Improvement in pH and Total Iron Concentration of Acid Mine Drainage after Backfilling: A Case Study of an Underground Abandoned Mine in Japan
Abstract
:1. Introduction
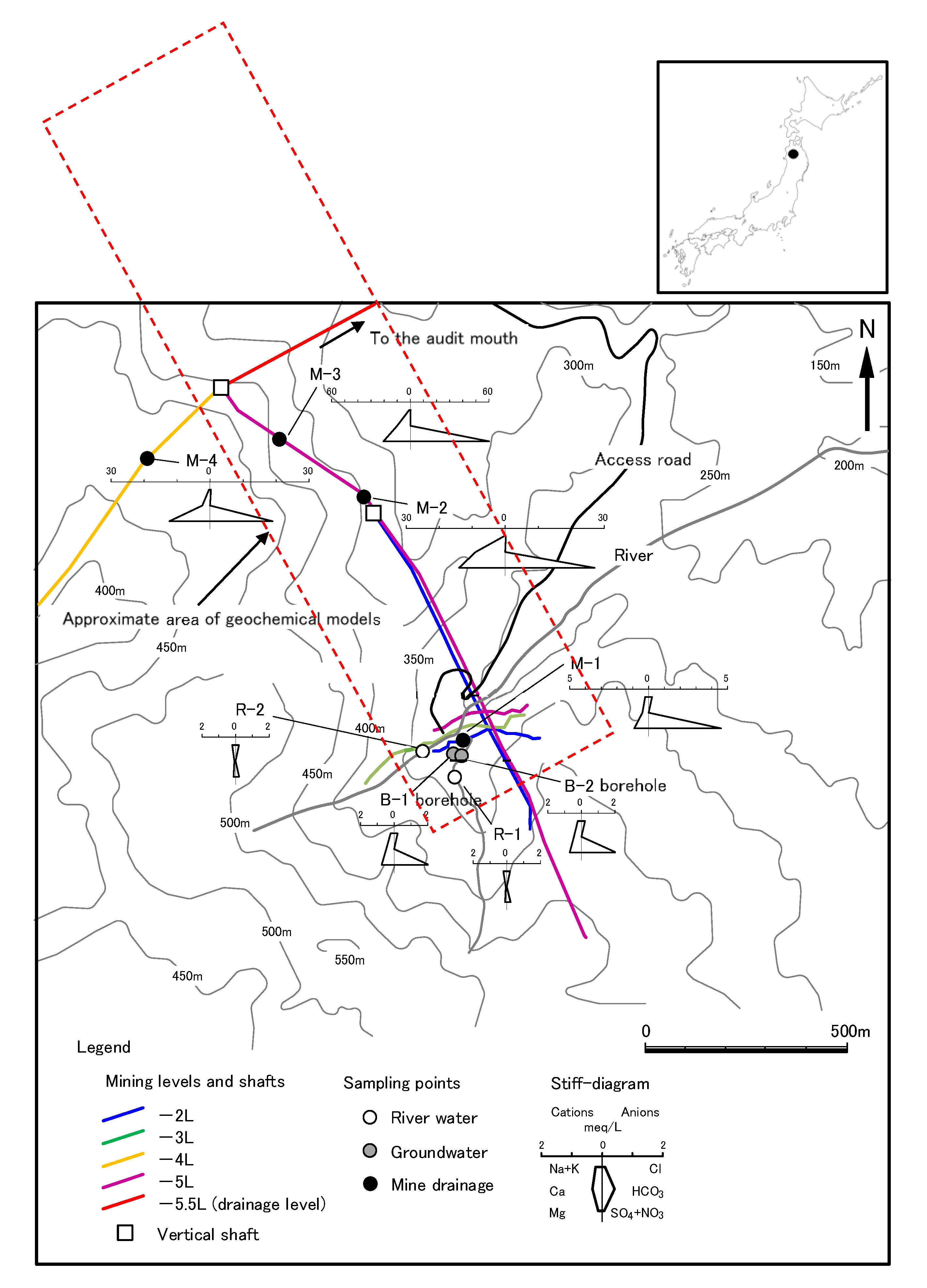
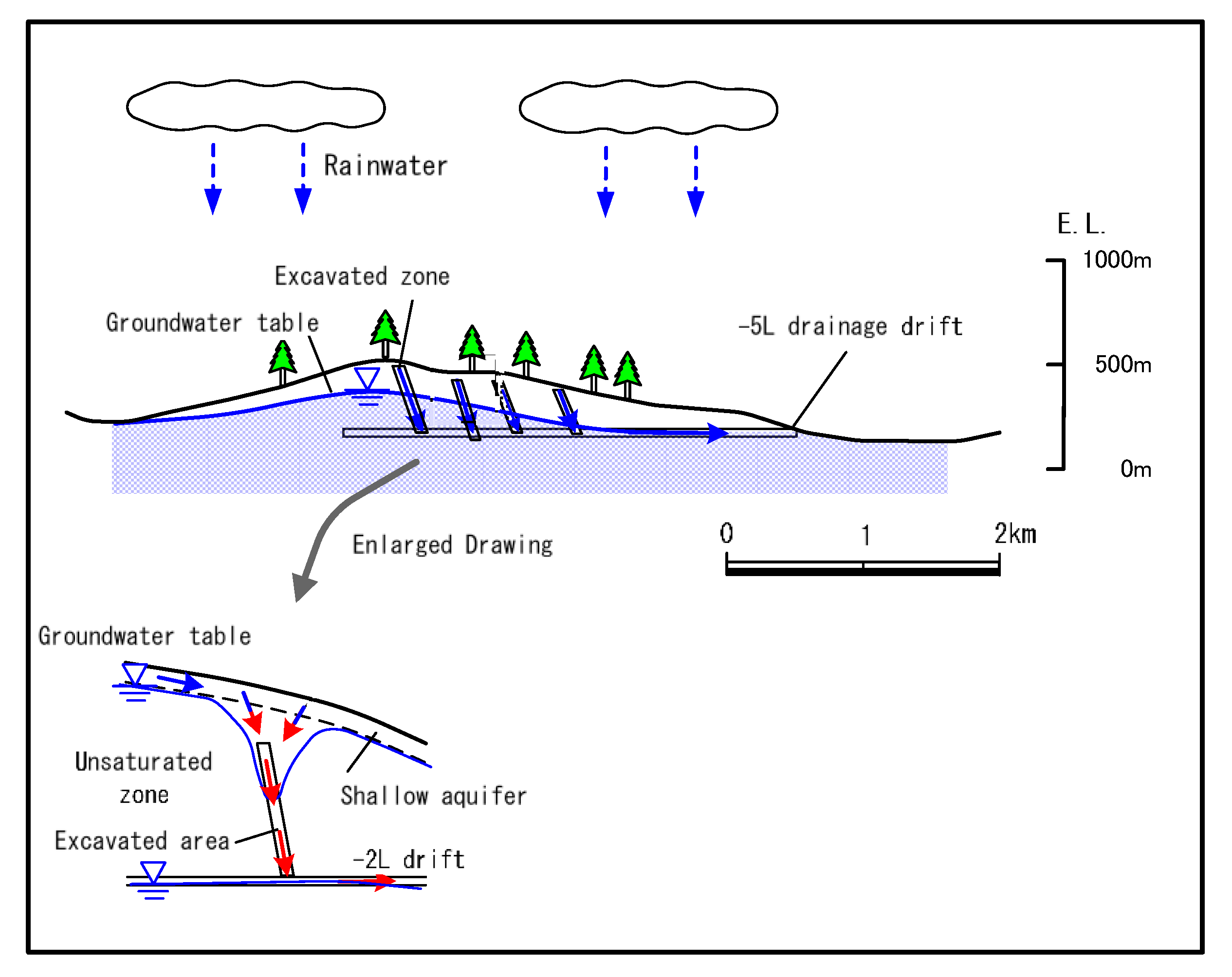
2. Geology and Hydrology of the Study Area
3. Materials and Methods
3.1. Sampling Location and Analysis
3.2. Properties of Rock Sample
3.3. Geochemical Simulation
| Materials | Hydraulic Conductivity | Porosity |
|---|---|---|
| Neogene | 9.2 × 10−9 m/s [11] | 0.036 [22] |
| Drift (before backfilling) | 1 × 10−3 m/s (The hydraulic conductivity was set to be significantly larger than that of the surrounding Neogene.) | 0.80 (Although the porosity in the old drift has not been measured, the wall surface has collapsed in some places, so 0.8 was assumed.) |
| Drift (after backfilling) | 1 × 10−3 m/s (Since the groundwater flow field after backfilling is controlled by the flow rate with the same boundary conditions, the hydraulic conductivity was set to be the same as before backfilling.) | 0.15 (Since there was no reference value of porosity after backfilling, the porosity in mortar [24] was used here.) |
| Initial minerals in the Neogene in cases 1, 2-1 and 2-2 | Pyrite | 10 wt% (Percentage estimated by the results of XRD) |
| Clinochlore (Chlorite identified by XRD is modeled.) | 5 wt% (same as above) | |
| Initial mineral in drift after backfilling in case 2-2 | Calcite | 65.5% [25] |
| Secondary minerals that appear when supersaturated in cases 1, 2-1 and 2-2 | Fe(OH)3 | - |
| Minerals | Reactions | Log of Equilibrium Constant | Kinetic Parameters 1 | ||
|---|---|---|---|---|---|
| k (mol/(m2·s)) | n | ||||
| Initial minerals (Neogene) | Pyrite | FeS2 + H2O = 0.25H+ + 0.25SO42− + Fe2+ + 1.75HS− | −24.6534 | 2 | |
| Clinochlore | Mg5Al2Si3O10(OH)8 + 16H+ = 2Al3+ + 3SiO2 + 5Mg2+ + 12H2O | 67.2391 | 10−11.11 [26] | 0.5 [26] | |
| Initial mineral (drift after backfilling) | Calcite | CaCO3 + H+ = Ca2+ + HCO3− | 1.8487 | 10−0.3 [26] | 1 [26] |
| Secondary mineral that appear if supersaturated | Fe(OH)3 | Fe(OH)3 + 3H+ = Fe3+ + 3H2O | 5.6556 | Reactions reaching equilibrium | |
4. Results
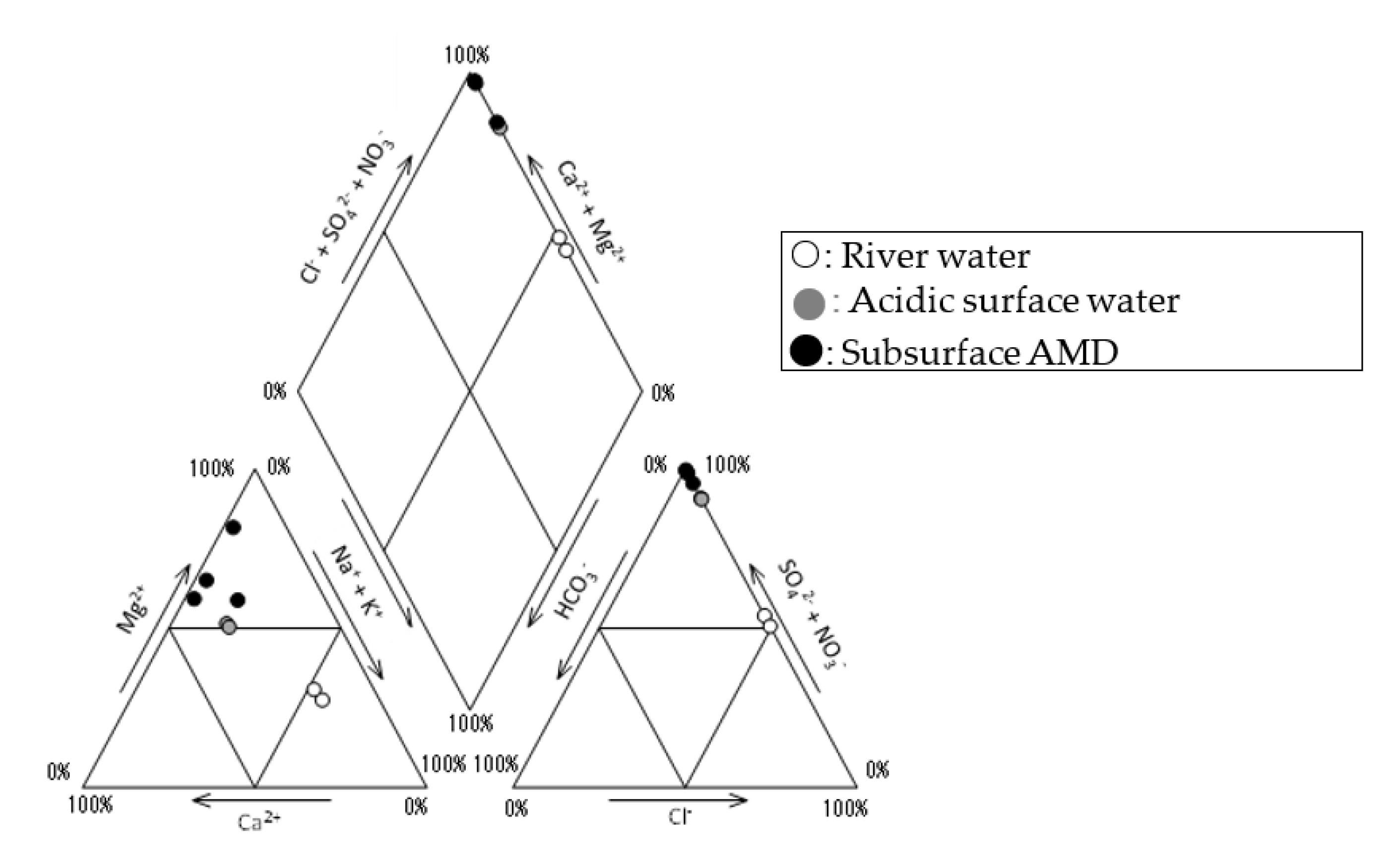
4.1. Quality of the Water Samples
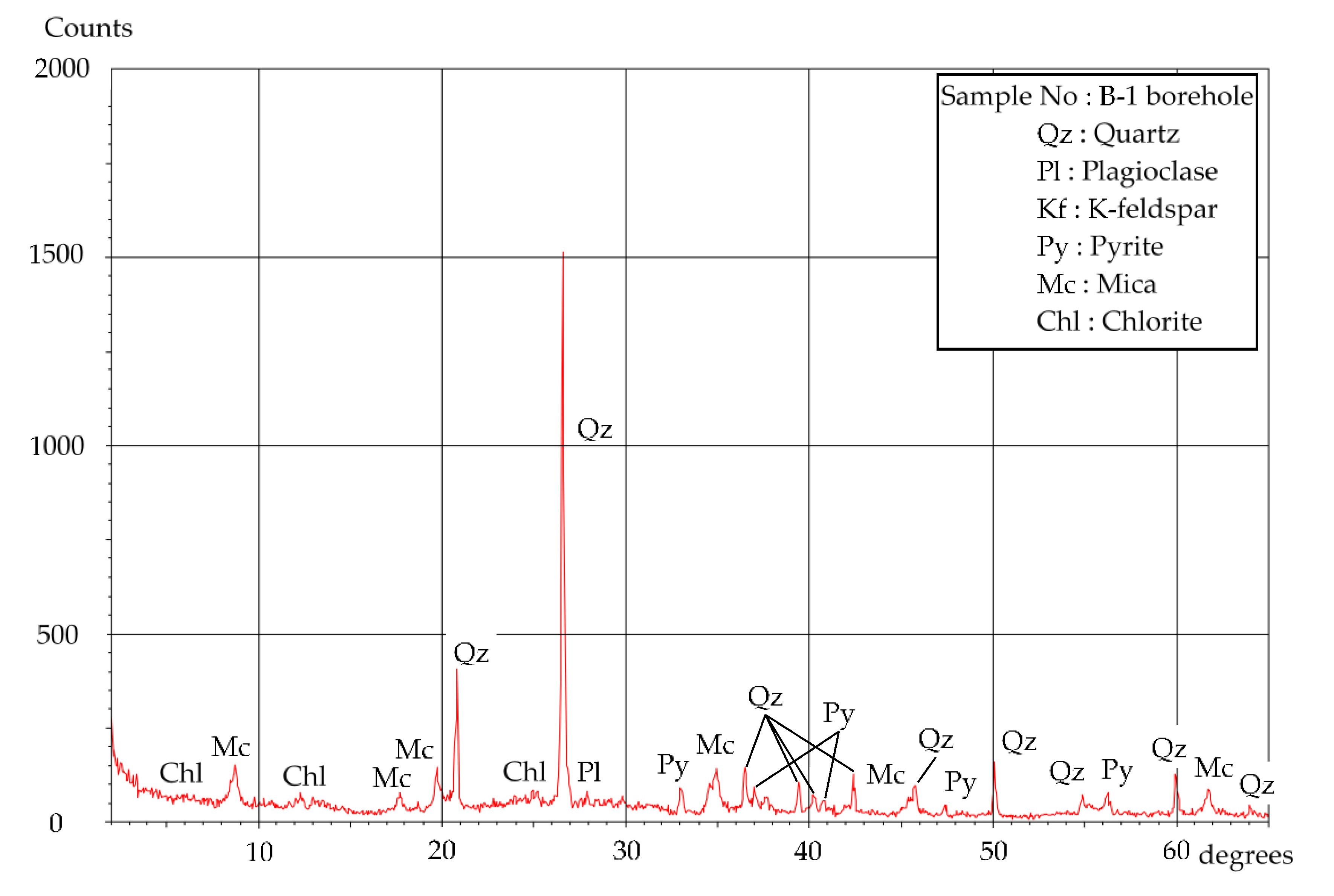
4.2. Mineral Composition
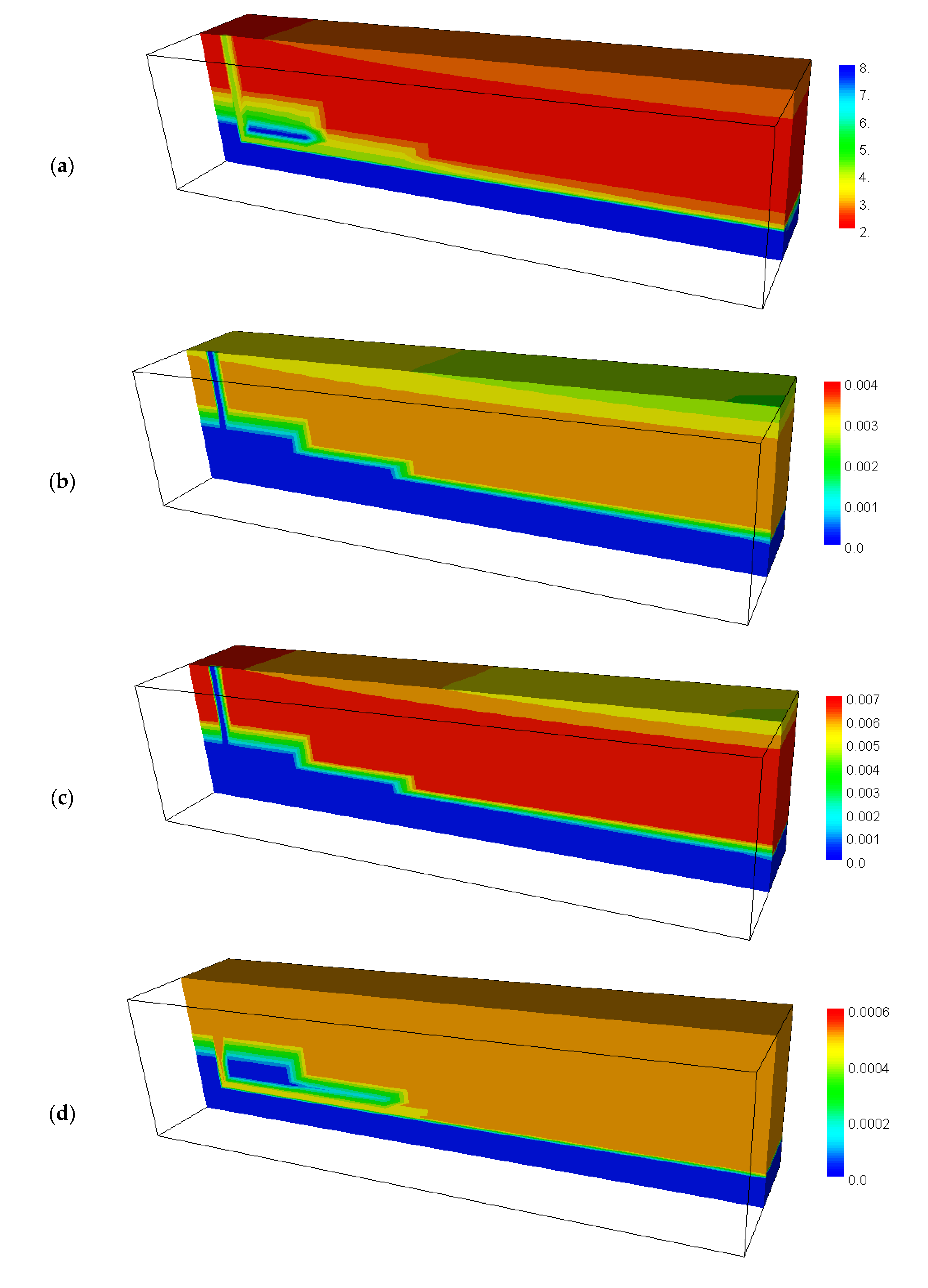
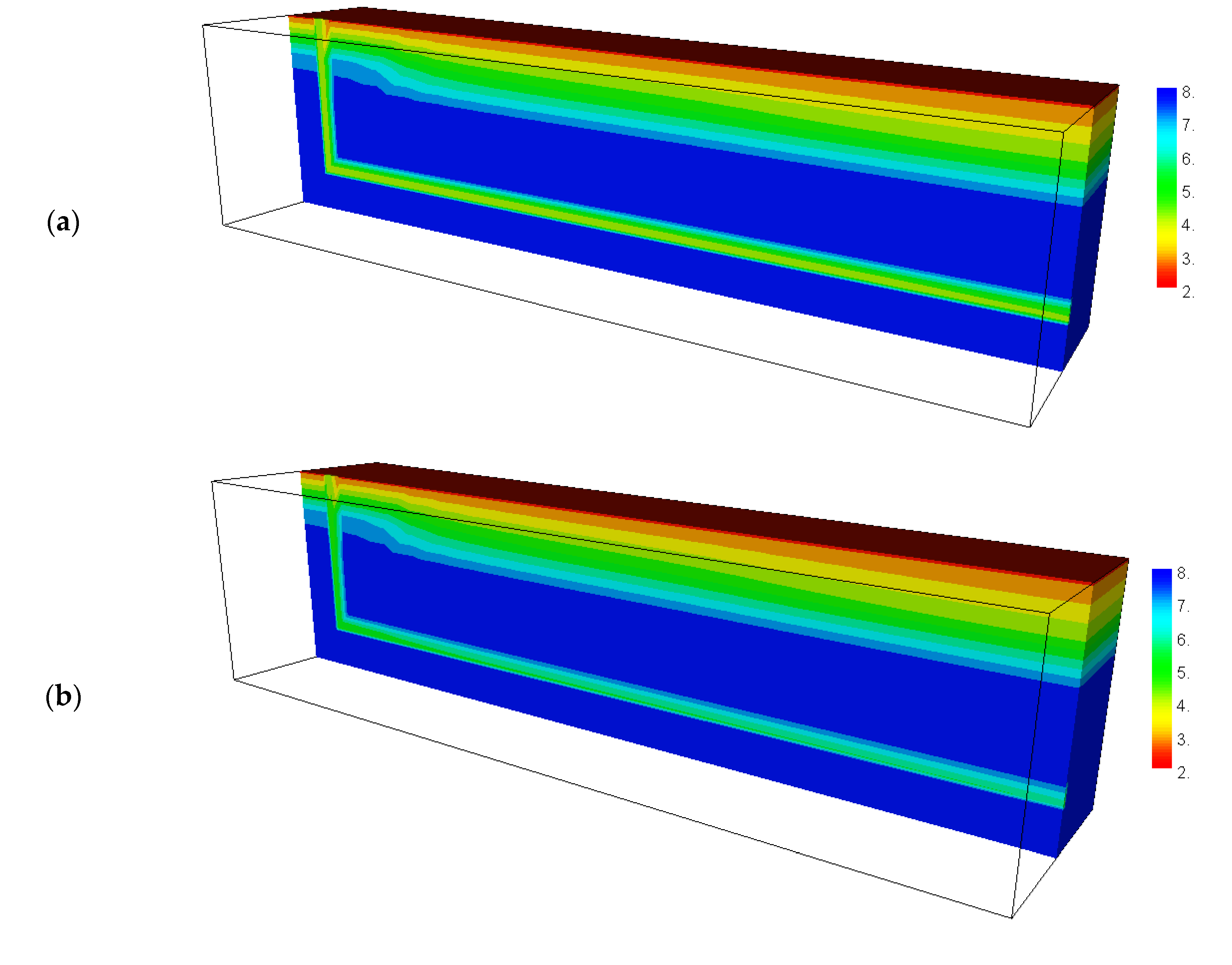
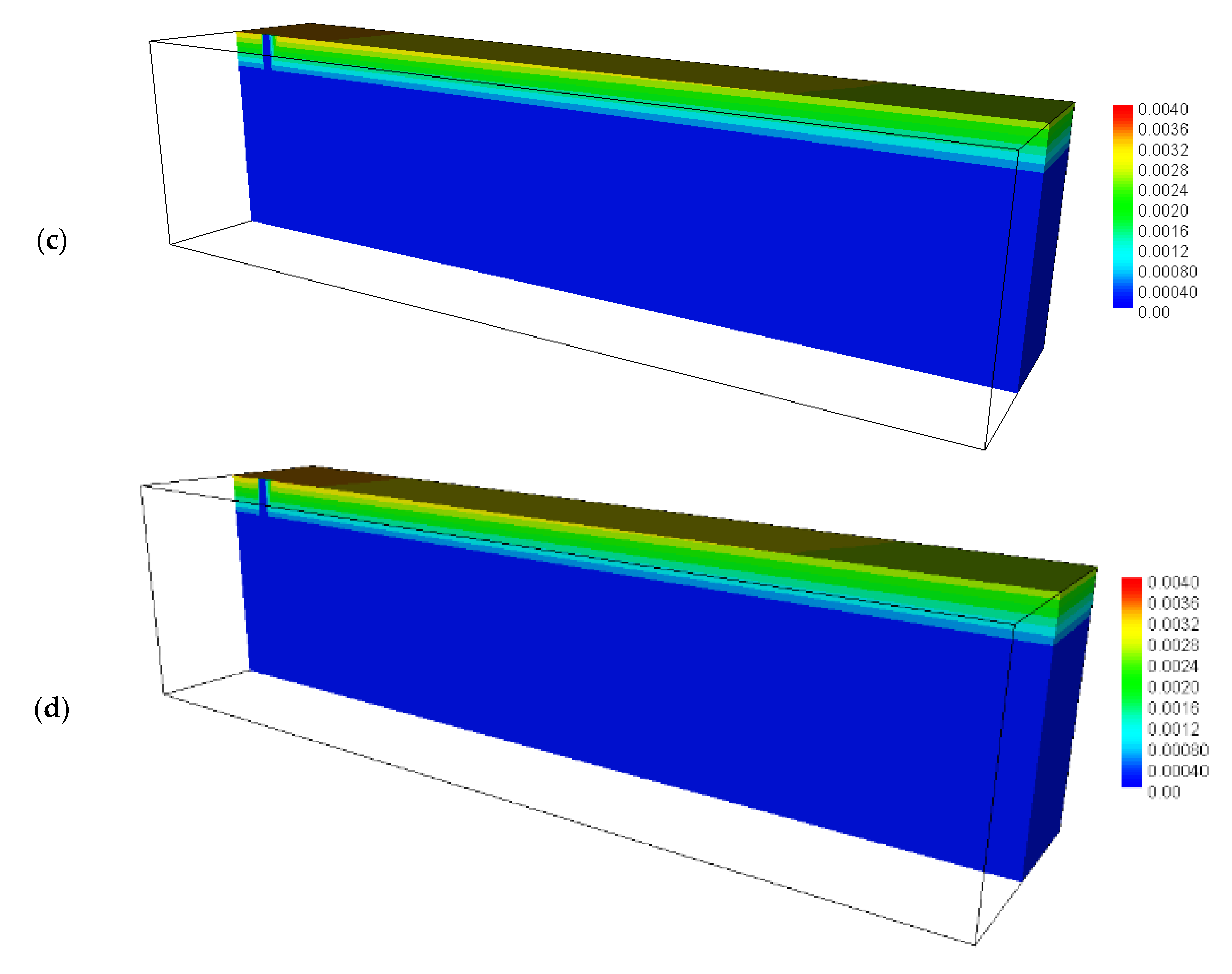
4.3. Calculated Results before Backfilling the Drift
4.4. Calculated Results after Backfilling the Drift
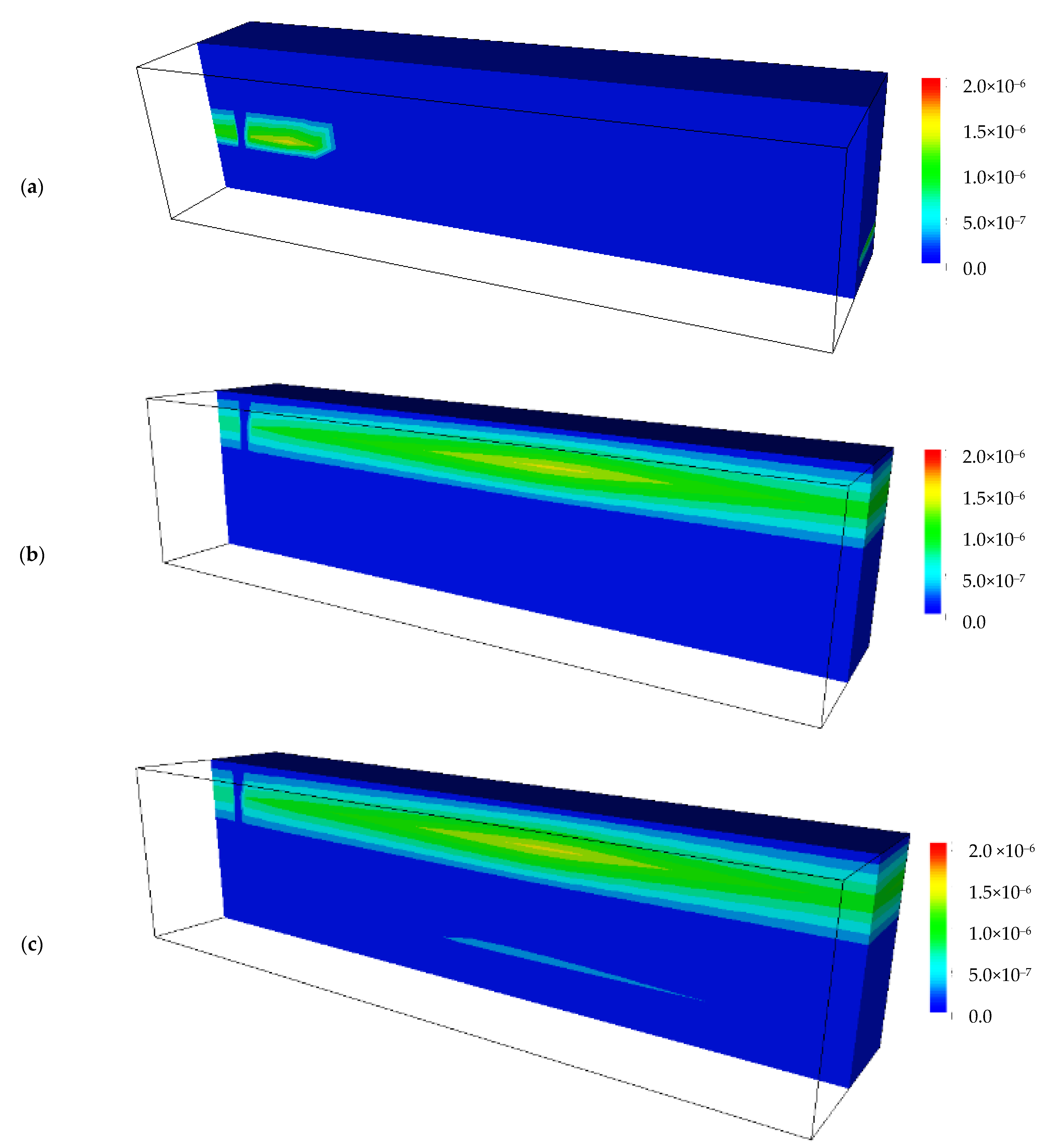
4.5. Calculated Results of the Secondary Minerals before and after Backfilling the Drift
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- JUDGIT! Available online: https://judgit.net/projects/3758 (accessed on 1 May 2021).
- Konno, Y.; Ikeda, H.; Sakata, T. Pollution control of Acid Mine Drainage at Matsuo sulphur-pyrite Mine. J. Min. Inst. Jpn. 1984, 100, 1031–1038. [Google Scholar] [CrossRef] [Green Version]
- Kosaka, K.; Noyori, K. About mine drainage treatment at Taishu mine. J. Min. Metall. Inst. Jpn. 1982, 98, 344–349. [Google Scholar]
- Razowska, L. Changes of groundwater chemistry caused by the flooding of iron mines (Czestochowa Region, Southern Poland). J. Hydrol. 2001, 244, 17–32. [Google Scholar] [CrossRef]
- Tomiyama, S.; Ueda, A.; Ii, H.; Nakamura, Y.; Koizumi, Y.; Saito, K. Sources and flow system of groundwater in the Hosokura mine, Miyagi prefecture, using geochemical method and numerical simulation. J. MMIJ 2010, 126, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Tomiyama, S.; Igarashi, T.; Ii, H.; Takano, H. Sources and flow system of groundwater in the Shimokawa mine, north Hokkaido, using geochemical method and numerical simulation. J. MMIJ 2016, 132, 80–88. [Google Scholar] [CrossRef] [Green Version]
- Tomiyama, S.; Igarashi, T.; Tabelin, C.B.; Tangviroon, P.; Ii, H. Acid mine drainage sources and hydrogeochemistry at the Yatani mine, Yamagata, Japan: A geochemical and isotopic study. J. Contam. Hydrol. 2019, 225, 103502. [Google Scholar] [CrossRef] [PubMed]
- Tomiyama, S.; Igarashi, T.; Tabelin, C.B.; Tangviroon, P.; Ii, H. Modeling of the groundwater flow system in excavated areas of an abandoned mine. J. Contam. Hydrol. 2020, 230, 103617. [Google Scholar] [CrossRef]
- Tomiyama, S.; Ii, H.; Koizumi, Y.; Metsugi, H. Modeling of groundwater recharge and discharge in Tomitaka mine, Miyazaki Prefecture. J. Groundw. Hydol. 2010, 52, 261–274. [Google Scholar] [CrossRef] [Green Version]
- Tokoro, C.; Fukaki, K.; Kadokura, M.; Fuchida, S. Forecast of AMD quantity by a series tank model in three stages: Case studies in two closed Japanese mines. Minerals 2020, 10, 430. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Tomiyama, S.; Igarashi, T.; Yamagata, S.; Ebato, M.; Sakoda, M. Effects of Backfilling Excavated Underground Space on Reducing Acid Mine Drainage in an Abandoned Mine. Minerals 2020, 10, 777. [Google Scholar] [CrossRef]
- Shen, B.; Poulsen, B.; Luo, X.; Qin, J.; Thiruvenkatachari, R.; Duan, Y. Remediation and monitoring of abandoned mines. Int. J. Min. Sci. Technol. 2017, 27, 803–811. [Google Scholar] [CrossRef]
- Sheshpari, M. A Review of Underground Mine Backfilling Methods with Emphasis on Cemented Paste Backfill. EJGE 2015, 20, 5183–5208. [Google Scholar]
- Arribas, A.; Mizuta, T. Potential for Porphyry Copper Deposits in Northern Tohoku (or the Exploration Potential for Base and Precious Metal Deposits in Japan 2020). Resour. Geol. 2018, 68, 144–163. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, H.; Matsunaga, E. Observations on the Tanosawa veins at the Osarizawa mine. Min. Geol. 1964, 14, 126–133. [Google Scholar]
- Saito, S. The Changes of a Community with the Decline of its Mining Industry—A Case of the Osarizawa Mining Industry Ltd. Ann. Tohoku Geogr. Assoc. 1979, 31, 1–7. [Google Scholar]
- Machinda, K.; Sugawara, K.; Nakamura, K. Effect of Various Metals on Human Body in Mine and the Districts (1) Regional variation of metals in stream water and drinking water. Jpn. J. Health Hum. Ecol. 1983, 49, 16–26. [Google Scholar] [CrossRef]
- Yokoyama, K.; Takeuchi, S.; Nakai, I.; Tsutsumi, Y.; Sano, T.; Shigeoka, M.; Miyawaki, R.; Matsubara, S. Chemical compositions of electrum grains in ore and placer deposits in the Japanese islands. Natl. Mus. Nat. Sci. Monogr. 2011, 42, 1–80. [Google Scholar]
- Parkhurst, D.L.; Kipp, K.L.; Charlton, S.R. PHAST Version 2—A Program for Simulating Groundwater Flow, Solute Transport, and Multicomponent Geochemical Reactions; U.S. Geological Survey: Denver, CO, USA, 2010; 235p.
- Parkhurst, D.L.; Appelo, C.A.J. User’s Guide to PHREEQC (Version 2)—A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations; Water-Resources Investigations Report 99-4259; U.S. Geological Survey: Denver, CO, USA, 1999; 312p.
- Kipp, K.L. Guide to the Revised Heat and Solute Transport Simulator HST3D—Version 2; Water-Resources Investigations Report 97-4157; U.S. Geological Survey: Denver, CO, USA, 1997; 149p.
- Kojima, K.; Nakao, K. Basics and Practices of Geological Technology; Kajima Institute Publishing Co., Ltd.: Tokyo, Japan, 1995; 391p. [Google Scholar]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry, 3rd ed.; Wiley: New York, NY, USA, 1995; pp. 690–691. [Google Scholar]
- Sugiyama, T.; Shimizu, S.; Ritthichauy, W.; Tsuji, Y. Determination of Pore Structure Characteristic of Mortar Using a Steady State Migration Test. Proc. JSCE 2004, 2004, 227–238. [Google Scholar] [CrossRef] [Green Version]
- Ito, K.; Fukushi, K.; Hashimoto, K.; Tanaka, C.; Ikeda, H.; Sato, T.; Yoneda, T. A Novel Remediation Method for Arsenic Bearing Acid Mine Drainage Learnt from Natural Attenuation Process. J. MMIJ 2008, 124, 519–528. [Google Scholar] [CrossRef]
- U.S. Geological Survey. A Compilation of Rate Parameters of Water-Mineral Interaction Kinetics for Application to Geological Modeling; U.S. Geological Survey: Denver, CO, USA, 2004; 64p.
- Lasaga, A.C. Chemical Kinetics of Water-Rock Interactions. J. Geophys. Res. 1984, 89, 4009–4025. [Google Scholar] [CrossRef]
- Williamson, M.A.; Rimstidt, J.D. The kinetics and electrochemical rate-determining step of aqueous pyrite oxidation. Geochim. Cosmochim. Acta 1994, 58, 5443–5454. [Google Scholar] [CrossRef]
- Brigatti, M.F.; Galán, E.; Theng, B.K.G. Chapter 2—Structure and Mineralogy of Clay Minerals. In Developments in Clay Science; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, pp. 21–81. [Google Scholar]
- Chandra, A.P.; Gerson, A.R. The mechanisms of pyrite oxidation and leaching: A fundamental perspective. Surf. Sci. Rep. 2010, 65, 293–315. [Google Scholar] [CrossRef]
- Herrera, P.S.; Uchiyama, H.; Igarashi, T.; Asakura, K.; Ochi, Y.; Ishizuka, F.; Kawada, S. Acid mine drainage treatment through a two-step neutralization ferrite-formation process in northern Japan: Physical and chemical characterization of the sludge. Miner. Eng. 2007, 20, 1309–1314. [Google Scholar] [CrossRef]
- Igarashi, T.; Herrera, S.P.; Uchiyama, H.; Miyamae, H.; Iyatomi, N.; Hashimoto, K.; Tabelin, C.B. The two-step neutralization ferrite-formation process for sustainable acid mine drainage treatment: Removal of copper, zinc and arsenic, and the influence of coexisting ions on ferritization. Sci. Total Environ. 2020, 715, 136877. [Google Scholar] [CrossRef] [PubMed]
- González, R.M.; Cánovas, C.R.; Olías, M.; Macías, F. Seasonal variability of extremely metal rich acid mine drainages from the Tharsis mines (SW Spain). Environ. Pollut. 2020, 259, 113829. [Google Scholar] [CrossRef]

| Case | Target | Content |
|---|---|---|
| 1 | Reproducing current quality of AMD | Before backfilling |
| 2-1 | Estimating improved quality of AMD | After backfilling |
| 2-2 | Same as above | After backfilling with calcite |
| Boundary | Groundwater Flow Calculation | Geochemical Calculations |
|---|---|---|
| Upper surface | Total head fixed | Fixed composition of river water (R-1 in Table 6) in equilibrium with atmospheric CO2 |
| The exit of the mine where AMD is released (5 m × 5 m) | Fixed flow rate (before backfilling: 0.091 m3/min; after backfilling: 0.059 m3/min) [11] | same as above |
| Side and bottom surfaces | Impermeable | Zero concentration gradient |
| Sample No | Locality | pH 1 | EC (mS/m) 1 | ORP (mV) 1 | Temp (°C) 1 | Chemical Composition (mg/L) 1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Na+ | K+ | Ca2+ | Mg2+ | Cl− | HCO3− | SO42− | NO3− | T-Fe | Pb | ||||||
| R-1 | River | 4.2 ± 0.1 | 9.0 ± 0.7 | 388 ± 36 | 13.8 ± 5.2 | 4.5 ± 0.3 | 0.5 ± 0.0 | 1.5 ± 0.2 | 1.3 ± 0.0 | 6.9 ± 0.2 | <5 | 11 ± 0.7 | 1.4 ± 0.1 | 0.15 ± 0.11 | 0.02 ± 0.01 |
| R-2 | River | 4.9 ± 0.2 | 6.5 ± 0.4 | 329 ± 31 | 13.1 ± 4.3 | 4.6 ± 0.1 | 0.3 ± 0.1 | 1.6 ± 0.2 | 1.6 ± 0.1 | 7.4 ± 0.1 | <5 | 11 ± 0.7 | 0.2 ± 0.0 | 0.05 ± 0.04 | <0.01 |
| B-1 | Deep depth well (GL– 46.0~51.5 m) | 3.5 | 33 | 530 | 10.3 | 4.8 | 0.9 | 9.4 | 9.0 | 7.5 | <5 | 100 | 0.1 | 4.6 | 0.08 |
| B-2 | Shallow depth well (GL– 2.0~15.2 m) | 3.7 | 37 | 504 | 9.7 | 5.0 | 0.8 | 8.9 | 8.4 | 7.7 | <5 | 99 | <0.1 | 4.8 | 0.34 |
| M-1 | Mining level (−2 L) | 3.3 | 46 | 523 | 9.8 | 5.0 | 0.9 | 7.9 | 11 | 7.8 | <5 | 220 | 0.7 | 300 | 0.58 |
| M-2 | Mining level (−5 L) | 3.6 ± 0.1 | 233 ± 8 | 404 ± 26 | 12.6 ± 0.5 | 9.7 ± 0.2 | 8.7 ± 0.5 | 200 ± 28 | 180 ± 14 | 6.9 ± 0.4 | <5 | 1250 ± 71 | <0.1 | 22 ± 15 | 0.38 ± 0.01 |
| M-3 | Mining level (−5 L) | 3.9 ± 0.1 | 375 ± 45 | 301 ± 7 | 15.0 ± 0.3 | 19 | 9.6 | 200 | 250 | 7.3 | <5 | 2900 | <0.1 | 610 ± 24 | 0.55 ± 0.04 |
| M-4 | Mining level (−4 L) | 3.5 ± 0.2 | 190 ± 10 | 407 ± 25 | 12.5 ± 0.3 | 7.8 ± 0.2 | 3.5 ± 0.5 | 49 ± 2 | 160 ± 14 | 10 ± 1 | <5 | 890 ± 28 | <0.1 | 27 ± 6 | 0.22 ± 0.01 |
| Sample No | Depth | Qz 1 | Pl | Kf | Py | Mc | Chl |
|---|---|---|---|---|---|---|---|
| B-1 borehole | GL −9.00~−9.07 m | ◎ 2 | − | + | + | − |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamaguchi, K.; Tomiyama, S.; Igarashi, T.; Yamagata, S.; Ebato, M.; Sakoda, M. Improvement in pH and Total Iron Concentration of Acid Mine Drainage after Backfilling: A Case Study of an Underground Abandoned Mine in Japan. Minerals 2021, 11, 1297. https://doi.org/10.3390/min11111297
Yamaguchi K, Tomiyama S, Igarashi T, Yamagata S, Ebato M, Sakoda M. Improvement in pH and Total Iron Concentration of Acid Mine Drainage after Backfilling: A Case Study of an Underground Abandoned Mine in Japan. Minerals. 2021; 11(11):1297. https://doi.org/10.3390/min11111297
Chicago/Turabian StyleYamaguchi, Kohei, Shingo Tomiyama, Toshifumi Igarashi, Saburo Yamagata, Masanori Ebato, and Masatoshi Sakoda. 2021. "Improvement in pH and Total Iron Concentration of Acid Mine Drainage after Backfilling: A Case Study of an Underground Abandoned Mine in Japan" Minerals 11, no. 11: 1297. https://doi.org/10.3390/min11111297
APA StyleYamaguchi, K., Tomiyama, S., Igarashi, T., Yamagata, S., Ebato, M., & Sakoda, M. (2021). Improvement in pH and Total Iron Concentration of Acid Mine Drainage after Backfilling: A Case Study of an Underground Abandoned Mine in Japan. Minerals, 11(11), 1297. https://doi.org/10.3390/min11111297






