Microscale Heterogeneous Distribution and Speciation of Phosphorus in Soils Amended with Mineral Fertilizer and Cattle Manure Compost
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Samples
2.2. Preparation of Soil Thin-Sections
2.3. Wet Chemical Analyses
2.4. 31P-NMR
2.5. Bulk P-K Edge XANES
2.6. Microscale Analyses
3. Results and Discussion
3.1. Soil Properties and P Concentrations
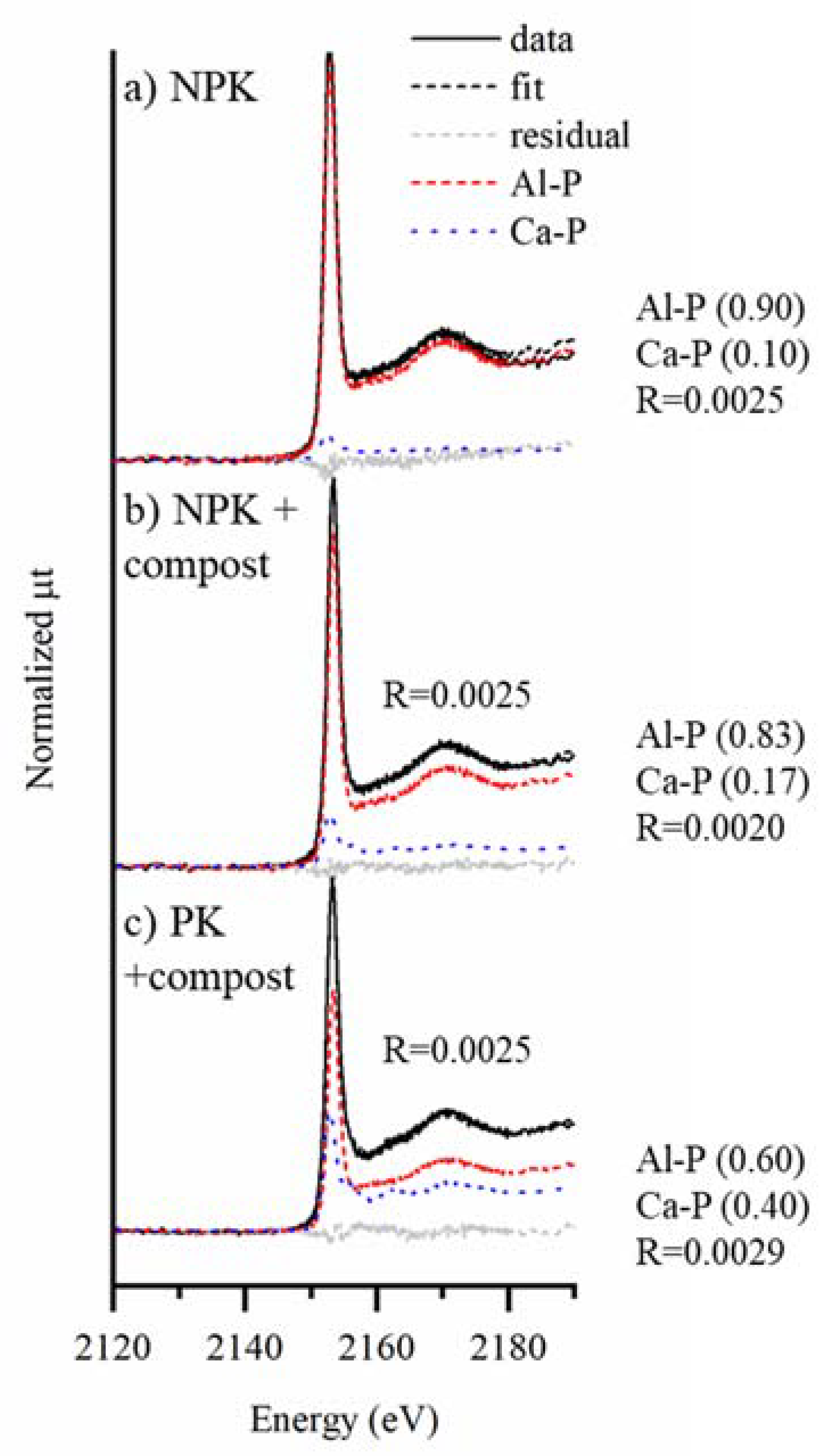
3.2. Bulk P K-Edge XANES
3.3. Phosphorus Speciation by 31P-NMR
3.4. Complementary P Speciation Using Extraction and Spectroscopic Techniques
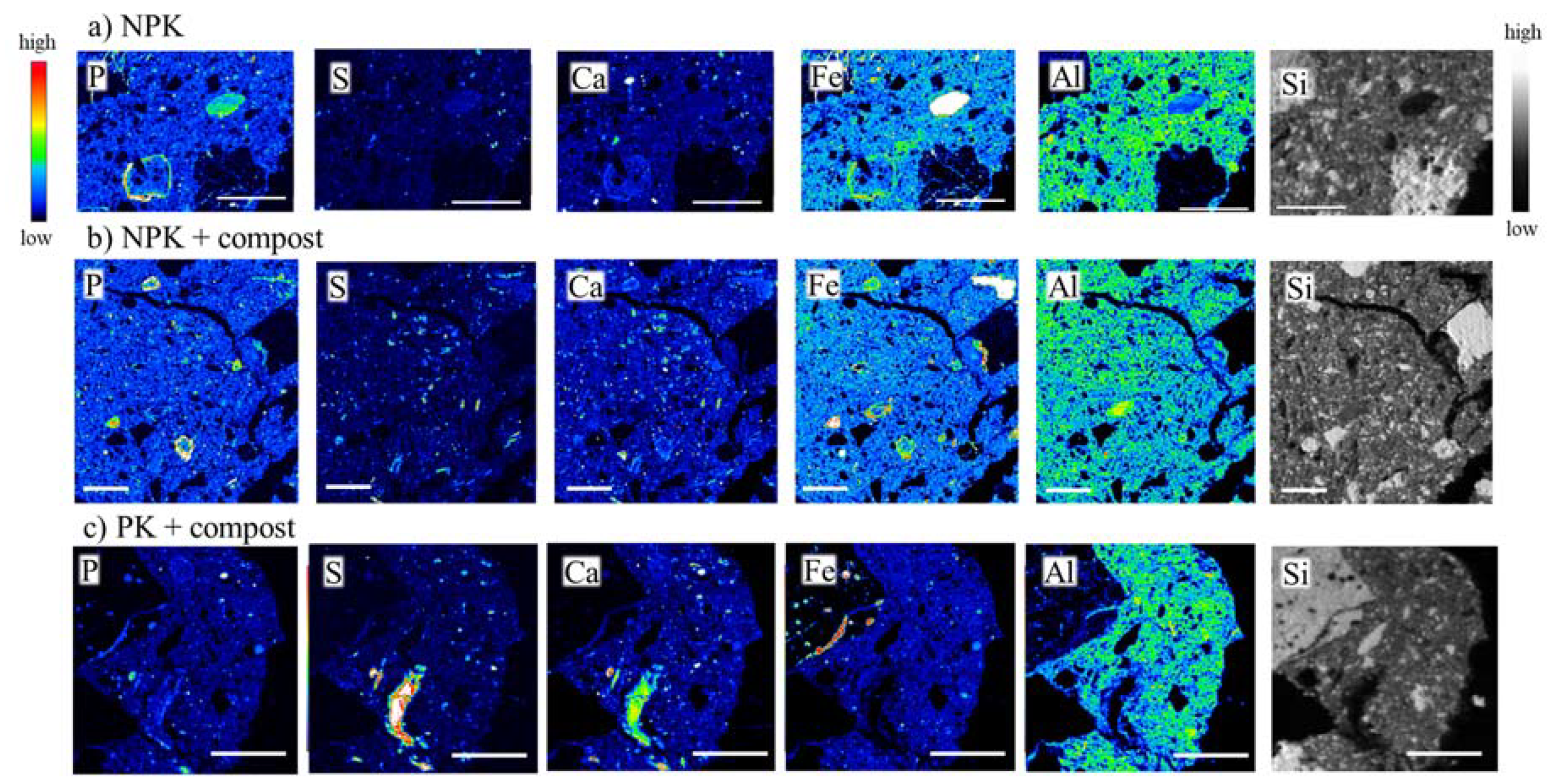
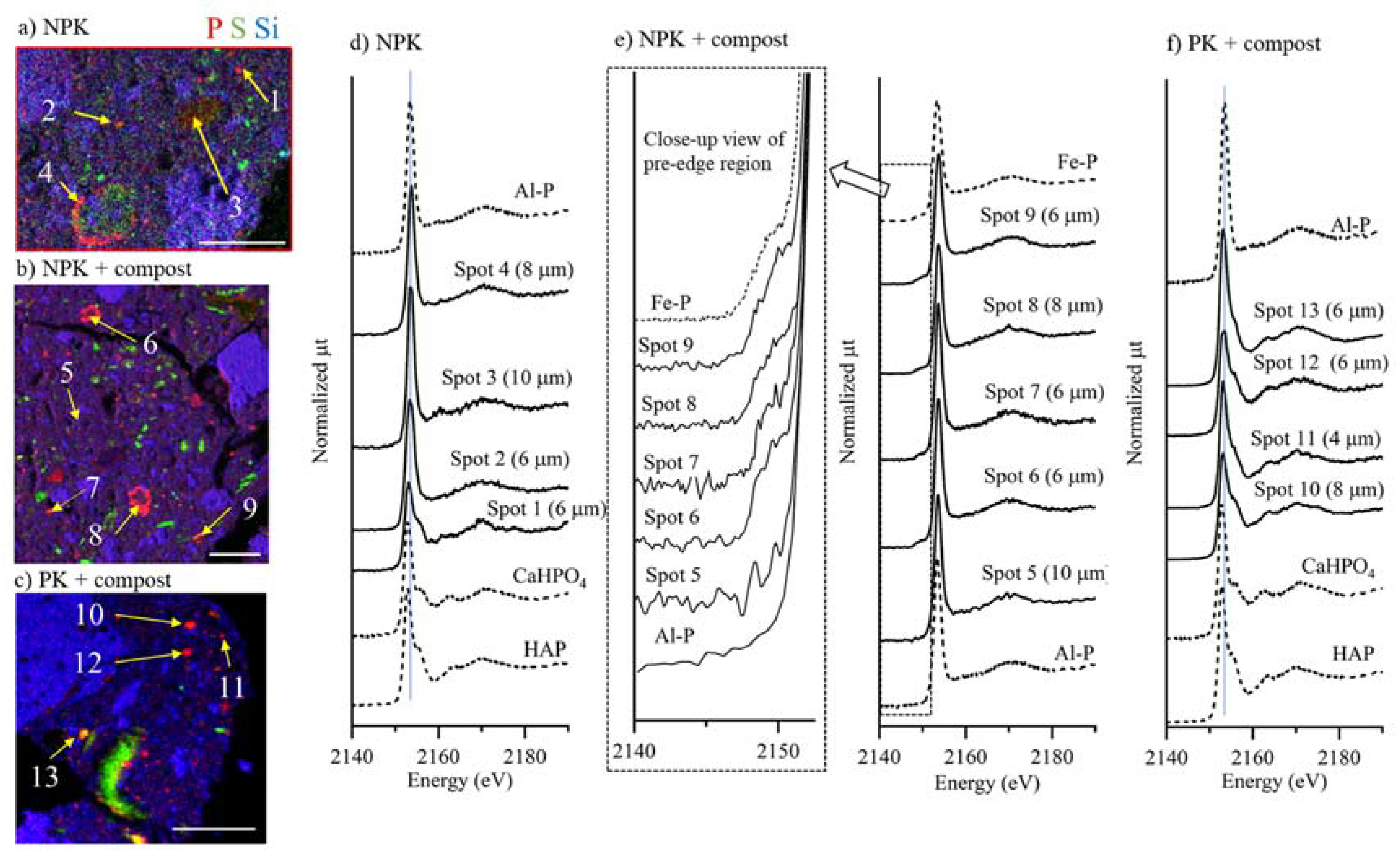
3.5. Microscale Distribution and Speciation of P
3.6. Accumulation Mechanisms of P in Soil Microsites
3.7. Complementary P Speciation Using Macro- and Micro-Spectroscopic Techniques
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Diacono, M.; Montemurro, F. Long-term effects of organic amendments on soil fertility. A review. Agron. Sustain. Dev. 2010, 30, 401–422. [Google Scholar] [CrossRef]
- Keesstra, S.D.; Bouma, J.; Wallinga, J.; Tittonell, P.; Smith, P.; Cerda, A.; Montanarella, L.; Quinton, J.N.; Pachepsky, Y.; van der Putten, W.H.; et al. The significance of soils and soil science towards realization of the united nations sustainable development goals. Soil 2016, 2, 111–128. [Google Scholar] [CrossRef]
- Daniel, T.C.; Sharpley, A.N.; Lemunyon, J.L. Agricultural phosphorus and eutrophication: A symposium overview. J. Environ. Qual. 1998, 27, 251–257. [Google Scholar] [CrossRef]
- Stutter, M.I.; Shand, C.A.; George, T.S.; Blackwell, M.S.A.; Dixon, L.; Bol, R.; MacKay, R.L.; Richardson, A.E.; Condron, L.M.; Haygarth, P.M. Land use and soil factors affecting accumulation of phosphorus species in temperate soils. Geoderma 2015, 257, 29–39. [Google Scholar] [CrossRef]
- Vaughan, R.E.; Needelman, B.A.; Kleinman, P.J.A.; Allen, A.L. Vertical distribution of phosphorus in agricultural drainage ditch soils. J. Environ. Qual. 2007, 36, 1895–1903. [Google Scholar] [CrossRef]
- Hedley, M.J.; Stewart, J.W.B.; Chauhan, B.S. Changes in organic and inorganic-phosphorus fractions induced by cultivation practices and laboratory incubations. Soil Sci. Soc. Am. J. 1982, 46, 970–976. [Google Scholar] [CrossRef]
- Chang, S.C.; Jackson, M.L. Fractionation of soil phosphorus. Soil Sci. 1957, 84, 133–144. [Google Scholar] [CrossRef]
- Sekiya, K. Phosphorus. In Methods of Soil Analysis; Bunsekihou, D.Y., Ed.; Yokendo: Tokyo, Japan, 1983. (In Japanese) [Google Scholar]
- Condron, L.M.; Newman, S. Revisiting the fundamentals of phosphorus fractionation of sediments and soils. J. Soils Sediments 2011, 11, 830–840. [Google Scholar] [CrossRef]
- Gu, C.; Dam, T.; Hart, S.C.; Turner, B.L.; Chadwick, O.A.; Berhe, A.A.; Hu, Y.; Zhu, M. Quantifying uncertainties in sequential chemical extraction of soil phosphorus using XANES spectroscopy. Environ. Sci. Technol. 2020, 54, 2257–2267. [Google Scholar] [CrossRef]
- Hinedi, Z.R.; Chang, A.C. Solubility and P-31 magic angle spinning nuclear magnetic resonance of phosphorus in sludge amended soils. Soil Sci. Soc. Am. J. 1989, 53, 1057–1061. [Google Scholar] [CrossRef]
- Beauchemin, S.; Hesterberg, D.; Chou, J.; Beauchemin, M.; Simard, R.R.; Sayers, D.E. Speciation of phosphorus in phosphorus-enriched agricultural soils using X-ray absorption near-edge structure spectroscopy and chemical fractionation. J. Environ. Qual. 2003, 32, 1809–1819. [Google Scholar] [CrossRef]
- Abdala, D.B.; da Silva, I.R.; Vergutz, L.; Sparks, D.L. Long-term manure application effects on phosphorus speciation, kinetics and distribution in highly weathered agricultural soils. Chemosphere 2015, 119, 504–514. [Google Scholar] [CrossRef]
- Koch, M.; Kruse, J.; Eichler-Lobermann, B.; Zimmer, D.; Willbold, S.; Leinweber, P.; Siebers, N. Phosphorus stocks and speciation in soil profiles of a long-term fertilizer experiment: Evidence from sequential fractionation, P K-edge XANES, and P-31 NMR spectroscopy. Geoderma 2018, 316, 115–126. [Google Scholar] [CrossRef]
- Sato, S.; Solomon, D.; Hyland, C.; Ketterings, Q.M.; Lehmann, J. Phosphorus speciation in manure and manure-amended soils using XANES spectroscopy. Environ. Sci. Technol. 2005, 39, 7485–7491. [Google Scholar] [CrossRef]
- Ando, K.; Yamaguchi, N.; Nakamura, Y.; Kasuya, M.; Taki, K. Speciation of phosphorus accumulated in fertilized cropland of Aichi prefecture in Japan with different soil properties by sequential chemical extraction and P K-edge XANES. Soil Sci. Plant Nutr. 2021. [Google Scholar] [CrossRef]
- Prietzel, J.; Harrington, G.; Hausler, W.; Heister, K.; Werner, F.; Klysubun, W. Reference spectra of important adsorbed organic and inorganic phosphate binding forms for soil P speciation using synchrotron-based K-edge XANES spectroscopy. J. Synchrotron Radiat. 2016, 23, 532–544. [Google Scholar] [CrossRef]
- Vogel, C.; Rivard, C.; Tanabe, I.; Adam, C. Microspectroscopy—Promising techniques to characterize phosphorus in soil. Commun. Soil Sci. Plant Anal. 2016, 47, 2088–2102. [Google Scholar] [CrossRef]
- Lombi, E.; Scheckel, K.G.; Armstrong, R.D.; Forrester, S.; Cutler, J.N.; Paterson, D. Speciation and distribution of phosphorus in a fertilized soil. Soil Sci. Soc. Am. J. 2006, 70, 2038–2048. [Google Scholar] [CrossRef]
- Rivard, C.; Lanson, B.; Cotte, M. Phosphorus speciation and micro-scale spatial distribution in North-American temperate agricultural soils from micro X-ray fluorescence and X-ray absorption near-edge spectroscopy. Plant Soil 2016, 401, 7–22. [Google Scholar] [CrossRef]
- Liu, J.; Sui, P.; Cade, B.; Hu, Y.F.; Yang, J.J.; Huang, S.M.; Ma, Y.B. Molecular-level understanding of phosphorus transformation with long-term phosphorus addition and depletion in an alkaline soil. Geoderma 2019, 353, 116–124. [Google Scholar] [CrossRef]
- Werner, F.; Mueller, C.W.; Thieme, J.; Gianoncelli, A.; Rivard, C.; Hoschen, C.; Prietzel, J. Micro-scale heterogeneity of soil phosphorus depends on soil substrate and depth. Sci. Rep. 2017, 7, 3203. [Google Scholar] [CrossRef] [PubMed]
- Gamble, A.V.; Northrup, P.; Sparks, D.L. Elucidation of soil phosphorus speciation in mid-Atlantic soils using synchrotron-based microspectroscopic techniques. J. Environ. Qual. 2020, 49, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Nobile, C.M.; Bravin, M.N.; Becquer, T.; Paillat, J.M. Phosphorus sorption and availability in an Andosol after a decade of organic or mineral fertilizer applications: Importance of pH and organic carbon modifications in soil as compared to phosphorus accumulation. Chemosphere 2020, 239. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Ma, Y.; Sanders, R.L.; Xu, C.; Li, J.; Myneni, S.C.B. Phosphorus speciation and transformation in long-term fertilized soil: Evidence from chemical fractionation and P K-edge XANES spectroscopy. Nutr. Cycl. Agroecosyst. 2017, 107, 215–226. [Google Scholar] [CrossRef]
- Lookman, R.; Geerts, H.; Grobet, P.; Merckx, R.; Vlassak, K. Phosphate speciation in excessively fertilized soil: A P-31 and Al-27 MAS NMR spectroscopy study. Eur. J. Soil Sci. 1996, 47, 125–130. [Google Scholar] [CrossRef]
- IUSS Working Group. WRB 2006. In World Reference Base for Soil Resources 2006; World Soil Resources Reports No.103; FAO: Rome, Italy, 2006. [Google Scholar]
- Yamamoto, T.; Tsuji, M.; Suzuki, R.; Kasuya, M.; Takeuchi, M. Changes of phosphate species in a cabbage field using cattle manure compost. Res. Bull. Aichi Agric. Res. Cent. 2016, 48, 101–107, (In Japanese with English summary). [Google Scholar]
- Sumner, M.E.; Miller, W.P. Cation exchange capacity and exchange coefficients. In Methods of Soil Analysis: Part 3 Chemical Methods; Sparks, D.L., Ed.; Soil Science Society of America: Fitchburg, WI, USA, 1996; pp. 1201–1229. [Google Scholar]
- Jackson, M.L.; Lim, C.H.; Zelazny, L.W. Oxides, hydroxides, and aluminosilicates. In Methods of Soil Analysis: Part 1—Physical and Mineralogical Methods; Klute, A., Ed.; Soil Science Society of America: Madison, WI, USA, 1986; pp. 101–142. [Google Scholar]
- Moriyama, T.; Ikeda, S.; Doi, M.; Fess, S. Trace element analysis using EDXRF with polarized optics. Adv. X Ray Anal. 2011, 54, 289–298. [Google Scholar]
- Gee, G.; Bauder, J. Particle-size analysis. In Methods of Soil Analysis: Part 1—Physical and Mineralogical Methods; Klute, A., Ed.; Soil Science Society of America: Madison, WI, USA, 1986; pp. 383–411. [Google Scholar]
- Truog, E. The determination of the readily available phosphorus of soils. J. Am. Soc. Agron. 1930, 22, 874–882. [Google Scholar] [CrossRef]
- Otani, T.; Ae, N. The status of inorganic and organic phosphorus in some soils in relation to plant availability. Soil Sci. Plant Nutr. 1997, 43, 419–429. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta. 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Cade-Menun, B.; Liu, C.W. Solution phosphorus-31 nuclear magnetic resonance spectroscopy of soils from 2005 to 2013: A review of sample preparation and experimental parameters. Soil Sci. Soc. Am. J. 2014, 78, 19–37. [Google Scholar] [CrossRef]
- Ingall, E.D.; Brandes, J.A.; Diaz, J.M.; de Jonge, M.D.; Paterson, D.; McNulty, I.; Elliott, W.C.; Northrup, P. Phosphorus K-edge XANES spectroscopy of mineral standards. J. Synchrotron Radiat. 2011, 18, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Ravel, B.; Newville, M. Athena, Artemis, Hephaestus: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Northrup, P. The TES beamline (8-BM) at NSLS-II: Tender-energy spatially resolved X-ray absorption spectroscopy and X-ray fluorescence imaging. J. Synchrotron Radiat. 2019, 26, 2064–2074. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, W.L. Chemical Equilibria in Soils; John Wiley: New York, NY, USA, 1979. [Google Scholar]
- Hesterberg, D.; Zhou, W.Q.; Hutchison, K.J.; Beauchemin, S.; Sayers, D.E. XAFS study of adsorbed and mineral forms of phosphate. J. Synchrotron Radiat. 1999, 6, 636–638. [Google Scholar] [CrossRef]
- Gustafsson, J.P.; Braun, S.; Tuyishime, J.R.M.; Adediran, G.A.; Warrinnier, R.; Hesterberg, D. Probabilistic approach to phosphorus speciation of soils using P K-edge XANES spectroscopy with linear combination fitting. Soil Syst. 2020, 4, 26. [Google Scholar] [CrossRef]
- Andersson, K.O.; Tighe, M.K.; Guppy, C.N.; Milham, P.J.; McLaren, T.I.; Schefe, C.R.; Lombi, E. XANES demonstrates the release of calcium phosphates from alkaline vertisols to moderately acidified solution. Environ. Sci. Technol. 2016, 50, 4229–4237. [Google Scholar] [CrossRef]
- Gerard, F. Clay minerals, iron/aluminum oxides, and their contribution to phosphate sorption in soils—A myth revisited. Geoderma 2016, 262, 213–226. [Google Scholar] [CrossRef]
- Komiyama, T.; Ito, T. The characteristics of phosphorus in animal manure composts. Soil Sci. Plant Nutr. 2019, 65, 281–288. [Google Scholar] [CrossRef]
- He, Z.Q.; Honeycutt, C.W.; Xing, B.; McDowell, R.W.; Pellechia, P.J.; Zhang, T.Q. Solid-state Fourier transform infrared and P-31 nuclear magnetic resonance spectral features of phosphate compounds. Soil Sci. 2007, 172, 501–515. [Google Scholar] [CrossRef]
- Li, W.; Pierre-Louis, A.M.; Kwon, K.D.; Kubicki, J.D.; Strongin, D.R.; Phillips, B.L. Molecular level investigations of phosphate sorption on corundum (alpha-Al2O3) by P-31 solid state NMR, ATR-FTIR and quantum chemical calculation. Geochim. Cosmochim. Acta 2013, 107, 252–266. [Google Scholar] [CrossRef]
- Frossard, E.; Tekely, P.; Grimal, J.Y. Characterization of phosphate species in urban sewage sludges by high-resolution solid-state P-31 NMR. Eur. J. Soil Sci. 1994, 45, 403–408. [Google Scholar] [CrossRef]
- Kruse, J.; Abraham, M.; Amelung, W.; Baum, C.; Bol, R.; Kühn, O.; Lewandowski, H.; Niederberger, J.; Oelmann, Y.; Rüger, C.; et al. Innovative methods in soil phosphorus research: A review. J. Plant Nutr. Soil Sci. 2015, 178, 43–88. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Takamoto, A.; Kikkawa, R.; Murakami, K.; Yamaguchi, N. Formations of hydroxyapatite and inositol hexakisphosphate in poultry litter during the composting period: Sequential fractionation, P K-edge XANES and solution 31P NMR investigations. Environ. Sci. Technol. 2014, 48, 5486–5492. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Hashimoto, Y.; Kang, J.; Kobayashi, K. Speciation of phosphorus zinc and copper in soil and water-dispersible colloid affected by a long-term application of swine manure compost. Environ. Sci. Technol. 2018, 52, 13270–13278. [Google Scholar] [CrossRef] [PubMed]
- Hesterberg, D. Macroscale chemical properties and X-ray absorption spectroscopy of soil phosphorus. In Synchrotron-Based Techniques in Soils and Sediments; Singh, B., Grafe, M., Eds.; Elsevier: Oxford, UK, 2010; pp. 313–356. [Google Scholar]
- Negassa, W.; Kruse, J.; Michalik, D.; Appathurai, N.; Zuin, L.; Leinweber, P. Phosphorus speciation in agro-industrial byproducts: Sequential fractionation, solution 31P NMR, and P K- and L2,3-edge XANES spectroscopy. Environ. Sci. Technol. 2010, 44, 2092–2097. [Google Scholar] [CrossRef]
- Kruse, J.; Leinweber, P.; Eckhardt, K.-U.; Godlinski, F.; Hu, Y.; Zuin, L. Phosphorus L2,3-edge XANES: Overview of reference compounds. J. Synchrotron Radiat. 2009, 16, 247–259. [Google Scholar] [CrossRef]
- Weyers, E.; Strawn, D.G.; Peak, D.; Moore, A.D.; Baker, L.L.; Cade-Menun, B. Phosphorus speciation in calcareous soils following annual dairy manure amendments. Soil Sci. Soc. Am. J. 2016, 80, 1531–1542. [Google Scholar] [CrossRef]
- Khare, N.; Hesterberg, D.; Beauchemin, S.; Wang, S.L. XANES determination of adsorbed phosphate distribution between ferrihydrite and boehmite in mixtures. Soil Sci. Soc. Am. J. 2004, 68, 460–469. [Google Scholar] [CrossRef]
- Abdala, D.B.; Moore, P.A.; Rodrigues, M.; Herrera, W.F.; Pavinato, P.S. Long-term effects of alum-treated litter, untreated litter and NH4NO3 application on phosphorus speciation, distribution and reactivity in soils using K-edge XANES and chemical fractionation. J. Environ. Manag. 2018, 213, 206–216. [Google Scholar] [CrossRef]
- Lehmann, J.; Lan, Z.; Hyland, C.; Sato, S.; Solomon, D.; Ketterings, Q.M. Long-term dynamics of phosphorus forms and retention in manure-amended soils. Environ. Sci. Technol. 2005, 39, 6672–6680. [Google Scholar] [CrossRef] [PubMed]
- Colombo, C.; Palumbo, G.; He, J.Z.; Pinton, R.; Cesco, S. Review on iron availability in soil: Interaction of Fe minerals, plants, and microbes. J. Soils Sediments 2014, 14, 538–548. [Google Scholar] [CrossRef]
- Werner, F.; Prietzel, J. Standard protocol and quality assessment of soil phosphorus speciation by P K-edge XANES spectroscopy. Environ. Sci. Technol. 2015, 49, 10521–10528. [Google Scholar] [CrossRef] [PubMed]
- Prietzel, J.; Dumig, A.; Wu, Y.H.; Zhou, J.; Klysubun, W. Synchrotron-based P K-edge XANES spectroscopy reveals rapid changes of phosphorus speciation in the topsoil of two glacier foreland chronosequences. Geochim. Cosmochim. Acta 2013, 108, 154–171. [Google Scholar] [CrossRef]
- Turner, B.L.; Mahieu, N.; Condron, L.M. Phosphorus-31 nuclear magnetic resonance spectral assignments of phosphorus compounds in soil NaOH-EDTA extracts. Soil Sci. Soc. Am. J. 2003, 67, 497–510. [Google Scholar] [CrossRef]
- Hesterberg, D.; McNulty, I.; Thieme, J. Speciation of soil phosphorus assessed by XANES spectroscopy at different spatial scales. J. Environ. Qual. 2017, 46, 1190–1197. [Google Scholar] [CrossRef]


| Cattle Manure Compost * | Chemical Fertilizer (kg·ha−1) | ||||||
|---|---|---|---|---|---|---|---|
| Winter Cabbage | Summer Cabbage | ||||||
| Mg·ha−1 | N | P2O5 | K2O | N | P2O5 | K2O | |
| NPK | 0 | 300 | 150 | 300 | 200 | 50 | 180 |
| NPK + compost | 15 | 300 | 150 | 300 | 200 | 50 | 180 |
| PK + compost | 15 | 0 | 150 | 300 | 0 | 50 | 180 |
| pH (H2O) | TC | TN | CEC | Exchangeable Cations | BSR | Alox | Feox | Total Al | Total Fe | Particle Size Distribution | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ca | Mg | K | Na | Coarse Sand | Fine Sand | Silt | Clay | ||||||||||
| g·kg−1 | cmolc·kg−1 | % | g·kg−1 | % | |||||||||||||
| NPK | 5.9 | 11.3 | 1.4 | 11.1 | 5.5 | 1.2 | 0.9 | 0.1> | 68 | 1.5 | 2.1 | 84 | 40 | 20.1 | 20.3 | 22.7 | 36.9 |
| NPK + compost | 6.0 | 16.9 | 1.6 | 12.5 | 6.9 | 1.8 | 1.3 | 0.1 | 81 | 1.7 | 2.2 | 81 | 39 | 21.4 | 21.5 | 22.3 | 34.8 |
| PK + compost | 7.4 | 16.5 | 1.6 | 13.7 | 14.5 | 3.1 | 2 | 0.1 | 144 | 1.8 | 2.0 | 81 | 38 | 21.9 | 20.1 | 22.1 | 35.9 |
| Total P | Water-Soluble P | Truog P | Sequential Extraction | EDTA-NaOH-P | ||||
|---|---|---|---|---|---|---|---|---|
| Ac-P | NH4F-P | NaOH-P | Residual-P | |||||
| mgP·kg−1 | ||||||||
| NPK | 1353 | 2.6 | 196 | 136 | 370 | 323 | 524 | 1346 |
| (0.19) | (14) | (10) | (27) | (24) | (39) | (100) | ||
| NPK + compost | 1641 | 4.4 | 298 | 265 | 343 | 332 | 701 | 1444 |
| (0.27) | (18) | (16) | (21) | (20) | (43) | (88) | ||
| PK + compost | 1672 | 6.7 | 480 | 526 | 354 | 322 | 470 | 1347 |
| (0.40) | (29) | (31) | (21) | (19) | (29) | (95) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamaguchi, N.; Ohkura, T.; Hikono, A.; Hashimoto, Y.; Suda, A.; Yamamoto, T.; Ando, K.; Kasuya, M.; Northrup, P.; Wang, S.-L.; et al. Microscale Heterogeneous Distribution and Speciation of Phosphorus in Soils Amended with Mineral Fertilizer and Cattle Manure Compost. Minerals 2021, 11, 121. https://doi.org/10.3390/min11020121
Yamaguchi N, Ohkura T, Hikono A, Hashimoto Y, Suda A, Yamamoto T, Ando K, Kasuya M, Northrup P, Wang S-L, et al. Microscale Heterogeneous Distribution and Speciation of Phosphorus in Soils Amended with Mineral Fertilizer and Cattle Manure Compost. Minerals. 2021; 11(2):121. https://doi.org/10.3390/min11020121
Chicago/Turabian StyleYamaguchi, Noriko, Toshiaki Ohkura, Atsuko Hikono, Yohey Hashimoto, Aomi Suda, Taku Yamamoto, Kaori Ando, Masahiro Kasuya, Paul Northrup, Shan-Li Wang, and et al. 2021. "Microscale Heterogeneous Distribution and Speciation of Phosphorus in Soils Amended with Mineral Fertilizer and Cattle Manure Compost" Minerals 11, no. 2: 121. https://doi.org/10.3390/min11020121
APA StyleYamaguchi, N., Ohkura, T., Hikono, A., Hashimoto, Y., Suda, A., Yamamoto, T., Ando, K., Kasuya, M., Northrup, P., Wang, S.-L., & Hesterberg, D. (2021). Microscale Heterogeneous Distribution and Speciation of Phosphorus in Soils Amended with Mineral Fertilizer and Cattle Manure Compost. Minerals, 11(2), 121. https://doi.org/10.3390/min11020121







