Infrared and Raman Spectroscopy of Ammoniovoltaite, (NH4)2Fe2+5Fe3+3Al(SO4)12(H2O)18
Abstract
1. Introduction
2. Materials and Methods
3. Theoretical Background
3.1. Crystal Structure
3.2. Local Symmetry; Infrared and Raman Band Activiation
4. Results
4.1. Infrared and Raman Spectra in the Region 4000 cm−1 to 2000 cm−1
4.1.1. Band Assignment
4.1.2. Hydrogen Bonding
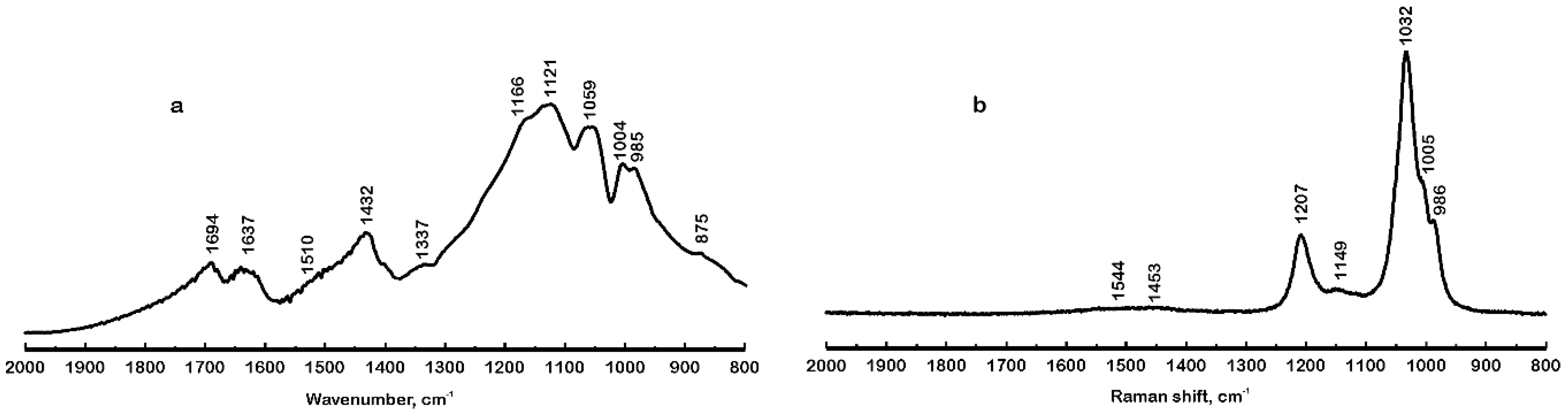
4.2. Infrared and Raman Spectra in the Region 2000 cm−1 to 800 cm−1
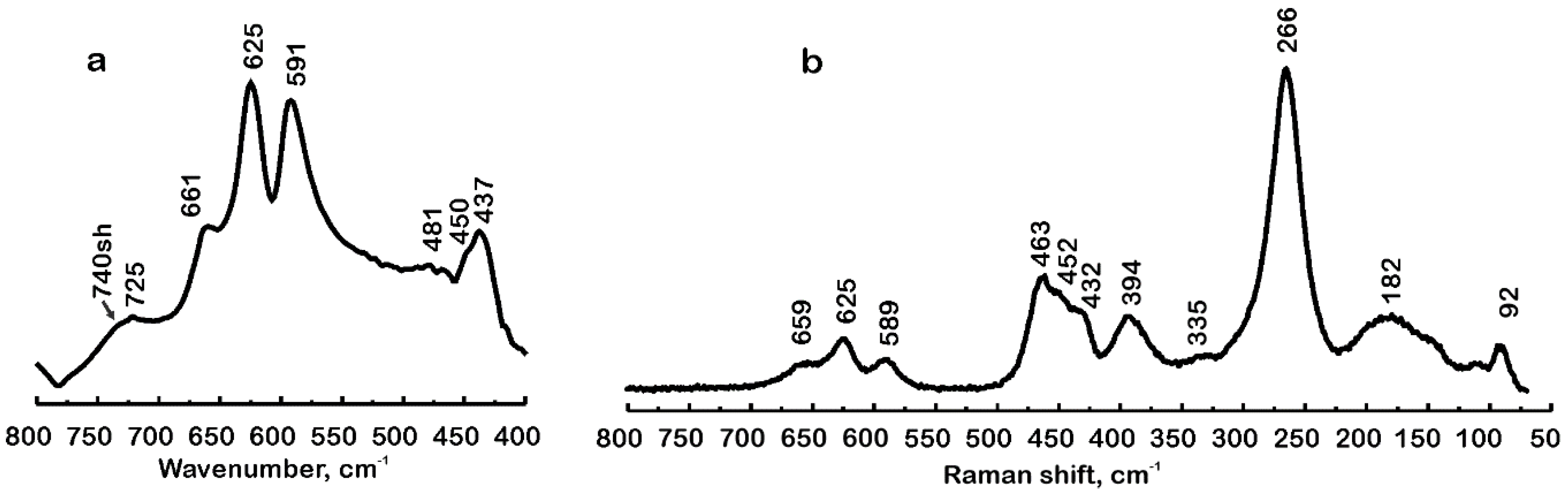
4.3. Infrared and Raman Spectra in the Region 800 cm−1 to 400(70) cm−1
5. Discussion
5.1. Infrared Spectroscopy of Voltaites
5.2. Raman Spectroscopy of Voltaites
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhitova, E.S.; Siidra, O.I.; Belakovsky, D.I.; Shilovskikh, V.V.; Nuzhdaev, A.A.; Ismagilova, R.M. Ammoniovoltaite, (NH4)2Fe2+5Fe3+3Al(SO4)12(H2O)18, a new mineral from the Severo-Kambalny geothermal field, Kamchatka, Russia. Mineral. Mag. 2018, 82, 1057–1077. [Google Scholar] [CrossRef]
- Sajó, I.E. Characterization of synthetic voltaite analogues. Eur. Chem. Bull. 2012, 1, 35–36. [Google Scholar]
- Majzlan, J.; Schlicht, H.; Wierzbicka-Wieczorek, M.; Giester, G.; Pöllmann, H.; Brömme, B.; Doyle, S.; Buth, G.; Koch, C.B. A contribution to the crystal chemistry of the voltaite group: Solid solutions, Mössbauer and infrared spectra, and anomalous anisotropy. Mineral. Petrol. 2013, 107, 221–233. [Google Scholar] [CrossRef]
- Breislak, S. Sulfate d’Alumine & de Fer. In Essais Mineralogiques sur la Solfatare de Pouzzole; Chez Janvier Giaccio: Naples, Italy, 1792; pp. 148–158. [Google Scholar]
- Ciesielczuk, J.; Żaba, J.; Bzowska, G.; Gaidzik, K.; Głogowska, M. Sulphate efflorescences at the geyser near Pinchollo, southern Peru. J. S. Am. Earth Sci. 2013, 42, 186–193. [Google Scholar] [CrossRef]
- Szakáll, S.; Sajó, I.; Fehér, B.; Bigi, S. Ammoniomagnesiovoltaite, a new voltaite-related mineral species from Pécs-Vasas, Hungary. Can. Mineral. 2012, 50, 65–72. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Aksenov, S.M.; Rastsvetaeva, R.K.; Möhn, G.; Rusakov, V.S.; Pekov, I.V.; Scholz, R.; Eremina, T.A.; Belakovskiy, D.I.; Lorenz, J.A. Magnesiovoltaite, K2Mg5Fe3+3Al(SO4)12·18H2O, a new mineral from the Alcaparrosa mine, Antofagasta region, Chile. Eur. J. Mineral. 2016, 28, 1005–1017. [Google Scholar] [CrossRef]
- Li, W.; Chen, G.; Sun, S. Zincovoltaite—A new sulfate mineral. Acta Mineral. Sinica 1987, 4, 307–312, (In Chinese with English abstract). [Google Scholar]
- Ertl, A.; Dyar, M.D.; Hughes, J.M.; Brandstätter, F.; Gunter, M.E.; Prem, M.; Peterson, R.C. Pertlikite, a new tetragonal Mg-rich member of the voltaite group from Madeni Zakh, Iran. Can. Mineral. 2008, 46, 661–669. [Google Scholar] [CrossRef]
- Zolotarev, A.A.; Krivovichev, S.V.; Panikorovskii, T.L.; Gurzhiy, V.V.; Bocharov, V.N.; Rassomakhin, M.A. Dmisteinbergite, CaAl2Si2O8, a Metastable Polymorph of Anorthite: Crystal-Structure and Raman Spectroscopic Study of the Holotype Specimen. Minerals 2019, 9, 570. [Google Scholar] [CrossRef]
- Zolotarev, A.A.; Zhitova, E.S.; Krzhizhanovskaya, M.G.; Rassomakhin, M.A.; Shilovskikh, V.V.; Krivovichev, S.V. Crystal chemistry and high-temperature behaviour of ammonium phases NH4MgCl3·6H2O and (NH4)2Fe3+Cl5·H2O from the burned dumps of the Chelyabinsk coal basin. Minerals 2019, 9, 486. [Google Scholar] [CrossRef]
- Chesnokov, B.V.; Shcherbakova, E.P.; Nishanbaev, T.P. Minerals of Burnt Dumps of the Chelyabinsk Coal Basin; Ural branch of RAS: Miass, Russia, 2008; pp. 1–139. (In Russian) [Google Scholar]
- Gendrin, A.; Mangold, N.; Bibring, J.P.; Langevin, Y.; Gondet, B.; Poulet, F.; Bonello, G.; Quantin, C.; Mustard, J.; Arvidson, R.; et al. Sulfates in Martian layered terrains: The OMEGA/Mars express view. Science 2005, 307, 1587–1591. [Google Scholar] [CrossRef]
- Carter, J.; Poulet, F.; Bibring, J.P.; Mangold, N.; Murchie, S. Hydrous minerals on Mars as seen by the CRISM and OMEGA imaging spectrometers: Updated global view. J. Geophys. Res. Planets 2013, 118, 831–858. [Google Scholar] [CrossRef]
- Ehlmann, B.L.; Edwards, C.S. Mineralogy of the Martian Surface. Annu. Rev. Earth Planet. Sci. 2014, 42, 291–315. [Google Scholar] [CrossRef]
- McCord, T.B.; Hansen, G.B.; Fanale, F.P.; Carlson, R.W.; Matson, D.L.; Johnson, T.V.; Smythe, W.D.; Crowley, J.K.; Martin, P.D.; Ocampo, A.; et al. Salts on Europa’s surface detected by Galileo’s near infrared mapping spectrometer. Science 1998, 280, 1242–1245. [Google Scholar] [CrossRef] [PubMed]
- McCord, T.B.; Hansen, G.B.; Hibbitts, C.A. Hydrated salt minerals on Ganymede’s surface: Evidence of an ocean below. Science 2001, 292, 1523–1525. [Google Scholar] [CrossRef] [PubMed]
- Mccord, T.B.; Teeter, G.; Hansen, G.B.; Sieger, M.T.; Orlando, T.M. Brines exposed to Europa surface conditions. J. Geophys. Res. Planets 2002, 107, 4-1. [Google Scholar] [CrossRef]
- Dalton, J.B.; Prieto-Ballesteros, O.; Kargel, J.S.; Jamieson, C.S.; Jolivet, J.; Quinn, R. Spectral comparison of heavily hydrated salts with disrupted terrains on Europa. Icarus 2005, 177, 472–490. [Google Scholar] [CrossRef]
- Poch, O.; Istiqomah, I.; Quirico, E.; Beck, P.; Schmitt, B.; Theulé, P.; Faure, A.; Hily-Blant, P.; Bonal, L.; Raponi, A.; et al. Ammonium salts are a reservoir of nitrogen on a cometary nucleus and possibly on some asteroids. Science 2020, 367, 7462. [Google Scholar] [CrossRef]
- Amils, R.; Fernández-Remolar, D.; IPBSL Team. Río Tinto: A Geochemical and Mineralogical Terrestrial Analogue of Mars. Life 2014, 4, 511–534. [Google Scholar] [CrossRef]
- Sobron, P.; Alpers, C.N. Raman spectroscopy of efflorescent sulfate salts from iron mountain mine superfund site, California. Astrobiol. 2013, 13, 270–278. [Google Scholar] [CrossRef]
- Makreski, P.; Jovanovski, G.; Dimitrovska, S. Minerals from Macedonia: XIV Identification of some sulfate minerals by vibrational (infrared and Raman) spectroscopy. Vib. Spectrosc. 2005, 39, 229–239. [Google Scholar] [CrossRef]
- Košek, F.; Culka, A.; Jehlička, J. Raman spectroscopic study of six synthetic anhydrous sulfates relevant to the mineralogy of fumaroles. J. Raman Spectrosc. 2018, 49, 1205–1216. [Google Scholar] [CrossRef]
- Žáček, V.; Škoda, R.; Laufek, F.; Kosek, F.; Jehlička, J. Complementing knowledge about rare sulphates lonecreekite, NH4Fe3+(SO4)2·12H2O and sabieite, NH4Fe3+(SO4)2: Chemical composition, XRD and RAMAN spectroscopy (Libušín near Kladno, the Czech Republic). J. Geosci. 2019, 64, 149–159. [Google Scholar] [CrossRef]
- Rull, F.; Guerrero, J.; Venegas, G.; Gázquez, F.; Medina, J. Spectroscopic Raman study of sulphate precipitation sequence in Rio Tinto mining district (SW Spain). Environ. Sci. Pollut. Res. 2014, 21, 6783–6792. [Google Scholar] [CrossRef]
- Blacksberg, J.; Alerstam, E.; Maruyama, Y.; Cochrane, C.J.; Rossman, G.R. Miniaturized time-resolved Raman spectrometer for planetary science based on a fast single photon avalanche diode detector array. Appl. Opt. 2016, 55, 739–748. [Google Scholar] [CrossRef]
- Jehlička, J.; Vítek, P.; Edwards, H.G.M.; Hargreaves, M.D.; Capoun, T. Fast detection of sulphate minerals (gypsum, anglesite, baryte) by a portable Raman spectrometer. J. Raman Spectrosc. 2009, 40, 1082–1086. [Google Scholar] [CrossRef]
- Rull, F.; Sansano, A.; Diaz, E.; Canora, C.P.; Moral, A.G.; Tato, C.; Colombo, M.; Belenguer, T.; Fernández, M.; Rodríguez-Manfredi, J.A.; et al. ExoMars Raman laser spectrometer for Exomars. Proc. SPIE 2011, 8152. [Google Scholar] [CrossRef]
- Plášil, J.; Škoda, R.; Fejfarová, K.; Čejka, J.; Kasatkin, A.V.; Dušek, M.; Talla, D.; Lapčák, L.; Machnovič, V.; Dini, M. Hydroniumjarosite, (H3O)+Fe3(SO4)2(OH)6, from Cerros Pintados, Chile: Single-crystal X-ray diffraction and vibrational spectroscopic study. Mineral. Mag. 2014, 78, 535–547. [Google Scholar] [CrossRef]
- Najorka, J.; Lewis, J.M.; Spratt, J.; Sephton, M.A. Single-crystal X-ray diffraction study of synthetic sodium-hydronium jarosite. Phys. Chem. Miner. 2016, 43, 377–386. [Google Scholar] [CrossRef]
- Libowitzky, E. Correlation of O-H stretching frequencies and O-HO hydrogen bond lengths in minerals. Monatsh. Chem. 1999, 130, 1047–1059. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Košek, F.; Edwards, H.G.M.; Jehlička, J. Raman spectroscopic vibrational analysis of the complex iron sulfates clairite, metavoltine, and voltaite from the burning coal dump Anna I, Alsdorf, Germany. J. Raman Spectrosc. 2019, 1–8. [Google Scholar] [CrossRef]
- Sergeeva, A.V.; Zhitova, E.S.; Bocharov, V.N. Infrared and Raman spectroscopy of tschermigite, (NH4)Al(SO4)2·12H2O. Vib. Spectrosc. 2019, 105, 102983. [Google Scholar] [CrossRef]
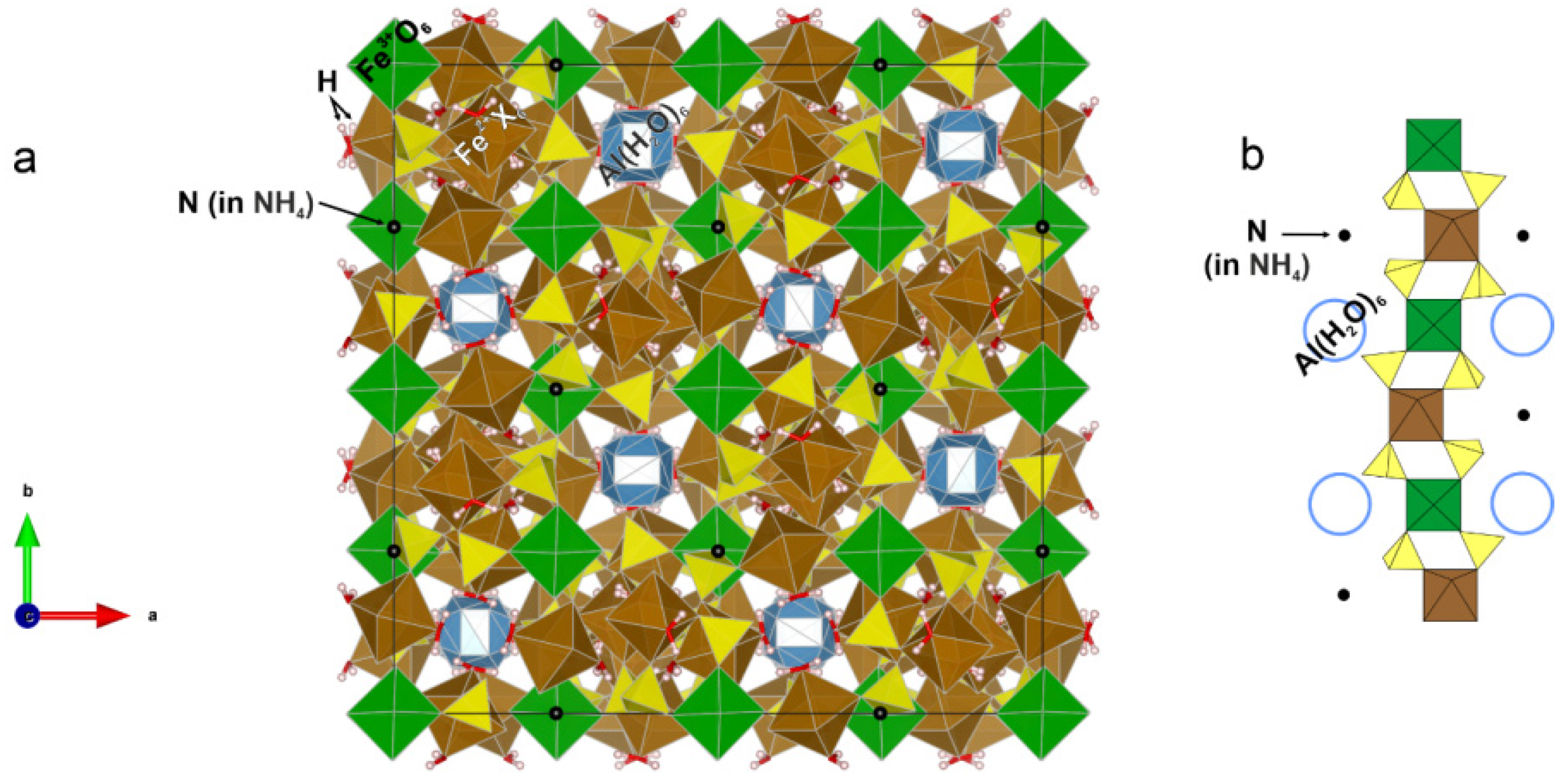
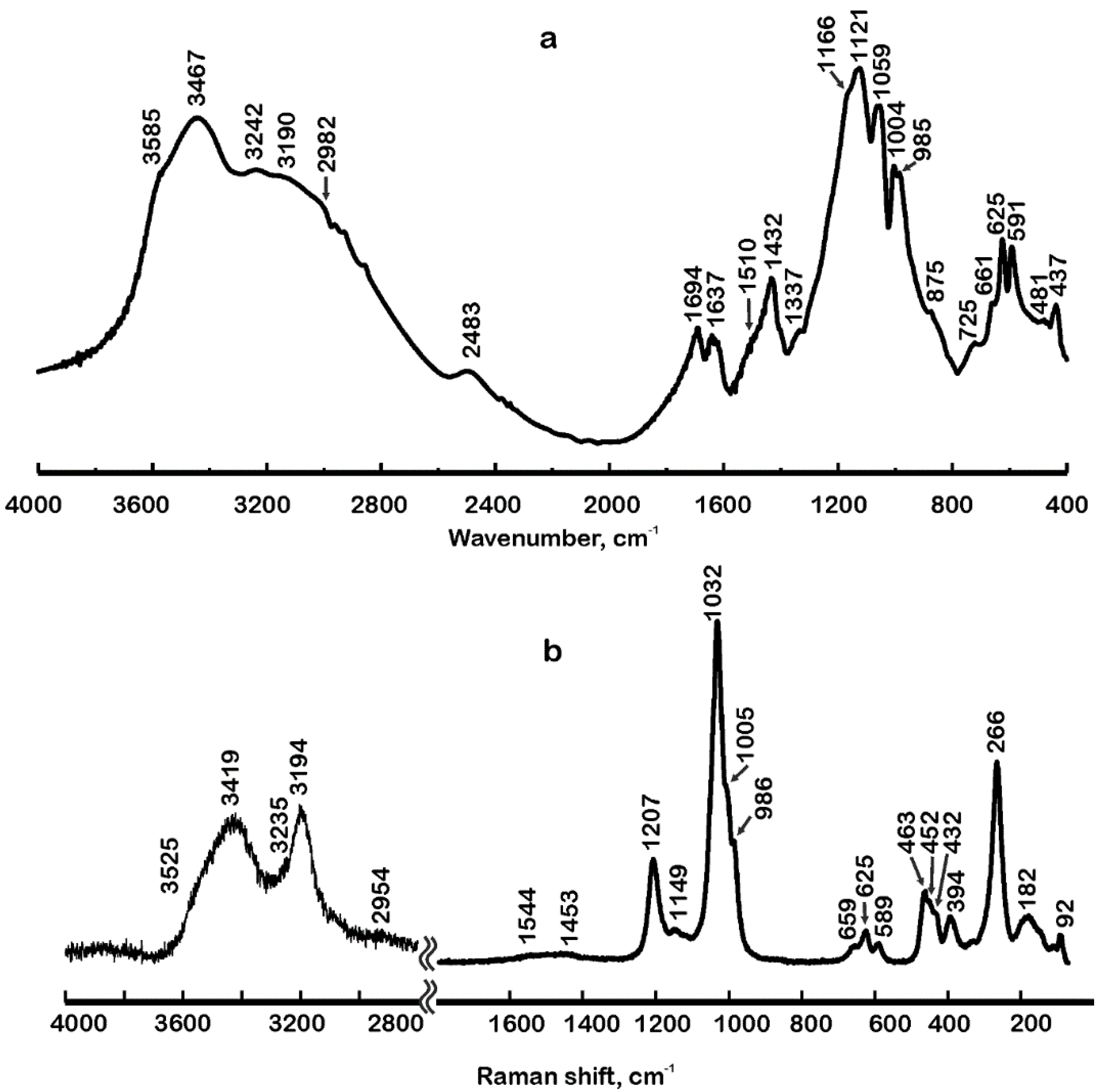
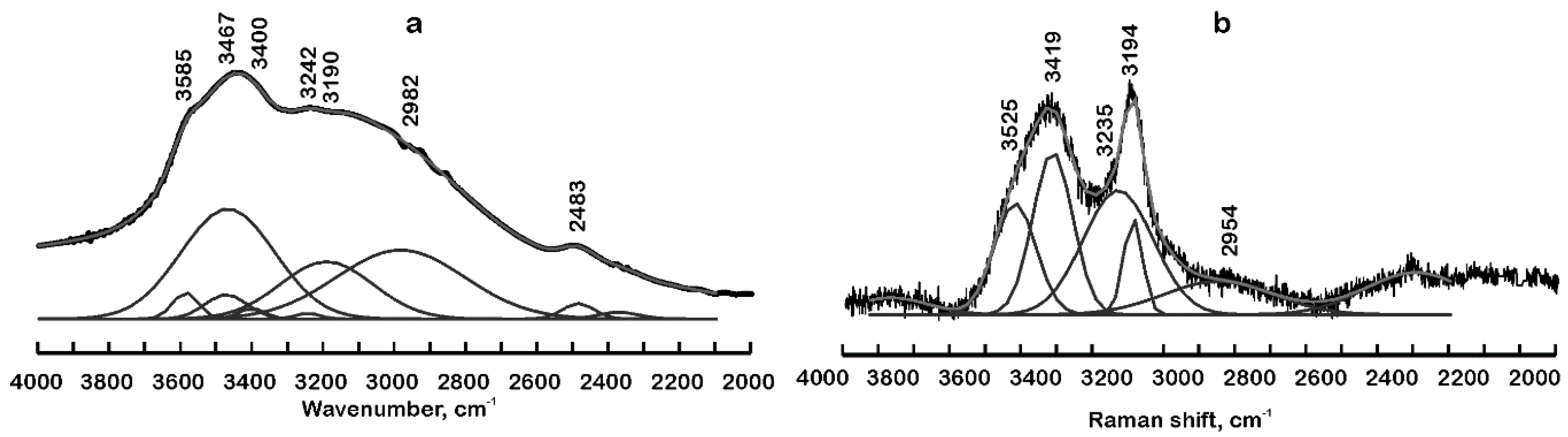
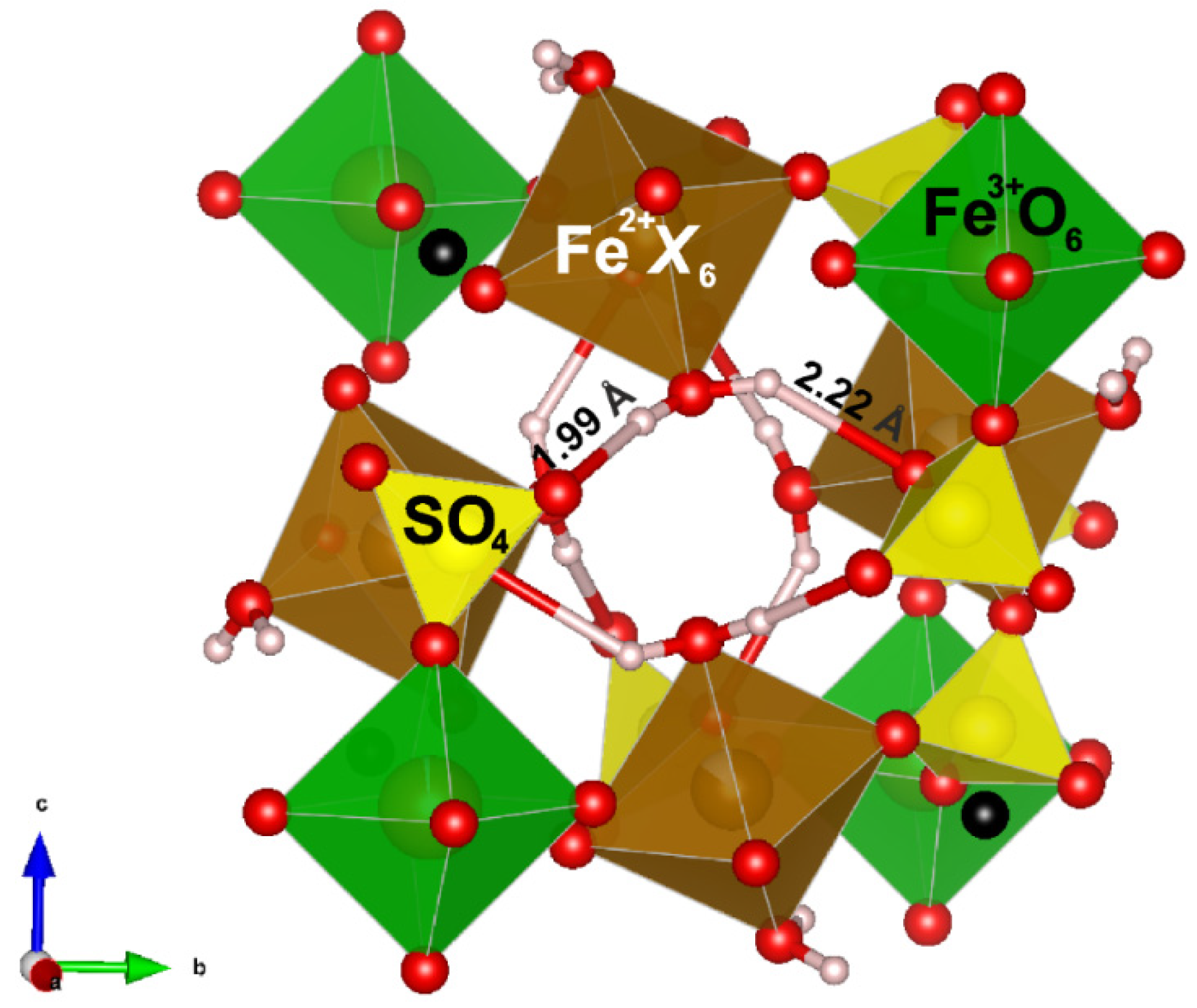
| Site | Dominantly Occupied by | Wyckoff Position | Site Symmetry | Structure Fragment | Infrared Active 1 | Raman Active 2 |
|---|---|---|---|---|---|---|
| A | N | 32b | D3 | NH4 | ν1, ν2, ν3, ν4 | ν1, ν2, ν3, ν4 |
| M1 | Fe3+ | 32c | S6 = C3i | Fe2+X6 | ν1, ν2, ν3, ν4, ν5, ν6 | ν1, ν2, ν3, ν4, ν5, ν6 |
| M2 | Fe2+ | 96g | C2 | Fe3+O6 | ν1, ν2, ν5 | ν3, ν4, ν6 |
| Al | Al3+ | 16a | T | Al(H2O)6 | ν1, ν2, ν3, ν4, ν5, ν6 | ν3, ν4, ν5, ν6 |
| S | S | 192h | C1 | SO4 | ν1, ν2, ν3, ν4 | ν1, ν2, ν3, ν4 |
| O1-O7 | O | 192h | C1 | - | - | - |
| H1, H2 | H | 192h | C1 | - | - | - |
| Infrared Spectra, cm−1 | Raman Spectra, cm−1 | Band Assignment |
|---|---|---|
| 3585 | 3525 | (Al–OH), (Fe–OH), ν3 (H2O)l 1 |
| 3467 | - | ν1 (H2O)l |
| 3400sh | 3419 | ν1 (H2O)s 1 |
| 3242 | 3235 | ν3 (NH4), 2ν2 (H2O), ν3 (H2O)s, |
| 3190 | 3194 | ν3 (NH4) |
| 2982 | 2954 | ν1 (NH4) |
| 2483 | - | ν3 (Al–H2O), ν (HSO4) |
| 1694 | - | ν2 (NH4) and/or ν2 (H2O) |
| 1637 | - | ν2 (H2O) |
| 1510sh, 1432 | 1544, 1453 | ν4 (NH4) |
| 1337sh | - | 2 ν4 (SO4), 2 ν3 (AlO6) |
| 1166sh, 1121 | 1207, 1149 | ν3 (SO4) |
| 1059, 1004, 985sh | 1032, 1005, 986 | ν1 (SO4) |
| 875sh, 740sh, 725 | - | (Me–H2O) |
| 661, 625, 591 | 659, 625, 589 | ν4 (SO4), ν3 (AlO6), ν3 (FeO6) |
| 481, 450 | 463, 452 | ν2 (SO4), ν3 (FeO6) |
| 437 | 432 | ν2 (SO4), ν3 (AlO6), ν3 (FeO6) |
| - | 394, 335, 266, 182, 92 | Lattice modes: (MeO6), (SO4), (NH4) |
| Type of Vibrations | Stretching | Bending | Deformation | ||
|---|---|---|---|---|---|
| Wavenumber, cm−1 | 3585 | 3585, 3467, 3400, 3242 | 2483 | 1637 | 875, 740, 725 |
| Band assignment | Al–OH, Fe–OH | H2O | Al–H2O | H2O | Me1–H2O |
| Structure fragment 1 | 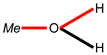 | 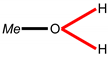 | 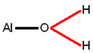 |  | 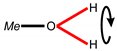 |
| Infrared Spectroscopy | Crystal Structure Data [1] | |||||
|---|---|---|---|---|---|---|
| Wavenumber, cm−1 | d(O···H) (Å) 1 | d(O···O) (Å) 1 | D2-H | d(O···H) (Å) 1 | D-D | d(O···O) (Å) 1 |
| 3585 | 2.25 | 3.24 | O2-H1 3 | 2.22 | O2-O5w | 2.95 |
| 3467 | 2.06 | 2.90 | ||||
| 3400sh | 1.99 | 2.83 | O3-H2 3 | 1.99 | O3-O5w | 2.94 |
| 3242 | 1.87 | 2.72 | Probably corresponds to H atoms of Al(H2O)6 or NH4 | |||
| 2483 | 1.59 | 2.53 | ||||
| Ammonio-Voltaite | Ammonio-Magnesio-Voltaite 1 | Synthetic Ammonio-Voltaite | Synthetic Mn-Ammonio-Voltaite | Synthetic Voltaite | Synthetic Magnesio-Voltaite | Band Assignment |
|---|---|---|---|---|---|---|
| 3585 | - | 3562 | 3560 | 3558 | 3579 | Me–OH, ν3 (H2O) |
| 3467 3400 | 3423 | 3417 | 3379 | 3417 | - | ν1 (H2O) |
| 3242 | 3263 | 3248 | 3261 | - | - | ν3 (NH4), ν3 (H2O), 2ν2 (H2O) |
| 3190 | - | 3091 | 3114 | 3074 | 3083 | ν3 (NH4) |
| 2982 | - | ν1 (NH4) | ||||
| 2483 | - | 2501 | 2501 | 2499 | 2497 | ν3 (Al–H2O), ν (HSO4) |
| 1694 | - | 1689 | 1686 | 1687 | 1691 | ν2 (NH4) and ν2 (H2O) |
| 1637 | 1641 | 1639 | 1641 | 1630 | 1635 | ν2 (H2O) |
| 1510sh | - | - | - | - | - | ν4 (NH4) |
| 1432 | 1431 | 1431 | 1431 | - | - | ν4 (NH4) |
| 1337sh | - | - | - | - | - | 2ν4 (SO4), 2ν3 (AlO6), 2ν (Al–H2O) |
| 1166 | - | 1153 | 1143 | 1157 | 1182 | ν3 (SO4) |
| 1121 | 1122 | 1130 | - | - | - | |
| 1059 | 1065 | 1055 | 1053 | 1055 | 1065 | ν1 (SO4) |
| 1004 | 1014 | 1007 | 1005 | 1007 | 1014 | |
| 985sh | - | - | - | - | - | |
| 875sh | - | 879 | 854 | 876 | 866 | Me2+–OH |
| 740sh | - | 731 | 729 | 735 | 733 | Al–H2O |
| 725 | ||||||
| 661sh | - | - | - | - | - | ν4 (SO4), ν3 (AlO6), ν3 (MeO6) |
| 625 | - | 627 | 627 | 627 | 634 | |
| 591 | 594 | 592 | 596 | 592 | 596 | |
| 481, 450, 437 | - | 442 | 444 | 442 | 445 | ν2 (SO4), ν3 (AlO6), ν3 (MeO6) |
| This work | [6] | [3] | Reference | |||
| Ammoniovoltaite | Voltaite | Tschermigite | Band Assignment |
|---|---|---|---|
| 3525 | 3583 | 3573 | ν (Al–OH), (Fe–OH), ν3 (H2O) |
| 3419 | 3441 | 3379 | ν1 (H2O) |
| 3235 | 3209 | - | ν3 (NH4), ν3 (H2O), 2ν2 (H2O) |
| 3194 | - | - | ν3 (NH4) |
| - | - | 3163 | ν3 (NH4) |
| - | - | 3124 | ν1 (H2O), ν1 (NH4) |
| 2954 | - | - | ν1 (NH4) |
| - | - | 2883 | 2ν4 (NH4) |
| - | - | 2562 | ν3 (H2O–Al) or possibly ν (HSO4) |
| - | - | 2461 | |
| - | - | 1680 | ν2 (NH4) |
| - | 1642 | 1600 | δ (H2O) |
| 1544 | - | - | ν4 (NH4) |
| 1453 | 1428 | 1445 | ν4 (NH4) |
| - | 1280 | - | ν3 (SO4) |
| 1207 | 1215 | - | ν3 (SO4) |
| 1149 | - | 1133 | ν3 (SO4) |
| - | - | 1100 | ν3 (SO4) |
| - | 1055 | - | ν1 (SO4) |
| 1032 | 1036 | - | ν1 (SO4) |
| 1005 | 1011 | - | ν1 (SO4) |
| 986 | 991 | 990 | ν1 (SO4) |
| 659 | 660 | - | ν4 (SO4), ν3 (AlO6), ν3 (FeO6) |
| 625 | 629 | 615 | ν4 (SO4), ν3 (AlO6), ν3 (FeO6) |
| 589 | 594 | - | ν4 (SO4), ν3 (AlO6), ν3 (FeO6) |
| - | - | 535 | ν2, ν4 (SO4); ν1, ν3 (AlO6) |
| 463 | 469 | 460 | ν2 (SO4), ν3 (FeO6) |
| 452 | - | - | ν2 (SO4), ν3 (FeO6) |
| 432 | 439 | 440 | ν2 (SO4), ν3 (AlO6), ν3 (FeO6) |
| 394 | 398 | - | ν2 (SO4), ν3 (AlO6), ν3 (FeO6) |
| 335 | 338 | 326 | Lattice modes: (MeO6), (SO4), (NH4) |
| - | 310 | - | |
| 266 | 268 | - | |
| 182 | 192 | 192 | |
| 92 | - | 80 | |
| This work | [34] | [35] | Reference |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sergeeva, A.V.; Zhitova, E.S.; Nuzhdaev, A.A.; Zolotarev, A.A.; Bocharov, V.N.; Ismagilova, R.M. Infrared and Raman Spectroscopy of Ammoniovoltaite, (NH4)2Fe2+5Fe3+3Al(SO4)12(H2O)18. Minerals 2020, 10, 781. https://doi.org/10.3390/min10090781
Sergeeva AV, Zhitova ES, Nuzhdaev AA, Zolotarev AA, Bocharov VN, Ismagilova RM. Infrared and Raman Spectroscopy of Ammoniovoltaite, (NH4)2Fe2+5Fe3+3Al(SO4)12(H2O)18. Minerals. 2020; 10(9):781. https://doi.org/10.3390/min10090781
Chicago/Turabian StyleSergeeva, Anastasia V., Elena S. Zhitova, Anton A. Nuzhdaev, Andrey A. Zolotarev, Vladimir N. Bocharov, and Rezeda M. Ismagilova. 2020. "Infrared and Raman Spectroscopy of Ammoniovoltaite, (NH4)2Fe2+5Fe3+3Al(SO4)12(H2O)18" Minerals 10, no. 9: 781. https://doi.org/10.3390/min10090781
APA StyleSergeeva, A. V., Zhitova, E. S., Nuzhdaev, A. A., Zolotarev, A. A., Bocharov, V. N., & Ismagilova, R. M. (2020). Infrared and Raman Spectroscopy of Ammoniovoltaite, (NH4)2Fe2+5Fe3+3Al(SO4)12(H2O)18. Minerals, 10(9), 781. https://doi.org/10.3390/min10090781







