Formation of Natural Silicate Hydrates by the Interaction of Alkaline Seepage and Sediments Derived from Serpentinized Ultramafic Rocks at Narra, Palawan, the Philippines
Abstract
1. Introduction
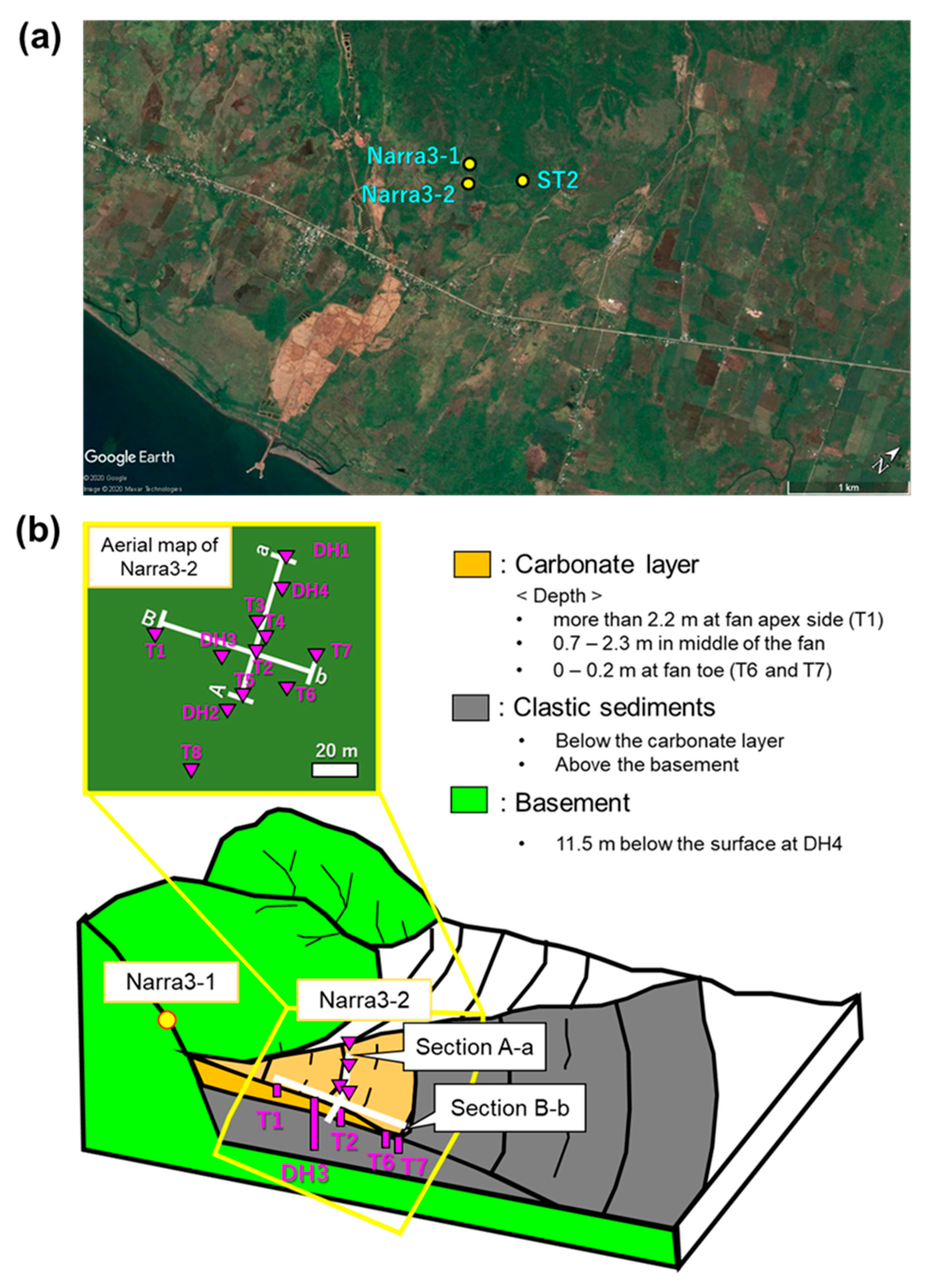
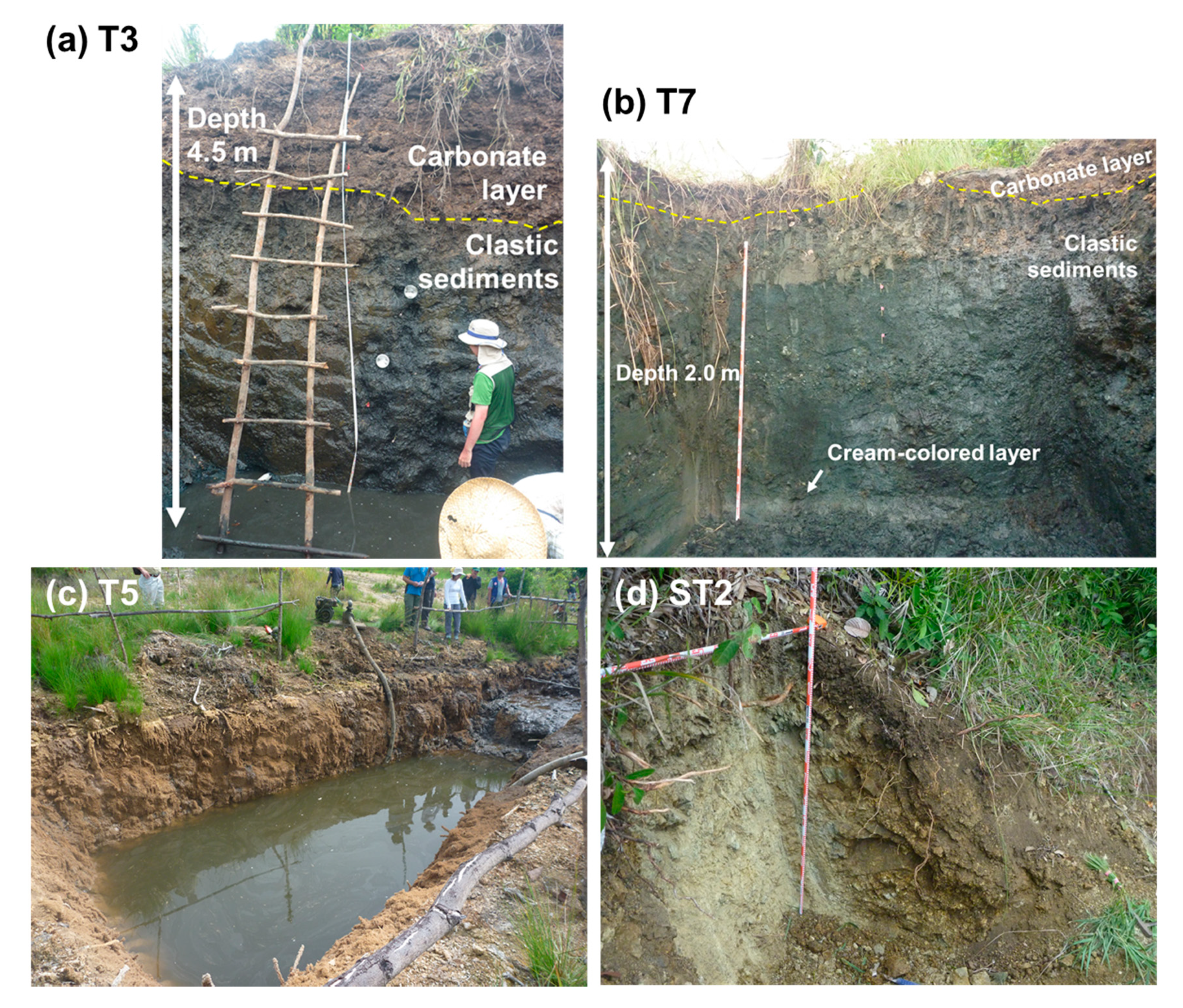
2. Site Description and Samples
3. Methods
4. Results
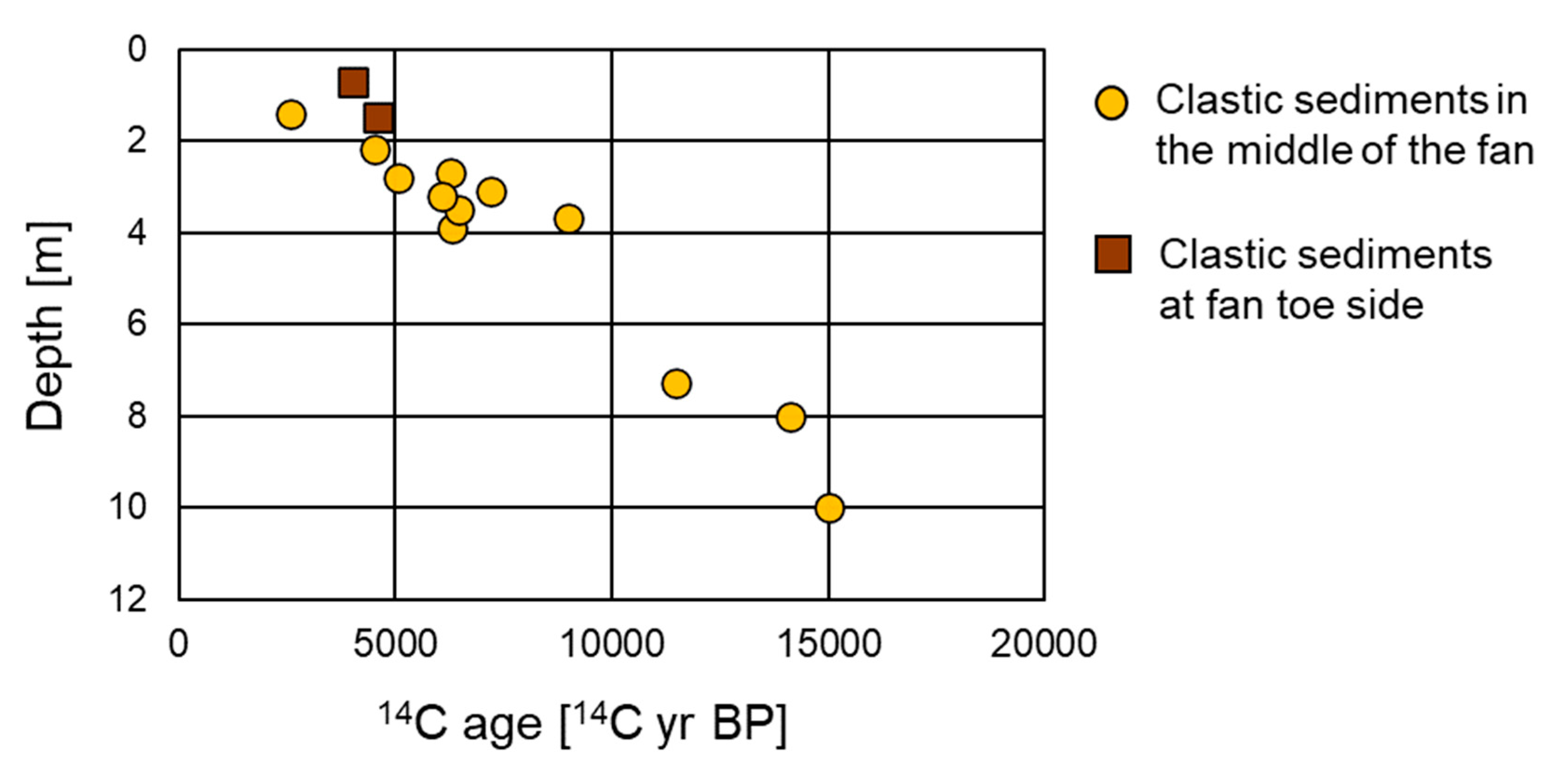
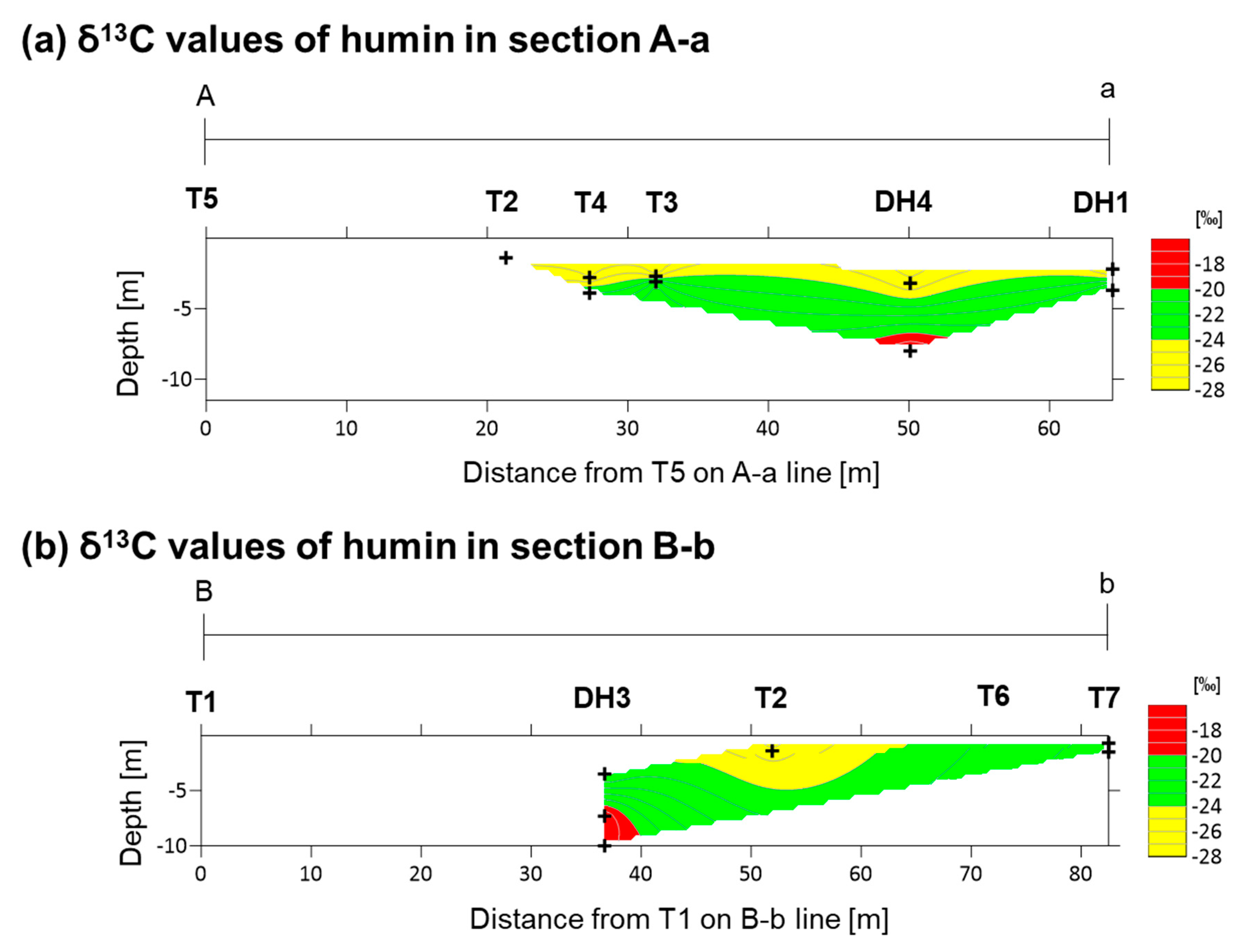
4.1. Carbon Isotope Measurements
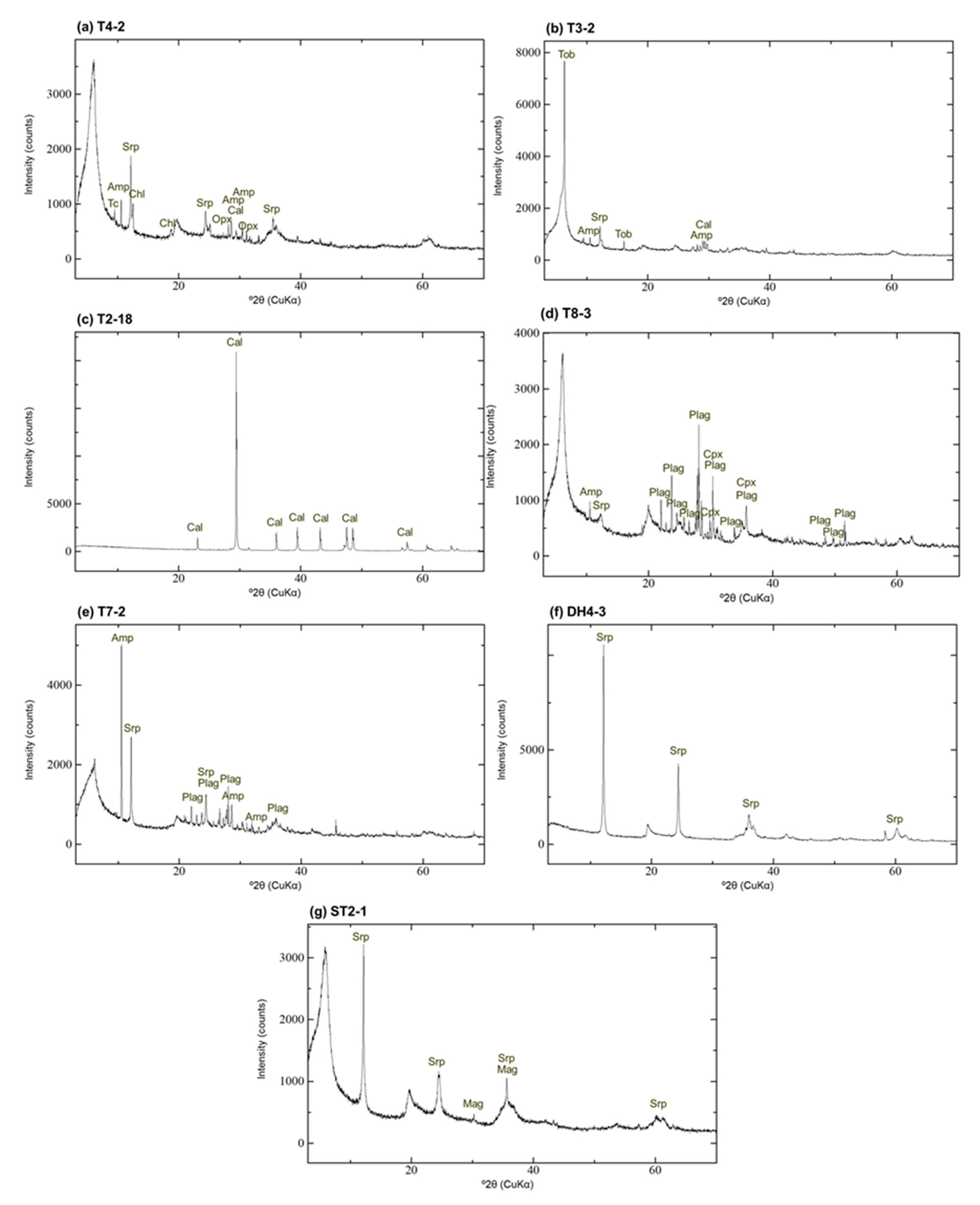
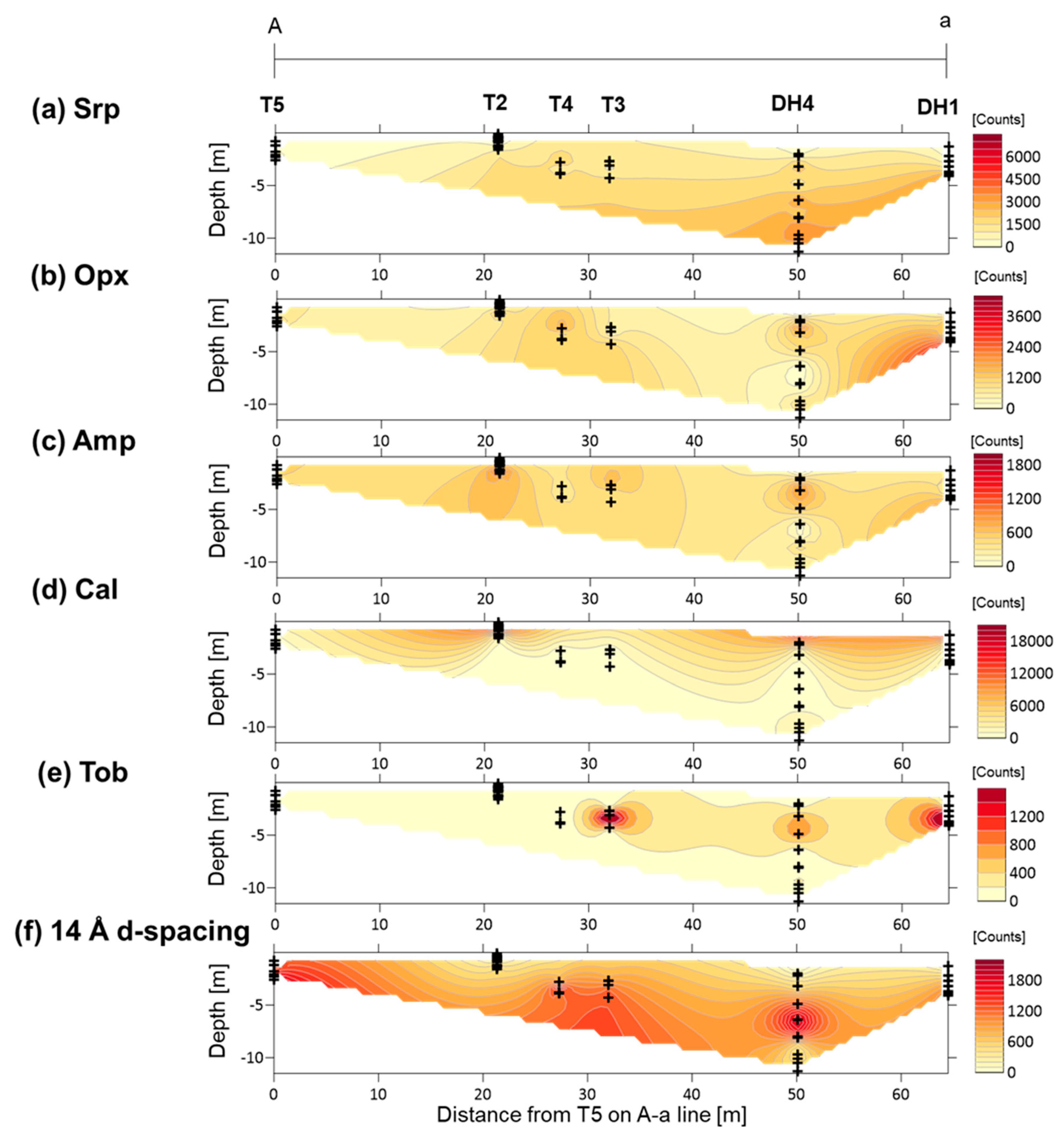
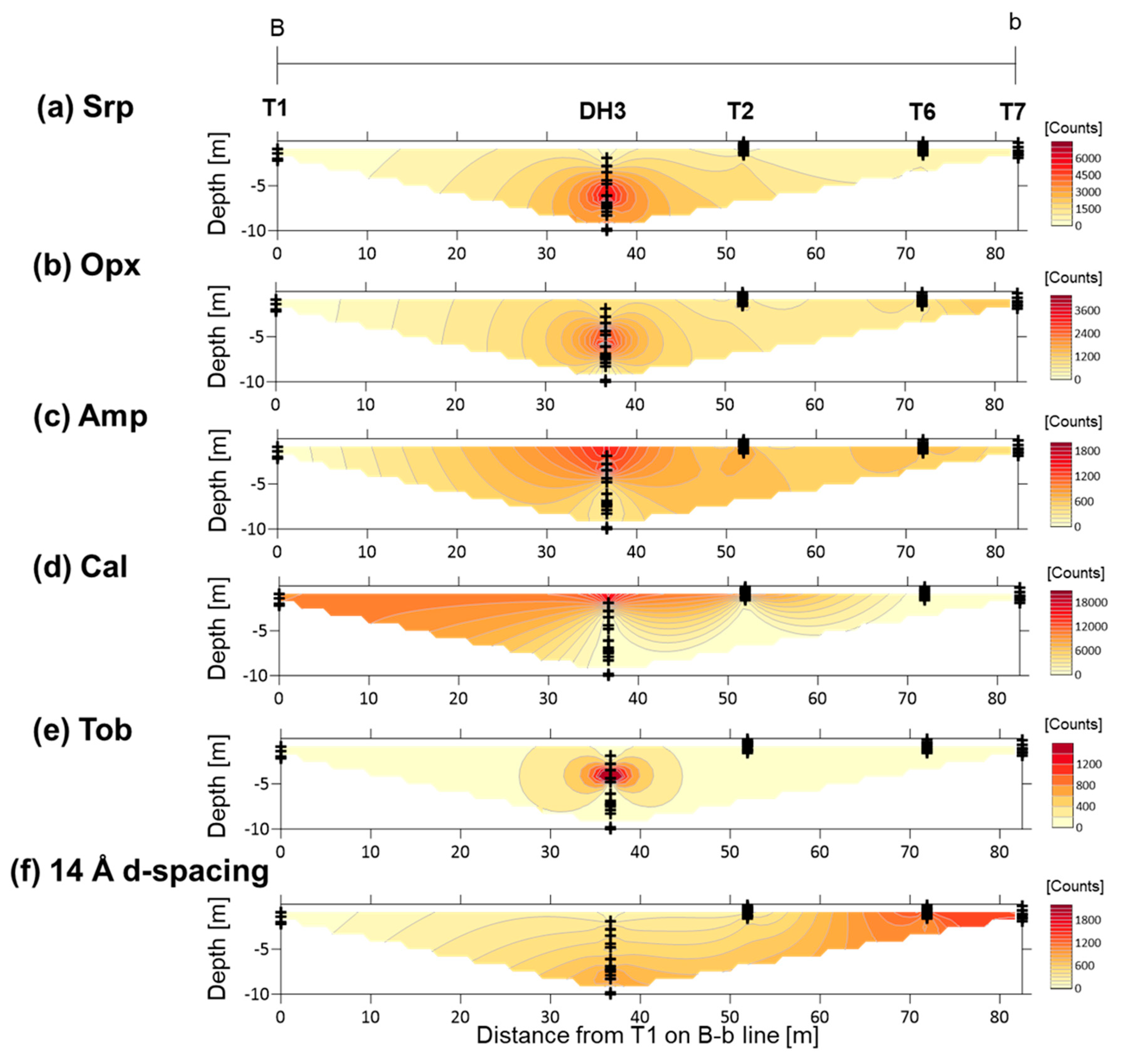
4.2. XRD Analysis of Randomly Oriented Samples
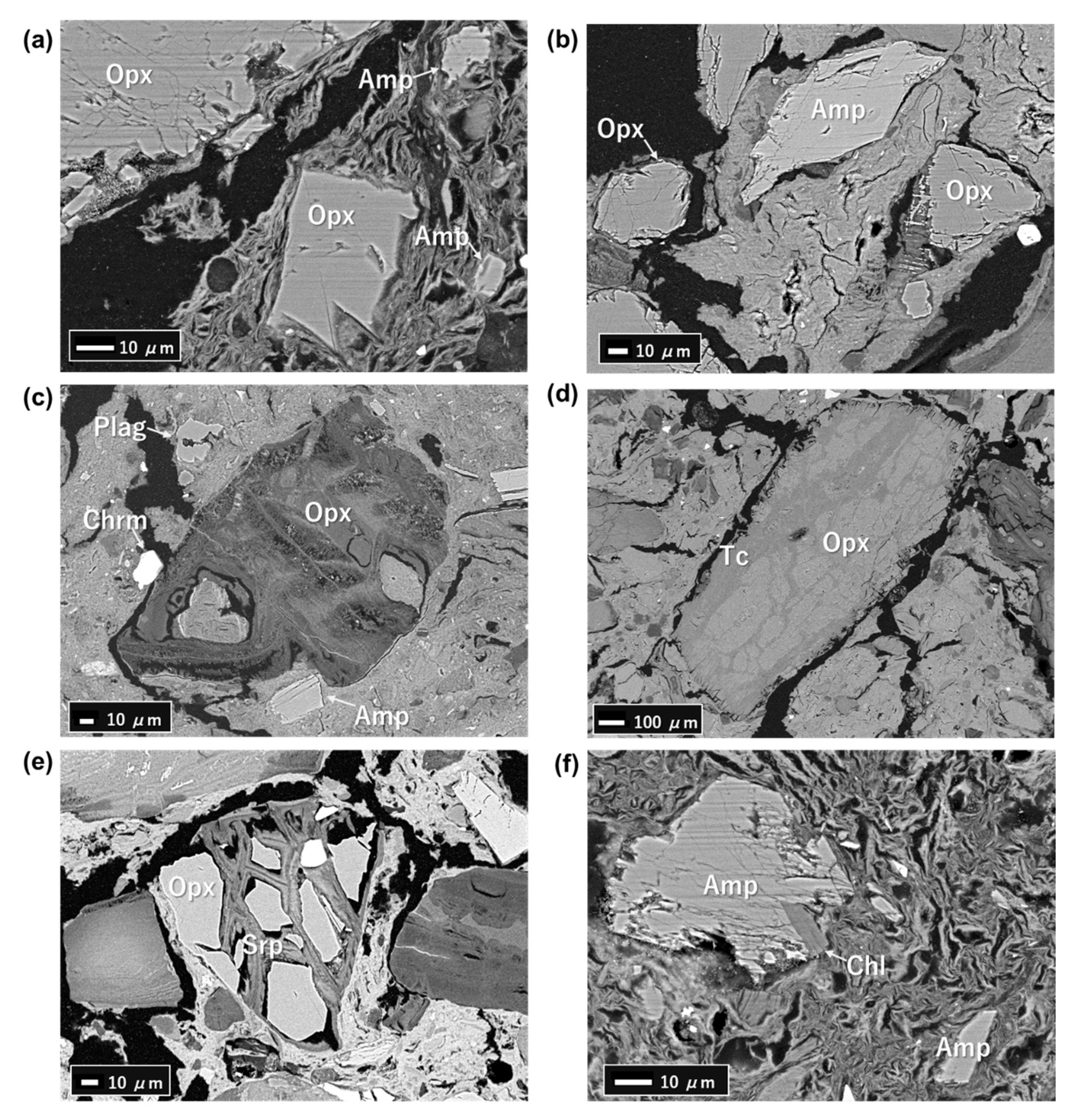
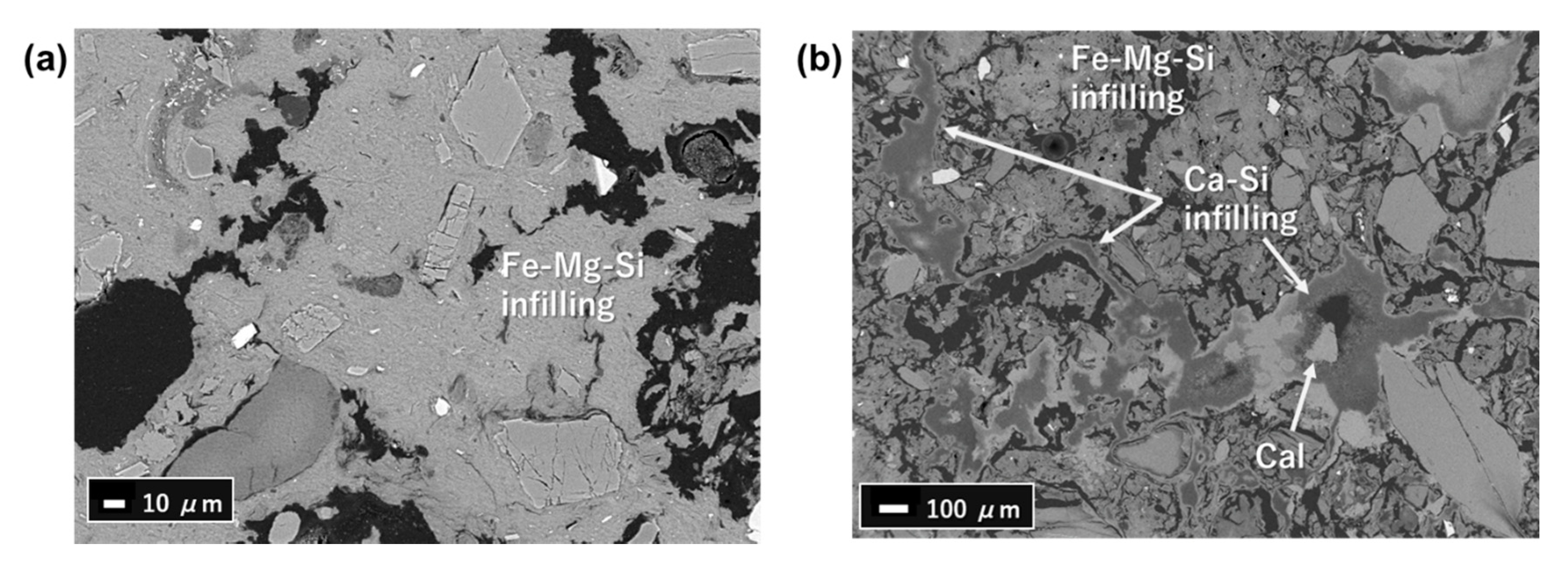
4.3. FESEM-EDS Analysis
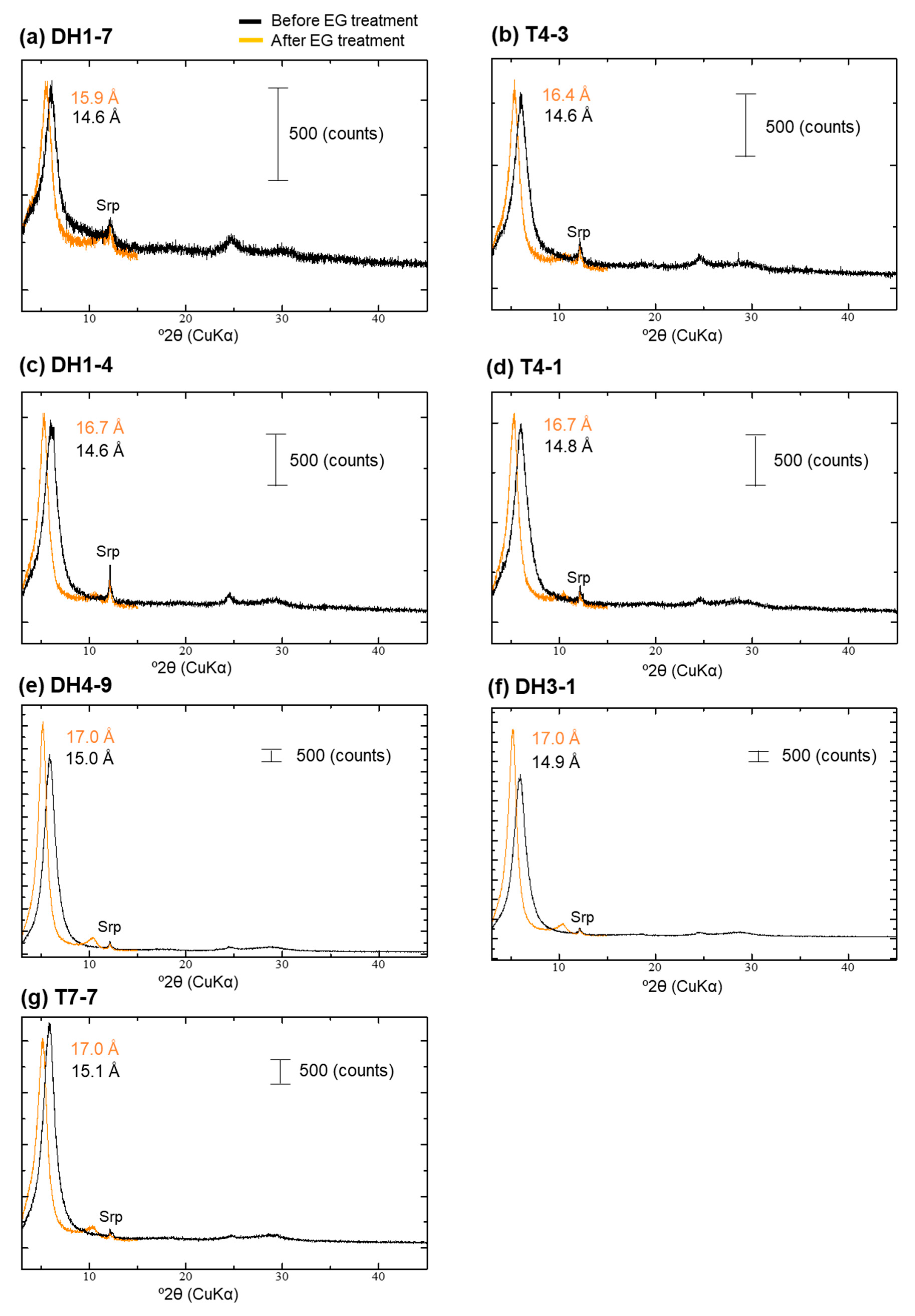
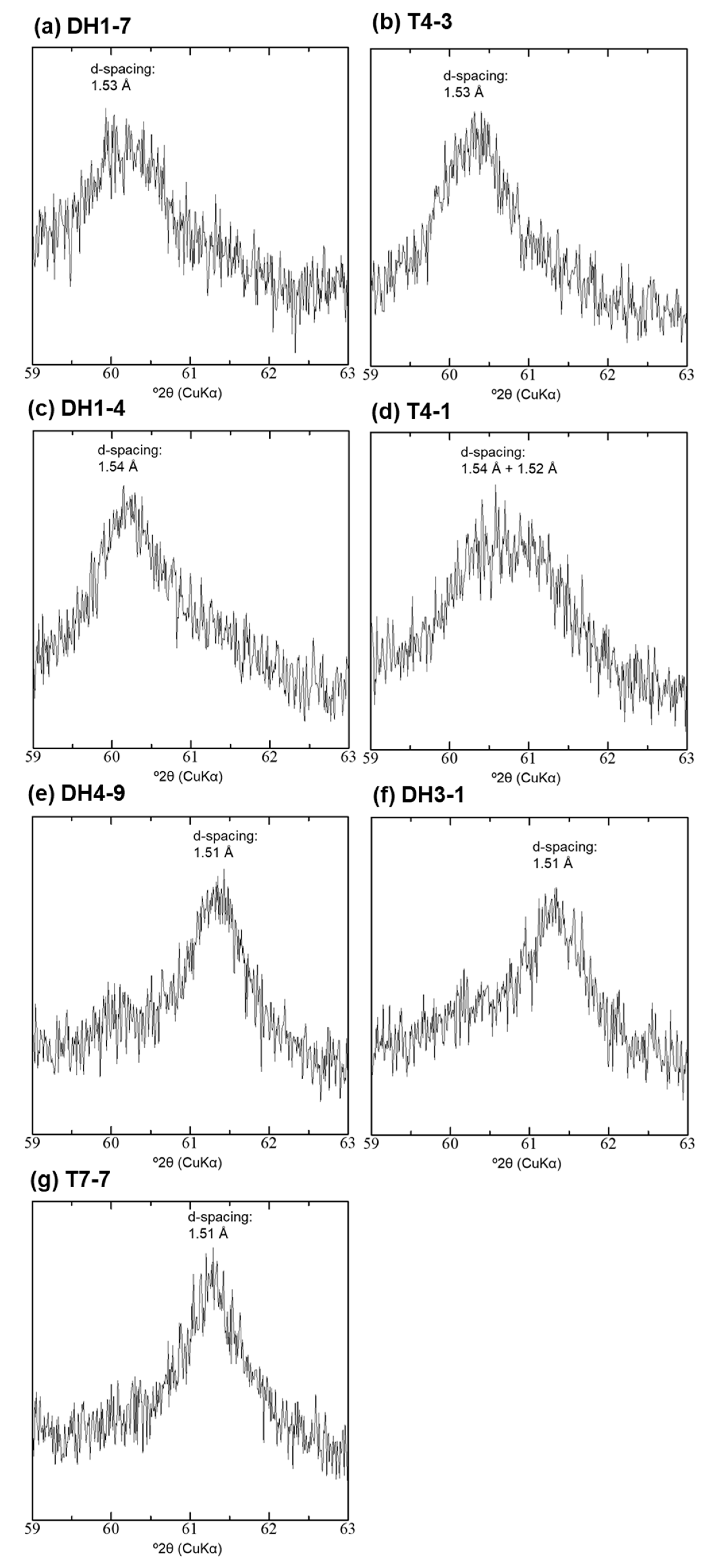
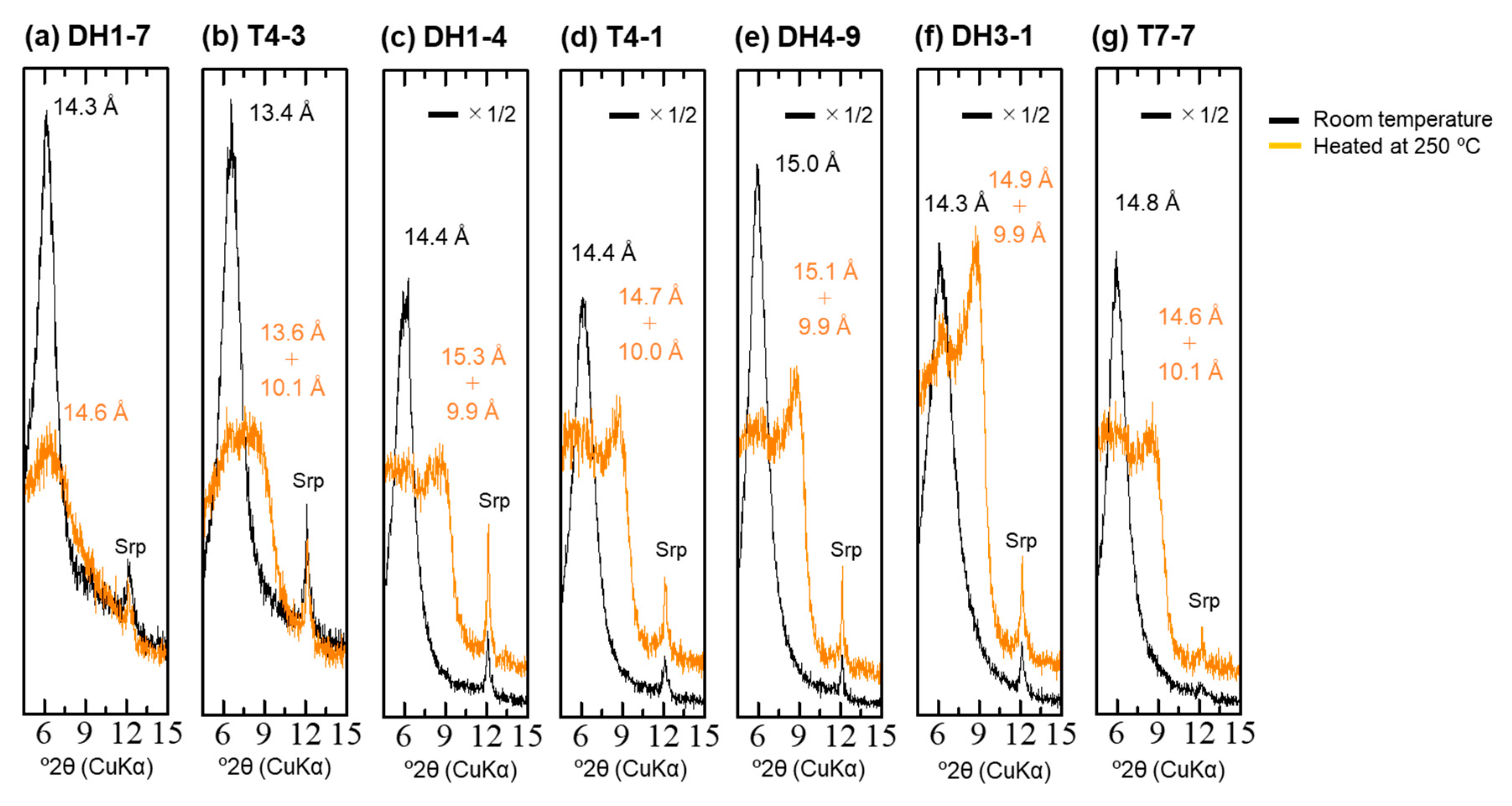
4.4. XRD Analysis of the <2-µm Fractions
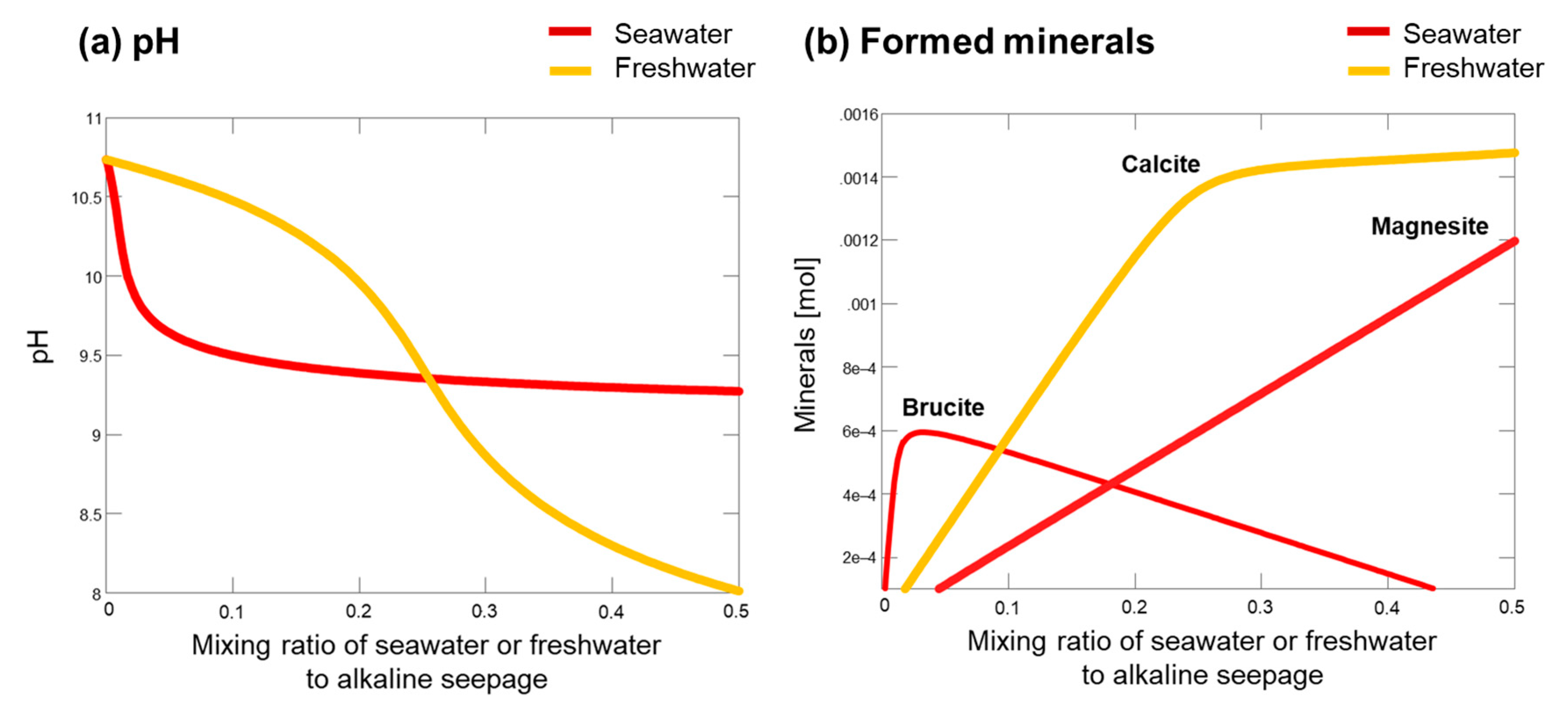
4.5. Thermodynamic Calculation
5. Discussion
5.1. Depositional Age and Environment
5.2. Primary Minerals in the Clastic Sediments
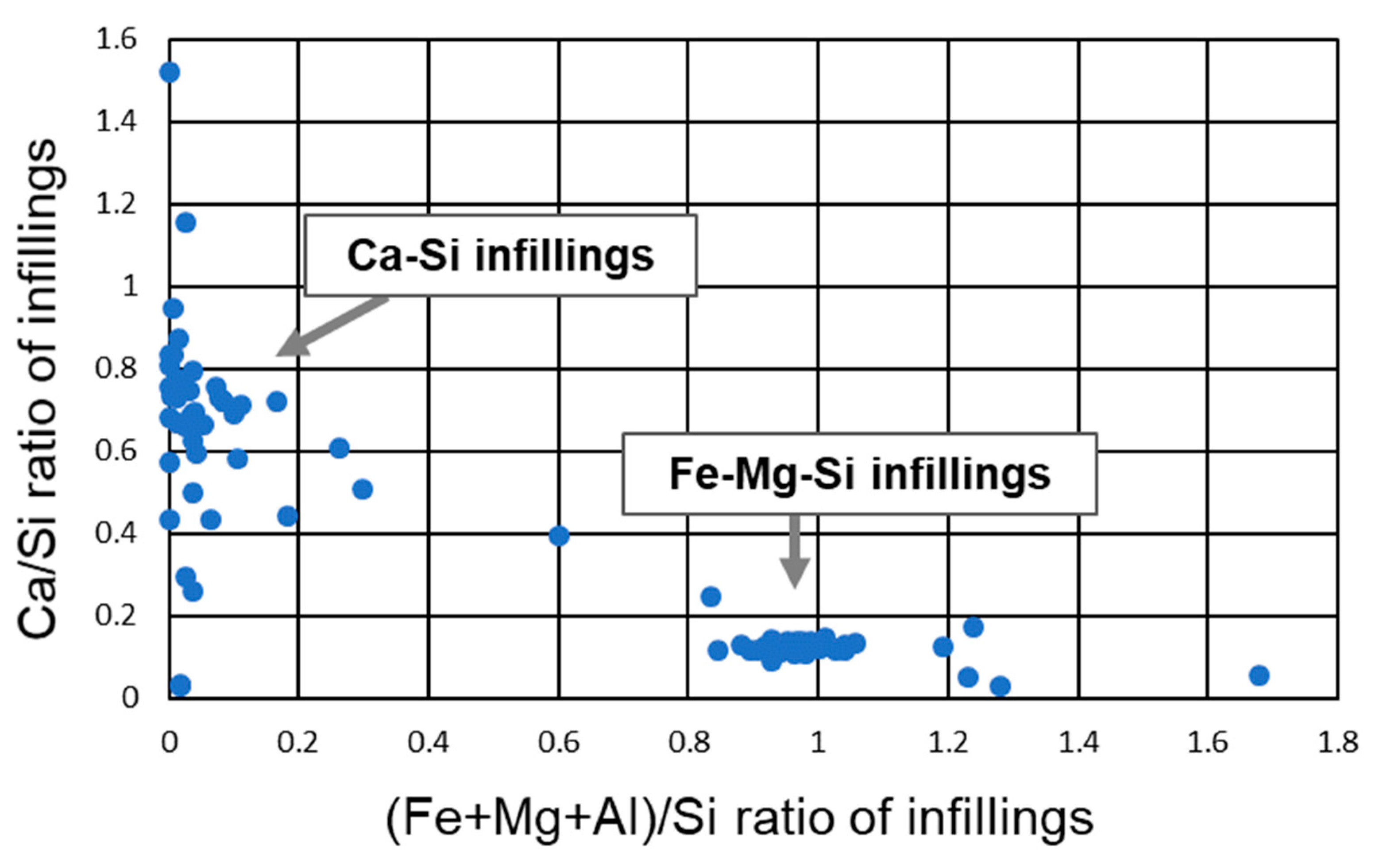
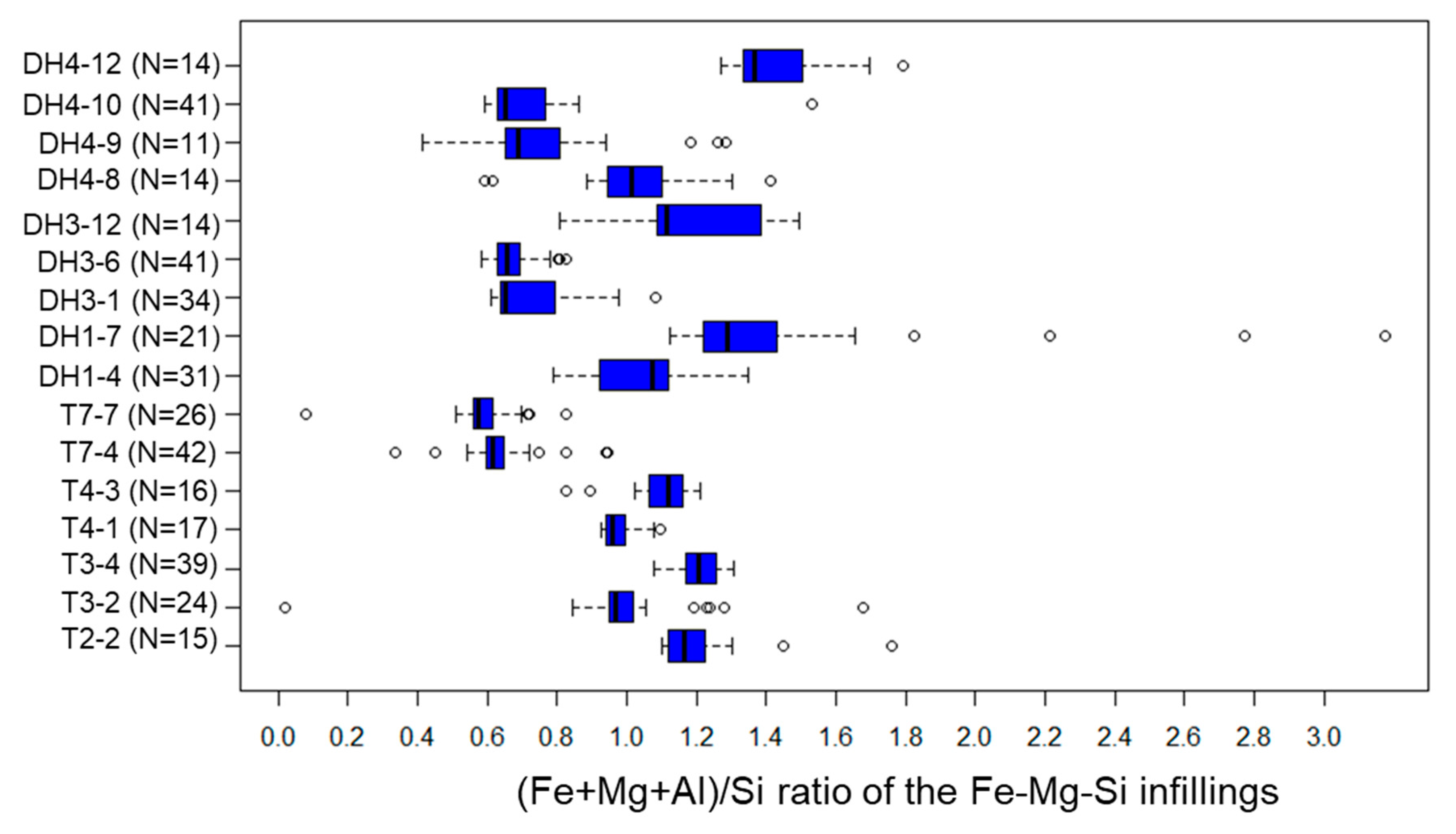
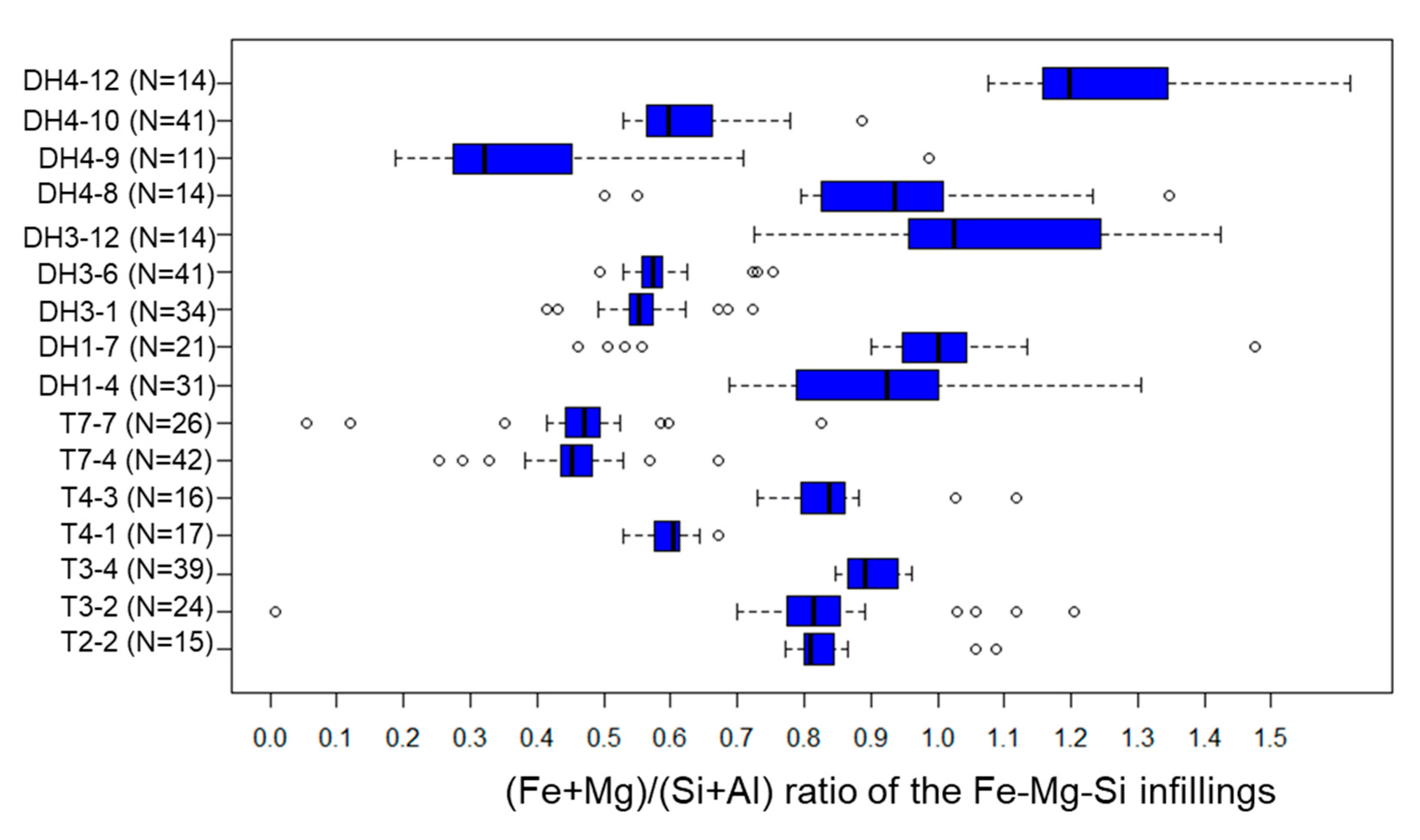
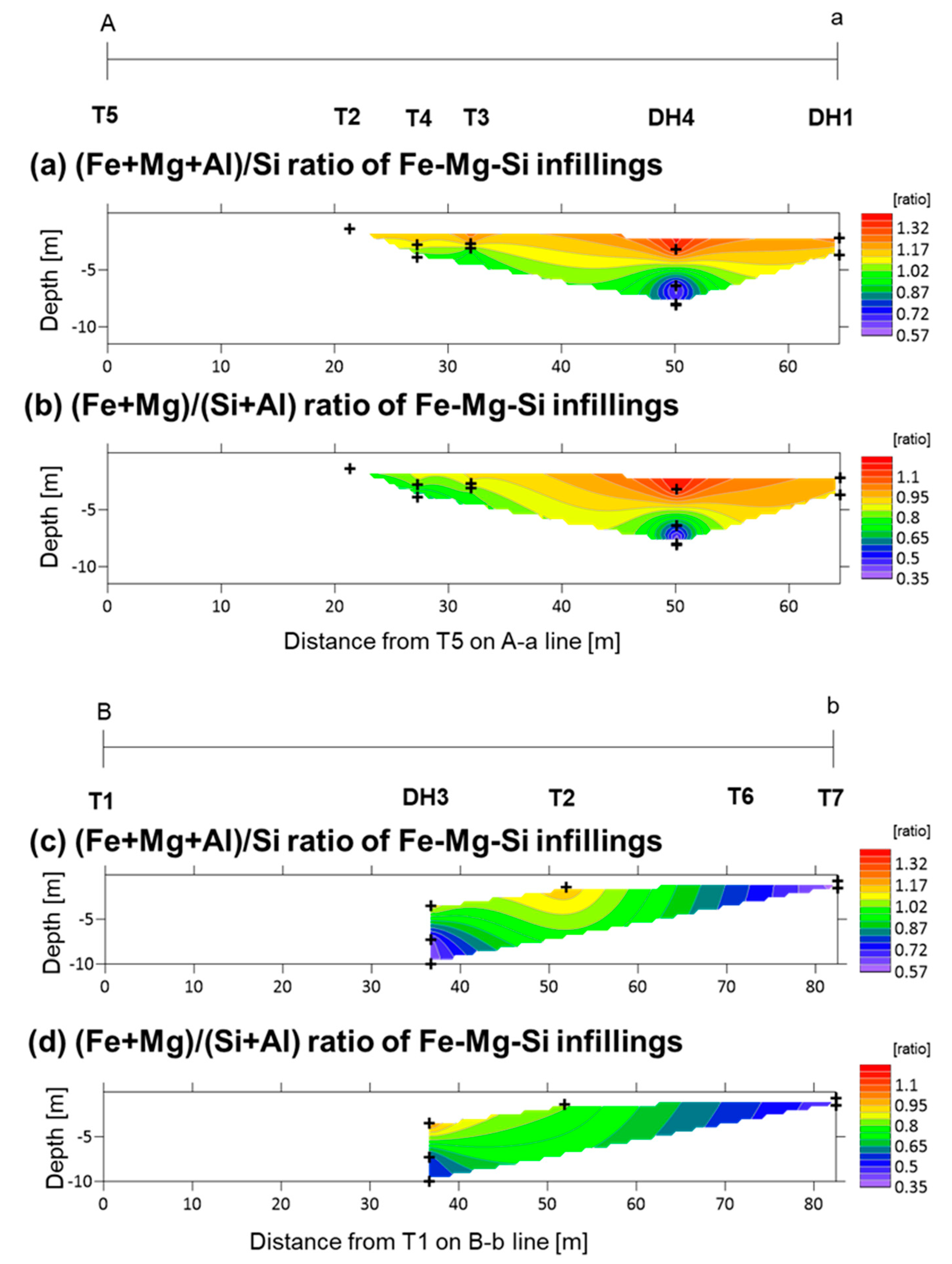
5.3. Fe-Mg-Si Infillings
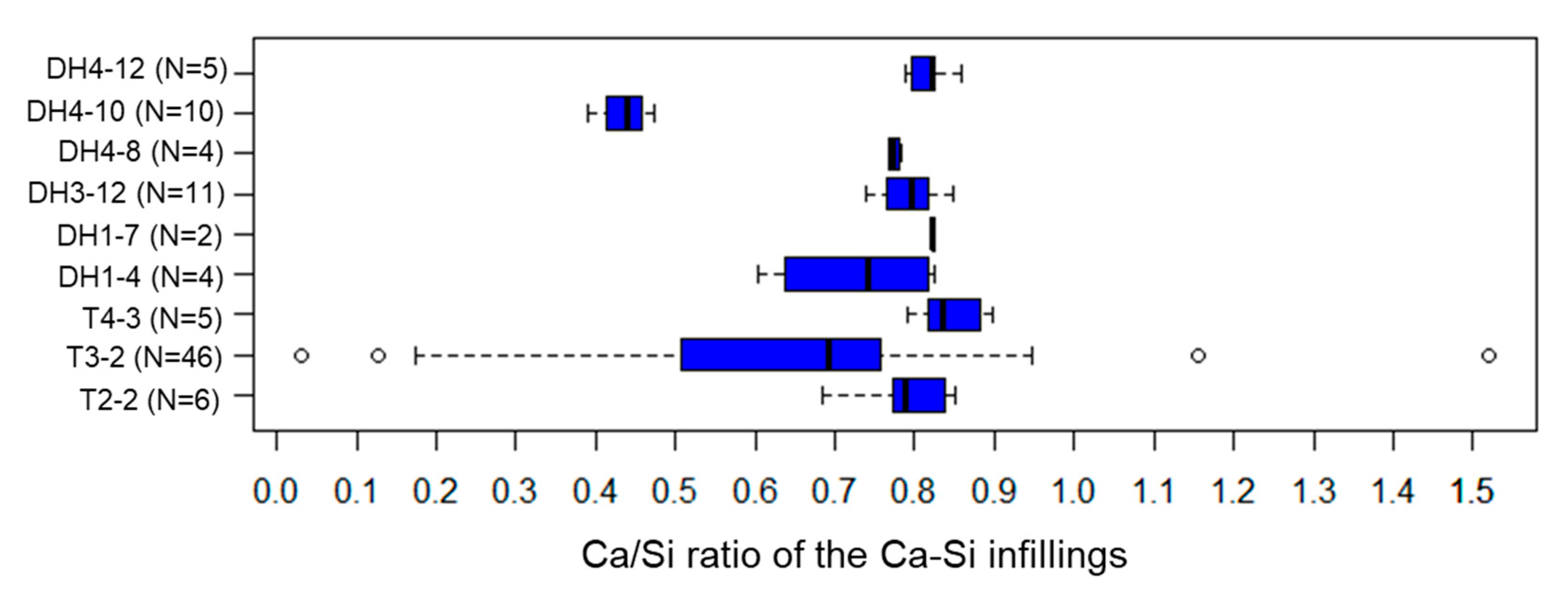
5.4. Ca-Si Infillings
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Minerals | Mineral Species in Database | Chemical Composition |
|---|---|---|
| Serpentine | Lizardite | Mg3Si2O5(OH)4 |
| Chrysotile | Mg3Si2O5(OH)4 | |
| Olivine | Forsterite | Mg2SiO4 |
| Fayalite | Fe2SiO4 | |
| Orthopyroxene | Enstatite, alpha | MgSiO3 |
| Ferrosilite, alpha | FeSiO3 | |
| Clinopyroxene | Diopside | CaMg(SiO3)2 |
| Amphibole | Tremolite | (Ca2Mg5)Si8O22(OH)2 |
| Ferrotremolite | (Ca2Fe5)Si8O22(OH)2 | |
| Pargasite | Na(Ca2Mg4Al)(Al2Si6)O22(OH)2 | |
| Ferropargasite | Na(Ca2Fe4Al)(Al2Si6)O22(OH)2 | |
| Cummingtonite | Mg7Si8O22(OH)2 | |
| Anthophyllite | Mg7Si8O22(OH)2 | |
| Talc | Talc | Mg3Si4O10(OH)2 |
| Chlorite | Clinochlore | Mg5Al(AlSi3)O10(OH)8 |
| Plagioclase | Anorthite | Ca(Al2Si2)O8 |
| Quartz | Quartz, alpha | SiO2 |
| Brucite | Brucite | Mg(OH)2 |
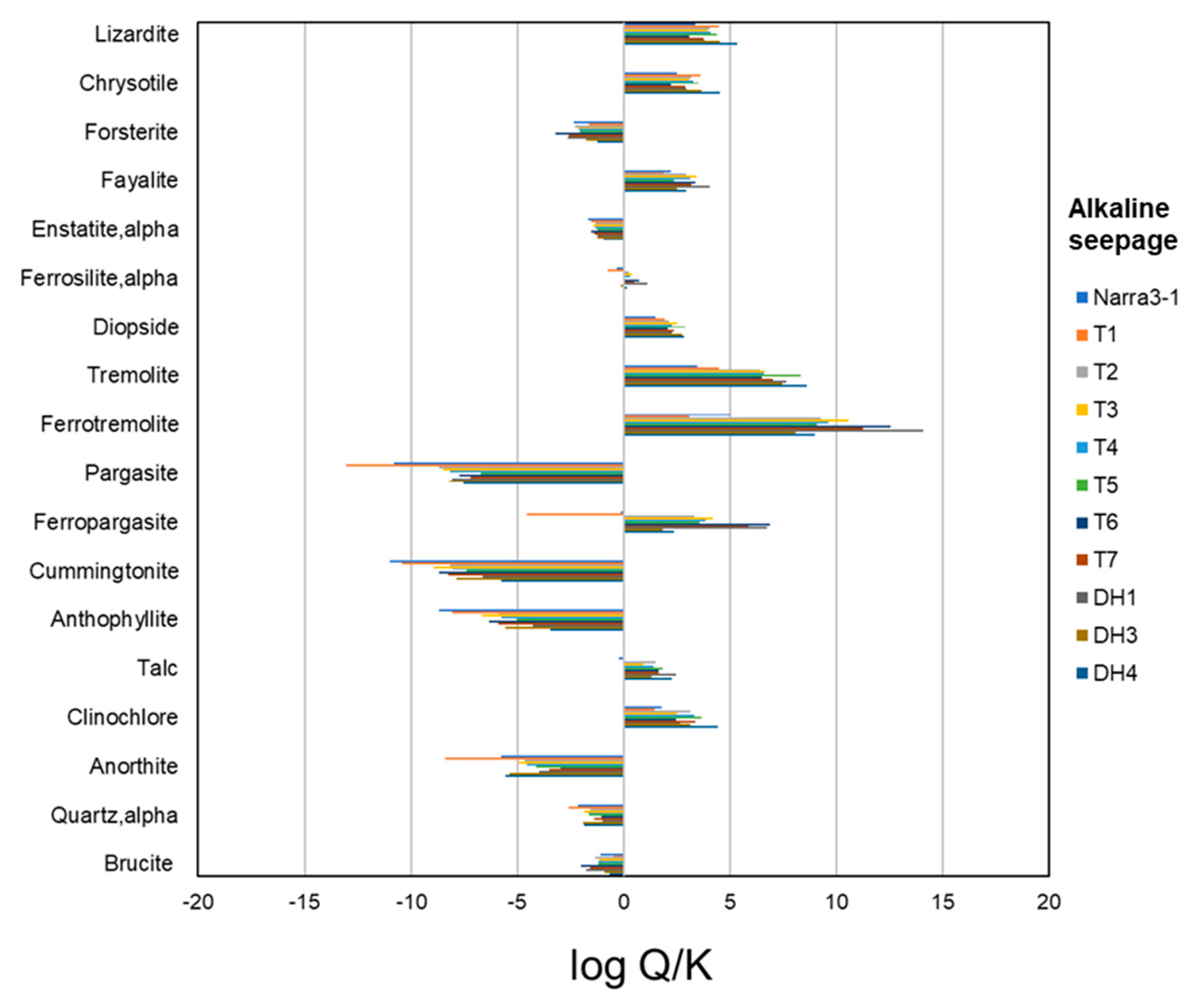
References
- Atkinson, A. The Time Dependence of pH within a Repository for Radioactive Waste Desposal; UKAEA: Harwell, UK, 1985.
- Nakayama, S.; Sakamoto, Y.; Yamaguchi, T.; Akai, M.; Tanaka, T.; Sato, T.; Iida, Y. Dissolution of montmorillonite in compacted bentonite by highly alkaline aqueous solutions and diffusivity of hydroxide ions. Appl. Clay Sci. 2004, 27, 53–65. [Google Scholar] [CrossRef]
- Rozalén, M.L.; Huertas, F.J.; Brady, P.V.; Cama, J.; García-Palma, S.; Linares, J. Experimental study of the effect of pH on the kinetics of montmorillonite dissolution at 25 °C. Geochim. Cosmochim. Acta 2008, 72, 4224–4253. [Google Scholar] [CrossRef]
- Taubald, H.; Bauer, A.; Schäfer, T.; Geckeis, H.; Satir, M.; Kim, J.I. Experimental investigation of the effect of high-pH solutions on the Opalinus Shale and the Hammerschmiede Smectite. Clay Miner. 2000, 35, 515–524. [Google Scholar] [CrossRef]
- Barnes, I.; O’Neil, J.R. Present day serpentinization in New Caledonia, Oman and Yugoslavia. Geochim. Cosmochim. Acta 1978, 42, 144–145. Available online: http://horizon.documentation.ird.fr/exl-doc/pleins_textes/pleins_textes_5/b_fdi_08-09/09131.pdf (accessed on 20 April 2020). [CrossRef]
- Bruni, J.; Canepa, M.; Chiodini, G.; Cioni, R.; Cipolli, F.; Longinelli, A.; Marini, L.; Ottonello, G.; Zuccolini, M.V. Irreversible water-rock mass transfer accompanying the generation of the neutral, Mg–HCO3 and high-pH, Ca–OH spring waters of the Genova province, Italy. Appl. Geochem. 2002, 17, 455–474. [Google Scholar] [CrossRef]
- Fujii, N.; Yamakawa, M.; Shikazono, N.; Sato, T. Geochemical and mineralogical characterizations of bentonite interacted with alkaline fluids generating in Zambales Ophiolite, Northwestern Luzons, Philippines. Geol. Soc. Jpn. 2014, 120, 361–375. [Google Scholar] [CrossRef]
- Milodowski, A.E.; Norris, S.; Alexander, W.R. Minimal alteration of montmorillonite following long-term interaction with natural alkaline groundwater: Implications for geological disposal of radioactive waste. Appl. Geochem. 2016, 66, 184–197. [Google Scholar] [CrossRef]
- Nishiki, Y.; Sato, T.; Katoh, T.; Otake, T.; Kikuchi, R. Precipitation of magnesium silicate hydrates in natural alkaline surface environments. Clay Sci. 2020, 24, 1–13. [Google Scholar] [CrossRef]
- Furquim, S.A.C.; Barbiéro, L.; Graham, R.C.; de Queiroz Neto, J.P.; Ferreira, R.P.D.; Furian, S. Neoformation of micas in soils surrounding an alkaline-saline lake of Pantanal wetland, Brazil. Geoderma 2010, 158, 331–342. [Google Scholar] [CrossRef]
- Hay, R.L.; Guldman, S.G.; Matthews, J.C.; Lander, R.H.; Duffin, M.E.; Kyser, T.K. Clay mineral diagenesis in core KM-3 of Searles Lake, California. Clays Clay Miner. 1991, 39, 84–96. [Google Scholar] [CrossRef]
- Shimbashi, M.; Sato, T.; Yamakawa, M.; Fujii, N.; Otake, T. Formation of Fe-and Mg-rich smectite under hyperalkaline conditions at narra in Palawan, the Philippines. Minerals 2018, 8, 155. [Google Scholar] [CrossRef]
- Aurelio, M.A.; Forbes, M.T.; Taguibao, K.J.L.; Savella, R.B.; Bacud, J.A.; Franke, D.; Pubellier, M.; Savva, D.; Meresse, F.; Steuer, S.; et al. Middle to Late Cenozoic tectonic events in south and central Palawan (Philippines) and their implications to the evolution of the south-eastern margin of South China Sea: Evidence from onshore structural and offshore seismic data. Mar. Pet. Geol. 2014, 58, 658–673. [Google Scholar] [CrossRef]
- RWMC. Advancement of Processing and Disposal Technique for the Geological Disposal of TRU Waste (FY2015). 2016. Available online: https://www.enecho.meti.go.jp/category/electricity_and_gas/nuclear/rw/library/library06.html (accessed on 20 April 2020).
- RWMC. Advancement of Processing and Disposal Technique for the Geological Disposal of TRU Waste (FY2016). 2017. Available online: https://www.enecho.meti.go.jp/category/electricity_and_gas/nuclear/rw/library/library06.html (accessed on 20 April 2020).
- RWMC. Advancement of Processing and Disposal Technique for the Geological Disposal of TRU Waste (FY2017). 2018. Available online: https://www.enecho.meti.go.jp/category/electricity_and_gas/nuclear/rw/library/library06.html (accessed on 20 April 2020).
- García Calvo, J.L.; Hidalgo, A.; Alonso, C.; Fernández Luco, L. Development of low-pH cementitious materials for HLRW repositories: Resistance against ground waters aggression. Cem. Concr. Res. 2010, 40, 1290–1297. [Google Scholar] [CrossRef]
- Degens, E.T. Biogeochemistry of Stable Carbon Isotopes. In Organic Geochemistry; Springer: Berlin/Heidelberg, Germany, 1969. [Google Scholar]
- Shimbashi, M.; Yokoyama, S.; Watanabe, Y.; Fujii, N.; Yamakawa, M.; Sato, T. Mineralogical evolution of Fe-Mg-Si phases under low-temperature, alkaline conditions at Narra in Palawan, Philippines. In Proceedings of the NAWG 16th Workshop, Yamagata, Japan, 15 October 2019. [Google Scholar]
- Appelo, C.A.J. Cation and proton exchange, pH variations, and carbonate reactions in a freshening aquifer. Water Resour. Res. 1994, 30, 2793–2805. [Google Scholar] [CrossRef]
- Bonaccorsi, E.; Merlino, S.; Kampf, A.R. The crystal structure of tobermorite 14 Å (plombierite), a C-S-H phase. J. Am. Ceram. Soc. 2005, 88, 505–512. [Google Scholar] [CrossRef]
| Sample | Depth [m] | 14C Age [14C yr BP] | Intensity of Broad 14 Å d-Spacing | d(001) after EG Treatment [Å] | d(060) [Å] | d(001) during Heating at 250 °C [Å] | Type |
|---|---|---|---|---|---|---|---|
| DH1-7 | 2.2 | 4520 | low | 15.9 | 1.53 | 14.6 | Type-I |
| T4-3 | 2.8 | 5060 | middle | 16.4 | 1.53 | 13.6 + 10.1 | Type-II |
| DH1-4 | 3.7 | 9010 | middle | 16.7 | 1.54 | 15.3 + 9.9 | Type-II |
| T4-1 | 3.9 | 6300 | middle | 16.7 | 1.54 + 1.52 | 14.7 + 10.0 | Type-II + Type-III |
| DH4-9 | 8 | 14,110 | high | 17.0 | 1.51 | 15.1 + 9.9 | Type-III |
| DH3-1 | 10 | 15,000 | high | 17.0 | 1.51 | 14.9 + 9.9 | Type-III |
| T7-7 | 0.7 | 4010 | middle–high | 17.0 | 1.51 | 14.6 + 10.1 | Type-III |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimbashi, M.; Yokoyama, S.; Watanabe, Y.; Sato, T.; Otake, T.; Kikuchi, R.; Yamakawa, M.; Fujii, N. Formation of Natural Silicate Hydrates by the Interaction of Alkaline Seepage and Sediments Derived from Serpentinized Ultramafic Rocks at Narra, Palawan, the Philippines. Minerals 2020, 10, 719. https://doi.org/10.3390/min10080719
Shimbashi M, Yokoyama S, Watanabe Y, Sato T, Otake T, Kikuchi R, Yamakawa M, Fujii N. Formation of Natural Silicate Hydrates by the Interaction of Alkaline Seepage and Sediments Derived from Serpentinized Ultramafic Rocks at Narra, Palawan, the Philippines. Minerals. 2020; 10(8):719. https://doi.org/10.3390/min10080719
Chicago/Turabian StyleShimbashi, Misato, Shingo Yokoyama, Yasutaka Watanabe, Tsutomu Sato, Tsubasa Otake, Ryosuke Kikuchi, Minoru Yamakawa, and Naoki Fujii. 2020. "Formation of Natural Silicate Hydrates by the Interaction of Alkaline Seepage and Sediments Derived from Serpentinized Ultramafic Rocks at Narra, Palawan, the Philippines" Minerals 10, no. 8: 719. https://doi.org/10.3390/min10080719
APA StyleShimbashi, M., Yokoyama, S., Watanabe, Y., Sato, T., Otake, T., Kikuchi, R., Yamakawa, M., & Fujii, N. (2020). Formation of Natural Silicate Hydrates by the Interaction of Alkaline Seepage and Sediments Derived from Serpentinized Ultramafic Rocks at Narra, Palawan, the Philippines. Minerals, 10(8), 719. https://doi.org/10.3390/min10080719






