Considerations About Bi and Pb in the Crystal Structure of Cu-Bearing Tourmaline
Abstract
1. Introduction
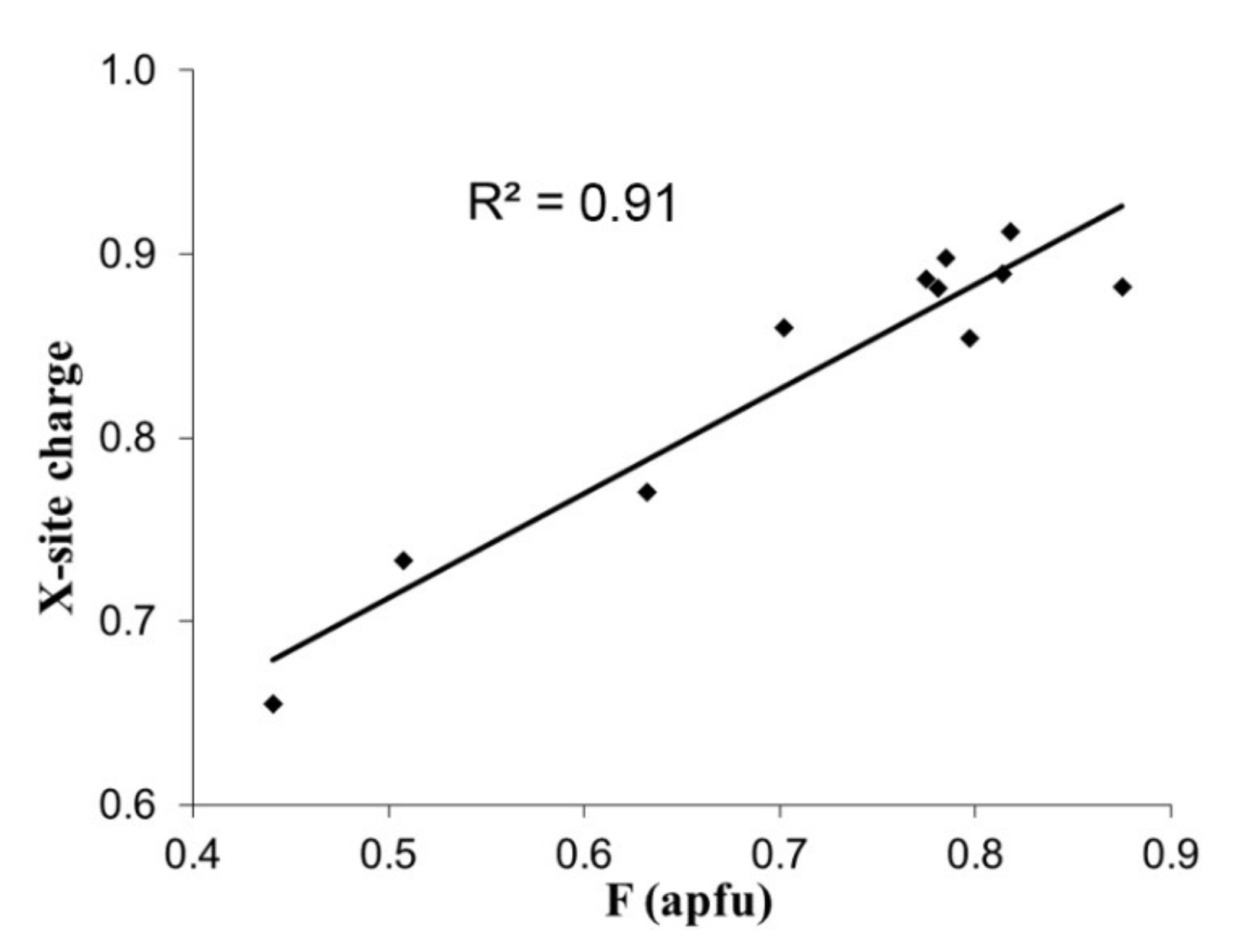
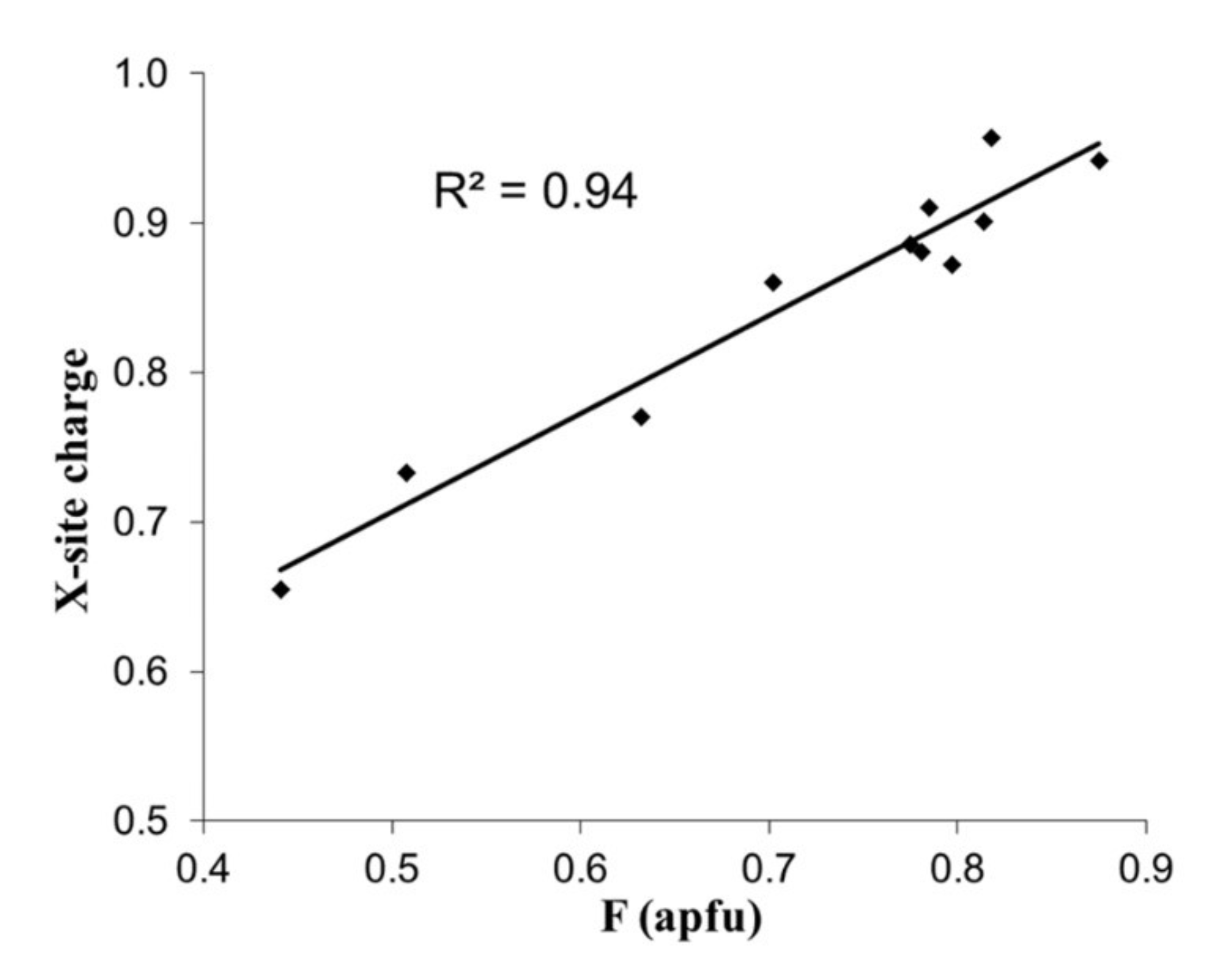
2. Theory and Correlations
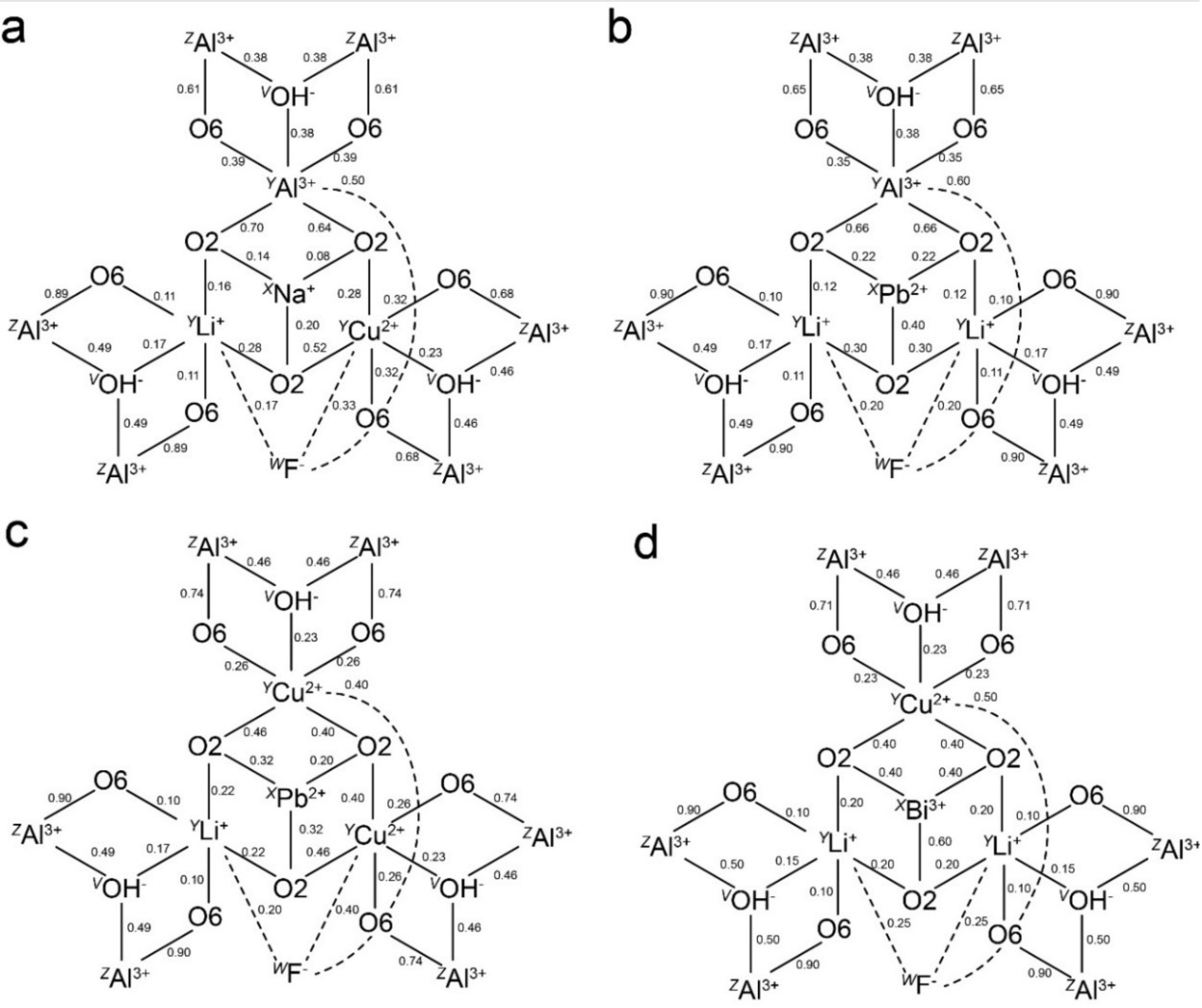
3. Bond Valence Calculations
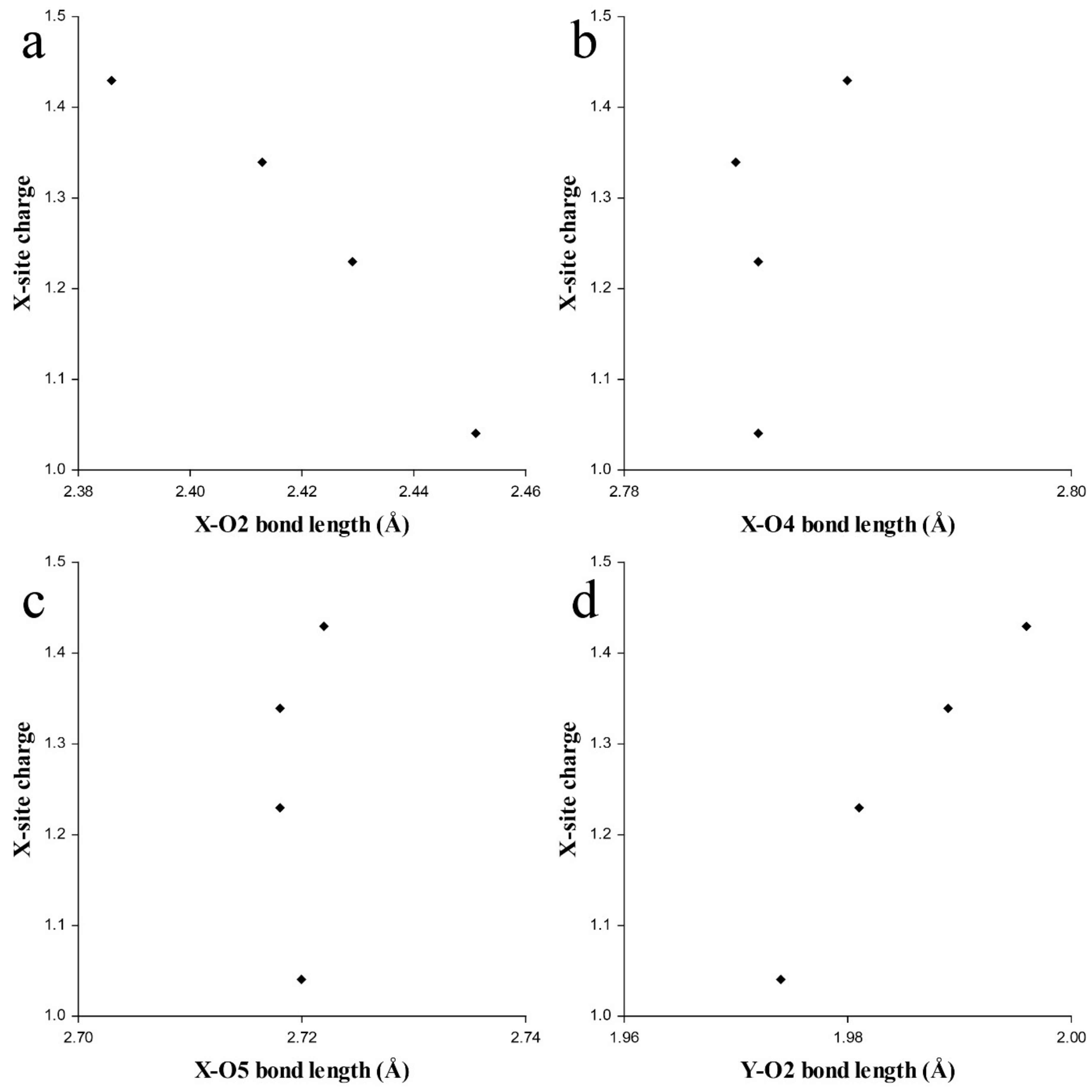
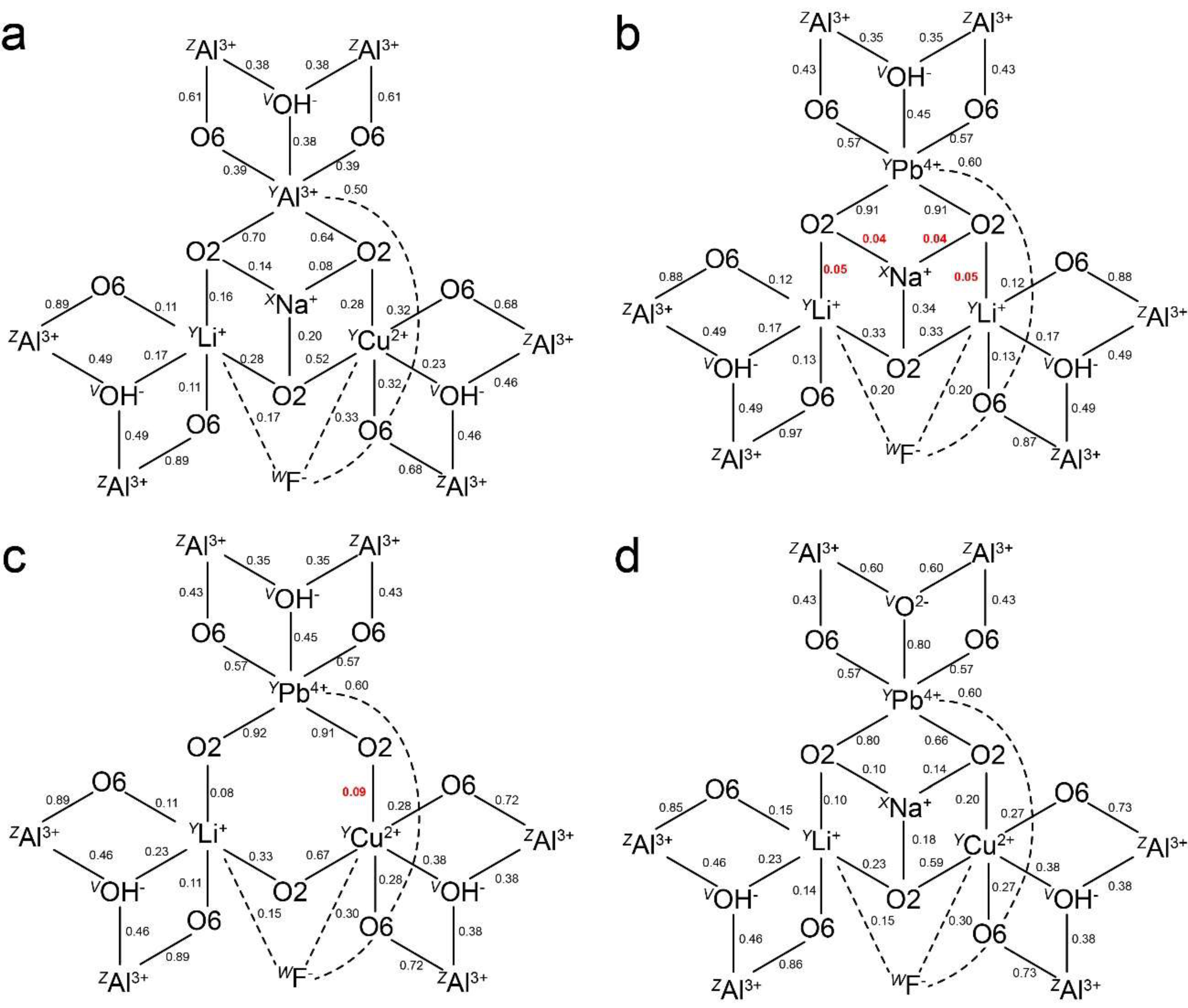
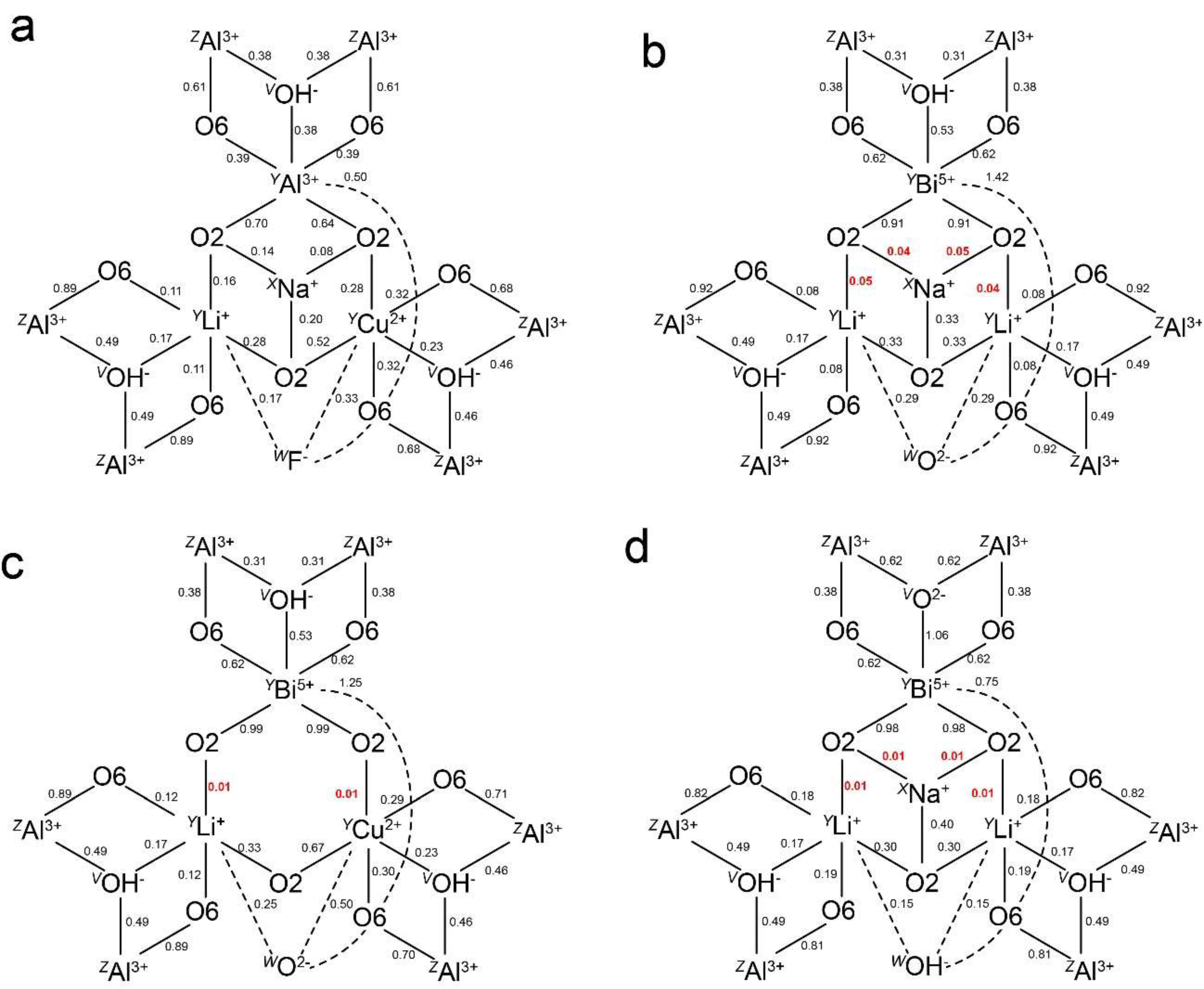
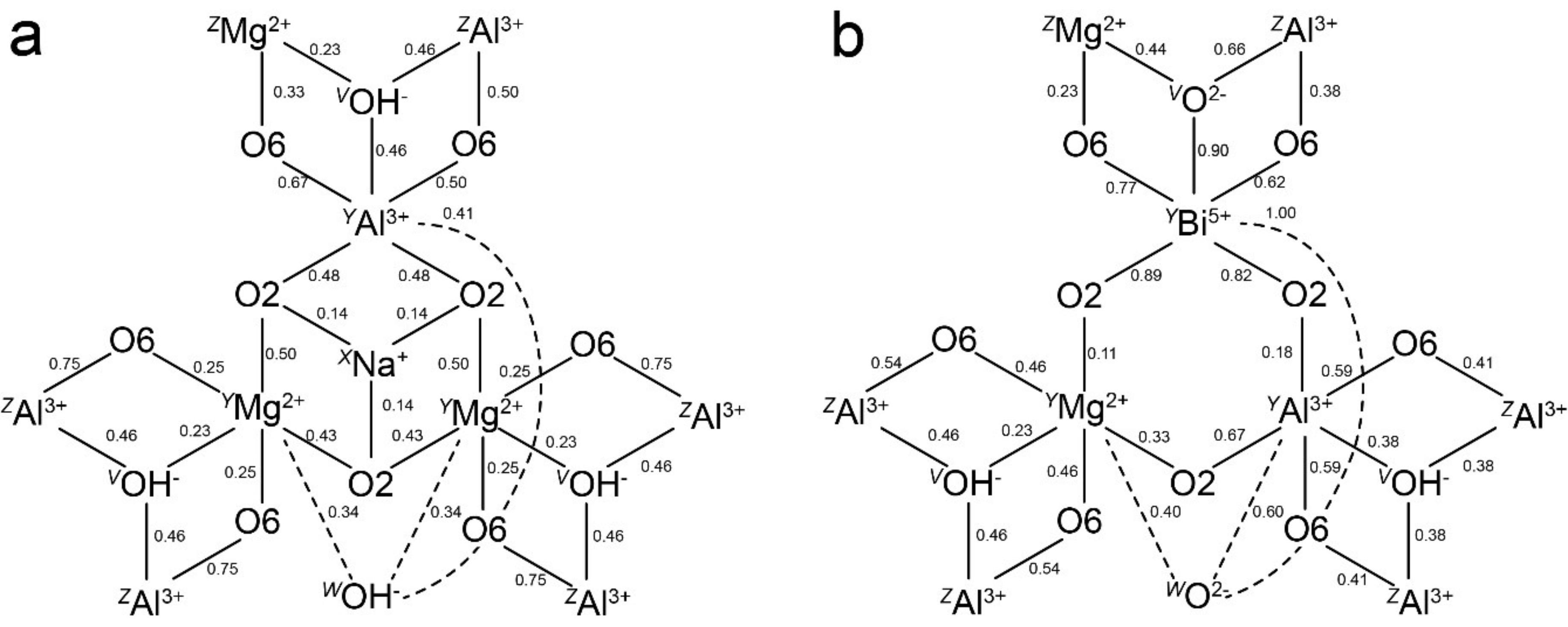
3.1. Pb2+ and Bi3+ at the X Site
3.2. Pb4+ and Bi5+ at the Octahedral Sites
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Povondra, P.; Čech, A. A method for the chemical analysis of tourmaline. Acta Univ. Carol. Geol. 1976, 1976, 209–218. [Google Scholar]
- Foit, F.F.; Rosenberg, P.E. The structure of vanadium-bearing tourmaline and its implications regarding tourmaline solid solutions. Am. Mineral. 1979, 64, 788–798. [Google Scholar]
- Deer, W.A.; Howie, R.A.; Zussman, J. Rock-Forming Minerals. Vol. 1B: Disilicates and Ring Silicates, 2nd ed.; Longman, Burnt Mill: Harlow, UK, 1986. [Google Scholar]
- Foit, F.F. Crystal chemistry of alkali-deficient schorl and tourmaline structual relationships. Am. Mineral. 1989, 74, 422–431. [Google Scholar]
- Hawthorne, F.C.; MacDonald, D.J.; Burns, P.C. Reassignment of cation site occupancies in tourmaline: Al-Mg disorder in the crystal structure of dravite. Am. Mineral. 1993, 78, 265–270. [Google Scholar]
- MacDonald, D.J.; Hawthorne, F.C. The crystal chemistry of Si = Al substitution in tourmaline. Can. Mineral. 1995, 33, 849–858. [Google Scholar]
- Hawthorne, F.C. Structural mechanisms for light-element variations in tourmaline. Can. Mineral. 1996, 34, 123–132. [Google Scholar]
- Henry, D.J.; Dutrow, B.L. Metamorphic Tourmaline and Its Petrologic Applications. Rev. Mineral. 1996, 33, 503–557. [Google Scholar]
- Ertl, A.; Pertlik, F.; Bernhardt, H.-J. Investigations on Olenite with Excess Boron from the Koralpe, Styria, Austria. Österr. Akad. Wiss. Math. Naturwiss. Kl. Abt. I Anz. 1997, 6, 3–10. [Google Scholar]
- Ertl, A.; Kolitsch, U.; Dyar, M.D.; Hughes, J.M.; Rossman, G.R.; Pieczka, A.; Henry, D.J.; Pezzotta, F.; Prowatke, S.; Lengauer, C.L.; et al. Limitations of Fe2+ and Mn2+ site occupancy in tourmaline: Evidence from Fe2+- and Mn2+-rich tourmaline. Am. Mineral. 2012, 97, 1402–1416. [Google Scholar] [CrossRef]
- Ertl, A.; Tillmanns, E. The [9]-coordinated X site in the crystal structure of tourmaline-group minerals. Z. Krist. 2012, 227, 456–459. [Google Scholar] [CrossRef]
- Ertl, A.; Schuster, R.; Hughes, J.M.; Ludwig, T.; Meyer, H.-P.; Finger, F.; Dyar, M.D.; Ruschel, K.; Rossman, G.R.; Klötzli, U.; et al. Li-bearing tourmalines in Variscan granitic pegmatites from the Moldanubian nappes, Lower Austria. Eur. J. Mineral. 2012, 24, 695–715. [Google Scholar] [CrossRef]
- Hawthorne, F.C.; Henry, D.J. Classification of the minerals of the tourmaline group. Eur. J. Mineral. 1999, 11, 201–216. [Google Scholar] [CrossRef]
- Bosi, F.; Lucchesi, S. Crystal chemistry of the schorl-dravite series. Eur. J. Mineral. 2004, 16, 335–344. [Google Scholar] [CrossRef]
- Bosi, F.; Lucchesi, S.; Reznitskii, L. Crystal chemistry of the dravite-chromdravite series. Eur. J. Mineral. 2004, 16, 345–352. [Google Scholar] [CrossRef]
- Bosi, F.; Andreozzi, G.B.; Federico, M.; Graziani, G.; Lucchesi, S. Crystal chemistry of the elbaite-schorl series. Am. Mineral. 2005, 90, 1784–1792. [Google Scholar] [CrossRef]
- Henry, D.J.; Novák, M.; Hawthorne, F.C.; Ertl, A.; Dutrow, B.L.; Uher, P.; Pezzotta, F. Nomenclature of the tourmaline-supergroup minerals. Am. Mineral. 2011, 96, 895–913. [Google Scholar] [CrossRef]
- Vereshchagin, O.S.; Rozhdestvenskaya, I.V.; Frank-Kamenetskaya, O.V.; Zolotarev, A.A.; Mashkovtsev, R.I. Crystal chemistry of Cu-bearing tourmalines. Am. Mineral. 2013, 98, 1610–1616. [Google Scholar] [CrossRef]
- Vereshchagin, O.S.; Frank-Kamenetskaya, O.V.; Rozhdestvenskaya, I.V. Crystal structure and stability of Ni-rich synthetic tourmaline. Distribution of divalent transition-metal cations over octahedral positions. Mineral. Mag. 2015, 79, 997–1006. [Google Scholar] [CrossRef]
- Bačík, P.; Kodĕra, P.; Uher, P.; Ozdín, D.; Jánošík, M. Chlorine-enriched tourmalines in hydrothermally altered diorite porphyry from the Biely vrch porphyry gold deposit (Slovakia). Can. Mineral. 2015, 53, 673–691. [Google Scholar] [CrossRef]
- MacDonald, D.J.; Hawthorne, F.C. Cu-bearing tourmaline from Paraiba, Brazil. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1995, 51, 555–557. [Google Scholar] [CrossRef]
- Ertl, A.; Hughes, J.M.; Brandstätter, F. Structural and Chemical Data of Cu-bearing elbaite from Alto dos Quintos mine, Brazil. 2002. Available online: http://members.a1.net/andreas.ertl/elbaite(Cu)_add_data.pdf (accessed on 29 May 2020).
- Ertl, A.; Vereshchagin, O.S.; Giester, G.; Tillmanns, E.; Meyer, H.-P.P.; Ludwig, T.; Rozhdestvenskaya, I.V.; Frank-Kamenetskaya, O.V. Structural and chemical investigation of a zoned synthetic Cu-rich tourmaline. Can. Mineral. 2015, 53, 209–220. [Google Scholar] [CrossRef]
- Ertl, A.; Hughes, J.M.; Prowatke, S.; Ludwig, T.; Brandstätter, F.; Körner, W.; Dyar, M.D. Tetrahedrally coordinated boron in Li-bearing olenite from “mushroom” tourmaline from Momeik, Myanmar. Can. Mineral. 2007, 45, 891–899. [Google Scholar] [CrossRef]
- Fritsch, E.; Shigley, J.E.; Rossman, G.R.; Mercer, M.E.; Muhlmeister, S.M.; Moon, M. Gem-quality cuprian tourmalines from São José da Batalha in Paraiba, Brazil. Gems Gemol. 1990, 26, 189–205. [Google Scholar] [CrossRef]
- Shigley, J.E.; Cook, B.C.; Laurs, B.M.; De Bernardes, M.O. An update on “Paraíba” tourmaline from Brazil. Gems Gemol. 2001, 37, 260–276. [Google Scholar] [CrossRef]
- Milisenda, C.C. “Paraiba-Turmaline” aus Quintos de Baixo, Rio Grande do Norte, Brasilien. Z. Dt. Gemmol. Ges. 2005, 54, 73–84. [Google Scholar]
- Okrusch, M.; Ertl, A.; Schüssler, U.; Tillmanns, E.; Brätz, H.; Bank, H. Major- and trace-element composition of Paraíba-type Tourmaline from Brazil, Mozambique and Nigeria. J. Gemmol. 2016, 35, 120–139. [Google Scholar] [CrossRef]
- Abduriyim, A.; Kitawaki, H.; Furuya, M.; Schwarz, D. “Paraíba”-type copper-bearing tourmaline from Brazil, Nigeria, and Mozambique: Chemical fingerprinting by LA-ICP-MS. Gems Gemol. 2006, 42, 4–21. [Google Scholar] [CrossRef]
- Johnson, M.L.; Wentzell, C.Y.; Elen, S. Multicolored bismuth-bearing tourmaline from Lundazi, Zambia. Gems Gemol. 1997, 33, 204–211. [Google Scholar] [CrossRef]
- Lussier, A.J.; Abdu, Y.; Hawthorne, F.C.; Michaelis, V.K.; Aguiar, P.M.; Kroekennnr, S. Oscillatory zoned liddicoatite from Anjanabonoina, Central Madagascar. I. Crystal chemistry and structure by SREF and 11B and 27Al MAS NMR spectroscopy. Can. Mineral. 2011, 49, 63–88. [Google Scholar] [CrossRef]
- Sokolov, M.; Martin, R.F. A Pb-dominant member of the tourmaline group, Minh Tien granitic pegmatite, Luc Yen district, Vietnam. Estud. Geol. 2009, 19, 352–353. [Google Scholar]
- Kubernátová, M. Composition of Pb-Rich Tourmaline from the Pegmatite Minh Tien, Vietnam; Masaryk University: Brno, Czech Republic, 2019. [Google Scholar]
- Kubernátová, M.; Cempírek, J. Crystal chemistry of Pb-rich tourmaline from pegmatite in Minh Tien, Vietnam. In Proceedings of the 9th European Conference on Mineralogy and Spectroscopy, Prague, Czech Republic, 11–13 September 2019. [Google Scholar]
- Henry, D.J. Fluorine—X-site vacancy avoidance in natural tourmaline: Internal vs external control. In Proceedings of the 2005 Goldschmidt Conference, Moscow, ID, USA, 20–25 May 2005; p. 1318. [Google Scholar]
- Ertl, A.; Kolitsch, U.; Meyer, H.P.; Ludwig, T.; Lengauer, C.L.; Nasdala, L.; Tillmanns, E. Substitution mechanism in tourmalines of the “Fluor-Elbaite”-rossmanite series from Wolkenburg, Saxony, Germany. Neues Jahrb. Mineral. Abh. 2009, 186, 51–61. [Google Scholar] [CrossRef]
- Ertl, A.; Rossman, G.R.; Hughes, J.M.; London, D.; Wang, Y.; O’Leary, J.A.; Dyar, M.D.; Prowatke, S.; Ludwig, T.; Tillmanns, E. Tourmaline of the elbaite-schorl series from the Himalaya Mine, Mesa Grande, California: A detailed investigation. Am. Mineral. 2010, 95, 24–40. [Google Scholar] [CrossRef]
- Shriver, D.F.; Atkins, P.W. Inorganic Chemistry, 3rd ed.; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Sadler, P.J.; Li, H.; Sun, H. Coordination chemistry of metals in medicine: Target sites for bismuth. Coord. Chem. Rev. 1999, 185, 689–709. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Ertl, A.; Hughes, J.M.; Pertlik, F.; Foit, F.F.; Wright, S.E.; Brandstätter, F.; Marler, B. Polyhedron distortions in tourmaline. Can. Mineral. 2002, 40, 153–162. [Google Scholar] [CrossRef]
- Sugiyama, K.; Arima, H.; Konno, H.; Mikouchi, T. XAFS study on the location of Cu and Mn in a greenish blue elbaite from Alto dos Quntos mine, Brazil. J. Mineral. Pet. Sci. 2017, 112, 139–146. [Google Scholar] [CrossRef][Green Version]
- Brown, I.D. The Chemical Bond in Inorganic Chemistry: The Bond Valence Model; Oxford University Press: Oxford, UK, 2010; ISBN 9780191708879. [Google Scholar]
- Gagné, O.C.; Hawthorne, F.C. Comprehensive derivation of bond-valence parameters for ion pairs involving oxygen. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2015, 71, 562–578. [Google Scholar] [CrossRef]
- Bačík, P.; Fridrichová, J. Cation partitioning among crystallographic sites based on bond-length constraints in tourmaline-supergroup minerals. Am. Mineral. 2020, 96, 895–913. [Google Scholar]
- Hawthorne, F.C. Bond-valence constraints on the chemical composition of tourmaline. Can. Mineral. 2002, 40, 789–797. [Google Scholar] [CrossRef]
- Bosi, F. Stereochemical constraints in tourmaline: From a short-range to a long-range structure. Can. Mineral. 2011, 49, 17–27. [Google Scholar] [CrossRef]
- Bačík, P. Cation ordering at octahedral sites in schorl-dravite series tourmalines. Can. Mineral. 2015, 53, 571–590. [Google Scholar] [CrossRef]
- Bačík, P. The crystal-chemical autopsy of octahedral sites in Na-dominant tourmalines: Octahedral metrics model unconstrained by the Y,Z-site disorder assignment. J. Geosci. (Czech Repub.) 2018, 63, 137–154. [Google Scholar] [CrossRef]
- Ertl, A.; Kolitsch, U.; Prowatke, S.; Dyar, M.D.; Henry, D.J. The F-analogue of schorl from Grasstein, Trentino South Tyrol, Italy: Crystal structure and chemistry. Eur. J. Mineral. 2006, 18, 583–588. [Google Scholar] [CrossRef]
- Ertl, A.; Giester, G.; Ludwig, T.; Meyer, H.P.; Rossman, G.R. Synthetic B-rich olenite: Correlations of single-crystal structural data. Am. Mineral. 2012, 97, 1591–1597. [Google Scholar] [CrossRef][Green Version]
- Ertl, A.; Hughes, J.M.; Prowatke, S.; Ludwig, T.; Prasad, P.S.R.; Brandstätter, F.; Körner, W.; Schuster, R.; Pertlik, F.; Marschall, H. Tetrahedrally coordinated boron in tourmalines from the liddicoatite-elbaite series from Madagascar: Structure, chemistry, and infrared spectroscopic studies. Am. Mineral. 2006, 91, 1847–1856. [Google Scholar] [CrossRef]
- Brandstätter, F.; Niedermayr, G. Einschlüsse von ged. Kupfer im Cu-Elbait von São José da Batalha in Paraiba, Brasilien. Z. Dt. Gemmol. Ges. 1993, 42, 37–41. [Google Scholar]
- Beurlen, H.; Müller, A.; Silva, D.; Da Silva, M.R.R. Petrogenetic significance of LA-ICP-MS trace-element data on quartz from the Borborema Pegmatite Province, northeast Brazil. Mineral. Mag. 2011, 75, 2703–2719. [Google Scholar] [CrossRef]
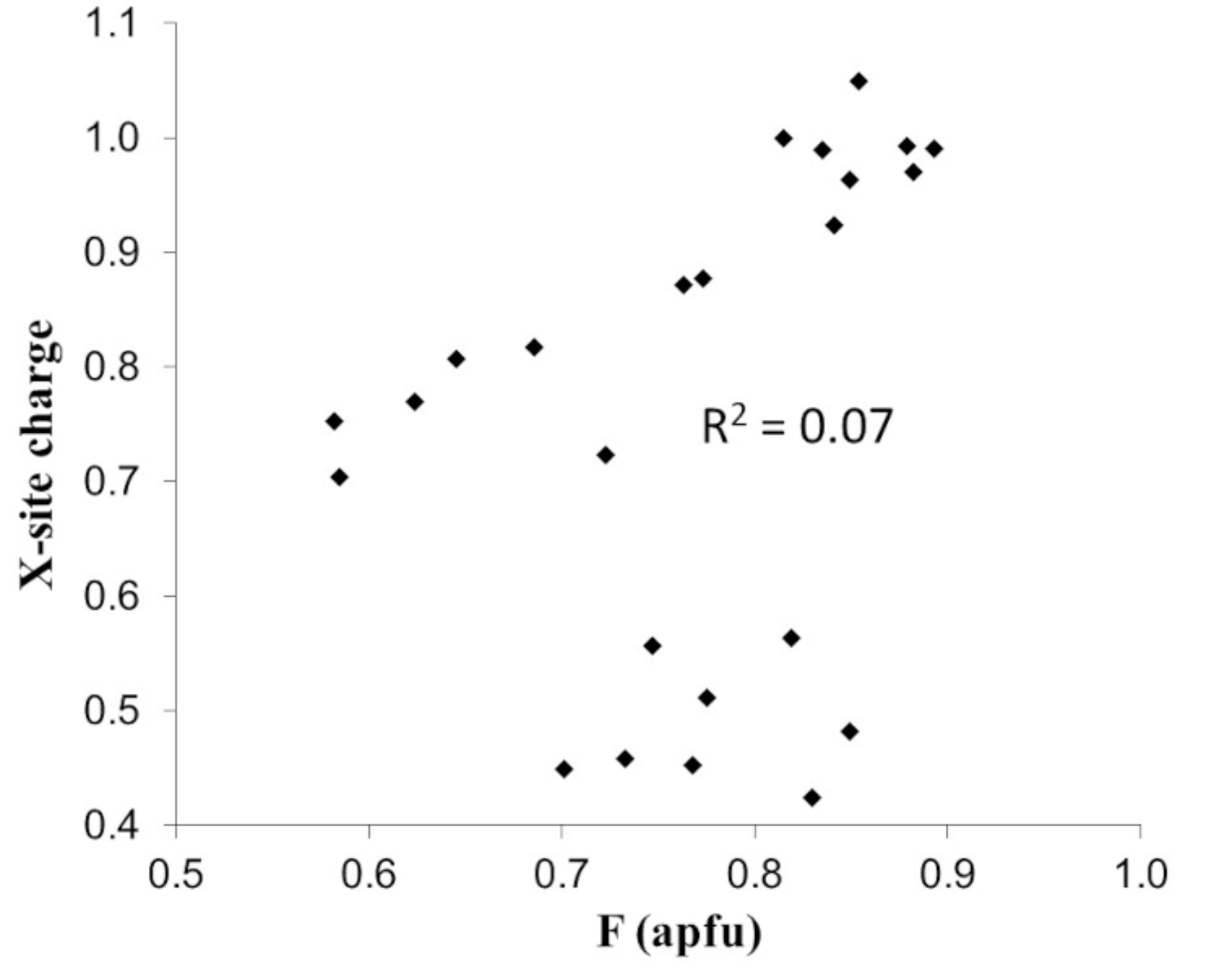
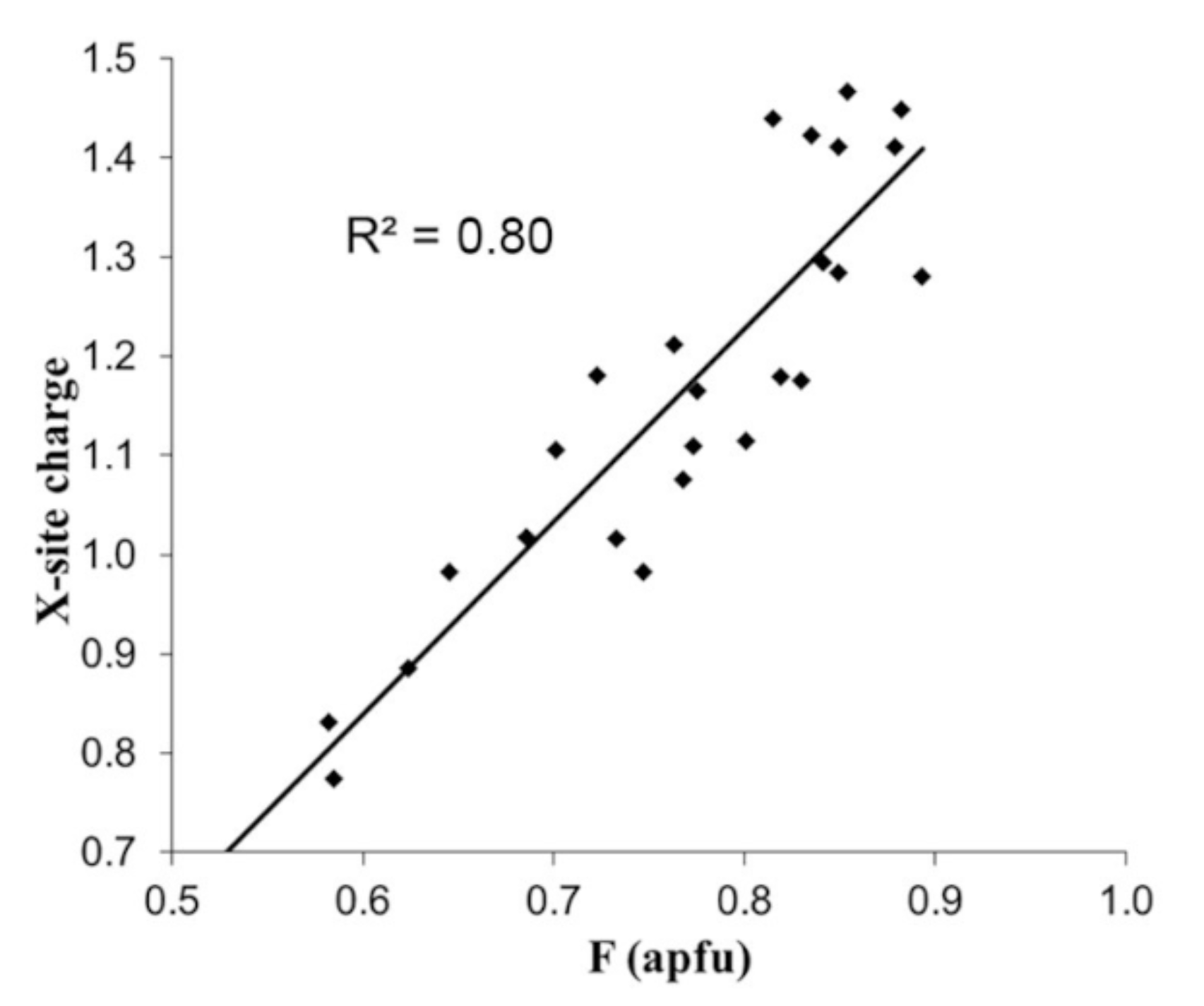
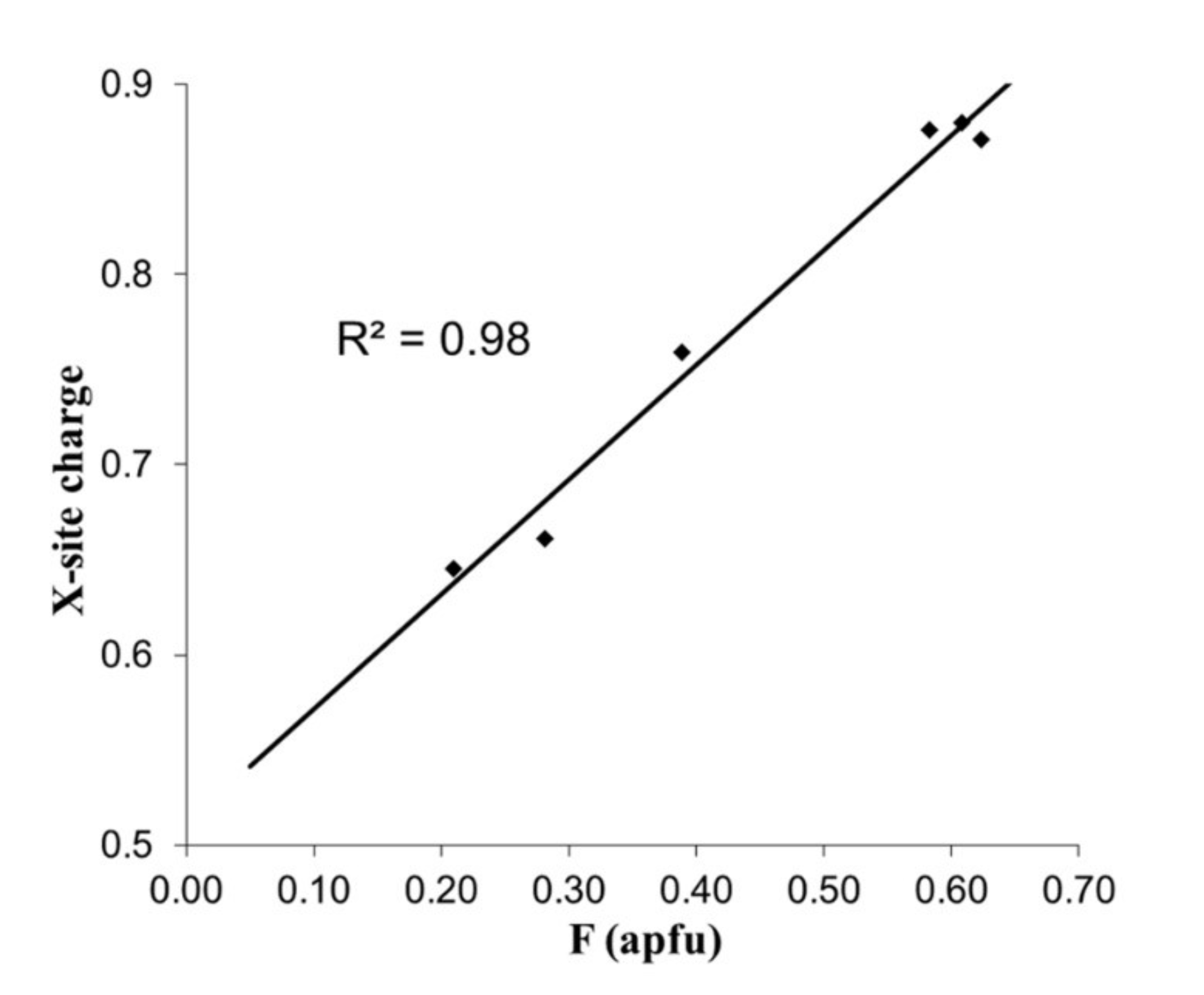
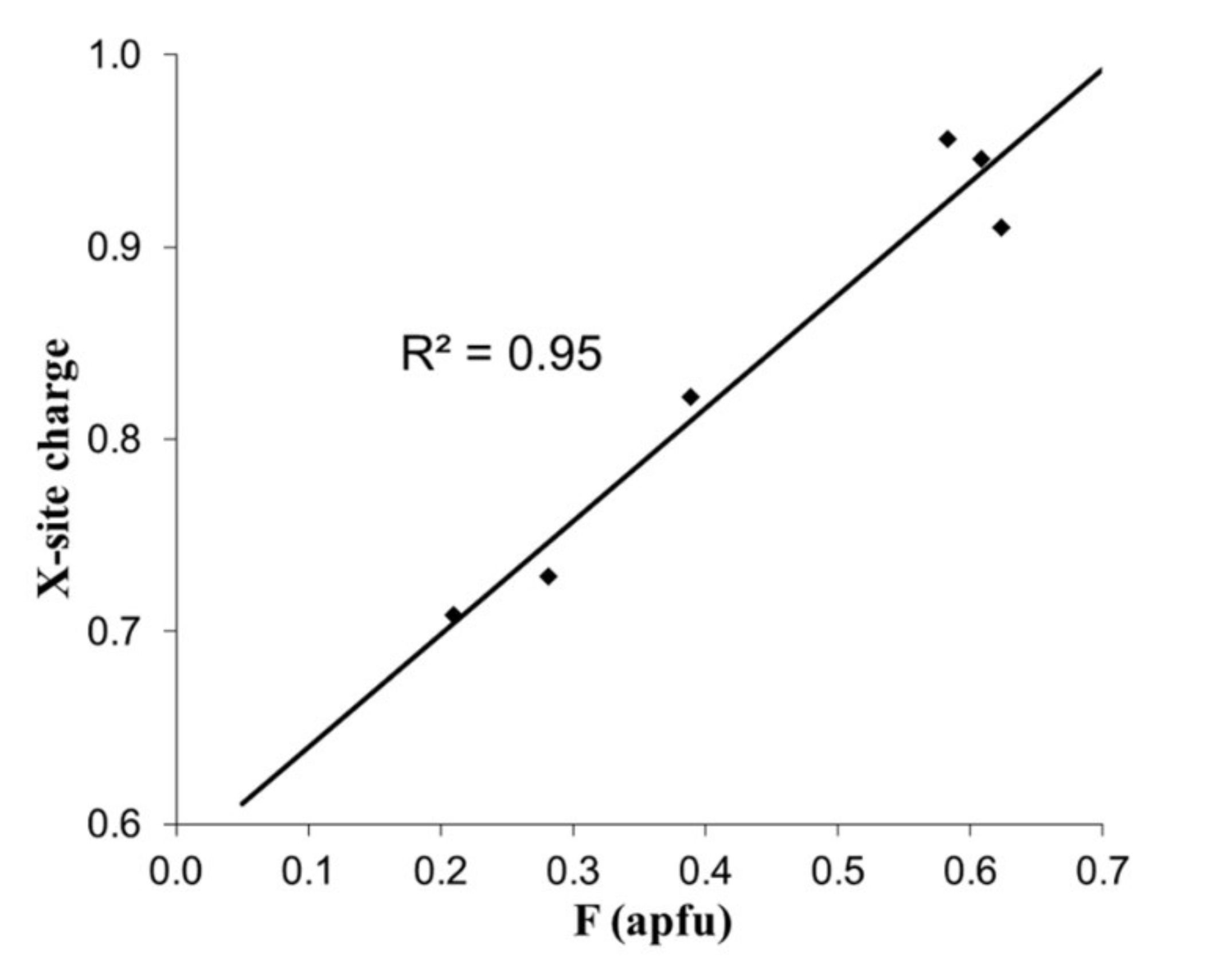

| Element | Bond Length [Å] | |
|---|---|---|
| Octahedral Sites | X Site | |
| Bi3+ | 2.3376 | 2.4954 |
| Bi5+ | 2.1389 | 2.2966 |
| Pb1+ | 2.8240 | 3.0032 |
| Pb2+ | 2.5176 | 2.6968 |
| Pb3+ | 2.3384 | 2.5176 |
| Pb4+ | 2.1695 | 2.2831 |
| “Goldilocks Zone” | ||
| +10% | 2.3335 | 2.9612 |
| −10% | 1.8274 | 2.4228 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ertl, A.; Bačík, P. Considerations About Bi and Pb in the Crystal Structure of Cu-Bearing Tourmaline. Minerals 2020, 10, 706. https://doi.org/10.3390/min10080706
Ertl A, Bačík P. Considerations About Bi and Pb in the Crystal Structure of Cu-Bearing Tourmaline. Minerals. 2020; 10(8):706. https://doi.org/10.3390/min10080706
Chicago/Turabian StyleErtl, Andreas, and Peter Bačík. 2020. "Considerations About Bi and Pb in the Crystal Structure of Cu-Bearing Tourmaline" Minerals 10, no. 8: 706. https://doi.org/10.3390/min10080706
APA StyleErtl, A., & Bačík, P. (2020). Considerations About Bi and Pb in the Crystal Structure of Cu-Bearing Tourmaline. Minerals, 10(8), 706. https://doi.org/10.3390/min10080706






