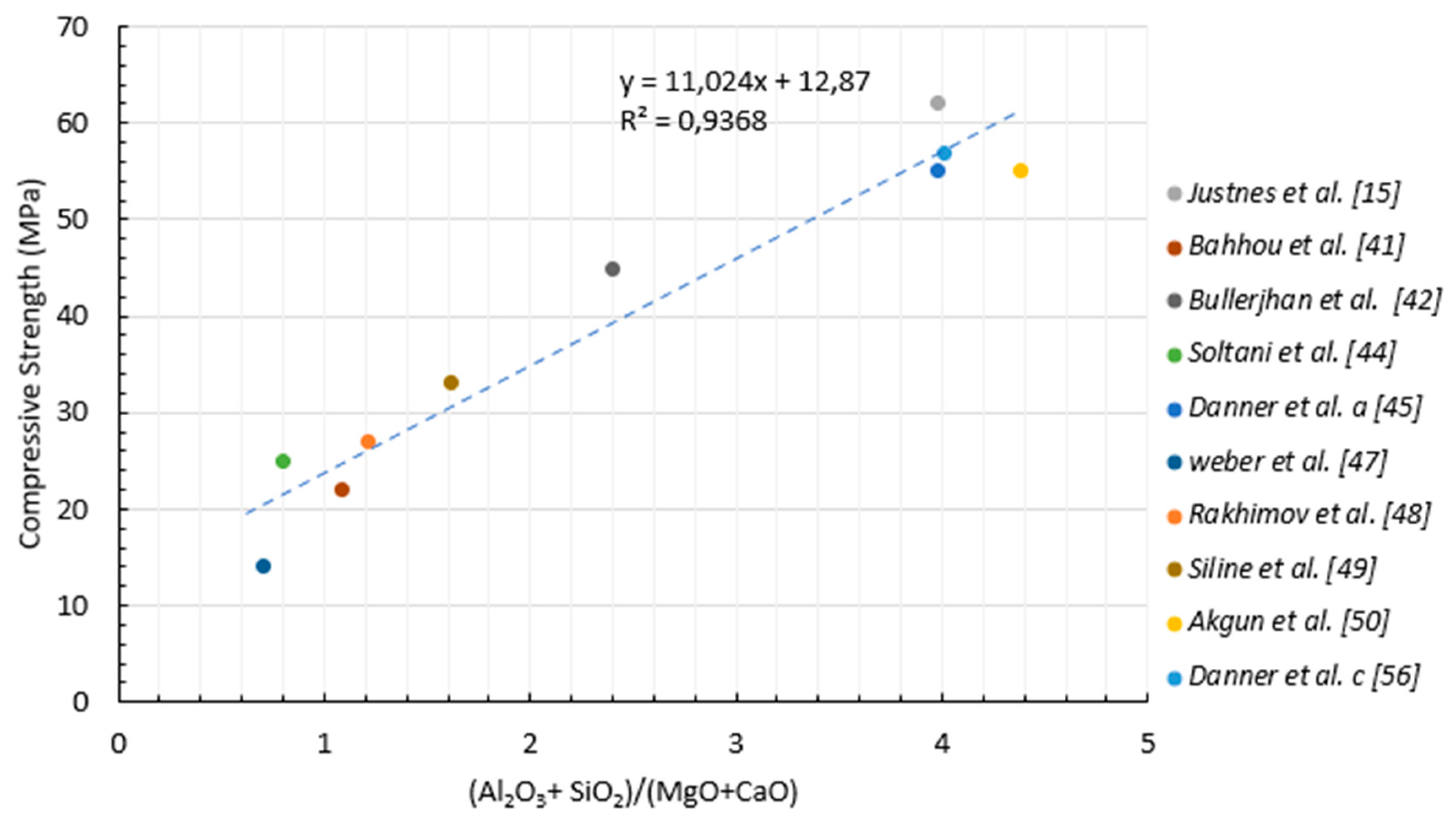
- A strong correlation between compressive strength values and (Al2O3 + SiO2) / (MgO + CaO) was established. Higher carbonate oxides at the expense of Al2O3 + SiO2 content caused a drop in CS values. A careful balance between the chemical composition of the marls should be considered in accordance with the proposed model to ensure the desired CS development.
- Corrosion and chloride resistance are the major advantages of using calcined marl as SCMs, while the carbonation seems to be the only drawback in terms of its durability.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Østnor, T.A.; Justnes, H. Durability of mortar with calcined marl as supplementary cementing material. Adv. Cem. Res. 2014, 26, 344–352. [Google Scholar] [CrossRef]
- Cembureau. World Statistical Review; Cembureau: Brussels, Belgium, 2010. [Google Scholar]
- FAO. Food Wastage Footprint Impacts on Natural Resources; Food and Agriculture Organisation of the United Nations: Rome, Italy, 2013. [Google Scholar]
- Li, N.; Ma, D.; Chen, W. Projection of Cement Demand and Analysis of the Impacts of Carbon Tax on Cement Industry in China. Energy Procedia 2015, 75, 1766–1771. [Google Scholar] [CrossRef]
- IFEP, Institut Français de L’énergie, de L’environnement et de la Francophonie. Diagnostic Energétique D’une Cimenterie; IFEP: Paris, France, 2001. [Google Scholar]
- Davidovits, J. The European Research Project GEOASH: Geopolymer Cement based on European Coal Fly Ash; Technical paper; Geopolymer Institute: Saint-Quentin, France, 2014. [Google Scholar]
- Steinberger, J.K.; Krausmann, F.; Eisenmenger, N. Global patterns of materials use: A socioeconomic and geophysical analysis. Ecol. Econ. 2010, 69, 1148–1158. [Google Scholar] [CrossRef]
- Cembureau. Best Available Techniques for the Cement Industry; Cembureau: Brussels, Belgium, 1999. [Google Scholar]
- IEA. Technology Roadmap: Cement—Foldout; International Energy Agency, OECD/IEA: Paris, France, 2009. [Google Scholar]
- Wirthwein, R.; Emberger, B. Burners for alternative fuels utilisation: Optimization of kiln firing systems for advanced alternative fuel co-firing. Cem. Int. 2010, 8, 42–46. [Google Scholar]
- ECRA. Deployment of CCS in the Cement Industry; ECRA: Duesseldorf, Germany, 2013.
- Schneider, M.; Romer, M.; Tschudin, M.; Bolio, H. Sustainable cement production-present and future. Cem. Concr. Res. 2011, 41, 642–650. [Google Scholar] [CrossRef]
- Barker, D.J.; Turner, S.A.; Napier-Moore, P.A.; Clark, M.; Davison, J.E. CO2 Capture in the Cement Industry. Energy Procedia 2009, 1, 87–94. [Google Scholar] [CrossRef]
- Smith, A.; El Hafiane, Y.; El Khessaimi, Y.; Faure, A. Some examples of mineral eco-materials. J. Eur. Ceram. Soc. 2019, 39, 3408–3415. [Google Scholar] [CrossRef]
- Justnes, H.; De Weerdt, K.; Vikan, H.; Østnor, T. Calcined marl and clay as mineral addition for more sustainable concrete structure. In Proceedings of the 36th Conference on Our World in Concrete & Structures, Singapore, 14–16 August 2011. [Google Scholar]
- Thomas, M. Supplementary Cementing Materials in Concrete; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Snellings, R.; Mertens, G.; Elsen, J. Supplementary cementitious materials. Rev. Mineral. Geochem. 2012, 74, 211–278. [Google Scholar] [CrossRef]
- Miller, S.A. Supplementary cementitious materials to mitigate greenhouse gas emissions from concrete: Can there be too much of a good thing? J. Clean. Prod. 2018, 178, 587–598. [Google Scholar] [CrossRef]
- Kupwade-Patil, K.; de Wolf, C.; Chin, S.; Ochsendorf, J.; Hajiah, A.E.; Al-Mumin, A.; Büyüköztürk, O. Impact of Embodied Energy on materials/buildings with partial replacement of ordinary Portland Cement (OPC) by natural Pozzolanic Volcanic Ash. J. Clean. Prod. 2018, 177, 547–554. [Google Scholar] [CrossRef]
- Scrivener, K.L.; John, V.M.; Gartner, E.M. Cement and Concrete Research Eco-e ffi cient cements: Potential economically viable solutions for a low-CO 2 cement-based materials industry. Cem. Concr. Res. 2018. [Google Scholar] [CrossRef]
- Juenger, M.C.G.; Snellings, R.; Bernal, S.A. Cement and Concrete Research Supplementary cementitious materials: New sources, characterization, and performance insights. Cem. Concr. Res. 2019, 122, 257–273. [Google Scholar] [CrossRef]
- Snellings, R. Assessing, Understanding and Unlocking Supplementary Cementitious Materials. RILEM Tech. Lett. 2016, 1, 50. [Google Scholar] [CrossRef]
- Martirena, F. Calcined Clays for Sustainable Concrete. In Proceedings of the 1st International Conference on Calcined Clays for Sustainable Concrete, Lausanne, Switzerland, 23–25 June 2015; Springer: Berlin/Heidelberg, Germany, 2015; Volume 10, ISBN 978-94-017-9938-6. [Google Scholar]
- Coppola, L.; Bellezze, T.; Belli, A.; Bignozzi, M.C.; Bolzoni, F.; Brenna, A.; Cabrini, M.; Candamano, S.; Cappai, M.; Caputo, D.; et al. Binders alternative to Portland cement and waste management for sustainable construction—Part 1. J. Appl. Biomater. Funct. Mater. 2018, 16, 186–202. [Google Scholar] [CrossRef] [PubMed]
- Flower, D.J.M.; Sanjayan, J.G. Green house gas emissions due to concrete manufacture. Int. J. Life Cycle Assess. 2007, 12, 282–288. [Google Scholar] [CrossRef]
- Scrivener, K.; Martirena, F.; Bishnoi, S.; Maity, S. Calcined clay limestone cements (LC3). Cem. Concr. Res. 2017, 114, 49–56. [Google Scholar] [CrossRef]
- Neville, A. Concrete: Neville’s Insights and Issues; ICE Publishing: London, UK, 2006. [Google Scholar]
- Behim, M.; Redjel, B.; Jauberthie, R. Réactivité du laitier de hauts fourneaux d’Annaba (Algérie) en substitution partielle du ciment. J. Phys. IV 2002, 12, 223–228. [Google Scholar] [CrossRef]
- Lognot, I. Etude de L’hydratation de Laitier de Hauts Fourneaux et de Ses Produits en Présence de Différents Activants. Applications Aux Coulis D’injection. Ph.D. Thesis, University of Burgundy, Dijon, France, 1996. [Google Scholar]
- Zhou, D. Developing Supplementary Cementitious Materials from Waste London Clay. Ph.D. Thesis, Imperial College, London, UK, October 2016. [Google Scholar]
- Crossin, E. The greenhouse gas implications of using ground granulated blast furnace slag as a cement substitute. J. Clean. Prod. 2015, 95, 101–108. [Google Scholar] [CrossRef]
- Mielenz, R.K.G. Natural pozzolans for concrete. Econ. Geol. 1951, 46, 311–328. [Google Scholar] [CrossRef]
- Mielenz, R.; Witte, L. Effect of calcination on natural pozzolans, use of pozzolanic materials in mortars. In Proceedings of the Symposium on Use of Pozzolanic Materials in Mortars and Concretes, San Francisco, CA, USA, 10–14 October 1949. [Google Scholar]
- Fernandez, R.; Martirena, F.; Scrivener, K.L. The origin of the pozzolanic activity of calcined clay minerals: A comparison between kaolinite, illite and montmorillonite. Cem. Concr. Res. 2011, 41, 113–122. [Google Scholar] [CrossRef]
- Taylor-Lange, S.C.; Lamon, E.L.; Riding, K.A.; Juenger, M.C.G. Calcined kaolinite–bentonite clay blends as supplementary cementitious materials. Appl. Clay Sci. 2015, 108, 84–93. [Google Scholar] [CrossRef]
- Menéndez, G.; Bonavetti, V.; Irassar, E.F. Strength development of ternary blended cement with limestone filler and blast-furnace slag. Cem. Concr. Compos. 2003, 25, 61–67. [Google Scholar] [CrossRef]
- Marchetti, G.; Pokorny, J.; Tironi, A.; Trezza, M.A.; Rahhal, V.F.; Pavlík, Z.; Černý, R.; Irassar, E.F. Blended Cements with Calcined Illitic Clay: Workability and Hydration; RILEM Bookseries; Springer: Dordrecht, The Netherlands, 2018; Volume 16, pp. 310–317. [Google Scholar]
- Scrivener, K.; Avet, F.; Zunino, F.; Ston, J. More sustainable constructions using limestone calcined clay cement (LC3). Sustain. Constr. Mater. Technol. 2019, 3, 2–6. [Google Scholar] [CrossRef]
- Yanguatin, H.; Ramírez, J.H.; Tironi, A.; Tobón, J.I. Effect of thermal treatment on pozzolanic activity of excavated waste clays. Constr. Build. Mater. 2019, 211, 814–823. [Google Scholar] [CrossRef]
- Ghafari, E.; Feys, D.; Khayat, K. Feasibility of using natural SCMs in concrete for infrastructure applications. Constr. Build. Mater. 2016, 127, 724–732. [Google Scholar] [CrossRef]
- Bahhou, A.; Taha, Y.; El Khessaimi, Y.; Idrissi, H.; Hakkou, R.; Amalik, J.; Benzaazoua, M. Use of phosphate mine by-products as supplementary cementitious materials. Mater. Today Proc. 2020, 37, 3781–3788. [Google Scholar] [CrossRef]
- Bullerjahn, F.; Zajac, M.; Pekarkova, J.; Nied, D. Novel SCM produced by the co-calcination of aluminosilicates with dolomite. Cem. Concr. Res. 2020, 134, 106083. [Google Scholar] [CrossRef]
- Kastis, D.; Kakali, G.; Tsivilis, S.; Stamatakis, M.G. Properties and hydration of blended cements with calcareous diatomite. Cem. Concr. Res. 2006, 36, 1821–1826. [Google Scholar] [CrossRef]
- Soltani, A.; Tarighat, A.; Varmazyari, M. Calcined Marl and Condensed Silica Fume as Partial Replacement for Ordinary Portland Cement. Int. J. Civ. Eng. 2018, 16, 1549–1559. [Google Scholar] [CrossRef]
- Danner, T.; Justnes, H.; Geiker, M.; Lauten, R.A. Phase changes during the early hydration of Portland cement with Ca-lignosulfonates. Cem. Concr. Res. 2015, 69, 50–60. [Google Scholar] [CrossRef]
- Danner, T.; Norden, G.; Justnes, H. Applied Clay Science Characterisation of calcined raw clays suitable as supplementary cementitious materials. Appl. Clay Sci. 2018, 162, 391–402. [Google Scholar] [CrossRef]
- Weber, J.; Gadermayr, N.; Kozłowski, R.; Mucha, D.; Hughes, D.; Jaglin, D.; Schwarz, W. Microstructure and mineral composition of Roman cements produced at defined calcination conditions. Mater. Charact. 2007, 58, 1217–1228. [Google Scholar] [CrossRef]
- Rakhimov, R.Z.; Rakhimova, N.R.; Gaifullin, A.R.; Morozov, V.P. Properties of Portland cement pastes enriched with addition of calcined marl. J. Build. Eng. 2017, 11, 30–36. [Google Scholar] [CrossRef]
- Mohammed, S.; Elhem, G.; Mekki, B. Valorization of pozzolanicity of Algerian clay: Optimization of the heat treatment and mechanical characteristics of the involved cement mortars. Appl. Clay Sci. 2016, 132–133, 711–721. [Google Scholar] [CrossRef]
- Akgün, Y. Alternatif puzolan kalsine marn içeren sürdürülebilir katkılı çimentolar. DÜMF Mühendislik Derg. 2019, 10, 779–789. [Google Scholar] [CrossRef]
- Trezza, M.A.; Tironi, A.; Irassar, E.F. Thermal Activation of Two Complex Clays (Kaolinite-Pyrophillite-Illite) from Tandilia System, Buenos Aires, Argentina. In Calcined Clays for Sustainable Concrete; RILEM Bookseries; Springer: Dordrecht, The Netherlands, 2018; Volume 16. [Google Scholar]
- Zhou, D.; Wang, R.; Tyrer, M.; Wong, H.; Cheeseman, C. Sustainable infrastructure development through use of calcined excavated waste clay as a supplementary cementitious material. J. Clean. Prod. 2017, 168, 1180–1192. [Google Scholar] [CrossRef]
- Antoni, M.; Rossen, J.; Martirena, F.; Scrivener, K. Cement and Concrete Research Cement substitution by a combination of metakaolin and limestone. Cem. Concr. Res. 2012, 42, 1579–1589. [Google Scholar] [CrossRef]
- Habert, G.; Choupay, N.; Escadeillas, G.; Guillaume, D.; Montel, J.M. Clay content of argillites: Influence on cement based mortars. Appl. Clay Sci. 2009, 43, 322–330. [Google Scholar] [CrossRef]
- He, C.; Osbaeck, B.; Makovicky, E. Pozzolanic reactions of six principal clay minerals: Activation, reactivity assessments and technological effects. Cem. Concr. Res. 1995, 25, 1691–1702. [Google Scholar] [CrossRef]
- Danner, T.; Norden, G.; Justnes, H. Calcareous smectite clay as a pozzolanic alternative to kaolin. Eur. J. Environ. Civ. Eng. 2019, 1–18. [Google Scholar] [CrossRef]
- Danner, T.; Norden, G.; Justnes, H. The Effect of Calcite in the Raw Clay on the Pozzolanic Activity of Calcined Illite and Smectite. In Calcined Clays for Sustainable Concrete; Springer: Singapore, 2019; Volume 25. [Google Scholar]
- Weerdt, D.; Building, S. Microstructure of binder from the pozzolanic reaction between lime and siliceous fly ash. In Proceedings of the 1st International Conference on Microstructure Related Durability of Cementitious Composites, Nanjing, China, 13–15 October 2008; pp. 107–116. [Google Scholar]
- Hollanders, S.; Adriaens, R.; Skibsted, J.; Cizer, Ö.; Elsen, J. Pozzolanic reactivity of pure calcined clays. Appl. Clay Sci. 2016, 132–133, 552–560. [Google Scholar] [CrossRef]
- Poussardin, V.; Paris, M.; Tagnit-Hamou, A.; Deneele, D. Potential for calcination of a palygorskite-bearing argillaceous carbonate. Appl. Clay Sci. 2020, 198, 105846. [Google Scholar] [CrossRef]
- Rakhimova, N.; Rakhimov, R.; Morozov, V.; Potapova, L. Marl as a supplementary material to alkali- activated blended cements. Eur. J. Environ. Civ. Eng. 2019, 1–18. [Google Scholar] [CrossRef]
- Mabroum, S.; Aboulayt, A.; Taha, Y.; Benzaazoua, M.; Semlal, N.; Hakkou, R. Elaboration of geopolymers based on clays by-products from phosphate mines for construction applications. J. Clean. Prod. 2020, 261, 121317. [Google Scholar] [CrossRef]
- Danner, T.; Østnor, T.; Justnes, H. Thermally activated marl as a pozzolan for cementitious based products. In Proceedings of the Twin Covilha International Conferences on Civil Engineering—Towards a Better Environment and the Concrete Future, Covilha, Portugal, 26–29 May 2013. [Google Scholar]
- Komadel, P. Chemically modified smectites. Clay Miner. 2003, 38, 127–138. [Google Scholar] [CrossRef]
- Sanz, J.; Massiot, D. Nuclear Magnetic Resonance Spectroscopy, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, ISBN 9780080982595. [Google Scholar]
- Provis, J.L. Activating solution chemistry for geopolymers. Geopolymers Struct. Process. Prop. Ind. Appl. 2009, 50–71. [Google Scholar] [CrossRef]
- Mabroum, S.; Moukannaa, S.; El Machi, A.; Taha, Y.; Benzaazoua, M.; Hakkou, R. Mine wastes based geopolymers: A critical review. Clean. Eng. Technol. 2020, 1, 100014. [Google Scholar] [CrossRef]
- Moukannaa, S.; Bagheri, A.; Benzaazoua, M.; Sanjayan, J.G.; Pownceby, M.I.; Hakkou, R. Elaboration of alkali activated materials using a non-calcined red clay from phosphate mines amended with fly ash or slag: A structural study. Mater. Chem. Phys. 2020, 256, 123678. [Google Scholar] [CrossRef]
- Baláž, P.; Achimovicová, M.; Baláž, M.; Billik, P.; Zara, C.Z.; Criado, J.M.; Delogu, F.; Dutková, E.; Gaffet, E.; Gotor, F.J.; et al. Hallmarks of mechanochemistry: From nanoparticles to technology. Chem. Soc. Rev. 2013, 42, 7571–7637. [Google Scholar] [CrossRef]
- Hennart, S.L.A.; Wildeboer, W.J.; van Hee, P.; Meesters, G.M.H. Identification of the grinding mechanisms and their origin in a stirred ball mill using population balances. Chem. Eng. Sci. 2009, 64, 4123–4130. [Google Scholar] [CrossRef]
- Aglietti, E.F.; Porto Lopez, J.M.; Pereira, E. Mechanochemical effects in kaolinite grinding. I. Textural and physicochemical aspects. Int. J. Miner. Process. 1986, 16, 125–133. [Google Scholar] [CrossRef]
- Danner, T. The Influence of Production Parameters on Pozzolanic Reactivity of Calcined Clays. Nord. Concr. Res. 2018, 1–12. [Google Scholar] [CrossRef]
- Cyr, M.; Lawrence, P.; Ringot, E. Efficiency of mineral admixtures in mortars: Quantification of the physical and chemical effects of fine admixtures in relation with compressive strength. Cem. Concr. Res. 2006, 36, 264–277. [Google Scholar] [CrossRef]
- Collepardi, M.; Monosi, S.; Moriconi, G.; Pauri, M. Influence of gluconate, lignosulfonate or glucose on the C3A hydration in the presence of gypsum with or without lime. Cem. Concr. Res. 1984, 14, 105–112. [Google Scholar] [CrossRef]
- Ng, S.; Justnes, H. Influence of dispersing agents on the rheology and early heat of hydration of blended cements with high loading of calcined marl. Cem. Concr. Compos. 2015, 60, 123–134. [Google Scholar] [CrossRef]
- Bich, C.; Ambroise, J.; Péra, J. Influence of degree of dehydroxylation on the pozzolanic activity of metakaolin. Appl. Clay Sci. 2009, 44, 194–200. [Google Scholar] [CrossRef]
- Moodi, F.; Ramezanianpour, A.A.; Safavizadeh, A.S. Evaluation of the optimal process of thermal activation of kaolins. Sci. Iran. 2011, 18, 906–912. [Google Scholar] [CrossRef]
- Chikouche, M.A.; Ghorbel, E.; Bibi, M. The possibility of using dredging sludge in manufacturing cements: Optimization of heat treatment cycle and ratio replacement. Constr. Build. Mater. 2016, 106, 330–341. [Google Scholar] [CrossRef]
- Berodier, E.; Scrivener, K. Understanding the filler effect on the nucleation and growth of C-S-H. J. Am. Ceram. Soc. 2014, 97, 3764–3773. [Google Scholar] [CrossRef]
- Ouyang, K.; Nayak, S.; Lee, Y.; Kim, E.; Wu, M.; Tallarida, C.S.; Rawls, S.M. Behavioral effects of Splenda, Equal and sucrose: Clues from planarians on sweeteners. Neurosci. Lett. 2017, 636, 213–217. [Google Scholar] [CrossRef][Green Version]
- Krishnan, S.; Bishnoi, S. Understanding the hydration of dolomite in cementitious systems with reactive aluminosilicates such as calcined clay. Cem. Concr. Res. 2018, 108, 116–128. [Google Scholar] [CrossRef]
- Skibsted, J.; Snellings, R. Reactivity of supplementary cementitious materials (SCMs) in cement blends. Cem. Concr. Res. 2019, 124, 105799. [Google Scholar] [CrossRef]
- ASTM International. ASTMC1709-18 Standard Guide for Evaluation of Alternative Supplementary Cementitious Materials (ASCM) for Use in Concrete; ASTM International: West Conshohocken, PA, USA, 2018. [Google Scholar]
- Keppert, M.; Scheinherrová, L.; Jerman, M.; Doušová, B.; Kobera, L.; Brus, J.; Černý, R. Hydration of Ordinary Portland Cement in Presence of lead sorbed on ceramic sorbent. Materials 2018, 12, 19. [Google Scholar] [CrossRef] [PubMed]
- Kerkhoff, B. Effects of Substances on Concrete and Guide to Protective Treatments; Concrete Technology; Portland Cement Association: Skokie, IL, USA, 2002. [Google Scholar]
- Quercia, G.; Lazaro, A.; Geus, J.W.; Brouwers, H.J.H. Characterization of morphology and texture of several amorphous nano-silica particles used in concrete. Cem. Concr. Compos. 2013, 44, 77–92. [Google Scholar] [CrossRef]
- ASTM International. ASTMC114 Standard Test Methods for Chemical Analysis of Hydraulic Cement; ASTM International: West Conshohocken, PA, USA, 2018. [Google Scholar]
- ASTM International. ASTMC311-04 Standard Test Method for Sampling and Testing Fly Ash or Natural Pozzolans for use in Portland-Cement Concrete; ASTM International: West Conshohocken, PA, USA, 2004. [Google Scholar]
- CSA. CSAA3004-E1 Standard Practice for the Evaluation of Alternative Supplementary Cementing Materials (ASCMs) for Use in Concrete (A3004-E1). In Cementitious Materials Compendium (A3000–18); CSA: Toronto, ON, Canada, 2018; pp. 182–192. [Google Scholar]
- ASTM International. ASTMC618 Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete; ASTM International: West Conshohocken, PA, USA, 2008. [Google Scholar]
- ASTM International. ASTMC1218/C1218M Standard Test Method for Water-Soluble Chloride in Mortar and Concrete; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- ASTM International. ASTMD3987 Standard Practice for Shake Extraction of Solid Waste with Water; ASTM International: West Conshohocken, PA, USA, 2012. [Google Scholar]
- ASTM International. ASTMC143/C143M-15a Standard Test Method for Slump of Hydraulic-Cement Concrete; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- ASTM International. ASTMC231/C231M-17a Standard Test Method for Air Content of Freshly Mixed Concrete by the Pressure Method; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- ASTM International. ASTMC403/C403M Standard Test Method for Time of Setting of Concrete Mixtures by Penetration Resistance; ASTM International: West Conshohocken, PA, USA, 2006. [Google Scholar]
- ASTM International. ASTMC138/C138M Standard Test Method for Density (Unit Weight), Yield, and Air Content (Gravimetric) of Concrete; ASTM International: West Conshohocken, PA, USA, 2012. [Google Scholar]
- ASTM International. ASTMC232/C232M Standard Test Method for Bleeding of Concrete; ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar]
- ASTM International. ASTMC39/C39M-18 Standard Test Method for Compressive Strength of Cylindrical Concrete Specimens; ASTM International: West Conshohocken, PA, USA, 2018. [Google Scholar]
- ASTM International. ASTMC78 Standard Test Method for Flexural Strength of Concrete (Using Simple Beam with Third-Point Loading; ASTM International: West Conshohocken, PA, USA, 2018. [Google Scholar]
- ASTM International. ASTMC157/C157M Standard Test Method for Length Change of Hardened Hydraulic-Cement Mortar and Concrete; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- ASTM International. ASTMC457/C457M Standard Test Method for Microscopical Determination of Parameters of the Air-Void System in Hardened Concrete; ASTM International: West Conshohocken, PA, USA, 2016. [Google Scholar]
- ASTM International. ASTMC469 Standard Test Method for Static Modulus of Elasticity and Poisson’s Ratio of Concrete in Compression; ASTM International: West Conshohocken, PA, USA, 2016. [Google Scholar]
- ASTM International. ASTMC1012/C1012M-18b Standard Test Method for Length Change of Hydraulic—Cement Mortars Exposed to a Sulfate Solution; ASTM International: West Conshohocken, PA, USA, 2018. [Google Scholar]
- ASTM International. ASTMC1567 Standard Test Method for Determining the Potential Alkali-Silica Reactivity of Combination of Cementitious Materials and Aggregates (Accelerated Mortar Bar Method); ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- ASTM International. ASTMC666/C666M-15 Standard Test Method for Resistance of Concrete to Rapid Freezing and Thawing; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- ASTM International. ASTMC186-17 Standard Test Method for Heat of Hydration of Hydraulic Cement; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- Avet, F.; Snellings, R.; Alujas Diaz, A.; Ben Haha, M.; Scrivener, K. Development of a new rapid, relevant and reliable (R3) test method to evaluate the pozzolanic reactivity of calcined kaolinitic clays. Cem. Concr. Res. 2016, 85, 1–11. [Google Scholar] [CrossRef]
- Snellings, R.; Salze, A.; Scrivener, K.L. Use of X-ray diffraction to quantify amorphous supplementary cementitious materials in anhydrous and hydrated blended cements. Cem. Concr. Res. 2014, 64, 89–98. [Google Scholar] [CrossRef]
- Durdziński, P.T.; Dunant, C.F.; Haha, M.B.; Scrivener, K.L. A new quantification method based on SEM-EDS to assess fly ash composition and study the reaction of its individual components in hydrating cement paste. Cem. Concr. Res. 2015, 73, 111–122. [Google Scholar] [CrossRef]
- Dai, Z.; Tran, T.T.; Skibsted, J. Aluminum incorporation in the C-S-H phase of white portland cement-metakaolin blends studied by 27 Al and 29 Si MAS NMR spectroscopy. J. Am. Ceram. Soc. 2014, 97, 2662–2671. [Google Scholar] [CrossRef]
- Garg, N.; Skibsted, J. Thermal activation of a pure montmorillonite clay and its reactivity in cementitious systems. J. Phys. Chem. C 2014, 118, 11464–11477. [Google Scholar] [CrossRef]
- Scrivener, K.L.; Lothenbach, B.; de Belie, N.; Gruyaert, E.; Skibsted, J.; Snellings, R.; Vollpracht, A. TC 238-SCM: Hydration and microstructure of concrete with SCMs: State of the art on methods to determine degree of reaction of SCMs. Mater. Struct. Constr. 2015, 48, 835–862. [Google Scholar] [CrossRef]
- Avet, F. Development of a New Rapid, Relevant and Reliable (R3) Testing Method to Evaluate the Pozzolanic Reactivity of Calcined Clays. In Calcined Clays for Sustainable Concrete; RILEM Bookseries; Springer: Dordrecht, The Netherlands, 2015; pp. 539–540. [Google Scholar] [CrossRef]
- Bediako, M.; Frimpong, A.O. Alternative Binders for Increased Sustainable Construction in Ghana—A Guide for Building Professionals. Mater. Sci. Appl. 2013, 4, 20–28. [Google Scholar] [CrossRef]
- Samad, S.; Shah, A. Role of binary cement including Supplementary Cementitious Material (SCM), in production of environmentally sustainable concrete: A critical review. Int. J. Sustain. Built Environ. 2017, 6, 663–674. [Google Scholar] [CrossRef]
- Stark, J. Recent advances in the field of cement hydration and microstructure analysis. Cem. Concr. Res. 2011, 41, 666–678. [Google Scholar] [CrossRef]
- Martirena, F. Blended Cements with Calcined Illitic Clay: Workability and Hydration. Calcined Clays Sustain. Concr. 2018. [Google Scholar] [CrossRef]
- Schwiete, H.E.; Ludwig, U.; Würth, K.E.; Grieshammer, G. Neubildungen bei der Hydratation von Hochofenschlacken. ZKG Int. 1969, 21, 154–160. [Google Scholar]
- Danner, T.; Justnes, H. Calcined Marl as Pozzolana for Sustainable Development of the Cement and Concrete Industry. In Proceedings of the 12th CANMET/ACI International Conference on Recent Advances in Concrete Technology and Sustainability Issues, Prague, Czech Republic, 31 October–1 November 2012. [Google Scholar]
- Taylor, H.F.W. Cement Chemistry; Thomas Telford Publishing: London, UK, 1997. [Google Scholar]
- Jansen, D.; Goetz-Neunhoeffer, F.; Lothenbach, B.; Neubauer, J. The early hydration of Ordinary Portland Cement (OPC): An approach comparing measured heat flow with calculated heat flow from QXRD. Cem. Concr. Res. 2012, 42, 134–138. [Google Scholar] [CrossRef]
- Scrivener, K.L.; Nonat, A. Hydration of cementitious materials, present and future. Cem. Concr. Res. 2011, 41, 651–665. [Google Scholar] [CrossRef]
- Lothenbach, B.; Le Saout, G.; Gallucci, E.; Scrivener, K. Influence of limestone on the hydration of Portland cements. Cem. Concr. Res. 2008, 38, 848–860. [Google Scholar] [CrossRef]
- Justnes, H. Aspects of replacing gypsum with other calcium salts in Portland cement. Adv. Cem. Res. 2013, 25. [Google Scholar] [CrossRef]
- Li, X.; Snellings, R.; Antoni, M.; Alderete, N.M.; Ben Haha, M.; Bishnoi, S.; Cizer, Ö.; Cyr, M.; De Weerdt, K.; Dhandapani, Y.; et al. Reactivity tests for supplementary cementitious materials: RILEM TC 267-TRM phase 1. Mater. Struct. Constr. 2018, 51. [Google Scholar] [CrossRef]
- EN European Standard EN 206-1; CEN: Brussels, Belgium, 2000.
- Østnor, T.A.; Justnes, H. Durability and Microstructure of Mortar Where Cement is Replaced by Calcined Marl. In Proceedings of the 1st Concrete Innovation Conference (CIC), Oslo, Norway, 11–13 June 2014. [Google Scholar]
- Varas, M.J.; de Buergo, M.A.; Fort, R. Natural cement as the precursor of Portland cement: Methodology for its identification. Cem. Concr. Res. 2005, 35, 2055–2065. [Google Scholar] [CrossRef]
- Gutteridge, A. Filler cement: The effect of the secondary component on the hydration of Portland cement. Cem. Concr. Res. 1990, 20, 853–861. [Google Scholar] [CrossRef]

| Natural SCMs | Structure | Features |
|---|---|---|
| Perlite | Siliceous and amorphous hydrated volcanic glass | Good pozzolanic reactivity |
| Pumice | Amorphous and porous volcanic rock | Low density and high absorption capacity |
| Zeolite | Hydrated aluminosilicate mineral | Chloride resistance |
| Activation Method | Technological Features | Advantages | Disadvantages |
|---|---|---|---|
| Mechanical | Different types of mills—ball, vibratory, vario-planetary | Simplicity | High energy costs |
| Chemical | The use of activators such as acid or alkaline compounds | Low curing time | Complexity, not scalable |
| Thermal | Heating with post-cooling following several patterns | A relatively simple and effective way | Environmental issues |
| Factors | Major Observation | References | |
|---|---|---|---|
| Grinding | Particle size | Finer particle size (50 to 10 μm) is followed by a high consumption of the lime which leads to a higher reactivity. | [72] |
| Dosage | Calcined marl Content | When adding calcined marl, the porosity and the water absorption increase, also the lack of C3S and C2S phases at higher substitution will alter the hydration properties. | [49] |
| W/C | -Low w/c: A well-developed compressive strength due to high dissolution rate of clinker phases. -High w/c: Lower compressive strength due to the weak pore refinement. | [73] [44] | |
| Calcination | Activation Temperature | The pozzolanic activity drops once the sintering temperature is reached, as a result of the inactive phases crystallization. The amount of periclase and free lime decreases as the calcination temperature increases. | [63] [42] |
| Resident time | -The dehydroxylation and decarbonation degrees increase with an extended holding time. -The time to retain the sample under treatment of the targeted temperature. It is a decisive factor against the pozzolanic aspect of the calcined marl. -Heating clays at lower residence times of 30 min claimed to be more useful and it leads to a higher pozzolanic reactivity. | [49] [76,77] | |
| Cooling rate | No significant influence on the pozzolanic reactivity of the calcined marls. | [72] | |
| Heating rate | To guarantee homogeneous spread of the temperature within the sample, the increase should be as low as possible. | [78] | |
| Additives | Superplasticizers | -The early hydration of the clinker phases is boosted when adding lingosulfanate as additive. -The retarder effect of plasticizers prevents the topochemical hydration being absorbed on the cement grain surfaces. -The poly(ethylene oxide) intercalates between the semectite interlayers of the calcined marl, the role of plasticizers is no longer shown. | [74,75] |
| Limestone | Systems with limestone is known to be an ettringite stabilizer, it enhances the formation of carboaluminate hydrate AFm phases (mono-sulphate phases). | [15] | |
| Stages | Why Going through this Step? | Tests | Standards | Recommendations |
|---|---|---|---|---|
| Characterization of the Material (Stage 1: ASTM C1709) | Some chemicals may affect negatively the cement hydration and they are also damaging to the environment and health. Specific tests are required to identify and dispose of these elements before proceeding with the evaluation. | Determination of: the loss on ignition water soluble alkalis total chloride ions Determination of the amount of available alkalis. Mineralogical characterization | ASTM C114 [87] ASTM C311 [88] A3004-E1 [89] | Proceed with the high-pressure method to extract the pore solutions. |
| Determination of Suitable Fineness (Stage II: ASTM C1709) | The fineness of the material is among the most key properties for improving concrete efficiency | No specific standard for testing the fineness. Measurement of compressive strength. | ------- ASTM C1709 [83] | Mortar samples are preferably stored in limewater to compensate for the difference in pH between the cement and the solution |
| Testing for Specification (Stage III: ASTM C1709) | Verify the conformity of the chemical and physical aspects of the ASCMs | The chemical properties: SO3, moisture, and LOI (Loss on ignition) contents. The sum of the three oxides content SiO2 + Al2O3 + Fe2O3 The physical properties: the fineness strength activity index water requirements volumetric stability The chloride contents. Free calcium oxide content, soluble alkali content. leachable heavy metals content. | ASTM C618 [90] ASTM C618 [90] ASTMC1218/C1218M [91] ASTMC114 [87] ASTM D3987 [92] | The author suggests that the standard should refer to the phase structure rather than focusing on the content of the three oxides. |
| Concrete Performance Tests (Stage IV: ASTM C1709) | Predict environmental behavior of concrete materials through empirical studies. | Fresh state of concrete: The slump test Air content The time of setting fresh density bleeding Hardened state of concrete: Compressive strength Flexural strength Length change Air void system parameters Modulus of elasticity Sulfate resistance Length change of the mortar bars owing to the alkali– silica reaction Additional tests: the resistance to rapid freezing and thawing the heat of hydration | ASTM C143/C143M [93] ASTM C231/C231M [94] ASTM C403 [95] ASTM C138/C138M [96] ASTM C232/C232M [97] ASTM C39/C39M [98] ASTM C78 [99] ASTM C157/C157M [100] ASTM C457/ C457M [101] ASTM C469 [102] ASTM C1012/C1012M [103] ASTM C1567 or C1293 [104] ASTM C666/C666M [105] STM C186 [106] | The present tests are mainly developed to be used in Portland cement systems. In certain cases, the curing process is not suitable for concrete containing ASCMs and will have a significant impact on the results analyzed. |
| Field Trials and Long-term Performance (Stage V: ASTM C1709) | This stage is intended to confirm the results collected in the laboratory experiments | --------------- | ---------- | Rapid assessment methods are urgently needed |
| Reference | Mineralogical composition (Content %) | Additives | Calcination | Hydration | |||
|---|---|---|---|---|---|---|---|
| Are the carbonates added or not? | Optimal activation T | Phase formed | Physical properties | W/C Ratio | Phase formed after hydration | ||
| Danner et al. [57] | Montmorillonite (54), Calcite (25), Kaolinite (8) | No | 800 °C | Anorthite CaAl2SiO8 Wollastonite CaSiO3 Diopside CaMgSi2O6 Gehlenite Ca2Al2SiO7 | d50 < 10 μm | 0.5 | Carbo-aluminate hydrates and Ettringite |
| Weber et al. [47] | Smectite (24), Calcite (71), Quartz (4). | No | 800 and 1100 °C | CS; C3S2; C2S, C2AS (gehlenite); C3A; C4AF | d50 = 30 μm SSA = 33.0 m2 /g | 0.65 | calcium silicate hydrates (CSH-phases) and calcium aluminate hydrates (AFm, AFt-phases) |
| Soltani et al. [44] | calcite, quartz, and clay minerals particularly illite | No | 750 °C | Crystalline silicate and aluminate minerals | D90 < 100 µm | 0.45 | C-S-H phases |
| Rakhimov et al. [48] | Kaolin (7.12), montmorillonite (12.4), chlorite (4.00), calcite (46.9), quartz (13.44), albite (7.83), mica (6.90), and gypsum (1.40) | No | 800 °C | Hatrurite Ca3SiO5 Larnite Ca2(SiO4) | SSA = 200 m2/Kg | Calcium silicate hydrate gel (CSH). Calcium hydrocarboaluminates | |
| Bahhou et al. [41] | Montmorillonite (27.4) Dolomite (56.2) Quartz (12.3) | Limestone (15) | 800 °C | Lime, periclase, amorphous phase | -- | -- | |
| Kastis et al. [43] | Calcite, Amorphous silica phase, Quartz | No | d50 = 6.3 μm. | 0.4 | calcium aluminate hydrates and carboaluminates, CaCO3, and Ca(OH)2 | ||
| Justen et al. [15] | Smectite (60), illite (30), kaolin (30) and calcium carbonate (10-20) | No | 800 °C | d50 ≈ 7 µm. | 0.5 | (CSH, CAH and CASH) | |
| Hollanders et al. [59] | Hectorite, Quartz | No | 900 °C | Enstatite and a triclinic MgSiO3 phase | SSA = 1.47 m2/g | Portlandite | |
| Siline et al. [49] | Calcite (30.5), Dolomite (16.5), Illite/muscovite (20), Chlorite (09), Quartz (7), Gypsum (02) | No | 700 °C | CaO | Blaine = 3630 cm2 /g | 0.5 | CSH, CaCO3 and CH products |
| Poussardin et al. [60] | Palygorskite (17.45) Smectite (15.58) Dolomite (53.58) | No | 800 °C | belite (C2S), Am (Amorphous), lime, periclase | -- | --- | |
| Danner et al. [45] | Smectite (70), calcium carbonate (20), the remainder being quartz and feldspars. | No | 800 °C | Silicate and aluminate phases | d50 = 7 µm | 0.5 | CAH, CASH, Hydrogarnet |
| Bullerjhan et al. [42] | Montmorillonite, Palygorskite, amorphous, Calcite | Marl co-calcined with Dolomite | 950 °C | Åkermanite types (melilite-type) C2MS2 Magnesium silicates | D90 <110 µm | 0.6 | Strätlingite, Ettringite, CASH |
| Marl blended with calcined Dolomite | D90 <110 µm | Hemi and mono carboaluminate/etteingite, CASH | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bahhou, A.; Taha, Y.; Khessaimi, Y.E.; Hakkou, R.; Tagnit-Hamou, A.; Benzaazoua, M. Using Calcined Marls as Non-Common Supplementary Cementitious Materials—A Critical Review. Minerals 2021, 11, 517. https://doi.org/10.3390/min11050517
Bahhou A, Taha Y, Khessaimi YE, Hakkou R, Tagnit-Hamou A, Benzaazoua M. Using Calcined Marls as Non-Common Supplementary Cementitious Materials—A Critical Review. Minerals. 2021; 11(5):517. https://doi.org/10.3390/min11050517
Chicago/Turabian StyleBahhou, Abdelmoujib, Yassine Taha, Yassine El Khessaimi, Rachid Hakkou, Arezki Tagnit-Hamou, and Mostafa Benzaazoua. 2021. "Using Calcined Marls as Non-Common Supplementary Cementitious Materials—A Critical Review" Minerals 11, no. 5: 517. https://doi.org/10.3390/min11050517
APA StyleBahhou, A., Taha, Y., Khessaimi, Y. E., Hakkou, R., Tagnit-Hamou, A., & Benzaazoua, M. (2021). Using Calcined Marls as Non-Common Supplementary Cementitious Materials—A Critical Review. Minerals, 11(5), 517. https://doi.org/10.3390/min11050517










