Incorporating Kinetic Modeling in the Development Stages of Hard Rock Mine Projects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Geological Background
2.2. Sample Preparation and Characterization
2.3. Weathering Cell Test
2.4. The Conceptual Model
2.4.1. Abiotic Kinetic Rates
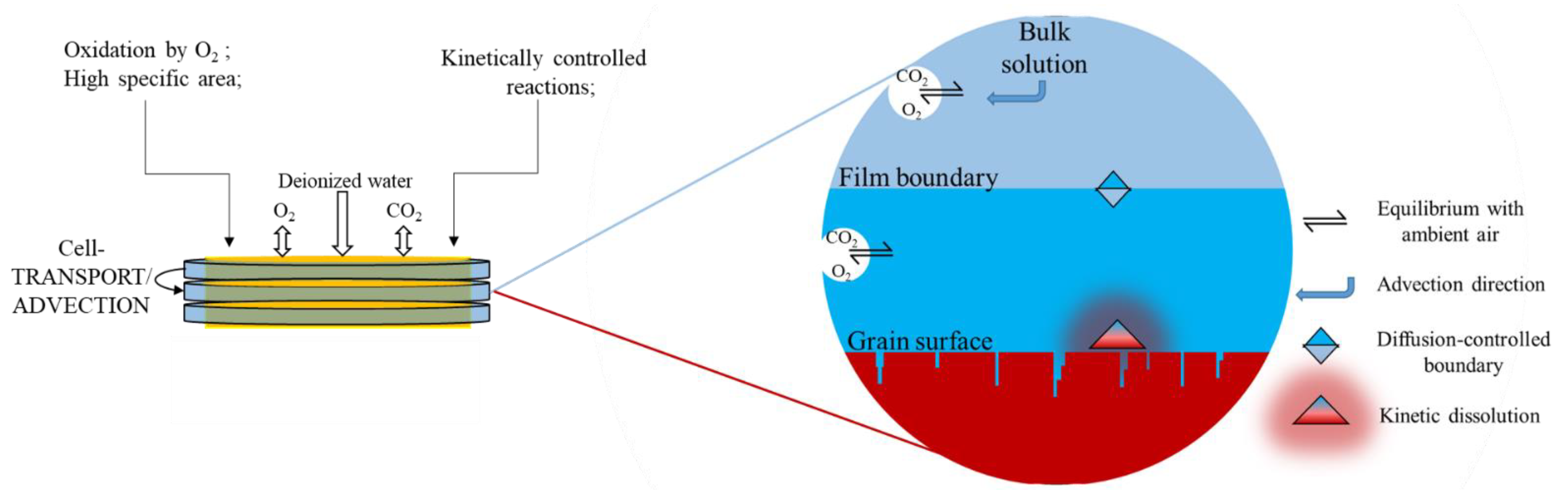
2.4.2. Diffusive Transport from the Film to the Pore Water
2.5. Calibration and Parameter Fitting
3. Results and Discussion
3.1. Experimental Datasets
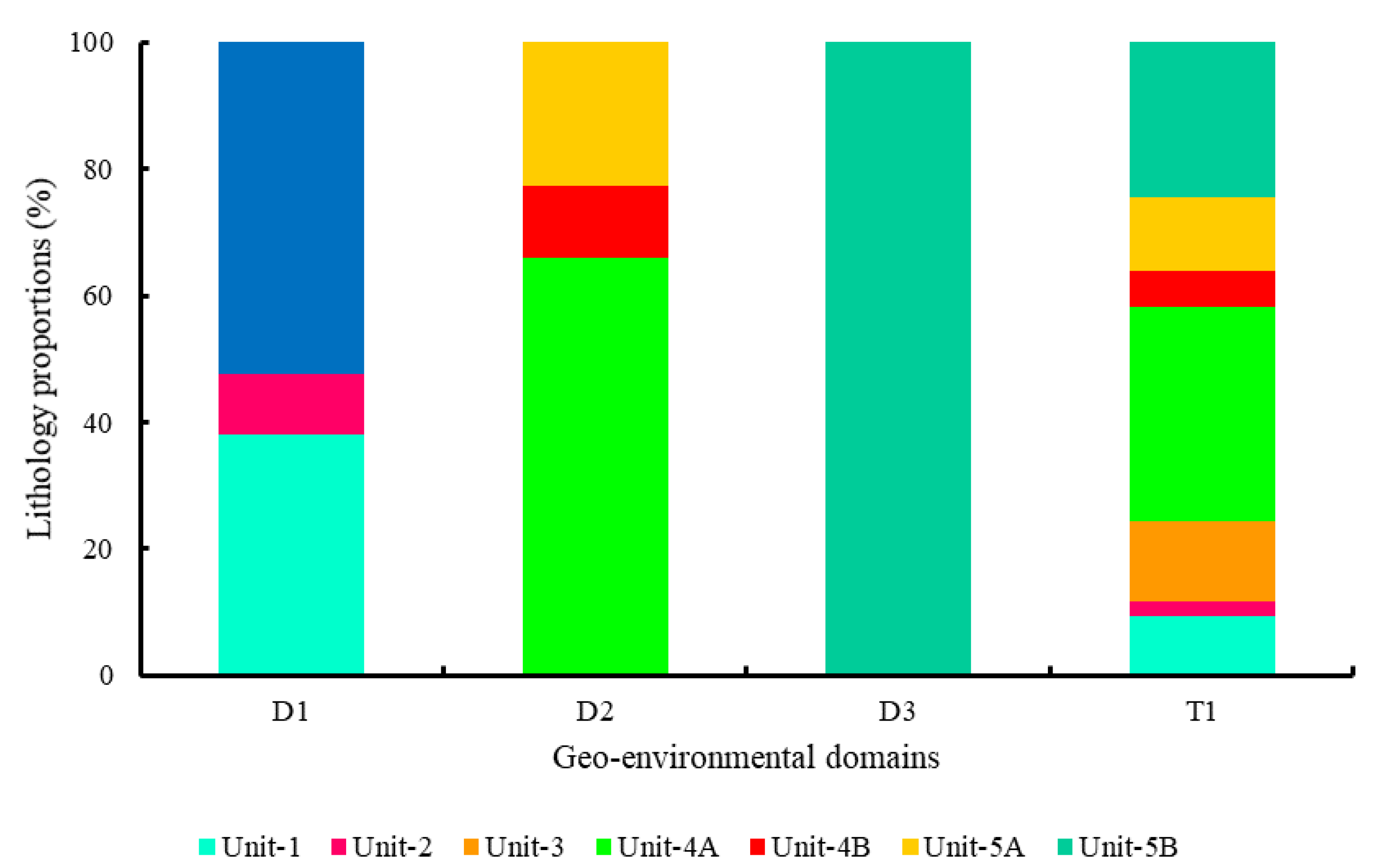
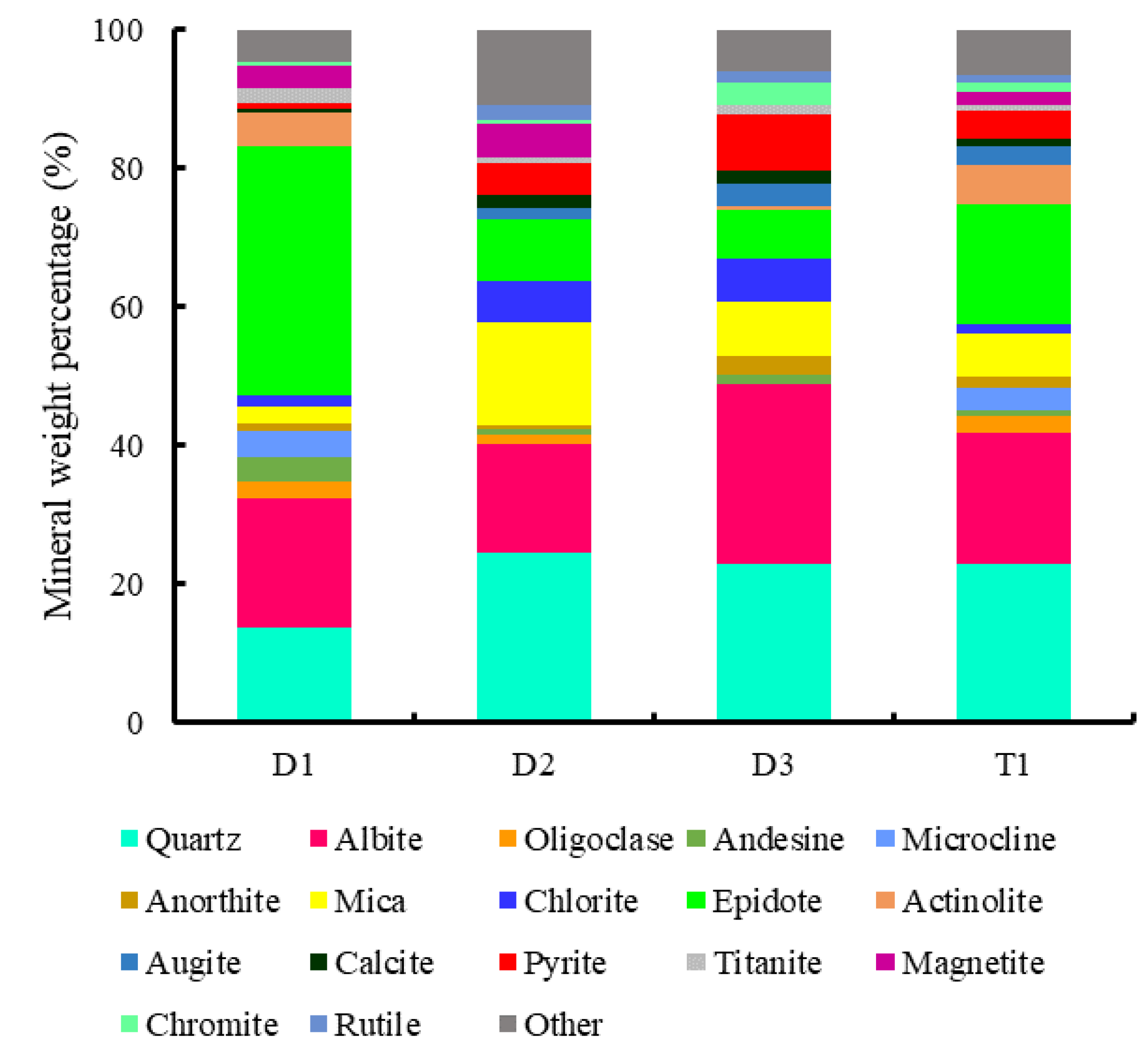
3.1.1. Characterization Results
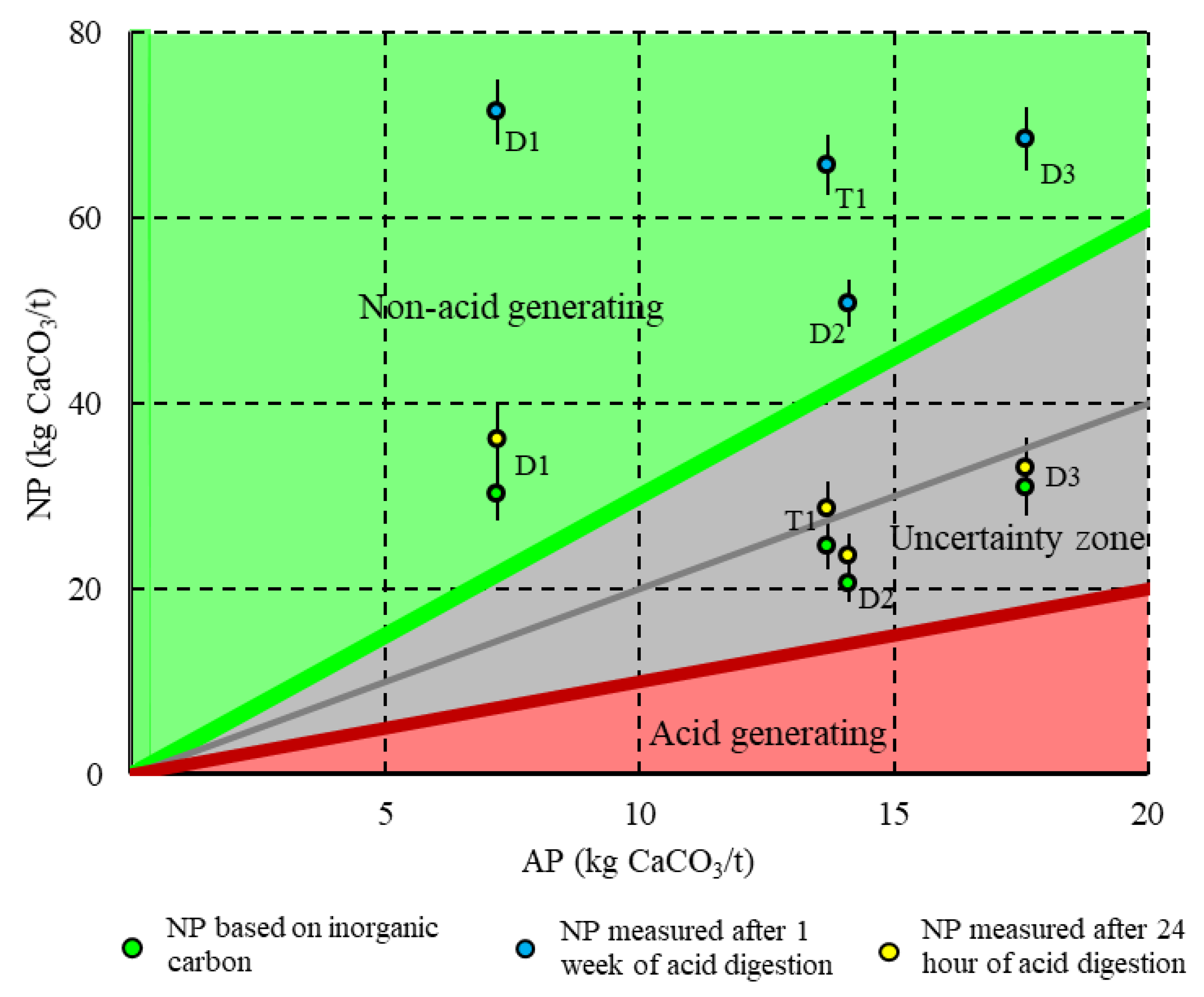
3.1.2. Weathering Cells Results
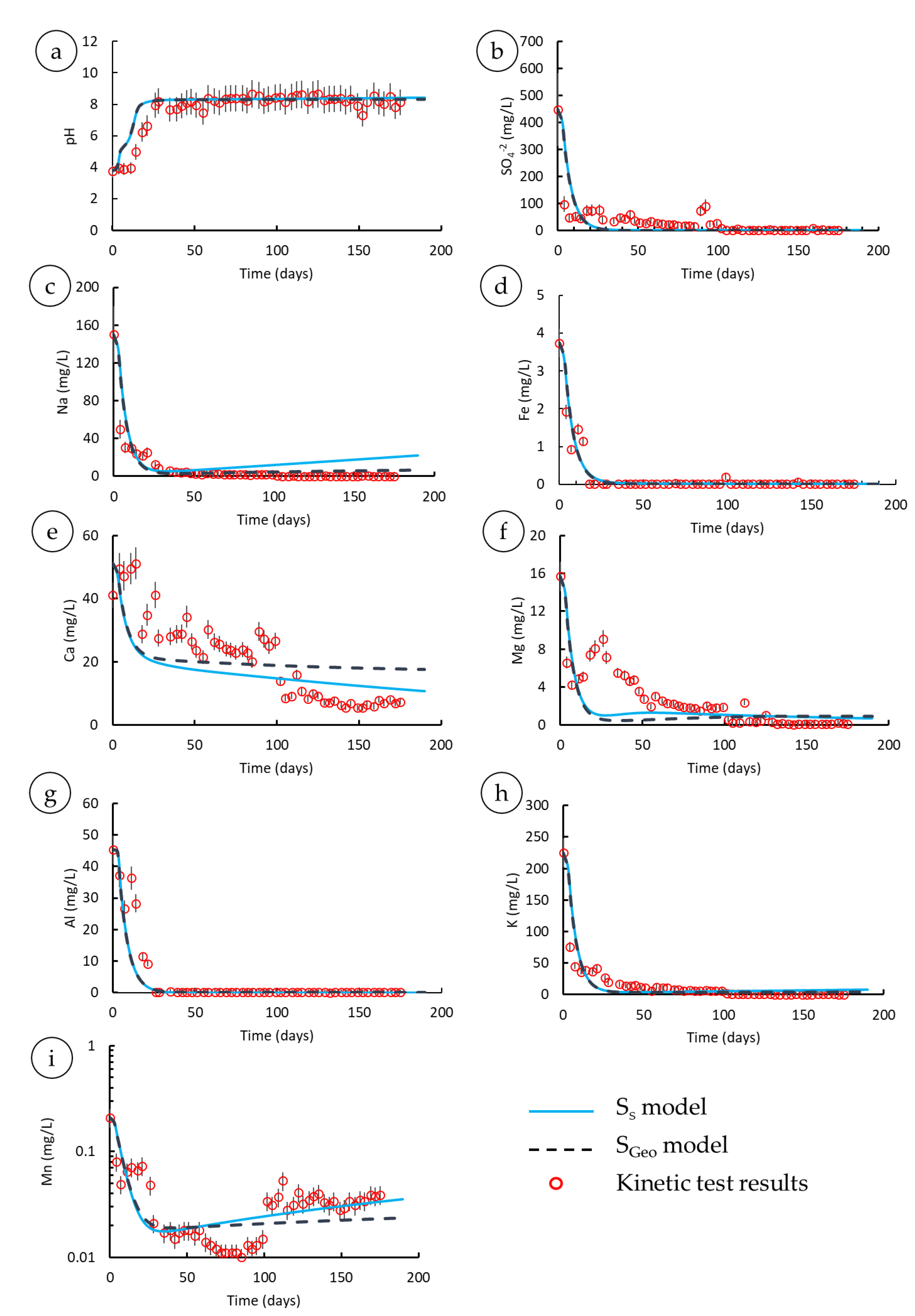
3.2. Modeling Results
3.2.1. Model Calibration
- (i)
- The active surface area of albite was at least fourfold smaller than the BET-based surface area, suggesting relatively low concentrations of Na compared to the model; however, there was no noteworthy deviation of modeled Na from the experimental results at acidic to neutral pH values (before day 50).
- (ii)
- The albite rate used in PHREEQC under subalkaline conditions (after day 50) expedited albite dissolution, thus yielding a high Na release rate compared to the kinetic test outcomes.
- (iii)
- The release rate of Na was substantially slower than the dissolution rate of albite; nevertheless, plagioclase dissolution has been known to entail preferential release of Na-Ca resulting in a Na-Ca poor thin layer at the plagioclase surface [89]. Therefore, the aforementioned factor is precluded, and the first two controlling factors may have occurred jointly to explain the geochemical behavior of Na.
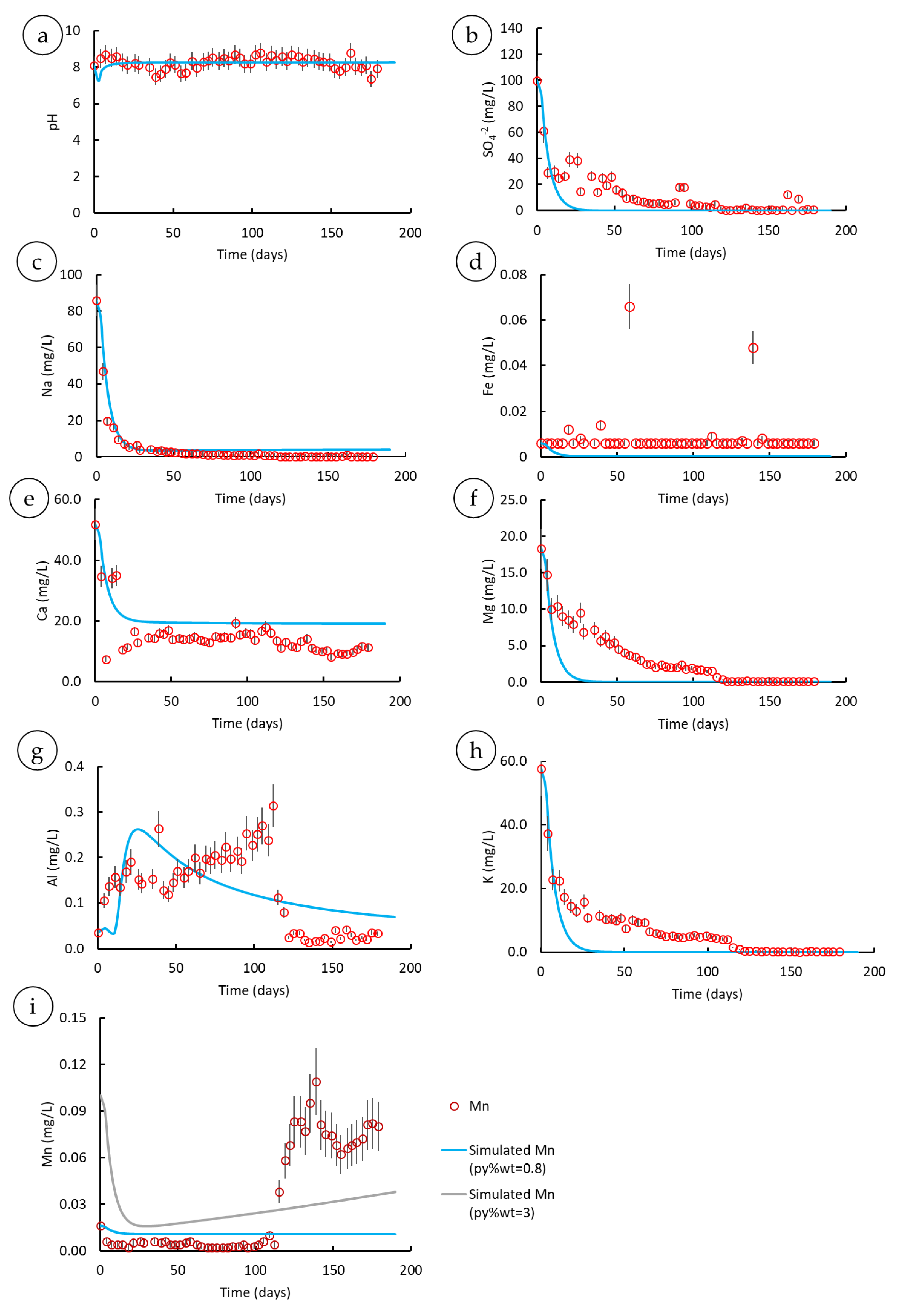
3.2.2. Model Benchmarking
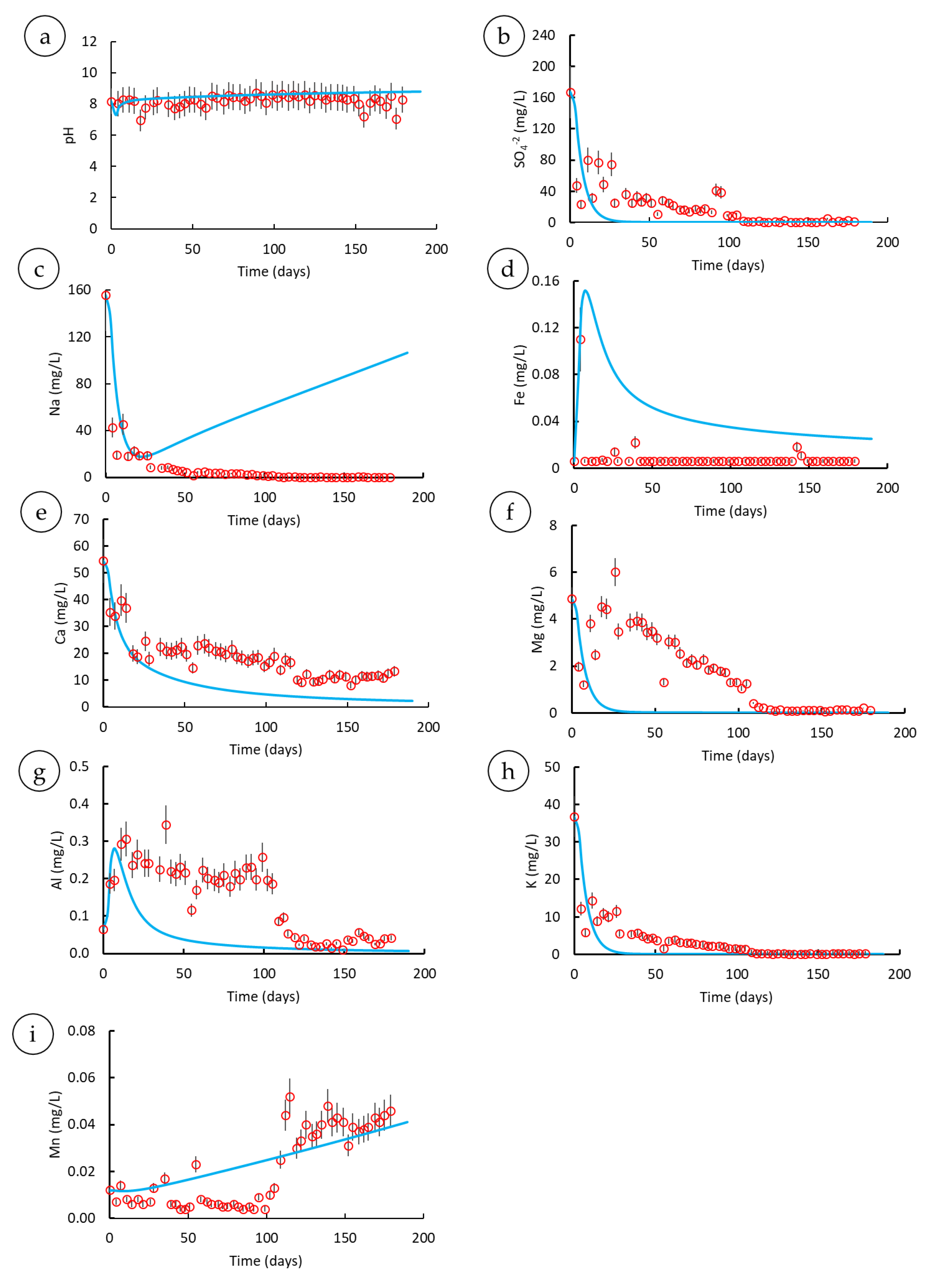
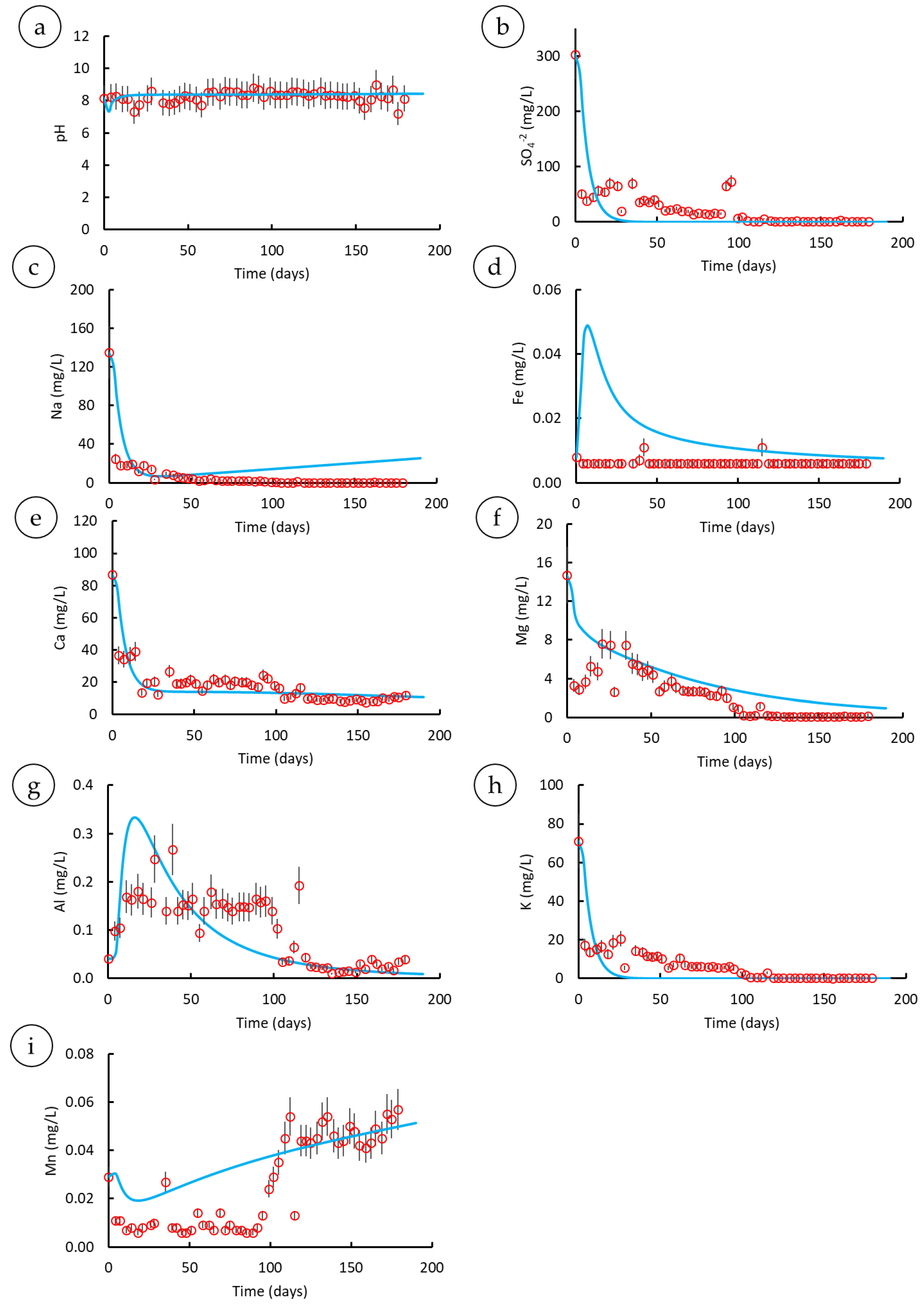
3.3. Parametric Analysis
3.3.1. Mineral Assemblages
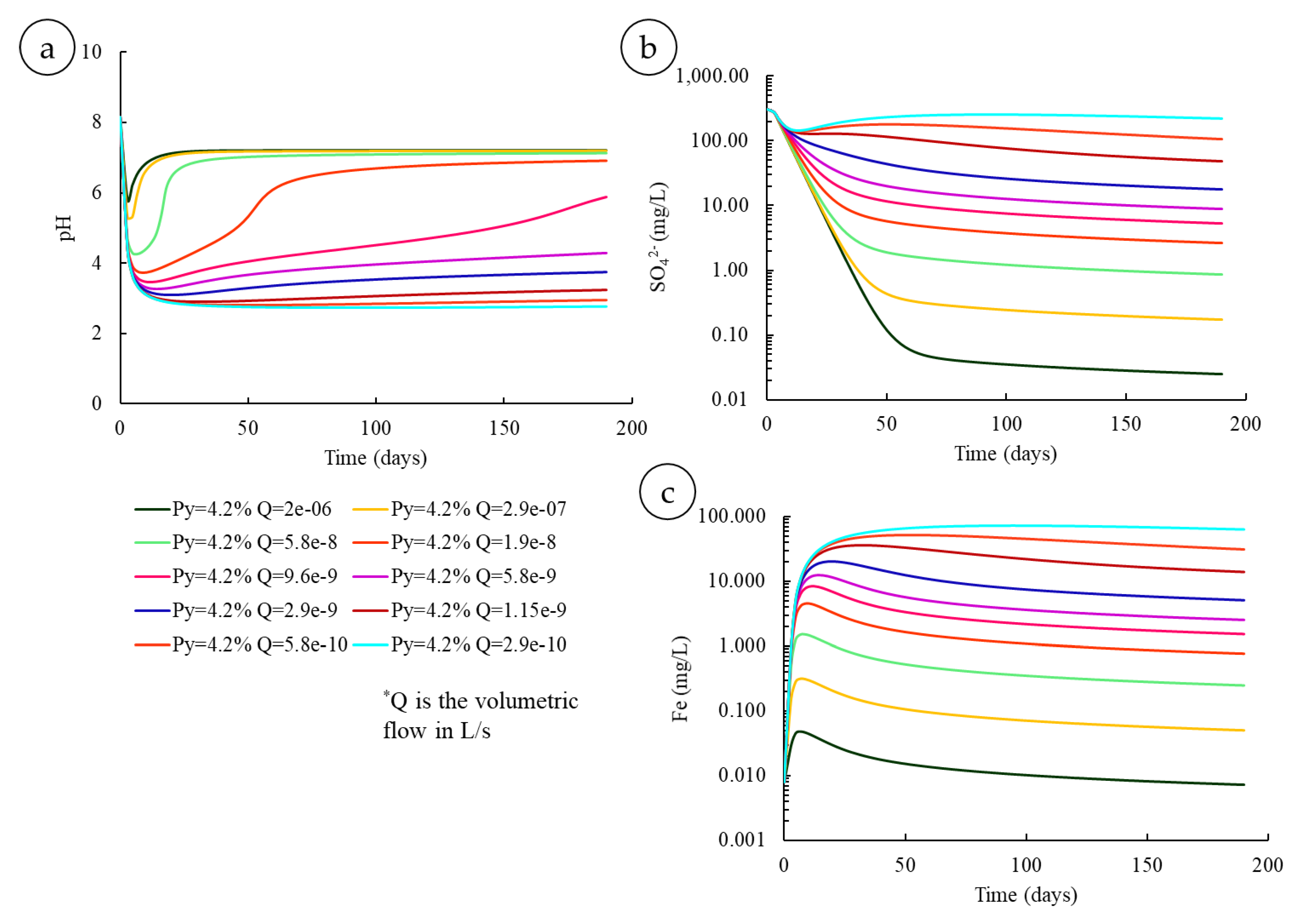
3.3.2. Various Residence Times
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mudd, G. Global trends in gold mining: Towards quantifying environmental and resource sustainability. Resour. Policy 2007, 32, 42–56. [Google Scholar] [CrossRef]
- Yilmaz, E. Advances in reducing large volumes of environmentally harmful mine waste rocks and tailings. Gospod. Surowcami Miner. 2011, 27, 89–112. [Google Scholar]
- Park, I.; Tabelin, C.B.; Jeon, S.; Li, X.; Seno, K.; Ito, M.; Hiroyoshi, N. A review of recent strategies for acid mine drainage prevention and mine tailings recycling. Chemosphere 2019, 219, 588–606. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Park, I.; Phengsaart, T.; Jeon, S.; Villacorte-Tabelin, M.; Alonzo, D.; Yoo, K.; Ito, M.; Hiroyoshi, N. Copper and critical metals production from porphyry ores and E-wastes: A review of resource availability, processing/recycling challenges, socio-environmental aspects, and sustainability issues. Resour. Conserv. Recycl. 2021, 170, 105610. [Google Scholar] [CrossRef]
- Akcil, A.; Koldas, S. Acid Mine Drainage (AMD): Causes, treatment and case studies. J. Clean. Prod. 2006, 14, 1139–1145. [Google Scholar] [CrossRef]
- Barfoud, L.; Pabst, T.; Zagury, G.J.; Plante, B. Effect of Dissolved Oxygen on The Oxidation of Saturated Mine Tailings. In Proceedings of the Geo-Environmental Engineering 2019, Concordia University, Montreal, QC, Canada, 30–31 May 2019. [Google Scholar]
- Blowes, D.; Ptacek, C.; Jambor, J.; Weisener, C. The geochemistry of acid mine drainage. Treatise Geochem. 2003, 9, 612. [Google Scholar]
- Evangelou, V.P.; Zhang, Y.L. A review: Pyrite oxidation mechanisms and acid mine drainage prevention. Crit. Rev. Environ. Sci. Technol. 1995, 25, 141–199. [Google Scholar] [CrossRef]
- Heikkinen, P.M.; Räisänen, M.L.; Johnson, R.H. Geochemical Characterisation of Seepage and Drainage Water Quality from Two Sulphide Mine Tailings Impoundments: Acid Mine Drainage versus Neutral Mine Drainage. Mine Water Environ. 2008, 28, 30–49. [Google Scholar] [CrossRef]
- Kleinmann, R.L.P.; Crerar, D.; Pacelli, R. Biogeochemistry of acid mine drainage and a method to control acid formation. Min. Eng. 1981, 33. Available online: https://www.osti.gov/biblio/6306683-biogeochemistry-acid-mine-drainage-method-control-acid-formation (accessed on 1 November 2021).
- Moses, C.O.; Nordstrom, D.K.; Herman, J.S.; Mills, A.L. Aqueous pyrite oxidation by dissolved oxygen and by ferric iron. Geochim. Et Cosmochim. Acta 1987, 51, 1561–1571. [Google Scholar] [CrossRef]
- Nicholson, R.V.; Gillham, R.W.; Reardon, E.J. Pyrite oxidation in carbonate-buffered solution: 1. Experimental kinetics. Geochim. Et Cosmochim. Acta 1988, 52, 1077–1085. [Google Scholar] [CrossRef]
- Nicholson, R.V.; Scharer, J.M. Laboratory Studies of Pyrrhotite Oxidation Kinetics; American Chemical Society (ACS): Washington, DC, USA, 1993; pp. 14–30. [Google Scholar]
- Nordstrom, D.K.; Alpers, C.N.; Ptacek, C.J.; Blowes, D.W. Negative pH and Extremely Acidic Mine Waters from Iron Mountain, California. Environ. Sci. Technol. 2000, 34, 254–258. [Google Scholar] [CrossRef] [Green Version]
- Nordstrom, D.K. Aqueous Pyrite Oxidation and the Consequent Formation of Secondary Iron Minerals; Wiley: Hoboken, NJ, USA, 2015; pp. 37–56. [Google Scholar]
- Nordstrom, D.K.; Alpers, C. Negative pH, efflorescent mineralogy, and consequences for environmental restoration at the Iron Mountain Superfund site, California. Proc. Natl. Acad. Sci. USA 1999, 96, 3455–3462. [Google Scholar] [CrossRef] [Green Version]
- Plante, B.; Benzaazoua, M.; Bussière, B.; Biesinger, M.; Pratt, A. Study of Ni sorption onto Tio mine waste rock surfaces. Appl. Geochem. 2010, 25, 1830–1844. [Google Scholar] [CrossRef]
- Singer, P.C.; Stumm, W. Acidic Mine Drainage: The Rate-Determining Step. Science 1970, 167, 1121–1123. [Google Scholar] [CrossRef] [PubMed]
- Weisener, C.; Weber, P. Preferential oxidation of pyrite as a function of morphology and relict texture. N. Z. J. Geol. Geophys. 2010, 53, 167–176. [Google Scholar] [CrossRef] [Green Version]
- Wiersma, C.; Rimstidt, J. Rates of reaction of pyrite and marcasite with ferric iron at pH 2. Geochim. Et Cosmochim. Acta 1984, 48, 85–92. [Google Scholar] [CrossRef]
- Ho, G.D.; Tabelin, C.B.; Tangviroon, P.; Tamamura, S.; Igarashi, T. Effects of cement addition on arsenic leaching from soils excavated from projects employing shield-tunneling method. Geoderma 2021, 385, 114896. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Igarashi, T.; Villacorte-Tabelin, M.; Park, I.; Opiso, E.M.; Ito, M.; Hiroyoshi, N. Arsenic, selenium, boron, lead, cadmium, copper, and zinc in naturally contaminated rocks: A review of their sources, modes of enrichment, mechanisms of release, and mitigation strategies. Sci. Total. Environ. 2018, 645, 1522–1553. [Google Scholar] [CrossRef]
- Tamoto, S.; Tabelin, C.B.; Igarashi, T.; Ito, M.; Hiroyoshi, N. Short and long term release mechanisms of arsenic, selenium and boron from a tunnel-excavated sedimentary rock under in situ conditions. J. Contam. Hydrol. 2015, 175, 60–71. [Google Scholar] [CrossRef]
- Aubertin, M.; Aachib, M.; Monzon, M.; Joanes, A.; Bussière, B.; Chapuis, R. Étude de laboratoire sur l’efficacité des barrières de recouvrement construites à partir de résidus miniers. Mine Environ. Neutral Drain. Rep. 1997, 2, 107. [Google Scholar]
- Benzaazoua, M.; Dagenais, A.-M.; Archambault, M. Kinetic tests comparison and interpretation for prediction of the Joutel tailings acid generation potential. Environ. Earth Sci. 2004, 46, 1086–1101. [Google Scholar] [CrossRef]
- Benzaazoua, M.; Bussière, B.; Demers, I.; Aubertin, M.; Fried, É.; Blier, A. Integrated mine tailings management by combining environmental desulphurization and cemented paste backfill: Application to mine Doyon, Quebec, Canada. Miner. Eng. 2008, 21, 330–340. [Google Scholar] [CrossRef]
- Benzaazoua, M.; Bussière, B.; Kongolo, M.; McLaughlin, J.; Marion, P. Environmental desulphurization of four Canadian mine tailings using froth flotation. Int. J. Miner. Process. 2000, 60, 57–74. [Google Scholar] [CrossRef]
- Bouzahzah, H.; Benzaazoua, M.; Bussiere, B.; Plante, B. Prediction of Acid Mine Drainage: Importance of Mineralogy and the Test Protocols for Static and Kinetic Tests. Mine Water Environ. 2013, 33, 54–65. [Google Scholar] [CrossRef]
- Bussière, B. Hydrogeotechnical properties of hard rock tailings from metal mines and emerging geoenvironmental disposal approaches. Can. Geotech. J. 2007, 44, 1019–1052. [Google Scholar] [CrossRef]
- Bussière, B.; Aubertin, M.; Mbonimpa, M.; Molson, J.; Chapuis, R.P. Field experimental cells to evaluate the hydrogeological behaviour of oxygen barriers made of silty materials. Can. Geotech. J. 2007, 44, 245–265. [Google Scholar] [CrossRef]
- Demers, I.; Bouda, M.; Mbonimpa, M.; Benzaazoua, M.; Bois, D.; Gagnon, M. Valorization of acid mine drainage treatment sludge as remediation component to control acid generation from mine wastes, part 2: Field experimentation. Miner. Eng. 2015, 76, 117–125. [Google Scholar] [CrossRef]
- Demers, I.; Bussière, B.; Benzaazoua, M.; Mbonimpa, M.; Blier, A. Column test investigation on the performance of monolayer covers made of desulphurized tailings to prevent acid mine drainage. Miner. Eng. 2008, 21, 317–329. [Google Scholar] [CrossRef]
- Demers, I.; Bussière, B.; Mbonimpa, M.; Benzaazoua, M. Oxygen diffusion and consumption in low-sulphide tailings covers. Can. Geotech. J. 2009, 46, 454–469. [Google Scholar] [CrossRef]
- Demers, I.; Mbonimpa, M.; Benzaazoua, M.; Bouda, M.; Awoh, S.; Lortie, S.; Gagnon, M. Use of acid mine drainage treatment sludge by combination with a natural soil as an oxygen barrier cover for mine waste reclamation: Laboratory column tests and intermediate scale field tests. Miner. Eng. 2017, 107, 43–52. [Google Scholar] [CrossRef]
- Elghali, A.; Benzaazoua, M.; Bouzahzah, H.; Bussière, B.; Villarraga-Gómez, H. Determination of the available acid-generating potential of waste rock, part I: Mineralogical approach. Appl. Geochem. 2018, 99, 31–41. [Google Scholar] [CrossRef]
- Elghali, A.; Benzaazoua, M.; Bussière, B.; Bouzahzah, H. Determination of the available acid-generating potential of waste rock, part II: Waste management involvement. Appl. Geochem. 2019, 100, 316–325. [Google Scholar] [CrossRef]
- Jouini, M.; Neculita, C.M.; Genty, T.; Benzaazoua, M. Environmental behavior of metal-rich residues from the passive treatment of acid mine drainage. Sci. Total Environ. 2020, 712, 136541. [Google Scholar] [CrossRef]
- Jouini, M.; Rakotonimaro, T.V.; Neculita, C.M.; Genty, T.; Benzaazoua, M. Prediction of the environmental behavior of residues from the passive treatment of acid mine drainage. Appl. Geochem. 2019, 110, 104421. [Google Scholar] [CrossRef]
- Michaud, M.L.; Plante, B.; Bussière, B.; Benzaazoua, M.; Leroux, J. Development of a modified kinetic test using EDTA and citric acid for the prediction of contaminated neutral drainage. J. Geochem. Explor. 2017, 181, 58–68. [Google Scholar] [CrossRef]
- Ouangrawa, M.; Aubertin, M.; Molson, J.W.; Bussière, B.; Zagury, G.J. Preventing Acid Mine Drainage with an Elevated Water Table: Long-Term Column Experiments and Parameter Analysis. Water Air Soil Pollut. 2010, 213, 437–458. [Google Scholar] [CrossRef]
- Parbhakar-Fox, A.K.; Edraki, M.; Walters, S.; Bradshaw, D. Development of a textural index for the prediction of acid rock drainage. Miner. Eng. 2011, 24, 1277–1287. [Google Scholar] [CrossRef]
- Plante, B.; Bussière, B.; Benzaazoua, M. Lab to field scale effects on contaminated neutral drainage prediction from the Tio mine waste rocks. J. Geochem. Explor. 2014, 137, 37–47. [Google Scholar] [CrossRef]
- Nordstrom, D.K.; Blowes, D.W.; Ptacek, C.J. Hydrogeochemistry and microbiology of mine drainage: An update. Appl. Geochem. 2015, 57, 3–16. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Veerawattananun, S.; Ito, M.; Hiroyoshi, N.; Igarashi, T. Pyrite oxidation in the presence of hematite and alumina: I. Batch leaching experiments and kinetic modeling calculations. Sci. Total Environ. 2017, 580, 687–698. [Google Scholar] [CrossRef]
- Tabelin, C.; Veerawattananun, S.; Ito, M.; Hiroyoshi, N.; Igarashi, T. Pyrite oxidation in the presence of hematite and alumina: II. Effects on the cathodic and anodic half-cell reactions. Sci. Total Environ. 2017, 581, 126–135. [Google Scholar] [CrossRef]
- Demers, I.; Molson, J.; Bussière, B.; Laflamme, D. Numerical modeling of contaminated neutral drainage from a waste-rock field test cell. Appl. Geochem. 2013, 33, 346–356. [Google Scholar] [CrossRef]
- Fala, O.; Aubertin, M.; Molson, J.; Bussière, B.; Wilson, G.; Chapuis, R.; Martin, V. Numerical modelling of unsaturated flow in uniform and heterogeneous waste rock piles. In Proceedings of the Sixth International Conference on Acid Rock Drainage (ICARD), Cairns, Australia, 12–18 July 2003; Publication Series. Australasian Institute of Mining and Metallurgy: Carlton, Australia; pp. 895–902. [Google Scholar]
- Fala, O.; Molson, J.; Aubertin, M.; Dawood, I.; Bussière, B.; Chapuis, R. A numerical modelling approach to assess long-term unsaturated flow and geochemical transport in a waste rock pile. Int. J. Min. Reclam. Environ. 2012, 27, 38–55. [Google Scholar] [CrossRef]
- Kalonji-Kabambi, A.; Demers, I.; Bussière, B. Reactive transport modeling of the geochemical behavior of highly reactive tailings in different environmental conditions. Appl. Geochem. 2020, 122, 104761. [Google Scholar] [CrossRef]
- Molson, J.; Aubertin, M.; Bussière, B.; Benzaazoua, M. Geochemical transport modelling of drainage from experimental mine tailings cells covered by capillary barriers. Appl. Geochem. 2008, 23, 1–24. [Google Scholar] [CrossRef]
- Molson, J.; Fala, O.; Aubertin, M.; Bussière, B. Numerical simulations of pyrite oxidation and acid mine drainage in unsaturated waste rock piles. J. Contam. Hydrol. 2005, 78, 343–371. [Google Scholar] [CrossRef]
- Nicholson, R.V.; Rinker, M.J.; Acott, G.; Venhuis, M.A. Integration of field data and a geochemical transport model to assess mitigation strategies for an acid-generating mine rock pile at a uranium mine. In Proceedings of the Mining and the Environment Conference, Sudbury, ON, Canada, 25 May 2003. [Google Scholar]
- Pabst, T.; Bussière, B.; Aubertin, M.; Molson, J. Comparative performance of cover systems to prevent acid mine drainage from pre-oxidized tailings: A numerical hydro-geochemical assessment. J. Contam. Hydrol. 2018, 214, 39–53. [Google Scholar] [CrossRef]
- Pabst, T.; Molson, J.; Aubertin, M.; Bussière, B. Reactive transport modelling of the hydro-geochemical behaviour of partially oxidized acid-generating mine tailings with a monolayer cover. Appl. Geochem. 2017, 78, 219–233. [Google Scholar] [CrossRef]
- Wunderly, M.D.; Frind, E.O.; Blowes, D.W.; Ptacek, C.J. Sulfide mineral oxidation and subsequent reactive transport of oxidation products in mine tailings impoundments: A numerical model. Water Resour. Res. 1996, 32, 3173–3187. [Google Scholar] [CrossRef]
- Romano, C.G.; Mayer, K.U.; Jones, D.R.; Ellerbroek, D.A.; Blowes, D.W. Effectiveness of various cover scenarios on the rate of sulfide oxidation of mine tailings. J. Hydrol. 2003, 271, 171–187. [Google Scholar] [CrossRef]
- Graupner, B.J.; Koch, C.; Prommer, H. Prediction of diffuse sulfate emissions from a former mining district and associated groundwater discharges to surface waters. J. Hydrol. 2014, 513, 169–178. [Google Scholar] [CrossRef]
- Abbasi, F.; Feyen, J.; van Genuchten, M. Two-dimensional simulation of water flow and solute transport below furrows: Model calibration and validation. J. Hydrol. 2004, 290, 63–79. [Google Scholar] [CrossRef]
- Fala, O.; Molson, J.; Aubertin, M.; Bussière, B.; Chapuis, R.P. Numerical Simulations of Long Term Unsaturated Flow and ACID Mine Drainage at Waste Rock Piles. J. Am. Soc. Min. Reclam. 2006, 2006, 582–597. [Google Scholar] [CrossRef]
- Kandelous, M.M.; Šimůnek, J. Numerical simulations of water movement in a subsurface drip irrigation system under field and laboratory conditions using HYDRUS-2D. Agric. Water Manag. 2010, 97, 1070–1076. [Google Scholar] [CrossRef]
- Šimůnek, J.; van Genuchten, M.T.; Šejna, M. Development and Applications of the HYDRUS and STANMOD Software Packages and Related Codes. Vadose Zone J. 2008, 7, 587–600. [Google Scholar] [CrossRef] [Green Version]
- Šimůnek, J.; Van Genuchten, M.T.; Šejna, M. The HYDRUS software package for simulating the two-and three-dimensional movement of water, heat, and multiple solutes in variably-saturated porous media. Tech. Man. Version 2012, 2, 258. [Google Scholar]
- Šimůnek, J.; Van Genuchten, M.T.; Šejna, M. Recent developments and applications of the HYDRUS computer software packages. Vadose Zone J. 2016, 15, vzj2016-04. [Google Scholar] [CrossRef] [Green Version]
- Mayer, K.U.; Frind, E.O.; Blowes, D.W. Multicomponent reactive transport modeling in variably saturated porous media using a generalized formulation for kinetically controlled reactions. Water Resour. Res. 2002, 38, 13-1–13-21. [Google Scholar] [CrossRef]
- Brookfield, A.; Blowes, D.; Mayer, K.U. Integration of field measurements and reactive transport modelling to evaluate contaminant transport at a sulfide mine tailings impoundment. J. Contam. Hydrol. 2006, 88, 1–22. [Google Scholar] [CrossRef]
- Jurjovec, J.; Blowes, D.W.; Ptacek, C.J.; Mayer, K.U. Multicomponent reactive transport modeling of acid neutralization reactions in mine tailings. Water Resour. Res. 2004, 40, 40. [Google Scholar] [CrossRef] [Green Version]
- Mayer, K.; Amos, R.; Molins, S.; Gerard, F. Reactive Transport Modeling in Variably Saturated Media with MIN3P: Basic Model Formulation and Model Enhancements; Bentham Science Publishers: Sharjah, United Arab Emirates, 2012; Volume 26. [Google Scholar]
- Ouangrawa, M.; Molson, J.; Aubertin, M.; Bussière, B.; Zagury, G. Reactive transport modelling of mine tailings columns with capillarity-induced high water saturation for preventing sulfide oxidation. Appl. Geochem. 2009, 24, 1312–1323. [Google Scholar] [CrossRef]
- Amar, H.; Benzaazoua, M.; Elghali, A.; Bussière, B.; Duclos, M. Upstream environmental desulphurisation and valorisation of waste rocks as a sustainable AMD management approach. J. Geochem. Explor. 2020, 215, 106555. [Google Scholar] [CrossRef]
- Bouzahzah, H. Modification et Amélioration des Tests Statiques et Cinétiques Pour Une Prédiction Fiable du Drainage Minier Acide; Université du Québec en Abitibi-Témiscamingue: Quebec, QC, Canada, 2013. [Google Scholar]
- Cruz, R.; Bertrand, V.; Monroy, M.; González, I. Effect of sulfide impurities on the reactivity of pyrite and pyritic concentrates: A multi-tool approach. Appl. Geochem. 2001, 16, 803–819. [Google Scholar] [CrossRef]
- Toubri, Y.; Demers, I.; Poirier, A.; Pépin, G.; Gosselin, M.-C.; Beier, N.A. Merging 3D geological modeling and stochastic simulation to foster waste rock upstream management. J. Geochem. Explor. 2021, 224, 106739. [Google Scholar] [CrossRef]
- Villeneuve, M.; Bussière, B.; Benzaazoua, M.; Aubertin, M. Assessment of interpretation methods for kinetic tests performed on tailings having a low acid generating potential. In Proceedings of the Securing the Future and 8th ICARD, Skelleftea, Sweden, 23–26 June 2009. [Google Scholar]
- Parkhurst, D.L.; Appelo, C. User’s guide to PHREEQC (Version 2): A computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. Water-Resour. Investig. Rep. 1999, 99, 312. [Google Scholar]
- Labus, K.; Grmela, A. A model of water chemistry forming in effect of pyrite oxidation in a coal mining waste pile. PODZEMNÁ VODA 2006, 1, 50–55. [Google Scholar]
- Embile, R.F., Jr.; Walder, I.F.; Mahoney, J.J. Multicomponent reactive transport modeling of effluent chemistry using locally obtained mineral dissolution rates of forsterite and pyrrhotite from a mine tailings deposit. Adv. Water Resour. 2019, 128, 87–96. [Google Scholar] [CrossRef]
- Vermette, D. Approche de Caractérisation Géoenvironnementale Axée Sur L’utilisation des Concepts Géométallurgiques; Université du Québec en Abitibi-Témiscamingue: Quebec, QC, Canada, 2018. [Google Scholar]
- Amar, H.; Benzaazoua, M.; Edahbi, M.; Villeneuve, M.; Joly, M.-A.; Elghali, A. Reprocessing feasibility of polymetallic waste rock for cleaner and sustainable mining. J. Geochem. Explor. 2021, 220, 106683. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Chapuis, R.P.; Aubertin, M. On the use of the Kozeny–Carman equation to predict the hydraulic conductivity of soils. Can. Geotech. J. 2003, 40, 616–628. [Google Scholar] [CrossRef]
- Lawrence, R.W.; Wang, Y. Determination of neutralization potential in the prediction of acid rock drainage. In Proceedings of the 4th International Conference on Acid Rock Drainage, Vancouver, BC, Canada, 1 June 1997; pp. 449–464. [Google Scholar]
- Bouzahzah, H.; Benzaazoua, M.; Plante, B.; Bussiere, B. A quantitative approach for the estimation of the “fizz rating” parameter in the acid-base accounting tests: A new adaptations of the Sobek test. J. Geochem. Explor. 2015, 153, 53–65. [Google Scholar] [CrossRef]
- Sobek, A.A. Field and Laboratory Methods Applicable to Overburdens and Minesoils; Industrial Environmental Research Laboratory, Office of Research and Development, US Environmental Protection Agency: Colombia, WA, USA, 1978.
- Bouzahzah, H.; Benzaazoua, M.; Bussière, B.; Plante, B. Revue de littérature détaillée sur les tests statiques et les essais cinétiques comme outils de prédiction du drainage minier acide. Déchets Sci. Et Tech. Tech. 2014, 66, 14–31. [Google Scholar] [CrossRef] [Green Version]
- Plante, B.; Benzaazoua, M.; Bussière, B. Kinetic Testing and Sorption Studies by Modified Weathering Cells to Characterize the Potential to Generate Contaminated Neutral Drainage. Mine Water Environ. 2010, 30, 22–37. [Google Scholar] [CrossRef]
- Park, I.; Tabelin, C.B.; Seno, K.; Jeon, S.; Inano, H.; Ito, M.; Hiroyoshi, N. Carrier-microencapsulation of arsenopyrite using Al-catecholate complex: Nature of oxidation products, effects on anodic and cathodic reactions, and coating stability under simulated weathering conditions. Heliyon 2020, 6, e03189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eary, L.E.; Williamson, M.A. Simulations of the Neutralizing Capacity of Silicate Rocks in Acid Mine Drainage Environments. J. Am. Soc. Min. Reclam. 2006, 2006, 564–577. [Google Scholar] [CrossRef]
- Salmon, S.U. Geochemical modelling of acid mine drainage in mill tailings: Quantification of kinetic processes from laboratory to field scale. Byggvetenskap 2003, 7, 33–39. [Google Scholar]
- Plante, B. Évaluation des Principaux Facteurs D’influence sur la Prédiction du Drainage Neutre Contaminé; Université du Québec à en Abitibi-Témiscamingue: Quebec, QC, Canada, 2010. [Google Scholar]
- Jerz, J.K.; Rimstidt, J. Pyrite oxidation in moist air. Geochim. Et Cosmochim. Acta 2004, 68, 701–714. [Google Scholar] [CrossRef]
- Chou, L.; Wollast, R. Steady-state kinetics and dissolution mechanisms of albite. Am. J. Sci. 1985, 285, 963–993. [Google Scholar] [CrossRef]
- Casey, W.H.; Ludwig, C. Chapter 3. Silicate Mineral Dissolution as a Ligand-Exchange Reaction. Chem. Weather. Rates Silic. Miner. 1995, 87–118. [Google Scholar] [CrossRef]
- Palandri, J.L.; Kharaka, Y.K. A Compilation of Rate Parameters of Water-Mineral Interaction Kinetics for Application to Geochemical Modeling; US Geological Survey: Liston, WV, USA, 2004.
- Marty, N.; Claret, F.; Lassin, A.; Tremosa, J.; Blanc, P.; Madé, B.; Giffaut, E.; Cochepin, B.; Tournassat, C. A database of dissolution and precipitation rates for clay-rocks minerals. Appl. Geochem. 2015, 55, 108–118. [Google Scholar] [CrossRef]
- Lammers, K.; Smith, M.M.; Carroll, S.A. Muscovite dissolution kinetics as a function of pH at elevated temperature. Chem. Geol. 2017, 466, 149–158. [Google Scholar] [CrossRef]
- Smith, M.M.; Carroll, S.A. Chlorite dissolution kinetics at pH 3–10 and temperature to 275 °C. Chem. Geol. 2016, 421, 55–64. [Google Scholar] [CrossRef] [Green Version]
- Parkhurst, D.L.; Appelo, C.A.J. Description of Input and Examples for PHREEQC Version 3—A Computer Program for Speciation, Batch-reaction, One-dimensional Transport, and Inverse Geochemical Calculations. In Techniques and Methods; U.S. Geological Survey: Reston, VA, USA, 2013. [Google Scholar]
- Tiruta-Barna, L. Using PHREEQC for modelling and simulation of dynamic leaching tests and scenarios. J. Hazard. Mater. 2008, 157, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Neuman, S.P. Universal scaling of hydraulic conductivities and dispersivities in geologic media. Water Resour. Res. 1990, 26, 1749–1758. [Google Scholar] [CrossRef]
- Kinniburgh, D.; Cooper, D. PhreePlot: Creating Graphical Output with PHREEQC. 2011. Available online: http://nora.nerc.ac.uk/id/eprint/19744/ (accessed on 23 October 2021).
- Appelo, C.A.J.; Postma, D. Geochemistry, Groundwater and Pollution; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Le Bourre, B.; Neculita, C.M.; Coudert, L.; Rosa, E. Manganese removal processes and geochemical behavior in residues from passive treatment of mine drainage. Chemosphere 2020, 259, 127424. [Google Scholar] [CrossRef]
- Hellmann, R. The albite-water system: Part II. The time-evolution of the stoichiometry of dissolution as a function of pH at 100, 200, and 300 °C. Geochim. Et Cosmochim. Acta 1995, 59, 1669–1697. [Google Scholar] [CrossRef]
- Reczek, L.; Michel, M.M.; Trach, Y.; Siwiec, T.; Tytkowska-Owerko, M. The Kinetics of Manganese Sorption on Ukrainian Tuff and Basalt—Order and Diffusion Models Analysis. Minerals 2020, 10, 1065. [Google Scholar] [CrossRef]
- Trach, Y.; Tytkowska-Owerko, M.; Reczek, L.; Michel, M.M. Comparison the Adsorption Capacity of Ukrainian Tuff and Basalt with Zeolite–Manganese Removal from Water Solution. J. Ecol. Eng. 2021, 22, 161–168. [Google Scholar] [CrossRef]
- Kimball, B.E.; Rimstidt, J.D.; Brantley, S.L. Chalcopyrite dissolution rate laws. Appl. Geochem. 2010, 25, 972–983. [Google Scholar] [CrossRef]

| Mineral | Acidic Mechanism | Neutral Mechanism | Alkaline Mechanism | |||||
|---|---|---|---|---|---|---|---|---|
| log k | E | n | log k | E | log k | E | n | |
| K-feldspar * (KAlSi3O8) | −10.06 | 51.7 | 0.5 | −12.41 | 38 | −21.2 | 94.1 | −0.823 a |
| Oligoclase * (Na0.8Ca0.2Al1.2 Si2.8O8) | −9.67 | 65 | 0.457 | −11.84 | 69.8 | - | - | - |
| Andesine * (Na0.6Ca0.4Al1.4 Si2.6O8) | −8.88 | 53.5 | 0.541 | −11.47 | 57.4 | - | - | - |
| Anorthite * (CaAl2Si2O8) | −3.5 | 16.6 | 1.411 | −9.12 | 17.8 | - | - | - |
| Augite * (Ca0.35Mg0.42Fe0.23SiO3) | −6.82 | 78 | 0.7 | −11.97 | 78 | - | - | - |
| Epidote * (Ca2FeAl2Si3O12OH) | −10.6 | 71.1 | 0.338 | −11.99 | 70.7 | −17.33 | 79.1 | −0.556 a |
| Calcite * (CaCO3) | −0.3 | 14.4 | 1 | −5.81 | 23.5 | −3.48 | 35.4 | 1 b |
| Tremolite * (Ca2Mg5Si8O22(OH)2) | −8.4 | 18.9 | 0.7 | −10.6 | 94.4 | - | - | - |
| Albite d ** (NaAlSi3O8) | −10.07 | 58 | 0.34 | −19.29 | 57 | −9.85 | 56 | 0.32 c |
| Muscovite *** (KAlSi3O10(OH)2) | −2.5 | 44 | 0.8 | −5.04 | 45 | −0.3 | 61 | 0.6 c |
| Chlorite **** (Mg5Al2Si3O10(OH)8) | −4 | 30 | 0.74 | −10.32 | 13 | −8.82 | 15 | 0.43 c |
| The Composite Samples | Gs | Ss (m2/g) | D10 (μm) | D50 (μm) | D90 (μm) |
|---|---|---|---|---|---|
| D1 | 2.95 | 1.46 | 2 | 12 | 56 |
| D2 | 2.73 | 2.03 | 2.4 | 12 | 47.1 |
| D3 | 2.69 | 1.77 | 2.28 | 11.85 | 46.2 |
| T1 | 2.77 | 1.82 | 2.3 | 11.8 | 48.5 |
| The Composite Samples | Mass Fraction (wt%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ctotal | Al | Ca | Cu | Fe | K | Mg | Mn | Na | Stotal | |
| Detection limit (ppm) | 60 | 90 | 60 | 10 | 10 | 1 | 15 | 15 | 1 | 90 |
| Absolute standard deviation (1SD) (wt%) | 0.03 | 0.49 | 0.075 | 0.016 | 0.4 | 0.26 | 0.043 | 0.004 | 0.12 | 0.048 |
| D1 | 0.36 | 9.4 | 9.09 | 0.04 | 8.1 | 1.1 | 1.9 | 0.06 | 1.78 | 0.23 |
| D2 | 0.25 | 8.97 | 2.07 | 0.13 | 4.5 | 1.5 | 1.4 | 0.03 | 2.78 | 0.45 |
| D3 | 0.37 | 13.5 | 2.34 | 0.02 | 3 | 0.9 | 0.75 | 0.02 | 3.46 | 0.56 |
| T1 | 0.3 | 9.08 | 3.29 | 0.08 | 3.8 | 1.2 | 1.36 | 0.03 | 2.66 | 0.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toubri, Y.; Vermette, D.; Demers, I.; Beier, N.; Benzaazoua, M. Incorporating Kinetic Modeling in the Development Stages of Hard Rock Mine Projects. Minerals 2021, 11, 1306. https://doi.org/10.3390/min11121306
Toubri Y, Vermette D, Demers I, Beier N, Benzaazoua M. Incorporating Kinetic Modeling in the Development Stages of Hard Rock Mine Projects. Minerals. 2021; 11(12):1306. https://doi.org/10.3390/min11121306
Chicago/Turabian StyleToubri, Youssef, Denys Vermette, Isabelle Demers, Nicholas Beier, and Mostafa Benzaazoua. 2021. "Incorporating Kinetic Modeling in the Development Stages of Hard Rock Mine Projects" Minerals 11, no. 12: 1306. https://doi.org/10.3390/min11121306
APA StyleToubri, Y., Vermette, D., Demers, I., Beier, N., & Benzaazoua, M. (2021). Incorporating Kinetic Modeling in the Development Stages of Hard Rock Mine Projects. Minerals, 11(12), 1306. https://doi.org/10.3390/min11121306








