Synthesis and Compressibility of Novel Nickel Carbide at Pressures of Earth’s Outer Core
Abstract
1. Introduction
2. Materials and Methods
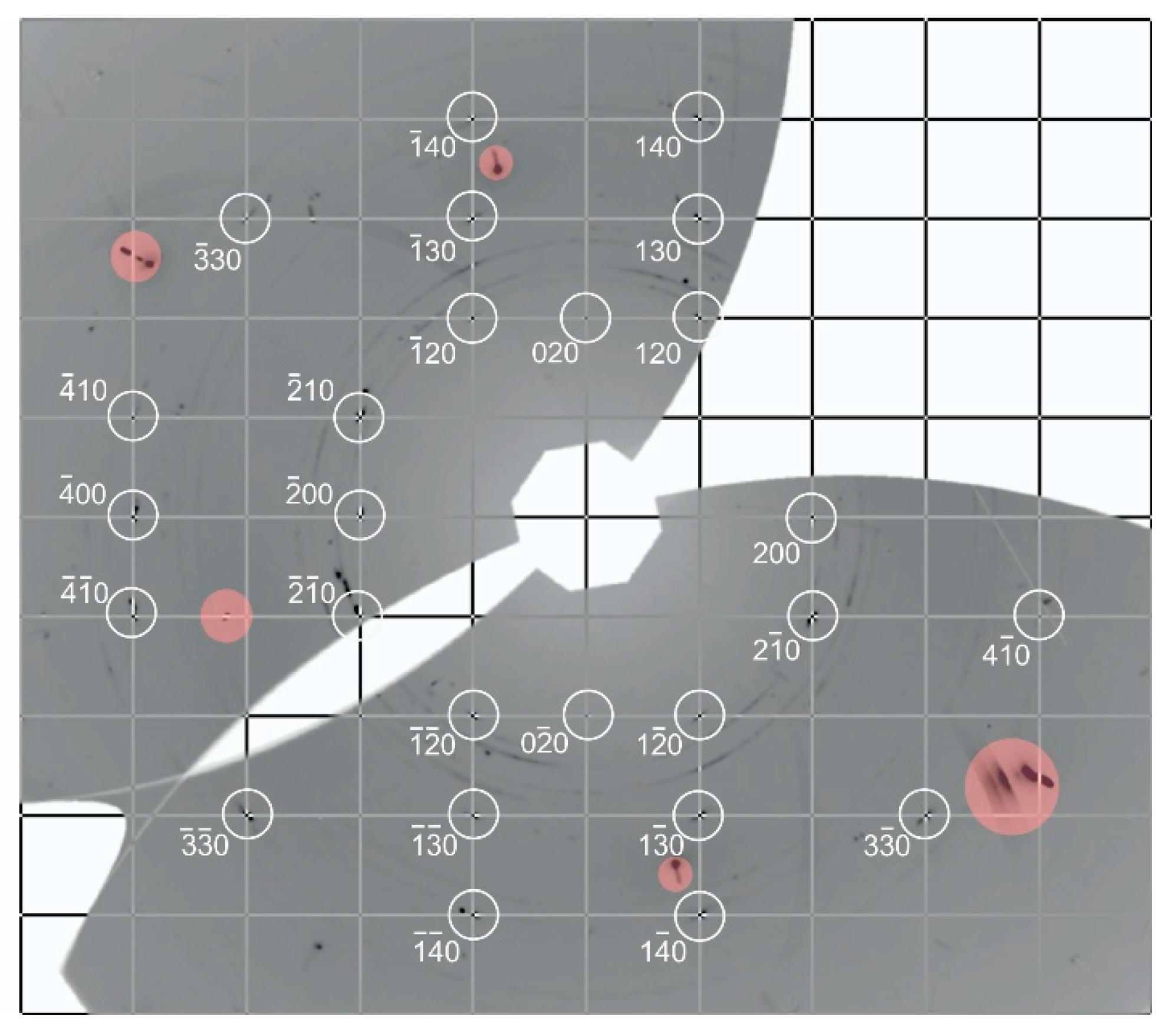
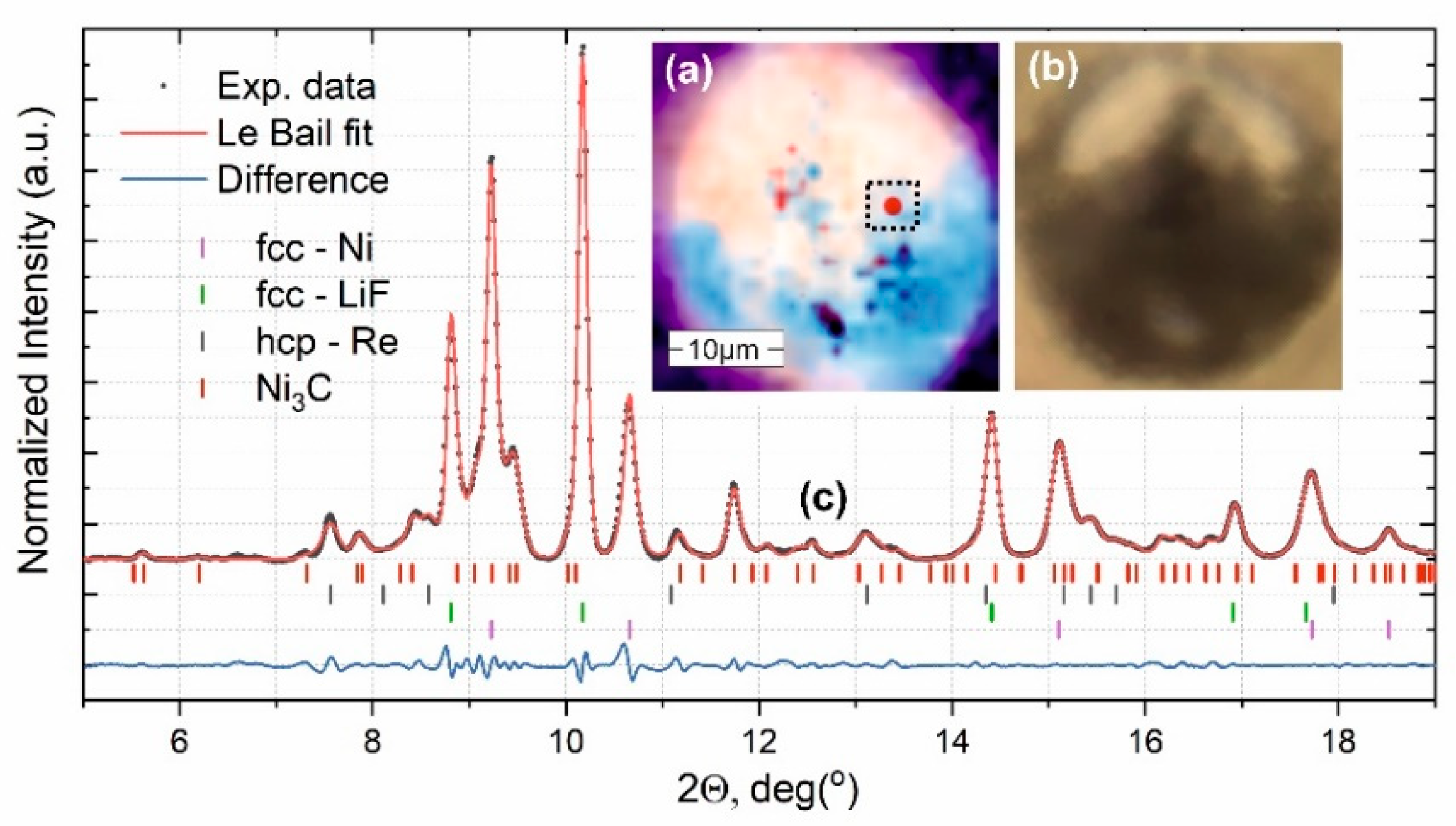
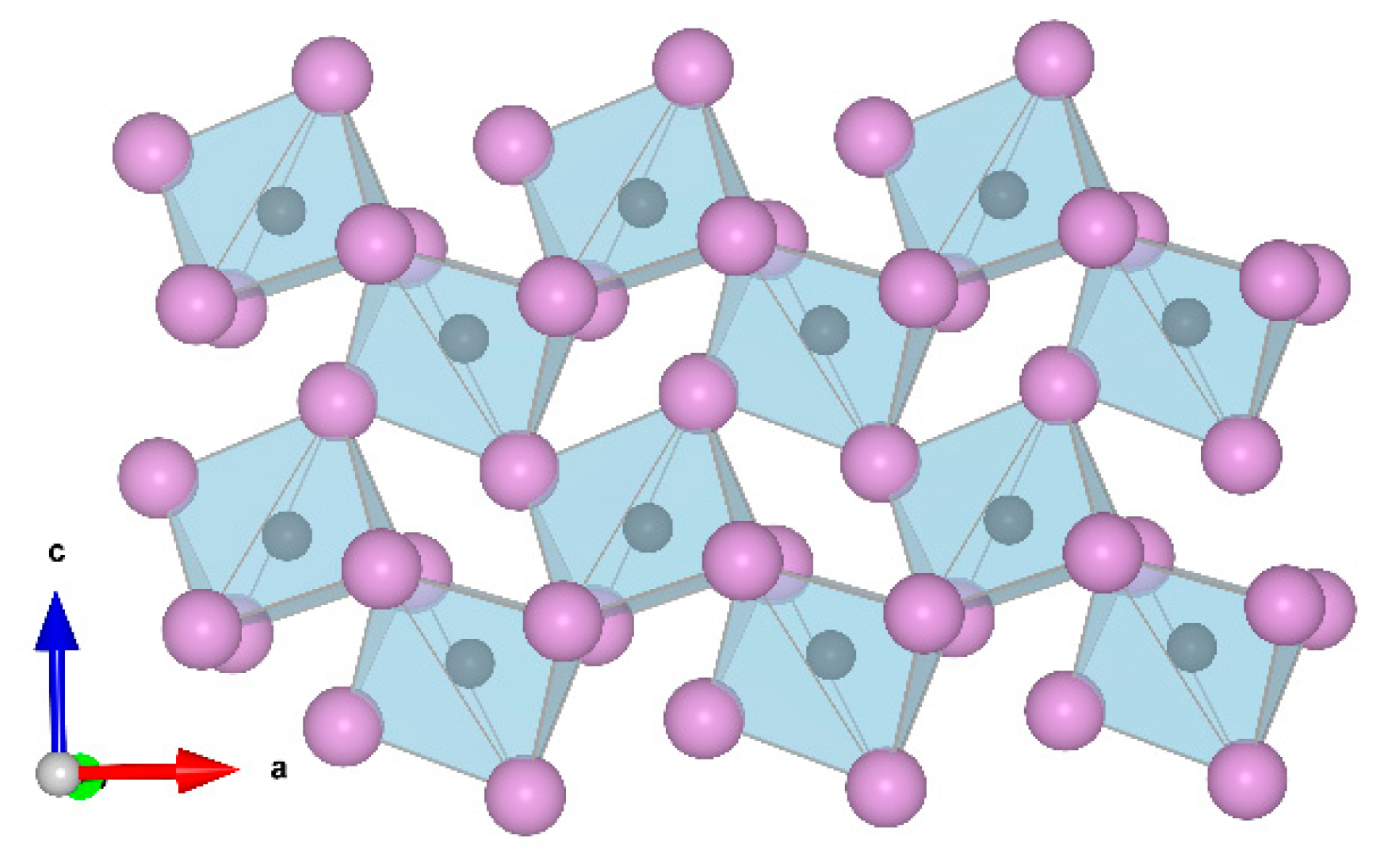
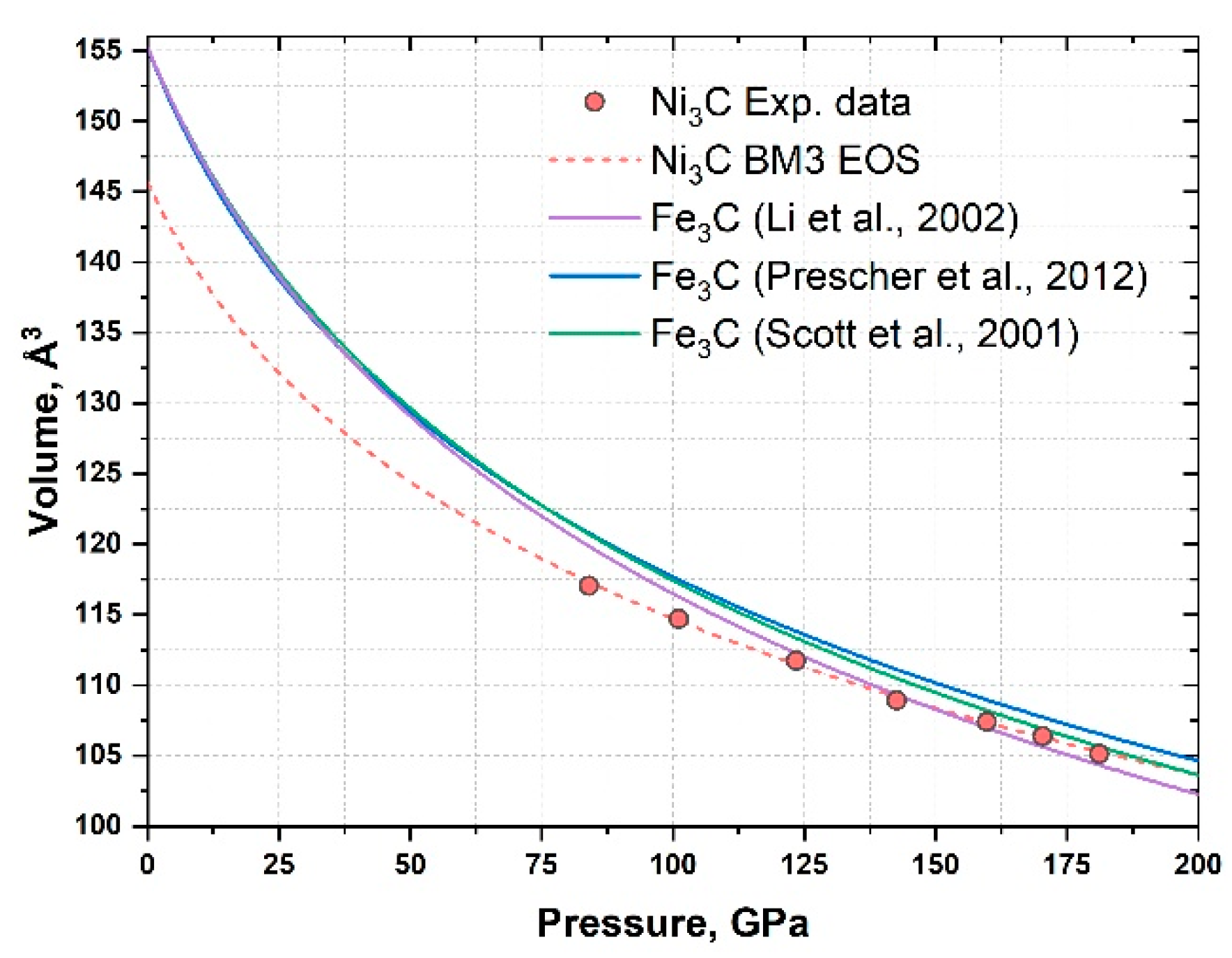
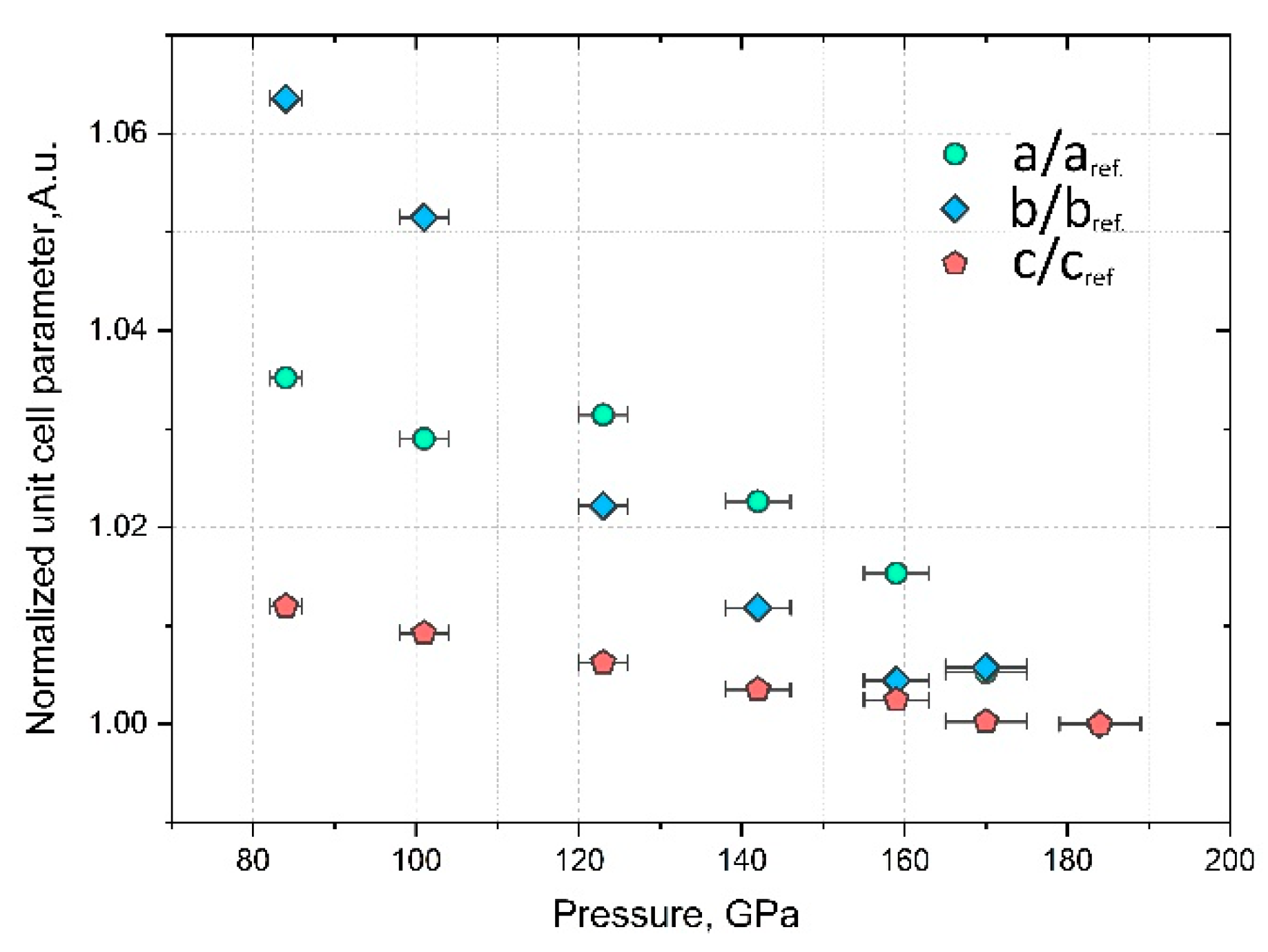
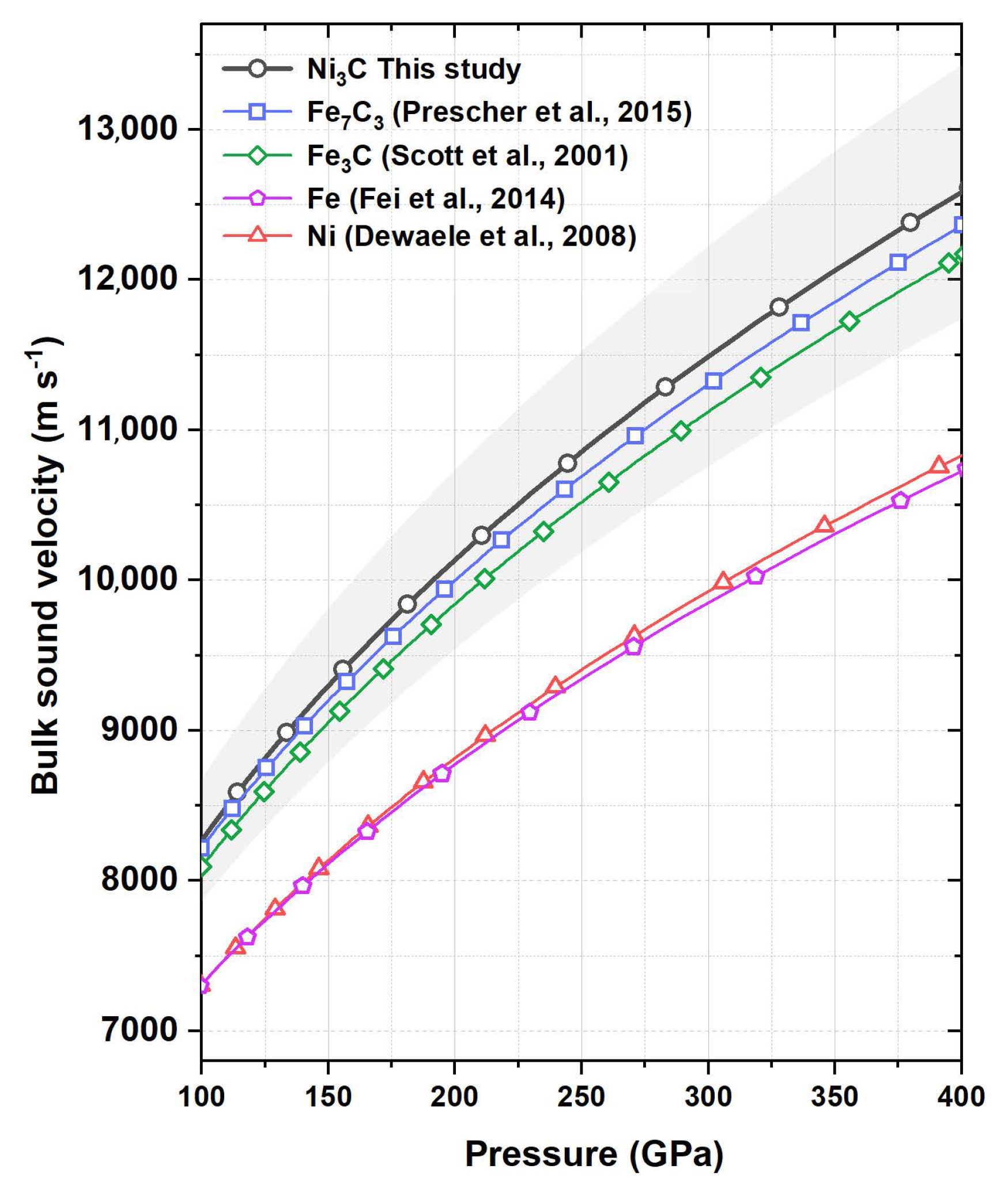
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Birch, F. Elasticity and constitution of the Earth’s interior. J. Geophys. Res. 1952, 57, 227–286. [Google Scholar] [CrossRef]
- Prescher, C.; Dubrovinsky, L.; Bykova, E.; Kupenko, I.; Glazyrin, K.; Kantor, A.; McCammon, C.; Mookherjee, M.; Nakajima, Y.; Miyajima, N.; et al. High Poisson’s ratio of Earth’s inner core explained by carbon alloying. Nat. Geosci. 2015, 8, 220–223. [Google Scholar] [CrossRef]
- Litasov, K.D.; Shatskiy, A.F. Composition of the Earth’s core: A review. Russ. Geol. Geophys. 2016, 57, 22–46. [Google Scholar] [CrossRef]
- McDonough, W.F. Compositional Model for the Earth’s Core. In Treatise on Geochemistry; Elsevier: Amsterdam, The Netherlands, 2003; pp. 547–568. [Google Scholar]
- Wood, B.J. Carbon in the core. Earth Planet. Sci. Lett. 1993, 117, 593–607. [Google Scholar] [CrossRef]
- Torchio, R.; Boccato, S.; Miozzi, F.; Rosa, A.D.; Ishimatsu, N.; Kantor, I.; Sévelin-Radiguet, N.; Briggs, R.; Meneghini, C.; Irifune, T.; et al. Melting Curve and Phase Relations of Fe-Ni Alloys: Implications for the Earth’s Core Composition. Geophys. Res. Lett. 2020, 47, e2020GL088169. [Google Scholar] [CrossRef]
- Poirier, J.-P. Light elements in the Earth’s outer core: A critical review. Phys. Earth Planet. Inter. 1994, 85, 319–337. [Google Scholar] [CrossRef]
- Bashir, N.; Beckett, J.R.; Hutcheon, I.D.; Stolper, E.M. Carbon in the Metal of Iron Meteorites. In Proceedings of the Lunar and Planetary Science Conference, Houston, TX, USA, 18–22 March 1996; Volume 27, p. 63. [Google Scholar]
- Hirayama, Y.; Fujii, T.; Kurita, K. The melting relation of the system, iron and carbon at high pressure and its bearing on the early stage of the Earth. Geophys. Res. Lett. 1993, 20, 2095–2098. [Google Scholar] [CrossRef]
- Lord, O.T.; Walter, M.J.; Dasgupta, R.; Walker, D.; Clark, S.M. Melting in the Fe–C system to 70 GPa. Earth Planet. Sci. Lett. 2009, 284, 157–167. [Google Scholar] [CrossRef]
- Nakajima, Y.; Takahashi, E.; Suzuki, T.; Funakoshi, K. “Carbon in the core” revisited. Phys. Earth Planet. Inter. 2009, 174, 202–211. [Google Scholar] [CrossRef]
- Chen, B.; Li, Z.; Zhang, D.; Liu, J.; Hu, M.Y.; Zhao, J.; Bi, W.; Alp, E.E.; Xiao, Y.; Chow, P.; et al. Hidden carbon in Earth’s inner core revealed by shear softening in dense Fe7C3. Proc. Natl. Acad. Sci. USA 2014, 111, 17755–17758. [Google Scholar] [CrossRef]
- Liu, J.; Lin, J.-F.; Prakapenka, V.B.; Prescher, C.; Yoshino, T. Phase relations of Fe3C and Fe7C3 up to 185 GPa and 5200 K: Implication for the stability of iron carbide in the Earth’s core. Geophys. Res. Lett. 2016, 43, 12415–12422. [Google Scholar] [CrossRef]
- Dziewonski, A.M.; Anderson, D.L. Preliminary reference Earth model. Phys. Earth Planet. Inter. 1981, 25, 297–356. [Google Scholar] [CrossRef]
- Morard, G.; Boccato, S.; Rosa, A.D.; Anzellini, S.; Miozzi, F.; Henry, L.; Garbarino, G.; Mezouar, M.; Harmand, M.; Guyot, F.; et al. Solving Controversies on the Iron Phase Diagram Under High Pressure. Geophys. Res. Lett. 2018, 45, 11-074. [Google Scholar] [CrossRef]
- Dubrovinsky, L.; Dubrovinskaia, N.; Abrikosov, I.A.; Vennström, M.; Westman, F.; Carlson, S.; van Schilfgaarde, M.; Johansson, B. Pressure-Induced Invar Effect in Fe-Ni Alloys. Phys. Rev. Lett. 2001, 86, 4851–4854. [Google Scholar] [CrossRef] [PubMed]
- Reagan, M.M.; Gleason, A.E.; Liu, J.; Krawczynski, M.J.; Van Orman, J.A.; Mao, W.L. The effect of nickel on the strength of iron nickel alloys: Implications for the Earth’s inner core. Phys. Earth Planet. Inter. 2018, 283, 43–47. [Google Scholar] [CrossRef]
- Brett, R. Cohenite in Meteorites: A Proposed Origin. Science 1966, 153, 60–62. [Google Scholar] [CrossRef]
- Ringwood, A.E. Cohenite as a pressure indicator in iron meteorites. Geochim. Cosmochim. Acta 1960, 20, 155–158. [Google Scholar] [CrossRef]
- Dong, H.; Dorfman, S.M.; Holl, C.M.; Meng, Y.; Prakapenka, V.B.; He, D.; Duffy, T.S. Compression of lithium fluoride to 92 GPa. High Press. Res. 2014, 34, 39–48. [Google Scholar] [CrossRef]
- Dewaele, A.; Torrent, M.; Loubeyre, P.; Mezouar, M. Compression curves of transition metals in the Mbar range: Experiments and projector augmented-wave calculations. Phys. Rev. B 2008, 78, 104102. [Google Scholar] [CrossRef]
- Akahama, Y.; Kawamura, H. Pressure calibration of diamond anvil Raman gauge to 310GPa. J. Appl. Phys. 2006, 100, 043516. [Google Scholar] [CrossRef]
- Fedotenko, T.; Dubrovinsky, L.; Aprilis, G.; Koemets, E.; Snigirev, A.; Snigireva, I.; Barannikov, A.; Ershov, P.; Cova, F.; Hanfland, M.; et al. Laser heating setup for diamond anvil cells for in situ synchrotron and in house high and ultra-high pressure studies. Rev. Sci. Instrum. 2019, 90, 104501. [Google Scholar] [CrossRef]
- Liermann, H.-P.; Konôpková, Z.; Morgenroth, W.; Glazyrin, K.; Bednarčik, J.; McBride, E.E.; Petitgirard, S.; Delitz, J.T.; Wendt, M.; Bican, Y.; et al. The Extreme Conditions Beamline P02.2 and the Extreme Conditions Science Infrastructure at PETRA III. J. Synchrotron Radiat. 2015, 22, 908–924. [Google Scholar] [CrossRef]
- CrysAlis Pro (v. 171.39.46). Available online: https://www.rigaku.com/products/smc/crysalis (accessed on 3 January 2020).
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Petříček, V.; Dušek, M.; Plášil, J. Crystallographic computing system Jana2006: Solution and refinement of twinned structures. Zeitschrift für Krist. Cryst. Mater. 2016, 231, 583–599. [Google Scholar] [CrossRef]
- Prescher, C.; Prakapenka, V.B. DIOPTAS: A program for reduction of two-dimensional X-ray diffraction data and data exploration. High Press. Res. 2015, 35, 223–230. [Google Scholar] [CrossRef]
- Gonzalez-Platas, J.; Alvaro, M.; Nestola, F.; Angel, R. EosFit7-GUI: A new graphical user interface for equation of state calculations, analyses and teaching. J. Appl. Crystallogr. 2016, 49, 1377–1382. [Google Scholar] [CrossRef]
- Huba, Z.J.; Carpenter, E.E. Monitoring the formation of carbide crystal phases during the thermal decomposition of 3d transition metal dicarboxylate complexes. Dalt. Trans. 2014, 43, 12236–12242. [Google Scholar] [CrossRef]
- Li, J.; Mao, H.K.; Fei, Y.; Gregoryanz, E.; Eremets, M.; Zha, C.S. Compression of Fe 3 C to 30 GPa at room temperature. Phys. Chem. Miner. 2002, 29, 166–169. [Google Scholar] [CrossRef]
- Prescher, C.; Dubrovinsky, L.; McCammon, C.; Glazyrin, K.; Nakajima, Y.; Kantor, A.; Merlini, M.; Hanfland, M. Structurally hidden magnetic transitions in Fe3C at high pressures. Phys. Rev. B 2012, 85, 140402. [Google Scholar] [CrossRef]
- Scott, H.P.; Williams, Q.; Knittle, E. Stability and equation of state of Fe 3 C to 73 GPa: Implications for carbon in the Earth’s core. Geophys. Res. Lett. 2001, 28, 1875–1878. [Google Scholar] [CrossRef]
- Mookherjee, M. Elasticity and anisotropy of Fe3C at high pressures. Am. Miner. 2011, 96, 1530–1536. [Google Scholar] [CrossRef]
- Fei, Y.; Murphy, C.; Shibazaki, Y.; Shahar, A.; Huang, H. Thermal equation of state of hcp-iron: Constraint on the density deficit of Earth’s solid inner core. Geophys. Res. Lett. 2016, 43, 6837–6843. [Google Scholar] [CrossRef]
| Chemical Formula | Ni3C |
|---|---|
| Crystal system | Orthorhombic |
| Space group | Pnma |
| Pressure (GPa) | 184(5) |
| Temperature (K) | 293 |
| a (Å) | 4.520(3) |
| b (Å) | 5.8014(17) |
| c (Å) | 4.009(4) |
| V (Å3) | 105.12(13) |
| Z | 4 |
| Density(g·cm−3) | 11.884 |
| Radiation type | synchrotron, λ = 0.2895 Å |
| Diffractometer | P02.2 @DESY |
| No. of measured, Independent and observed [I > 3σ(I)] reflections | 366, 182, 83 |
| Rint | 7.3% |
| Refinement method | Full matrix least-squares on F |
| R [F > 3σ(F)], wR(F), S | 6.43, 8.42, 1.43 |
| No. of parameters | 19 |
| Δρmax, Δρmin(e·Å−3) | 3.09, −3.51 |
| Pressure, GPa | Volume, Å3 |
|---|---|
| 84 (2) | 117.1 (6) |
| 101 (2) | 114.7 (3) |
| 123 (3) | 111.7 (4) |
| 142 (3) | 108.9 (4) |
| 160 (4) | 107.4 (4) |
| 170 (4) | 106.3 (3) |
| 184 (5) | 105.1 (2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedotenko, T.; Khandarkhaeva, S.; Dubrovinsky, L.; Glazyrin, K.; Sedmak, P.; Dubrovinskaia, N. Synthesis and Compressibility of Novel Nickel Carbide at Pressures of Earth’s Outer Core. Minerals 2021, 11, 516. https://doi.org/10.3390/min11050516
Fedotenko T, Khandarkhaeva S, Dubrovinsky L, Glazyrin K, Sedmak P, Dubrovinskaia N. Synthesis and Compressibility of Novel Nickel Carbide at Pressures of Earth’s Outer Core. Minerals. 2021; 11(5):516. https://doi.org/10.3390/min11050516
Chicago/Turabian StyleFedotenko, Timofey, Saiana Khandarkhaeva, Leonid Dubrovinsky, Konstantin Glazyrin, Pavel Sedmak, and Natalia Dubrovinskaia. 2021. "Synthesis and Compressibility of Novel Nickel Carbide at Pressures of Earth’s Outer Core" Minerals 11, no. 5: 516. https://doi.org/10.3390/min11050516
APA StyleFedotenko, T., Khandarkhaeva, S., Dubrovinsky, L., Glazyrin, K., Sedmak, P., & Dubrovinskaia, N. (2021). Synthesis and Compressibility of Novel Nickel Carbide at Pressures of Earth’s Outer Core. Minerals, 11(5), 516. https://doi.org/10.3390/min11050516







