Numerical Modelling of Radiogenic Ingrowth and Diffusion of Pb in Apatite Inclusions with Variable Shape and U-Th Zonation
Abstract
1. Introduction
- (1)
- radiogenic isotopes are completely lost form the system once they reach crystal boundaries,
- (2)
- parent isotopes are homogeneously distributed within a crystal,
- (3)
- the crystal geometry can be approximated by a simple shape, such as a sphere.
2. Previous Work
2.1. Behaviour of Radiogenic Isotopes at Crystal Boundaries
2.2. Complex Geometry and Parent Isotope Zonation
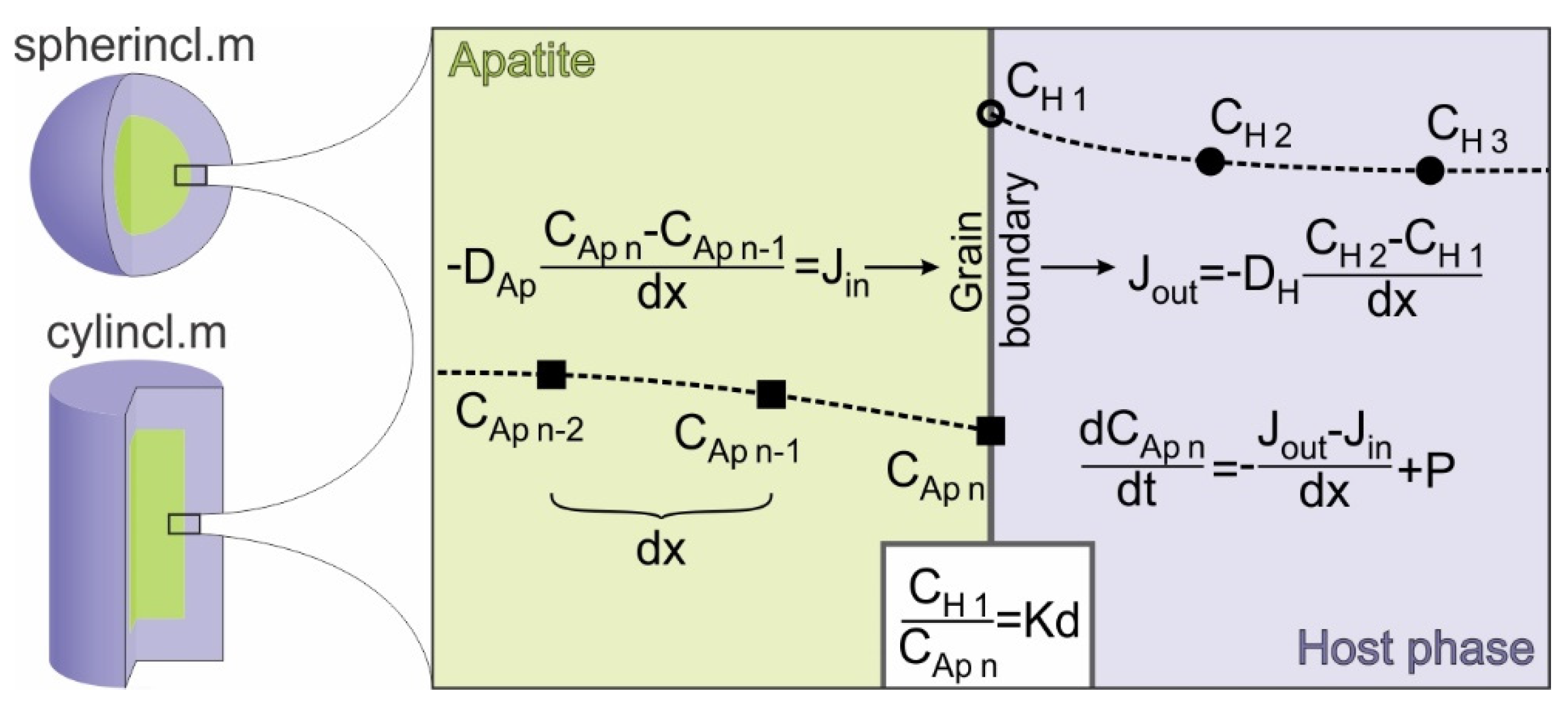
3. Method
4. Results and Discussion
4.1. Chemically Homogeneous Spheres
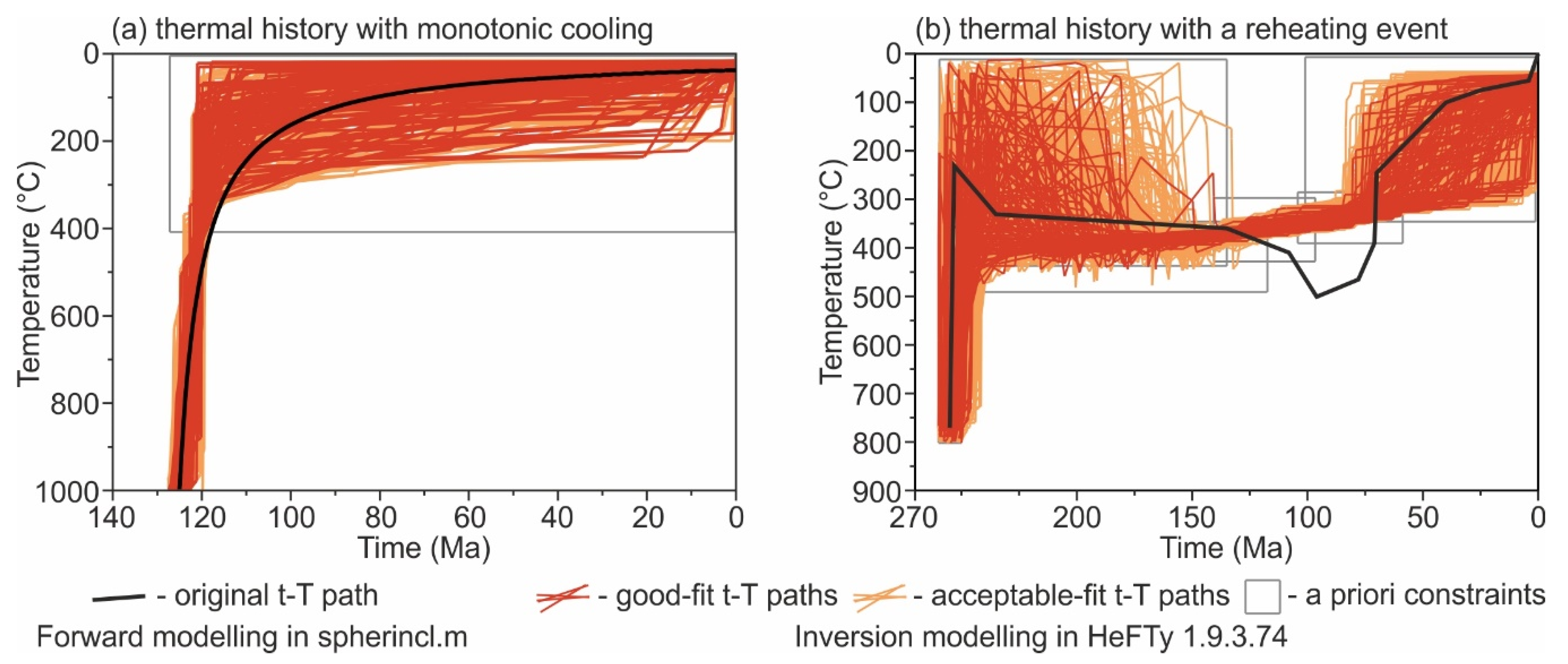
4.1.1. Forward Modelling: Do U-Th-Pb Dates of Apatite Inclusions Depend on Their Host Minerals?
4.1.2. Inversion Modelling: Is the Knowledge of Boundary Behaviour Needed to Infer t-T Paths?
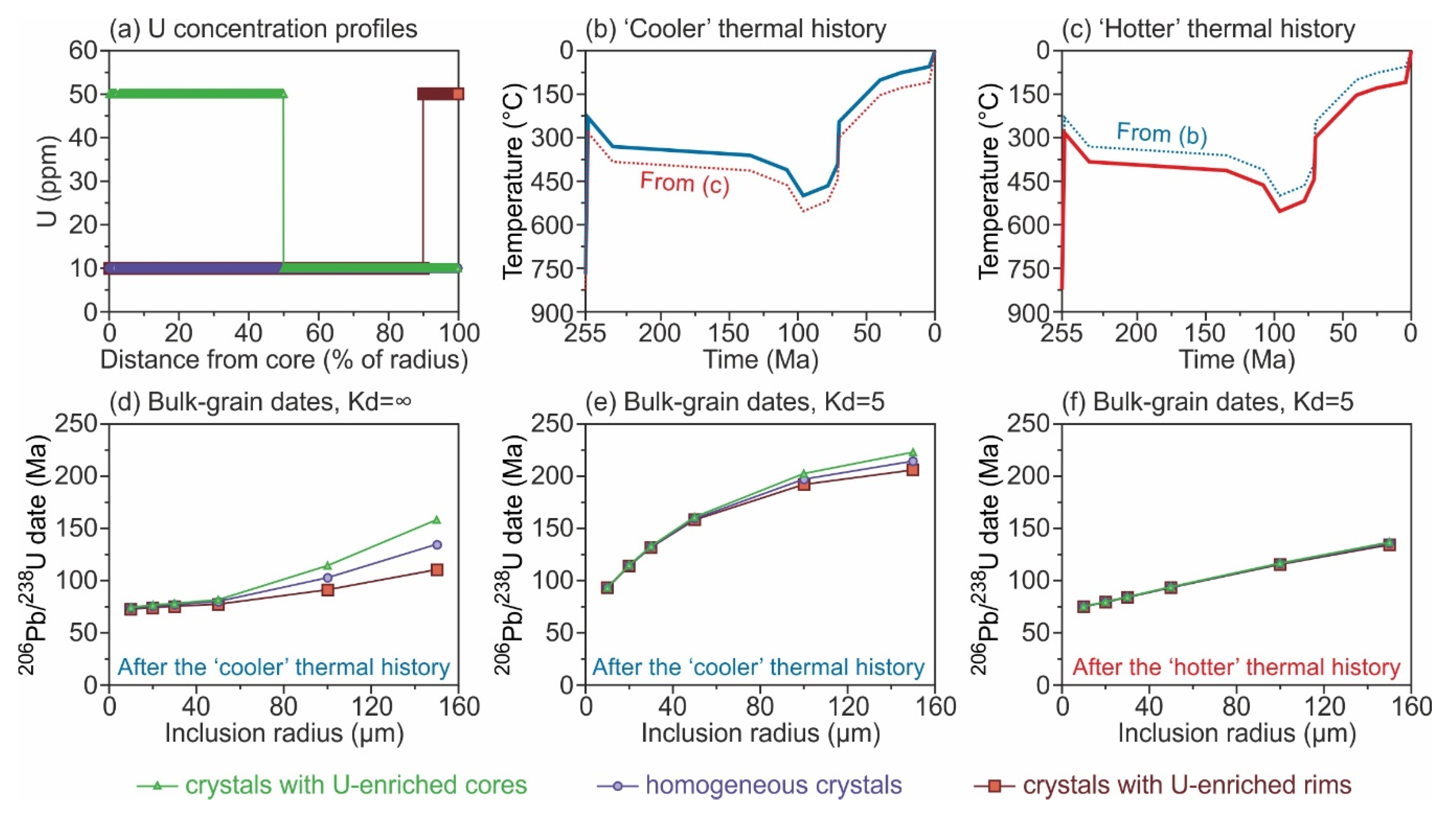
4.2. Chemically Zoned Spheres: What Is the Effect of U and Th Zonation on Bulk-Grain U-Th-Pb Dates?
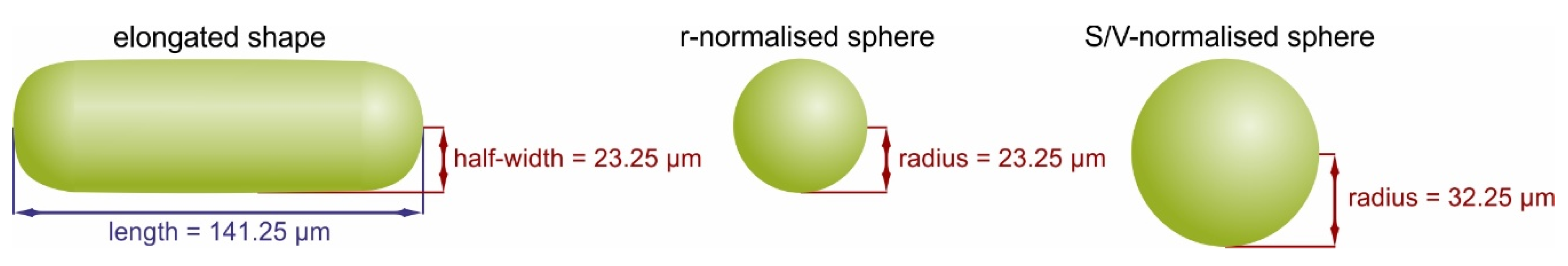
4.3. Spheres and Spherocylinders: What Is the Effect of Crystal Geometry on Intra-Grain U-Th-Pb Dates?
5. Further Considerations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dodson, M.H. Closure Temperature in Cooling Geochronological and Petrological Systems. Contrib. Mineral. Petrol. 1973, 40, 259–274. [Google Scholar] [CrossRef]
- Dodson, M.H. Closure Profiles in Cooling Systems. Mater. Sci. Forum 1986, 7, 145–154. [Google Scholar] [CrossRef]
- Ganguly, J.; Tirone, M. Diffusion Closure Temperature and Age of a Mineral with Arbitrary Extent of Diffusion: Theoretical Formulation and Applications. Earth Planet. Sci. Lett. 1999, 170, 131–140. [Google Scholar] [CrossRef]
- Guralnik, B.; Jain, M.; Herman, F.; Paris, R.B.; Harrison, T.M.; Murray, A.S.; Valla, P.G.; Rhodes, E.J. Effective Closure Temperature in Leaky and/or Saturating Thermochronometers. Earth Planet. Sci. Lett. 2013, 384, 209–218. [Google Scholar] [CrossRef]
- Ketcham, R.A. Forward and Inverse Modeling of Low-Temperature Thermochronometry Data. Rev. Mineral. Geochem. 2005, 58, 275–314. [Google Scholar] [CrossRef]
- Gallagher, K. Transdimensional Inverse Thermal History Modeling for Quantitative Thermochronology. J. Geophys. Res. Solid Earth 2012, 117. [Google Scholar] [CrossRef]
- Chamberlain, K.R.; Bowring, S.A. Apatite–Feldspar U–Pb Thermochronometer: A Reliable, Mid-Range (450 °C), Diffusion-Controlled System. Chem. Geol. 2001, 172, 173–200. [Google Scholar] [CrossRef]
- Schoene, B.; Bowring, S.A. Determining Accurate Temperature–Time Paths from U–Pb Thermochronology: An Example from the Kaapvaal Craton, Southern Africa. Geochim. Cosmochim. Acta 2007, 71, 165–185. [Google Scholar] [CrossRef]
- Cochrane, R.; Spikings, R.A.; Chew, D.; Wotzlaw, J.-F.; Chiaradia, M.; Tyrrell, S.; Schaltegger, U.; Van der Lelij, R. High Temperature (>350 °C) Thermochronology and Mechanisms of Pb Loss in Apatite. Geochim. Cosmochim. Acta 2014, 127, 39–56. [Google Scholar] [CrossRef]
- Paul, A.N.; Spikings, R.A.; Ulianov, A.; Ovtcharova, M. High Temperature (>350 °C) Thermal Histories of the Long Lived (>500 Ma) Active Margin of Ecuador and Colombia: Apatite, Titanite and Rutile U-Pb Thermochronology. Geochim. Cosmochim. Acta 2018, 228, 275–300. [Google Scholar] [CrossRef]
- Paul, A.N.; Spikings, R.A.; Chew, D.; Daly, J.S. The Effect of Intra-Crystal Uranium Zonation on Apatite U-Pb Thermochronology: A Combined ID-TIMS and LA-MC-ICP-MS Study. Geochim. Cosmochim. Acta 2019, 251, 15–35. [Google Scholar] [CrossRef]
- Reiners, P.W.; Ehlers, T.A.; Zeitler, P.K. Past, Present, and Future of Thermochronology. Rev. Mineral. Geochem. 2005, 58, 1–18. [Google Scholar] [CrossRef]
- Smye, A.J.; Stockli, D.F. Rutile U–Pb Age Depth Profiling: A Continuous Record of Lithospheric Thermal Evolution. Earth Planet. Sci. Lett. 2014, 408, 171–182. [Google Scholar] [CrossRef]
- Smye, A.J.; Marsh, J.H.; Vermeesch, P.; Garber, J.M.; Stockli, D.F. Applications and Limitations of U-Pb Thermochronology to Middle and Lower Crustal Thermal Histories. Chem. Geol. 2018, 494, 1–18. [Google Scholar] [CrossRef]
- Villa, I.M. From Nanometer to Megameter: Isotopes, Atomic-Scale Processes, and Continent-Scale Tectonic Models. Lithos 2006, 87, 155–173. [Google Scholar] [CrossRef]
- Harrison, T.M.; Heizler, M.T.; McKeegan, K.D.; Schmitt, A.K. In Situ 40K–40Ca ‘Double-plus’ SIMS Dating Resolves Klokken Feldspar 40K–40Ar Paradox. Earth Planet. Sci. Lett. 2010, 299, 426–433. [Google Scholar] [CrossRef]
- Villa, I.M.; Hanchar, J.M. K-Feldspar Hygrochronology. Geochim. Cosmochim. Acta 2013, 101, 24–33. [Google Scholar] [CrossRef]
- Villa, I.M. Diffusion in Mineral Geochronometers: Present and Absent. Chem. Geol. 2016, 420, 1–10. [Google Scholar] [CrossRef]
- Holder, R.M.; Hacker, B.R. Fluid-Driven Resetting of Titanite Following Ultrahigh-Temperature Metamorphism in Southern Madagascar. Chem. Geol. 2019, 504, 38–52. [Google Scholar] [CrossRef]
- Popov, D.V.; Spikings, R.A. Diffusion vs. Fluid Alteration in Alkali Feldspar 40Ar/39Ar Thermochronology: Does Cross-Correlation of Log(r/R₀) and Age Spectra Validate Thermal Histories? Chem. Geol. 2020, 539, 119506. [Google Scholar] [CrossRef]
- Popov, D.V.; Spikings, R.A.; Scaillet, S.; O’Sullivan, G.; Chew, D.; Badenszki, E.; Daly, J.S.; Razakamanana, T.; Davies, J.H.F.L. Diffusion and Fluid Interaction in Itrongay Pegmatite (Madagascar): Evidence from in Situ 40Ar/39Ar Dating of Gem-Quality Alkali Feldspar and U-Pb Dating of Protogenetic Apatite Inclusions. Chem. Geol. 2020, 556, 119841. [Google Scholar] [CrossRef]
- Kirkland, C.L.; Yakymchuk, C.; Szilas, K.; Evans, N.; Hollis, J.; McDonald, B.; Gardiner, N.J. Apatite: A U-Pb Thermochronometer or Geochronometer? Lithos 2018, 318–319, 143–157. [Google Scholar] [CrossRef]
- Glorie, S.; Jepson, G.; Konopelko, D.; Mirkamalov, R.; Meeuws, F.; Gilbert, S.; Gillespie, J.; Collins, A.S.; Xiao, W.; Dewaele, S.; et al. Thermochronological and Geochemical Footprints of Post-Orogenic Fluid Alteration Recorded in Apatite: Implications for Mineralisation in the Uzbek Tian Shan. Gondwana Res. 2019, 71, 1–15. [Google Scholar] [CrossRef]
- Antoine, C.; Bruand, E.; Guitreau, M.; Devidal, J.-L. Understanding Preservation of Primary Signatures in Apatite by Comparing Matrix and Zircon-Hosted Crystals from the Eoarchean Acasta Gneiss Complex (Canada). Geochem. Geophys. Geosyst. 2020, 21. [Google Scholar] [CrossRef]
- Albarède, F. The Thermal History of Leaky Chronometers above Their Closure Temperature. Geophys. Res. Lett. 2003, 30, 15-1–15-4. [Google Scholar] [CrossRef]
- Gardés, E.; Montel, J.-M. Opening and Resetting Temperatures in Heating Geochronological Systems. Contrib. Mineral. Petrol. 2009, 158, 185–195. [Google Scholar] [CrossRef]
- Arnaud, N.O.; Kelley, S.P. Evidence for Excess Argon during High Pressure Metamorphism in the Dora Maira Massif (Western Alps, Italy), Using an Ultra-Violet Laser Ablation Microprobe 40Ar-39Ar Technique. Contrib. Mineral. Petrol. 1995, 121, 1–11. [Google Scholar] [CrossRef]
- Scaillet, S. Excess 40Ar Transport Scale and Mechanism in High-Pressure Phengites: A Case Study from an Eclogitized Metabasite of the Dora-Maira Nappe, Western Alps. Geochim. Cosmochim. Acta 1996, 60, 1075–1090. [Google Scholar] [CrossRef]
- Pickles, C.; Kelley, S.; Reddy, S.; Wheeler, J. Determination of High Spatial Resolution Argon Isotope Variations in Metamorphic Biotites. Geochim. Cosmochim. Acta 1997, 61, 3809–3833. [Google Scholar] [CrossRef]
- Kelley, S. Excess Argon in K–Ar and Ar–Ar Geochronology. Chem. Geol. 2002, 188, 1–22. [Google Scholar] [CrossRef]
- Camacho, A.; Lee, J.K.; Hensen, B.J.; Braun, J. Short-Lived Orogenic Cycles and the Eclogitization of Cold Crust by Spasmodic Hot Fluids. Nature 2005, 435, 1191–1196. [Google Scholar] [CrossRef]
- Warren, C.; Sherlock, S.; Kelley, S. Interpreting High-Pressure Phengite 40Ar/39Ar Laserprobe Ages: An Example from Saih Hatat, NE Oman. Contrib. Mineral. Petrol. 2011, 161, 991–1009. [Google Scholar] [CrossRef]
- McDonald, C.S.; Warren, C.J.; Mark, D.F.; Halton, A.M.; Kelley, S.P.; Sherlock, S.C. Argon Redistribution during a Metamorphic Cycle: Consequences for Determining Cooling Rates. Chem. Geol. 2016, 443, 182–197. [Google Scholar] [CrossRef]
- McDonald, C.S.; Regis, D.; Warren, C.J.; Kelley, S.P.; Sherlock, S.C. Recycling Argon through Metamorphic Reactions: The Record in Symplectites. Lithos 2018, 300, 200–211. [Google Scholar] [CrossRef]
- Baxter, E.F. Quantification of the Factors Controlling the Presence of Excess 40Ar or 4He. Earth Planet. Sci. Lett. 2003, 216, 619–634. [Google Scholar] [CrossRef]
- Kühn, A.; Glodny, J.; Iden, K.; Austrheim, H. Retention of Precambrian Rb/Sr Phlogopite Ages through Caledonian Eclogite Facies Metamorphism, Bergen Arc Complex, W-Norway. Lithos 2000, 51, 305–330. [Google Scholar] [CrossRef]
- Glodny, J.; Kühn, A.; Austrheim, H. akon Diffusion versus Recrystallization Processes in Rb–Sr Geochronology: Isotopic Relics in Eclogite Facies Rocks, Western Gneiss Region, Norway. Geochim. Cosmochim. Acta 2008, 72, 506–525. [Google Scholar] [CrossRef]
- Giletti, B.J. Rb and Sr Diffusion in Alkali Feldspars, with Implications for Cooling Histories of Rocks. Geochim. Cosmochim. Acta 1991, 55, 1331–1343. [Google Scholar] [CrossRef]
- Jenkin, G.R.; Rogers, G.; Fallick, A.E.; Farrow, C.M. Rb-Sr Closure Temperatures in Bi-Mineralic Rocks: A Mode Effect and Test for Different Diffusion Models. Chem. Geol. 1995, 122, 227–240. [Google Scholar] [CrossRef]
- Ault, A.K.; Flowers, R.M.; Mahan, K.H. Quartz Shielding of Sub-10 μm Zircons from Radiation Damage-Enhanced Pb Loss: An Example from a Metamorphosed Mafic Dike, Northwestern Wyoming Craton. Earth Planet. Sci. Lett. 2012, 339–340, 57–66. [Google Scholar] [CrossRef]
- Ibanez-Mejia, M.; Bloch, E.M.; Vervoort, J.D. Timescales of Collisional Metamorphism from Sm-Nd, Lu-Hf and U-Pb Thermochronology: A Case from the Proterozoic Putumayo Orogen of Amazonia. Geochim. Cosmochim. Acta 2018, 235, 103–126. [Google Scholar] [CrossRef]
- Kohn, M.J.; Penniston-Dorland, S.C.; Ferreira, J.C.S. Implications of Near-Rim Compositional Zoning in Rutile for Geothermometry, Geospeedometry, and Trace Element Equilibration. Contrib. Mineral. Petrol. 2016, 171. [Google Scholar] [CrossRef]
- Meesters, A.G.C.A.; Dunai, T.J. Solving the Production–Diffusion Equation for Finite Diffusion Domains of Various Shapes: Part I. Implications for low-temperature (U–Th)/He thermochronology. Chem. Geol. 2002, 186, 333–344. [Google Scholar] [CrossRef]
- Meesters, A.G.C.A.; Dunai, T.J. Solving the Production–Diffusion Equation for Finite Diffusion Domains of Various Shapes: Part II. Application to cases with a-ejection and nonhomogeneous distribution of the source. Chem. Geol. 2002, 186, 347–363. [Google Scholar] [CrossRef]
- Watson, E.B.; Wanser, K.H.; Farley, K.A. Anisotropic Diffusion in a Finite Cylinder, with Geochemical Applications. Geochim. Cosmochim. Acta 2010, 74, 614–633. [Google Scholar] [CrossRef]
- Gautheron, C.; Tassan-Got, L. A Monte Carlo Approach to Diffusion Applied to Noble Gas/Helium Thermochronology. Chem. Geol. 2010, 273, 212–224. [Google Scholar] [CrossRef]
- Farley, K.A.; Shuster, D.L.; Ketcham, R.A. U and Th Zonation in Apatite Observed by Laser Ablation ICPMS, and Implications for the (U–Th)/He System. Geochim. Cosmochim. Acta 2011, 75, 4515–4530. [Google Scholar] [CrossRef]
- Huber, C.; Cassata, W.S.; Renne, P.R. A Lattice Boltzmann Model for Noble Gas Diffusion in Solids: The Importance of Domain Shape and Diffusive Anisotropy and Implications for Thermochronometry. Geochim. Cosmochim. Acta 2011, 75, 2170–2186. [Google Scholar] [CrossRef]
- Fox, M.; McKeon, R.E.; Shuster, D.L. Incorporating 3-D Parent Nuclide Zonation for Apatite 4He/3He Thermochronometry: An Example from the Appalachian Mountains. Geochem. Geophys. Geosyst. 2014, 15, 4217–4229. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Oxford University Press: Oxford, UK, 1975; p. 414. [Google Scholar]
- Cherniak, D.J. Diffusion in Accessory Minerals: Zircon, Titanite, Apatite, Monazite and Xenotime. Rev. Mineral. Geochem. 2010, 72, 827–869. [Google Scholar] [CrossRef]
- Cherniak, D.J. Cation Diffusion in Feldspars. Rev. Mineral. Geochem. 2010, 72, 691–733. [Google Scholar] [CrossRef]
- Cherniak, D.J.; Dimanov, A. Diffusion in Pyroxene, Mica and Amphibole. Rev. Mineral. Geochem. 2010, 72, 641–690. [Google Scholar] [CrossRef]
- Cherniak, D.J. Pb Diffusion in Rutile. Contrib. Mineral. Petrol. 2000, 139, 198–207. [Google Scholar] [CrossRef]
- Bindeman, I.N.; Davis, A.M.; Drake, M.J. Ion Microprobe Study of Plagioclase-Basalt Partition Experiments at Natural Concentration Levels of Trace Elements. Geochim. Cosmochim. Acta 1998, 62, 1175–1193. [Google Scholar] [CrossRef]
- Nielsen, R.L.; Ustunisik, G.; Weinsteiger, A.B.; Tepley, F.J.; Johnston, A.D.; Kent, A.J.R. Trace Element Partitioning between Plagioclase and Melt: An Investigation of the Impact of Experimental and Analytical Procedures. Geochem. Geophys. Geosyst. 2017, 18, 3359–3384. [Google Scholar] [CrossRef]
- Prowatke, S.; Klemme, S. Trace Element Partitioning between Apatite and Silicate Melts. Geochim. Cosmochim. Acta 2006, 70, 4513–4527. [Google Scholar] [CrossRef]
- Blundy, J. Mineral-Melt Partitioning of Uranium, Thorium and Their Daughters. Rev. Mineral. Geochem. 2003, 52, 59–123. [Google Scholar] [CrossRef]
- Fedele, L.; Lustrino, M.; Melluso, L.; Morra, V.; Zanetti, A.; Vannucci, R. Trace-Element Partitioning between Plagioclase, Alkali Feldspar, Ti-Magnetite, Biotite, Apatite, and Evolved Potassic Liquids from Campi Flegrei (Southern Italy). Am. Mineral. 2015, 100, 233–249. [Google Scholar] [CrossRef]
- Arzilli, F.; Fabbrizio, A.; Schmidt, M.W.; Petrelli, M.; Maimaiti, M.; Dingwell, D.B.; Paris, E.; Burton, M.; Carroll, M.R. The Effect of Diffusive Re-Equilibration Time on Trace Element Partitioning between Alkali Feldspar and Trachytic Melts. Chem. Geol. 2018, 495, 50–66. [Google Scholar] [CrossRef]
- Joesten, R. Grain-Boundary Diffusion Kinetics in Silicate and Oxide Minerals. In Diffusion, Atomic Ordering, and Mass Transport; Ganguly, J., Ed.; Advances in Physical Geochemistry; Springer: New York, NY, USA, 1991; Volume 8, pp. 345–395. ISBN 978-1-4613-9021-3. [Google Scholar]
- Dohmen, R.; Milke, R. Diffusion in Polycrystalline Materials: Grain Boundaries, Mathematical Models, and Experimental Data. Rev. Mineral. Geochem. 2010, 72, 921–970. [Google Scholar] [CrossRef]
- Kaur, I.; Mishin, Y.; Gust, W. Fundamentals of Grain and Interphase Boundary Diffusion, 3rd ed.; John Wiley: Chichester, UK; New York, NY, USA, 1995; ISBN 978-0-471-93819-4. [Google Scholar]
- Mishin, Y.; Herzig, C. Diffusion in Fine-Grained Materials: Theoretical Aspects and Experimental Possibilities. Nanostruct. Mater. 1995, 6, 859–862. [Google Scholar] [CrossRef]
- Mishin, Y.; Herzig, C. Grain Boundary Diffusion: Recent Progress and Future Research. Mater. Sci. Eng. A 1999, 260, 55–71. [Google Scholar] [CrossRef]
- Hiraga, T.; Kohlstedt, D.L. Equilibrium Interface Segregation in the Diopside–Forsterite System I: Analytical Techniques, Thermodynamics, and Segregation Characteristics. Geochim. Cosmochim. Acta 2007, 71, 1266–1280. [Google Scholar] [CrossRef]
- Farver, J.R.; Yund, R.A. Grain Boundary Diffusion of Oxygen, Potassium and Calcium in Natural and Hot-Pressed Feldspar Aggregates. Contrib. Mineral. Petrol. 1995, 118, 340–355. [Google Scholar] [CrossRef]



| Radius (μm) | 206Pb/238U Date ± 1σ (Ma) Monotonic Cooling | 206Pb/238U Date ± 1σ (Ma) History with Reheating |
|---|---|---|
| 10 | 120.0 ± 1.2 | 93.1 ± 0.9 |
| 20 | 120.4 ± 1.2 | 114.4 ± 1.1 |
| 30 | 120.7 ± 1.2 | 132.3 ± 1.3 |
| 50 | 121.0 ± 1.2 | 159.4 ± 1.6 |
| 100 | 121.4 ± 1.2 | 196.9 ± 2.0 |
| 150 | 121.7 ± 1.2 | 214.4 ± 2.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popov, D.V.; Spikings, R.A. Numerical Modelling of Radiogenic Ingrowth and Diffusion of Pb in Apatite Inclusions with Variable Shape and U-Th Zonation. Minerals 2021, 11, 364. https://doi.org/10.3390/min11040364
Popov DV, Spikings RA. Numerical Modelling of Radiogenic Ingrowth and Diffusion of Pb in Apatite Inclusions with Variable Shape and U-Th Zonation. Minerals. 2021; 11(4):364. https://doi.org/10.3390/min11040364
Chicago/Turabian StylePopov, Daniil V., and Richard A. Spikings. 2021. "Numerical Modelling of Radiogenic Ingrowth and Diffusion of Pb in Apatite Inclusions with Variable Shape and U-Th Zonation" Minerals 11, no. 4: 364. https://doi.org/10.3390/min11040364
APA StylePopov, D. V., & Spikings, R. A. (2021). Numerical Modelling of Radiogenic Ingrowth and Diffusion of Pb in Apatite Inclusions with Variable Shape and U-Th Zonation. Minerals, 11(4), 364. https://doi.org/10.3390/min11040364







