The Occurrence of Authigenic Clay Minerals in Alkaline-Saline Lakes, Pantanal Wetland (Nhecolândia Region, Brazil)
Abstract
1. Introduction
2. Geological Setting
3. Materials and Methods
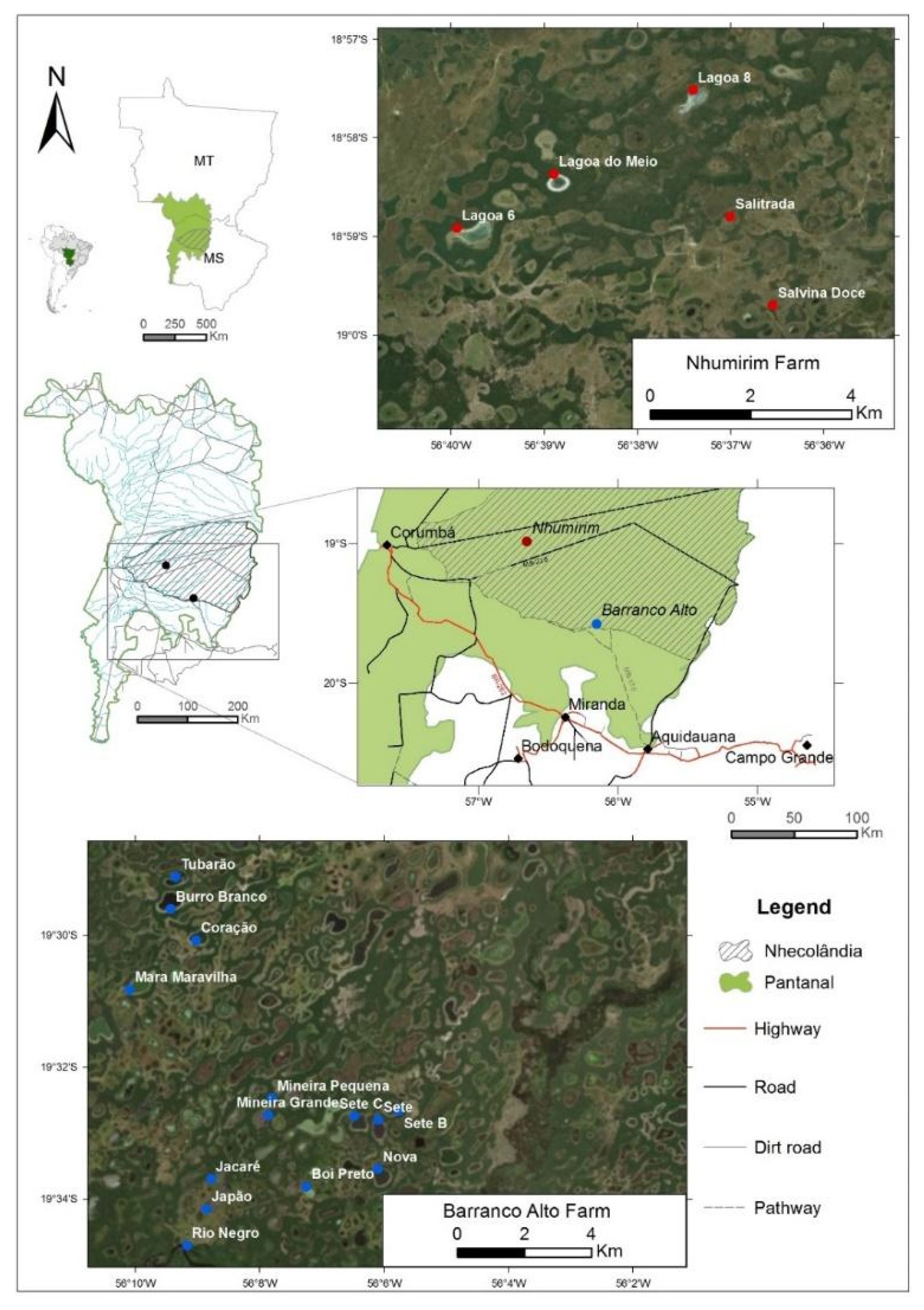
3.1. Study Area and Sampling
3.2. Water Analyses
3.3. Mineralogical Analyses
4. Results
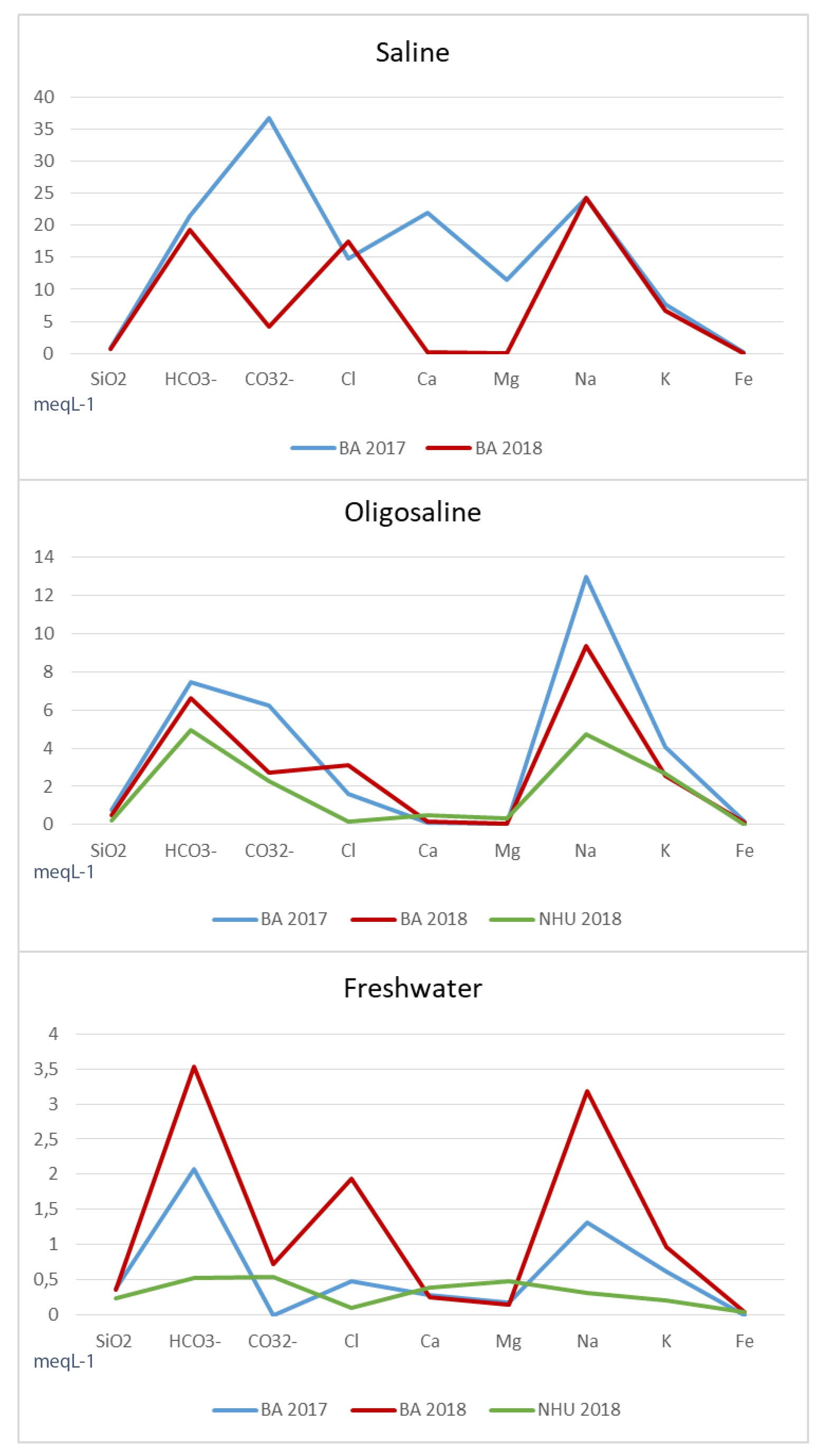
4.1. Water Chemistry
4.2. Occurrence of Microbial Mats
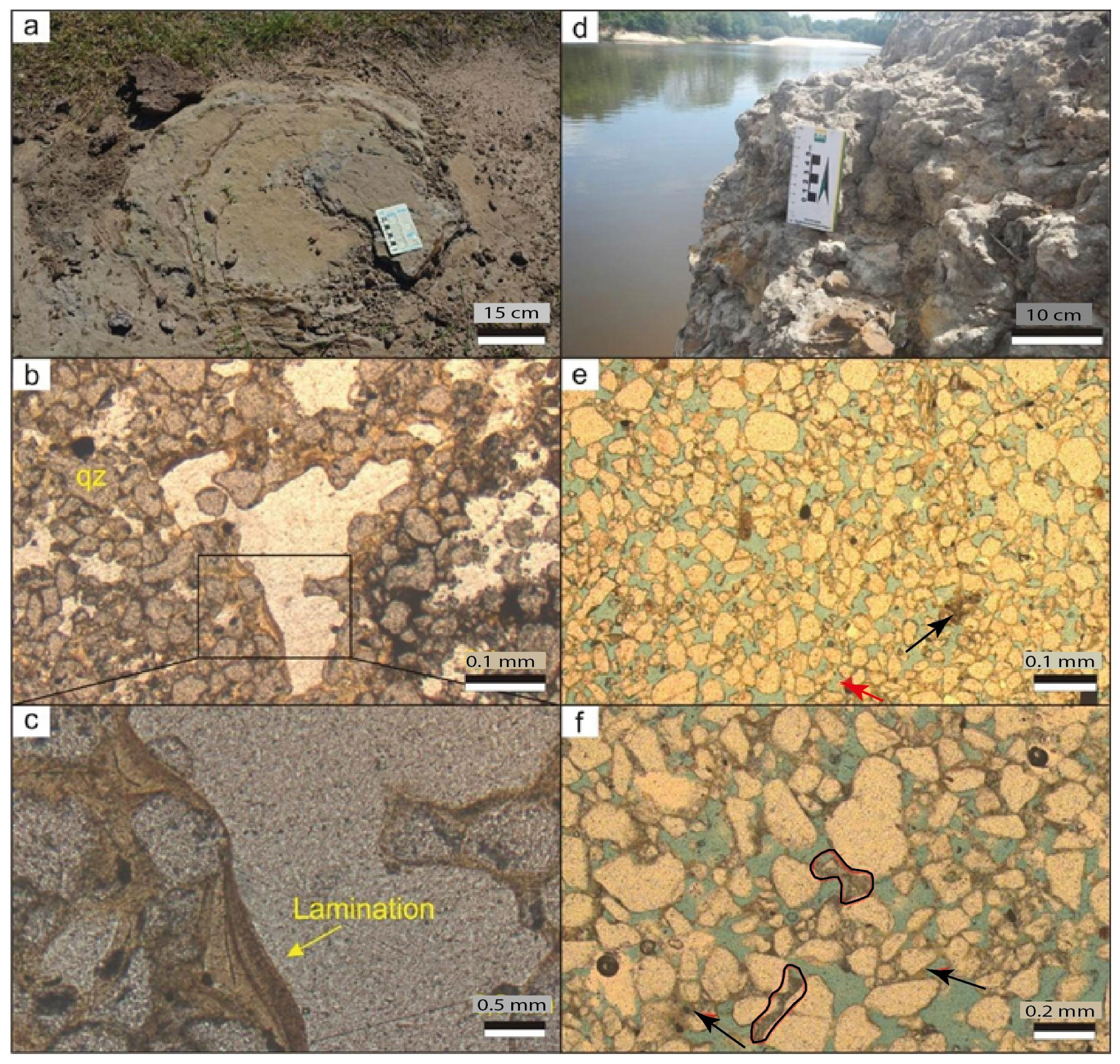
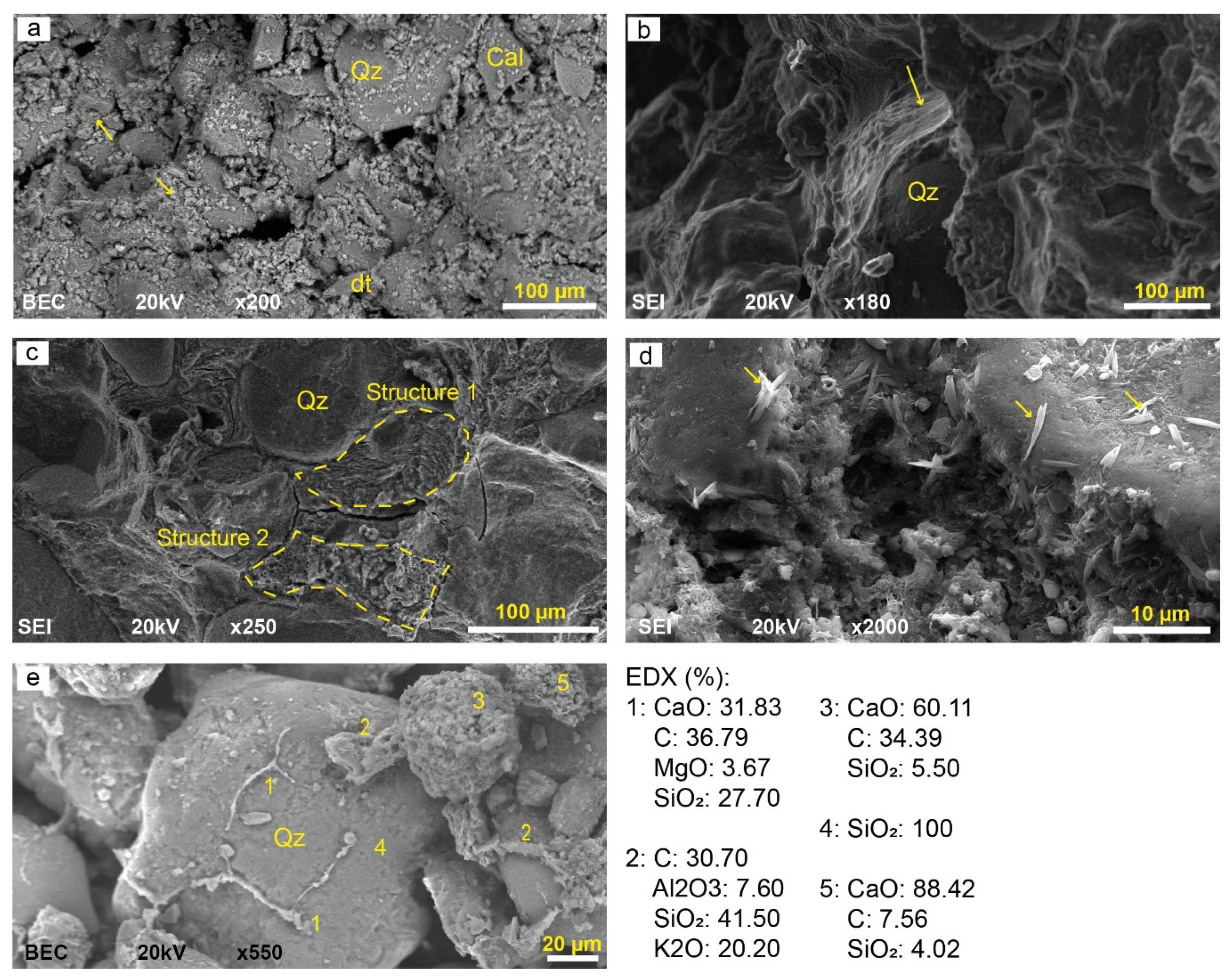
4.3. Petrographic Characterization
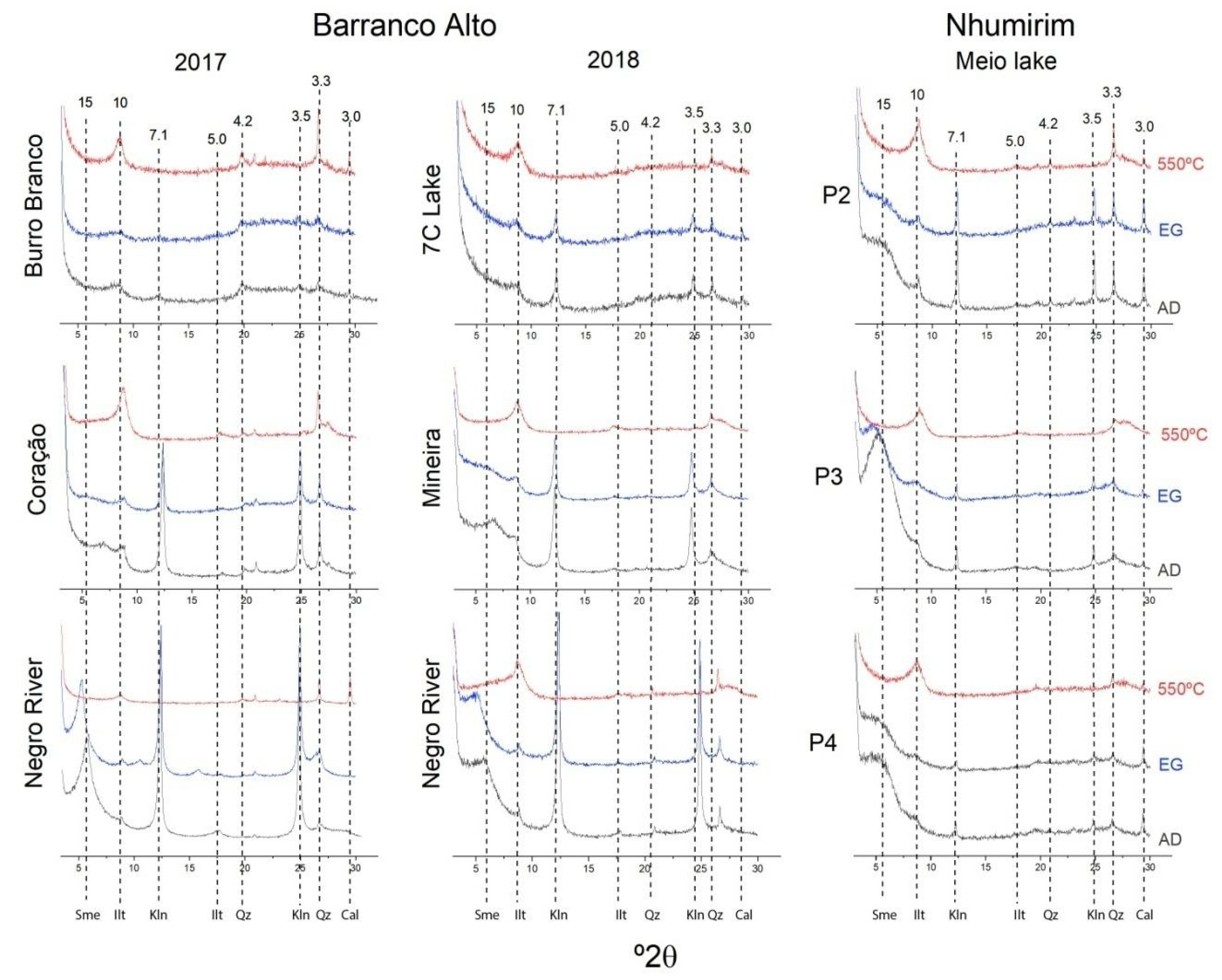
4.4. Mineral Characterization
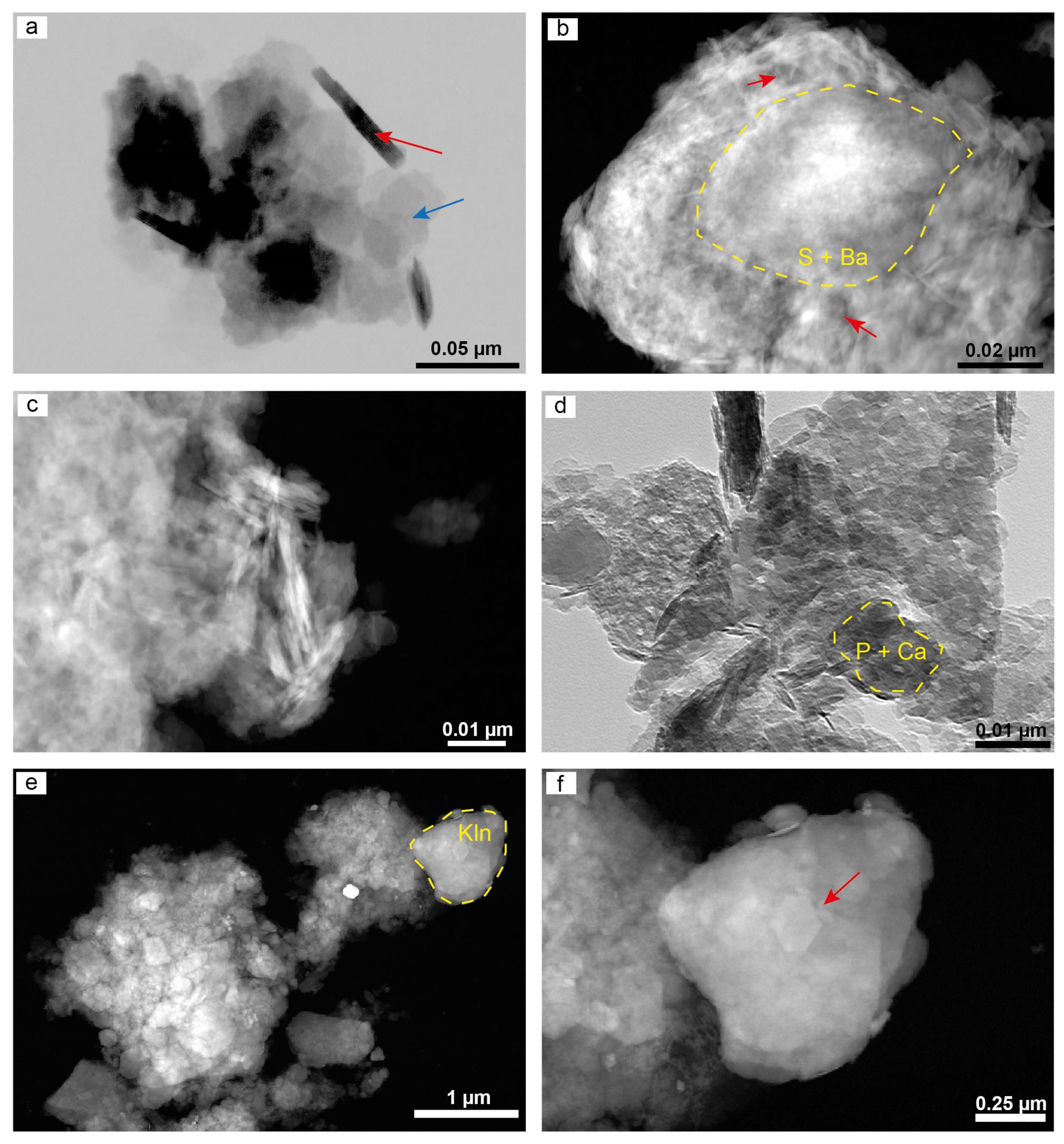
4.5. Microstructure of the Clay Minerals
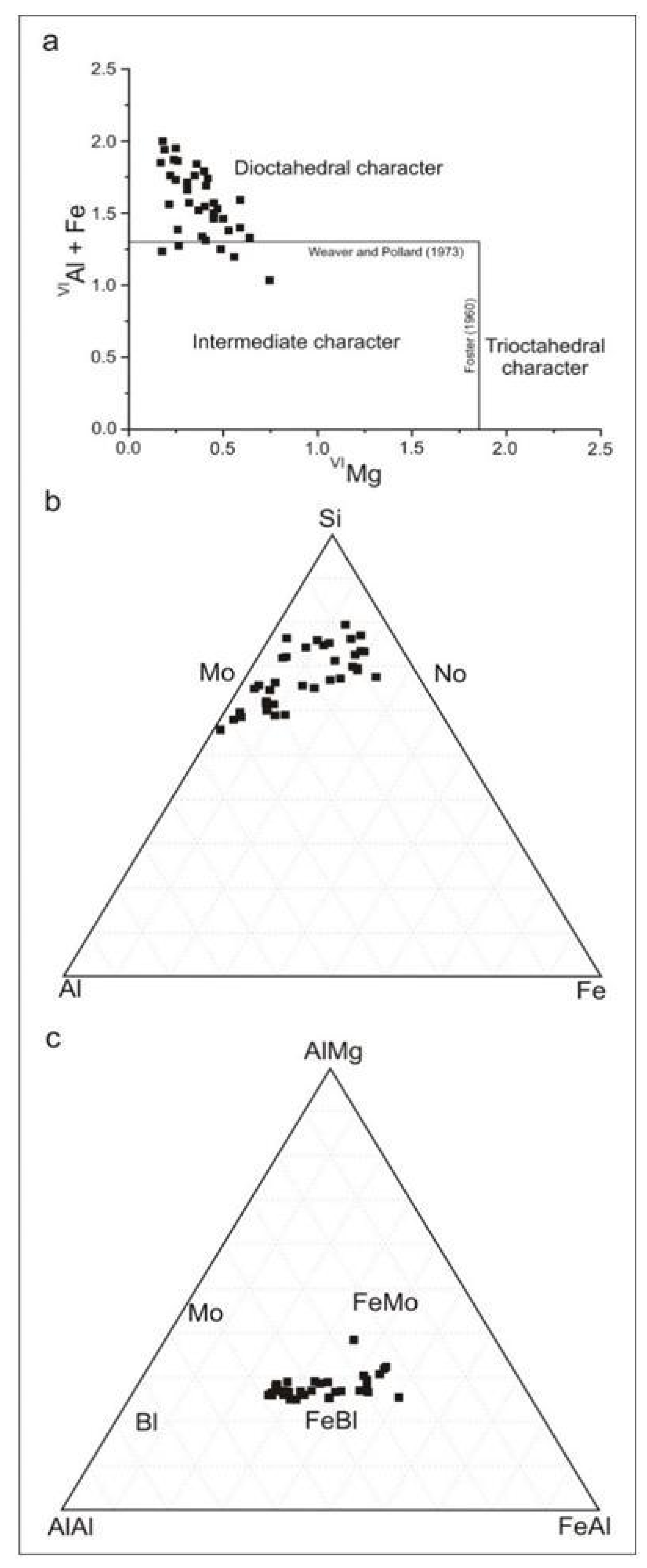
4.6. Chemical Composition and Structural Formulae of the Clay Minerals
5. Discussion
5.1. The Distinct Clay Minerals from Pantanal
5.2. A Primary Precursor as a Responsible for the Precipitation of Clay Minerals Associated with Calcite
5.3. Genesis of di and Trioctahedral Clay Minerals
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Galán, E. Genesis of Clay Minerals. In Handbook of Clay Science, 1st ed.; Bergaya, F., Theng, B.K.G., Lagaly, G., Eds.; Elsevier: Oxford, UK, 2006; pp. 1129–1162. [Google Scholar]
- Meunier, A. Clays; Springe: Oxford, UK, 2005; p. 472. [Google Scholar]
- Galán, E.; Pozo, M. Palygorskite and Sepiolite deposits in Continental Environments: Description, genetic patterns and sedimentary settings. In Developments in Clay Science; Galán, E., Singer, A., Eds.; Elsevier: Oxford, UK, 2011; Volume 3, pp. 125–173. [Google Scholar]
- Faure, G. Principles and Applications of Geochemistry: A Comprehensive Textbook for Geology Students, 2nd ed.; Prentice Hall: New Jersey, NJ, USA, 1998; p. 600. [Google Scholar]
- Tosca, N.J.; Masterson, A.L. Chemical controls on incipient Mg-silicate crystallization at 25 °C: Implications for early and late diagenesis. Clay Miner. 2014, 49, 165–194. [Google Scholar] [CrossRef]
- Calvo, J.P.; Blanc-Valleron, M.M.; Rodríguez-Arandía, J.P.; Rouchy, J.M.; Sanz, M.E. Authigenic clay minerals in continental evaporitic environments. In Palaeoweathering, Palaeosurfaces and Related Continental Deposits; Thiry, M., Simon-Coinçon, R., Eds.; Springer: Oxford, UK, 1999; pp. 129–151. [Google Scholar]
- Furquim, S.A.C.; Graham, R.C.; Barbiero, L.; Queiroz Neto, J.P.; Vallès, V. Mineralogy and Genesis of smectites in an alkaline-saline environment of Pantanal wetland, Brazil. Clays Clay Miner. 2008, 56, 579–595. [Google Scholar] [CrossRef]
- Deocampo, D.M. Authigenic clay minerals in lacustrine mudstones. In Paying Attention to Mudrocks: Priceless; Egenhoff, S., Larsen, D., Fishman, N., Eds.; Geological Society of America Special Paper 515: Colorado, CO, USA, 2015; pp. 49–64. [Google Scholar]
- García-Romero, E.; MaríaManchado, E.; Suárez, M.; García-Rivas, J. Spanish Bentonites: A Review and New Data on Their Geology, Mineralogy, and Crystal Chemistry. Minerals 2019, 9, 696. [Google Scholar] [CrossRef]
- Darragi, F.; Tardy, Y. Authigenic trioctahedral smectites controlling pH, alkalinity, silica and magnesium concentrations in alkaline lakes. Chem. Geol. 1987, 63, 59–72. [Google Scholar] [CrossRef]
- Furquim, S.A.C. Formação de Carbonatos e Argilominerais em Solos Sódicos do Pantanal Sul Mato-Grossense. Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 2007. [Google Scholar]
- Assine, M.L. Sedimentação na Bacia do Pantanal Mato-Grossense, Centro-Oeste do Brasil. Ph.D. Thesis, Universidade Estadual Paulista, Rio Claro, Brazil, 2003. [Google Scholar]
- Barbiéro, L.; Queiroz Neto, J.P.; Ciornei, G.; Sakamoto, A.Y.; Capellari, B.; Fernandes, E.; Valles, V. Geochemistry of water and ground water in the Nhecolândia, Pantanal of Mato Grosso, Brazil: Variability and associated processes. Wetlands 2002, 22, 528–540. [Google Scholar] [CrossRef]
- Barbiero, L.; Berger, G.; Rezende Filho, A.T.; Meunier, F.; Martins-Silva, E.R.; Furian, S. Organic Control of Dioctahedral and Trioctahedral clay formation in an Alkaline Soil System in the Pantanal Wetland of Nhecolândia, Brazil. PLoS ONE 2016, 11, e0159972. [Google Scholar] [CrossRef]
- Warren, L.V.; Quaglio, F.; Simões, M.G.; Freitas, B.T.; Assine, M.L.; Riccomini, C. Underneath the Pantanal Wetland: A Deep-Time history of Gondwana assembly, climate change, and the dawn of Metazoan Life. In Dynamics of the Pantanal Wetland in South America. The Handbook of Environmental Chemistry; Bergier, I., Assine, M.L., Eds.; Springer: Oxford, UK, 2015; Volume 37, pp. 1–21. [Google Scholar]
- Horton, B.K.; DeCelles, P.G. The modern foreland basin system adjacent to the Central Andes. Geology 1997, 25, 895–898. [Google Scholar] [CrossRef]
- Ussami, N.; Shiraiwa, S.; Domingues, J.M.L. Basement reactivation in a sub-Andean foreland flexural bulge: The Pantanal wetland, SW Brazil. Tectonics 1999, 18, 25–39. [Google Scholar] [CrossRef]
- Almeida, T.I.R.; Sígolo, J.B.; Fernandes, E.; Queiroz Neto, J.P.; Barbiero, L.; Sakamoto, A.Y. Proposta de classificação e gênese das lagoas da Baixa Nhecolândia-MS com base em sensoriamento remoto e dados de campo. Braz. J. Geol. 2003, 33, 83–90. [Google Scholar] [CrossRef]
- Assine, M.L.; Soares, P.C. Quaternary of the Pantanal, wet-central Brazil. Quat. Int. 2004, 114, 23–34. [Google Scholar] [CrossRef]
- Almeida, T.I.R.; Calijuri, M.C.; Falco, P.B.; Casali, S.P.; Kupriyanova, E.; Paranhos Filho, A.C.; Sígolo, J.B.; Bertolo, R.A. Biogeochemical process and the diversity of Nhecolândia lakes, Brazil. An. Acad. Bras. Ciênc. 2011, 83, 391–407. [Google Scholar] [CrossRef] [PubMed]
- Almeida, T.I.R.; Fernandes, E.; Mendes, D.; Branco, F.C.; Sígolo, J.B. Distribuição espacial de diferentes classes de lagoas no Pantanal da Nhecolândia, MS, a partir de dados vetoriais e SRTM: Uma contribuição ao estudo de sua compartimentação e gênese. IGc USP 2007, 7, 95–107. [Google Scholar] [CrossRef]
- Soares, A.P.; Soares, P.C.; Assine, M.L. Areiais e lagoas do Pantanal, Brasil: Herança paleoclimática? Braz. J. Geol. 2003, 33, 211–224. [Google Scholar]
- Almeida, T.I.R.; Karmann, I.; Paranhos Filho, A.C.; Sígolo, J.B.; Bertolo, R.A. Os diferentes graus de isolamento da água subterrânea como origem de sua variabilidade: Evidências isotópicas, hidroquímicas e da variação sazonal do nível da água no Pantanal da Nhecolândia. IGc USP 2010, 10, 37–47. [Google Scholar] [CrossRef]
- Almeida, T.I.R.; Calijuri, M.C.; Falco, P.B.; Casali, S.P.; Paranhos Filho, A.C.; Queiroz, L.A. A atividade biogeoquímica como agente da diversidade das lagoas salino-alcalinos e hipossalinos do Pantanal da Nhecolândia, Brasil. In Anais 2° Simpósio de Geotecnologias no Pantanal; Embrapa Informática Agropecuária/INPE: Corumbá, Brazil, 2009; pp. 31–40. [Google Scholar]
- Bergier, I.; Krusche, A.; Guérin, F. Alkaline Lake Dynamics in the Nhecolândia Landscape. In Dynamics of the Pantanal Wetland in South America—The Handbook of Environmental Chemistry; Bergier, I., Assine, M.L., Eds.; Springer: São Paulo, Brazil, 2015; Volume 37, pp. 145–162. [Google Scholar]
- Conselho Nacional do Meio Ambiente (CONAMA). Dispõe Sobre a Classificação dos Corpos de Água e Diretrizes Ambientais para o seu Enquadramento, bem como estabelece as Condições e Padrões de Lançamento de Efluentes, e dá outras providências; Diário Oficial da União (DOU): Brasília, Brazil, 2005.
- Dias, I.A. Mineralogia e Geoquímica de Argilominerais da Nhecolândia—Pantanal Sul-Mato-Grossense. Master Thesis, Universidade Federal do Paraná, Curitiba, Brazil, 2019. [Google Scholar]
- Moore, D.M.; Reynolds, R.C. X-Ray Diffraction and Identification and Analysis of Clay Minerals, 2nd ed.; Oxford University Press: New York, NY, USA, 1997; p. 378. [Google Scholar]
- Zhang, F.; Xu, H.; Konishi, H.; Roden, E.E. A relationship between d104 value and composition in the calcite-disordered dolomite solid-solution series. Am. Mineral. 2010, 95, 1650–1656. [Google Scholar] [CrossRef]
- Welton, J.E. SEM Petrology Atlas: Methods in Exploration Series, 2nd ed.; Amer Assn of Petroleum Geologists: Tulsa, OK, USA, 2003; p. 240. [Google Scholar]
- Buey, C.S.; Barrios, M.S.; Romero, E.G.; Diaz, M.C.D.; Montoya, M.D. Electron microscopic study of the illite-smectite transformation in the bentonites from Cerro del Aguila (Toledo, Spain). Clay Miner. 1998, 33, 501–510. [Google Scholar]
- Sánchez-Navas, A.; Martín-Algarra, A.; Nieto, F. Bacterially-Mediated authigenesis of clays in phosphate stromatolites. Sedimentology 1998, 45, 519–533. [Google Scholar] [CrossRef]
- Singh, B.; Gilkes, R.J. Application of analytical transmission electron microscopy to identifying intercrystal variations in the composition of Clay Minerals. Analyst 1995, 120, 1335–1339. [Google Scholar] [CrossRef]
- Lorimer, G.W.; Cliff, J.N. Clark: Determination of the thickness and spatial resolution for the quantitative analysis of thin foils. In Developments in Electron Microscopy and Analysis; Venables, J.A., Ed.; Academic: London, UK, 1976; p. 153. [Google Scholar]
- Poppe, L.J.; Paskevich, V.F.; Hathaway, J.C.; Blackwood, D.S. A laboratory Manual for X-Ray Powder Diffraction; U.S. Geological Survey: Virginia, VA, USA, 2001; p. 88.
- Mas, A.; Guisseau, D.; Patrier Mas, P.; Beaufort, D.; Genter, A.; Sanjuan, B.; Girard, J.P. Clay Minerals related to the hydrothermal activity of the Bouillante geothermal field (Guadeloupe). J. Volcanol. Geotherm. Res. 2006, 158, 380–400. [Google Scholar] [CrossRef]
- Güven, N. Smectites. In Hydrous Phyllosilicates; Bailey, S.W., Ed.; Reviews in Mineralogy: Washington, DC, USA, 1988; Volume 19, pp. 497–559. [Google Scholar]
- Nieto, F.; Ortega-Huertas, M.N.; Peacor, D.R.; Arostegui, J. Evolution of illite/smectite from early diagenesis through incipient metamorphism in sediments of the Basque-Cantabrian Basin. Clays Clay Miner. 1996, 44, 304–323. [Google Scholar] [CrossRef]
- Cuadros, J.; Altaner, S.P. Characterization of mixed-layer illite-smectite from bentonites using microscopic, chemical, and X-Ray methods: Constraints on the smectite-to-illite transformation mechanism. Am. Mineral. 1998, 83, 762–774. [Google Scholar] [CrossRef]
- Alt, J.C.; Laverne, C.; Muehlenbachs, K. Alteration of the Upper Oceanic Crust: Mineralogy and Processes in Deep Sea drilling project hole 504B, LEG 83. In Initial Reports of the Deep Sea Drilling Project; Anderson, R.N., Honnorez, J., Adamson, A.C., Alt, J.C., Emmermann, R., Kempton, P.D., Kinoshita, H., Laverne, C., Mottl, M.J., Newmark, R., et al., Eds.; U.S. Govt. Printing Office: Washington, DC, USA, 1985; pp. 217–247. [Google Scholar]
- Weaver, C.E.; Pollard, L.D. The Chemistry of Clay Minerals; Elsevier: Amsterdam, The Netherlands, 1973; p. 213. [Google Scholar]
- Foster, M.D. Interpretation of the composition of trioctahedral micas. US Geol. Surv. Prof. Paper 1960, 354B, 11–43. [Google Scholar]
- Pozo, M.; Casas, J. Origin of Kerolite and associated Mg clays in palustrine environments. The Esquivias deposit (Neogene Madrid Basin, Spain). Clay Miner. 1999, 34, 395–418. [Google Scholar] [CrossRef]
- Setti, M.; Marinoni, L.; López-Galindo, A. Mineralogical and geochemical characteristics (major, minor, trace elements and REE) of detrital and authigenic clay minerals in a Cenozoic sequence from Sea, Antarctica. Clay Miner. 2004, 39, 405–421. [Google Scholar] [CrossRef]
- Tosca, N.J.; Wright, V.P. Diagenetic pathways linked to labile Mg-clays in lacustrine carbonate reservoirs: A model for the origin of secondary porosity in the Cretaceous pre-salt Barra Velha Formation, offshore Brazil. Geol. Soc. Lond. 2015, 435, 33–46. [Google Scholar] [CrossRef]
- Tosca, N.J. Geochemical pathways to Mg-clay formation. In Magnesian Clays: Characterization, Origins and Applications; Pozo, M., Galán, E., Eds.; AIPEA: St Andrews, UK, 2015; Volume 2, pp. 283–329. [Google Scholar]
- Dong, H. Clay-Microbe Interactions and Implications for Environmental Mitigation. Elements 2012, 8, 113–118. [Google Scholar] [CrossRef]
- Wright, V.P. Lacustrine carbonates in rift settings: The interaction of volcanic and microbial processes on carbonate deposition. In Advances in Carbonate Exploration and Reservoir Analysis; Garland, J., Neilson, J.E., Laubach, S.E., Whidden, K.J., Eds.; Geological Society: London, UK, 2012; Volume 370, pp. 39–47. [Google Scholar]
- Burne, R.V.; Moore, L.S.; Christy, A.G.; Troitzsch, U.; King, P.L.; Carnerup, A.M.; Hamilton, P.J. Stevensite in the modern thrombolites of Lake Clifton, Western Australia: A missing link in microbialitemineralization? Geology 2014, 42, 575–578. [Google Scholar] [CrossRef]
- Hay, R.L.; Huches, R.E.; Kyser, T.K.; Glass, H.D.; Liu, J. Magnesium-Rich Clays of the Meerschaum Mines in the Amboseli Basin, Tanzania and Kenya. Clays Clay Miner. 1995, 43, 455–466. [Google Scholar] [CrossRef]
- Buey, C.S.; Barrios, M.S.; Romero, E.G.; Montoya, M.D. Mg-rich Smectite “precursor” phase in the Tagus Basin, Spain. Clays Clay Miner. 2000, 48, 366–373. [Google Scholar] [CrossRef]
- Arp, G.; Reimer, A.; Reitner, J. Calcification in cyanobacterial biofimls of alkaline salt lakes. Eur. J. Phycol. 1999, 34, 393–403. [Google Scholar] [CrossRef]
- Bontognali, T.R.R.; Vasconcelos, C.; Warthmann, R.J.; Bernasconi, S.M.; Dupraz, C.; Strohmenger, C.J.; Mckenzie, J.A. Dolomite formation within microbial mats in the coastal sabkha of Abu Dhabi (United Arab Emirates). Sedimentology 2010, 57, 824–844. [Google Scholar] [CrossRef]
- Del Buey, P.; Cabestrero, O.; Arroyo, X.; Sanz-Montero, M.E. Microbially induced palygorskite-sepiolite authigenesis in modern hypersaline lakes (Central Spain). Appl. Clay Sci. 2018, 160, 9–21. [Google Scholar] [CrossRef]


| Element | Equipment | Methodology | Quantification Limit |
|---|---|---|---|
| Alkalinity | Automatic burette | Titration | 0.10 mg L−1 CaCO3 |
| HCO3 | 0.10 mg L−1 HCO3 | ||
| CO3 | 0.10 mg L−1 CO3 | ||
| Ca | Atomic absorption Spectrometer | Atomic absorption Spectrometer | 0.10 mg L−1 |
| Mg | |||
| Cl | Spectrophotometer UV/VIS II (190−1100 nm) | REF 918 20 NANOCOLOR® | 0.20 mg L−1 Cl |
| Si | REF 936 225 VISOCOLOR® | 2.00 mg L−1 SiO2 | |
| Fe | REF 918 36 NANOCOLOR® | 0.01 mg L−1 Fe | |
| SO4 | Turbidimetric determination | 1.00 mg L−1 SO4 | |
| Na | Flame photometer | Flame photometer | 0.10 Na mg L−1 |
| K | 0.10 K mg L−1 |
| Clay Minerals | d(001) | d(060) Å | ||
|---|---|---|---|---|
| Air Dried | Ethylene-Glycol | 550 °C | ||
| Kaolinite | 7.0–7.1 | No change | Collapse | 1.49 |
| Illite | 10.1 | No change | No change | 1.50 |
| Glauconite | 10.1 | No change | No change | 1.51–1.52 |
| Smectite (dioc) | 15.0 | 16.9 | 10.0 | 1.49–1.52 |
| Smectite (trioc) | 15.0 | 16.9 | 10.0 | 1.52–1.54 |
| Vermiculite | ~14.0 | No change | No change | 1.54 |
| Chlorite | ~14.0 | No change | Increase the intensity | 1.54 |
| Farm | Year | Lake | WT | pH | EC | TDS | Alkalinity | Si | HCO3− | CO32− | Cl− | Ca | Mg | Na | K | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (°C) | (mS cm−1) | (mg L−1) | (mmol L−1) | (meq L−1) | ||||||||||||
| Barranco Alto | 2017 | Tubarão | 28.77 | 10.10 | 3.20 | 1811.00 | 1560.65 | 0.7706 | 18.1514 | 13.0547 | 1.7860 | 0.2031 | 0.1094 | 24.0107 | 5.9849 | 0.4244 |
| Burro Branco | 30.93 | 11.15 | 8.30 | 4596.00 | 4456.80 | 2.2219 | 40.2987 | 48.8194 | 7.2011 | 32.1373 | 22.5468 | 25.2286 | 8.4403 | 0.0645 | ||
| Coração | 26.70 | 11.00 | 4.25 | 1987.00 | 1836.40 | 0.0083 | 1.7176 | 35.0048 | 7.5396 | 16.8671 | 7.5705 | 16.9641 | 4.0922 | 0.0501 | ||
| Mara Maravilha | 26.88 | 11.35 | 9.07 | 5570.00 | 3788.60 | 0.7739 | 25.8535 | 49.9059 | 42.8878 | 38.8243 | 16.0461 | 30.6658 | 11.8931 | 0.0125 | ||
| Mineira G. | 28.77 | 7.80 | 0.78 | 1071.00 | 723.36 | 0.7739 | 9.4010 | 5.0629 | 1.7742 | 0.1856 | 0.1111 | 13.8322 | 4.9107 | 0.0716 | ||
| Mineira P. | 29.31 | 7.82 | 0.56 | 369.00 | 262.58 | 0.4497 | 5.2502 | 0.00 | 0.2547 | 0.2675 | 0.1267 | 3.3058 | 1.2942 | 0.0004 | ||
| Sete | 23.15 | 9.78 | 1.50 | 845.00 | 646.50 | 0.7223 | 5.5503 | 7.3772 | 1.4916 | 0.0589 | 0.0214 | 12.1793 | 3.2482 | 0.2944 | ||
| Boi Preto | 20.82 | 6.81 | 0.11 | 89.00 | 54.79 | 0.3332 | 1.0956 | 0.00 | 0.0860 | 0.1722 | 0.1070 | 0.5959 | 0.1709 | 0.0002 | ||
| Jacaré | 21.23 | 9.90 | 1.40 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | ||
| Japão | NM | 10.70 | 1.90 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | ||
| Negro River | NM | NM | NM | 369.00 | 17.72 | 0.1012 | 0.3543 | 0.00 | 0.2462 | 0.1856 | 0.1736 | 0.1087 | 0.0726 | 0.0082 | ||
| Well | NM | NM | NM | 224.00 | 81.02 | 0.6141 | 1.6199 | 0.00 | 1.3350 | 0.4875 | 0.2864 | 1.2701 | 0.9438 | 0.0007 | ||
| 2018 | Tubarão | 32.40 | 9.52 | 0.53 | 471.32 | 365.70 | 0.3678 | 6.7921 | 0.4999 | 0.2905 | 0.1597 | 0.0494 | 3.2275 | 1.4374 | 0.1450 | |
| Burro Branco | 30.80 | 9.31 | 1.91 | 1203.15 | 245.10 | 0.7746 | 0.7418 | 4.1594 | 3.4327 | 0.2675 | 0.0387 | 15.4851 | 3.7342 | 0.0376 | ||
| Coração | 31.80 | 10.86 | 0.92 | 806.00 | 545.20 | 0.6511 | 9.7812 | 1.1198 | 2.7010 | 0.2051 | 0.0642 | 7.8296 | 1.9950 | 0.0398 | ||
| Mara Maravilha | 33.10 | 10.40 | 2.78 | 2522.00 | 1092.50 | 0.7406 | 19.2447 | 4.1594 | 17.5139 | 0.2001 | 0.0634 | 24.2717 | 6.6499 | 0.0290 | ||
| Mineira G. | 30.60 | 9.53 | 0.60 | 263.77 | 390.00 | 0.4986 | 7.7978 | 0.00 | 1.7663 | 0.5065 | 0.0848 | 5.5677 | 2.0461 | 0.0437 | ||
| Sete C | 32.40 | 10.09 | 1.42 | 1272.70 | 860.30 | 0.4492 | 15.6417 | 1.5598 | 4.6439 | 0.1432 | 0.0592 | 11.6574 | 2.4042 | 0.0057 | ||
| Sete B | 31.40 | 10.18 | 0.49 | 447.53 | 149.00 | 0.3217 | 0.2599 | 2.7196 | 0.5272 | 0.0419 | 0.0485 | 1.4833 | 0.5422 | 0.0140 | ||
| Sete | 31.80 | 9.39 | 0.79 | 729.30 | 514.00 | 0.6424 | 8.8376 | 1.4398 | 1.2806 | 0.0948 | 0.0617 | 8.1776 | 2.7623 | 0.4154 | ||
| Nova | 30.80 | 10.13 | 1.25 | 1160.25 | 512.90 | 0.3974 | 4.7767 | 5.4792 | 0.0231 | 0.2001 | 0.0568 | 11.3964 | 4.8084 | 0.0061 | ||
| Boi Preto | 37.2 | 8.3 | 0.07 | 50.0 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | ||
| Jacaré | 31.10 | 10.48 | 1.49 | 1387.60 | 583.80 | 0.3938 | 6.5543 | 5.1192 | 9.6677 | 0.1747 | 0.0625 | 10.9614 | 2.1996 | 0.0179 | ||
| Japão | 30.40 | 9.81 | 1.29 | 325.33 | 500.50 | 0.3542 | 8.1278 | 1.8797 | 8.3612 | 0.1367 | 0.0568 | 10.5264 | 2.0973 | 0.0118 | ||
| Negro River | 32.80 | 7.44 | 0.02 | 339.37 | 14.10 | 0.0449 | 0.2819 | 0.0000 | 0.1077 | 0.0778 | 0.0905 | 0.1131 | 0.1228 | 0.0444 | ||
| Well | NM | NM | NM | 179.14 | 74.90 | 0.3981 | 1.4976 | 0.00 | 1.2495 | 0.5439 | 0.3818 | 0.9134 | 0.3581 | 0.0050 | ||
| Nhumirim | 2018 | Oito | 15.60 | 8.60 | 1.09 | 651.00 | 453.26 | 0.2447 | 8.9828 | 0.0800 | 0.1089 | 0.7885 | 0.4921 | 5.4807 | 3.1971 | 0.00 |
| Meio | 20.30 | 10.18 | 1.36 | 926.00 | 353.31 | 0.2670 | 3.5451 | 3.5195 | 1.6966 | 0.2630 | 0.2617 | 6.1332 | 2.4362 | 0.0004 | ||
| Seis | 20.03 | 10.00 | 0.35 | 350.00 | 267.12 | 0.1731 | 0.9017 | 4.4393 | 0.2036 | 0.1841 | 0.1563 | 3.9583 | 2.1356 | 0.00 | ||
| Salitrada | 28.80 | 7.73 | 0.31 | 163.00 | 133.66 | 0.3062 | 0.5336 | 2.1390 | 0.0578 | 0.9317 | 1.2499 | 0.5742 | 0.4834 | 0.0047 | ||
| SalvinaDoce | 25.40 | 6.57 | 0.14 | 53.00 | 34.79 | 0.0766 | 0.6955 | 0.00 | 0.0062 | 0.2670 | 0.3259 | 0.2175 | 0.1458 | 0.0004 | ||
| Paraguay River | NM | NM | NM | 30.00 | 25.46 | 0.1082 | 0.5189 | 0.00 | 0.0138 | 0.2021 | 0.2321 | 0.1000 | 0.0384 | 0.0072 | ||
| Farms | Year | Lakes | Bulk Rock (%) | Clay Fraction (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Qz | Kfs | Ilt | Kln | Cal | Ilt | Kln | Sme | Cal | |||
| Barranco Alto | 2017 | Burro Branco | 78 | 17 | - | - | 5 | 66 | 29 | - | 5 |
| Coração | 84 | 12 | - | - | 4 | 26 | 71 | 3 | - | ||
| Negro River | 69 | 16 | - | 15 | - | 25 | 70 | 4 | - | ||
| 2018 | Sete C | 90 | 10 | - | - | - | 70 | 28 | - | 2 | |
| Mineira | 89 | 11 | - | - | - | 41 | 59 | * | - | ||
| Negro River | 85 | 15 | - | - | - | 32 | 52 | 16 | - | ||
| Nhumirim | 2018 | Meio Lake-P2 | 92 | 8 | - | - | - | 63 | 30 | 5 | 2 |
| Meio Lake-P3 | 91 | 8 | - | - | 1 | 51 | 24 | 25 | - | ||
| Meio Lake-P4 | 90 | 8 | - | - | 2 | 85.9 | 8 | * | 6 | ||
| Farm | Year | Lake | SiO2 | TiO2 | Al2O3 | Fe2O3 | MnO | MgO | CaO | Na2O | K2O | P2O5 | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Barranco Alto | 2017 | Tubarão | 88.33 | 0.20 | 2.18 | 1.98 | 0.27 | 0.63 | 0.78 | 0.98 | 1.73 | 0.10 | 2.80 |
| Burro Branco | 82.20 | 0.20 | 3.36 | 2.10 | 0.28 | 0.60 | 1.28 | 2.14 | 1.84 | 0.16 | 5.70 | ||
| Coração | 87.43 | 0.28 | 3.33 | 2.28 | 0.90 | 0.50 | 0.98 | 0.38 | 1.28 | 0.17 | 2.47 | ||
| Mara Maravilha | 69.17 | 0.36 | 5.63 | 4.04 | 0.77 | 2.09 | 4.01 | 1.20 | 3.04 | 0.12 | 9.00 | ||
| Mineira G. | 78.40 | 0.30 | 3.30 | 1.50 | 0.20 | 100 | 3.00 | 0.40 | 1.40 | 0.50 | 9.65 | ||
| Mineira P. | 78.60 | 0.40 | 4.60 | 2.20 | 0.10 | 0.50 | 0.80 | 0.20 | 1.20 | 0.20 | 11.08 | ||
| Sete B | 66.00 | 0.30 | 4.90 | 5.80 | 0.40 | 1.50 | 5.60 | 0.20 | 2.60 | 0.10 | 11.90 | ||
| Sete | 94.83 | 0.13 | 1.90 | 0.53 | 0.00 | 0.17 | 0.20 | 0.13 | 0.90 | 0.10 | 1.05 | ||
| Boi Preto | 96.90 | 0.10 | 1.20 | 0.20 | 0.00 | <0.1 | <0.1 | <0.1 | 0.60 | 0.10 | 0.76 | ||
| Jacaré | 95.70 | 0.20 | 1.40 | 0.20 | 0.00 | <0.1 | 0.10 | 0.10 | 0.90 | 0.10 | 1.21 | ||
| Japão | 97.80 | 0.10 | 0.90 | 0.20 | 0.00 | 0.00 | <0.1 | 0.10 | 0.50 | 0.10 | 0.40 | ||
| Negro River | 71.95 | 0.80 | 14.80 | 4.80 | 0.20 | 0.85 | 0.30 | 0.10 | 1.65 | 0.10 | 4.30 | ||
| 2018 | Tubarão | 88.99 | 0.15 | 1.82 | 1.94 | 0.17 | 0.43 | 0.61 | 0.14 | 1.27 | 0.04 | 4.48 | |
| Burro Branco | 97.10 | 0.09 | 0.85 | 0.38 | 0.03 | 0.04 | 0.36 | 0.10 | 0.53 | 0.05 | 0.80 | ||
| Coração | 97.25 | 0.08 | 0.67 | 0.17 | 0.03 | 0.13 | 0.54 | 0.09 | 0.41 | 0.02 | 0.83 | ||
| Mara Maravilha | 89.37 | 0.21 | 2.13 | 1.51 | 0.25 | 0.49 | 1.18 | 0.38 | 1.31 | 0.04 | 3.42 | ||
| Mineira G. | 83.70 | 0.25 | 2.82 | 1.12 | 0.09 | 0.38 | 0.84 | 0.18 | 0.85 | 0.10 | 9.98 | ||
| Sete C | 97.22 | 0.13 | 1.28 | 0.34 | 0.01 | 0.07 | 0.07 | 0.10 | 0.55 | 0.01 | 0.76 | ||
| Sete B | 82.43 | 0.24 | 3.11 | 3.43 | 0.20 | 0.67 | 1.67 | 0.12 | 1.61 | 0.03 | 6.55 | ||
| Sete | 97.41 | 0.10 | 1.04 | 0.32 | 0.01 | 0.07 | 0.05 | 0.06 | 0.50 | 0.01 | 0.50 | ||
| Nova | 98.30 | 0.06 | 0.53 | 0.16 | 0.01 | 0.05 | 0.04 | 0.05 | 0.31 | 0.01 | 0.61 | ||
| Boi Preto | 97.99 | 0.08 | 0.66 | 0.16 | 0.01 | <0.01 | 0.03 | 0.03 | 0.32 | 0.01 | 0.76 | ||
| Jacaré | 98.83 | 0.07 | 0.51 | 0.11 | <0.01 | 0.01 | 0.02 | 0.04 | 0.26 | 0.01 | 0.24 | ||
| Japão | 98.24 | 0.09 | 0.65 | 0.14 | 0.01 | 0.01 | 0.03 | 0.05 | 0.32 | 0.02 | 0.56 | ||
| Negro River | 92.54 | 0.23 | 3.01 | 1.00 | 0.03 | 0.18 | 0.09 | 0.06 | 0.68 | 0.02 | 1.74 | ||
| Nhumirim | 2018 | Oito Lake | 96.75 | 0.10 | 0.72 | 0.37 | 0.07 | 0.13 | 0.31 | 0.07 | 0.47 | 0.02 | 0.99 |
| Meio Lake | 96.45 | 0.13 | 0.81 | 0.19 | 0.04 | 0.20 | 0.52 | 0.12 | 0.49 | 0.02 | 1.02 | ||
| Seis Lake | 88.57 | 0.21 | 1.14 | 3.33 | 0.28 | 0.52 | 1.16 | 0.11 | 1.58 | 0.27 | 3.18 | ||
| Salitrada | 93.39 | 0.25 | 1.51 | 0.38 | 0.02 | 0.05 | 0.10 | 0.06 | 0.56 | 0.03 | 3.66 | ||
| SalvinaDoce | 95.59 | 0.12 | 0.85 | 0.43 | 0.04 | 0.06 | 0.07 | 0.05 | 0.42 | 0.02 | 2.35 |
| Detrital Micas | Illite | I/S | Smectites |
|---|---|---|---|
| Si = 3.05–3.15 | Si = ≈ 3.20 | K = 0.40–0.52 | Si = 3.50–4.00 |
| Mg: 0.10–0.25 | Mg: 0.30–0.70 | ||
| Fe: 0.05–0.10 | ∑(VI): 1.95–2.20 or 2.80–3.05 | ||
| K: 0.57–0.70 | |||
| ∑(XII): <0.65 |
| Lakes | Si | Al(IV) | Al(VI) | Fe ** | Mg | Mn | ∑(VI) | K | Na | Ca | ∑(XII) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MM (P2) | 3.58 | 0.42 | 1.82 | 0.18 | 0.18 | 0.02 | 2.20 | 0.02 | 0.00 | 0.00 | 0.02 |
| Coração (P1) | 3.75 | 0.25 | 1.04 | 0.67 | 0.31 | 2.02 | 0.40 | 0.05 | 0.03 | 0.48 | |
| 3.80 | 0.20 | 1.37 | 0.36 | 0.25 | 1.98 | 0.29 | 0.16 | 0.03 | 0.48 | ||
| 4.00 | 1.24 | 0.31 | 0.40 | 1.95 | 0.19 | 0.03 | 0.22 | ||||
| 3.97 | 0.03 | 0.39 | 1.01 | 0.59 | 0.13 | 2.12 | 0.34 | 0.02 | 0.36 | ||
| 3.64 | 0.36 | 0.80 | 0.79 | 0.59 | 0.09 | 2.27 | 0.26 | 0.02 | 0.28 | ||
| 4.00 | 0.32 | 0.88 | 0.56 | 0.13 | 1.89 | 0.24 | 0.01 | 0.25 | |||
| 3.73 | 0.27 | 0.67 | 0.90 | 0.32 | 0.03 | 1.92 | 0.77 | 0.04 | 0.81 | ||
| 4.00 | 0.39 | 0.64 | 0.75 | 0.08 | 1.86 | 0.27 | 0.01 | 0.28 | |||
| 3.91 | 0.09 | 0.30 | 1.03 | 0.64 | 0.12 | 2.09 | 0.55 | 0.02 | 0.57 | ||
| 3.45 | 0.55 | 1.79 | 0.16 | 0.25 | 2.20 | 0.15 | 0.02 | 0.17 | |||
| Burro Branco (P2) | 4.00 | 0.75 | 0.59 | 0.39 | 0.08 | 1.80 | 0.42 | 0.11 | 0.53 | ||
| 4.00 | 0.44 | 0.81 | 0.49 | 0.08 | 1.81 | 0.50 | 0.07 | 0.01 | 0.58 | ||
| 4.00 | 1.06 | 0.18 | 0.18 | 1.41 | 0.23 | 0.10 | 0.01 | 0.34 | |||
| 4.00 | 0.78 | 0.49 | 0.27 | 0.03 | 1.57 | 0.36 | 0.09 | 0.01 | 0.46 | ||
| 4.00 | 0.95 | 0.43 | 0.26 | 0.05 | 1.69 | 0.26 | 0.08 | 0.02 | 0.36 | ||
| 4.00 | 1.29 | 0.27 | 0.21 | 0.02 | 1.80 | 0.19 | 0.11 | 0.02 | 0.31 | ||
| Tubarão (P2) | 3.64 | 0.36 | 1.47 | 0.4 | 0.24 | 0.02 | 2.13 | 0.19 | 0.02 | 0.21 | |
| 3.66 | 0.34 | 1.31 | 0.35 | 0.31 | 0.02 | 1.99 | 0.62 | 0.08 | 0.70 | ||
| 3.94 | 0.06 | 0.7 | 0.82 | 0.37 | 0.1 | 1.99 | 0.5 | 0.04 | 0.54 | ||
| 4.00 | 0.68 | 0.63 | 0.41 | 0.05 | 1.77 | 0.44 | 0.03 | 0.47 | |||
| 3.91 | 0.09 | 0.41 | 0.97 | 0.53 | 0.10 | 2.01 | 0.62 | 0.04 | 0.66 | ||
| 3.26 | 0.74 | 1.77 | 0.08 | 0.17 | 0.01 | 2.03 | 0.83 | 0.83 | |||
| Sete B (P2) | 3.78 | 0.22 | 0.36 | 1.1 | 0.5 | 0.05 | 2.01 | 0.62 | 0.07 | 0.69 | |
| 3.82 | 0.18 | 0.43 | 1.03 | 0.45 | 0.04 | 1.95 | 0.51 | 0.16 | 0.67 | ||
| 3.74 | 0.26 | 0.18 | 1.35 | 0.47 | 0.05 | 2.05 | 0.55 | 0.04 | 0.59 | ||
| 3.83 | 0.17 | 0.4 | 1.1 | 0.45 | 0.06 | 2.01 | 0.58 | 0.03 | 0.61 | ||
| 3.75 | 0.25 | 0.57 | 1.0 | 0.45 | 0.03 | 2.05 | 0.49 | 0.05 | 0.54 | ||
| 3.61 | 0.39 | 1.45 | 0.41 | 0.26 | 0.03 | 2.15 | 0.18 | 0.03 | 0.21 | ||
| 3.4 | 0.60 | 1.08 | 0.68 | 0.22 | 0.11 | 2.09 | 0.27 | 0.2 | 0.47 | ||
| Negro River (P2) | 3.74 | 0.26 | 1.49 | 0.2 | 0.41 | 2.10 | 0.17 | 0.17 | |||
| 3.73 | 0.27 | 1.56 | 0.18 | 0.42 | 2.16 | 0.05 | 0.08 | 0.13 | |||
| 3.54 | 0.46 | 1.27 | 0.49 | 0.35 | 2.11 | 0.4 | 0.05 | 0.45 | |||
| 3.5 | 0.50 | 1.35 | 0.59 | 0.19 | 2.13 | 0.08 | 0.11 | 0.19 | |||
| 3.39 | 0.61 | 1.57 | 0.22 | 0.4 | 2.19 | 0.47 | 0.47 | ||||
| 3.51 | 0.49 | 1.38 | 0.46 | 0.36 | 2.20 | 0.17 | 0.05 | 0.22 |
| Farm | Year | Lakes | d(001)Å | d(060) Å | Clay Minerals | ||
|---|---|---|---|---|---|---|---|
| Air-Dried | Ethylene-Glycol | 550 °C | |||||
| Barranco Alto | 2017 | Burro Branco | 10.0–12.0 | No change | 10 Å more intense | - | I/S |
| Coração | 10.0/13.0 | No change | 10 Å more intense | 1.541 | I/S | ||
| Negro River | 15.0 | 17.0 | 10.0 | - | Sme | ||
| 2018 | Sete C | 10.0–12.0 | 17.0 | 10 Å more intense | 1.529 | I/S | |
| Mineira | 10.0/14.0 | No change | 10 Å more intense | 1.504 | I/S | ||
| Negro River | 15.0 | 17.0 | 10.0 | 1.541 | Sme (tri) | ||
| Nhumirim | 2018 | Meio Lake—P2 | 15.0–16.0 | No change | 10.0 | - | I/S |
| Meio Lake—P3 | 15.0–16.0 | 17.0 | 10.0 | 1.521 | Sme (di/tri) | ||
| Meio Lake—P4 | 15.0–16.0 | No change | 10.0 | 1.524 | I/S | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dias, I.A.; Cury, L.F.; Titon, B.G.; Athayde, G.B.; Fedalto, G.; da Rocha Santos, L.; Soares, A.P.; de Vasconcelos Müller Athayde, C.; Manuela Bahniuk Rumbeslperger, A. The Occurrence of Authigenic Clay Minerals in Alkaline-Saline Lakes, Pantanal Wetland (Nhecolândia Region, Brazil). Minerals 2020, 10, 718. https://doi.org/10.3390/min10080718
Dias IA, Cury LF, Titon BG, Athayde GB, Fedalto G, da Rocha Santos L, Soares AP, de Vasconcelos Müller Athayde C, Manuela Bahniuk Rumbeslperger A. The Occurrence of Authigenic Clay Minerals in Alkaline-Saline Lakes, Pantanal Wetland (Nhecolândia Region, Brazil). Minerals. 2020; 10(8):718. https://doi.org/10.3390/min10080718
Chicago/Turabian StyleDias, Isis Armstrong, Leonardo Fadel Cury, Bruno Guimarães Titon, Gustavo Barbosa Athayde, Guilherme Fedalto, Larissa da Rocha Santos, Ana Paula Soares, Camila de Vasconcelos Müller Athayde, and Anelize Manuela Bahniuk Rumbeslperger. 2020. "The Occurrence of Authigenic Clay Minerals in Alkaline-Saline Lakes, Pantanal Wetland (Nhecolândia Region, Brazil)" Minerals 10, no. 8: 718. https://doi.org/10.3390/min10080718
APA StyleDias, I. A., Cury, L. F., Titon, B. G., Athayde, G. B., Fedalto, G., da Rocha Santos, L., Soares, A. P., de Vasconcelos Müller Athayde, C., & Manuela Bahniuk Rumbeslperger, A. (2020). The Occurrence of Authigenic Clay Minerals in Alkaline-Saline Lakes, Pantanal Wetland (Nhecolândia Region, Brazil). Minerals, 10(8), 718. https://doi.org/10.3390/min10080718






